Abstract
Purpose:
The comparability between serum, plasma, and urinary measurements of estrogen metabolites via liquid chromatography-tandem mass spectrometry (LC-MS/MS) has not been largely explored, and it is unclear if urinary LC-MS/MS measurements are suitable surrogates of circulating levels.
Methods:
Serum, plasma (EDTA and heparin), and urinary estrogen/estrogen metabolite levels were measured via LC-MS/MS in paired samples from 64 healthy volunteers (18 men, 20 premenopausal women, 26 postmenopausal women). Geometric means and Spearman correlation coefficients were used to compare individual and combined pathway levels of estrogens/estrogen metabolites across biologic matrices by sex/menopausal status.
Results:
Measured concentrations of estrogens/estrogen metabolites across blood matrices were almost identical (percent differences<4.8%). Parent estrogen concentrations measured in serum and urine were moderately correlated in postmenopausal women (estrone: r=0.69, estradiol: r=0.69). Correlations were similar comparing unconjugated serum estradiol to urinary estrone (r=0.76) and urinary estradiol (r=0.65) in postmenopausal women but were moderate to low in premenopausal women (r=0.60, 0.40, respectively)/men (r=0.33, 0.53, respectively). Comparing metabolite ratios, proportionally higher concentrations of 16-pathway metabolites were measured in urine versus serum across sex/menopausal status groups (e.g. postmenopausal women: 50.3% 16-pathway metabolites/total in urine vs. 35.3% in serum).
Conclusions:
There is strong agreement between estrogen/estrogen metabolites measurements in serum, heparin plasma, and EDTA plasma. Individual estrogen metabolite concentrations were moderately correlated between urine and serum, but were not well correlated when evaluating pathway- or relative estrogen concentrations. Differences between serum and urine are likely explained by differences in metabolism and/or excretion.
Keywords: estrogens, estrogen metabolites, urine, serum, plasma, comparison
INTRODUCTION
Endogenous estrogens have been associated with the risk of several female cancers including those of the breast [1–4], endometrium [4, 5], and ovaries [4, 6] and have been implicated in the development of prostate and testicular cancer among men [7, 8]. Studies assessing estrogen as a risk factor have measured levels in serum and plasma, as well as in urine. Urine has often been utilized because of higher estrogen concentrations than in blood, it is less costly to collect, and it is more acceptable for study participants [9]. Earlier studies utilizing radioimmunoassays (RIAs) or enzyme immunoassays (EIAs), found urinary measurements of estrogens are useful in predicting ovulation and assessing reproductive function in premenopausal women. Specifically, serum estradiol concentrations have been demonstrated to be highly correlated with conjugated urinary estrone [10–12], urinary estrone-3-glucuronide [13–17], urinary estradiol-17β-glucuronide [13, 18] and estradiol-3-glucuronide [13], as well as urinary estriol-3-glucuronide [13] and estriol-16α-glucuronide [13, 17].
However, there are ongoing concerns as to whether urinary estrogens provide a reasonable surrogate for circulating levels in epidemiologic studies. Though urine was traditionally utilized to measure estrogen metabolites [19–23], recent advances in liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays allow estrogens and estrogen metabolites to be measured in urine and serum with high sensitivity and accuracy [24, 25]. The RIA and EIA assays measured the concentration of the individual estrogen glucuronides directly, whereas the MS assays employ an enzymatic hydrolysis step resulting in the measurement of combined estrogen concentrations, for example for estradiol that is, sulfated estradiol plus glucuronidated estradiol plus unconjugated estradiol in serum and sulfated estradiol plus glucuronidated estradiol plus negligible amounts of unconjugated estradiol in urine. The comparability between serum, plasma, and urinary measurements of estrogen metabolites via LC-MS/MS has not been extensively explored, and it is not clear if urinary LC-MS/MS measurements correlate with serum/plasma. In the only previous study evaluating correlations between serum and urine estrogen/estrogen metabolite levels in premenopausal women using LC-MS/MS, correlations were modest (r ranged from 0.23–0.39) [26], and correlations between the relative concentrations of 2-, 4-, or 16-alpha hydroxylation pathway metabolites, which are relevant metrics for breast cancer risk [27], were not presented. No study has assessed urine compared to serum estrogen metabolite correlations in men or postmenopausal women, persons in whom estrogen concentrations are much lower and among whom the preponderance of epidemiologic studies of endogenous estrogens and cancer risk have been conducted.
Using high-quality LC-MS/MS assays, the current study sought to determine whether measured concentrations of estrogens/estrogen metabolites, individually and as pathway ratios, were comparable across the biological matrices of serum, plasma, and urine by sex and menopausal status.
MATERIALS/METHODS
Study Design
Estrogens and estrogen metabolite levels were measured in 64 healthy volunteers enrolled in the Research Donor Program conducted at the Frederick National Laboratory for Cancer Research’s Occupational Health Services (Frederick, MD) under an approved protocol. Blood and urine (first morning void) specimens were collected. Blood specimens were processed into in serum, EDTA plasma, heparin plasma. Donors included 18 men ages 32–60, 20 premenopausal women ages 30–51, and 26 postmenopausal women ages 44–69. Participants were de-identified via code, and all specimens were labelled using laboratory accession numbers. Individuals who reported use of testosterone or other hormonal supplements, oral contraceptives, or menopausal hormone therapy within three months of the blood draw were excluded. For this analysis, three postmenopausal women who were suspected to be on menopausal hormone therapy were excluded based on high levels of unconjugated estradiol (>30 pg/mL) in serum, leaving 23 postmenopausal women for analysis. As the motivation of this study was to assess the comparability of hormone levels over various collection types and specimen types, donors were not additionally selected based on factors that could affect estrogen profiles (e.g. body mass).
Laboratory Assays
A validated and reproducible stable isotope dilution LC-MS/MS assay was utilized to measure 15 estrogens and estrogen metabolites, including estrone, estradiol, 2-hydroxyestrone, 2-methoxyestrone, 2-hydroxyestradiol, 2-methoxyestradiol, 2-hydroxyestrone-3-methyl ether, 4-hydroxyestrone, 4-methoxyestrone, 4-methoxyestradiol, 16α-hydroxyestrone, 16-ketoestradiol, estriol, 17-epiestriol, and 16-epiestriol, in serum, plasma, and urine as previously described (18–19, 23) with updated instrumentation and additional stable isotope labeled estrogen metabolites. Briefly, LC-MS/MS analysis was performed using a Thermo TSQ Quantiva™ triple quadrupole mass spectrometer (Thermo Scientific, San Jose, CA) coupled with a Nexera XR LC system (Shimadzu Scientific Instruments, Columbia, MD). Both LC and mass spectrometer were controlled by Xcalibur™ software (Thermo Scientific). Twelve stable isotopically labeled estrogens and estrogen metabolites were used to account for losses during sample preparation and LC-MS/MS assays, which included deuterated estriol, (C/D/N Isotopes, Inc., Pointe-Claire, Quebec, Canada); deuterated 16-epiestriol (Medical Isotopes, Inc., Pelham, NH); and 13C-labeled estrone, estradiol, 2-hydroxyestrone, 2-methoxyestrone, 2-hydroxyestradiol, 2-methoxyestradiol, 2-hydroxyestrone-3-methyl ether, 4-hydroxyestrone, 4-methoxyestrone, and 4-methoxyestradiol (Cambridge Isotope Laboratories, Andover, MA). The calibration curves were linear over a concentration range of 1 pg/mL to 5000 pg/mL for all estrogens and estrogen metabolites. The assay limit of detection providing estrogen signal-to-noise ratio greater than 3-to-1 was 100 fg/mL (approximately 0.33–0.37 pmol/L). The assay lower limit of quantitation was 1 pg/mL for each estrogen and estrogen metabolite with intra-and inter-batch CV <10%, and assay accuracy between 85–115% of known targeted values.
