Abstract
Background:
Lung ventilation defects identified by hyperpolarized 3He lung magnetic resonance imaging (MRI) are prevalent in patients with asthma but the clinical importance of ventilation defects is poorly understood.
Objectives:
To correlate the lung defect volume quantified by 3He MRI with clinical features in children with mild and severe asthma.
Methods:
31 children with asthma (median age 10, range 3–17 years) underwent detailed characterization and hyperpolarized 3He lung MRI. Quantification of the 3He signal defined ventilation defect, hypoventilated, ventilated, and well ventilated volumes.
Results:
The ventilation defect to total lung volume fraction ranged from 0.1% to 11.6%. Children with ventilation defect % in the upper tercile were more likely to have severe asthma than children in the lower terciles (p=0.005). The ventilation defect % correlated (p < 0.05 for all) positively with the inhaled corticosteroid (ICS) dose, total number of controller medications, total blood eosinophils, and negatively with the asthma control test score, FEV1 (% predicted), FEV1/FVC (% predicted), and FEF25–75 (% predicted).
Conclusion:
The lung defect volume % measured by hyperpolarized 3He MRI correlates with several clinical features of asthma including severity, symptom score, medication requirement, airway physiology, and atopic markers.
Keywords: Hyperpolarized 3He MRI, ventilation defects, ventilation heterogeneity, severe asthma, childhood asthma
Short Summary:
Magnetic resonance imaging with inhaled hyperpolarized 3He gas can identify and measure lung volume compartments in children with asthma. The magnitude of the non-ventilated compartment highly correlated with clinical features including lung function and asthma control.
Introduction
Lung ventilation defects are regions of absent 3He gas signal identified by hyperpolarized noble gas MRI in healthy volunteers and patients with asthma (1–3). Proposed mechanisms of ventilation defects vary from obstruction of proximal airways to closure of acinar lung structures (4–6). Ventilation defects form more often in the lung bases in asthma, and correspond spatially to regions of air trapping mapped by multi-detector computed tomography (CT) (7). In repeat MRI studies of defects in adults with asthma, approximately two-thirds of ventilation defects do not change in size or location over time (8). Ventilation defects increase in size following methacholine-induced bronchospasm (9), and decrease in size but do not completely resolve following inhalation of the bronchodilator albuterol (2, 6).
Lung ventilation defects occur with increased prevalence in adults and children with asthma (10–11). Whereas much is known about the spatio-temporal features of lung ventilation defects in asthma, the clinical implications of ventilation defects are not well understood. In an original report the number of defects per image slice correlated with asthma severity, and the degree of airflow limitation (10). In a recent report the ventilation defect volume % correlated significantly with older age, lower FEV1/FVC ratio, higher expired nitric oxide, greater methacholine responsiveness, and airway wall thickness estimated by chest CT (12). However these studies did not include children and important clinical features of asthma including symptom control, treatment, and markers of inflammation were not evaluated. This is an important gap in so far as even children with mild asthma and normal spirometry may have ventilation heterogeneity and closure of peripheral airways (13). Young children may be at increased risk for the formation of ventilation defects due to the small size of their airways, and in children before two years of age, the relative instability of the chest wall (14).
Advances in rapid image sequencing methods have facilitated the acquisition of high-quality hyperpolarized gas MR images in pre-school children (15). Furthermore improvements in image processing and signal intensity analysis can accurately measure lung volume compartments (16). We applied these innovations in a sample of children with asthma to study whether the lung defect volume % as measured by hyperpolarized lung MRI correlates with a range of clinical features. We hypothesized the ventilation defect volume % would be higher in children with severe asthma, and correlate not only with the degree of airflow limitation, but indicators of asthma control, treatment, and inflammation.
Methods
The study sample included children with a physician diagnosis of asthma referred for evaluation to a regional medical center. The study was approved by the Institutional Review Board of the University of Virginia Health System (IRB #157200). Hyperpolarized 3He gas was administered under an IND # 57866 held by Dr. E. de Lange. The parents of enrollees provided informed consent and children of appropriate age provided assent.
Asthma Confirmation and Characterization Procedures
To fit inclusion criteria enrollees were 3–17 years of age, mature enough to cooperate with the MRI procedures, and had current asthma based on physician diagnosis, symptoms of reversible airflow obstruction, and current treatment (17). Enrollees 5 years of age or older also had a 12% or greater improvement in FEV1 post-bronchodilator from baseline, or a methacholine PC20 < 16 mg/ml. Severe asthma was defined according to American Thoracic Society (ATS) (18) and European Respiratory Society (ERS) Task Force (19) guidelines as either controlled or un-controlled despite six months of treatment by an asthma specialty physician with high-dose inhaled or systemic corticosteroids and a second controller. For enrollees less than 6 years of age severe asthma was defined by treatment with high-dose corticosteroids according to the ATS/ERS high-dose definitions for children (19). Lung function was measured according to ATS guidelines for precision and reproducibility and expressed according to standard population reference values (20). Criteria for poor asthma control included an asthma control test (ACT) or children’s asthma control test (cACT) value less than 20 units (21). Exclusion criteria included a diagnosis of significant congenital or medical disorders apart from asthma.
