Abstract
Purpose:
Somatostatin receptor antagonists have shown promise for imaging neuroendocrine tumors (NETs) in preclinical studies, but clinical data is still very limited. In this study, we assess the feasibility of using the novel somatostatin antagonist 68Ga-DOTA-JR11 for PET imaging of NETs.
Methods:
Twenty patients with advanced NETs underwent whole-body PET/CT imaging 60 min after injection of 169 MBq (median) 68Ga-DOTA-JR11 as part of a prospective study. Volumes of interest were drawn around up to four 68Ga-DOTA-JR11-avid lesions per patient (with uptake greater than liver) and standardized uptake values were estimated. Additionally, target-to-normal tissue ratios were calculated. A subset of 6 patients had additional imaging (25-min dynamic scan of the upper abdomen including, at least partly, cardiac left ventricle, liver, spleen, and kidney and a whole-body PET/CT scan at 30 min post-injection) to determine the time course of tracer distribution and facilitate radiation dose estimates. Absorbed doses were calculated using OLINDA/EXM 1.0.
Results:
In contrast to the known biodistribution of somatostatin receptor agonists, little or no uptake above background was seen in the pituitary gland, spleen, adrenals, and uninvolved liver; e.g., median spleen SUVmean 1.4 (range: 0.7-1.8), liver SUVmean 1.1 (0.7-1.9). A total of 42 tumor lesions were analyzed with median SUVmax 13.0 (range: 2.9-94), TNR blood 9.3 (1.8-87), TNR spleen 4.9 (1.9-48), TNR kidney 2.2 (0.52-28), and TNR liver 10.5 (2.3-107). Tumor uptake reached plateau levels by 20-30 min post-injection. The highest absorbed dose estimates (mGy/MBq) to normal tissues were: urinary bladder wall (0.30; SD 0.06) and kidneys (0.050; SD 0.013). The effective dose (ICRP 103) was 0.022 (SD 0.003) mSv/MBq.
Conclusions:
68Ga-DOTA-JR11 demonstrated rapid tumor uptake, high tumor/background ratios, and rapid clearance from blood. The low liver background is advantageous and may facilitate detection of liver metastases. Dosimetric data compare favorably with published data for 68Ga-DOTATATE and 68Ga-DOTATOC.
Keywords: Somatostatin receptor antagonists, JR11, PET/CT, neuroendocrine tumors
INTRODUCTION
Neuroendocrine tumors (NETs) represent a heterogenous group of tumors with significantly increasing incidence. For example, gastroenteropancreatic neuroendocrine tumors (GEP-NETs), the largest subgroup of NETs, underwent a striking increase in incidence from 1.0/100,000 in 1973 to 3.65/100,000 in the period 2003-2007 [1] in the US.
Overexpression of somatostatin receptors and specifically the somatostatin receptor subtype 2 (SSTR2) is a common feature of NETs. Radiolabeled, metabolically stable somatostatin receptor binding peptides have been used clinically for imaging of NETs since 1994 [2]. The first FDA-approved agent was 111In-octreotide (Octreoscan®). More recently, several 68Ga-labeled somatostatin analogues enabling SSTR positron emission tomography/computed tomography (PET/CT) with improved binding affinity and biodistribution have been introduced. One of these agents, 68Ga-DOTA-TATE, was FDA-approved in 2016 [3]. All of these imaging agents are somatostatin agonists; i.e., they stimulate the somatostatin receptor and are consequently internalized.
Preclinical studies have indicated that SSTR antagonists bind to significantly more receptor sites than the agonists currently used for peptide receptor radionuclide therapy (PRRT) [4]. This finding was confirmed by quantitative autoradiography of patient-derived tumor samples [5], demonstrating a more than four-fold increase in the ex vivo binding of the somatostatin receptor antagonist 177Lu-DOTA-BASS compared to the agonist 177Lu-DOTA-TATE. Wild et al. performed a preliminary clinical study comparing the dosimetry of a 177Lu-labeled therapeutic SSTR antagonist 177Lu-DOTA-JR11 with 177Lu-DOTA-TATE in four patients. In this study, the antagonist demonstrated, on average, a three-fold higher tumor dose and a two-fold higher tumor-to-kidney ratio [6]. PET and biodistribution studies in mice bearing SSTR2-expressing xenografts also demonstrated higher tumor uptake of 68Ga-DOTA-JR11 and 68Ga-NODAGA-JR11 (68Ga-OPS202) than the high-affinity SSTR2 agonist 68Ga-DOTA-TATE [7], supporting further clinical evaluation of these imaging agents.
Nicolas et al. recently reported two clinical studies [8, 9] on the use of 68Ga-NODAGA-JR11 (68Ga-OPS202) to image GEP-NETs. The first of these studies [8] focused on biodistribution, safety, and radiation dosimetry, while the second directly compared the biodistribution of 68Ga-NODAGA-JR11 (68Ga-OPS202) with that of the SSTR2 agonist 68Ga-DOTA-TOC, and found the antagonist to be superior in terms of lesion contrast, detection, and sensitivity [9].
The purpose of this study was to assess the utility of the novel somatostatin antagonist 68Ga-DOTA-JR11 for PET imaging of NETs. Patients with advanced NETs and positive 111In-pentetreotide (OctreoScan®) scans were imaged with 68Ga-DOTA-JR11 prior to PRRT with 177Lu-DOTA-JR11. Detailed biodistribution and radiation dosimetry was investigated on a subset of these patients.
