Abstract
Stress-activated MAP kinases (SAPKs) respond to a wide variety of stressors. In most cases, the pathways through which specific stress signals are transmitted to the SAPKs are not known. Our recent findings have begun to address two important and related questions. First, do various stresses activate a SAPK through common pathways initiated at the cell surface, or through alternative, intracellular inputs? Second, how does an activated SAPK mount a specific response appropriate to the particular stress experienced? Our work has uncovered the mechanisms by which two stresses, arsenite treatment and DNA damage, stimulate the yeast SAPKs Hog1 and Mpk1, respectively. We found that these stresses activate the SAPKs through intracellular inputs that modulate their basal phosphorylation, rather than by activation of the protein kinase cascades known to stimulate them. Both stresses act through targeting, in different ways, the tyrosine-specific or dual-specificity protein phosphatases that normally maintain the SAPKs in a low activity state. Previous work has demonstrated that basal signal flux through SAPK pathways is important for the sensitivity and dynamic response to external signals. Our work reveals that basal activity of SAPKs is additionally important to allow SAPK activation by intracellular inputs that modulate that activity. Additionally, because different stressors may activate SAPKs by modulation of basal signal through inputs at distinct nodes along the canonical activation pathway, stress-specific SAPK outputs may be controlled, in part, by the specific intracellular mechanisms of their activation. Thus, understanding the intracellular pathways through which various stressors activate SAPKs is likely to provide insight into how they elicit physiologically coherent responses to the specific stress experienced.
Keywords: Hog1, Mpk1, protein tyrosine phosphatase, dual-specificity phosphatase, arsenite, genotoxic stress, basal signal
Stress-activated MAP kinases (SAPKs), like Mitogen-activated protein kinases (MAPKs), are stimulated through protein kinase cascades connected to sensors or receptors on the cell surface that culminate in the dual phosphorylation of the SAPK on neighboring Tyr and Thr residues. It is intriguing that SAPKs, which are activated by a wide variety of stressors, subsequently regulate coherent, stress-specific outputs. In most cases, neither the pathways through which specific stress signals are transmitted to the SAPKs, nor the mechanisms that underlie their outputs, are known. There is a tendency to view SAPK pathways through the same lens as growth factor-activated MAPK pathways in which signals are initiated from the cell surface. However, with the large list of diverse stressors that activate a relatively small number of SAPKs, it seems increasingly likely that many of these stressors activate signaling through intracellular mechanisms, rather than by top-down signaling through their canonical kinase cascades. This is particularly true for the yeast Saccharomyces cerevisiae, which possesses only two SAPK activation pathways - the High Osmolarity Glycerol (HOG) pathway, which responds to hyper-osmotic stress, and the Cell Wall Integrity (CWI) pathway, which responds to cell wall stress. Both of these pathways are well characterized from their plasma membrane sensors to their kinase cascades and the transcription factors that act in response to these stresses. Yet both yeast SAPKs, Hog1 and Mpk1, are activated by a diverse collection of stressors that appear unlikely to act through the known cell surface sensors and do not activate the recognized transcriptional programs of these pathways.
Recently, we discovered two distinct, but related mechanisms by which different stress signals are transmitted to the two SAPKs of budding yeast S. cerevisiae (Lee and Levin, 2018; Liu and Levin, 2018). Our findings reveal that stressors can activate SAPKs through intracellular pathways that amplify the basal activity of the SAPK through action on its protein phosphatases, rather than by activation of their cognate protein kinase cascades. This work sheds new light on the importance of basal flux through SAPK cascades.
Activation of Hog1 by arsenite.
In the first study, we explored the mechanism by which the toxic metalloid arsenite (As[III]) activates Hog1, the SAPK of the High Osmolarity Glycerol (HOG) pathway (Lee and Levin, 2018), the functional ortholog of mammalian p38 SAPK (Han et al., 1994). The HOG pathway has been well characterized with regard to its response to hyper-osmotic shock (Saito and Posas, 2012). However, its mode of activation by a wide variety of stressors, including organic acids (Laurence et al., 2004; Mollapour and Piper, 2006), oxidative stress (Bilsland et al., 2004), heavy metals (Jiang et al., 2014), methylglyoxal (Aguilera et al., 2005), curcumin (Azad et al., 2014), arsenicals (Sotelo and Rodriguez-Gabriel, 2006; Thorsen et al., 2006; Lee and Levin, 2018), etc., is poorly understood. Where tested, the upstream components of the canonical HOG signaling pathway, including the cell surface osmosensors, were shown to be required for Hog1 activation by these various stressors. Although such results are consistent with the interpretation that signaling is initiated from the cell surface, they do not distinguish between this model and the possibility that upstream pathway components are required merely to provide basal signal to Hog1, which might be modulated by intracellular inputs.