3He MRI of the Chest
3He MR imaging studies were performed within a few weeks of enrollment according to a protocol developed for children. Inhaled beta agonists were withheld for four hours before the MRI procedure. Enrollees were evaluated on the day of imaging for clinical stability by a research nurse coordinator. Criteria for exclusion were exposure to oral corticosteroids in the past four weeks before imaging, conditions for which an MRI procedure was contraindicated, the presence of any non-MRI compatible metallic materials in the body, chest circumference larger than the MRI coil, and self-reported pregnancy. Children underwent the imaging protocol without sedation using a 1.5-T commercial whole-body scanner (TIM Avanto, Siemens Medical Solutions) and either a flexible, vest-shaped chest radio frequency (RF) coil (Clinical MR Solutions, Brookfield, WI) or a fixed geometry RF coil (Rapid Biomedical, Rimpar, Germany) tuned to the 3He resonant frequency. Details of the 3He MRI image acquisition procedures are found in the On-line Data Supplement.
Quantification of Lung Volume Compartments
The lung images were subdivided into regions defined by the level of 3He ventilation using an automated scoring platform (23, 24). To define the outline of the lungs, six investigators individually traced whole lung masks for each image which were then combined into a single mask to help minimize rater bias (25). To prepare the images for automated scoring, bias correction was next performed to remove artifacts in the images due to imperfections in the RF coil homogeneity (16). The lung images then underwent an automated computer scoring in which the relative signal intensity in each voxel was used to classify the voxel into one of 4 classes: ventilation defect (black areas), hypoventilated (gray areas), ventilated (white areas), and well-ventilated (bright white areas) (26). The sum of all of the voxels in each class times the voxel volume is the volume of the lung in each class. The sum of the four class volumes equaled the total lung volume for any one participant, and each of the volumes was expressed as a ratio of its value to total lung volume. The co-investigator performing the image analysis (NT) was not aware of the clinical features and asthma severity of the participants.
Data Analysis
The ventilation volumes and clinical variables were analyzed for normal distribution by frequency histogram plots. Results were stratified for comparison by the tercile distribution (lower, middle, and upper) of the ventilation defect volume to total lung volume ratio. Differences in non-normally distributed measurements across the terciles were tested by the Kruskal Wallis test and for normally distributed measurements by ANOVA with adjustment for more than two groups by the post-hoc Bonferroni adjustment. Non-scaled variables were tested with contingency tables and Pearson’s chi square statistic. Spearman’s rho statistic was used to test correlations among non-normally distributed data.
Results
Thirty-one children (median age 10, minimum 3, maximum 17 years) were enrolled. The features of enrollees (Table I) were similar to those not enrolled (results not shown). The sample included 12/31 (39%) pre-school children (3–6 years of age) and 19/31 (61%) school-age children (7–17 years of age). The free breathing protocol allowed safe acquisition with no adverse events of high-quality images even in pre-school children with severe asthma (Figure 1). Ventilation defects were classified according to three general patterns, no visible ventilation defects (7/31, 23%), diffuse, small defects (9/31, 29%), and large focal defects (15/31, 48%) (Figure 1, On-line Data Supplement). Large ventilation defects were found only in children with severe asthma (n = 15/20, 75%) and none (0/11) in mild to moderate asthma (p < 0.001).
Table I.
Features of the Study Sample Stratified by the Ventilation Defect /Total Lung Volume Tercile Distribution
| Variable | Sample | Lower Tercile (0.13 – 0.81%) | Middle Tercile (1.0–2.1%) | Upper Tercile (2.5–11.6%) | p |
|---|---|---|---|---|---|
| n | 31 | 10 | 11 | 10 | |
| Enrollment Age (yr) | 10 (3–17) | 9 (4–16) | 11 (3–17) | 9 (3–17) | 0.97** |