Each serum/plasma sample was split into two aliquots, one to measure the combined concentration (the sum of conjugated plus unconjugated forms after a β-glucuronidase/sulfatase hydrolysis) of each of the 15 estrogens (listed above); the other prepared without enzymatic hydrolysis, to measure the unconjugated forms of estrone, estradiol, estriol, 2-methoxyestrone, and 2-methoxyestradiol. The combined concentration of serum (or plasma) estrogen represents, e.g., estradiol=estradiol-3-glucuronide+estradiol-3-sulfate+estradiol-17-glucuronide+estradiol-17-sulfate+unconjugated estradiol. Absolute recovery after hydrolysis and extraction ranged from 85%−92% [25]. Sex hormone binding globulin (SHBG) was measured in serum and plasma specimens using an electrochemiluminescence immunoassay on the Elecsys 2010 autoanalyzer (Roche Diagnostics, Indianapolis, IN). Free estradiol was calculated utilizing a formula including unconjugated estradiol, SHBG, and a constant for albumin (43 g/L) [28].
In urine, parent estrogens and their metabolites are present primarily in conjugated form. Thus, the combined concentration of each of the 15 estrogens was measured after β-glucuronidase/sulfatase hydrolysis, and the combined concentration of urinary estrogen represents, e.g., estradiol=estradiol-3-glucuronide+estradiol-3-sulfate+estradiol-17-glucuronide+estradiol-17-sulfate. Total urinary creatinine was measured in each sample by the Jaffé alkaline picrate method. We accounted for differences in urine concentration by standardizing molar quantities to creatinine (pmol/mg) [29, 30].
Statistical analysis
To quantify the comparability of estrogen levels in serum, plasma (heparin and EDTA), and urine, the geometric mean and range of each estrogen and estrogen metabolite was tabulated by biologic matrices and stratified by sex/menopausal status (male, premenopausal, or postmenopausal). Estrogens were then ranked by biologic matrix and sex/menopausal status accordingly. One-way ANOVA was conducted to determine differences between biological matrices utilizing blood (serum, EDTA plasma, and heparin plasma), and between all biological matrices. Hormone levels were log-transformed to normalize distributions and stabilize the variances. To compare results across specimens, Spearman’s rank correlation coefficients were calculated for continuous estrogen measurements and tertiles based on cutpoints within each sex/menopausal status group. Given the association between breast cancer and the ratios of estrogen metabolite pathway to combined estrogen concentrations [27], the ratio of pathway to combined (summed) estrogen concentrations in serum were compared to the same ratio in urine. Spearman’s correlation coefficients were also calculated to compare pathway:combined estrogen ratios in serum versus urine, and interpreted based on an established scale (negligible (<0.30), low (0.30–0.49), moderate (0.50–0.69), high (0.70–0.89), very high (0.90–1.0) [31]. Age and sex/menopausal status adjusted linear regression models were utilized to evaluate a potential relationship between serum and urine measurements of estrogens (modelled to represent the change in urinary estrogen concentration (dependent variable) per one-unit increase in serum estrogen concentration (independent variable)). Analyses were conducted in SAS Version 9.3 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA).
RESULTS
Study participants were predominantly Non-Hispanic white (~90%, Supplemental Table 1). The proportion with obese BMI varied by subgroup representing 30% of postmenopausal women, 25% of premenopausal women, and 22% of men. Among premenopausal women, 60% of women donated a blood and urine sample during the follicular phase of their menstrual cycle.
Estrogen and estrogen metabolite levels by serum, EDTA plasma, heparin plasma, and creatinine adjusted urine are presented across sex/menopausal groups in Table 1. For all groups, estrogen concentrations and their respective rankings were similar when comparing serum, EDTA plasma, and heparin plasma specimens (percent differences ranged from 0.25%−3.1% between serum and EDTA, and 0.53%−4.8% between serum and heparin plasma). SHBG concentrations were lower in EDTA plasma compared to serum and heparin plasma (p<0.01) among all subgroups, and this drove the variation seen in the calculated free estradiol concentrations across the blood matrices (although these differences were not statistically significant).
Table 1.
Geometric mean concentration (pmol/L), range, and rank order by sex/menopausal status group and collection method.
| Serum | EDTA Plasma | Heparin plasma | Urine (creatinine adjusted; pmol/mg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | min-max | Rank | GM | min-max | Rank | GM | min-max | Rank | Pa | GM | min-max | Rank | Pb | |
