Abstract.
Neurotropism and infiltration by Mycobacterium leprae of peripheral nerves causing neuropathy are well established, but reports of central nervous system (CNS) damage are exceptional. We report CNS magnetic resonance imaging (MRI) abnormalities of the brain and spinal cord as well as lesions in nerve roots and plexus in leprosy patients. Eight patients aged between 17 and 41 years underwent detailed clinical, histopathological, and MRI evaluation. All had prominent sensory–motor deficits with hypopigmented and hypo/anesthetic skin patches and thickened peripheral nerves. All demonstrated M. Leprae DNA in affected peripheral nerve tissue. All received multidrug therapy (MDT). Two patients had brainstem lesions with enhancing facial nuclei and nerves, and one patient had a lesion in the nucleus ambiguus. Two patients had enhancing spinal cord lesions. Follow-up MRI performed in four cases showed resolution of brainstem and cord lesions after starting on MDT. Thickened brachial and lumbosacral plexus nerves were observed in six and two patients, respectively, which partially resolved on follow-up MRI in the two cases who had reimaging. The site and side of the MRI lesions corresponded with the location and side of neurological deficits. This precise clinico-radiological correlation of proximal lesions could be explained by an immune reaction in the gray matter corresponding to the involved peripheral nerves, retrograde axonal and gray matter changes, or infection of the CNS and plexus by lepra bacilli. Further study of the CNS in patients with leprous neuropathy is needed to establish the exact nature of these CNS MRI findings.
INTRODUCTION
Hansen’s disease/leprosy is the leading infectious cause of disability, and the neurological involvement may commence before, during, or after treatment, leading to significant functional impairments and deformities.1,2 Although the number of new leprosy cases detected globally is reported to have reduced by 46% from 2004 to 2011, the incidence of the disease is still comparatively high in the South Asian region, with new case detection of > 10/100,000 in India.3 The public health problem of leprosy also continues in several regions of the world such as Brazil and the Oceania region. Leprosy is also known to occur in the United States with cases from Louisiana, Texas, Mississippi, Georgia, and Florida.4
Skin and peripheral nerve affliction in leprosy has been extensively studied and published, but reports on involvement of the central nervous system (CNS) and proximal nerves are extremely rare.5–7 Clinical and histopathological findings on skin and nerve tissue are usually sufficient to diagnose leprosy. However, polymerase chain reaction (PCR) is reported to be the most sensitive and specific test to confirm Mycobacterium leprae DNA in any tissue/fluid sample.8–10 With the mobility of patients across the world, physicians need be aware of leprosy mimicking other disorders, and hence, understanding rare findings in leprosy is essential for a high level of suspicion and an early diagnosis.
In the present series, we describe eight cases of leprosy with magnetic resonance imaging (MRI) abnormalities in the brainstem, spinal cord, and brachial and lumbosacral plexuses.
MATERIALS AND METHODS
All patients provided written informed consent to publish their medical data and clinical photographs. Detailed clinical and histopathological testing, MRI, and PCR testing of peripheral nerve tissue for M. leprae DNA were performed in all eight cases.
Magnetic resonance imaging protocol.
Magnetic resonance imaging was performed on 1.5 or 3.0 Tesla machines, and the protocol consisted of T1 and T2 axial and sagittal images of the spine and coronal short inversion time inversion recovery (STIR) sequences for the plexus. For the brain, it was T1 axial, T2 sagittal and axial, diffusion-weighted imaging (DWI), and post-contrast T1 magnetization-prepared rapid gradient-echo (MPRAGE).
Polymerase chain reaction–based gene amplification was performed in biopsied peripheral nerve tissue using primers according to the WHO “Global Surveillance of Drug Resistance in Leprosy” (2009) guidelines for detection of mutation in rpoB, gyrA, and folP genes in M. leprae genome (Supplemental Data 1).11 In addition, PCR for 16S ribosomal RNA (rRNA) gene was performed in the cerebrospinal fluid (CSF) samples (Supplemental Data 2).
RESULTS
Case details.
Case 1.
A 32-year-old man was evaluated in July 2015. He hailed from a higher socioeconomic status and had no known contact with an infected individual. In 2007, he developed hypo-pigmented anesthetic patches over the trunk and upper extremities for 6 months. He was diagnosed with leprosy and received standard multidrug therapy (MDT) for leprosy for 1 year and had complete clinical recovery. In 2012, he developed burning paresthesias and numbness over the trunk and extremities for 2 months and had thickened tender nerves. Fine-needle aspiration cytology from the right common peroneal nerve swelling demonstrated acid-fast bacilli (AFB). He again received MDT for 2 years and had complete clinical recovery. In July 2015, he presented with progressive sensory loss in the left upper limb, severe bifacial weakness, and skin lesions on the legs and gluteal regions. He had profound distal weakness and wasting of the left upper limb and both lower limbs for 4 months, sensory loss and burning paresthesias in stocking distribution for 2 months, hesitancy of micturition, poor stream and sensation of post-void residual urine for 3 months, and sensory loss with severe weakness and wasting of the right upper limb for 1 month. On examination, he had skin lesions consisting of palmar erythema; scaly, circular reddish maculopapular rashes over the forearms and anterior legs; and trophic ulcers over the feet. Neurological examination revealed thickened, nodular, markedly tender superficial nerves; severe bifacial weakness; claw hands; foot drop; and glove and stocking sensory loss (Figure 1). Slit skin smear was negative for AFB. Superficial radial nerve (SRN) and skin biopsy from the leg demonstrated leprous neuritis—borderline tuberculoid (BT) type (Figure 1). The serum angiotensin converting enzyme (ACE) level was high (80.7 U/L; 136 U/L; range, 8–52 U/L). Magnetic resonance imaging demonstrated symmetrical T1W hypo- and T2W hyperintense facial nuclei with enhancement of facial nuclei and exiting nerves on post-contrast MPRAGE (Figure 2). Coronal STIR showed bilateral brachial plexus thickening (Figure 2). Lumbar CSF showed a few transformed lymphocytes, and reactive monocytes, protein of 153 mg/dL, and a normal glucose level. The cerebrospinal fluid venereal disease research laboratory (VDRL) was nonreactive. The patient was treated with steroids along with minocycline 100 mg/day and clofazimine 100 mg/day, the regimen advised by the dermatologist, presumably because he was thought to have drug-resistant leprosy. At the 16-month follow-up, MRI showed complete resolution of the brain lesions but brachial plexus thickening showed only partial improvement (Figure 3).
