Abstract
Macular xanthophylls (MXs) are distinguished from other dietary carotenoids by their high membrane solubility and preferential transmembrane orientation. Additionally, these properties enhance the chemical and physical stability of MXs in the eye retina, and maximize their protective activities. The effectiveness of MXs’ protection is also enhanced by their selective accumulation in the most vulnerable domains of retinal membranes. The retina is protected by MXs mainly through blue-light filtration, quenching of the excited triplet states of potent photosensitizers, and physical quenching of singlet oxygen. To perform these physical, photo-related actions, the structure of MXs should remain intact. However, the conjugated double-bond structure of MXs makes them highly chemically reactive and susceptible to oxidation. Chemical quenching of singlet oxygen and scavenging of free radicals destroy their intact structure and consume MXs. Consequently, their physical actions, which are critical to the protection of retina, are diminished. Thus, it is timely and important to identify mechanisms whereby the chemical destruction (bleaching) of MXs in retinal membranes can be reduced. It was shown that nitroxide free radicals (spin labels) located in membranes protect MXs against destruction, and their effect is especially pronounced during the light-induced formation of singlet oxygen. That should extend and enhance their positive action in the retina through physical processes. In this review, we will discuss possible applications of this new strategy during ophthalmological procedures, which can cause acute bleaching of MXs and damage the retina through oxidative processes.
Keywords: macular xanthophylls, oxidative stress, lipid peroxidation, antioxidants, age-related macular degeneration
1. Introduction
One of the major process that leads to eye retina degradation is oxidative stress. During that process, through formation of reactive oxygen species (ROS), lipids and proteins are oxidized and destroyed. It is commonly accepted that macular xanthophylls (MXs) (see Fig. 1 for their structures) protect the retina against degenerative processes (Gale et al., 2003; Koushan et al., 2013; Landrum et al., 1996; Obana et al., 2008). Because humans cannot synthesize carotenoids, they acquire them through dietary intake. During biological evolution, only two carotenoids, namely lutein (Lut) and zeaxanthin (Zea), were chosen from those available in the human diet and available in blood plasma to protect the retina. The reason for that selection is not clear, but as discussed previously (Widomska and Subczynski, 2014), the major factors leading to their selection are their high membrane solubility and their transmembrane orientation.
Fig. 1.

Chemical structures of MXs (Lut, Zea, meso-Zea). Chemical structures of phospholipids (DLPC, PAPC, DMPC) and nitroxide free radicals (CTPO, TEMPONE, 16-PC) mentioned in this review.
Major protection of the retina against oxidative damage occurs through the involvement of MXs in photo-related processes, including blue-light absorption, quenching of excited triplet states of photosensitizers (PSs), and physical quenching of singlet oxygen. These processes require the structure of MXs to be intact. Also, the retina can be protected through the dark processes that involve MXs in chemical reactions with singlet oxygen and interception of ROS. These chemical reactions, however, destroy carotenoids and diminish the protection provided through photo-related physical processes. Most of MXs’ protective activities are related to photo-related physical processes; in order to enhance and prolong these actions, MXs’ structure must remain intact.
In this review, we discuss the mechanisms through which the protective actions of MXs are enhanced in the retina membranes. We also indicate mechanisms that were developed during biological evolution to keep the structures of MXs intact, so that their protective actions are more effective and longer lasting. Finally, we propose a new strategy for maintaining intact MXs structures during oxidative stress, which should enhance their protective activity in the retina.
2. Physical antioxidant actions
2.1. Blue-light filtration
The human eye possesses short-wavelength light-blocking filters formed by the chromophores of the cornea, iris, lens, and macula lutea. These filters protect the retina from the short-wavelength light-associated oxidative damage to the photoreceptors cells and the retinal pigment epithelium (RPE). First, the cornea absorbs all the UV light below 295 nm (Sliney, 2002). Next, the light is filtrated by the melanin located in iridial melanocytes, with an absorption increasing monotonically from visible-NIR range to UV light (Borovansky and Riley, 2011). The low-molecular-weight tryptophan metabolite is the major lens pigment that provides the third level of protection by absorbing light between 295 and 400 nm with a peak at 365 nm (Dillon, 1995). Finally, MXs of the macula lutea, which form the last filter, exhibit an absorption spectrum with the maximum around 450 nm (which reaches minimum at 360 and 520 nm) (Fig. 2). However, at high concentrations, MX molecules tend to aggregate in a card-packed manner, which causes a shift in their light absorption toward short wavelengths, whereas binding with protein shifts its absorption toward longer wavelengths. Thus, the cornea, and lens block most of the light below 400 nm, whereas the MX filter absorbs about 40% of the blue light part of the visible spectrum (Schwartz et al., 2010; Youssef et al., 2011).
Fig. 2.

Absorption spectra of MXs (Zea and Lut) in ethanol.
The spatial distribution of MXs in the retina is probably dictated by the specific distribution of photoreceptors, which should be protected by this carotenoid pigment. It should be noted that the highest concentration of MXs is only in the central part of the retina—in the foveola—which occupies less than 0.01% of the total area of the retina (see yellow color in Fig. 3). The central part of fovea (foveola) contains only cones (Purves et al., 2001). The long-wavelength sensitive “red” cones and the middle-wavelength sensitive “green” cones are concentrated in the human foveola. The short-wavelength sensitive “blue” cones are mostly found outside the central region of the fovea. Therefore, MXs protect cones responsible for photopic vision, primarily “red” and “green” cones (but not “blue” cones and rods). High concentrations of MXs outside the fovea would reduce the blue light input on this part of retina and cause the loss of scotopic vision by the retina. It is worth noting that early pathology of age-related macular degeneration occurs in the rod-rich region of retina (Curcio et al., 1996; Okano et al., 2012). The parafoveal and perifoveal regions of the human macula are affected first, with the relative preservation of the cone-rich region. These different susceptibilities to light-induced degeneration of cones and rods can be related to the accumulation of toxic PSs (all-trans retinal) occurring in rods (Masutomi et al., 2012) and the lack of the protection by the blue-light MX filter (fewer MXs are present in the rod-rich region).
Fig. 3.
