Abstract.
We analyzed the association between insulin-like growth factor-I (IGF-I) and the pathogenesis of anemia during active visceral leishmaniasis (VL). Serum levels of IGF-I, IGF-binding protein 3 (IGFBP3), and cytokines were measured in samples from individuals with active VL and cured VL, asymptomatic Leishmania-infected, and noninfected individuals. Then, we extended our analysis to VL dogs to evaluate hematimetric parameters, bone marrow alterations, and cytokine and IGF-I expression. We identified a positive correlation between lower IGF-I and IGFBP3 levels in active VL patients and lower hemoglobin levels. In infected dogs, there was a positive correlation between lower IGF-I expression in the bone marrow and lower peripheral blood hematocrit and hemoglobin levels. There was no correlation between decreased IGF-I level/expression and any measured cytokine serum levels in either host. The data suggest that low IGF-I expression is associated with pathogenesis of anemia in active VL, primarily in severe cases, by mechanisms other than alterations in cytokine production.
INTRODUCTION
In Brazil, most of the Leishmania (L.) infantum–infected individuals in visceral leishmaniasis (VL)-endemic areas do not develop active disease and can be identified by their delayed-type hypersensitivity (DTH) response to the Leishmania antigen and/or the presence of anti-Leishmania antibodies.1 However, susceptible individuals develop active VL disease that involves fever, weight loss, hepatosplenomegaly, pancytopenia, hypergammaglobulinemia, and dyslipidemia and may result in death if untreated.2
In active VL, anemia is considered an important marker for the evolution to fatal outcomes.3,4 Leishmania infection may contribute to anemia via different mechanisms that are not clearly known, including splenic sequestration, immune mechanisms, and ineffective erythropoiesis.3 The effects of some growth factors/cytokines in the bone marrow have also been studied in experimental VL.3,5–7 Among growth factors, insulin-like growth factor-I (IGF-I) has been implicated in hematopoiesis,8 and many studies have demonstrated that the growth hormone/IGF-I/IGF-binding protein 3 (IGFBP-3) axis can be downregulated during infections/immune responses through pro-inflammatory cytokine effects.9 During active VL, intense immune inflammatory activation occurs with an overproduction of pro-inflammatory cytokines,10 including interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α).11 In certain cases, these molecules may promote the inhibition of IGF-I expression and production12,13 and may also influence erythropoiesis.14 In this context, we raised the hypothesis that during active VL, infection may modulate IGF-I production and may consequently contribute to the development of anemia under inflammatory cytokine environment. We studied this relationship between IGF-I and anemia in human and canine VL.
We initially examined IGF-I, its binding protein IGFBP3, and cytokine levels in samples from individuals living in a VL-endemic area, including individuals with either active VL or cured VL, asymptomatic Leishmania-infected individuals, and noninfected controls when we observed low IGF-I levels in active VL cases. Then to verify the similar association between IGF-I and anemia in canine VL, we studied dogs presenting with different severities of anemia assessing hematimetric parameters and bone marrow alterations. The IGF-I level profile was similar in both VL patients and VL dogs in the severe stage of anemia, suggesting that IGF-I may contribute to the development of anemia.
MATERIALS AND METHODS
Human subjects from a VL–endemic area, and data and sample collection.