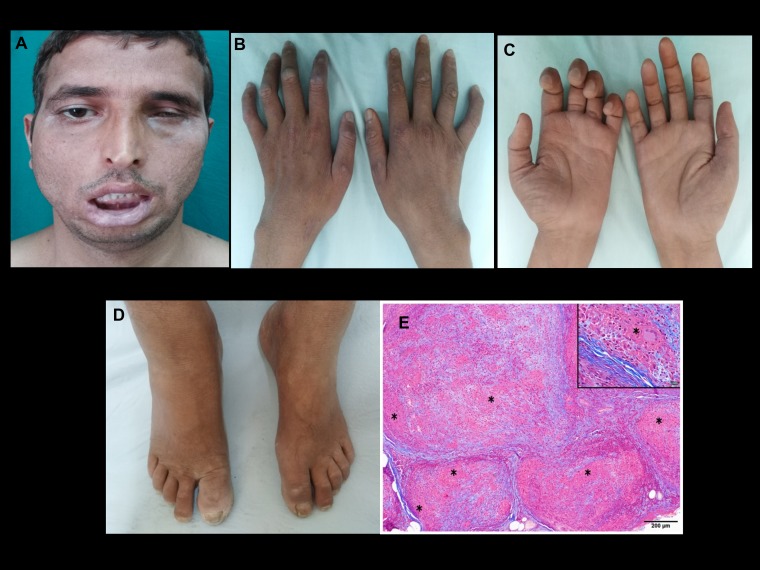
Figure 1.
Case 1: Clinical photographs showing (A) severe bifacial weakness and (B) macular healing lesions over interphalangeal joints. Note the wasting of the interossei muscles; (C) note the asymmetrical wasting and weakness of the hand. Radial cutaneous nerve biopsy; (D) note mild pedal edema and trophic changes of the toes; (E) multiple epithelioid granulomas (*) expanding the nerve fascicle, replacing the contents. Inset shows granuloma with Langhans giant cell (*). Note the fibrosis of the endoneurium. (Masson trichrome, magnification = scale bar). This figure appears in color at www.ajtmh.org.
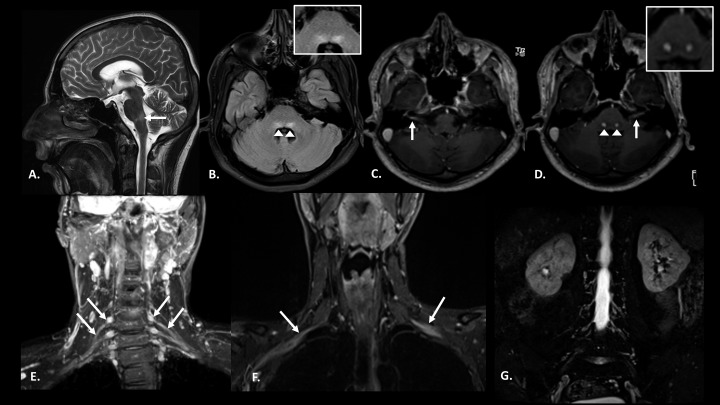
Figure 2.
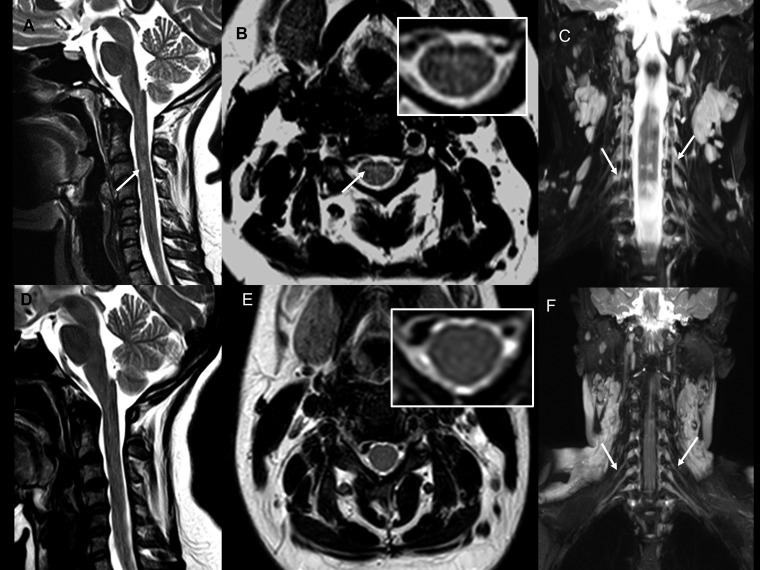
Case 1: Pretreatment magnetic resonance imaging images: Pretreatment: (A) T2 sagittal section of the brain showing hyperintensity in dorsal pons. (B) Fluid-attenuated inversion recovery (FLAIR) axial section of the brain at the level of facial colliculus shows bilateral symmetrical hyperintensity in the facial nerve nuclei (inset). (C and D) Post-contrast T1 magnetization-prepared rapid gradient-echo axial sections show enhancement of bilateral facial nerves along with their nuclei (inset) in pons. (E and F) Coronal short inversion time inversion recovery (STIR) images reveal thickening of bilateral brachial plexus roots, trunks (E), and divisions (F). (G) Coronal STIR image of the lumbar plexus does not reveal any nerve thickening.
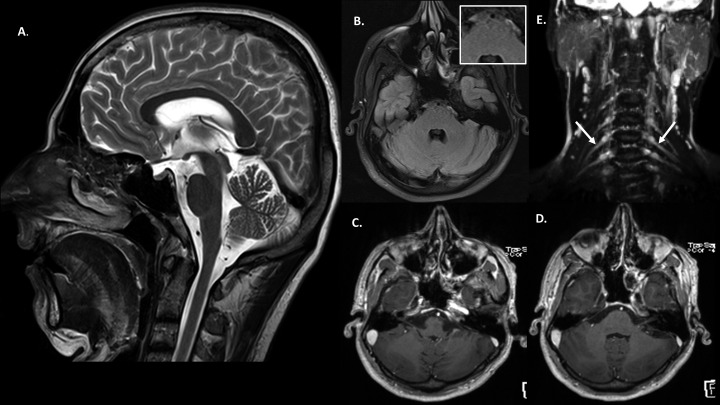
Figure 3.