| Male sex n (%) | 21 (68%) | 7 (70%) | 7 (64%) | 7 (70%) | 0.94 |
| African American n (%) | 10 (32%) | 2 (20%) | 4 (36%) | 4 (40%) | 0.48 |
| Body Mass Index (kg/m2) | 18.3 (14.7–33.6) | 18.5 (17.1–29.0) | 17.5 (14.7–33.6) | 19.6 (14.7–31.5) | 0.77 |
| Birth Gestational Age (mo) | 39 (24–39) | 38 (28–39) | 38 (27–39) | 39 (24–39) | 0.66 |
| Age at First Wheeze (mo) | 12 (2–108) | 12 (2–60) | 12 (2–24) | 8 (2–108) | 0.89 |
| Hospital Admissions for Asthma (n) | 1 (0–10) | 1 (0–6) | 1 (0–10) | 6 (1–6) | 0.16 |
| Any ICU Admission for Asthma n (%) | 8 (26%) | 2 (20%) | 2 (18%) | 4 (40%) | 0.46 |
| Any Past Pneumonia n (%) | 26 (84%) | 8 (80%) | 10 (91%) | 8 (80%) | 0.73 |
| Family History Asthma n (%) | 24 (77%) | 9 (90%) | 8 (73%) | 7 (70%) | 0.51 |
| Daily ICS Dose (mcg fluticasone equiv.) | 800 (0–2000) | 445 (0–920) | 460 (320 −2000) | 860 (80–1600) | 0.08 |
| High Dose ICS n (%) | 25 (81%) | 6 (60%) | 10 (91%) | 9 (90%) | 0.13 |
| Daily Prednisone n (%) | 7 (23%) | 0 (0%) | 2 (18%) | 5 (50%) | 0.03 |
| Daily LABA n (%) | 22 (71%) | 5 (50%) | 8 (73%) | 9 (90%) | 0.14 |
| Montelukast n (%) | 20 (64%) | 5 (50%) | 8 (73%) | 7 (70%) | 0.50 |
| # Daily Controllers | 3 (1–4) | 2 (1–4) | 3 (1–4) | 4 (1–4) | 0.05 |
| cACT/ACT score | 12 (8–25) | 14 (11–25) | 11 (8–23) | 12 (9–14) | 0.21 |
| Asthma Event Score | 0.83 (0.0–3.0) | 0.83 (0.25–1.5) | 0.66 (0.0–2.8) | 0.87 (0.0–3.0) | 0.84 |
| Severe Asthma n (%) | 20 (64%) | 3 (30%) | 7 (64%) | 10 (100%) | 0.005 |
Scaled variables shown as the median (min-max values);
Kruskal Wallis test corrected for multiple group comparisons.
Figure 1.
Hyperpolarized 3He lung MRI in a 4 year old with severe asthma. This gray scale image illustrates the spectrum of signal intensities attained in a small child with the free breathing acquisition protocol. Note that with the first inhalation of hyperpolarized 3He, a large focal ventilation defect is visible in the right middle and right lower lobes (A.). With subsequent inhalations the defect persists, and there is delayed filling of a region in the left upper lobe (B.). The region with delayed filling eventually fills (C.) and during the washout phase (D.) demonstrates relatively “bright” signal intensity consistent with trapped 3He gas.
Ventilation Volume Compartments
Automated analysis of the corrected gray scale MRI defined four ventilation volumes labeled as red/green/blue/yellow regions in the image sections (Figure 2). A composite image derived from the individual hand-scored images defined a “ground truth” image for each subject. The automated image corresponded to this “ground truth” image better than any individual hand-scored image. The four volumes were expressed according to the percentage of the total lung volume and adjusted for height (On-line Data Supplement, Table I). The ventilation defect volume % distributed asymmetrically to lower values (Figure 2, On-line Data Supplement).
Figure 2.
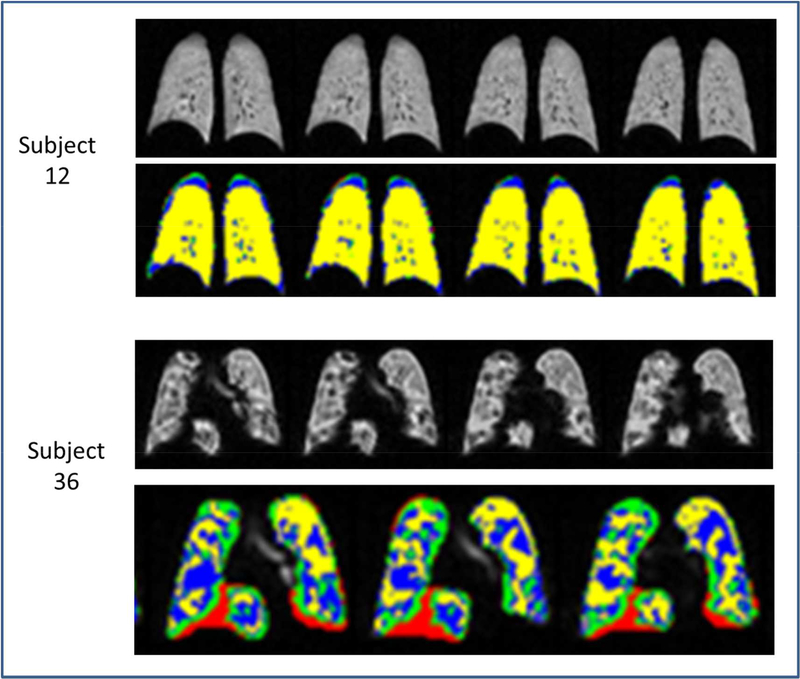
Coronal hyperpolarized 3He lung MRI slices in two children with asthma. The gray scale images are shown above the corresponding labeled images which have undergone automated analysis of the 3He signal intensity. The ventilation defect volume is labeled black on the gray scale images and red on the automated images, the hypoventilated volume is gray on the gray scale images and green on the automated images, the ventilated volume is white on the gray scale images and blue on the automated images, and the well-ventilated volume is bright white on the gray scale images and yellow on the automated images. Subject 12 has mild to moderate asthma and normal lung function. The gray scale images show primarily white to bright white regions and the automated images show mostly yellow regions. This subject had a relatively low defect volume to total lung volume ratio of 0.3% Subject 36 by contrast has severe asthma, with significant airflow obstruction at baseline. The MRI shows visible contrasts in both the gray scale and automated images. Note the relative abundance of red and green regions on the automated images compared to subject 12. This subject had a relatively high defect volume to total lung volume ratio of 8.6%.