MATERIALS AND METHODS
Clinical study with 68Ga-DOTA-JR11
Under the auspices of a protocol approved by the Institutional Review Board and an Investigational New Drug application approved by the FDA, 20 patients (10 male, 10 females, age: 22-73 years, mean 54 ± 14 years) with progressive, histologically proven, unresectable NETs (18 GEP-NETS, 1 bronchopulmonary neuroendocrine neoplasm, and 1 renal neuroendocrine carcinoma) were imaged with the SSTR2 antagonist 68Ga-DOTA-JR11 as a prelude to planned therapy with 177Lu-DOTA-JR11 after providing written informed consent (trial registration ID NCT02609737). Of these 20 patients, the first 6 had extended imaging to facilitate kinetic analysis and normal tissue radiation dose estimates.
68Ga-DOTA-JR11 preparation and administration
68Ga-DOTA-JR11 was manufactured by the MSK Radiochemistry and Molecular Imaging Probe Core Facility in compliance with an FDA-approved IND (FDA IND #128,082). The DOTA-JR11 precursor (OctreoPharm, Sciences GmbH, Berlin, Germany) (Supplementary Figure 1) was labeled with 68Ga using an EZ-102 reagent kit and a 1.85 GBq/50 mCi 68Ga generator supplied by Eckert & Ziegler Radiopharma GmbH, Germany. Briefly, 68Ga was eluted from the generator with ~5 ml of 0.1N HCl and adsorbed onto an SCX cartridge. The concentrated 68Ga was then eluted from the SCX cartridge using a 5M NaCl/HCl solution directly into the reactor containing 100 μg of DOTA-JR11 peptide and dissolved in sodium acetate buffer, for a final reaction mixture pH of ~3.7. The reaction mixture was heated to 105 °C for 7 minutes to allow for radionuclide incorporation. The reaction mixture was then loaded onto a C18 light SEP-PAK cartridge and washed with normal saline to remove unincorporated radionuclide. Finally, 68Ga-DOTA-JR11 was eluted off the purification cartridge with 1 ml of a 1:1 solution of ethanol:water, followed by 9 mL of normal saline, through a 0.22 μm filter, into a sterile, apyrogenic, USP Type I glass vial, sealed with rubber septum and crimped.
The final drug product underwent quality control (QC) testing prior to batch release for patient administration in accordance with acceptance specifications for radiochemical purity, endotoxin content, sterilizing filter integrity, pH, appearance, and radionuclide identity confirmation. Sterility testing was performed post-release.
68Ga-DOTA-JR11 was administered to subjects at a planned activity of 185 MBq (5 mCi) ±10% by slow bolus injection.
Toxicity monitoring
Toxicity monitoring consisted of pre-injection and post-scan vital signs (heart rate, blood pressure, body temperature), pulse oximetry, 3-lead electrocardiography, and monitoring of clinical symptoms.
PET/CT imaging with 68Ga-DOTA-JR11
All 20 patients underwent whole-body (vertex of skull to mid-thigh) PET/CT imaging (GE PET/CT 710 scanner with time-of-flight) at 60 minutes (mean 64 min; SD 6) post-injection. Patients were asked to void their bladders before imaging. PET scanning was performed in 3-D mode with a 3-min emission time per bed position. A low-dose CT scan (120 kVp, 80 mA tube current, helical pitch 1.5:1; estimated radiation dose 9.0 mGy) was acquired prior to PET imaging for attenuation correction and anatomical localization. Total acquisition time was 18 min or 21 min for 6 or 7 bed positions, respectively. Images were reconstructed using a 700-mm field of view into a 128×128 matrix with iterative ordered subset expectation maximization (OSEM: 16 subsets; 2 iterations) with a 6.4-mm 2D Gaussian post-filter and “heavy” axial 3-point smoothing ([1 2 1]/4). All corrections per manufacturer including CT-based attenuation, scatter, and SharpIR (point spread function correction) were utilized. PET scanner quality assurance as per ACR guidelines, including an 18F well counter calibration, was performed quarterly. Activity quantification for 68Ga PET scans was based on the 18F calibration and knowledge of ratio of positron yields for 68Ga and 18F (i.e., 0.889 to 0.987).
For the purpose of radiation-absorbed dose estimation, additional images were acquired in the first 6 patients of the 20 accrued (3 female and 3 male). These consisted of a 25-min/19-frame dynamic acquisition initiated at the time of administration and centered on the upper abdomen to include, at least partly, cardiac left ventricle, liver, spleen, and kidney. This was immediately followed at approximately 30 min (mean 34 min; SD 2) post-injection by a whole-body PET/CT scan identical to that acquired at 60 min. Total acquisition time for the dosimetry patient subset was 61-67 min for 6-7 bed positions, respectively.
Image interpretation, lesion detection, and data analysis
The whole-body static PET/CT scans acquired at 60 min post-injection were first interpreted without reference to other imaging modalities. Any lesions with focal radiotracer uptake not explained by physiologic SSTR2 expression were interpreted as metastatic disease.
Volumes of interest (VOI) were generated over lesions using a Hermes imaging workstation (Hermes medical solutions, Chicago, IL, USA). Typically, a value of 40-50% of maximum tracer uptake was used as a threshold but this was guided by interpretation of the CT scan. A total of 42 lesions were analyzed with a maximum of four lesions per patient.
