Abstract
It is well established that dendritic cells and macrophages play a role in antigen presentation to B and T cells and in shaping B and T cell responses via cytokines they produce. We have previously reported that depletion of neutrophils improves the production of mucosal IgA after sublingual immunization with Bacillus anthracis edema toxin as adjuvant. These past studies also demonstrated that an inverse correlation exists between the number of neutrophils and production of IgA by B cells. Using specific inhibitors of elastase, we addressed whether the elastase activity of neutrophil could be the factor that interferes with production of IgA and possibly other immunoglobulin isotypes. We found that murine splenocytes and mesenteric lymph node cells cultured for 5 days in the presence of neutrophil elastase inhibitors secreted higher levels of IgG and IgA than cells cultured in the absence of inhibitor. The effect of the inhibitors was dose-dependent and was consistent with increased frequency of CD138+ cells expressing IgG or IgA. Finally, neutrophil elastase inhibitors increased transcription of mRNA for AID, IL-10, BAFF and APRIL, factors involved in B cell differentiation. These findings identify inhibitors of elastase as potential adjuvants for increasing production of antibodies.
Keywords: B cells, Elastase, neutrophil elastase inhibitor, immunoglobulin class switching, AID, IL-10, BAFF, APRIL
Introduction
A series of finely regulated events lead to maturation of B cells and their differentiation into plasma cells that secrete antibodies [1]. Thus, after antigen stimulation of IgM-bearing inactivated B cells, these cells undergo Ig class switch recombination (CSR) to produce antibodies of different isotypes but with the same antigen specificity than the IgM. The activation-induced cytidine deaminase (AID or AICD) plays a crucial role in CSR and somatic hypermutation [2]. Additional signals for immunoglobulin CSR are provided by the ligation of CD40L on B cells and other factors such as B cell-activating factor of the TNF family (BAFF), and a proliferation-inducing ligand (APRIL). Furthermore, an array of cytokines including IL-4, IL-13, IFN-γ, TGF-β, and IL-10 regulate the Ig class switching toward selected isotype and subclasses, while other provide survival and proliferation signals (IL-5, and IL-6), or enhance antibody affinity maturation in the germinal centers (i.e., IL-21) [3]. A number of cells contribute co-stimulatory and cytokines signals required for Ig CSR and production of antibodies by B cells. Macrophages and dendritic cells contribute via their expression of CD40 and secretion of BAFF, APRIL, as well as pro-inflammatory (i.e., IL-6, IFN-γ) and anti-inflammatory (i.e., TGF-β, IL-10) cytokines. Epithelial cells can produce BAFF and APRIL, as well as cytokines, including IL-6 and TGF-β. Cytokines produced by T helper cell, and innate lymphoid cells in mucosal tissues, play an important role in both Ig CSR and affinity maturation. Mast cells produce IL-6 and IL-10 and a mast cell activator compound (i.e., compound 48/80) was shown to promote IgA responses in vivo by stimulating the migration of dendritic cells (DC) into T cell area [4].
Neutrophils represent the largest number of myeloid cells in the blood stream and the major phagocytic cells that eliminate invading pathogens [5,]. We have reported an inverse relationship between IgA response and the early recruitment of neutrophils in sublingual tissues and cervical lymph nodes after sublingual immunization with Bacillus anthracis edema toxin as an adjuvant [6]. Neutrophils were also found to suppress IgA production in vitro via mechanisms independent of NF-κB pathway [6]. The primary (or azurophilic) granules of neutrophils contain defensins, myeloperoxidase, lysozymes, and three serine proteases: neutrophil elastase, cathepsin G and protease 3 [5, 7]. Neutrophil elastase (NE) is a cationic glycoprotein stored in readily active form in primary granules at concentrations exceeding millimolar range and thus, making it a major antimicrobial enzyme of neutrophils [8, 9].
We addressed whether the elastase activity of neutrophils could mediate their suppressive effect on IgA production. Here we show that inhibitors of NE activity stimulate production of IgG and IgA by spleen and mesenteric lymph node cells in vitro, which indicates that elastase-like serine proteases modulate Ig CSR by B cells and production of antibodies.
Results and discussion
Neutrophil elastase inhibitors stimulate production of IgG and IgA.