Active VL and cured VL patients and asymptomatic L. (L.) infantum–infected individuals and healthy noninfected subjects from a VL-endemic area in Natal, Rio Grande do Norte state in Northeastern Brazil were studied. All participants or their legal representatives signed the informed consent before participation in the study, and the project protocol was approved by the Conselho Nacional de Ética e Pesquisa (Ethical Certificate 0313.0.015.000-08). Visceral leishmaniasis patients were those treated at the Hospital Giselda Trigueiro, Hospital Infantil Varela Santiago, and Hospital de Pediatria of Universidade Federal do Rio Grande do Norte. Nineteen active VL and 29 cured VL patients (at least 6 months after the end of treatment) were included in this study. Visceral leishmaniasis diagnoses were confirmed via the identification of Leishmania amastigotes in bone marrow aspirates and/or the presence of serum anti-Leishmania antibodies detected by ELISA using whole Leishmania promastigote lysate as an antigen according to Braz et al.15 Thirty-seven L. (L.) infantum–infected asymptomatic control subjects living in the same household as VL patients were selected based on the positive anti-Leishmania DTH test, and we refer to these subjects as control (C) DTH+ subjects. Forty-five individuals with a negative anti-Leishmania DTH test and who were negative for anti-Leishmania antibodies were selected in the neighborhood of these patients, and we refer to these as C DTH− subjects. Demographic, clinical, and epidemiological data were recorded from all subjects. Serum samples were obtained for IGF-I, IGFBP3, and cytokine level measurements and biochemical analysis of each group. Serum samples from active VL patients were collected before treatment.
Serum IGF-I and IGFBP3 levels in human subjects.
Insulin-like growth factor-I and IGFBP3 assays were performed using the IMMULITE kit (DPC-Diagnostics Products Corporation, Los Angeles, CA) in an automated two-site sandwich immunoassay with a chemiluminescent immunometric assay in IMMULITE 2000 (DPC-Diagnostics Products Corporation) according to the manufacturer’s instructions at the Laboratorio de Hormonios do Hospital das Clinicas, Universidade de São Paulo. The IGF-I results are expressed in ng/mL (detection level: 20.0 ng/mL), and the IGFBP3 levels are expressed in µg/mL (detection level: 0.5 µg/mL).
Fluorescent bead–based measurement of human cytokines.
A Bio-Plex Pro Human Cytokine 17-plex Assay (Bio-Rad Laboratories, Inc., Hercules, CA) was used in conjunction with the Bio-Plex system (Bio-Rad Laboratories, Hercules, CA) array reader according to the manufacturer’s directions. Interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 p70, IL-13, IL-17, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, monocyte chemotactic protein 1(MCP-1), macrophage inflammatory protein 1β (MIP)-1β, and TNF-α levels were quantified. Standard curves for each of the analyzed cytokines were included in each run, and sample concentrations were calculated using Bio-Plex Manager software version 4.0. Standard curves ranged from 32,000 to 1.95 pg/mL.
Animals and experimental procedures.
Ethical procedures.
Protocols for animal experiments were prepared and conducted according to the guidelines of CONCEA—CONSELHO NACIONAL DE CONTROLE DE EXPERIMENTAÇÃO ANIMAL (Brazilian Council of Animal Experimentation) and strictly followed the Brazilian law for “Procedures for the Scientific Use of Animals” (11.794/2008). Experimental methods were previously approved by the Research Ethics Committee of Instituto de Medicina Tropical de São Paulo, Universidade de São Paulo (Protocol 2010/077), and Universidade Federal do Piauí (Protocol 010/2010). All dog owners signed the informed consent before participating in the study.
Dogs.
Fourteen mixed-breed adult male and female dogs of unknown ages naturally infected with VL were selected from the Zoonosis Control Center from a VL-endemic area in Teresina City, Piaui State, Northeastern Brazil. The VL diagnosis was confirmed by the identification of Leishmania parasites in the popliteal lymph node and/or sternal bone marrow samples. Then healthy dogs from Sao Paulo city, Sao Paulo state, Brazil, a nonendemic area for VL, were included in the study as controls. All dogs were serologically tested to exclude ehrlichiosis.16 Ten milliliters of peripheral blood was obtained for complete blood cell counts and IGF-I level evaluation. Classification of severity of anemia in dogs was based on hematocrit values as follows: mild (30–37%), moderate (20–29%), and severe (< 19%).17
Bone marrow samples were obtained by aspiration biopsy from the sternum after anesthesia with acepromazine (0.05 mg/kg-intramuscular) and butorphanol (0.2 mg/kg-intramuscular) followed by propofol (5 mg/kg-intravenous). Bone marrow samples were collected for myelogram and analysis of IGF-I and cytokine mRNA expression.