To identify the clinical correlates of ventilation defects, characteristics of the study participants were stratified according to the tercile distribution of the defect volume % (Table I). Children with ventilation defect volume % in the upper tercile had more severe asthma, received more daily prednisone, and took more daily controller medications compared to children with ventilation defect volume % in the middle or lower terciles. Children with ventilation defect volume % in the upper and middle terciles had lower FEV1 %, FEV1/FVC %, and FEF25–75 % than participants in the lower tercile (Table II).
Table II.
Lung Function Stratified by the Ventilation Defect/Total Lung Volume Tercile Distribution
| Variable | Sample | Lower Tercile | Middle Tercile | Upper Tercile | p |
|---|---|---|---|---|---|
| n | 31 | 10 | 11 | 10 | |
| SaO2 in Room Air (%) | 98 ± 1 | 97 ± 1 | 98 ± 2 | 98 ± 32 | 0.83 |
| n | 16 | 6 | 6 | 4 | |
| FEV1 (l) | 1.87 ± 0.68 | 2.32 ± 0.64 | 1.78 ± 0.61 | 1.33 ± 0.49 | 0.07 |
| FEV1 (% pred.) | 79 ± 24 | 102 ± 9 | 64 ± 6 | 65 ± 32 | 0.004** |
| FVC (l) | 2.75 ± 1.29 | 2.88 ± 1.04 | 3.08 ± 1.78 | 2.08 ± 0.61 | 0.49 |
| FVC (% pred.) | 99 ± 25 | 109 ± 5 | 94 ± 24 | 91 ± 41 | 0.47 |
| FEV1/FVC | 0.70 ± 0.13 | 0.82 ± 0.08 | 0.63 ± 0.12 | 0.63 ± 0.07 | 0.008** |
| FEV1/FVC (% pred.) | 78 ± 14 | 91 ± 9 | 70 ± 13 | 70 ± 9 | 0.006** |
| FEF25–75 (l/sec) | 1.56 ± 0.94 | 2.49 ± 0.79 | 1.13 ± 0.29 | 0.84 ± 0.47 | 0.004** |
| FEF25–75 (% pred.) | 56 ± 38 | 93 ± 37 | 32 ± 5 | 32 ± 19 | 0.007** |
Mean ± one standard deviation;
p < 0.05 lower tercile versus middle and upper terciles, ANOVA corrected for multiple comparisons.
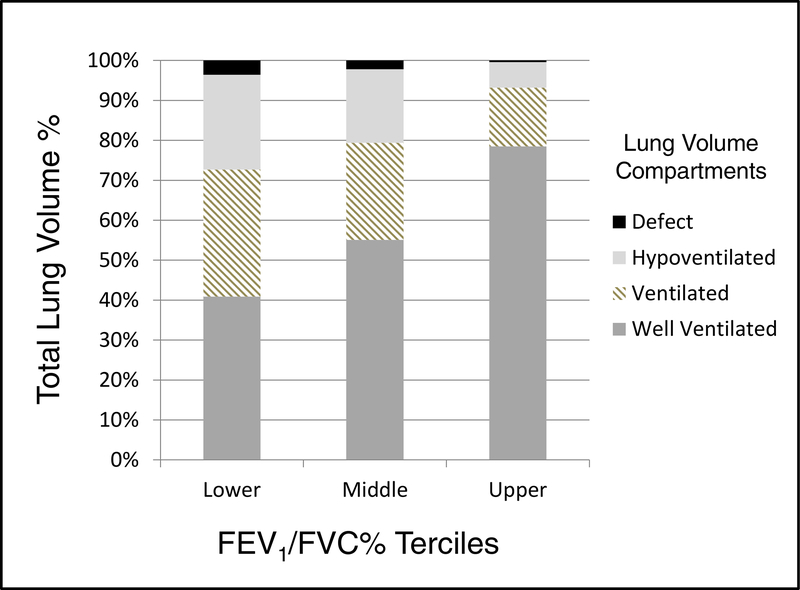
To further evaluate the effects of airflow limitation on distribution of ventilation, we compared lung volume compartments according to the tercile distribution of the FEV1/FVC ratio. Children with FEV1 /FVC % in the lower tercile (< 66% predicted) had significantly higher ventilation defect, hypoventilated, and ventilated volume ratios, but lower well-ventilated to total lung volume ratios versus children with FEV1/FVC % in the middle and upper terciles (p < 0.05 for all comparisons, Figure 3).
Figure 3.