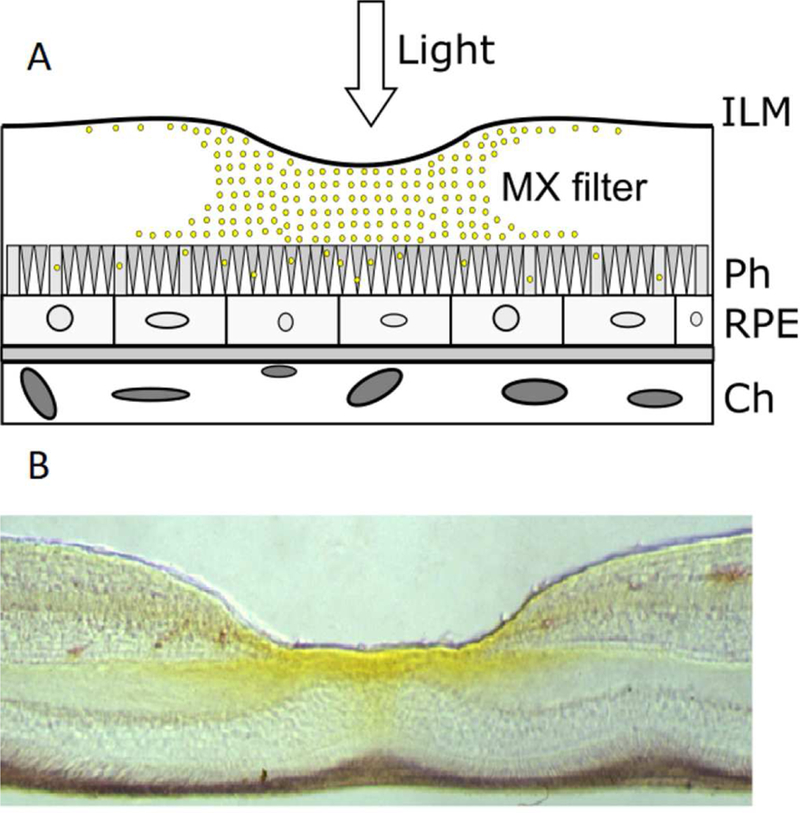
(A) Schematic representation of a section through the retina at the fovea. The symbols are MXs - macular xanthophylls; ILM - inner limiting membrane; Ph - photoreceptor; RPE - retinal pigmented epithelium; Ch - choroid. (B) Vertical section through a monkey fovea showing the distribution of MXs (yellow colors). MXs are most concentrated within the inner part of the foveola. (Adapted with permission from Snodderly, D.M., 1995. Am J. Clin. Nutr. 62, 1448S-1461S.)
MXs are distributed on the retina surface to protect “red” and “green” cones, which are mostly used for precision vision, from photodamage. Also, the exposure to light of cones in the foveola is significantly greater than the exposure of rods on periphery of the retina. This distribution of MXs also allows rods to perform their function in vision because the blue light is not filtered in the area where they are located. As shown in Fig. 3, yellow pigmentation is more intense in inner retinal layers and Henle fibers (Snodderly, 1995). Yellow pigment is also evident in the central part of the foveola in the photoreceptor outer layer. Thus, the MX filter decreases the blue light exposure in that region where the antioxidant and protective action of carotenoids in lipid phase is most needed. This can be considered as a self-defense mechanism against the toxic effect of blue-violet light exposure, also protecting MXs in the most vulnerable regions of the retina.
2.2. Quenching of excited triplet states of photosensitizers
The retina contains a large number of triplet PSs that, when activated, could lead to visual cell damage. They include vitamin A derivatives, melanin, and the lipofuscin-chromophore A2E, as well as mitochondrial flavins, flavoproteins, and cytochromes (Wood et al., 2007). Triplet PSs can be efficiently excited by visible light to the short-lived (nanoseconds) singlet state that, through the process of intersystem crossing, can flip into the long-lived (microseconds) triplet excited state. The lifetime of the excited triplet state of PSs is long enough to significantly increase the probability of collisions with other molecules, including molecular oxygen. During collisions with molecular oxygen (in its ground triplet state), energy from the triplet excited state of the PS is transferred to molecular oxygen, producing highly reactive and toxic singlet oxygen. This type II photosensitization is schematically illustrated in Fig. 4B. The second possible channel of deactivation of PSs (type I photosensitization) involves electron-transfer or hydrogen-atom abstraction between the excited PS and other intracellular molecule, yielding free radicals. Xanthophylls, due to the lower energy level of their excited triplet state, are efficient quenchers of excited triplet states of PSs, therefore preventing the generation of singlet oxygen (Fig. 4A). Quenching of excited triplet states of chlorophyll by carotenoids is well known and was intensively studied in higher plants and bacteria (Mozzo et al., 2008). Two molecules of Lut in the light-harvesting complex II of higher plants quench the long-lived chlorophyll excited triplet state efficiently, with efficiency close to 100% (Nechushtai et al., 1988).
Fig. 4.

Diagram illustrating the physical antioxidant actions of MXs: blue-light filtration and physical quenching of excited triplet state of PSs (A) and blue-light filtration and physical quenching of singlet oxygen (B).
Although, the fact that xantophylls play an important protective role in photosynthesis is well-known, the quenching of excited triplet states of endogenous retinal PSs by MXs has not been investigated. To perform effective quenching, MXs and potent retinal PSs should be co-localized. Sommerburg et al. have shown that about 25% of the total amount of MXs present in the entire retina is located in the human rod outer segments (Sommerburg et al., 1999). In another study, it was shown that approximately 10% to 15% of MXs were found in rod outer segments of the perifoveal retina (Rapp et al., 2000). These were the first studies showing that Lut and Zea are also associated with photoreceptor outer segments. However, these two studies do not correlate well with cross-sectional imaging studies that show that the concentration of MXs decreases with the increasing distance from the fovea (i.e., more than 100-fold just a few millimeters from the foveal center) (Bone et al., 1997). There is an additional factor that ensures the close proximity of MXs and potent retinal PSs. One such potent retinal PS is all-trans retinal that, with its long-lifetime excited triplet state, effectively generates singlet oxygen (Masutomi et al., 2012; Różanowska and Sarna, 2005). It is known that photoactivation of rhodopsin leads to isomerization of its chromophore, 11-cis-retinal to all-trans-retinal, which under certain conditions can act as a photosensitizer. Rhodopsin, which is the source of free all-trans retinal, is located in the bulk domain of outer photoreceptor segments enriched with polyunsaturated phospholipids (Boesze-Battaglia et al., 2002; Polozova and Litman, 2000; Stinson et al., 1991) (rhodopsin requires the presence of polyunsaturated lipids for its activity (Litman and Mitchell, 1996; Mitchell et al., 1992)). Also, MXs are concentrated in this bulk domain and are substantially excluded from raft domains enriched in saturated phospholipids and cholesterol (Wisniewska and Subczynski, 2006a, 2006b). Thus, co-localization of MXs with rhodopsin may allow PSs in photoreceptor membranes to be effectively neutralized.