| Postmenopausal women | ||||||||||||||
| Unconjugated estrogens | ||||||||||||||
| Unconjugated Estrone | 41.2 | (15.6, 90.9) | 41.1 | (15.7, 89.5) | 41.3 | (15.7, 89.8) | 0.99 | . | - | |||||
| Unconjugated Estradiol | 13.6 | (4.2, 40.6) | 13.6 | (4.2, 40.5) | 13.6 | (4.3, 40.8) | 0.99 | . | - | |||||
| SHBG (nmol/L) | 63.3 | (16.2, 181) | 33.3 | (10.4, 80.8) | 60.1 | (15.9, 174) | <0.01 | . | - | |||||
| free estradiol | 0.3 | (0.1, 1.3) | 0.4 | (0.1, 1.3) | 0.3 | (0.1, 1.3) | 0.56 | . | - | |||||
| Unconjugated 2-Methoxyestrone | 11.7 | (1.2, 32.7) | 11.6 | (1.1, 32.8) | 11.5 | (1.2, 32.7) | 0.99 | . | - | |||||
| Unconjugated 2-Methoxyestradiol | 12.6 | (7.1, 24.5) | 13.1 | (7.0, 24.5) | 13.0 | (7.0, 24.0) | 0.93 | . | - | |||||
| Unconjugated Estriol | 6.8 | (2.4, 14.5) | 6.7 | (2.4, 14.5) | 6.7 | (2.4, 14.4) | 0.99 | . | - | |||||
| Combined unconjugated and conjugated estrogens | ||||||||||||||
| Parent Estrogens | ||||||||||||||
| Estrone | 253 | (56.5, 1070) | 1 | 254 | (57.7, 1070) | 1 | 255 | (57.2, 1069) | 1 | 0.99 | 5.0 | (0.4, 17.0) | 2 | <0.01 |
| Estradiol | 31.2 | (10.3, 90.9) | 8 | 31.4 | (10.4, 89.4) | 8 | 31.4 | (10.4, 89.9) | 8 | 0.99 | 0.7 | (0.05, 4.0) | 9 | <0.01 |
| 2-Hydroxylation Pathway | ||||||||||||||
| 2-Hydroxyestrone | 67.3 | (34.6, 168) | 3 | 67.2 | (34.4, 168) | 3 | 67.3 | (34.4, 168) | 3 | 0.99 | 2.1 | (0.4, 25.6) | 3 | <0.01 |
| 2-Hydroxyestradiol | 35.1 | (15.4, 125) | 6 | 35.3 | (15.7, 121) | 6 | 35.2 | (15.7, 120) | 6 | 0.99 | 0.9 | (0.2, 30.0) | 8 | <0.01 |
| 2-Methoxyestrone | 32.1 | (11.7, 76.0) | 7 | 32.1 | (11.6, 75.7) | 7 | 31.8 | (11.6, 75.0) | 7 | 0.99 | 1.4 | (0.1, 4.4) | 5 | <0.01 |
| 2-Methoxyestradiol | 35.8 | (20.3, 89.6) | 5 | 35.4 | (20.2, 89.7) | 5 | 35.3 | (20.2, 89.6) | 5 | 0.99 | 0.5 | (0.1, 2.0) | 10 | <0.01 |
| 2-Hydroxyestrone-3-methyl ether | 4.6 | (1.1, 15.3) | 12 | 4.6 | (1.1, 15.3) | 12 | 4.6 | (1.2, 15.3) | 12 | 0.99 | 0.2 | (0.02, 1.2) | 13 | <0.01 |
| 4-Hydroxylation Pathway | ||||||||||||||
| 4-Hydroxyestrone | 6.6 | (1.8, 25.4) | 10 | 6.5 | (1.8, 25.3) | 10 | 6.5 | (1.8, 25.3) | 10 | 0.99 | 0.4 | (0.04, 9.2) | 11 | <0.01 |
| 4-Methoxyestrone | 3.3 | (1.0, 21.4) | 14 | 3.3 | (1.0, 21.4) | 14 | 3.2 | (0.9, 21.4) | 14 | 0.99 | 0.2 | (0.04, 0.9) | 12 | <0.01 |
| 4-Methoxyestradiol | 1.4 | (0.6, 3.3) | 15 | 1.4 | (0.5, 3.3) | 15 | 1.4 | (0.6, 3.2) | 15 | 0.99 | 0.1 | (0.002, 0.2) | 15 | <0.01 |
| 16alpha-Hydroxylation Pathway | ||||||||||||||
| 16alpha-Hydroxyestrone | 39.2 | (18.3, 111) | 4 | 39.3 | (18.2, 111) | 4 | 39.2 | (18.3, 110) | 4 | 0.99 | 1.1 | (0.2, 9.8) | 7 | <0.01 |
| Estriol | 186 | (85.0, 600) | 2 | 186 | (84.6, 601) | 2 | 186 | (84.2, 598) | 2 | 0.99 | 9.2 | (3.4, 40.7) | 1 | <0.01 |
| 16-Ketoestradiol | 21.7 | (7.5, 78.2) | 9 | 21.7 | (7.5, 78.5) | 9 | 21.6 | (7.4, 78.0) | 9 | 0.99 | 1.4 | (0.4, 7.5) | 4 | <0.01 |
| 16-Epiestriol | 6.1 | (2.7, 14.5) | 11 | 6.1 | (2.7, 14.3) | 11 | 6.1 | (2.7, 14.3) | 11 | 0.99 | 1.2 | (0.3, 11.2) | 6 | <0.01 |
| 17-Epiestriol | 3.5 | (1.4, 7.2) | 13 | 3.5 | (1.4, 7.3) | 13 | 3.5 | (1.4, 7.4) | 13 | 0.99 | 0.2 | (0.03, 1.3) | 14 | <0.01 |
| Premenopausal women | ||||||||||||||
| Unconjugated estrogens | ||||||||||||||
| Unconjugated Estrone | 119 | (38.2,411) | 119 | (38.6, 420) | 119 | (38.0, 425) | 0.99 | . | - | |||||
| Unconjugated Estradiol | 181 | (26.9, 1067) | 182 | (27.1, 1072) | 182 | (27.0, 1076) | 0.99 | . | - | |||||
| SHBG (nmol/L) | 77.0 | (22.6, 166) | 40.1 | (12.8, 79.7) | 72.6 | (21.4, 158) | <0.01 | . | - | |||||
| free estradiol | 3.4 | (0.5, 17.4) | 4.5 | (0.7, 23.0) | 3.5 | (0.5, 17.6) | 0.62 | . | - | |||||
| Unconjugated 2-Methoxyestrone | 47.0 | (8.8, 550) | 47.0 | (8.8, 550) | 46.7 | (8.7, 553) | 0.99 | . | - | |||||
| Unconjugated 2-Methoxyestradiol | 19.6 | (8.2, 58.6) | 19.5 | (7.7, 58.6) | 19.4 | (7.7, 58.6) | 0.99 | . | - | |||||
| Unconjugated Estriol | 6.4 | (3.1, 19.4) | 6.3 | (3.1, 19.3) | 6.3 | (3.0, 19.1) | 0.99 | . | - | |||||
| Combined unconjugated and conjugated estrogens | ||||||||||||||
| Parent Estrogens | ||||||||||||||
| Estrone | 1301 | (322, 5238) | 1 | 1303 | (325, 5253) | 1 | 1305 | (325, 5260) | 1 | 0.99 | 18.7 | (3.7, 71.5) | 1 | <0.01 |
| Estradiol | 287 | (55.7, 1507) | 2 | 287 | (55.4, 1522) | 2 | 288 | (56.0, 1524) | 2 | 0.99 | 3.7 | (1.1, 12.5) | 7 | <0.01 |
| 2-Hydroxylation Pathway | ||||||||||||||
| 2-Hydroxyestrone | 228 | (55.0, 1349) | 4 | 229 | (53.9, 1362) | 4 | 229 | (53.9, 1365) | 4 | 0.99 | 7.6 | (0.8, 37.8) | 3 | <0.01 |
| 2-Hydroxyestradiol | 68.8 | (18.8, 543) | 7 | 68.9 | (19.3, 537) | 7 | 68.6 | (19.3, 535) | 7 | 0.99 | 1.9 | (0.1, 17.9) | 9 | <0.01 |
| 2-Methoxyestrone | 92.4 | (12.1, 771) | 6 | 92.1 | (12.0, 771) | 6 | 92.0 | (12.0, 769) | 6 | 0.99 | 4.6 | (1.3, 15.4) | 6 | <0.01 |