NE contributes to tissue destruction in inflammatory diseases including emphysema, glomerulonephritis, rheumatoid arthritis [10] and colitis [11]. NE also exacerbates lung pathologies via stimulation of goblet cell metaplasia and mucus production [12]. The latter effect of NE was shown to be associated with stimulation of cytokine responses (i.e., KC and IL-5) and recruitment of lymphocytes and eosinophils in the airways [12]. Another study has shown that NE regulates lung inflammatory responses after infection with P. aeruginosa, and that purified NE stimulates IL-6, TNF-α and MIP-2 responses by murine macrophages [13]. The effects of NE extend beyond regulation of cytokine responses, because uptake of NE by breast cancer cells upregulates their MHC class I molecule expression and enhances the presentation of tumor-associated antigen [14].
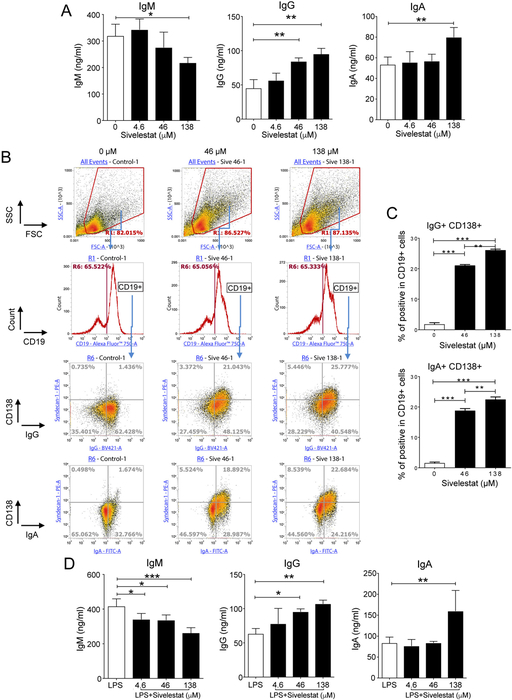
To address whether NE regulates immunoglobulin production, spleen cells were cultures for 5 days in the presence of doses of the Sivelestat (ONO-5046), a potent and specific inhibitor of neutrophil elastase [15]. Analysis of immunoglobulin isotypes in culture supernatants showed that Sivelestat reduced secretion of IgM in a dose-dependent manner (Figure 1A). This effect more likely reflected a stimulation of immunoglobulin class switching because it correlated with a dose-dependent increase of IgG and IgA secretion (Figure 1A). In order to elucidate how the inhibitor stimulated secretion of immunoglobulins, splenocytes were analyzed by flow cytometry after 2 days of culture. The presence of Sivelestat did not affect the frequency of myeloid cells (CD11b+) Figure S1A), T cell subsets (Figure S1B) or B cells (CD19+) (Figure 1B). Interestingly, this inhibitor increased the frequency of CD138+ among CD19+ cells (Figures 1B, S1C, and S1D). A significant number of CD138+CD19+ cells induced by Sivelestat expressed surface IgG and a low but significant percentage of CD138+CD19+ cells expressed surface IgA (Figures S1D). Intracellular cellular staining of immunoglobulin isotypes confirmed induction of CD138+CD19+IgG+ and CD138+CD19+IgA+ cells by Sivelestat (Figures 1B and 1C). Finally, addition of Sivelestat also increased secretion of IgG and IgA by splenocytes cultured in the presence of LPS (Figure 1D).
Figure 1. The neutrophil elastase inhibitor Sivelestat stimulates immunoglobulin class switching.
Spleen cells were cultured for 5 days (A and D) or 2 days (B and C) in the presence of increasing doses of Sivelestat. (A) IgM, IgG and IgA secreted in culture supernanants were measured by ELISA. (B and C) Flow cytometry analysis. Cells were stained extracellularly with anti-CD19 and anti-CD138 and intracellularly with anti-IgG and anti-IgA Abs. (B) Gating strategy for analysis of IgG+CD138+ and IgA+CD138+ cells among CD19+ cells. (C) Percentage of CD138+IgG+ and CD138+IgA+ among CD19+ B cells. (D) Spleen cells were cultured for 5 days in the presence of LPS (0.05 μg/ml) and increasing doses of Sivelestat. The amounts of IgM, IgG and IgA secreted culture supernanants were measured by ELISA. Results are expressed as means ± SD and are from at least 4 independent experiments. *P≤ 0.05, **P≤ 0.01, ***P≤ 0.05.