Bone marrow aspirates in dogs.
Smears of bone marrow aspirates were stained with May–Grünwald Giemsa for evaluation of the number of myeloid and eryhtroid cells among 500 cells according to the methods of Harvey.18
Serum IGF-I levels in dogs.
Insulin-like growth factor-I levels were determined in dog serum samples using an ELISA kit for IGF-1 (E90050Ca, Cloud-Clone Corp., Houston, TX) according to the protocol provided by the manufacturer.
Insulin-like growth factor-I and cytokine mRNA expression in bone marrow samples from dogs.
Differential mRNA expression was assessed in bone marrow samples of dog by real-time PCR. Total RNA from bone marrow cells (5 × 106 cells/mL) was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) as recommended by the manufacturer. cDNA synthesis was performed using 1 µg of total RNA plus 10 µL of High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, CA) as recommended by the manufacturer. PCR amplification was performed using Sybr Green (2×) (Life Technologies, Carlsbad, CA) and 150 nM primers (see Supplemental Table 1). The samples were incubated for an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, 58–61°C for 1 minute, and 70°C for 1 minute on an Applied Biosystems 7500 Real-Time PCR System thermal cycler. The expression levels were analyzed by the relative expression method and presented as 2−∆Ct relative to endogenous gene expression.19
Statistical analysis.
Data were analyzed using parametric or nonparametric tests using GraphPad Prism 5.0 (GraphPad Software, Inc., Irvine, CA). The results were expressed as the means ± standard deviation or median and percentiles of 25% and 75%. In all cases, the level of significance was set at P < 0.05.
RESULTS
Characteristics of human subjects from a VL–endemic area.
We studied four groups of subjects: active VL, cured VL, asymptomatic Leishmania-infected (C DTH+), and noninfected healthy (C DTH−) individuals. The gender distribution was similar between groups. However, the age range was different in the active VL group, which primarily included younger subjects compared with the cured VL and C DTH+ groups (Table 1).
Table 1.
Demographic data of subjects from endemic area grouped according to infection or disease conditions
| Groups | Active VL | Cured VL | C DTH+ | C DTH− |
|---|---|---|---|---|
| Number of subjects | 19 | 29 | 37 | 45 |
| Male | 9 | 10 | 17 | 21 |
| Female | 10 | 19 | 20 | 24 |
| Median age (years) | 3.3* | 10.7 | 11.6 | 8.4† |
| 25–75 percentiles | 1.9–7.4 | 8.6–12.6 | 9.7–13.4 | 6.0–9.9 |
C DTH = control delayed-type hypersensitivity; VL = visceral leishmaniasis. Significant values are indicated in bold.
* P < 0.05 (ANOVA and Dunn’s tests) in relation to the cured VL and C DTH+ groups.
† P < 0.05 (ANOVA and Dunn’s tests) in relation to C DTH+ group.
Serum IGF-I and IGFBP3 levels in human subjects from the VL-endemic area and their correlation with laboratory parameters in the active VL group.