| 2-Methoxyestradiol | 59.6 | (30.5, 121) | 8 | 59.2 | (30.7, 120) | 8 | 58.8 | (30.6, 120) | 8 | 0.99 | 0.6 | (0.1, 1.7) | 10 | <0.01 |
| 2-Hydroxyestrone-3-methyl ether | 9.6 | (2.8, 91.8) | 12 | 9.6 | (2.8, 90.7) | 12 | 9.6 | (2.8, 90.5) | 12 | 0.99 | 0.4 | (0.05, 2.4) | 14 | <0.01 |
| 4-Hydroxylation Pathway | ||||||||||||||
| 4-Hydroxyestrone | 17.3 | (2.3, 56.3) | 11 | 17.3 | (2.4, 56.3) | 11 | 17.3 | (2.4, 56.3) | 11 | 0.99 | 0.6 | (0.04, 2.6) | 11 | <0.01 |
| 4-Methoxyestrone | 6.8 | (2.6, 21.2) | 13 | 6.8 | (2.6, 21.2) | 13 | 6.8 | (2.6, 21.1) | 13 | 0.99 | 0.4 | (0.1, 1.1) | 13 | <0.01 |
| 4-Methoxyestradiol | 2.0 | (0.7, 4.3) | 15 | 1.9 | (0.7, 4.2) | 15 | 1.9 | (0.7, 4.1) | 15 | 0.99 | 0.1 | (0.02, 0.3) | 15 | <0.01 |
| 16alpha-Hydroxylation Pathway | ||||||||||||||
| 16alpha-Hydroxyestrone | 112 | (15.6, 407) | 5 | 112 | (15.6, 407) | 5 | 112 | (15.6, 410) | 5 | 0.99 | 5.5 | (1.1, 32.8) | 4 | <0.01 |
| Estriol | 261 | (101, 513) | 3 | 260 | (100, 512) | 3 | 260 | (100, 514) | 3 | 0.99 | 15.0 | (2.3, 185) | 2 | <0.01 |
| 16-Ketoestradiol | 52.2 | (8.9, 146) | 9 | 52.0 | (8.9, 146) | 9 | 51.8 | (8.7, 144) | 9 | 0.99 | 5.1 | (1.2, 22.3) | 5 | <0.01 |
| 16-Epiestriol | 18.9 | (3.7, 63.6) | 10 | 18.8 | (3.7, 64.1) | 10 | 18.8 | (3.7, 63.1) | 10 | 0.99 | 3.4 | (1.0, 8.6) | 8 | <0.01 |
| 17-Epiestriol | 5.9 | (2.2, 40.2) | 14 | 5.9 | (2.2, 40.2) | 14 | 5.9 | (2.2, 39.2) | 14 | 0.99 | 0.5 | (0.04, 3.4) | 12 | <0.01 |
| Men | ||||||||||||||
| Unconjugated estrogens | ||||||||||||||
| Unconjugated Estrone | 56.6 | (36.2, 94.9) | 56.1 | (35.7, 93.5) | 56.2 | (35.5, 93.6) | 0.99 | . | - | |||||
| Unconjugated Estradiol | 54.2 | (30.5, 105) | 53.8 | (30.5, 105) | 54.1 | (30.9, 105) | 0.99 | . | - | |||||
| SHBG (nmol/L) | 33.7 | (13.2, 70.5) | 19.5 | (8.5, 36.9) | 31.8 | (13.2,60.2) | <0.01 | . | - | |||||
| free estradiol | 1.4 | (0.7, 2.8) | 1.6 | (0.9, 3.1) | 1.4 | (0.8, 2.8) | 0.58 | . | - | |||||
| Unconjugated 2-Methoxyestrone | 6.4 | (1.9, 21.9) | 6.3 | (1.8, 21.8) | 6.4 | (1.8, 21.7) | 0.99 | . | - | |||||
| Unconjugated 2-Methoxyestradiol | 18.3 | (10.2, 32.3) | 18.3 | (10.2, 32.3) | 18.2 | (10.2, 32.2) | 0.99 | . | - | |||||
| Unconjugated Estriol | 5.4 | (2.9, 17.7) | 5.4 | (2.9, 17.7) | 5.4 | (2.9, 18.0) | 0.99 | . | - | |||||
| Combined unconjugated and conjugated estrogens | ||||||||||||||
| Parent Estrogens | ||||||||||||||
| Estrone | 660 | (319, 1878) | 1 | 661 | (318, 1878) | 1 | 661 | (318, 1876) | 1 | 0.99 | 5.5 | (1.9, 14.6) | 2 | <0.01 |
| Estradiol | 104 | (58.7, 245) | 3 | 104 | (58.8, 245) | 3 | 104 | (59.2, 245) | 3 | 0.99 | 1.5 | (0.3, 6.9) | 4 | <0.01 |
| 2-Hydroxylation Pathway | ||||||||||||||
| 2-Hydroxy estrone | 62.8 | (30.0, 107) | 4 | 62.5 | (29.8, 107) | 4 | 62.5 | (29.8, 107) | 4 | 0.99 | 1.7 | (0.4, 5.2) | 3 | <0.01 |
| 2-Hydroxyestradiol | 47.0 | (28.3, 402) | 6 | 47.2 | (28.3, 400) | 6 | 47.1 | (27.9, 402) | 6 | 0.99 | 0.6 | (0.1, 5.9) | 9 | <0.01 |
| 2-Methoxyestrone | 31.6 | (16.6, 74.7) | 8 | 31.6 | (16.5, 74.3) | 8 | 31.4 | (16.1, 73.6) | 8 | 0.99 | 0.9 | (0.2, 1.8) | 8 | <0.01 |
| 2-Methoxyestradiol | 37.1 | (17.2, 77.7) | 7 | 37.0 | (17.1, 77.0) | 7 | 36.9 | (17.1,76.5) | 7 | 0.99 | 0.4 | (0.1, 5.8) | 11 | <0.01 |
| 2-Hydroxyestrone-3-methyl ether | 4.8 | (0.8, 12.4) | 12 | 4.7 | (0.8, 12.4) | 12 | 4.7 | (0.8, 12.3) | 12 | 0.99 | 0.1 | (0.03, 0.8) | 14 | <0.01 |
| 4-Hydroxylation Pathway | ||||||||||||||
| 4-Hydroxyestrone | 6.3 | (1.8, 23.4) | 11 | 6.3 | (1.8, 23.4) | 11 | 6.3 | (1.8, 23.4) | 11 | 0.99 | 0.4 | (0.1, 2.4) | 10 | <0.01 |
| 4-Methoxyestrone | 2.7 | (1.0, 6.4) | 14 | 2.7 | (1.0, 6.4) | 14 | 2.7 | (1.0, 6.4) | 14 | 0.99 | 0.2 | (0.02, 0.6) | 13 | <0.01 |
| 4-Methoxyestradiol | 1.5 | (1.0, 3.0) | 15 | 1.5 | (1.0, 3.0) | 15 | 1.5 | (1.0, 2.9) | 15 | 0.99 | 0.1 | (0.01, 0.2) | 15 | <0.01 |
| 16alpha-Hydroxylation Pathway | ||||||||||||||
| 16alpha-Hydroxyestrone | 55.2 | (9.8, 214) | 5 | 55.1 | (9.8, 214) | 5 | 55.1 | (9.8, 213.0) | 5 | 0.99 | 1.2 | (0.5, 2.5) | 5 | <0.01 |
| Estriol | 226 | (82.3, 1273) | 2 | 226 | (81.8, 1273) | 2 | 226 | (81.9, 1274) | 2 | 0.99 | 7.0 | (3.6, 20.8) | 1 | <0.01 |
| 16-Ketoestradiol | 31.3 | (10.4, 68.7) | 9 | 31.3 | (10.4, 68.4) | 9 | 31.2 | (10.4, 68.4) | 9 | 0.99 | 1.2 | (0.5, 2.6) | 6 | <0.01 |
| 16-Epiestriol | 12.2 | (3.5, 47.2) | 10 | 12.1 | (3.6, 47.2) | 10 | 12.1 | (3.6, 47.1) | 10 | 0.99 | 1.1 | (0.2, 3.5) | 7 | <0.01 |
| 17-Epiestriol | 4.3 | (1.6, 10.4) | 13 | 4.3 | (1.6, 10.6) | 13 | 4.3 | (1.6, 10.6) | 13 | 0.99 | 0.2 | (0.04, 0.9) | 12 | <0.01 |
p-value from one-way ANOVA to determine if estrogen measurements vary across any of the blood collection methods.
p-value from one-way ANOVA to determine if estrogen measurements vary comparing all collection methods.