Case 1: Posttreatment: (A and B) T2 sagittal and FLAIR axial images of brain showing resolution of the signal changes in the facial nerve nuclei (inset). (C and D) Post-contrast T1 magnetization-prepared rapid gradient-echo axial sections at the level of facial colliculus do not show enhancement of the facial nerves or their nuclei. (E) Coronal short inversion time inversion recovery image of the brachial plexus shows decreased thickness of the nerves as compared with the pretreatment images.
Case 2.
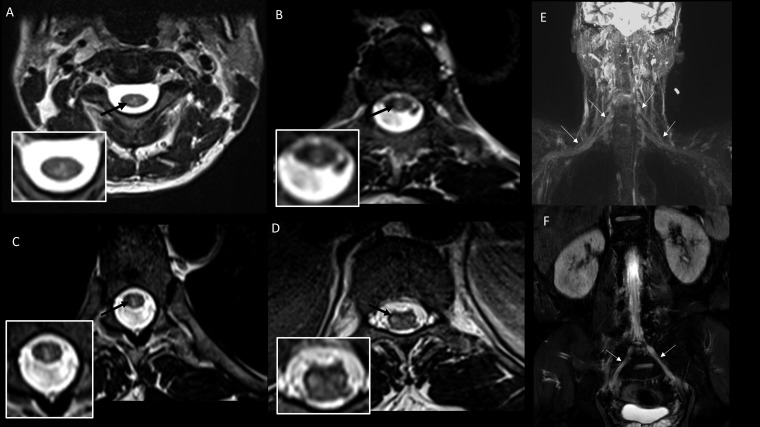
A 26-year-old man presented in September 2016 with painless, clear, fluid-filled bullous skin lesions; loss of touch, pain, and temperature sensation over the left palm for 6 months; and weakness and wasting of the left hand for 3 months. The bullous lesions ruptured and healed spontaneously (Figure 4). Examination revealed multiple hypopigmented/hypoesthetic patches over the thumb and trunk, thickened peripheral nerves, severe wasting of small muscles of the left hand and forearm, profound weakness of the left finger flexors and small muscles of the hand, and sensory loss over the left hand. Superficial radial nerve biopsy showed BT-type leprosy. T2-Weighted (T2W) images showed cord hyperintensity involving the gray matter at C6 and C7 levels with left brachial plexus thickening on coronal STIR sequences (Figure 4). Standard MDT was started, and a follow-up MRI at 8 months showed complete resolution of the cord lesion but persistent brachial plexus abnormality (Figure 4).
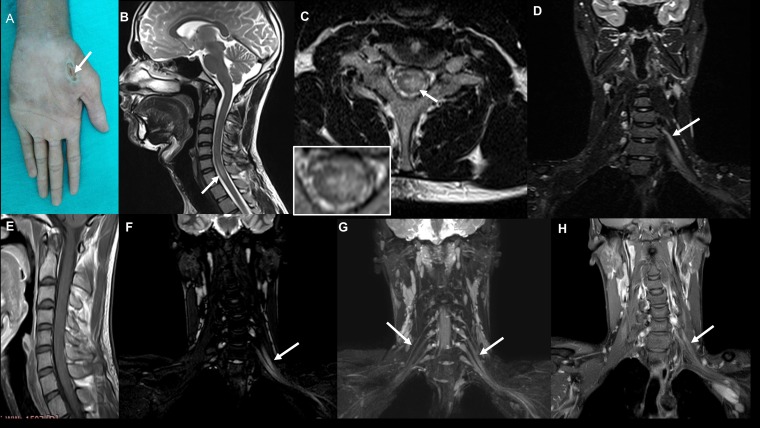
Figure 4.
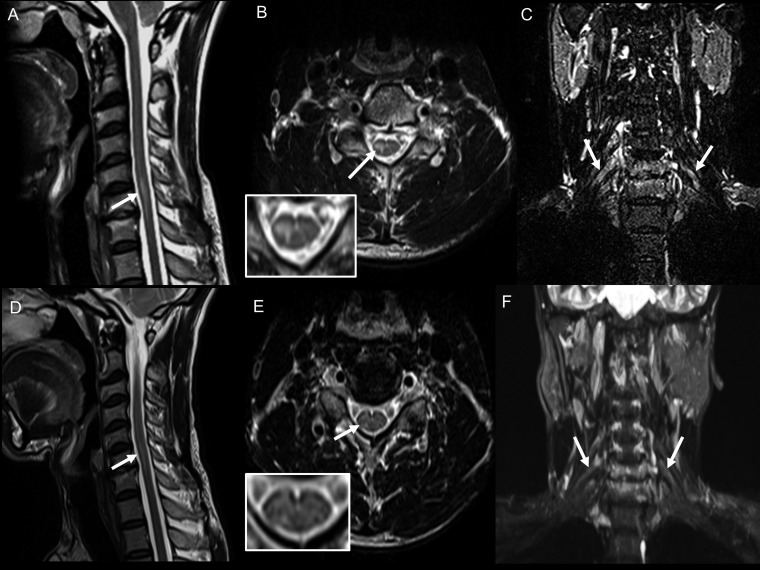
Case 2: Clinical photograph (A) showing chronic ulcer in the left thenar eminence (C7 dermatome). Pretreatment magnetic resonance imaging (MRI) images: (B) T2-weighted sagittal image of the cervical spine showing linear hyperintensity in the cervical cord at the C6 and C7 levels; (C) T2-weighted axial image of the cervical spine at the C7 level showing hyperintensity located predominantly in the left hemicord with extension across the midline slightly to the right side (inset); (D) coronal short inversion time inversion recovery (STIR) image showing thickening of the left brachial plexus. Posttreatment MRI images: (E) Post-contrast T1-weighted sagittal image of the cervical spine does not show any enhancement; (F) coronal 3D STIR imaging of the brachial plexus shows persistent thickening of the brachial plexus on the left side; (G) maximum intensity projection images of the coronal 3D STIR showing normal right and thickened left brachial plexus; (H) post-contrast coronal Dixon fat saturated image shows enhancement of the left brachial plexus. This figure appears in color at www.ajtmh.org.
Case 3.