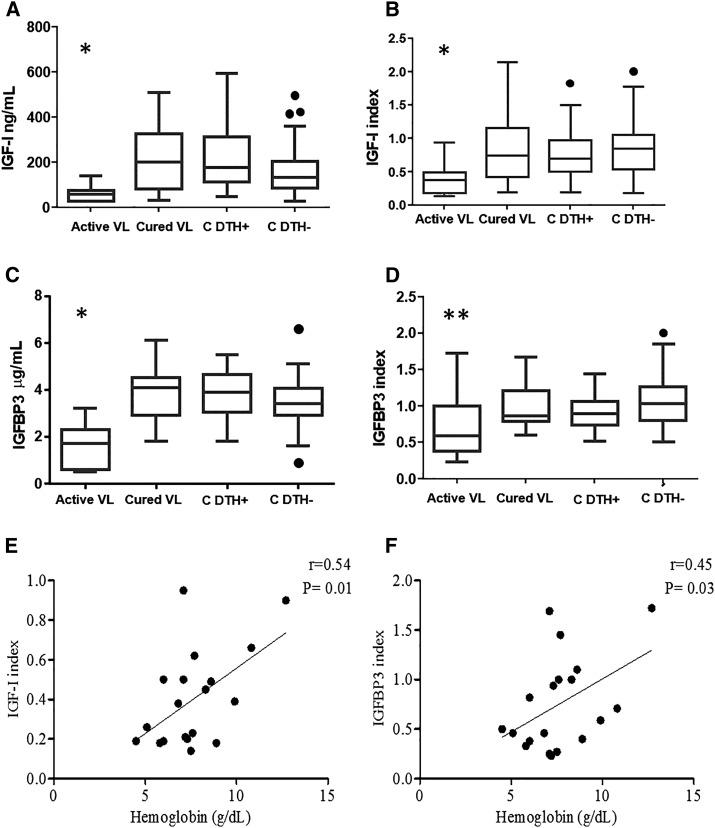
To assess the IGF-I and IGPBP3 production on Leishmania infection, serum levels in subjects with symptomatic or asymptomatic infection were quantified and compared with those from healthy noninfected subjects. Serum IGF-I and IGFBP3 levels were significantly reduced in active VL patients compared with the other groups (Figure 1A and C). In Figure 1A, we directly present the measured levels but because the levels vary considerably according to age and gender, we analyzed the data after transformation into the index obtained by dividing the measured value by the corresponding median value from reference value ranges,20 and we present the data in Figure 1B to confirm the low level of IGF-I in active VL patients. With regard to the IGFBP3 index, the levels were significantly reduced only in the active VL group compared with the C DTH− group (Figure 1D). Thus, data from this study show decreased levels of both IGF-I and IGFBP3 in active VL.
Figure 1.
Insulin-like growth factor-I (IGF-I) and IGF-binding protein 3 (IGFBP3) levels in human subjects from the visceral leishmaniasis (VL)-endemic area and their correlation with laboratory parameters in the active VL group. The IGF-I level (ng/mL) (A), IGF-I index (B), IGFBP3 level (µg/mL) (C), and IGFBP3 index (D) in patients with active VL and cured VL and asymptomatic Leishmania (L.) infantum–infected (C DTH+ [control delayed-type hypersensitivity]) individuals and healthy noninfected (C DTH−) subjects from the VL-endemic area. *P < 0.05 (Kruskal–Wallis and Dunn’s tests) compared with other groups. **P < 0.05 (Kruskal–Wallis and Dunn’s tests) compared with the C DTH− group. Correlations between hemoglobin concentration (g/dL) and the IGF-I index (E), hemoglobin concentration (g/dL) and IGFBP3 index (F) in the active VL group using Pearson’s test.
Does low IGF-I levels associate with anemia?
Most patients with active VL presented with anemia and leukopenia and tended to have thrombocytopenia. We observed a positive correlation between lower hemoglobin levels and lower IGF-I (r = 0.54, P = 0.01; Pearson’s correlation) (Figure 1E) and IGFBP3 indices (r = 0.45, P = 0.03; Pearson’s correlation) (Figure 1F).
In addition, the vast majority had hypoalbuminemia and hyperglobulinemia, with a mean albumin/globulin ratio of 0.5 (see Supplemental Table 2). Given that these parameters likely reflect the severity of the disease, we also analyzed their possible correlation with IGF-I and IGFBP3 indices. No correlations were observed between albumin levels and either IGF-I or IGFBP3 (data not shown).
Serum cytokine levels and their correlation with IGF-I and IGFBP3 levels in human subjects from the VL-endemic area.
To search for one of the known mechanisms of IGF-I–mediated anemia, we next investigated whether inflammatory and anti-inflammatory cytokines modulate IGF-I and IGFPB3 production in VL. IL-1β, IL-2, IL-4, IL-5, IL-8, IL-13, IL-17, G-CSF, MCP-1 (monocyte chemotactic and activating factor [MCAF]), and MIP-1β serum levels did not differ between the groups of individuals from the VL-endemic area. However, IFN-γ, IL-6, IL-10, IL-12 p70, and GM-CSF levels were increased in active VL cases compared with non-diseased individuals. Furthermore, within active VL groups, seven of 19 individuals exhibited very high levels of TNF-α compared with the healthy control group. Only IL-7 was decreased in active VL (Table 2).