Hog1 controls a coordinated adaptive response to hyper-osmotic shock that leads to the accumulation of intracellular glycerol (Saito and Posas, 2012). As part of this response, Hog1 closes the glycerol channel Fps1 through phosphorylation and eviction of a pair of redundant regulators, Rgc1 and Rgc2, whose presence in complex with the channel normally maintain it in an open state (Lee et al., 2013). Importantly, trivalent arsenic, or arsenite, uses Fps1 as its main entry port into the cell (Thorsen et al., 2006). Hog1 is important for survival of arsenite treatment, in part through closure of Fps1 (Thorsen et al., 2006). We found that Hog1 activated by arsenite closes Fps1 through phosphorylation of Rgc1 and Rgc2 on the same residues that are targeted in response to hyper-osmotic shock (Lee and Levin, 2018), revealing an important connection between the responses to these two stresses. However, the pathway leading to Hog1 activation by arsenite is quite different from that of hyper-osmotic shock. The HOG pathway is activated in response to hyper-osmotic shock through stimulation of the cell surface osmo-sensors, which trigger activation of two parallel protein kinase cascades that converge upon Hog1 (Fig. 1A). In contrast to this, we found that arsenite must enter the cell to activate Hog1, suggesting that this stressor does not act at the cell surface. Cells that are blocked for Fps1 function not only display tolerance to arsenite, but fail to activate Hog1 in response to exposure.
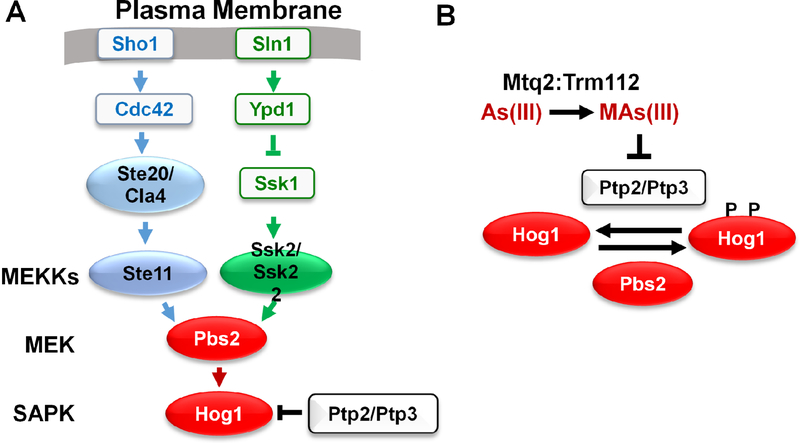
Fig 1.
A. The High Osmolarity Glycerol (HOG) pathway. In response to hyper-osmotic shock, two pathway branches are activated at the cell surface by plasma membrane osmosensors, and converge upon Pbs2, the MEK of the HOG pathway. B. Mechanism of Hog1 activation by arsenite. Arsenite (As[III]) is converted to methylarsenite (MAs[III]) by the dimeric methyl transferase Mtq2:Trm122. MAs inhibits Ptp2 and Ptp3, the tyrosine-specific protein phosphatases that maintain Hog1 in a low-activity state. This shifts the equilibrium to phosphorylated (active) Hog1 and relies on basal phosphorylation of Hog1 by MEK Pbs2.