For normal tissues (liver, kidney, spleen, lung, and cardiac left ventricle), regions of interest (ROI) were drawn within organs over at least five consecutive transaxial PET slices and combined to generate VOIs using a Hermes imaging workstation. For liver, ROI were drawn to exclude, as far as possible, enhanced uptake in disease foci.
Tracer uptake was quantified by standardized uptake values (SUV) normalized to patients’ body weight. For lesion VOI, SUVmax and SUVpeak were recorded in addition to SUVmean. SUVmax referred to the maximum voxel in the VOI, whereas SUVpeak was defined as the mean SUV of the hottest 1 cubic centimeter in the VOI. For normal tissues, only SUVmean within the VOI was used. In the special case of cardiac left ventricle, the representative value used was the average SUVmax over five slices. We have found this quantity to correspond closely to ex vivo measured blood activity concentration in prior studies.
Comparative lesion uptake was quantified using target-to-normal (TNR) SUV ratios defined as SUVmax (lesion)/SUV (tissue) for blood, spleen, kidney, and liver. As 4 of the 20 patients had prior splenectomy, TNR (spleen) was calculated for only 16 patients.
Absorbed dose calculations
Absorbed radiation doses to normal tissues were estimated in a subset of 6 patients who had additional imaging as described above. Absorbed doses to whole body (WB) and individual organs were calculated based on the combined dynamic and static PET/CT images. Activity concentration-time curves for all organs displaying 68Ga-DOTA-JR11 accumulation were generated by VOI analysis. For liver, this curve was for normal (uninvolved) liver. It was assumed that the red marrow activity concentration was equal to that of blood [10]. WB activity-time curves were generated using the 3 points defined by the administered activity (time zero) and the total activities in the two whole-body PET scans. These data were used to calculate mono-exponential biological half-times for WB clearance.
The areas under activity concentration-time curves (AUC) were estimated by trapezoidal integration of the combined data from dynamic and static images with a terminal contribution calculated by extrapolation from the last measured value using the shorter of apparent terminal clearance rate or physical decay. Whole-organ AUCs were estimated by multiplying the activity concentration AUC by organ mass as taken from the OLINDA/EXM 1.0 software application [11]. We did not use patient mass-based rescaling in this study, as the actual patients’ masses did not differ substantially from OLINDA/EXM 1.0 standard values (73.7 kg for male; 56.9 kg for female). The number of disintegrations in normal organs (i.e., residence times) were derived by dividing whole-organ AUC by the administered activity. For urinary bladder contents, residence times were derived using the voiding bladder model built into OLINDA/EXM 1.0, the estimated mono-exponential whole-body biological half-time, and an assumed voiding interval of 1 hour. This relatively short time was deemed appropriate for this short-lived radiopharmaceutical, considering that patients were instructed to void their bladders before the 60-min PET scan and encouraged to hydrate and void frequently thereafter. Residence times for the remainder of body were derived by subtracting all the individually estimated residence times from the whole-body residence time. Absorbed radiation doses to the whole body and various organs were calculated using the OLINDA/EXM 1.0 software application [11]. Subsequently, effective doses were recalculated using the tissue weighting factors promulgated in ICRP Report 103 [12].
RESULTS
68Ga-DOTA-JR11 administration and safety
Patient characteristics are summarized in Table 1. All 20 enrolled patients were administered 68Ga-DOTA-JR11, underwent at least one whole-body PET/CT, and are included in the analysis. The mean administered activity was 169 MBq (4.6 mCi); range: 137-192 MBq (3.7-5.2 mCi) with a mean radiochemical purity of 99.95% (range: 99-100%). The mean injected peptide mass was 81 μg (range: 60-97 μg) (Supplementary Table 1).
Table 1.
Demographic and clinical characteristics of patients
| Patient | Sex | Age (years) | Site of primary | Grade |
|---|---|---|---|---|
| 1 | F | 22 | Small intestine | G2 |
| 2 | F | 29 | Pancreas | G2 |
| 3 | M | 61 | Pancreas | G1 |
| 4 | M | 42 | Stomach | G2 |
| 5 | M | 59 | BP | atypical |
| 6 | F | 56 | Pancreas | G2 |
| 7 | M | 73 | Rectum | G1 |
| 8 | M | 72 | Pancreas | G2 |
| 9 | F | 49 | Small intestine | G2 |
| 10 | F | 56 | Small intestine | G2 |
| 11 | F | 54 | Pancreas | G2 |
| 12 | F | 45 | Pancreas | G2 |
| 13 | F | 59 | Small intestine | G2 |
| 14 | F | 68 | Small intestine | G1 |
| 15 | M | 59 | Pancreas | G1 |
| 16 | F | 64 | Pancreas | G2 |
| 17 | M | 65 | Pancreas | G2 |
| 18 | M | 63 | Small intestine | G2 |
| 19 | M | 37 | Renal | G3 |
| 20 | M | 44 | Pancreas | G1 |
F: female; M: male; BP: bronchopulmonary; G1: low grade (well differentiated); G2: intermediate grade (moderately differentiated); G3: high grade (poorly differentiated)
Of the 20 patients, 18 experienced no infusion-related symptoms. Two patients with hormone-secreting tumors experienced flushing (G3/2), hypotension (G3/G1), and nausea (G1). Of these, one experienced additional tachycardia (G1), abdominal pain (G2), and diarrhea (G1), requiring injection of 150 μg Octreotide s.c. No significant changes (pre- vs. post-injection) were evident in blood glucose, body temperature, peripheral oxygen saturation, or EKG. No subsequent adverse events were observed in the follow-up period (mean 16 days; range 2-30 days) prior to PRRT.