Bar plot of 4 ventilation volume compartments as measured by automated analysis of the inhaled 3He MRI signal and stratified by the tercile distribution of the FEV1/FVC ratio % predicted in 31 children with asthma. The volume compartments are expressed as the median values of the volume compartments expressed as % of the total lung volume. Children with FEV1 /FVC % in the lower tercile (< 66% predicted) had significantly higher ventilation defect, hypoventilated, and ventilated volume ratios, but lower well-ventilated to total lung volume ratios versus children with FEV1/FVC % in the middle and upper terciles (p < 0.05 for all comparisons).
For markers of atopic inflammation the number of positive allergens and the peripheral blood eosinophil % were significantly higher in children whose ventilation defect volume % distributed to the upper tercile (Table III). However the ventilation defect volume % was no greater in children sensitized with one or more allergens versus those with no allergen sensitization and was no greater in children with sensitization to one or more foods versus those with no food sensitization (On-line Data Supplement, Table III).
Table III.
Markers of Atopic Inflammation Stratified by the Ventilation Defect/Total Lung Volume Tercile Distribution
| Variable | Sample | Lower Tercile | Middle Tercile | Upper Tercile | p |
|---|---|---|---|---|---|
| n | 27 | 8 | 9 | 10 | |
| Serum IgEα (IU/ ml) | 210 (1–3638) | 266 (24–3638) | 113 (1–594) | 637 (21–2047) | 0.07 |
| Number of Positiveα Allergens (#) | 2 (0–16) | 1 (0–8) | 1 (0–8) | 8 (0–16) | 0.016* |
| Peripheral Blood Eosinophilα (%) | 4 (0–18) | 1 (0–6) | 6 (2–14) | 8 (0–18) | 0.03** |
| Total Blood Eosinophilα Count (cells/μL) | 0.42 (0.0–2.05) | 0.21 (0.0–0.47) | 0.74 (0.17–2.05) | 0.61 (0.0–1.82) | 0.08 |
| Self-reported wheeze post allergen (%) | 21 (70%) | 6 (60%) | 6 (60%) | 9 (90%) | 0.240 |
| Atopic n (%) | 22 (71%) | 7 (70%) | 6 (55%) | 9 (90%) | 0.20 |
,median (min-max values);
p < 0.05 lower tercile versus middle and upper terciles, Kruskal Wallis corrected for multiple comparisons;
p < 0.05 lower tercile versus upper tercile, Kruskal Wallis corrected for multiple comparisons.
Correlations between Ventilation Defects and Clinical Features of Asthma
There were significant correlations (Table IV, Figure 4) between the ventilation defect volume % and the daily ICS dose, the number of asthma controllers, and the ACT/cACT scores. Lung function variables with significant correlations with the ventilation defect % included FEV1 %, FEV1/FVC % and FEF25–75 %, but not FVC%. Markers of inflammation which correlated significantly with the ventilation defect % included the peripheral blood eosinophil % and the total number of peripheral eosinophils.
Table IV.
Correlation of the Ventilation Defect/Total Lung Volume Ratio with Clinical Features of Asthma in Children
| Variable | Spearman’s rho | p |
|---|---|---|
| Age | 0.09 | 0.61 |
| Body Mass Index | −0.01 | 0.99 |
| Birth Gestational Age | 0.19 | 0.31 |
| Age at First Wheeze | −0.02 | 0.91 |
| Daily ICS dose | 0.51 | 0.003 |
| # Daily Controllers | 0.52 | 0.003 |
| Daily Prednisone Dose | 0.54 | 0.11 |
| cACT/ACT scores | −0.45 | 0.05 |
| Asthma Event Score | 0.09 | 0.63 |
| SaO2 | −0.18 | 0.35 |
| FEV1 | −0.71 | 0.002 |
| FVC | −0.47 | 0.08 |
| FEV1/FVC | −0.76 | 0.001 |
| FEF25–75 | −0.90 | 0.0001 |
| Serum IgE | 0.23 | 0.24 |
| # Positive Allergens | 0.33 | 0.10 |
| Peripheral Eosinophils % | 0.45 | 0.02 |
| Total Blood Eosinophils | 0.39 | 0.04 |
Figure 4.
Scatter plots showing the correlation between the ventilation defect volume ratio with selected variables in children with asthma. There was a strong inverse correlation between ventilation defect volume ratio and the FEV1/FVC % (A.) and the FEF25–75 % predicted (B.) The ventilation defect volume ratio also correlated significantly with other clinical markers including the percentage of blood eosinophils (C) and the asthma control test (D).
Clinical Features and Ventilation Volume Ratios by Asthma Severity
Children with severe asthma (n=20) had significantly more hospitalizations, more intensive care admissions, received more daily controller therapies, and more maintenance prednisone versus children with mild to moderate asthma (n=11) (On-line Data Supplement, Table II ). Children with severe asthma had lower FEV1%, FEV-1/FVC%, and FEF25–75% but no difference in FVC% versus children with mild to moderate asthma. There was no difference in total lung volume or lung volume adjusted by height in children with severe versus mild to moderate asthma. However children with severe asthma had significantly higher ventilation defect, higher hypoventilated, higher ventilated, but lower well ventilated to total lung volume ratios compared to those volumes in children with mild to moderate asthma (On-line Data Supplement, Figure 3).