Abbreviations: Ethylenediaminetetraacetic acid, EDTA; GM, geometric mean; min, minimum; max, maximum; p, p-value; Sex hormone-binding globulin, SHBG.
Both unconjugated and total serum metabolite concentrations are being compared to total urinary metabolite concentrations.
The rank order of estrogen/estrogen metabolites did not vary between serum or plasma specimens, but differences between men and women and between urine and serum were seen (Table 1). In serum, geometric mean estrone concentrations were highest: 253 pmol/L in postmenopausal women, 1301 pmol/L in premenopausal women, and 660 pmol/L in men. In postmenopausal women and men, this was followed by estriol (186 pmol/L and 226 pmol/L respectively), while in premenopausal women, estradiol ranked second (287 pmol/L). The highest concentrations were in the 2-hydroxylation (2-pathway) followed by the 16-hydroxylation pathway (16-pathway), while concentrations of 4-hydroxylation pathway (4-pathway) metabolites were lowest. Urinary geometric mean concentrations diverged from this pattern: among postmenopausal women and men, the highest concentration was estriol (9.2 pmol/mg creatinine and 7.0 pmol/mg respectively) followed by estrone (5.0 pmol/mg and 5.5 pmol/mg respectively), whereas in premenopausal women, estrone concentration was highest at 18.7 pmol/mg, followed by estriol (15.0 pmol/mg). Additionally, following the parent estrogens, the 16-pathway metabolites were highest in urine, followed by 2- and 4-pathway metabolites, respectively.
Given the comparable findings between serum and plasma estrogens, further analyses stratified by sex/menopausal status group and adjusted for age were conducted among serum and urinary estrogens only. Among postmenopausal women, serum and urinary concentrations of estrone and estradiol were indicative of a modest increase in urinary estrogen level for each unit increase in serum [estrone: β=0.87, p <0.01; estradiol: β=1.33, p<0.01] (Table 2). Unconjugated serum estrone (β=1.11, p<0.01), and unconjugated estradiol (β=1.18, p<0.01) were positively associated with their corresponding urinary conjugate. Unconjugated serum estradiol was also associated with urinary estrone in postmenopausal women (β=0.92, p<0.01). Similar positive associations were also found for 2-hydroxyestrone (β=2.04, p<0.01), and 2-hydroxyestradiol (β=1.33, p<0.01).
Table 2.
Comparison between log transformed serum and urinary (adjusted for creatinine) estrogen measurements by sex/menopausal statusa
| Age adjusted βb,c | SE | P | Spearman (continuous) | Spearman (tertiles) | |
|---|---|---|---|---|---|
| Postmenopausal women | |||||
| Unconjugated Estrone | 1.11 | 0.33 | <0.01 | 0.68 | 0.59 |
| Unconjugated Estradiol (w/ urinary estradiol) | 1.18 | 0.31 | <0.01 | 0.65 | 0.53 |
| Unconjugated Estradiol (w/ urinary estrone) | 0.92 | 0.22 | <0.01 | 0.76 | 0.66 |
| Unconjugated Estradiol (w/ urinary estriol) | 0.16 | 0.22 | 0.47 | 0.18 | 0.20 |
| Unconjugated 2-Methoxyestrone | 0.36 | 0.29 | 0.22 | 0.22 | 0.33 |
| Unconjugated 2-Methoxyestradiol | −0.29 | 0.36 | 0.43 | 0.05 | 0.07 |
| Unconjugated Estriol | 0.24 | 0.31 | 0.45 | 0.11 | 0.19 |
| Parent Estrogens | |||||
| Estrone | 0.87 | 0.21 | <0.01 | 0.69 | 0.54 |
| Estradiol | 1.33 | 0.28 | <0.01 | 0.69 | 0.53 |
| 2-Hydroxylation Pathway | |||||
| 2-Hydroxyestrone | 2.04 | 0.45 | <0.01 | 0.76 | 0.67 |
| 2-Hydroxyestradiol | 1.33 | 0.36 | <0.01 | 0.54 | 0.33 |
| 2-Methoxyestrone | 0.22 | 0.49 | 0.66 | −0.10 | −0.01 |
| 2-Methoxyestradiol | −0.51 | 0.44 | 0.26 | −0.19 | −0.14 |
| 2-Hydroxyestrone-3-methyl ether | 0.44 | 0.42 | 0.32 | 0.15 | 0.19 |
| 4-Hydroxylation Pathway | |||||
| 4-Hydroxyestrone | 0.74 | 0.36 | 0.06 | 0.34 | 0.20 |
| 4-Methoxyestrone | −0.26 | 0.31 | 0.40 | −0.09 | −0.07 |
| 4-Methoxyestradiol | −0.09 | 0.62 | 0.89 | −0.03 | −0.13 |
| 16alpha-Hydroxylation Pathway | |||||
| 16alpha-Hydroxyestrone | 0.76 | 0.37 | 0.05 | 0.40 | 0.20 |
| Estriol | 0.20 | 0.29 | 0.49 | 0.29 | 0.39 |
| 16-Ketoestradiol | 0.20 | 0.33 | 0.55 | 0.23 | 0.20 |
| 16-Epiestriol | −0.46 | 0.42 | 0.28 | −0.23 | −0.14 |
| 17-Epiestriol | −0.27 | 0.58 | 0.65 | −0.13 | −0.27 |
| Premenopausal women | |||||
| Unconjugated Estrone | 0.72 | 0.24 | <0.01 | 0.63 | 0.61 |
| Unconjugated Estradiol (w/ urinary estradiol) | 0.35 | 0.20 | 0.10 | 0.44 | 0.30 |
| Unconjugated Estradiol (w/ urinary estrone) | 0.42 | 0.16 | 0.02 | 0.60 | 0.60 |
| Unconjugated Estradiol (w/ urinary estriol) | 0.25 | 0.24 | 0.31 | 0.29 | 0.23 |
| Unconjugated 2-Methoxyestrone | 0.36 | 0.16 | 0.03 | 0.41 | 0.30 |
| Unconjugated 2-Methoxyestradiol | 0.51 | 0.24 | 0.05 | 0.23 | 0.14 |
| Unconjugated Estriol | 0.59 | 0.46 | 0.21 | 0.34 | 0.38 |
| Parent Estrogens | |||||
| Estrone | 0.61 | 0.20 | 0.01 | 0.59 | 0.60 |
| Estradiol | 0.46 | 0.21 | 0.05 | 0.52 | 0.53 |
| 2-Hydroxylation Pathway | |||||
| 2-Hydroxyestrone | 0.07 | 0.28 | 0.81 | 0.14 | 0.15 |
| 2-Hydroxyestradiol | 0.70 | 0.27 | 0.02 | 0.60 | 0.68 |
| 2-Methoxyestrone | 0.42 | 0.17 | 0.02 | 0.55 | 0.45 |
| 2-Methoxyestradiol | 0.35 | 0.37 | 0.36 | 0.22 | 0.15 |
| 2-Hydroxyestrone-3-methyl ether | 0.54 | 0.22 | 0.02 | 0.34 | 0.21 |
| 4-Hydroxylation Pathway | |||||
| 4-Hydroxyestrone | −0.07 | 0.28 | 0.81 | 0.01 | 0.00 |
| 4-Methoxyestrone | 0.05 | 0.27 | 0.85 | −0.06 | 0.00 |