A 26-year-old woman presented in April 2017 with numbness, inadvertent burns, and nodular eruptions of the right little finger for 3 years, ring finger for 2.5 years, medial forearm for 1 month, left medial hand and forearm for 2 weeks, and pain in bilateral shoulder regions and mild weakness for 1 week. Examination revealed healed scars over the fingers, mild weakness of small muscles of the hands, and sensory loss to all modalities up to the lower arm. Dorsal cutaneous branch of the ulnar nerve biopsy revealed borderline lepromatous (BL)–type leprous neuritis with abundant AFB. Magnetic resonance imaging showed hyperintense signal affecting the gray matter at C3 on T2W images and mild symmetrical thickening of brachial plexus on STIR sequence (Figure 5). After MDT (2 months), there was resolution of the cord signals, but persistent brachial plexus abnormality (Figure 5).
Figure 5.
Case 3: Pretreatment magnetic resonance imaging (MRI) images: (A) T2 sagittal cervical spine showing focal hyperintense signal in the cord at the C3 vertebral level; (B) T2 axial cervical spine at the C3 vertebral level showing hyperintense signal in the central cord (inset); (C) maximum intensity projection (MIP) image of coronal short inversion time inversion recovery (STIR) of the cervical spine shows mild symmetrical thickening of the brachial plexus nerve roots bilaterally; post-treatment MRI images: (D) T2 sagittal cervical spine showing resolution of the signal change in the cord at the C3 vertebral level; (E) T2 axial cervical spine at the C3 vertebral level showing the same (inset); (F) MIP image of coronal STIR of the cervical spine shows mild symmetrical thickening of the brachial plexus nerve roots bilaterally—with no significant change as compared with pretreatment imaging.
Case 4.
A 40-year-old man presented in February 2017 with right upper limb impaired sensation up to the mid-arm, difficulty in gripping objects, and urgency and precipitancy of micturition for 3 years. He took MDT for 1 year from September 2015, but symptoms were persistent. Examination revealed a hypoesthetic, hypopigmented patch over the right arm, mild wasting and weakness of the right-hand muscles, and thickened nerves. Magnetic resonance imaging showed a short segment, enhancing gray matter lesion at the C4–C5 region with thickened brachial plexus (Figure 6). Left SRN biopsy showed BT-type leprosy. The patient was started on MDT, and at the 5-month follow-up the neurological deficits were the same; however, MRI showed reduction in cord signal changes and brachial plexus abnormality (Figure 6).
Figure 6.
Case 4: Pretreatment magnetic resonance imaging (MRI) images: (A) T2W sagittal image showing linear hyperintensity in the cervical cord at C6 and C7 levels; (B) axial image at C6 shows right hemicord hyperintensity (inset); (C) coronal short inversion time inversion recovery (STIR) image shows thickening of bilateral brachial plexus roots (right > left). Posttreatment MRI images: (D) T2 sagittal image of cervical spine shows that linear hyperintensity in the cervical cord at the C6 and C7 levels has decreased as compared with the pretreatment image; (E) T2-weighted axial image at the C6 level shows a decrease in the signal change in the right hemicord (inset); (F) coronal STIR image shows decrease in the thickening of brachial plexus roots as compared with the pretreatment image.
Case 5.
A 21-year-old man presented in October 2013 with pain in the right forearm and wrist joint for 12 months and paresthesias and numbness with weakness and wasting of the right hand for 4 months. Examination revealed wasting and weakness of the right hand with impaired sensations in the distal forearm and hand. Left sural nerve biopsy showed BL leprosy with AFB. Magnetic resonance imaging showed gray matter signal changes from C4 to D2 levels with mildly thickened brachial plexus (Figure 7). On telephone discussion in October 2017, he reported having completed MDT and subjectively had no deficits.
Figure 7.
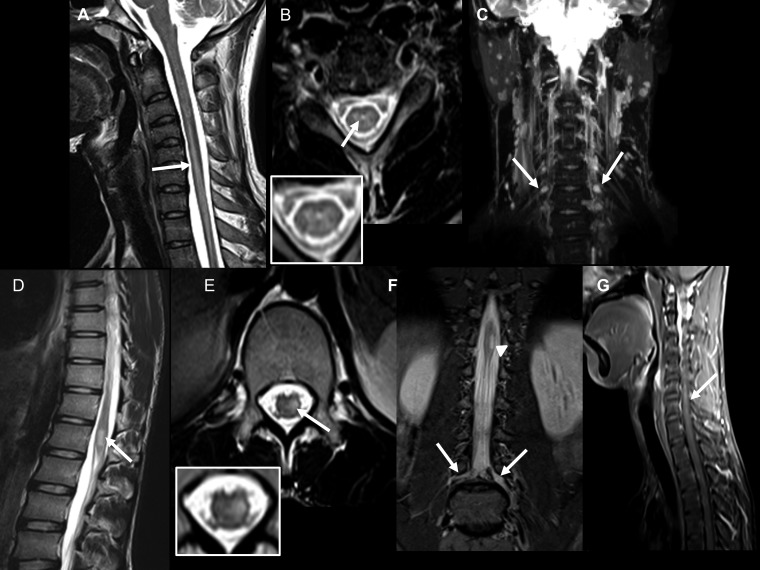
Case 5: (A) T2 sagittal image of the cervical spine shows a long-segment signal change in the cord from the C4 to D2 levels. (B) T2 axial image at the C6 vertebral level shows hyperintensity in the central cord (inset). (C) Maximum intensity projection image of coronal short inversion time inversion recovery (STIR) of the cervical spine shows mild bilateral symmetrical thickening of the brachial plexus nerve roots. Case 6: (D) T2-weighted sagittal image of the lumbar spine showing linear hyperintensity at the conus. (E) T2-weighted axial image at the level of the conus showing hyperintensity located in the left hemicord (inset). (F) Coronal STIR images of the lumbar spine showing hyperintensity at the conus (arrowhead) with thickening of bilateral lumbar plexus nerve roots (left > right). Case 7: (G) T1-weighted post-contrast fat-suppressed left parasagittal image showing linear enhancing lesion at the C5–C6 level.
Case 6.
A 17-year-old man presented in November 2014 with acute left foot drop, knee joint pain, and absent sensations below the knee for 2 months. He had grade 0 strength in foot dorsiflexion, reduced sensation in left L4, L5 dermatomal distribution, and asymmetrical hyperactive knee and ankle jerks. Left sural nerve and anterior leg skin biopsies showed BT-type leprosy with AFB. Magnetic resonance imaging showed left hemi-cord gray matter hyperintensity at conus with asymmetrical thickening (left > right) of the lumbar plexus (Figure 7). At telephone follow-up 12 months later, he reported having completed the course of MDT and had subjectively recovered.
Case 7.