To confirm that the effect of Sivelestat on IgG and IgA production was a characteristic of elastase inhibitors, we next evaluated Alvelestat (AZD9668), a fluorinated inhibitor of NE. The presence of Alvelestat reduced IgM and increased IgG and IgA secretion by splenocytes in vitro (Figure 2A). This effect was dose-dependent and significant increase in IgA production was also seen when Alvelestat was added to spleen cells cultured in the presence of LPS (Figure 2B). We next analyzed the cellular changes induced by Alvelestat to support IgG and IgA production. The frequencies of CD11b+ (Figure S2A) or CD19+ spleen cells (Figure 2C), were not affected after 2 days culture in the presence of Alvelestat. This treatment however, increased the frequency of CD4+ T cells (Figure S2B). Of interest for antibody secretion, Alvelestat increased the frequency of CD138+ CD19+ cells, which expressed surface IgG or a low but significant percentage of surface IgA (Figures 2C, S2C and S2D). Production of IgG or IgA by Alvelestat-induced CD138+CD19+ was further confirmed by intracellular cellular staining of immunoglobulin isotypes (Figures 2C and 2D). We also established that the regulatory effects of NE inhibitors on B cell differentiation and IgG and IgA production were not limited to spleen cells. Thus, a similar pattern of responses was seen in mesenteric lymph node cells cultured in the presence of Alvelestat, which produced higher levels of IgG and IgA in culture supernatants (Figure 2E).
Figure 2. The fluorinated elastase inhibitor Alvelestat stimulates immunoglobulin class switching and production of IgG and IgA.
Spleen cells were cultured for 5 days (A and B) or 2 days (C and D) in the presence of increasing doses of Alvelestat. (A) IgM, IgG and IgA secreted in supernatants of cells cultured alone, or (B) IgA secreted in supernatants of cells cultured in the presence of LPS (0.05 μg/ml) were measured by ELISA. (C and D) Flow cytometry analysis. Cells were stained extracellularly with anti-CD19 and anti-CD138, and intracellularly with anti-IgG and anti-IgA Abs. (C) Gating strategy for analysis of IgG+CD138+ and IgA+CD138+ cells among CD19+ cells. (D) Percentage of CD138+IgG+ and CD138+IgA+ among CD19+ B cells. (E) IgM, IgG and IgA secreted in supernatants of mesenteric lymph node cells after 5-days culture in the presence of Alvelestat were measured by ELISA. Results are expressed as means ± SD and are from at least 4 independent experiments. *P≤ 0.05, **P≤ 0.01, ***P≤ 0.05.
These findings are in line with a previous report by others that depletion of neutrophils before systemic injection of vaccines increased the magnitude of antigen-specific CD4+ T cell responses, and the levels of antigen-specific serum IgG responses [16]. These authors showed that neither reactive oxygen species, nor nitric oxide, were involved in the suppressive effect of neutrophils. It should be noted that their study identified antigen presentation by macrophages and dendritic cells as the step limited by neutrophils and that neutrophils could mediate this effect in the absence physical interactions with dendritic cells or T cells [16], suggesting that factors secreted by neutrophils were responsible for the effect. Our new finding that inhibition of NE activity stimulates secretion of IgG and IgA indicates that elastase is the molecule (or one of the molecules) secreted by neutrophils that impairs antibody production, and perhaps adaptive immunity. This notion is consistent with the report that increased levels of NE in hematopoietic stem cells and progenitor cells in the bone marrow of mice fed high-fat diet was associated with reduced B lymphocytes production [17].