PET/CT imaging with 68Ga-DOTA-JR11
68Ga-DOTA-JR11 PET/CT revealed positive lesions in all 20 patients. On visual inspection, images demonstrated favorable biodistribution with little or no uptake in the pituitary, parotid, and salivary glands; thyroid; spleen; adrenals; and uninvolved liver (Figs. 1–3). Quantitative analysis indicated little uptake above blood pool in organs such as spleen (SUVmean 1.4; range 0.7-1.8; n=16) and uninvolved liver (SUVmean 1.1; range 0.7-1.9; n=19). Renal uptake was higher with an SUVmean of 4.5; range 1.3-7.2 (Table 2). The main route of excretion was renal and substantial urinary bladder activity was observed in patients who had whole-body PET/CT images at 30 min post-administration, amounting to a median of 15% of the administered activity (range 12-26%; n=6). However, in most cases, this was significantly reduced at the time of the 60-min image before which patients were instructed to void.
Fig. 1.
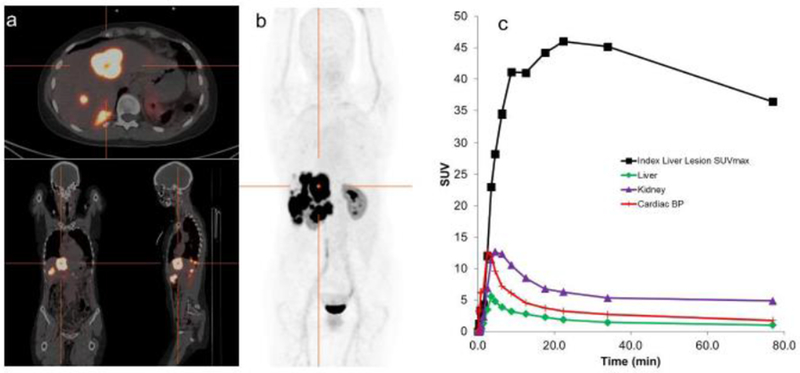
Patient with predominant liver involvement. (a) Transverse, coronal, sagittal PET/CT image set at the level of the crosshairs shown in (b) Whole-body maximum-intensity projection images showing the overall distribution of 68Ga-DOTA-JR11. Images acquired at 77 minutes post-injection demonstrate the lack of significant uptake in any normal tissues, apart from kidney, and show high uptake in hepatic disease. Minimal activity was present in the urinary bladder following pre-scan voiding. (c) SUV-time curves illustrate the rapid uptake of 68Ga-DOTA-JR11 into liver disease with prolonged retention.
Fig. 3.
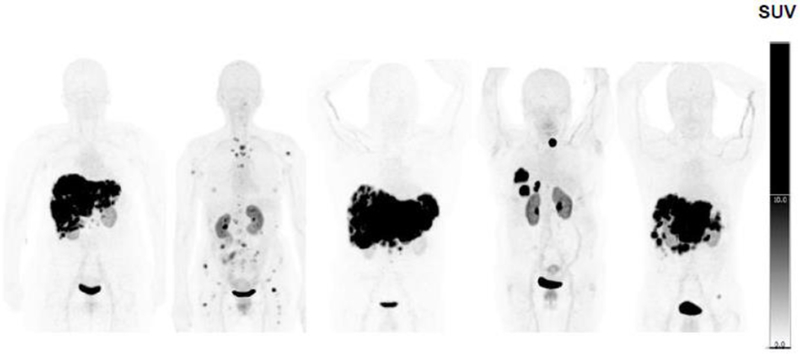
Panel of five whole-body maximum-intensity projection images acquired at 60 minutes post-injection demonstrating intense focal uptake in metastases and minimal background uptake, resulting in excellent image contrast.
Table 2.
SUVmean of organs for the 60-min whole-body scan (normal liver, spleen, kidney)
| Organ | SUVmean |
|---|---|
| Liver (n=19) | 1.1 ± 0.3 |
| Spleen (n=16) | 1.4 ± 0.3 |
| Kidney (n=20) | 4.5 ± 1.5 |
Data are mean ± SD
A total of 42 lesions were examined on whole-body 68Ga-DOTA-JR11 PET/CT (n=4, 3 pts; n=3, 3 pts; n=2, 7 pts; n=1, 7 pts). Liver was the most common site of disease with 30 lesions, followed by lymph nodes (n=8), bone (n=3), and lung (n=1). Lesion uptake at 60 min was variable with median SUVmax of 13 (range: 2.9-94) and SUVpeak of 10 (2.5-84) (see also Table 3). There were a wide range of tumor sizes ranging from a small vertebral lesion (1.4 ml) up to a very large lesion that encompassed almost the entire liver (1100 ml) with a median lesion volume of 15 ml. Although partial volume averaging effects inevitably lead to an underestimate of lesion uptake at small sizes, there was no statistically significant trend between lesion volume and SUVmax (r2 = 0.16). A full list of lesion volumes and associated SUVmax is provided in Supplementary Table 2.