2.3. Quenching of singlet oxygen
If the quenching of the excited triplet states of retinal PSs by MXs is inefficient, toxic singlet oxygen is formed. Carotenoids are effective quenchers of singlet oxygen. Physical quenching is the major process of deactivation of singlet oxygen by MXs. It involves energy transfer from the excited state of oxygen to the MX molecule forming the ground state of oxygen and the excited triplet state of the MX. The MX molecule converts excess energy into heat and returns it to its ground state (see schematic drawing in Fig. 4B). It is important that the structure of MXs is not destroyed during this process. Retaining an intact structure ensures that MX molecules can be reused, thus increasing the efficiency of the quenching process. The physical quenching of singlet oxygen by MXs is much more effective than chemical quenching (see Sect. 3.1). It should be noted that, in organic solvents, the efficiency of singlet oxygen quenching by MXs changes with the number of conjugated double bonds in the molecule. Zea, with its 11 conjugated double bonds, is a more efficient singlet oxygen quencher than Lut, with only 10 conjugated double bonds in the molecule. In benzene (Edge et al., 1997) and in lipid bilayers (Cantrell et al., 2003), Lut quenches singlet oxygen at a rate equal to half that at which Zea quenches singlet oxygen (Edge and Truscott, 2018).
A very important aspect of MX’s antioxidant action is its presence in the lipid bilayer portion of photoreceptor membranes, because generation of singlet oxygen straightforwardly depends on the local oxygen concentration and oxygen diffusion in the place where PSs are located. PSs located in the membrane interior can activate a significantly greater number of oxygen molecules than in water because the oxygen-diffusion-concentration product in the membrane center is two–three times greater than in water (Fig. 5). This process of enhanced reactivity in the membrane is called “membrane lens effect” (Cordeiro, 2014; Liu et al., 1998). MXs, with their rigid structure and the transmembrane location in the lipid bilayers, reduce the oxygen diffusion-concentration product in the lipid bilayer center (see Fig. 5), reduce the rates of singlet oxygen formation (Subczynski et al., 1991), and thus reduce the destruction of lipid molecules and MXs themselves.
Fig. 5.
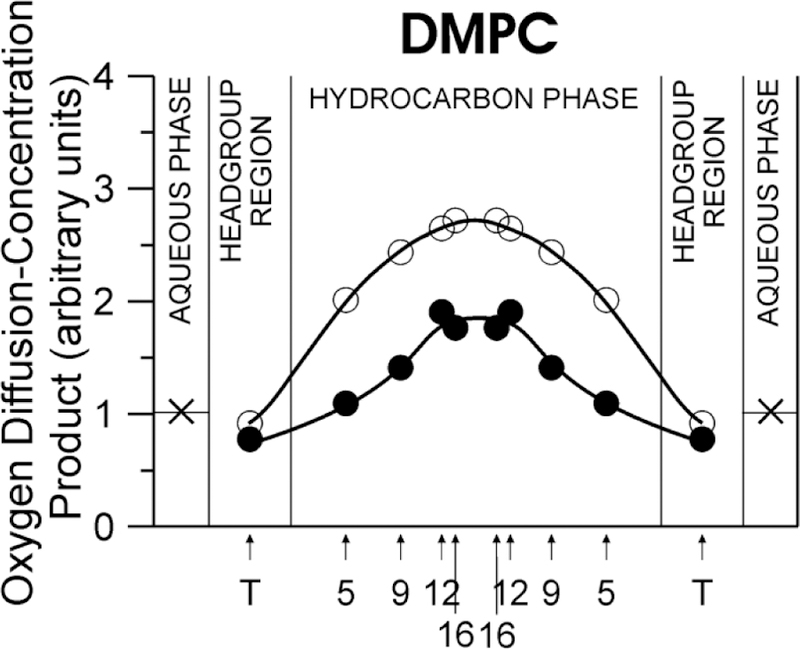
Profiles of the relative oxygen-diffusion-concentration product across the DMPC bilayer containing 0 (○) and 10 mol% Zea (●) at 25°C. (Obtained with permission from Subczynski et al., 1991. Biochim. Biophys. Acta 1068, 68–72.)
3. Chemical antioxidant actions
In the retina, MXs are associated with membranes subjected to light and a high level of oxygen. Additionally, MXs are selectively located in membrane domains that are very rich in long-chain polyunsaturated fatty acids, especially docosahexaenoic acid (Wisniewska and Subczynski, 2006b). All these promote production of ROS and increase the probability of involving MXs in chemical reactions. As a result of these reactions, MXs are consumed, their intact structure is destroyed, and their physical actions, which are critical to protecting the retina, are diminished. Below, we identify mechanisms of the chemical destruction (bleaching) of MXs in retinal membranes.
3.1. Reactions with singlet oxygen
There are two kinds of singlet oxygen quenching by MXs: chemical quenching and physical quenching (described in Sect.2.3). Although, only a minority portion (less than 0.05% (Stahl and Sies, 2003)) of singlet oxygen is deactivated through chemical quenching, this reaction is significant because it consumes MXs and destroys their intact structure. Although, the chemical reaction of MXs with singlet oxygen protects lipids and proteins in retina membranes, the degradation of MXs that accompanied this process leads to the loss of their ability to protect retina through the physical action, including filtration of blue light, quenching of excited states of PSs, and physical quenching of singlet oxygen. Chemical reactions of MXs with singlet oxygen in the retina tissue are not well described or understood. However, analogous to the reaction of β-carotene with singlet oxygen, we can conclude that endoperoxides are also the specific products of the oxidation of MXs by singlet oxygen (Fig. 6). Formation of carotenoid endoperoxides is specific to the chemical reaction of carotenoid with singlet oxygen, and cannot occur through enzymatic pathways or by reactions with free radicals (Havaux, 2014; Ramel et al., 2012). In fact, some oxidative metabolites of MXs were found in the retinal tissue (Bhosale et al., 2004; Khachik et al., 1997).
Fig. 6.

Diagram illustrating the chemical antioxidant actions of MXs during which the intact structure of MXs is destroyed.
3.2. ROS interception
Some of the MX molecules are lost by sacrificing mechanisms while protecting the retina. This may be the result of singlet oxygen chemical quenching as well as reactions of MXs with other ROS. The conjugated polyene chain is responsible for the susceptibility of MXs to free-radical attacks. In many reactions with ROS, MXs are broken down into shorter compounds, e.g., apo-carotenoids (Sui et al., 2013). The absorption spectrum of apo-carotenoids is shifted toward shorter wavelengths. It is assumed that, during ROS interception, the high antioxidant properties of MXs decrease, and they lose their blue-light filtration abilities.