Discussion
We report that the magnitude of the lung ventilation defect % in children correlates significantly with asthma severity, symptom control scores, treatment, lung function, and biomarkers of atopy. Furthermore this is the first report of lung volume compartments measured by automated analysis of the inhaled hyperpolarized 3He signal in children with asthma. We learned that the relative contribution of the ventilation defect and hypoventilated volumes to the total lung volume, estimates of the degree of ventilation heterogeneity, were greatest in children with severe asthma, and both volumes increased in children with airflow obstruction as indicated by low FEV1/FVC ratios. Thus precise measurement of ventilation volumes by hyperpolarized noble gas MRI not only resolved the spatial and temporal characteristics of gas distribution in children with asthma, but was informative in regards to asthma severity and its clinical features.
Hyperpolarized gas imaging has significant promise as a safe and innovative clinical tool to non-invasively assess the regional distribution of ventilation in adults and children with asthma. This is possible through recent advances in automated signal analysis as described in this and other reports (16), wherein the intensity of the inhaled 3He signal in the airspaces is quantified to precisely map regional volume compartments and oxygen tension (27). In the past simple computer-assisted systems (28–29) or hand counts of visual defects were used to estimate the ventilation defect volume (2). In this study we compared hand-scoring of visible defect regions by manual segmentation to an automated analysis platform and found that the automated analysis yielded volume estimates which corresponded best to the “ground-truth” or gold-standard composite image. These automated scoring methods are important in so far as they could be used to facilitate rapid conversion of complex hyperpolarized gas signal data into volume compartments for clinical applications.
We found differences in the pattern and clinical correlates of ventilation defects in children with asthma compared to those reported previously in adults. Although the fraction of children with asthma with visible defects was 77% versus 65% in adults, the ventilation defect % was comparable in the two samples, 2.3% in children versus a range of 1.3 to 4.3% in adults (12). Svenningsen et al found that adults with visible defects were significantly older than those with no defects (12). However we found no significant correlation between age and the ventilation defect % in children with asthma. In our study asthma severity highly informed the prevalence of defects in children, but severe asthma was more prevalent in school-age versus pre-school enrollees. Our study was not powered to determine the effects of age on ventilation defect % in children; hence this question is not resolved. De Lange et al reported a significant negative correlation between the numbers of lung defects per image slice and the FEV1-/FVC and the FEF25–75 in adults (10). Svenningsen et al found no difference in the FEV-1% in asthmatics with and without apparent ventilation defects, but did report a strong negative correlation with ventilation defect % and FEV1 % (12). In contrast we found the relationship between defect % and asthma features was strongest for the FEF25–75 -%. This result and those of Svenningsen (12) and De Lange (10) supports the evolving evidence pointing to pathological changes in the lung periphery as contributing in an important way to the maldistribution of ventilation and pathobiology of asthma (30).
The present study has design and sample size disadvantages which limit broad application of the findings to individuals with asthma. Foremost the study sample features are enriched in children with severe, poorly-controlled asthma. The burden of inhaled corticosteroid exposure was high and asthma control poor even in the mild to moderate group, although mild to moderate enrollees did have relatively normal lung function compared to severe participants. As a result we may over-estimate the magnitude of ventilation defect% in the general population of children with asthma. This is the likely reason our findings are different from those of Cadman et al (11) who studied older children with mild to moderate asthma and less airflow limitation based on a relatively high FEV1/FVC ratio in their sample. A second limitation of the study is that there is no control group for quantification of ventilation defects in healthy children. Apparent ventilation defects were not identified in healthy adults in the report of Svenningsen et al (12) but in reports from de Lange et al small peripheral ventilation defects were commonly seen in healthy adult volunteers (10). The prevalence and volume of ventilation defects identified by 3He MRI in healthy children have not been widely reported. A third limitation of the study is that it is a cross-sectional study and thus it is not possible to infer causality between the defects and clinical features or vice versa. This will require an interventional study designed to reverse ventilation defects to see which clinical features of asthma if any improve. The fourth disadvantage is that many of the images were obtained in younger children who were tidally breathing and who were not able to perform a standard volume maneuver. For this reason we did not attempt to measure the helium diffusion path constant as has been reported in older children with asthma by Cadman et al (11).
The feasibility of hyperpolarized 3He MRI as a tool in the clinical management of patients with asthma has promise but will require further clinical studies. This will depend in part on advances in automated signal analysis as we have reported so that quantification of lung volume compartments will be accessible to practitioners. MRI has significant advantages over chest CT in so far as it does not involve exposure to ionizing radiation, a highly important consideration in children. Hyperpolarized noble gas lung MRI may have particular application in the evaluation of the peripheral airways in asthma (31). Spirometry, forced oscillometry, and nitrogen clearance are available methods to assess the peripheral lung, but these methods address global lung function and are less sensitive to regional patterns of disease. The advent of hyperpolarized gas methods has changed the way we think about lung function in patients with asthma, from an understanding of asthma as a uniform disorder of airways obstruction to a disorder more akin to regional ileitis with patchy involvement. Inhaled corticosteroids, especially those with coarse particle features, likely do not penetrate ventilation defect regions in patients with asthma (32). Inhaled hyperpolarized 3He lung MRI is ideally suited to evaluate regional ventilation changes after treatment, as has been shown recently for bronchial thermoplasty (33). By assessment of the 3He diffusion path, lung MRI has also been used to assess lung development and alveolar growth in ex-premature children (34).