Table 3.
SUV and TNR for 42 reference lesions at 60 minutes post-administration
| Site | N | SUVmax | SUVmean | SUVpeak | TNR-B | TNR-S | TNR-K | TNR-L |
|---|---|---|---|---|---|---|---|---|
| Lung | 1 | 17 | 7.4 | 11 | 11 | 9 | 2.3 | 15 |
| Liver | 30 | 25 ± 22 | 11 ± 9 | 21 ± 19 | 22 ± 20 | 12 ± 12 | 6 ± 6 | 23 ± 23 |
| LN | 8 | 14 ± 20 | 7 ± 6 | 12 ± 19 | 10 ± 14 | 4 ± 3 | 2.3 ± 3.1 | 12 ± 17 |
| Bone | 3 | 6 ± 3 | 4 ± 2 | 5 ± 2 | 5 ± 3 | 4 ± 2 | 1.4 ± 0.3 | 6 ± 1 |
| Total | 42 | 21 ± 21 | 10 ± 8 | 18 ± 19 | 18 ± 19 | 10 ± 11 | 5 ± 6 | 20 ± 21 |
TNR: tumor-to-normal tissue (SUVmax/SUVmean) ratio; B: blood; S: spleen; K: kidney; L: normal liver. Data are mean ± SD
In the 6 patients who had dynamic images, lesion uptake was observed to be rapid (Figs. 1 and 2), typically reaching the highest levels (SUVmax of up to 50) by 20-30 min post-injection and in most instances remaining close to plateau thereafter. Blood clearance was generally rapid but with a low-level terminal component that cleared more slowly, corresponding to a median time-zero SUV intercept of 3.7 clearing with a median biological halftime of 60 min.
Fig. 2.
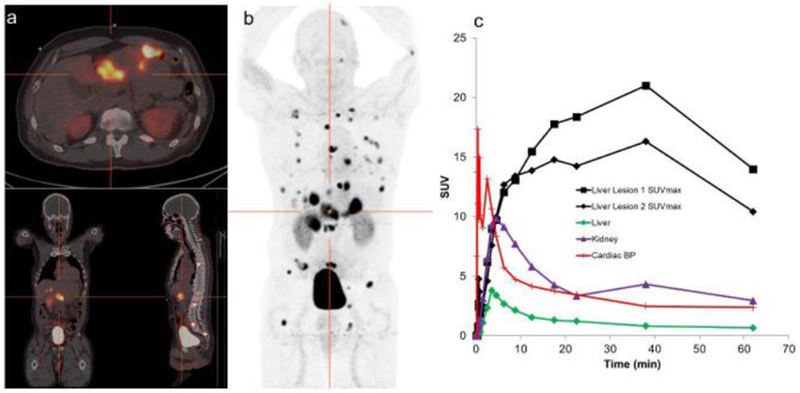
Patient with atypical broncho-pulmonary NET with hepatic and osseous metastases. (a) Transverse, coronal, sagittal PET/CT image set at the level of the crosshairs shown in (b) Whole-body maximum-intensity projection images showing overall 68Ga-DOTA-JR11 distribution. Images acquired at 62 minutes post-injection demonstrate the lack of significant normal tissue uptake, apart from kidney. Focal uptake is seen in hepatic and osseous metastases. There is significant activity (approximately 15% of the administered amount) in the urinary bladder, illustrating the primary route of excretion. (c) SUV-time curves show rapid uptake of 68Ga-DOTA-JR11 into lesions.
Absorbed dose estimates for 68Ga-DOTA-JR11
Normal tissue residence times for 6 patients are provided in Table 4. Those for red marrow and cardiac contents were generated from the blood activity-time curves. The full set of normal tissue absorbed dose estimates (mGy/MBq) are shown in Table 5. The highest values were (mean ± SD): urinary bladder wall (0.30 ± 0.06), kidneys (0.050 ± 0.013), liver (0.023 ± 0.014), lungs (0.021 ± 0.05), heart wall (0.020 ± 0.04), and spleen (0.018 ± 0.002). The estimated effective dose (ICRP 103) was 0.022 ± 0.003 mSv/MBq.
Table 4.
Residence times (hr) in selected organs (n = 6)
| Patient | Kidney | Liver | Spleen | Lung | RM | C Cont | UB | RoB | WB |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.028 | 0.049 | 0.0050 | 0.024 | 0.053 | 0.015 | 0.24 | 0.40 | 0.82 |
| 2 | 0.017 | 0.032 | 0.0058 | 0.027 | 0.070 | 0.032 | 0.20 | 0.57 | 0.95 |
| 3 | 0.040 | 0.050 | N/A | 0.045 | 0.088 | 0.025 | 0.22 | 0.42 | 0.88 |
| 4 | 0.027 | 0.133 | 0.0041 | 0.031 | 0.067 | 0.019 | 0.18 | 0.53 | 1.00 |
| 5 | 0.030 | 0.048 | 0.0071 | 0.040 | 0.056 | 0.025 | 0.26 | 0.31 | 0.77 |
| 6 | 0.033 | 0.079 | 0.0071 | 0.053 | 0.078 | 0.035 | 0.20 | 0.45 | 0.93 |
RM: red marrow; C Cont: cardiac contents; UB: urinary bladder; RoB: remainder of body; WB: whole body; N/A: not available
Table 5.