3.3. Chain-breaking action
Another sacrificing mechanism that occurs during protection of the retina is the lipid chain-breaking action through which MXs protect lipid molecules; however, this causes MXs to lose their intact structure. The reaction of carotenoid molecules with lipid-derived radicals may stop the propagation of lipid peroxidation. This reaction has been demonstrated to occur via three possible paths: adduct formation, hydrogen abstraction, and electron transfer. During the first path (adduct formation), the carotenoid radical adduct is formed. When the chain-breaking mechanism involves he hydrogen abstraction process, a neutral carotenoid radical is produced. When carotenoids exert their chain-breaking action through electron transfer, carotenoid radical cations and anions are formed. In all three processes, the unpaired electron of the free radical is transferred to the MX molecule, so a new MX radical (or MX radical adduct) is formed (Fig. 6). Reactions of this MX radical with other lipid-derived radicals result in additional nonradical products. Additionally, MX radicals can react with oxygen, producing a chain-carrying peroxyl radical. MX radicals may also decay to MX epoxides when a new alkoxyl radical is formed (Fig. 6). All the reactions that occur between MXs and lipid radicals involve sacrificial consumption of pigment to produce less-reactive molecules and reduce lipid peroxidation. Both rod and cone photoreceptors have outer segment disc membranes that are highly enriched with polyunsaturated fatty acids, particularly docosahexaenoic acid, which are susceptible to oxidation (Fliesler and Anderson, 1983). Colocalization of lipid-soluble antioxidants such as MXs and polyunsaturated fatty acids (Wisniewska and Subczynski, 2006b) protect that environment by suppressing lipid oxidation. MXs may protect lipids directly by consuming lipid radicals and indirectly by quenching singlet oxygen, which can react with the electron-rich double bonds of unsaturated phospholipids.
4. Mechanisms enhancing the antioxidant actions of MXs
4.1. Distribution in the retina
It is unclear why MXs are distributed differently in the plane of the retina, with a significantly greater concentration of Zea in the center (with Zea/Lut = 2.4/1) as compared with the periphery region (with Zea/Lut = 1/2) (Bone et al., 1997, 1988). This distribution is very different from the natural abundance of Zea in the human diet (with Zea/Lut = 1/7) (Landrum and Bone, 2001) and in human blood plasma (with Zea/Lut = 1/4) (Curran-Celentano et al., 2001). For some reason, the retina increases the pool of Zea and additionally “developed” special enzymatic machinery, which transfers directly in the retina the part of Lut into the isomer of Zea (meso-Zea). Gruszecki’s group (Gruszecki and Strzałka, 2005; Sujak et al., 1999) provided a promising explanation for this phenomenon with the suggestion that Zea and Lut orient differently in the lipid bilayer, with Zea resembling only transmembrane orientations and Lut resembling both transmembrane and parallel-to-membrane surface orientations. However, in their recent work, Grudzinski et al. indicate that both Zea and Lut only remain in transmembrane orientations in the lipid bilayer membranes (Grudzinski et al., 2017). So, the reasons for the high concentration of Zea in the central region of the retina and the transformation of some of Lut into meso-Zea has been unclear until now.
Easiest to understand is the reason for the distribution of MXs in the front of the photoreceptors (Fig. 3A), which significantly enhances the protective power of MXs. The location of a significant amount of MXs between incoming light and the photoreceptors ensures that the blue-light filtration forms the first line of defense (Trieschmann et al., 2008). Decreasing the intensity of blue light prior to potentially involving it in the formation of ROS through the PSs is very effective. Possibly because of that, nature used most of the MX molecules available in the retina for this defense mechanism. These MXs are predominantly located in the fibers of Henle and the plexiform layers (Trieschmann et al., 2008). This location is ideal if MXs have to act as blue-light filters, effectively protecting the photoreceptors located in the foveal pit. It has been estimated that MXs absorb approximately 40% of blue light before it is incident on the photoreceptors (Loane et al., 2008; Sabour-Pickett et al., 2012).
4.2. Transmembrane localization in lipid-bilayer portions of retina membranes
Conclusions about the effects of the transmembrane localization of MXs in the lipid bilayer portion of retina membranes on their antioxidant actions in the retina can be made when their antioxidant properties are compared with the antioxidant properties of other dietary carotenoids in organic solvents and in membranes. These comparisons show that, in organic solvents, the antioxidant properties of both MXs (Zea) and β-carotene are similar. However, when incorporated into lipid bilayer membranes, they show very different antioxidant properties. In membranes, dipolar Zea reacts with free radicals more effectively than monopolar β-cryptoxanthin and much more effectively than nonpolar β-carotene (Woodall et al., 1997, 1995). Nonpolar carotenoids such as β-carotene and lycopene can react only with free radicals presented (generated) inside the lipid bilayer. Due to the presence of polar hydroxyl groups at the ends of molecules and their exposure to an aqueous environment, dipolar MXs can also scavenge free radicals presented and generated in the aqueous phase (Britton, 1995). All of these observations suggest that the anchoring of MX molecules at opposite membrane surfaces helps to maximize their protective actions in the retina.
4.3. Localization in the most vulnerable membrane regions
We think that the protective activity of MXs is significantly enhanced by their preferential location in the most vulnerable domains of retina membranes. In membranes with raft domains, it was shown that MXs are 8–14 times more concentrated in the bulk domain as compared with the raft domain (Wisniewska and Subczynski, 2006a, 2006b). It should be noted that raft domains are rich in stable, saturated phospholipids and cholesterol, while bulk domains contain polyunsaturated phospholipids and a much lower concentration of cholesterol. Thus, MXs are excluded from the membrane region that does not need their protection and concentrated in regions that will prompt lipid peroxidation. This is especially significant in photoreceptor cells (membranes of rods and cones), where raft domains and bulk domains coexist and where MXs’ membrane concentration is low. Excluding MXs from stable rafts and concentrating them in vulnerable bulk domains should enhance their protective actions. Bulk domains are rich in polyunsaturated phospholipids such as PUFAS and VLPUFAS. Also, photoreceptor machinery (rhodopsin) is located in these domains, increasing the damaging actions of the light. Thus, the preferential location of MXs in bulk domains encourages their antioxidant actions.
4.4. Xanthophyll-binding proteins
The selective uptake of MXs into the retina from blood plasma suggests involvement of specific xanthophyll-binding proteins. However, there is no consensus as to whether these proteins are only selective transporters of MXs or whether they can also store MXs. Two different human ocular proteins, which are involved in Lut and Zea transport, were identified (Li et al., 2010)). They include the high-affinity Lut-binding protein StARD3 (steroidogenic acute regulatory domain protein 3) (Li et al., 2011) and the Zea-binding protein (one form of glutathione S-transferases [GSTs]) (Bhosale et al., 2004). The Lut-binding protein StARD3 belongs to a group of transport proteins that regulates cholesterol transfer within the mitochondria. GSTs, by contrast, are a well-known, diverse family of phase II detoxification enzymes that catalyze the conjugation of glutathione. One of the classes of human cytosolic GST is the pi isoform of GST (GSTP1). GSTP1 was identified as a Zea-binding protein with a high-affinity for Zea and meso-Zea but a low affinity for Lut. The interaction of MXs with GSTP1 enhances the antioxidant effect of MXs in model lipid membranes (Bhosale and Bernstein, 2005). It was shown that the synergistic effect of Zea and GSTP1 is involved in the prevention of unsaturated lipid membranes against the oxidation induced by two lipid peroxyl radical generators, AAPH (2,2′-Azobis(2-amidinopropane) dihydrochloride) and AMVN (2,2′-azobis (2,4-dimethylvaleronitrile)) (Bhosale and Bernstein, 2005). Their data indicate that some part of this synergistic activity is derived from the protein’s ability to bind and preserve the intact structure of MXs. Thus, the interaction of Zea (and meso-Zea) with GSTP1 enhances the antioxidant effect of these MXs. We can conclude that most likely both membrane-located, Lut- and Zea-binding proteins can stabilize MXs chemically and enhance their protective activity (Bhosale and Bernstein, 2005).