A 23-year-old man presented during July 2017 with weakness, wasting, and impaired sensations in the left medial two fingers and multiple hypopigmented, hypoesthetic lesions for 9 months. Examination revealed thickened ulnar nerves and SRNs, wasting and weakness of the small muscles of the left hand and finger extensors, profound sensory loss in the left ulnar nerve distribution, and hyperactive tendon reflexes. Left SRN biopsy showed BT-type leprosy. Linear enhancement of the gray matter at the C5–C6 level was seen on the MRI (Figure 7). He was lost to follow-up.
Case 8.
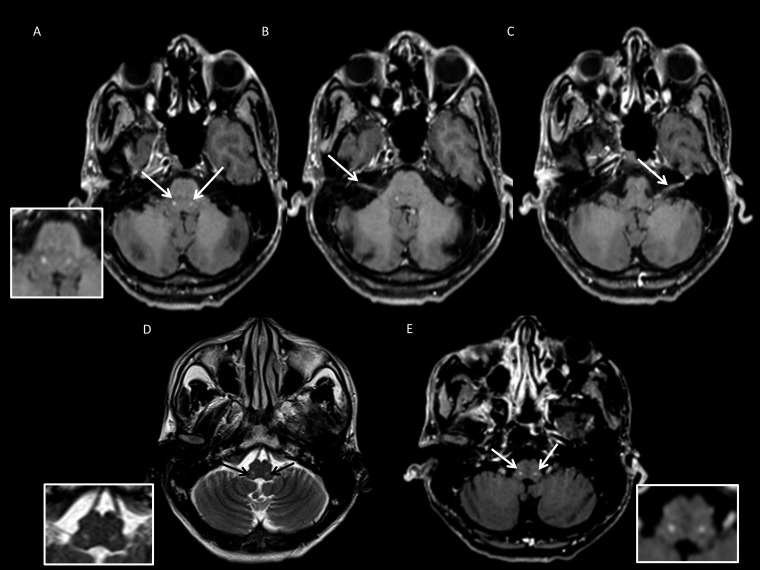
A 41-year-old man presented in August 2018 with progressive paresthesias, severe weakness, and wasting of both upper limbs for 8 months; facial paresthesias for 6 months; and bilateral foot weakness with gait ataxia, nonhealing foot ulcers, dysphagia, and nasal regurgitation for 2 months (Figure 8). Examination revealed multiple skin lesions characteristic of type 1 lepra reaction, generalized thickened and tender peripheral nerves, severe bilateral lower motor neuron (LMN) facial weakness, impaired facial and mucosal sensations, absent gag reflex with pooling of secretions, symmetrical weakness and wasting of distal limbs, glove and stocking sensory loss with impaired joint position sense in all limbs, and absent tendon reflexes. Left SRN biopsy and lesional skin biopsy showed BT-type leprosy. Cerebrospinal fluid showed elevated protein (96 mg/dL) and normal glucose levels. Magnetic resonance imaging showed enhancing facial nuclei, exiting facial nerves, and bilateral nucleus ambiguus (Figure 9). Cord signal changes were observed at multiple levels with thickened brachial and lumbosacral plexus (Figure 10).
Figure 8.
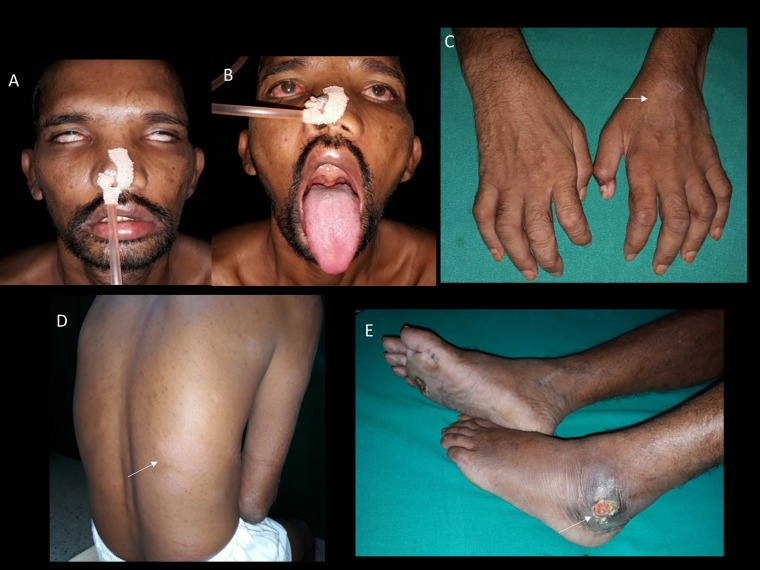
Case 8: Clinical photographs showing (A) severe bifacial weakness; (B) palatal weakness; (C) bilateral hand weakness and wasting with macular skin lesion on the dorsum of the left hand; (D) large macular skin lesions on the lower back; (E) nonhealing ulcer over the lateral malleolus. This figure appears in color at www.ajtmh.org.
Figure 9.
Magnetic resonance imaging images. (A–C) Post-contrast axial T1-weighted images show enhancement in the region of the facial nucleus bilaterally (A-inset), right (B) and left (C) facial nerve. (D and E) Axial T2 image at the level of the medulla showing discrete focal hyperintensity in the region of bilateral nucleus ambiguus (D-inset), with focal enhancement on post-contrast axial T1 (E-inset).
Figure 10.
Case 8: (A–D) Sequential axial T2-weighted images with corresponding reference images of the spine showing hyperintense lesions predominantly involving the central gray matter of the cord at the C2–C3 (A-inset), D4–D5 (B-inset), and D7–D8 (C-inset) levels with hyperintense lesions also seen in the conus medullaris at the D11–D12 level (D-inset). (E) Maximum intensity projection (MIP) image constructed from T2 sampling perfection with application optimized contrasts using different flip angle evolution (SPACE) coronal sequence with fat-sat shows thickening and hyperintensity in the cords, divisions, and trunks of bilateral brachial plexus; (F) MIP image from T2 SPACE coronal sequence shows thickening and hyperintensity of the lumbosacral plexus.
A summary of the clinical, electrophysiological, pathological, and MRI findings is shown in Table 1. Only one patient (Case 4) gave a history of contact with an infected individual. All had prominent thickening of many superficial nerves. Some had tender, nodular nerve swellings. Nerve conduction studies had revealed severe sensory–motor demyelinating and axonal neuropathy predominantly involving clinically affected nerves. According to nerve histopathology findings, patients were classified into BT = 6 and BL = 2. Three cases (Cases 3, 5, and 6) demonstrated AFB in the biopsied nerve. All patients were negative for HIV, hepatitis B surface antigen (HBsAg), and hepatitis C virus (HCV) antibodies.