To assess the level at which arsenite interfaces with the HOG pathway, we mutationally severed Hog1 from the upstream components of the canonical HOG pathway and restored a low-level of basal signal to the SAPK by expression of a constitutive form of Pbs2, which encodes the MEK that functions immediately above Hog1. Arsenite treatment activated Hog1 in this setting, suggesting that its action was at the level of Hog1 itself. This interpretation was corroborated by the finding that arsenite treatment did not activate Pbs2. These results suggested the possibility that Hog1 is activated in response to arsenite through inhibition of Ptp2 and Ptp3, the tyrosine-specific protein phosphatases that normally maintain this SAPK in an inactive state. Although arsenite can react with cysteine residues in proteins, it did not inhibit Ptp3 activity. However, a methylated metabolite of arsenite (methylarsenite; MAs) had been shown previously to inhibit mammalian tyrosine phosphatases PTPB1 and CD45 through reaction with cysteine residues at their active sites (Rehman et al., 2012). We found that, to activate Hog1, arsenite must be metabolically activated to MAs by the dimeric methyltransferase Mtq2:Trm112, and that MAs is a potent inhibitor of Ptp3 (Fig. 1B). It may be that the methylated arsenical is able to access the phosphatase active site, whereas arsenite cannot. Thus, arsenite activates Hog1 by modulating the basal activity of Hog1 through action on its inhibitory phosphatases, rather than by stimulating signaling through the HOG pathway. It is important to note that basal flux through the kinase cascade is a critical aspect of this mode of regulation. Without it, regulation of a counteracting protein phosphatase would be without effect.
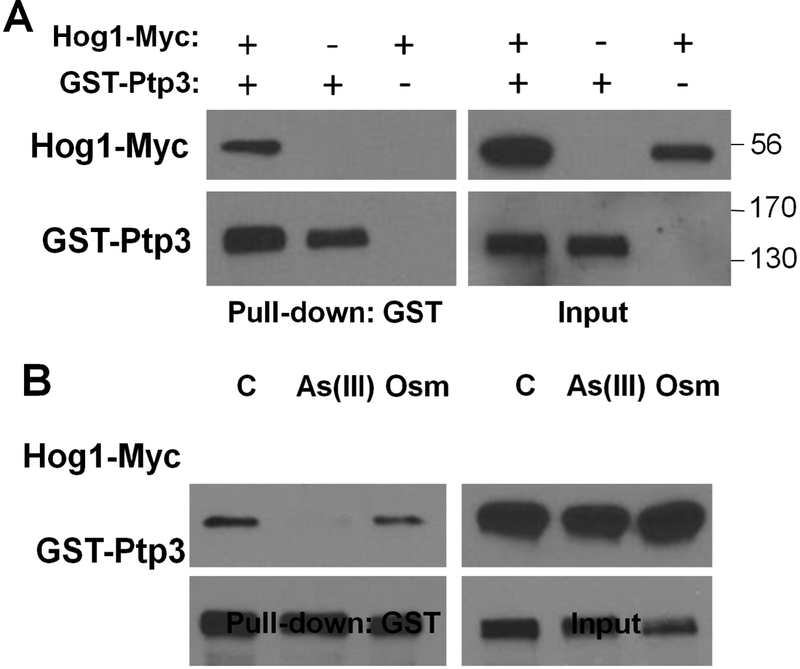
It is interesting that the cellular response to arsenite overlaps with, but is not identical to, the response to hyper-osmotic shock. For example, Hog1 activated in response to arsenite treatment fails to drive gene expression in support of glycerol production, but contributes to arsenic-specific gene expression through transcription factor Acr1 (Sotelo and Rodríguez-Gabriel, 2006). How a SAPK that is stimulated by different stresses can initiate coherent, stress-specific responses remains an important and largely unexplored question. At least two possible explanations are worthy of consideration. One is that SAPKs activated through various intracellular inputs may engage in the formation of distinct complexes that alter their target specificity or their subcellular localization. Our recent finding that the association between Ptp3 and Hog1 is disrupted by arsenite treatment, but not by hyper-osmotic shock, may be relevant to this model. Hog1-Myc was co-precipitated with GST-Ptp3 (Fig. 2A). Although this association remained intact during hyper-osmotic shock, it was lost in response to arsenite treatment (Fig. 2B). Such changes in SAPK complex formation may play an important role in dictating signal output. Another likely explanation for stress-specific SAPK outputs is that each intracellular stressor may have additional targets that modify the output of the activated SAPK. For example, as noted above, arsenite reacts with cysteine thiols in proteins. Arsenic adducts may modify the activity or interactions of SAPK target proteins in a manner that alters their regulation by the active kinase.