4.5. Mechanisms maintaining the intact structure of MXs
4.5.1. Natural mechanisms (endogenous)
Most of the preventive functions of MXs occur through their physical actions; equally significant is the protection of highly unsaturated retina membranes through the trapping of chain-initiating and/or chain-propagating peroxyl radicals. These chemical actions consume MXs. It is possible that some portion of carotenoid radicals can be regenerated by their interaction with α-tocopherol and ascorbate to keep MXs intact (Burke et al., 2001; Edge et al., 1998; Mortensen and Skibsted, 1997). It is postulated that natural lipid-soluble antioxidants, MXs and α-tocopherol, provide synergistic protection against the photo-induced oxidative damage of photoreceptor membranes (Koga and Terao, 1995; Thiyam et al., 2006). Another natural water-soluble antioxidant, ascorbate, can be added to this synergistic chain because, during lipid oxidation, the α-tocopherol radical can be repaired by ascorbate (Traber and Stevens, 2011). This synergistic effect may be explained by effective scavenging of free radicals in photoreceptor membranes by the interaction between α-tocopherol and ascorbate, by keeping MXs’ structure intact, and by allowing MXs to protect membranes through very efficient physical processes. Nature diminished MXs’ involvement in dark chemical actions through many physical processes. Preventing blue light from penetrating deep into the retina decreases the efficiency of PSs excitation and singlet oxygen formation. Also, the majority of singlet oxygen is quenched by MXs through a physical quenching process. All of these mechanisms can be considered as “self-protection” for MXs.
MXs are dipolar carotenoids that are more chemically stable than nonpolar carotenoids (Socaciu et al., 2000). This stability manifests itself through great resistance of MXs to autoxidation. This is an important feature because MXs perform most of their protective actions in their intact structure. Also, evidence exists that their unique localization and transmembrane orientation in lipid bilayer membranes increases their chemical stability. Less chemically stable carotenes can be oxidized easier in lipid bilayer membranes (Socaciu et al., 2000) and can even function as prooxidant compounds (Palozza et al., 2003; Zhang and Omaye, 2001). MXs are also physically stable in retina membranes. This stability is manifest by their very long retention time in the retina.
4.5.2. Chemical interventions (exogenous)
The turnover of the MXs pool is very slow. In a study where healthy volunteers were given xanthophyll supplements, the removal of MXs from the retina after discontinuation of the supplements was observed to be very slow (about 50 days) (Landrum et al., 1997). In similar studies, an increase in MX concentration in the blood plasma was observed one to two days after beginning supplementation, whereas it was increased in the retina after several weeks (Berendschot et al., 2000; Hammond et al., 1997; Johnson et al., 2000). Thus, because of this slow recycling, the chemical reactions occurring in the retina membranes can destroy a significant portion of MXs. Destroyed MX molecules must be replaced by new xanthophyll molecules, which are supplied from the food and absorbed through blood plasma. Thus, for MXs to protect the retina, it is imperative that their structure remains intact. As discussed in Sect. 4.5.1, there are few natural endogenous mechanisms that protect the intact structure of MXs. However, the acute need for additional protection is encountered during eye surgery and laser procedures, when partial pressure of oxygen in the retina is elevated and the retina is exposed to intensive light. Destruction of MXs during these oxidative conditions decreases their photoreceptor protection at a time when this protection is especially needed. Thus, it is timely and important to develop additional exogeneous mechanisms whereby the chemical destruction (bleaching) of MXs in retinal membranes can be diminished.
Promising results were obtained by Zareba et al. (Zareba et al., 2016), which indicate that membrane-located nitroxide free radicals (spin labels) protect MXs located in DLPC membranes against chemical destruction (bleaching) during autoxidation of DLPC molecules (Fig. 7A). The strongest effect was observed with phospholipid nitroxide spin label 1-palmitoyl-2-(16-doxylstearoyl)phosphatidylcholine (16-PC). The nitroxide free radical CTPO, located mainly in the water phase, had virtually no effect on the rate of bleaching of MXs. The water soluble TEMPONE, which can also partition into the lipid bilayer, provides partial protection of MXs (see Fig. 1 for structures of nitroxide free radicals). The protection of MXs by 16-PC was especially pronounced during the light-induced formation of singlet oxygen and the singlet-oxygen-induced lipid peroxidation of polyunsaturated membranes (made of 1-palmitoyl-2-arachidonoylphosphochatidyline (PAPC)) (Fig. 7B). The protective effect of 16-PC was compared with the protection provided by α-tocopherol, which is the natural antioxidant and protector of MXs within the retina. Results presented in Fig. 7B indicate that α-tocopherol was about two times less effective in protecting MXs than 16-PC.
Fig. 7.
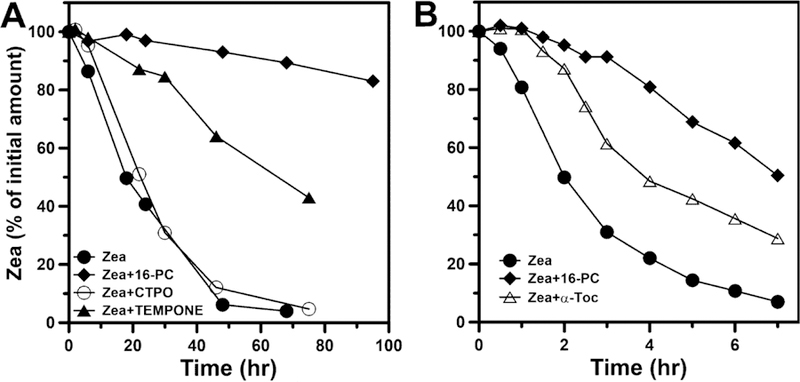
(A) Bleaching of Zea (25 「M) in DLPC membranes subjected to autoxidation in the absence and presence of nitroxide free radicals: CTPO (250 「M), TEMPONE (250 「M), and 16-PC (25 「M). (B) Bleaching of Zea (8 「M) in PAPC membranes subjected to peroxidation induced by PS (Rose Bengal [10 「M]) and green light in the absence and presence of 16-PC (25 「M) and 〈-tocopherol (25 「M). (Adapted with permission from Zareba et al., 2016. Free Radic. Biol. Med. 101, 446–454.)