Table 1.
Summary of the salient clinical, histopathological, and imaging characteristics of the eight patients
| Case | Affected limb | Skin lesions | Cranial nerve involvement | Site of biopsy; nerve /skin lesion | Duration of illness | Histological diagnosis | Acid-fast bacilli in nerve tissue/skin | PCR for lepra DNA (nerve) | PCR for 16S rRNA gene (cerebrospinal fluid) | Magnetic resonance imaging lesion (s) | On multidrug therapy before biopsy (duration) | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | All four limbs | Yes | VII | Left SRN; skin from leg | 8 years | BT | No | Positive | Negative | Facial nuclei and nerve; bilateral BP (Figure 2) | Yes | 16 months |
| 2 | Left hand; skin | Yes | No | Left SRN | 6 months | BT | No | Positive | Negative | C6–C7; left BP (Figure 4) | No | 8 months |
| 3 | Upper limbs | Yes | No | Left DCUN | 3 years | BL | Yes | Positive | Not done | C3; bilateral BP | No | 2 months |
| 4 | Right upper limb | Yes | No | Left SRN | 3 years | BT | No | Positive | Positive | C4–C5 hemicord lesion; bilateral BP | Yes | 4 months |
| 5 | Right upper limb | No | No | Left sural nerve | 1 year | BL | Yes | Positive | Not done | C4–D2; bilateral BP (Figure 7) | No | No |
| 6 | Left lower limb | Yes | No | Left sural; skin from leg | 2 months | BT | Yes | Positive | Not done | Left conus, lumbar plexus (Figure 7) | No | No |
| 7 | Left upper limb | Yes | No | Left SRN | 9 months | BT | No | Positive | Not done | Left C5–C6 (Figure 7) | No | No |
| 8 | All four limbs | Yes | V, VII, IX, and X | Left SRN | 8 months | BT | No | Positive | Negative | Facial nuclei and nerves, cord C2–C3, D4–D5, D7–D8, D11–D12; bilateral brachial plexus and lumbar plexus (Figure 8) | No | No |
BL = bilateral; BT = borderline tuberculoid; DCUN = dorsal cutaneous ulnar nerve; PCR = polymerase chain reaction; SRN = superficial radial nerve.
Magnetic resonance imaging findings.
Magnetic resonance imaging showed brainstem gray matter lesions in facial nuclei and nerves with contrast enhancement in Cases 1 and 8, whereas Case 8 had bilateral nucleus ambiguus lesions (Table 1, Figures 2 and 9). Cases 2–8 had enhancing gray matter spinal cord lesions as described (Table 1, Figures 4–7 and 10). Thickening of BP was observed in Cases 1–5 and Case 8 (Figures 2, 4–7, and 10). Lumbar plexus thickening was present in Cases 6 and 8 (Figures 7 and 10).
Repeat MRI was performed in four patients after MDT. In Case 1, the brain lesions completely resolved (Figure 3). The spinal cord lesions disappeared in Cases 2 and 3 (Figures 4 and 5), whereas faint cord signal was still observed in Case 4 (Figure 6). The brachial plexus showed partial resolution in Cases 1 and 4, whereas no change was noticed in Cases 2 and 3 at 8- and 2-month follow-ups, respectively (Figures 3–6). These changes corresponded well with clinical improvement, with Cases 1, 2, and 4 showing significant improvement in both sensory and motor symptoms and Case 3 reporting improvement in sensory symptoms.
Polymerase chain reaction result.
Polymerase chain reaction detected M. leprae DNA in peripheral nerve tissue, and assays for rpoB, folp, and gyrA gene showed amplifications in all cases. Sequencing of the amplified PCR products for the three genes revealed that all patients were fully sensitive to rifampicin, dapsone, and ofloxacin. Polymerase chain reaction for 16S rRNA was performed in CSF samples in four cases (Cases 1, 2, 4, and 8) and was found to be positive in only Case 4.
DISCUSSION
In the present case series, we describe MRI brainstem and spinal cord lesions and brachial and lumbosacral plexus abnormalities in eight patients with leprosy. Clinically, all had the cardinal manifestations of leprosy, including hypopigmented and hypoesthetic or anesthetic skin lesions and enlarged peripheral nerves. All demonstrated M. leprae–specific genomic DNA in peripheral nerve tissue by PCR method, which confirmed the presence of M. leprae.8–10
We found only three case reports in the English literature on living patients with leprosy demonstrating CNS involvement on MRI. In two, there were intramedullary cervical cord lesions and in one, a 2-cm frontal cystic lesion in a patient with BL-type leprosy. Although infiltration by M. leprae bacilli was not pathologically confirmed in cervical cord lesions, the specimen from the frontal cystic lesion demonstrated abundant scattered foamy macrophages with fragmented bacilli on Fite acid-fast stain, and PCR showed positive bands with sequence homology to the M. leprae genome.5–7
Our Case 1 had relapsing leprosy with severe facial weakness and demonstrated pontine lesions. We believe that this is the first report to document involvement of the facial nuclei along with enhancing facial nerves by MRI in a living patient with leprosy. Although the facial nerve is frequently affected in leprosy, radiological evidence for involvement of its nucleus and nerves is conspicuously lacking. Nevertheless, as early as 1952, Mitsuda identified lepra bacilli in the neurons of facial nucleus, cervical anterior horns, and nucleus ambiguus in autopsy specimens, confirming infection of the brain and spinal cord by M. leprae.12 In another report from Japan, involvement of the CNS was explored in 67 autopsy cases of clinically cured lepromatous leprosy. Of these, 44 had vacuolar changes of motor neurons either in the medulla oblongata (nucleus ambiguus or hypoglossal nucleus) or spinal cord which were positive for anti-phenolic glycolipid-I (PGL-I) immunostaining but negative by Fite acid-fast staining. Polymerase chain reaction had revealed M. leprae–specific genomic DNA in 95% of the cases.13 Yamada14 in their series of 173 cases also have shown that M. leprae–specific PGL-I antigen was expressed in motor neurons of the spinal cord at all levels in 12/173 cases and in the nucleus ambiguus in the medulla oblongata in 55/173 cases. Antibodies against 35-kDa and PGL-1 antigens have also been demonstrated in the CSF of leprosy patients with features of upper motor neuron involvement.15 These findings on autopsy studies support the hypothesis that lepra bacilli can infect the brain but do not establish that the infection has any clinical consequences. Three studies from India have reviewed the evidence for clinical involvement of cranial nerves I, V, VII, VIII IX, X, and XII in leprosy, and remarked that the CNS may be involved in leprosy.16,17 These were purely peripheral clinical findings and presumptions were made about probable intracranial involvement.