Fig. 2.
Co-precipitation of Hog1 with Ptp3. A. Extracts from a wild-type yeast strain BY4741 (Research Genetics) expressing Myc-tagged Hog1 (Hog1-Myc), or Glutathione-S-transferase-tagged Ptp3 (GST-Ptp3), or both, were subjected to affinity purification of GST-Ptp3 with glutathione agarose beads followed by SDS-PAGE and immunoblot detection of Hog1-Myc (using mouse monoclonal α-Myc antibody 9E10), or GST-Ptp3 (using mouse monoclonal α-GST antibody B-14). Molecular mass markers (in kDa) are on the right. Details of the expression plasmids and purification methods can be found in Lee and Levin (2018). B. Ptp3 dissociates from Hog1 in response to arsenite treatment. Wild-type cells co-expressing Hog1-Myc and GST-Ptp3 were subjected to 1 mM arsenite (As[III]) for 5 min, 1 M sorbitol (Osm) for 5 min as a hyper-osmotic shock, or untreated (C). Extracts were processed as above for detection of Hog1 association with Ptp3.
Activation of Mpk1 by genotoxic stress.
In the second study, we examined the mechanism by which genotoxic stress activates Mpk1, the SAPK of the Cell Wall Integrity (CWI) signaling pathway (Liu and Levin, 2018). As its name implies, this pathway responds to cell wall stress during growth and morphogenesis (Klis et al., 2002; Lesage and Bussey, 2006; Levin, 2011). Although activation of the CWI pathway and its response to cell wall stress have been studied extensively, the mechanism of Mpk1 activation in response to other stressors, including oxidative stress (Vilella et al., 2005), heat shock (Kamada et al., 1995), endoplasmic reticulum stress (Chen et al., 2005; Babour et al., 2010), and genotoxic stress (Queralt and Igual, 2005; Soriano-Carot et al., 2012), is much less well understood.
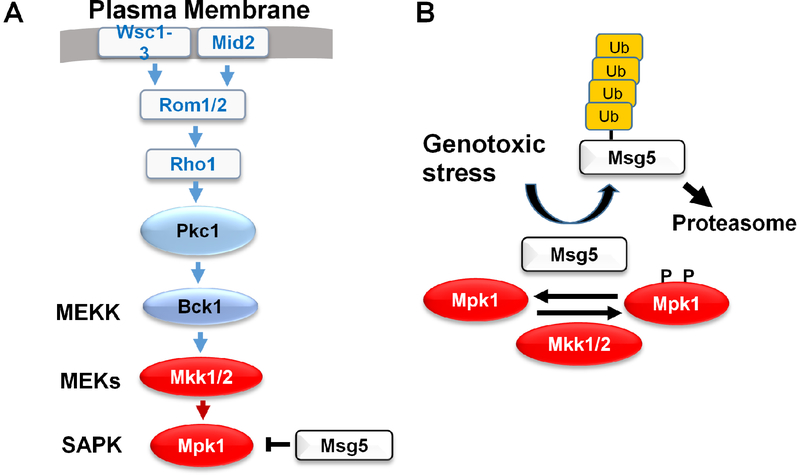
Mpk1 is the ortholog of mammalian ERK5/BMK1 (Truman et al., 2006) and is important for survival of genotoxic stress. Mpk1 is activated by a variety of DNA damaging agents, including double-strand breaks induced by the HO endonuclease (Soriano-Carot et al., 2012), strongly suggesting the existence of an intracellular pathway to Mpk1 activation. We approached this problem in a manner similar to our studies of Hog1 activation by arsenite. Specifically, we mutationally severed Mpk1 from its upstream activators and restored to it a low-level of basal signal by expression of a constitutive form of Mkk1, one of two redundant MEKs that act on this SAPK (Fig. 3A). In this setting, Mpk1 was activated in response to treatment either with hydroxyurea (HU) or methylmethane sulfonate (MMS), agents that act in very different ways to induce genotoxic stress. Moreover, in contrast to cells treated with calcofluor white, a cell wall stress-inducing agent, MEK Mkk1 was not activated in response to genotoxic stress. These findings implicated the dual-specificity phosphatase, Msg5, which forms a complex with Mpk1 and maintains this SAPK in a low-activity state in the absence of stress. We found that genotoxic stress induced ubiquitin-mediated proteolysis of Msg5. Inhibition of the proteasome with MG-132 during HU treatment resulted in the accumulation of highly ubiquitinated forms of Msg5 and prevented activation of Mpk1 (Fig. 3B). Taken together, these findings revealed that genotoxic stress activates Mpk1 by modulating basal activity of the SAPK through destruction of Msg5, rather than by stimulating signaling through the CWI pathway. The targets of Mpk1 activity that are important for survival of genotoxic stress have yet to be identified. However, as was the case for arsenite activation of Hog1, Mpk1 activation by DNA damage does not drive the transcriptional program understood to be associated with the signaling pathway, indicating the existence of mechanisms that evoke stress-specific outputs from the SAPK.