In the case of the singlet-oxygen-induced lipid peroxidation, MXs clearly showed protection against peroxidation of polyunsaturated membranes. The most impressive observation is the very strong synergistic, inhibitory effect of MXs and 16-PC on lipid peroxidation of PAPC membranes (Fig. 8), which is much stronger than the sum of the effects of the individual agents. Several studies have postulated that Zea and α-tocopherol synergistically protect the human retina against chronic oxidative damage caused by photo-induced lipid peroxidation in photoreceptor and RPE cell membrane (Olchawa et al., 2015; Wrona et al., 2004). The synergistic protection provided by MXs and 16-PC is much stronger than that provided by MXs and α-tocopherol (see Fig. 8). Thus, 16-PC not only protects MXs from destruction (as shown in Fig. 7), it also enhances the effect of MXs as a lipid-soluble antioxidant during light-induced lipid peroxidation (as shown in Fig. 8). Theoretically, new strategies, like the one described above, aimed at keeping the structure of MXs intact, should enhance their protective potential during acute oxidative stress (e.g., surgery, laser treatments).
Fig. 8.
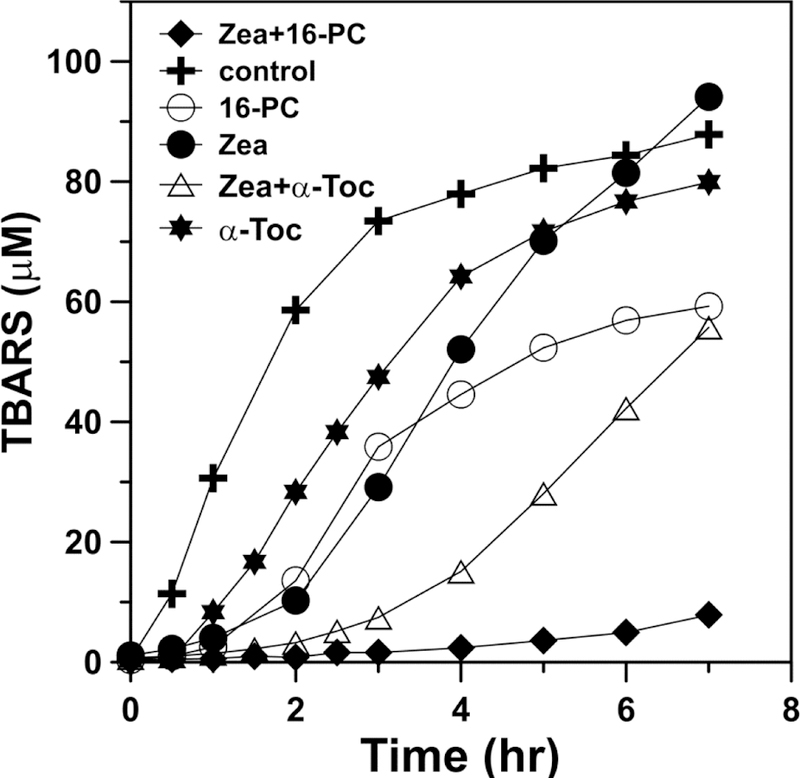
The effect of 16-PC (25 「M) or α-tocopherol (25 「M), in combination with Zea (8 「M), on the accumulation of phospholipid peroxidation products (TBARS). Model membranes were made of the polyunsaturated phospholipid (PAPC, 2.5 mM) and subjected to peroxidation induced by PS (Rose Bengal [10 「M]) and green light. (Adapted with permission from Zareba et al., 2016. Free Radic. Biol. Med. 101, 446–454.)
5. Concluding remarks
In the review titled “Why has nature chosen lutein and zeaxanthin to protect the retina?” we indicated properties of MXs that explain their selective accumulation in the primate retina (Widomska and Subczynski, 2014). In that review, we focused on xanthophyll–membrane interactions. Now we are aware of additional significant properties of MXs that distinguish them from other carotenoids available in the primate (human) diet and present in human blood plasma, namely, their resistance to degradation. Most of the time, MXs protect the retina through physical mechanisms, namely, through blue-light filtration and quenching of excited triplet states of PSs and singlet oxygen. During these actions, the structure of MXs is unaltered. This is also what distinguishes them from other lipid antioxidants (e.g., α-tocopherol). However, certain retinal MXs undergo chemical degradation, especially during excessive exposure to light. As discussed in Sect. 4.5.1, during biological evolution, nature developed a number of endogenous mechanisms (including “self-protection” mechanisms) to keep MXs’ structure intact. Also, nature developed some chemical mechanisms that allow partially destroyed MXs to regenerate their structure.
In Sect. 4.5.2, we indicate that MXs can be also protected through exogeneous chemical interactions. As shown by Zareba et al. (Zareba et al., 2016), membrane-located nitroxide free radicals effectively protect MXs against destruction, and their effect is especially pronounced during the light-induced formation of singlet oxygen. During that process, nitroxides are reduced to the appropriate hydroxylamines. However, it does not affect the protective process because both nitroxides and their reduced forms, hydroxylamines, are equally effective in protecting against degradation of MXs (Zareba et al., 2016). This protective recycling mechanism (nitroxides–hydroxyloamines–nitroxides) seems to be a great advantage over the traditional action of α-tocopherol. Moreover, a combination of MXs and lipid-soluble nitroxides exerts strong synergistic protection against singlet-oxygen-induced lipid peroxidation. This synergistic protection is much greater than that provided by the combination of MXs with α-tocopherol. These results indicate that the MXs protection provided by membrane-located nitroxides preserves and enhances the photo-related protective actions of MXs. The strategies employed here may be used to protect MXs, and thus provide better protection to retina during ophthalmological procedures (surgeries, different laser treatments) in which acute increases of retinal oxygenation and (damaging) exposure to light may occur. Administration of nitroxides before such processes (through injections or eye drops) can help.
Highlights.
Macular xanthophylls protect retina mainly through physical actions.
To perform physical, photo-related protection, macular xanthophylls should remain intact.
Nitroxide free radicals protect macular xanthophylls against destruction (bleaching).
Maintaining of the intact structure of macular xanthophylls enhance their protection actions in the retina through physical processes.
Acknowledgements
This work was supported by grants R01 EY015526 and P30 EY001931 from the National Institutes of Health.