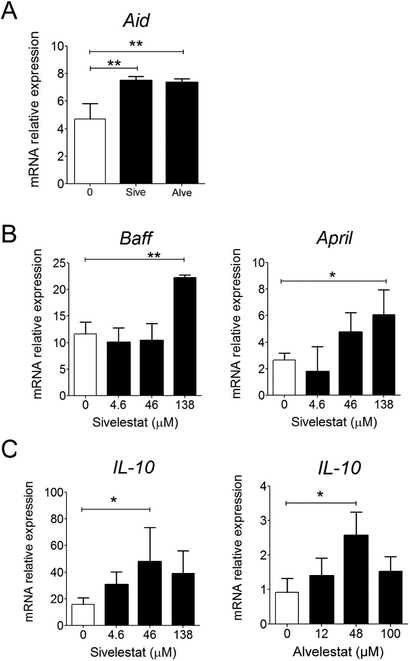
The neutrophil elastase inhibitors stimulate cytokine signals for Ig class switch recombination
To gain insight into mechanisms used by NE inhibitors to facilitate production of IgG and IgA, we analyzed the expression of AID, BAFF and APRIL, and IL-10, transcripts in spleen cells cultured 24 hrs in the presence of NE inhibitors. Both Alvelestat and Sivelestat were found to stimulate AID mRNA synthesis (Figure 3A). The NE inhibitors also induced expression of BAFF, APRIL (Figure 3B) and IL-10 mRNA in a dose-dependent manner (Figure 3C).
Figure 3. Mechanisms underlying the stimulatory effect of neutrophil elastase inhibitor on IgG and IgA production.
(A) Spleen cells were cultured for 24 hrs in the presence of Alvelestat (48 μM) or Sivelestat (48 μM) and AID mRNA responses were measured by quantitative real-time RT-PCR. (B and C) Spleen cells were cultured for 24 hrs in the presence of Alvelestat or Sivelestat at the indicated doses. BAFF, APRIL (B) and IL-10 mRNA responses were measured by quantitative real-time RT-PCR. Results are expressed as means ± SD and are from at least 3 independent experiments. *P≤ 0.05, **P≤ 0.01, ***P≤ 0.05 compared to negative control.
Neutrophil elastase and the related serine proteases cathepsin G and proteinase 3, were shown to regulate cytokine responses through activation or degradation of cytokines, cytokine receptors, or toll-like receptor [5, 18]. This is consistent with our finding that NE inhibitors stimulate IL-10 expression, but also BAFF and APRIL in cultures of splenocytes. IL-10 was reported to regulate expression of AID for induction of Ig CSR [19] and it is well-established that IL-10 facilitates Ig class switching for production of IgA [20]. Thus, our data suggest that NE inhibitors stimulate antibody production through stimulation of AID and inhibition of the activation/degradation of cytokines and cytokine receptors by elastase. NE was also shown to inhibit maturation and function of dendritic cells, including expression of the costimulatory molecules CD40, CD80 and CD86 [21]. Thus, the presence of neutrophil inhibitors may have promoted the maturation of dendritic cells and their expression BAFF and APRIL and perhaps costimulatory molecules (i.e., CD40) for enhanced antibody production. Although Alvelestat and Sivelestat are specific NE inhibitors, one should consider that they might also inhibit related elastase-like enzymes. In this regard, activated human B cells were reported to express a trypsin-like serine protease on the cell surface [22].
Concluding remarks
It was previously shown that neutrophil peptide defensins enhance IgG, but not IgA responses against nasally co-administered vaccine antigens [23]. Here, we have shown that the serine protease activity of neutrophil elastase, and possibly other serine protease such as B cell elastase, also participate in the regulation of B cell biology and adaptive immunity. However, unlike neutrophil peptide defensins, NE negatively regulate differentiation of B lymphocytes into IgG and IgA secreting cells. We have previously reported that depletion of neutrophils enhances IgA responses to sublingual immunization with Bacillus anthracis edema toxin as adjuvant [6]. Our new finding show that inhibitors of serine proteases could represent an alternative to neutrophil depletion as a strategy for enhancing IgG and IgA responses.
Material and methods
Mice
C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and were used at 8–12 weeks of age. All experiments were in accordance with both National Institutes of Health and Institutional Animal Care and Use Committee guidelines to avoid pain and destress.
In vitro cultures
Single cell suspensions of spleen cells, mesenteric lymph nodes were resuspended (2×106 cells/ml) in RPMI 1640 supplemented with L-glutamine, HEPES, penicillin, streptomycine and 10 % heat-inactivated fetal calf serum. Cells were cultured for 1, 5, or 6 days at 37°C and 5% CO2 in the presence of various doses of the neutrophil elastase inhibitor Sivelestatt (ONO-5046, C20H22N2O7S) or Alvelestat (AZD9668, C25H22F3N5O4S) (Selleckchem, Houston, TX). In selected experiments, neutrophil elastase inhibitors were added to cells cultured in the presence of Salmonella typhimurium LPS (0.05 μg/ml, Sigma, Saint Louis, MO).