Table 2.
Serum cytokine levels in human subjects from VL-endemic area
| Groups | Active VL | Cured VL | C DTH + | C DTH− |
|---|---|---|---|---|
| IFN-γ | 90.3*† | 20.9 | 16.3 | 34.3 |
| 46.3–947.5 | 5.1–36.9 | 7.5–68.4 | 9.4–103.0 | |
| IL-2 | 25.0‡ | 4.1 | 3.9 | 4.2 |
| 4.9–102.7 | 1.5–9.0† | 1.41–6.4 | 2.2–14.1 | |
| IL-6 | 20.8§ | 3.5 | 4.6 | 5,3 |
| 5.5–82.0 | 1.8–7.5 | 2.4–26.8 | 2.2–25.0 | |
| IL-7 | 1.2‖ | 3.9 | 3.6 | 3.4 |
| 0.5–2.6 | 2.5–5.7 | 2.3–4.8 | 2.2–5.8 | |
| IL-10 | 13.2‖ | 0.8 | 0.9 | 1.1 |
| 3.0–27.8 | 0.5–1.4 | 0.7–1.2 | 0.7–2.1 | |
| IL-12 p70 | 1.6‡ | 0.6 | 0.4 | 0.7 |
| 0.5–12.8 | 0.4–1.1 | 0.4–0.7 | 0.4–1.4 | |
| GM-CSF | 248.1‡ | 24.5 | 46.3 | 85.7 |
| 56.4–448.6 | 7.9–58.8 | 20.8–457.2 | 22.8–175.6 |
C DTH = control delayed-type hypersensitivity; IL = Interleukin; VL = visceral leishmaniasis. Significant values are indicated in bold.
* Data (pg/mL) expressed as median, 25th and 75th percentiles.
† P < 0.05 (Kruskal–Wallis and Dunn’s tests) in relation to Cured VL and C DTH−.
‡ P < 0.05 (Kruskal–Wallis and Dunn’s tests) in relation to C DTH+.
§ P < 0.05 (Kruskal-Wallis and Dunn’s tests) in relation to Cured VL.
‖ P < 0.05 (Kruskal-Wallis and Dunn’s tests) in relation to Cured VL, C DTH+, and C DTH−.
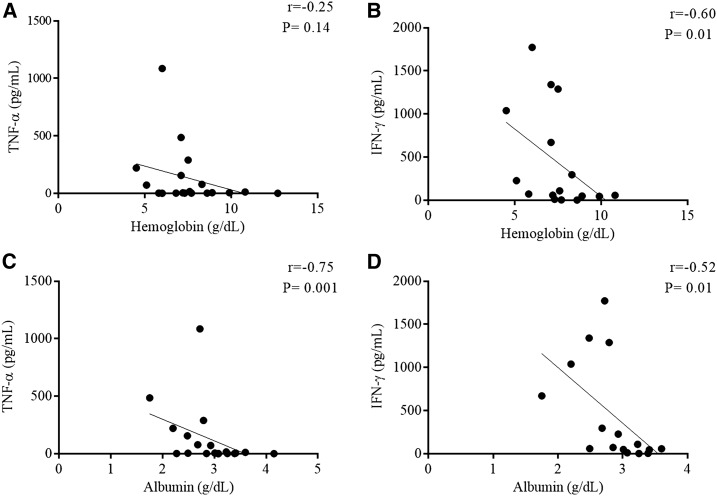
None of the cytokine levels exhibited any correlation with the IGF-I and IGFBP3 levels; however, a negative correlation was noted between the hemoglobin concentration and IFN-γ level (Figure 2A and B) as well as between the albumin concentration and TNF-α (Figure 2C) and IFN-γ levels (Figure 2D) in the active VL group.