The authors wish to thank Mariusz Zareba, PhD, William O’Brien, PhD, and Thomas Connor, MD, for their critical reading of, and thoughtful comments about, the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RE, Benolken RM, Dudley PA, Landis DJ, Wheeler TG, 1974. Proceedings: polyunsaturated fatty acids of photoreceptor membranes. Exp. Eye Res 18, 205–213. [DOI] [PubMed] [Google Scholar]
- Berendschot TT, Goldbohm RA, Klöpping WA, van de Kraats J, van Norel J, van Norren D, 2000. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest. Ophthalmol. Vis. Sci 41, 3322–3326. [PubMed] [Google Scholar]
- Bhosale P, Bernstein PS, 2005. Synergistic effects of zeaxanthin and its binding protein in the prevention of lipid membrane oxidation. Biochim. Biophys. Acta 1740, 116–121. [DOI] [PubMed] [Google Scholar]
- Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS, 2004. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem 279, 49447–49454. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Dispoto J, Kahoe MA, 2002. Association of a photoreceptor-specific tetraspanin protein, ROM-1, with Triton X-100-resistant membrane rafts from rod outer segment disk membranes. J. Biol. Chem 277, 41843–41849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Fernandez L, Tarsis SL, 1988. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest. Ophthalmol. Vis. Sci 29, 843–849. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W, 1997. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res 64, 211–218. [DOI] [PubMed] [Google Scholar]
- Borovansky J, Riley PA, 2011. Melanins and melanosomes: biosynthesis, structure, physiological and pathological functions John Wiley & Sons. [Google Scholar]
- Britton G, 1995. Structure and properties of carotenoids in relation to function. FASEB J 9, 1551–1558. [PubMed] [Google Scholar]
- Burke M, Edge R, Land EJ, Truscott TG, 2001. Characterisation of carotenoid radical cations in liposomal environments: interaction with vitamin C. J. Photochem. Photobiol. B 60, 1–6. [DOI] [PubMed] [Google Scholar]
- Cantrell A, McGarvey DJ, Truscott TG, Rancan F, Böhm F, 2003. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch. Biochem. Biophys 412, 47–54. [DOI] [PubMed] [Google Scholar]
- Cordeiro RM, 2014. Reactive oxygen species at phospholipid bilayers: Distribution, mobility and permeation. Biochim. Biophys. Acta 1838, 438–444. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Medeiros NE, Millican CL, 1996. Photoreceptor loss in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 37, 1236–1249. [PubMed] [Google Scholar]
- Curran-Celentano J, Hammond BR, Ciulla TA, Cooper DA, Pratt LM, Danis RB, 2001. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am. J. Clin. Nutr 74, 796–802. [DOI] [PubMed] [Google Scholar]
- Dillon J, 1995. UV-B as a pro-aging and pro-cataract factor. Doc. Ophthalmol 88, 339–344. [DOI] [PubMed] [Google Scholar]
- Edge R, Land EJ, McGarvey D, Mulroy L, Truscott TG, 1998. Relative one-electron reduction potentials of carotenoid radical cations and the interactions of carotenoids with the vitamin E radical cation. J. Am. Chem. Soc 120, 4087–4090. [Google Scholar]
- Edge R, McGarvey DJ, Truscott TG, 1997. The carotenoids as anti-oxidants — a review. J. Photochem. Photobiol. B 41, 189–200. [DOI] [PubMed] [Google Scholar]
- Edge R, Truscott TG, 2018. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids—A Review. Antioxidants 7 10.3390/antiox7010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE, 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res 22, 79–131. [DOI] [PubMed] [Google Scholar]
- Gale CR, Hall NF, Phillips DIW, Martyn CN, 2003. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci 44, 2461–2465. [DOI] [PubMed] [Google Scholar]
- Grudzinski W, Nierzwicki L, Welc R, Reszczynska E, Luchowski R, Czub J, Gruszecki WI, 2017. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep 7, 9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszecki WI, Strzałka K, 2005. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 1740, 108–115. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM, 1997. Dietary modification of human macular pigment density. Invest. Ophthalmol. Vis. Sci 38, 1795–1801. [PubMed] [Google Scholar]
- Havaux M, 2014. Carotenoid oxidation products as stress signals in plants. Plant J 79, 597–606. [DOI] [PubMed] [Google Scholar]
- Johnson EJ, Hammond BR, Yeum KJ, Qin J, Wang XD, Castaneda C, Snodderly DM, Russell RM, 2000. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am. J. Clin. Nutr 71, 1555–1562. [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL, 1997. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest. Ophthalmol. Vis. Sci 38, 1802–1811. [PubMed] [Google Scholar]
- Koga T, Terao J, 1995. Phospholipids increase radical-scavenging activity of vitamin E in a bulk oil model system. J. Agric. Food Chem 43, 1450–1454. [Google Scholar]
- Koushan K, Rusovici R, Li W, Ferguson LR, Chalam KV, 2013. The role of lutein in eye-related disease. Nutrients 5, 1823–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, 2001. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys 385, 28–40. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE, 1997. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp. Eye Res 65, 57–62. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bonet RA, Kilburn MD, 1996. The Macular Pigment: A Possible Role in Protection from Age-Related Macular Degeneration, in: Sies H (Ed.), Advances in Pharmacology. Academic Press, pp. 537–556. [DOI] [PubMed] [Google Scholar]
- Li B, Vachali P, Bernstein PS, 2010. Human ocular carotenoid-binding proteins. Photochem. Photobiol. Sci 9, 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Vachali P, Frederick JM, Bernstein PS, 2011. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 50, 2541–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman BJ, Mitchell DC, 1996. A role for phospholipid polyunsaturation in modulating membrane protein function. Lipids 31 Suppl, S193–197. [DOI] [PubMed] [Google Scholar]
- Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR, 1998. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. U. S. A 95, 2175–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane E, Kelliher C, Beatty S, Nolan JM, 2008. The rationale and evidence base for a protective role of macular pigment in age-related maculopathy. Br. J. Ophthalmol 92, 1163–1168. [DOI] [PubMed] [Google Scholar]
- Masutomi K, Chen C, Nakatani K, Koutalos Y, 2012. All-trans retinal mediates light-induced oxidation in single living rod photoreceptors. Photochem. Photobiol 88, 1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DC, Straume M, Litman BJ, 1992. Role of sn-1-saturated,sn-2-polyunsaturated phospholipids in control of membrane receptor conformational equilibrium: effects of cholesterol and acyl chain unsaturation on the metarhodopsin I in equilibrium with metarhodopsin II equilibrium. Biochemistry 31, 662–670. [DOI] [PubMed] [Google Scholar]