Absorbed doses to normal tissues from 68Ga-DOTA-JR11 (n = 6)
| Patient |
|||||||
|---|---|---|---|---|---|---|---|
| Target organ | 1 | 2 | 3 | 4 | 5 | 6 | Mean ± SD |
| Adrenals | 0.007 | 0.007 | 0.008 | 0.009 | 0.005 | 0.007 | 0.007 ± 0.002 |
| Brain | 0.004 | 0.005 | 0.005 | 0.006 | 0.003 | 0.004 | 0.004 ± 0.001 |
| Breasts | 0.005 | 0.005 | 0.005 | 0.006 | 0.003 | 0.004 | 0.005 ± 0.001 |
| GB wall | 0.007 | 0.007 | 0.007 | 0.010 | 0.005 | 0.007 | 0.007 ± 0.002 |
| LLI wall | 0.009 | 0.008 | 0.009 | 0.010 | 0.007 | 0.007 | 0.008 ± 0.001 |
| Small intestine | 0.007 | 0.007 | 0.007 | 0.008 | 0.005 | 0.006 | 0.007 ± 0.001 |
| Stomach wall | 0.006 | 0.006 | 0.006 | 0.008 | 0.004 | 0.005 | 0.006 ± 0.001 |
| ULI wall | 0.007 | 0.007 | 0.007 | 0.009 | 0.004 | 0.006 | 0.007 ± 0.001 |
| Heart wall | 0.014 | 0.023 | 0.021 | 0.019 | 0.017 | 0.024 | 0.020 ± 0.004 |
| Kidneys | 0.049 | 0.029 | 0.070 | 0.048 | 0.048 | 0.053 | 0.050 ± 0.013 |
| Liver | 0.020 | 0.010 | 0.020 | 0.050 | 0.015 | 0.023 | 0.023 ± 0.014 |
| Lungs | 0.016 | 0.015 | 0.027 | 0.021 | 0.020 | 0.026 | 0.021 ± 0.005 |
| Muscle | 0.006 | 0.006 | 0.006 | 0.007 | 0.004 | 0.005 | 0.006 ± 0.001 |
| Ovaries | 0.009 | N/A | 0.009 | 0.010 | N/A | N/A | 0.009 ± 0.000 |
| Pancreas | 0.006 | 0.007 | 0.007 | 0.009 | 0.005 | 0.006 | 0.007 ± 0.001 |
| Red marrow | 0.013 | 0.017 | 0.019 | 0.017 | 0.013 | 0.018 | 0.016 ± 0.003 |
| Osteogenic cells | 0.015 | 0.015 | 0.021 | 0.020 | 0.011 | 0.015 | 0.016 ± 0.004 |
| Skin | 0.004 | 0.005 | 0.005 | 0.006 | 0.003 | 0.004 | 0.004 ± 0.001 |
| Spleen | 0.018 | 0.017 | N/A | 0.016 | 0.020 | 0.020 | 0.018 ± 0.002 |
| Testes | N/A | 0.007 | N/A | N/A | 0.005 | 0.006 | 0.006 ± 0.001 |
| Thymus | 0.005 | 0.006 | 0.006 | 0.007 | 0.004 | 0.005 | 0.006 ± 0.001 |
| Thyroid | 0.005 | 0.005 | 0.005 | 0.006 | 0.003 | 0.004 | 0.005 ± 0.001 |
| UB wall | 0.378 | 0.230 | 0.343 | 0.289 | 0.297 | 0.237 | 0.30 ± 0.06 |
| Uterus | 0.013 | N/A | 0.013 | 0.013 | N/A | N/A | 0.013 ± 0.000 |
| Total body | 0.007 | 0.007 | 0.008 | 0.010 | 0.005 | 0.007 | 0.007 ± 0.001 |
| Effective dose (mSv/MBq) | 0.024 | 0.018 | 0.025 | 0.023 | 0.020 | 0.020 | 0.022 ± 0.003 |
GB: gall bladder; LLI: lower large intestine; ULI: upper large intestine; UB: urinary bladder. Effective doses were calculated using ICRP 103 weighting factors.
Data are in mGy/MBq unless otherwise specified.
DISCUSSION
This first-in-human study of 68Ga-DOTA-JR11 demonstrated very favorable biodistribution with only minimal tracer uptake in normal parenchymal organs except for the kidneys. Tumor uptake was rapid and resulted in high-contrast images of metastatic NETs. In particular, the low background activity in liver was advantageous and facilitated detection of liver metastases. These characteristics make 68Ga-DOTA-JR11 an attractive companion diagnostic for patient selection for therapy with 177Lu-DOTA-JR11.
The reasons for differences in normal tissue uptake of 68Ga-DOTA-JR11 and the known biodistribution of somatostatin receptor antagonists are currently not well understood. Specifically, the lack of uptake by organs with known SSTR2 expression, such as the adrenals and the pituitary gland is intriguing and requires further study. Contributory factors may include the SSTR2 antagonistic properties of 68Ga-DOTA-JR11, the possibility of large disease “sinks” reducing agent availability and the higher peptide mass administered. It should be noted however that adrenal/pituitary uptake was not seen, even for patients with low disease burdens, while the rationale for using a higher peptide mass was for compatibility with subsequent PRRT using 177Lu-DOTA-JR11, which all patients went on to have.