Enhancing gray matter lesions in the spinal cord were observed in seven of our eight patients. Follow-up MRI performed in three of these seven cases showed complete resolution of the cord lesion in two cases, whereas faint enhancement was still observed in one case. There are two earlier case reports of MRI findings with spinal cord abnormalities suspected to be secondary to leprosy without definitive pathological confirmation.5,6 Khadilkar et al.5 reported a 20-year-old Indian man with a hypo-aesthetic patch and ulnar neuropathy. Superficial radial nerve biopsy showed lepra bacilli. Cervical spine MRI revealed focal T1W hyperintense central signal within a larger area of T2W hyperintensity at the C5–C6 level along with smooth thickening of ventral and dorsal roots and enlarged enhancing dorsal root ganglia.5 Rice et al.6 reported a 27-year-old Brazilian woman with skin lesions, progressive pain, tingling, numbness, and weakness of the left upper limb. Magnetic resonance imaging showed expansion of the cervical cord with an intramedullary, enhancing area of high signal at C5–C7, and the corresponding dorsal root ganglia on the same side were swollen and demonstrated high T2 signal compatible with ganglionitis.6
Yamada14 identified para-rosaniline–positive material in the brain and spinal cord more in patients with tuberculoid leprosy than lepromatous leprosy. This is in concordance with the majority our patients (6/8) with CNS lesions having features of BT-type leprosy in their peripheral nerves. Spinal cord autopsies in long survivors of posttreated leprosy have shown degeneration of posterior columns and amyloid bodies in the gray and white matter.18,19 Koya and Arakawa20 examined the spinal cord of leprosy patients and found marked degeneration of the posterior column, especially the gracile fasciculus. These authors ruled out other causes leading to posterior column disease. Case 8 from our present series had profound kinesthetic impairment, although the cord lesions did not appear to involve the posterior columns and the ganglia.
Six of our cases had MRI evidence of either asymmetrical or symmetrical brachial or lumbosacral plexus thickening, and follow-up MRI showed partial resolution with MDT treatment in some. However, we did not perform pathological studies of the thickened plexus to identify the exact cause for the abnormality. Furthermore, reports of histopathological findings at biopsy or autopsy demonstrating involvement of proximal nerves and plexus are conspicuously lacking.
The pathogenesis of these MRI abnormalities is uncertain. Possible explanations include direct infection, reactive gray matter changes related to axonal transection of proximal nerves and roots, or an inflammatory immunological reaction related to the infection outside the CNS. All our patients except Case 6 had more than 6-month duration of illness, and this long duration of active disease and relapses (Case 1) with persistent bacillemia could have contributed to the changes noted in the more proximal nerves and possibly the CNS.21 It is also known that in advanced cases M. leprae can grow in the bone marrow, liver, and spleen, where body temperature is as high as that in the CNS.22–24 In contrast to these reports, we have no direct evidence of M. leprae in the proximal nerves or CNS lesions, but it is noteworthy that these restricted MRI lesions in the brainstem, spinal cord, and plexus corresponded precisely to the neurological symptoms and signs. This clinico-radiological correlation requires confirmation of the pathophysiological basis and needs further tissue-specific studies in such cases. It may be noteworthy that we identified the presence of M. leprae–specific16S rRNA gene in the CSF of one patient (Case 4) indicating the presence of lepra bacilli or its protein within the subarachnoid space. However, because of a lack of tissue confirmation by histopathological studies of the proximal lesions, direct invasion of the CNS by lepra bacilli would be a mere speculation.
An alternative explanation for these CNS gray matter lesions could be reactive changes secondary to retrograde degeneration from infection or inflammation leading to axonal injury close to the root entry or exit zones. Magnetic resonance imaging signal changes are known to occur in the spinal cord in about 20% of cases with brachial plexus injury resulting from edema in the acute phase, and transforming into irreversible myelomalacia in the chronic phase.25 However, the reversal of the CNS changes in our cases on follow-up without the development of myelomalacia may suggest a dynamic process or acute phase reaction within the spinal cord and brainstem resulting from the exaggerated immune response as noted in tuberculoid- and BT-type leprosy. In fact, to support this hypothesis, both of our patients with brainstem lesions had type 1 lepra reaction at the time of MRI. Nevertheless, even with a chronic disease course in most of our patients, there was complete or partial resolution of MRI lesions after MDT. This resolution could possibly be because of the anti-inflammatory action of MDT itself.
Symptoms and signs of leprosy and the associated immunologic reactions can mimic other more common conditions such as Lyme disease, neurosarcoidosis, lupus vulgaris, rheumatologic diseases, and others affecting the skin and peripheral nerves. Our Case 1 with pontine lesions had elevated serum ACE levels and could easily be misdiagnosed as sarcoidosis. In a case report from Italy, an Indian man with leprosy was treated as sarcoidosis based on elevated serum ACE levels.26 As early as 1977, Lieberman27 had studied 42 patients with leprosy and identified elevated serum ACE levels in 71.4%. Corticosteroid therapy had an immediate and dramatic effect on reducing the elevated ACE level.27 Similarly, the ACE level reduced to normal in our case following therapy with steroids.
In conclusion, the present series of eight cases shows MRI evidence of CNS lesions in patients with leprosy. Also, hitherto unreported, we have demonstrated lower brainstem MRI lesions involving the seventh cranial nucleus and its nerve and the nucleus ambiguus corresponding with clinical findings. Furthermore, there was variable thickening of the brachial or lumbosacral plexus which continued to be abnormal even when the CNS lesions resolved. We propose that the gray matter lesions could be due to retrograde reaction to proximal axonal damage or a phenomenon of immune reaction. We also acknowledge the limitation of this study in that pathological confirmation of the CNS or proximal nerve lesions is lacking. Nonetheless, these radiological findings may have implications for the understanding of the disease pathophysiology and therapeutic considerations in leprosy. Magnetic resonance imaging could also play an important role during the follow-up of patients with nerve function impairment for rehabilitation measures.