- Mortensen A, Skibsted LH, 1997. Relative stability of carotenoid radical cations and homologue tocopheroxyl radicals. A real time kinetic study of antioxidant hierarchy. FEBS Lett 417, 261–266. [DOI] [PubMed] [Google Scholar]
- Mozzo M, Dall’Osto L, Hienerwadel R, Bassi R, Croce R, 2008. Photoprotection in the antenna complexes of photosystem II. Role of individual xanthophylls in chlorophyll triplet quenching J. Biol. Chem 283, 6184–6192. [DOI] [PubMed] [Google Scholar]
- Nechushtai R, Thornber JP, Patterson LK, Fessenden RW, Levanon H, 1988. Photosensitization of triplet carotenoid in photosynthetic light-harvesting complex of photosystem II. J. Phys. Chem 92, 1165–1168. [Google Scholar]
- Obana A, Hiramitsu T, Gohto Y, Ohira A, Mizuno S, Hirano T, Bernstein PS, Fujii H, Iseki K, Tanito M, Hotta Y, 2008. Macular carotenoid levels of normal subjects and age-related maculopathy patients in a Japanese population. Ophthalmology 115, 147–157. [DOI] [PubMed] [Google Scholar]
- Okano K, Maeda A, Chen Y, Chauhan V, Tang J, Palczewska G, Sakai T, Tsuneoka H, Palczewski K, Maeda T, 2012. Retinal cone and rod photoreceptor cells exhibit differential susceptibility to light–induced damage. J. Neurochem 121, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olchawa MM, Herrnreiter AM, Pilat AK, Skumatz CMB, Niziolek-Kierecka M, Burke JM, Sarna TJ, 2015. Zeaxanthin and α-tocopherol reduce the inhibitory effects of photodynamic stress on phagocytosis by ARPE-19 cells. Free Radic. Biol. Med 89, 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palozza P, Serini S, Di Nicuolo F, Piccioni E, Calviello G, 2003. Prooxidant effects of beta-carotene in cultured cells. Mol. Aspects Med 24, 353–362. [DOI] [PubMed] [Google Scholar]
- Polozova A, Litman BJ, 2000. Cholesterol dependent recruitment of di22:6-PC by a G protein-coupled receptor into lateral domains. Biophys. J 79, 2632–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A-S, McNamara JO, Williams SM, 2001. Anatomical distribution of rods and cones, second ed Sunderland. [Google Scholar]
- Ramel F, Birtic S, Cuiné S, Triantaphylidès C, Ravanat J-L, Havaux M, 2012. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 158, 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp LM, Maple SS, Choi JH, 2000. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest. Ophthalmol. Vis. Sci 41, 1200–1209. [PubMed] [Google Scholar]
- Różanowska M, Sarna T, 2005. Light-induced Damage to the Retina: Role of Rhodopsin Chromophore Revisited. Photochem. Photobiol 81, 1305–1330. [DOI] [PubMed] [Google Scholar]
- Sabour-Pickett S, Nolan JM, Loughman J, Beatty S, 2012. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol. Nutr. Food Res 56, 270–286. [DOI] [PubMed] [Google Scholar]
- Schwartz SG, Gombos DS, Schneider S, 2010. Light Toxicity in the Posterior Segment, in: Tasman W, Jaeger EA (Eds.), Duane’s Ophthalmology, Lippincott Williams and Wilkins, Philadelphia. [Google Scholar]
- Sliney DH, 2002. How Light Reaches the Eye and Its Components. Int. J. Toxicol 21, 501–509. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, 1995. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr 62, 1448S–1461S. [DOI] [PubMed] [Google Scholar]
- Socaciu C, Jessel R, Diehl HA, 2000. Carotenoid incorporation into microsomes: yields, stability and membrane dynamics. Spectrochim. Acta 56, 2799–2809. [DOI] [PubMed] [Google Scholar]
- Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ, 1999. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr. Eye Res 19, 491–495. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H, 2003. Antioxidant activity of carotenoids. Mol. Aspects Med 24, 345–351. [DOI] [PubMed] [Google Scholar]
- Stinson AM, Wiegand RD, Anderson RE, 1991. Fatty acid and molecular species compositions of phospholipids and diacylglycerols from rat retinal membranes. Exp. Eye Res 52, 213–218. [DOI] [PubMed] [Google Scholar]
- Subczynski WK, Markowska E, Sielewiesiuk J, 1991. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim. Biophys. Acta 1068, 68–72. [DOI] [PubMed] [Google Scholar]
- Sui X, Kiser PD, von Lintig J, Palczewski K, 2013. Structural basis of carotenoid cleavage: from bacteria to mammals. Arch. Biochem. Biophys 539, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujak A, Gabrielska J, Grudziński W, Borc R, Mazurek P, Gruszecki WI, 1999. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch. Biochem. Biophys 371, 301–307. [DOI] [PubMed] [Google Scholar]
- Thiyam U, Stöckmann H, Schwarz K, 2006. Antioxidant activity of rapeseed phenolics and their interactions with tocopherols during lipid oxidation. J. Am. Oil Chem. Soc 83, 523–528. [Google Scholar]
- Traber MG, Stevens JF, 2011. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med 51, 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieschmann M, van Kuijk FJGM, Alexander R, Hermans P, Luthert P, Bird AC, Pauleikhoff D, 2008. Macular pigment in the human retina: histological evaluation of localization and distribution. Eye 22, 132–137. [DOI] [PubMed] [Google Scholar]
- Widomska J, Subczynski WK, 2014. Why has nature chosen lutein and zeaxanthin to protect the retina? J. Clin. Exp. Ophthalmol 5, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska A, Subczynski WK, 2006a. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic. Biol. Med 40, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Wisniewska A, Subczynski WK, 2006b. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic. Biol. Med 41, 1257–1265. [DOI] [PubMed] [Google Scholar]
- Wood JPM, Lascaratos G, Bron AJ, Osborne NN, 2007. The influence of visible light exposure on cultured RGC-5 cells. Mol. Vis 14, 334–344. [PMC free article] [PubMed] [Google Scholar]
- Woodall AA, Britton G, Jackson MJ, 1995. Antioxidant activity of carotenoids in phosphatidylcholine vesicles: chemical and structural considerations. Biochem. Soc. Trans 23, 133S. [DOI] [PubMed] [Google Scholar]
- Woodall AA, Lee SW-M, Weesie RJ, Jackson MJ, Britton G, 1997. Oxidation of carotenoids by free radicals: relationship between structure and reactivity. Biochim. Biophys. Acta 1336, 33–42. [DOI] [PubMed] [Google Scholar]
- Wrona M, Rózanowska M, Sarna T, 2004. Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic. Biol. Med 36, 1094–1101. [DOI] [PubMed] [Google Scholar]
- Youssef PN, Sheibani N, Albert DM, 2011. Retinal light toxicity. Eye 25, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareba M, Widomska J, Burke JM, Subczynski WK, 2016. Nitroxide free radicals protect macular carotenoids against chemical destruction (bleaching) during lipid peroxidation. Free Radic. Biol. Med 101, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Omaye ST, 2001. Antioxidant and prooxidant roles for beta-carotene, alpha-tocopherol and ascorbic acid in human lung cells. Toxicol. Vitro Int. J. Publ 15, 13–24. [DOI] [PubMed] [Google Scholar]