Figure 2.
Hemoglobin and albumin levels correlate with TNF-α and IFN-γ levels in the active visceral leishmaniasis (VL) group. Correlation between hemoglobin and TNF-α (A), hemoglobin and IFN-γ (B) and albumin and TNF-α (C), and albumin and IFN-γ (D) levels in the active VL group based on Spearman’s correlation.
Investigating a possible association between anemia and IGF-I levels in naturally infected dogs with VL.
We initially studied the hematological measures and the bone marrow of VL dogs. Adult male and female stray dogs, of unknown age, from an endemic Brazilian area (Teresina, state of Piaui) with VL (N = 14) and VL-negative controls (N = 10), were included in this study.
Hematological alterations in naturally infected dogs with VL.
The hematological profiles of 14 dogs with VL primarily exhibited anemia and thrombocytopenia. Red blood cell counts, hemoglobin, hematocrit, and platelet numbers were significantly reduced compared with healthy dogs (Table 3). Mean corpuscular hemoglobin concentration and mean corpuscular volume were similar to controls, indicating normocytic normochromic anemia. In addition, the total leukocyte count was normal in the present study.
Table 3.
Hematological profile of naturally infected visceral leishmaniasis dogs
| Hematological profile | Healthy dogs (N = 10) | Infected dogs (N = 14) |
|---|---|---|
| Red blood cells (×106/mm3) | 7.0 (6.4–7.3)* | 3.5 (2.8–4.1)† |
| Hemoglobin (g/dL) | 15.0 (14.3–16.3) | 7.8 (4.5–8.8)† |
| Hematocrit (%) | 45.1 (42.2–48.8) | 21.5 (17.6–23.8)† |
| Mean corpuscular volume (fL) | 66.1 (63.8–67.8) | 61.5 (58.3–68.5) |
| Mean corpuscular hemoglobin concentration (g/dL) | 33.3 (32.8–34.0) | 36.3 (32.3–38.3) |
| Platelets (×103/mm3) | 272.0 (224.5–285.8) | 111.5 (84.7–181.3)† |
| Total leukocytes (×103/mm3) | 6.8 (5.6–9.5) | 6.2 (4.0–12.5) |
Significant data are indicated in bold.
* Median (25–75 percentiles).
† P < 0.05 (Mann–Whitney test).
Bone marrow aspirate profile of VL dogs.
Alterations in the bone marrow during the progression of VL are consistently examined in human VL.22 Thus, we evaluated bone marrow alterations in dogs with VL and compared these assessments with anemia.
Bone marrow aspirates from infected dogs exhibited an increased myeloid:erythroid ratio because of myeloid hyperplasia, which was characterized by a substantial increase in mature myeloid precursor cells. We also observed a significant decrease in erythroid cells, with lower percentages of nucleated erythroid precursor cells. In addition, considerably reduced numbers of nucleated erythroid mature cells were noted in infected dogs compared with healthy dogs (Table 4).
Table 4.
Myeloid and erythroid cells from the bone marrow of dogs with visceral leishmaniasis
| Healthy dogs (N = 10) | Infected dogs (N = 14) | |
|---|---|---|
| Myeloid:erythroid | 1.0 (0.9–1.2)* | 2.6 (1.8–5.5)† |
| Precursor myeloid cells:mature myeloid cells | 0.02 (0.01–0.03) | 0.02 (0.01–0.03) |
| Nucleated erythroid precursor cells: nucleated erythroid mature cells | 0.03 (0.02–0.1) | 0.05 (0.01–0.1) |
| Precursor myeloid cells (%) | 1.0 (0.5–1.5) | 1.2 (0.7–1.5) |
| Mature myeloid cells (%) | 46.8 (43.1–48.8) | 62.7 (56.6–74.8)† |
| Nucleated precursor erythroid cells (%) | 1.6 (0.9–2.9) | 1.2 (0.2–1.8)† |
| Nucleated mature erythroid cells (%) | 44.8 (42.1–46.5) | 24.3 (12.0–31.1)† |
Significant data are indicated in bold.