Real-time RT- PCR
For analysis of mRNA responses, cells were collected after 1 day culture and washed once in RPMI before addition of TRIzol (Invitrogen, Carlsbad, CA). RNA was isolated and cDNA was synthesized by using Superscript III (Invitrogen). Real-time PCR was performed as previously described [24] and data were expressed as relative mRNA expression = 2−ΔΔCt where ΔCt = Ctunknown – CtHKG, and normalized against two house-keeping genes (HKG): β-actin and HPRT1.
Flow cytometry analysis
Cell suspensions were collected after 2 days of culture and stained with combinations of the following Abs: APC anti-CD11b (eBioscience), AF700 anti-CD3e, AF750 anti CD4, PerCp-Cy5.5 anti-CD8a, BV421 anti-IgG, AF750 anti-CD19 (Biolegend), PE anti-CD138 (Syndecan-1, R&D Systems), FITC anti-IgA (Southern Biotech). For intracellular detection of IgG and IgA, golgi-stop was added to cell cultures 4 hours before harvest and extracellular staining with anti-CD19 and anti-CD138 Abs. Cells were then fixed and permeabilized before intracellular staining with anti-IgG and anti-IgA Abs. Stained cells were analyzed with an Attune NxT flow cytometer (Thermo Fisher Scientific, Waltham, MA).
Immunoglobulin isotype ELISA
The amount of immunoglobulin isotype secreted in culture supernatants after 6 days of culture was measured as previously described [25] by ELISA, using goat anti-murine IgM, IgG, and IgA antibodies (Southern Biotech, Birmingham, AL) and extrapolation against standards.
Statistical analysis
Results were expressed as mean ± one standard deviation. Statistical significance was determined by ANOVA. Results were considered significant at p < 0.05. Statistical tests were performed using GraphPad Prism 6 (GraphPad Software Inc, La Jolla, CA).
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants DK101323 and AI123661.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Shapiro-Shelef M, Calame K, Regulation of plasma-cell development, Nat Rev Immunol, 5 (2005) 230–242. [DOI] [PubMed] [Google Scholar]
- [2].Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T, Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme, Cell, 102 (2000) 553–563. [DOI] [PubMed] [Google Scholar]
- [3].Acosta-Rodriguez EV, Merino MC, Montes CL, Motran CC, Gruppi A, Cytokines and chemokines shaping the B-cell compartment, Cytokine Growth Factor Rev, 18 (2007) 73–83. [DOI] [PubMed] [Google Scholar]
- [4].McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN, Mast cell activators: a new class of highly effective vaccine adjuvants, Nat Med, 14 (2008) 536–541. [DOI] [PubMed] [Google Scholar]
- [5].Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A, Neutrophil function: from mechanisms to disease, Annu Rev Immunol, 30 (2012) 459–489. [DOI] [PubMed] [Google Scholar]
- [6].Jee J, Bonnegarde-Bernard A, Duverger A, Iwakura Y, Cormet-Boyaka E, Martin TL, Steiner HE, Bachman RC, Boyaka PN, Neutrophils negatively regulate induction of mucosal IgA responses after sublingual immunization, Mucosal Immunol, 8 (2015) 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Borregaard N, Sorensen OE, Theilgaard-Monch K, Neutrophil granules: a library of innate immunity proteins, Trends Immunol, 28 (2007) 340–345. [DOI] [PubMed] [Google Scholar]
- [8].Liou TG, Campbell EJ, Nonisotropic enzyme--inhibitor interactions: a novel nonoxidative mechanism for quantum proteolysis by human neutrophils, Biochemistry, 34 (1995) 16171–16177. [DOI] [PubMed] [Google Scholar]
- [9].Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW, Killing activity of neutrophils is mediated through activation of proteases by K+ flux, Nature, 416 (2002) 291–297. [DOI] [PubMed] [Google Scholar]
- [10].Dallegri F, Ottonello L, Tissue injury in neutrophilic inflammation, Inflamm Res, 46 (1997) 382–391. [DOI] [PubMed] [Google Scholar]