Fig. 3.
The Cell Wall Integrity (CWI) pathway. In response to cell wall stress, cell surface sensors initiate signaling though the SAPK cascade. B. Mechanism of Mpk1 activation by genotoxic stress. A DNA damage signal drives ubiquitination and proteolysis of Msg5, the dual-specificity protein phosphatase that maintains Mpk1 in a low-activity state. This shifts the equilibrium to phosphorylated (active) Mpk1 and relies on basal phosphorylation of Mpk1 by the redundant MEKs, Mkk1 and Mkk2.
Macia et al. (2009) demonstrated the importance of basal signal through the HOG pathway with regard to the sensitivity and response time for activation by hyper-osmotic shock. High basal signal is driven specifically through the Sln1 osmo-sensor branch of the HOG pathway and poises the system to respond rapidly to small changes in osmolarity. It is now clear that basal activity of yeast SAPKs is also important for their activation by intracellular inputs that modulate this activity through inhibition of tyrosine-specific or dual-specificity phosphatases. Moreover, intracellular inputs to SAPKs may extend beyond regulation of these phosphatases. It is possible that a multitude of inputs interface with SAPK pathways at various levels and that distinct mechanisms of SAPK activation contribute to the diversity of stress-specific outputs. Mammalian JNK, p38, and ERK5 SAPKs similarly respond to a wide variety of stressors. However, it is not yet clear if basal signal is likewise important for the activation of these SAPKs in response to intracellular stress signals.
Acknowledgements
Supported by NIH grant R01GM048533 to D.E.L.
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- Aguilera J, Rodrígues-Vargas S, Prieto JA (2005) The HOG MAP kinase pathway is required for the induction of methylglyoxal-responsive genes and determines methylglyoxal resistance in Saccharomyces cerevisiae. Mol Microbiol 56:228–239. 10.1111/j.1365-2958.2005.04533.x [DOI] [PubMed] [Google Scholar]
- Azad GK, Singh V, Thakare MJ, Barawal S, Tomar RS (2014) Mitogen-activated protein kinase Hog1 is activated in response to curcumin exposure in the budding yeast Saccharomyces cerevisiae. BMC Microbiol 14:317–327. 10.1186/s12866-014-0317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babour A, Bicknell AA, Tourtellotte J, Niwa M (2010) A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell 142:256–269. 10.1016/j.cell.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland E, Molin C, Swaminathan S, Ramne A, Sunnerhagen P (2004) Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol Micro 53:1743–1756. 10.1111/j.1365-2958.2004.04238.x [DOI] [PubMed] [Google Scholar]
- Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC (2005) Identification of mitogen-activated protein kinase signaling pathways that confer resistance to endoplasmic reticulum stress in Saccharomyces cerevisiae. Mol Cancer Res 3:669–677. 10.1158/1541-7786.MCR-05-0181 [DOI] [PubMed] [Google Scholar]
- Han J, Lee J-D, Bibbs L, Ulevitch RJ (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808–811. 10.1126/science.7914033 [DOI] [PubMed] [Google Scholar]
- Jiang L, Cao C, Zhang L, Lin W, Xia J, Xu H, Zhang Y (2014) FEMS Yeast Res. Cadmium-induced activation of high osmolarity pathway through its Sln1 branch is dependent on the MAP kinase kinase kinase Ssk2, but not its paralog Ssk22, in budding yeast. FEMS Yeast Res 14:1263–1272. 10.1111/1567-1364.12220 [DOI] [PubMed] [Google Scholar]
- Kamada Y, Jung US, Piotrowski J, Levin DE (1995) The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev 9:1559–1571. 10.1101/gad.9.13.1559 [DOI] [PubMed] [Google Scholar]
- Klis FM, Mol P, Hellingwerf K, Brul S (2002) Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Micro Rev 26:239–256. 10.1111/j.1574-6976.2002.tb00613.x [DOI] [PubMed] [Google Scholar]