Supplementary Material
Acknowledgments
The authors wish to acknowledge the efforts of Kristin Wavell and Donna Wolf, PhD, who participated in the validation of the manual segmentation volume estimates with the ANTs-derived volumes. Denise Thompson-Batt, RRT assisted in the recruiting and enrollment of participants in the study.
This work supported by the NIH/NHLBI Severe Asthma Research Program (SARP), 1U01HL109250-1(W. Gerald Teague, MD), The Ivy Foundation (W. Gerald Teague, MD), and the Hartwell Foundation (Talissa A. Altes, MD).
Abbreviations/Acronyms
- 3He
hyperpolarized 3- helium gas
- ACT
asthma control test
- cACT
children’s asthma control test
- CT
computed tomography
- Expired NO
expired nitric oxide
- FEF25–75
Forced expired flow rate from 25 to 75% expired volume
- FEV1
Forced expired volume in one second
- FVC
forced expired vital capacity
- MRI
magnetic resonance imaging
- PC20
provocative concentration of methacholine with 20% decline in FEV1
- RF
radio frequency
- T
Tesla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article has an online data supplement, which is accessible from this issue’s table of contents.
The study is listed on clinicaltrials.gov.
References
- 1.de Lange EE, Mugler JP 3rd, Brookeman JR, Knight-Scott J, Truwitt JD, Teates CD, Daniel TM, Bogorad PL, Cates GD. Lung air spaces: MR imaging evaluation with hyperpolarized 3He gas. Radiology 1999; 210: 851–7. [DOI] [PubMed] [Google Scholar]
- 2.Altes T, Powers P, Knight-Scott J, Rakes G, Platts-Mills T, Lange E, Alford BA, Mugler JP, Brookeman JR. Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging 2001; 13: 378–384. [DOI] [PubMed] [Google Scholar]
- 3.Castro M, Fain SB, Hoffman EA, Gierada D, Erzurum SC, Wenzel S. Lung imaging in asthma: The picture is clearer. J Allergy Clin Immunol 2011; 128: 467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irvin CG, Bates JHT. Physiologic dysfunction of the asthmatic lung. What’s going on down there anyway? Proc Am Thorac Soc 2009; 6: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venegas JG, Winkler T, Musch G, Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 2005; 434: 777–82. [DOI] [PubMed] [Google Scholar]
- 6.Harris RS, Winkler T, Tgavelekos N, Musch G, Melo MCFV, Schroeder T, Chang Y, Venegas JG. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am J Respir Crit Care Med 2006; 174: 245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fain SB, Gonzalez-Fernandez G, Peterson ET, Evans MD, Sorkness RL, Jarjour NN, Busse WW, Kuhlman JE. Evaluation of structure-function relationships in asthma using multi-detector CT (MDCT) and hyperpolarized (HP) 3He MRI. Acad Radiol 2008; 15: 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lange EE, Altes TA, Patrie JT, et al. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology 2009; 250:567–75. [DOI] [PubMed] [Google Scholar]
- 9.Costella S, Kirby M, Maksym GN, McCormack DG, Paterson NA, Parraga G. Regional pulmonary response to a methacholine challenge using hyperpolarized 3He magnetic resonance imaging. Respirology 2012; 17:1237–46. [DOI] [PubMed] [Google Scholar]
- 10.de Lange EE, Altes TA, Patrie JT, Gaare JD, Knake JJ, Mugler JP, Platts-Mills TA. Evaluation of asthma with hyperpolarized 3helium MRI: Correlation with clinical severity and spirometry. Chest 2006; 130: 1055–62. [DOI] [PubMed] [Google Scholar]
- 11.Cadman RV, Lemanske RF, Evans MD, Jackson DJ, Gern JE, Sorkness RL, Fain SB. Pulmonary 3He magnetic resonance imaging of childhood asthma. J Allergy Clin Immunol 2013; 131: 369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svenningsen S, Kirby M, Starr D, Coxson HO, Paterson NA, McCormack DG, Parraga G. What are ventilation defects in asthma? Thorax 2014; 69: 63–71 [DOI] [PubMed] [Google Scholar]
- 13.Macleod KA, Horsley AR, Bell NJ, Greening AP, Innes JA, Cunningham S. Ventilation heterogeneity in children with well controlled asthma with normal spirometry indicates residual airways disease. Thorax 2009; 64: 33–7. [DOI] [PubMed] [Google Scholar]
- 14.Papastamelos C, Panitch HB, England DE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. J Apply Physiol 1995; 78: 179–84. [DOI] [PubMed] [Google Scholar]
- 15.Altes TA, Mata J, de Lange EE, Brookeman JR, Mugler JP 3rd. Assessment of lung development using hyperpolarized 3helium diffusion MR imaging. Magn Reson Imaging 2006; 24:1277–83. [DOI] [PubMed] [Google Scholar]