In a recent preclinical study, Nicolas et al. evaluated the effect of peptide mass on the biodistribution and dosimetry of 177Lu-DOTA-JR11 [13]. Escalating amounts (10, 200, and 2000 pmol) did not lead to saturation of binding sites on tumor, but greatly suppressed uptake in normal SSTR2-expressing organs, thereby increasing tumor-to-background ratios.
Irrespective of its cause, the low uptake by most normal parenchymal organs resulted in favorable radiation dosimetry for 68Ga-DOTA-JR11 when compared with published data on Gallium-labeled somatostatin receptor agonists. For example, liver and kidney doses were up to 50% lower than those reported for 68Ga-DOTA-TOC or 68Ga-DOTA-TATE [3, 14], while spleen dose was less by a factor of 6-15 (Table 6). Here, it should be noted that the existence of large disease sinks, especially in the liver, could lead to an underestimate of absorbed dose to normal liver, kidney and spleen as any contribution due to photon irradiation from liver disease-associated activity was not considered. The impact of this effect will vary from patient to patient, depending on the amount and biodistribution of disease and will be alleviated, to some extent, by the inclusion of the cross-dose contribution from activity in the remainder-of-body compartment.
Table 6.
Absorbed doses in selected organs using selected SSTR2 tracers
| Target organ | 68Ga-DOTA-JR11 | 68Ga-DOTA-TATE [3] | 68Ga-DOTA-TATE [14] | 68Ga-DOTA-TOC [14] | 68Ga-NODAGA-JR11 [8] |
|---|---|---|---|---|---|
| Kidney | 0.050 ± 0.013 | 0.092 ± 0.028 | 0.093 ± 0.016 | 0.082 ± 0.020 | 0.084 ± 0.031 |
| Liver | 0.023 ± 0.014 | 0.045 ± 0.015 | 0.050 ± 0.015 | 0.041 ± 0.014 | 0.022 ± 0.009 |
| Spleen | 0.018 ± 0.002 | 0.28 ± 0.12 | 0.109 ± 0.058 | 0.108 ± 0.065 | 0.06 ± 0.05 |
| Adrenals | 0.007 ± 0.002 | 0.015 ± 0.001 | 0.086 ± 0.052 | 0.077 ± 0.028 | 0.03 ± 0.01 |
| Lungs | 0.021 ± 0.005 | 0.012 ± 0.0004 | 0.006 ± 0.001 | 0.007 ± 0.001 | 0.021 ± 0.007 |
| UB wall | 0.30 ± 0.06 | 0.12 ± 0.06 | 0.098 ± 0.048 | 0.12 ± 0.058 | 0.10 ± 0.04 |
| Red marrow | 0.016 ± 0.003 | 0.010 ± 0.0003 | 0.015 ± 0.003 | 0.016 ± 0.003 | 0.011 ± 0.003 |
| Total body | 0.007 ± 0.001 | 0.014 ± 0.0003 | 0.014 ± 0.002 | 0.014 ± 0.002 | N/A |
| Effective dose (mSv/MBq) | 0.022 ± 0.003 | 0.026 ± 0.003 | 0.021 ± 0.003 | 0.021 ± 0.003 | 0.024 ± 0.002 |
Mean ± SD are provided. Data are absorbed dose in mGy/MBq unless otherwise specified.
Because of large variabilities in tumor uptake of SSTR2-targeting imaging agents, it is not straightforward to objectively compare 68Ga-DOTA-JR11 with somatostatin receptor agonists. However, the high image contrast observed in this study with SUVmax up to 94 is certainly comparable to SUVmax reported for somatostatin receptor agonists.
Nicolas et al. [8, 9] recently reported the clinical use of 68Ga-NODAGA-JR11 (68Ga-OPS202) to image GEP-NETs. The use of 68Ga-NODAGA-JR11 is supported by preclinical studies [7], which indicated that labeling DOTA-JR11 with 68Ga reduced its SSTR2 binding affinity by a factor of approximately 80, whereas labeling NODAGA-JR11 with 68Ga had no impact on SSTR2 binding. Interestingly, our data indicate that despite the lower affinity of 68Ga-DOTA-JR11, substantial tumor uptake and high-contrast images are feasible. Our findings suggest that, in the range studied, receptor binding affinity may not be the key factor determining in vivo tumor uptake. The normal tissue distributions of 68Ga-DOTA-JR11 and 68Ga-NODAGA-JR11 were broadly similar, with somewhat higher uptake of 68Ga-NODAGA-JR11 by kidney and around three times higher for the spleen, resulting in higher radiation doses for these organs. Additionally, despite its lower affinity, 68Ga-DOTA-JR11 at a peptide mass dose of 80 μg (±10) had somewhat higher uptake in liver metastases (median SUVmax 18) than 68Ga-NODAGA-JR11 (median SUVmax 10.9, 12.6 at peptide mass doses of 15 and 50 μg) and was broadly similar for all lesions combined (median SUVmax 13 for 68Ga-DOTA-JR11 vs 12.3, 14.4 for 68Ga-NODAGA-JR11 at 15 and 50 μg).