| 4-Methoxyestradiol | 0.27 | 0.40 | 0.51 | 0.18 | 0.07 |
| 16alpha-Hydroxylation Pathway | |||||
| 16alpha-Hydroxyestrone | 0.12 | 0.30 | 0.69 | 0.28 | 0.22 |
| Estriol | 0.87 | 0.49 | 0.09 | 0.33 | 0.22 |
| 16-Ketoestradiol | 0.09 | 0.34 | 0.81 | 0.21 | 0.24 |
| 16-Epiestriol | 0.26 | 0.14 | 0.08 | 0.46 | 0.46 |
| 17-Epiestriol | 0.54 | 0.39 | 0.19 | 0.23 | 0.22 |
| Men | |||||
| Unconjugated Estrone | 0.55 | 0.43 | 0.22 | 0.27 | 0.08 |
| Unconjugated Estradiol (w/ urinary estradiol) | 1.14 | 0.49 | 0.03 | 0.53 | 0.42 |
| Unconjugated Estradiol (w/ urinary estrone) | 0.35 | 0.36 | 0.35 | 0.33 | 0.42 |
| Unconjugated Estradiol (w/ urinary estriol) | 0.55 | 0.37 | 0.16 | 0.42 | 0.42 |
| Unconjugated 2-Methoxyestrone | 0.42 | 0.26 | 0.12 | 0.22 | 0.08 |
| Unconjugated 2-Methoxyestradiol | −0.78 | 0.77 | 0.33 | −0.37 | −0.17 |
| Unconjugated Estriol | 0.38 | 0.27 | 0.18 | 0.28 | 0.25 |
| Parent Estrogens | |||||
| Estrone | 0.76 | 0.20 | <0.01 | 0.58 | 0.58 |
| Estradiol | 0.85 | 0.44 | 0.08 | 0.33 | 0.17 |
| 2-Hydroxylation Pathway | |||||
| 2-Hydroxyestrone | −0.16 | 0.43 | 0.72 | 0.04 | 0.25 |
| 2-Hydroxyestradiol | 0.28 | 0.36 | 0.45 | 0.05 | −0.08 |
| 2-Methoxyestrone | 0.29 | 0.39 | 0.48 | −0.06 | −0.33 |
| 2-Methoxyestradiol | −0.29 | 0.64 | 0.66 | 0.02 | 0.17 |
| 2-Hydroxyestrone-3-methyl ether | 0.12 | 0.31 | 0.72 | −0.01 | 0.08 |
| 4-Hydroxylation Pathway | |||||
| 4-Hydroxyestrone | −0.03 | 0.27 | 0.92 | 0.07 | 0.00 |
| 4-Methoxyestrone | 0.43 | 0.37 | 0.26 | 0.26 | 0.17 |
| 4-Methoxyestradiol | −0.38 | 0.57 | 0.51 | −0.19 | 0.00 |
| 16alpha-Hydroxylation Pathway | |||||
| 16alpha-Hydroxyestrone | 0.10 | 0.14 | 0.49 | 0.29 | 0.17 |
| Estriol | 0.02 | 0.21 | 0.94 | 0.07 | 0.17 |
| 16-Ketoestradiol | 0.11 | 0.17 | 0.56 | 0.20 | 0.00 |
| 16-Epiestriol | 0.79 | 0.16 | <0.01 | 0.79 | 0.83 |
| 17-Epiestriol | 1.12 | 0.33 | <0.01 | 0.65 | 0.50 |
Adjusted for age (continuous).
Statistically significant at p <0.05.
Beta coefficients are modelled to represent the change in urinary estrogen concentration (dependent variable) per one-unit increase in serum estrogen concentration (independent variable).
Abbreviations: SE, standard error; p, p-value.
In premenopausal women, the association between serum and urinary estrone was attenuated relative to what was found in postmenopausal women, but remained significant (β=0.61, p<0.01). An association between unconjugated serum estradiol and urinary estrone was observed (β=0.42, p=0.02), as well as an association between 2-hydroxyestradiol and its urinary conjugate (β=0.70, p=0.02). Additional associations were found between 2-methoxyestrone, and 2-hydroxyestrone-3-methyl ether (p=0.02). In men, serum estrone was similarly positively associated with urinary estrone (β=0.76, p<0.01), and unconjugated serum estradiol was associated with its urinary compliment (β=1.14, p=0.03). In contrast to post- and premenopausal women, metabolites of estriol in serum from men were positively associated with their complimentary measure in urine [16-Epiestriol (β=0.79, p<0.01), and 17-Epiestriol (β=1.12, p<0.01)].
Positive correlations, when present, ranged from moderately positive (e.g. estrone, r=0.69; estradiol, r=0.69), to negligible (e.g. estriol, r=0.29) in postmenopausal women, with the strongest correlation observed between unconjugated serum estradiol and urinary estrone (r=0.76), and serum 2-hydroxyestrone with its urinary compliment (r=0.76). In premenopausal women, correlations were generally lower than in postmenopausal women, ranging from moderately positive to negligible (e.g. estrone, r=0.59, estradiol, r=0.52), with the strongest correlation between unconjugated serum estrone and its urinary conjugate (r=0.63). Positive correlations in men were moderately positive to negligible, except for 16-epiestriol, which was strongly correlated with its urinary compliment (r=0.79). Ranked correlations did not change substantially when estrogens were categorized into tertiles (Table 2). Further, correlations for premenopausal women did not change when stratified by menstrual phase (follicular or luteal; results not shown).
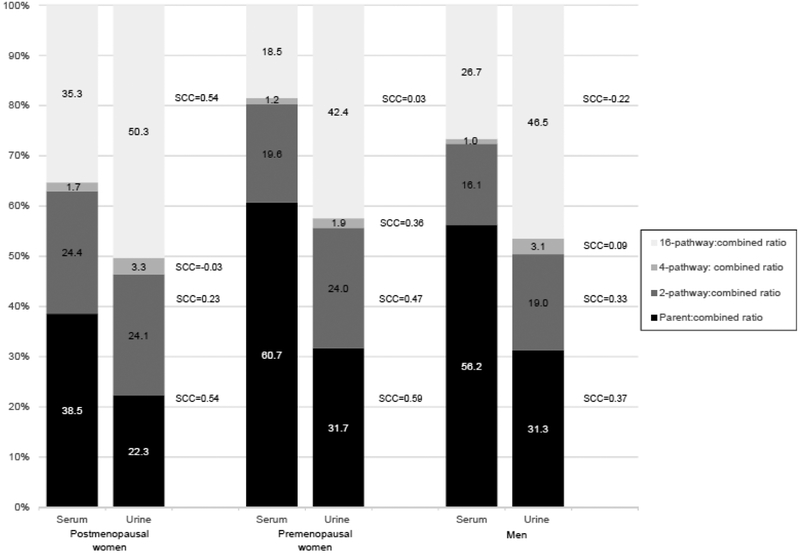
Figure 1 depicts the percentage of parent estrogens and pathway estrogen metabolites in serum compared to urine in each sex/menopausal group. Two patterns were observed: first, the relative contribution of parent and pathway estrogens differed in serum and urine; and second, parent estrogens were proportionately lower in postmenopausal women in comparison to men or premenopausal women. In serum, parent estrogens were highest in each group (comprising 61% of the total in premenopausal women, 56% in men and ~39% in postmenopausal women), followed by 16-pathway metabolites (proportions ranged from ~19%−35%). The proportion of 2- and 4-pathway metabolites was similar across groups (ranging from 16%−24% for 2-pathway and <2% for 4-pathway metabolites). In urine, 16-pathway metabolites were the largest contributors, accounting for 42% and 50% of total metabolites in pre- and postmenopausal women, respectively, and 47% of the total in men. Parent estrogens comprised 22%−31% of the total, while 2- and 4-pathway metabolites comprised 19%−24% and 2%−3%, respectively.