- Lawrence CL, Botting CH, Antrobus R, Coote PJ (2004) Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: regulating adaptation to citric acid stress. Mol Cell Biol 24:3307–3323. 10.1128/MCB.24.8.3307-3323.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Levin DE (2018) Intracellular mechanism by which arsenite activates yeast stress MAPK Hog1. Mol Biol Cell 29:1904–1915. 10.1091/mbc.E18-03-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Reiter W, Dohnal I, Gregori C, Beese-Sims S, Kuchler K, Ammerer G, Levin DE (2013) MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes Dev 27:2590–2601. 10.1101/gad.229310.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE (2011) Regulation of cell wall biosynthesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 189:1145–1175. 10.1534/genetics.111.128264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage G, Bussey H (2006) Cell wall assembly in Saccharomyces cerevisiae. Micro Mol Biol Rev 70:317–343. 10.1128/MMBR.00038-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Levin DE (2018) Intracellular mechanism by which genotoxic stress activates yeast SAPK Mpk1. Mol Biol Cell Epub ahead of print. 10.1091/mbc.E18-07-0441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia J, Regot S, Peeters T, Conde N, Solé R, Posas F (2009) Dynamic signaling in the Hog1 MAPK pathway relies on high basal signal transduction. 10.1126/scisignal.2000056 [DOI] [PubMed] [Google Scholar]
- Mollapour M, Piper PW (2006) Hog1p mitogen-activated protein kinase determines acetic acid resistance in Saccharomyces cerevisiae. FEMS Yeast Res 6:1274–1280. 10.1128/MCB.02205-06 [DOI] [PubMed] [Google Scholar]
- Queralt E, Igual JC (2005) Functional connection between the Clb5 cyclin, the protein kinase C pathway and the Swi4 transcription factor in Saccharomyces cerevisiae. Genetics 171:1485–1498. 10.1534/genetics.105.045005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K, Chen Z, Wang WW, Wang YW, Sakamoto A, Zhang YF, Naranmandura H, Suzuki N (2012) Mechanisms underlying the inhibitory effects of arsenic compounds on protein tyrosine phosphatase (PTP). Toxicol Appl Pharmacol 263:273–280. 10.1016/j.taap.2012.06.019 [DOI] [PubMed] [Google Scholar]
- Saito H, Posas F (2012) Response to hyperosmotic stress. Genetics 192:289–318. 10.1534/genetics.112.140863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Carot M, Bañó MC, Igual JC (2012) The yeast mitogen-activated protein kinase Slt2 is involved in the cellular response to genotoxic stress. Cell Div 7, 10.1186/1747-1028-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo J, Rodríguez-Gabriel MA (2006) Mitogen-activated protein kinase Hog1 is essential for the response to arsenite in Saccharomyces cerevisiae. Euk Cell 5, 1826–1830. 10.1128/EC.00225-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen M, Di Y, Tängemo C, Morillas M, Ahmadpour D, Van der Does C, Wagner A, Johansson E, Boman J, Posas F, et al. (2006) The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol Biol Cell 17:4400–4410. 10.1091/mbc.e06-04-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman AW, Millson SH, Nuttall JM, King V, Mollapour M, Prodromou C, Pearl LH, Piper PW (2006) Expressed in the yeast Saccharomyces cerevisiae, human ERK5 is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpk1p) cell integrity stress-activated protein kinase. Euk Cell 5:1914–1924. 10.1128/EC.00263-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella F, Herrero E, Torres J, de la Torre-Ruiz MA (2005) Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. J Biol Chem 280:9149–9159. 10.1074/jbc.M411062200 [DOI] [PubMed] [Google Scholar]