68Ga-DOTA-JR11 administration was well tolerated in 18 out of 20 patients. However, two patients with functional NETs experienced symptoms with flushing, grade 3 hypotension, nausea, and lightheadedness, possibly related to the SSTR2 antagonistic properties of 68Ga-DOTA-JR11. To our knowledge, this is in contrast to studies evaluating the safety of 68Ga-labeled agonists such as DOTA-TATE [3], which did not report any physiologic responses to the radiopharmaceutical. Therefore, administration of 68Ga-DOTA-JR11 by infusion may be preferable to slow bolus injection.
Conclusion
68Ga-DOTA-JR11 is a promising companion diagnostic for selection of patients for therapy with 177Lu-DOTA-JR11. The unexpected yet favorable biodistribution of 68Ga-DOTA-JR11 requires further studies to better understand the impact of peptide mass, disease burden and SSTR2 antagonistic properties of 68Ga-DOTA-JR11. Because of the large variability of SSTR2 expression in NETs, head-to-head comparisons in specific patient groups would likely be required to determine whether 68Ga-DOTA-JR11 or 68Ga-NODAGA-JR11 are preferable for detection and staging of NETs.
Supplementary Material
Acknowledgements:
This study was supported in part by the Geoffrey Beene Cancer Research Center at MSK and the MSK Radiochemistry and Molecular Imaging Probe Core, which is funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. We gratefully acknowledge funding by Caring for the Carcinoid/NETRF. SK was supported in part by the NIH/NCI Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746. The precursor used in this study was provided by Ipsen.
We gratefully acknowledge Rashid Ghani and members of the Nuclear Medicine Pharmacy; nuclear medicine nurses Ann Longing and Louise Harris for their help in patient management; RSAs Alicia Lashley, Hanh Pham, and Martha Ziolkowska and Clinical Research Manager Bolorsukh Gansukh for their excellent support with patient flow and protocol management; the radiation safety officers and nuclear medicine technologists for their excellent technical assistance; and members of the Department of Medicine at MSK for patient referral. We also thank Leah Bassity for her assistance in editing this manuscript.
Funding: This study was supported in part by the Geoffrey Beene Cancer Research Center at MSK and the MSK Radiochemistry and Molecular Imaging Probe Core was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. We gratefully acknowledge the funding by Caring for the Carcinoid/NETRF. SK was supported in part by NIH/NCI Paul Calabresi Career Development Award for Clinical Oncology K12 CA184746. The precursor used in this study was provided by Ipsen.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Compliance with ethical standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed written consent was obtained from all individual participants included in the study.
References
- 1.Lawrence B, Gustafsson Bl, Chan A, Svejda B, Kidd M, Modlin IM The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011. ;40:1–18, vii. [DOI] [PubMed] [Google Scholar]
- 2.Kaltsas GA, Mukherjee JJ, Grossman AB. The value of radiolabelled MIBG and octreotide in the diagnosis and management of neuroendocrine tumours. Ann Oncol. 2001; 12 Suppl 2:S47–50. [DOI] [PubMed] [Google Scholar]
- 3.Walker RC, Smith GT, Liu E, Moore B, Clanton J, Stabin M. Measured human dosimetry of 68Ga-DOTATATE. J Nucl Med. 2013;54:855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, et al. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cescato R, Waser B, Fani M, Reubi JC. Evaluation of 177Lu-DOTA-sst2 antagonist versus 177Lu-DOTA-sst2 agonist binding in human cancers in vitro. J Nucl Med. 2011;52:1886–90. [DOI] [PubMed] [Google Scholar]
- 6.Wild D, Fani M, Fischer R, Del Pozzo L, Kaul F, Krebs S, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55:1248–52. [DOI] [PubMed] [Google Scholar]
- 7.Fani M, Braun F, Waser B, Beetschen K, Cescato R, Erchegyi J, et al. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J Nucl Med. 2012;53:1481–9. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas GP, Beykan S, Bouterfa H, Kaufmann J, Bauman A, Lassmann M, et al. Safety, biodistribution, and radiation dosimetry of (68)Ga-OPS202 ((68)Ga-NODAGA-JR11) in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase I imaging study. J Nucl Med. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Nicolas GP, Schreiter N, Kaul F, Uiters J, Bouterfa H, Kaufmann J, et al. Sensitivity comparison of (68)Ga-OPS202 and (68)Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase II imaging study. J Nucl Med. 2018;59:915–21. [DOI] [PubMed] [Google Scholar]
- 10.Forrer F, Krenning EP, Kooij PP, Bernard BF, Konijnenberg M, Bakker WH, et al. Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0),Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7. [PubMed] [Google Scholar]
- 12.The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Annals of the ICRP. 2007;37:1–332. [DOI] [PubMed] [Google Scholar]
- 13.Nicolas GP, Mansi R, McDougall L, Kaufmann J, Bouterfa H, Wild D, et al. Biodistribution, pharmacokinetics, and dosimetry of (177)Lu-, (90)Y-, and (111 )In-labeled somatostatin receptor antagonist OPS201 in comparison to the agonist (177)Lu-DOTATATE: the mass effect. J Nucl Med. 2017;58:1435–41. [DOI] [PubMed] [Google Scholar]
- 14.Sandstrom M, Velikyan I, Garske-Roman U, Sorensen J, Eriksson B, Granberg D, et al. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med. 2013;54:1755–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