- 16.Tustison NJ, Altes TA, Song G, de Lange EE, Mugler JP, Gee JC. Feature analysis of hyperpolarized 3helium MRI: A study of asthmatics versus nonasthmatics. Magnetic Resonance in Med 2010; 63: 1448–1455. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines for the diagnosis and management of asthma Expert panel report 3. National Asthma Education and Prevention Program. Bethesda, MD: National Heart, Lung, and Blood Institute. National Institute of Health, 1997. [Google Scholar]
- 18.American Thoracic Society. Proceedings of the American Thoracic Society Workshop on Refractory Asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med 2000; 162: 2341–2351. [DOI] [PubMed] [Google Scholar]
- 19.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk P, Adcock IM, Bateman E, Bel E, Bleecker E, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jarjour N, Mauad T, Sorkness R, Teague WG. International ERS/ATS Consensus Definition, Mechanisms, Evaluation and Treatment of Severe Asthma. Eur Respir J 2013; Dec (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159: 179–187. [DOI] [PubMed] [Google Scholar]
- 21.National Institutes of Allergy, Asthma, and Infectious Diseases. Standardizing Asthma Outcomes in Clinical Research: Report of the Asthma Outcomes Workshop. J Allergy Clin Immunol 2012; 130: 1227–1442. [Google Scholar]
- 22.Mooney KE, Miller GW, Dolph PA, et al. A 3-liter capacity, hybrid spin-exchange 3He polarizer for medical imaging In: Proceedings of the 17th Annual Meeting of ISMRM. Honolulu: 2009; p. 2166. [Google Scholar]
- 23.Tustison NJ, Avants BB, Flors L, Altes TA, de Lange EE, Mugler JP, Gee JC. Ventilation-based segmentation of the lungs using hyperpolarized 3He MRI. J Magn Reson Imaging 2011; 34: 831–41. [DOI] [PubMed] [Google Scholar]
- 24.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006; 31:1116–28. [DOI] [PubMed] [Google Scholar]
- 25.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of manual segmentation. IEEE Trans Med Imaging 2004; 23: 903–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avants BB, Tustison N, Wu J, Cook PA, Gee JC. An open source multivariate framework of n-tissue segmentation with evaluation on public data. Neuroinformatics 2011; 9: 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GW, Mugler JP 3rd, Altes TA, Cai J, Mata JF, de Lange EE, Tobias WA, Cates GD, Brookeman JR. A short-breath-hold technique for lung pO2 mapping with 3He MRI. Magn Reson Med. 2010; 63: 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parraga G, Ouriadov A, Evans A, McKay S, Lam WW, Fenster A, Etemad Rezai R, McCormack D, Giles S. Hyperpolarized 3He ventilation defects and apparent diffusion coefficients in chronic obstructive pulmonary disease: preliminary results at 3.0 tesla. Invest Radiol 2007; 42:384–391. [DOI] [PubMed] [Google Scholar]
- 29.Woodhouse N, Wild JM, Paley MNJ, Fichele S, Said Z, Swift AJ, van Beek EJR. Combined 3helium/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging 2005; 21:365–369. [DOI] [PubMed] [Google Scholar]
- 30.Thompson BR, Douglass JA, Ellis MJ, Kelly VJ, O’Hehir RE, King GG, Verbanck S. Peripheral lung function in patients with stable and unstable asthma. J Allergy Clin Immunol 2013; 131 (5):1322–28. [DOI] [PubMed] [Google Scholar]
- 31.Castro M, Woods J. Insights into pediatric asthma with hyperpolarized magnetic resonance imaging of the lung. J Allergy Clin Immunol 2013; 131:377–8. [DOI] [PubMed] [Google Scholar]
- 32.Goldin JG, Tashkin DP, Kleerup EC, Greaser LE, Haywood UM, Sayre JW, Simmons MD, Suttorp M, Colice GL, Vanden Burgt JA, Aberle DR. Comparative effects of hydrofluoroalkane and chlorofluorocarbon beclomethasone dipropionate inhalation on small airways: assessment with functional helical thin-section computed tomography. J Allergy Clin Immunol 1999; 104: S258–67. [DOI] [PubMed] [Google Scholar]
- 33.Thomen RP, Sheshadri A, Quirk JD, Ellison HD, Szczesniak RD, Castro M, Woods JC. Regional ventilation changes in severe asthma after bronchial thermoplasty. Radiology 2015; 274: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan M, Beardsmore CS, Owers-Bradley J, Dogaru CM, Mada M, Ball I, Garipov RR, Kuehni CE, Spycher BD, Silverman M. Catch up alveolarization in ex-preterm children: Evidence from 3He magnetic resonance. Am J Respir Crit Care Med 2013; 187:1104–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.