Figure 1.
Percentage of parent estrogen (estrone and estradiol) and 2-, 4- and 16-hydroxylation pathway metabolites in serum and creatinine adjusted urine measurements by sex and menopausal status. Spearman correlation coefficients (SCC) comparing serum and urinary measurements
Ratios of parent estrogens to combined estrogens/estrogen metabolite levels were consistently higher in serum than urine by sex/menopausal status, and were moderately correlated in pre- and postmenopausal women [pre: 60.7% in serum versus 31.7% in urine r=0.59; post: 38.5% in serum versus 22.3% in urine, r=0.54] and low correlation in men [56.2% in serum versus 31.3% in urine, r=0.37]. By contrast, the ratio of 16-pathway metabolites to the total combined levels was much higher in urine than serum by sex/menopausal status and correlations remained moderate [35.3% in serum versus 50.3% in urine, r=0.54; premenopausal women: 18.5% in serum versus 42.4% in urine r=0.03; men: 26.7% in serum versus 46.5% in urine, r=0.22]. The ratio of the 2-pathway metabolites to 16-pathway metabolites in serum compared to urine was low in postmenopausal women [r=0.36] and negligible in premenopausal women [r=−0.12] and men [r=0.06] (results not tabled). When comparing serum to urine the 2- and 4-pathway ratios were similar, though correlations ranged from negligible to moderate.
DISCUSSION
In the present analysis comparing serum, plasma, and urinary estrogen measurements, urinary concentrations of individual metabolites were correlated with serum concentrations, and plasma concentrations were commensurate with serum concentrations. As anticipated, prior to creatinine adjustment, urinary concentrations of estrogens were universally higher than concentrations in all blood matrices (results not reported). After creatinine adjustment, urinary concentrations were universally lower than concentrations in blood matrices. Absolute values and rankings of parent estrogens and estrogen metabolites in serum and plasma (heparin- or EDTA-preserved) were almost identical, and levels of SHBG were lower in EDTA plasma when compared to serum or heparin plasma (a finding that has been well-established in the literature [32]). By contrast, rankings for urinary estrogens deviated from measurements in blood, with a much higher proportion of 16-pathway metabolites observed in urine versus serum. Spearman’s correlation coefficients by sex and menopausal status comparing serum and urine ranged from negligible to moderate. Positive linear, age-adjusted associations were found between urinary and serum parent estrogen levels (which remained when using unconjugated serum estrone and estradiol compared to urinary estrone), and 2-hydroxyestradiol levels in pre- and postmenopausal women, as well as 2-methoxyestrone, and 3-hydroxyestrone-3-methyl ether in premenopausal women only, and 16-epiestriol and 17-epiestriol (p<0.05) in men indicating an increase in concentration of urine for a 1-unit increase in serum.
These findings are supported by a similar study assessing serum and urinary estrogen measurements among premenopausal women, although correlations were lower in the prior study. Maskarinec et al. reported correlations between urinary and serum estradiol of 0.35 while we found a relatively higher correlation of 0.52 [26]; however, the correlation between unconjugated serum estradiol and urinary estradiol in the present study was attenuated (r=0.44). This difference may be due to the inclusion of adjustment factors or the larger sample size (n=249) of the Maskarinec study, compared with 20 premenopausal women in the current analysis. In both the present analysis and the study conducted by Maskarinec et al., stratification by menstrual phase did not change correlations. The difference between the results from the Maskarinec et al. study and the present study may also be due to methodologic differences in the LC-MS/MS assays, e.g., differences in internal standards, hydrolysis efficiency, etc. Earlier works found strong correlations between urinary conjugated estrone (the sum of estrone sulfate and estrone glucuronide) and serum estradiol in women younger than 50 years old [11, 12]. However, the studies did not compare individual estrogens directly (i.e. serum estradiol to urinary estradiol) and were conducted before the implementation of high-performance LC-MS/MS which have substantially improved the precision and sensitivity of measurements in both serum and urine.
With respect to correlations between unconjugated serum and urinary metabolites of estrogens, the present findings differ in magnitude to what has been observed in the literature, predominantly among premenopausal women. In prior studies, serum estradiol and urinary estrone-3-glucuronide were highly correlated (r ranging from 0.60–0.93) [11, 12]. In the current analysis, the correlation between unconjugated serum estradiol and urinary estrone was 0.60 (for premenopausal women). Similarly, a correlation between serum estradiol and urinary estradiol-17β-glucuronide of 0.93 has been reported [13, 18], though our sample yielded a correlation of 0.44 between unconjugated serum estradiol and urinary estradiol in premenopausal women. Lastly, moderately high correlations between serum estradiol and urinary estriol (estriol-3-glucuronide r=0.81, estriol-16α-glucuronide r=0.83) were observed in one study [13], while the present study found a weak correlation of 0.29 between unconjugated serum estradiol and urinary estriol. Relative to prior findings, differences in observed correlations may be partly attributed to the timing of measurements in the menstrual cycle, and selection of samples within populations of women undergoing gonadotropin therapy in certain studies. Rather, our samples may be more representative of correlations seen in an unselected population. The modest correlations observed between urine and serum concentrations may be explained by how estrogens are metabolized and/or excreted, though it is unclear what mechanism drives this discrepancy. The LC-MS/MS assays in both the current study and the study by Maskarinec et al. measured the combined concentration of glucuronidated and sulfated urinary metabolites, which is a limitation of the current assay methodology. Thus, the modest correlations observed between urine and serum concentrations may also be due to the assay methods, as the prior comparisons using RIA and EIA assays directly quantified the individual urinary estrogen conjugates.
Utilizing a high-performing LC-MS/MS assay, this analysis presents novel findings on the comparability of serum, plasma, and urine among postmenopausal women and men, which had not been previously assessed. Additionally, as a result of utilizing broad selection criteria for this study sample, findings from this analysis provide evidence that is relevant to epidemiologic inquiry into hormone levels and health outcomes. Circulating levels of estrogen metabolites reflect metabolism in the liver, with some conjugates reentering circulation and transported to the kidney for excretion. Urinary levels therefore, are strongly influenced by kidney function, as well as diet and drug use. Lower SHBG measurements in EDTA plasma compared with serum and heparin plasma were to be anticipated since SHBG in EDTA plasma is denatured at room/refrigerator temperatures and the SHBG assay only measures non-denatured SHBG [32, 33]. Although peripheral blood and urinary estrogen measurements represent proxies of the intraorgan or tissue environment, they are frequently used in epidemiologic investigations of health outcomes. As such our study provides valuable information for the comparison of health outcome associations across studies utilizing different biospecimens.
Overall, the current study offers insight into the agreement between serum and urinary estrogens/estrogen metabolites, as well as the high degree of agreement between measurements in serum, EDTA plasma, and heparin plasma; however, our analyses were constrained by small sample sizes, and thus must be interpreted cautiously. Though urine was found to be correlated with serum estrogen concentrations when comparing individual markers one at a time, urine was less well correlated with serum when considering the estrogen profile or evaluating pathway and/or relative estrogen concentrations (e.g. proportion of 2-pathway of combined estrogen concentration or proportion of 2-pathway compared to parent estrogen measures). Given this, comparisons of studies utilizing pathway or relative estrogen concentration measurements with different specimen types (blood vs. urine) should be interpreted carefully.