- [11].Morohoshi Y, Matsuoka K, Chinen H, Kamada N, Sato T, Hisamatsu T, Okamoto S, Inoue N, Takaishi H, Ogata H, Iwao Y, Hibi T, Inhibition of neutrophil elastase prevents the development of murine dextran sulfate sodium-induced colitis, J Gastroenterol, 41 (2006) 318–324. [DOI] [PubMed] [Google Scholar]
- [12].Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM, Neutrophil elastase induces mucus cell metaplasia in mouse lung, Am J Physiol Lung Cell Mol Physiol, 287 (2004) L1293–1302. [DOI] [PubMed] [Google Scholar]
- [13].Benabid R, Wartelle J, Malleret L, Guyot N, Gangloff S, Lebargy F, Belaaouaj A, Neutrophil elastase modulates cytokine expression: contribution to host defense against Pseudomonas aeruginosa-induced pneumonia, J Biol Chem, 287 (2012) 34883–34894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chawla A, Alatrash G, Philips AV, Qiao N, Sukhumalchandra P, Kerros C, Diaconu I, Gall V, Neal S, Peters HL, Clise-Dwyer K, Molldrem JJ, Mittendorf EA, Neutrophil elastase enhances antigen presentation by upregulating human leukocyte antigen class I expression on tumor cells, Cancer Immunol Immunother, 65 (2016) 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kawabata K, Suzuki M, Sugitani M, Imaki K, Toda M, Miyamoto T, ONO-5046, a novel inhibitor of human neutrophil elastase, Biochem Biophys Res Commun, 177 (1991) 814–820. [DOI] [PubMed] [Google Scholar]
- [16].Yang CW, Strong BS, Miller MJ, Unanue ER, Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants, J Immunol, 185 (2010) 2927–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang JY, Zhou QL, Huang CH, Song Y, Sharma AG, Liao Z, Zhu K, Massidda MW, Jamieson RR, Zhang JY, Tenen DG, Jiang ZY, Neutrophil Elastase Regulates Emergency Myelopoiesis Preceding Systemic Inflammation in Diet-induced Obesity, J Biol Chem, 292 (2017) 4770–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Meyer-Hoffert U, Wiedow O , Neutrophil serine proteases: mediators of innate immune responses, Curr Opin Hematol, 18 (2011) 19–24. [DOI] [PubMed] [Google Scholar]
- [19].Fairfax KA, Gantier MP, Mackay F, Williams BR, McCoy CE, IL-10 regulates Aicda expression through miR-155, J Leukoc Biol, 97 (2015) 71–78. [DOI] [PubMed] [Google Scholar]
- [20].Pabst O, New concepts in the generation and functions of IgA, Nat Rev Immunol, 12 (2012) 821–832. [DOI] [PubMed] [Google Scholar]
- [21].Roghanian A, Drost EM, MacNee W, Howie SE, Sallenave JM, Inflammatory lung secretions inhibit dendritic cell maturation and function via neutrophil elastase, Am J Respir Crit Care Med, 174 (2006) 1189–1198. [DOI] [PubMed] [Google Scholar]
- [22].Biro A, Sarmay G, Rozsnyay Z, Klein E, Gergely J, A trypsin-like serine protease activity on activated human B cells and various B cell lines, Eur J Immunol, 22 (1992) 2547–2553. [DOI] [PubMed] [Google Scholar]
- [23].Lillard JW Jr., Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR, Mechanisms for induction of acquired host immunity by neutrophil peptide defensins, Proc Natl Acad Sci U S A, 96 (1999) 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Duverger A, Carre JM, Jee J, Leppla SH, Cormet-Boyaka E, Tang WJ, Tome D, Boyaka PN, Contributions of edema factor and protective antigen to the induction of protective immunity by Bacillus anthracis edema toxin as an intranasal adjuvant, J Immunol, 185 (2010) 5943–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Duverger A, Jackson RJ, van Ginkel FW, Fischer R, Tafaro A, Leppla SH, Fujihashi K, Kiyono H, McGhee JR, Boyaka PN, Bacillus anthracis edema toxin acts as an adjuvant for mucosal immune responses to nasally administered vaccine antigens, J Immunol, 176 (2006) 1776–1783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.