Supplementary Files
Acknowledgments:
We immensely thank all the patients for permitting us to publish the medical data. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1.Rodrigues LC, Lockwood DNj, 2011. Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis 11: 464–470. [DOI] [PubMed] [Google Scholar]
- 2.Lockwood DN, Saunderson PR, 2012. Nerve damage in leprosy: a continuing challenge to scientists, clinicians and service providers. Int Health 4: 77–85. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization , 2012. Global Leprosy Situation Vol. 87, Geneva, Switzerland: WHO, 317–328. Available at: http://www.who.int/wer/2012/wer8734/en/. Accessed June 12, 2018. [Google Scholar]
- 4.Leon KE, Jacob JT, Franco-Paredes C, Kozarsky PE, Wu HM, Fairley JK, 2016. Delayed diagnosis, leprosy reactions, and nerve injury among individuals with Hansen’s disease seen at a United States clinic. Open Forum Infect Dis 3: ofw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khadilkar SV, Kasegaonkar PS, Ursekar M, 2007. Spinal cord involvement and ganglionitis in leprosy. Neurol India 55: 427–428. [DOI] [PubMed] [Google Scholar]
- 6.Rice CM, Oware A, Klepsch S, Wright B, Bhatt N, Renowden SA, Jenkins MH, Rajan S, Bovill BA, 2016. Leprous ganglionitis and myelitis. Neurol Neuroimmunol Neuroinflamm 3: e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K-H, Moon K-S, Yun SJ, Won YH, Lee J-H, Lee M-C, Jung S, 2014. Brain involvement by leprosy presenting as a frontal cystic lesion. J Neurosurg 121: 184–188. [DOI] [PubMed] [Google Scholar]
- 8.Santos AR, De Miranda AB, Sarno EN, Suffys PN, Degrave WM, 1993. Use of PCR-mediated amplification of Mycobacterium leprae DNA in different types of clinical samples for the diagnosis of leprosy. J Med Microbiol 39: 298–304. [DOI] [PubMed] [Google Scholar]
- 9.Gillis TP, Williams DL, 1991. Polymerase chain reaction and leprosy. Int J Lepr Other Mycobact Dis 59: 311–316. [PubMed] [Google Scholar]
- 10.Williams DL, Scollard DM, Gillis TP, 2003. PCR-based diagnosis of leprosy in the United States. Clin Microbiol Newsl 25: 57–61. [Google Scholar]
- 11.World Health Organization, Regional Office for South-East Asia , 2009. Guidelines for Global Surveillance of Drug Resistance in Leprosy. Available at: http://www.searo.who.int/leprosy/documents/SEA_GLP_2009_2/en/. Accessed October 10, 2017. [Google Scholar]
- 12.Mitsuda K, 1952. Atlas of Leprosy. Available at: https://www.cabdirect.org/cabdirect/abstract/19532902231. Accessed July 1, 2017. [Google Scholar]
- 13.Aung T, Kitajima S, Nomoto M, En J, Yonezawa S, Arikawa I, Goto M, 2007. Mycobacterium leprae in neurons of the medulla oblongata and spinal cord in leprosy. J Neuropathol Exp Neurol 66: 284–294. [DOI] [PubMed] [Google Scholar]
- 14.Yamada N, 1984. Histopathological investigation of the central nervous system in leprosy. I. Distribution of para-rosanilin positive materials in leprosy with special relation to tissue injury [in Japanese]. Nihon Rai Gakkai Zasshi 53: 67–80. [PubMed] [Google Scholar]
- 15.Patil SA, Katoch K, Ramu G, Sengupta U, 1995. Detection of antibodies against phenolic glycolipid-1 (PGL-1), 35-kDa and 30-40-kDa components of Mycobacterium leprae in the cerebrospinal fluid of of leprosy patients. J Med Microbiol 43: 115–119. [DOI] [PubMed] [Google Scholar]
- 16.Katoch K, Ramu G, Sengupta U, Bharadwaj VP, 1984. Central nervous system involvement in leprosy. Indian J Lepr 56: 813–818. [PubMed] [Google Scholar]
- 17.Gopinath DV, Thappa DM, Jaishankar TJ, 2004. A clinical study of the involvement of cranial nerves in leprosy. Indian J Lepr 76: 1–9. [PubMed] [Google Scholar]
- 18.Woit O, 1952. The spinal cord, peripheral nerves and the skin patches of leprosy macules. Lepr India 24: 133–157. [Google Scholar]
- 19.Ermakóva N, 1936. Studies on leprosy. I. The central, sympathetic and peripheral nervous systems. Int J Lepr 4: 325–336. [Google Scholar]
- 20.Koya G, Arakawa I, 1979. Pathology of spinal cord in leprosy [in Japanese]. Nihon Rai Gakkai Zasshi 48: 27–36. [DOI] [PubMed] [Google Scholar]
- 21.Raval S, Sengupta U, Ramu G, Prabhune P, Desikan K, 1982. A study of continuous bacillaemia in borderline and lepromatous type of leprosy. Lepr India 54: 623–633. [PubMed] [Google Scholar]
- 22.Bernard JC, Vazquez CA, 1973. Visceral lesions in lepromatous leprosy. Study of sixty necropsies. Int J Lepr Other Mycobact Dis 41: 94–101. [PubMed] [Google Scholar]
- 23.Chen TS, Drutz DJ, Whelan GE, 1976. Hepatic granulomas in leprosy. Their relation to bacteremia. Arch Pathol Lab Med 100: 182–185. [PubMed] [Google Scholar]
- 24.Suster S, Cabello-Inchausti B, Robinson MJ, 1989. Nongranulomatous involvement of the bone marrow in lepromatous leprosy. Am J Clin Pathol 92: 797–801. [DOI] [PubMed] [Google Scholar]
- 25.Park HR, Lee GS, Kim IS, Chang J-C, 2017. Brachial plexus injury in adults. J Korean Soc Peripher Nerv Syst 3: 1–11. [Google Scholar]
- 26.Simeoni S, Puccetti A, Tinazzi E, Codella OM, Sorleto M, Patuzzo G, Colato C, Tessari G, Lunardi C, 2011. Leprosy initially misdiagnosed as sarcoidosis, adult-onset still disease, or autoinflammatory disease. J Clin Rheumatol 17: 432–435. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman J, Rea TH, 1977. Serum angiotensin-converting enzyme in leprosy and coccidioidomycosis. Ann Intern Med 87: 423–425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.