Supplementary Material
Financial support:
This work was supported in part by the Intramural Research Program of the National Cancer Institute.
Footnotes
Conflicts of interest: All authors declare they have no conflicts of interest.
REFERENCES
- 1.Key T, Appleby P, Barnes I, Reeves G, Endogenous Hormones and Breast Cancer Collaborative G (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. Journal of the National Cancer Institute. 94: 606–16. [DOI] [PubMed] [Google Scholar]
- 2.Eliassen AH, Missmer SA, Tworoger SS, et al. (2006) Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 98: 1406–15. [DOI] [PubMed] [Google Scholar]
- 3.Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE (2013) Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses’ Health Study II. Breast cancer research : BCR. 15: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown SB, Hankinson SE (2015) Endogenous estrogens and the risk of breast, endometrial, and ovarian cancers. Steroids. 99: 8–10. [DOI] [PubMed] [Google Scholar]
- 5.Allen NE, Key TJ, Dossus L, et al. (2008) Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocrine-related cancer. 15: 485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborative Group On Epidemiological Studies Of Ovarian Cancer, Beral V, Gaitskell K, et al. (2015) Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 385: 1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannandrea F, Paoli D, Figa-Talamanca I, Lombardo F, Lenzi A, Gandini L (2013) Effect of endogenous and exogenous hormones on testicular cancer: the epidemiological evidence. The International journal of developmental biology. 57: 255–63. [DOI] [PubMed] [Google Scholar]
- 8.Ho SM, Lee MT, Lam HM, Leung YK (2011) Estrogens and prostate cancer: etiology, mediators, prevention, and management. Endocrinology and metabolism clinics of North America. 40: 591–614, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasley BL, Mobed K, Gold EB (1994) The use of urinary hormonal assessments in human studies. Annals of the New York Academy of Sciences. 709: 299–311. [DOI] [PubMed] [Google Scholar]
- 10.Munro CJ, Stabenfeldt GH, Cragun JR, Addiego LA, Overstreet JW, Lasley BL (1991) Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin Chem. 37: 838–44. [PubMed] [Google Scholar]
- 11.Alper MM, Halvorson L, Lasley B, Mortola J (1994) Relationship between urinary estrone conjugates as measured by enzyme immunoassay and serum estradiol in women receiving gonadotropins for in vitro fertilization. J Assist Reprod Genet. 11: 405–8. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor KA, Brindle E, Holman DJ, et al. (2003) Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research. Clin Chem. 49: 1139–48. [DOI] [PubMed] [Google Scholar]
- 13.Stanczyk FZ, Miyakawa I, Goebelsmann U (1980) Direct radioimmunoassay of urinary estrogen and pregnanediol glucuronides during the menstrual cycle. American journal of obstetrics and gynecology. 137: 443–50. [DOI] [PubMed] [Google Scholar]
- 14.Denari JH, Farinati Z, Casas PR, Oliva A (1981) Determination of ovarian function using first morning urine steroid assays. Obstetrics and gynecology. 58: 5–9. [PubMed] [Google Scholar]
- 15.Kesner JS, Knecht EA, Krieg EF Jr., et al. (1994) Validations of time-resolved fluoroimmunoassays for urinary estrone 3-glucuronide and pregnanediol 3-glucuronide. Steroids. 59: 205–11. [DOI] [PubMed] [Google Scholar]
- 16.Kesner JS, Wright DM, Schrader SM, Chin NW, Krieg EF Jr. (1992) Methods of monitoring menstrual function in field studies: efficacy of methods. Reproductive toxicology. 6: 385–400. [DOI] [PubMed] [Google Scholar]
- 17.Brown JB, Blackwell LF, Cox RI, Holmes JM, Smith MA (1988) Chemical and homogeneous enzyme immunoassay methods for the measurement of estrogens and pregnanediol and their glucuronides in urine. Prog Clin Biol Res. 285: 119–38. [PubMed] [Google Scholar]
- 18.Miyakawa I, Stanczyk FZ, March CM, March AD, Goebelsmann U (1981) Urinary estradiol-17-beta-glucuronide assay for gonadotropin therapy. Obstetrics and gynecology. 58: 142–7. [PubMed] [Google Scholar]
- 19.Bradlow HL, Sepkovic DW, Klug T, Osborne MP (1998) Application of an improved ELISA assay to the analysis of urinary estrogen metabolites. Steroids. 63: 406–13. [DOI] [PubMed] [Google Scholar]
- 20.Ball P, Reu G, Schwab J, Knuppen R (1979) Radioimmunoassay of 2-hydroxyesterone and 2-methoxyestrone in human urine. Steroids. 33: 563–76. [DOI] [PubMed] [Google Scholar]
- 21.Emons G, Mente C, Knuppen R, Ball P (1981) Radioimmunoassay for 4-hydroxyoestrone in human urine. Acta endocrinologica. 97: 251–7. [DOI] [PubMed] [Google Scholar]
- 22.McGuinness BJ, Power MJ, Fottrell PF (1994) Radioimmunoassay of 2-hydroxyestrone in urine. Clinical chemistry. 40: 80–5. [PubMed] [Google Scholar]
- 23.Xu X, Veenstra TD, Fox SD, et al. (2005) Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Analytical chemistry. 77: 6646–54. [DOI] [PubMed] [Google Scholar]
- 24.Santen RJ, Demers L, Ohorodnik S, et al. (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 72: 666–71. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG (2007) Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal.Chem. 79: 7813–21. [DOI] [PubMed] [Google Scholar]
- 26.Maskarinec G, Beckford F, Morimoto Y, Franke AA, Stanczyk FZ (2015) Association of estrogen measurements in serum and urine of premenopausal women. Biomark Med. 9: 417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson JN, Falk RT, Schairer C, et al. (2017) Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer research. 77: 918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Södergård R, Backstrom T, Shanbhag V, Carstensen H (1982) Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 16: 801–10. [DOI] [PubMed] [Google Scholar]
- 29.Falk RT, Xu X, Keefer L, Veenstra TD, Ziegler RG (2008) A liquid chromatography-mass spectrometry method for the simultaneous measurement of 15 urinary estrogens and estrogen metabolites: assay reproducibility and interindividual variability. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 17: 3411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Keefer LK, Ziegler RG, Veenstra TD (2007) A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nature protocols. 2: 1350–5. [DOI] [PubMed] [Google Scholar]
- 31.Mukaka MM (2012) Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 24: 69–71. [PMC free article] [PubMed] [Google Scholar]
- 32.Fillmore CM, Fears TR, Hoover RN, et al. (2000) METHODOLOGICAL NOTE: Biomarkers (sex-hormone binding globulin (SHBG), bioavailable oestradiol, and bioavailable testosterone) and processing of blood samples in epidemiological studies. Biomarkers. 5: 395–8. [DOI] [PubMed] [Google Scholar]
- 33.Bocchinfuso WP, Hammond GL (1994) Steroid-Binding and Dimerization Domains of Human Sex Hormone-Binding Globulin Partially Overlap - Steroids and Ca2+ Stabilize Dimer Formation. Biochemistry-Us. 33: 10622–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.