* Median (25–75 percentiles).
† P < 0.05 (Mann–Whitney test).
Do low IGF-I levels correlate with anemia in VL dogs?
Few studies have explored the role of cytokines/growth factors in the canine bone marrow, which may contribute to VL anemia.5,7 Thus, we evaluated IGF-I, TNF-α, and IFN-γ expression in the bone marrow of dogs with VL.
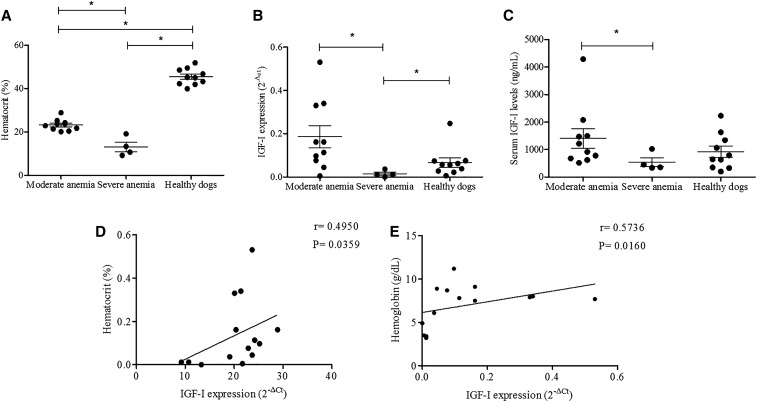
To better understand the possible role of IGF-I and anemia in canine VL, we classified the dogs into groups according to the severity of anemia. Ten dogs had moderate anemia and four had severe anemia (Figure 3A). None of the infected dogs exhibited mild anemia. Notably, diminished IGF-I mRNA expression in the bone marrow and IGF-I serum levels was observed in the dogs with severe anemia (Figure 3B and C). Furthermore, a positive correlation was noted between lowered IGF-I expression in the bone marrow and low hematocrit in dogs (r = 0.49, P = 0.03; Spearman’s correlation) and hemoglobin (r = 0.57, P = 0.016; Spearman’s correlation) levels (Figure 3D and E); there was no significant correlation between anemia and serum IGF-I levels. Thus, infected VL dogs with severe anemia had low IGF-I mRNA and protein levels in the bone marrow/serum, but serum IGF-I did not correlate with measures of anemia.
Figure 3.
Hematocrit values and insulin-like growth factor-I (IGF-I) profile in canine visceral leishmaniasis (VL). Hematocrit values in VL dogs based on the severity of anemia and in healthy dogs (A). IGF-I expression (B) and serum IGF-I levels (C) in infected dogs based on the severity of anemia and healthy dogs. * P < 0.05 (Kruskal–Wallis and Dunn’s tests). Correlation between hematocrit (%) and IGF-I expression (D), and hemoglobin concentration (g/dL) and IGF-I expression (E) in the bone marrow of VL dogs based on Spearman’s test.
High TNF-α and IFN-γ expression is observed in the bone marrow of dogs with moderate anemia but no correlation with IGF-I levels in the bone marrow/serum.
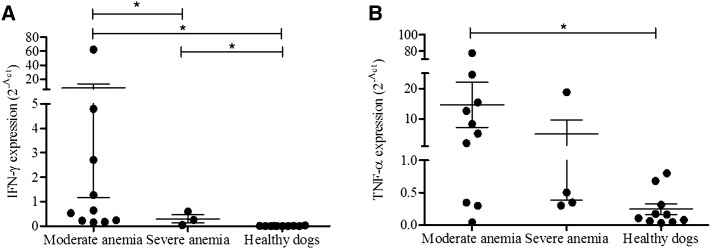
To search for one of the likely mechanisms, we also investigated whether cytokines influence the IGF-I production in canine VL, similar to human approach. In this way, TNF-α and IFN-γ expression in the bone marrow of VL dogs was determined according to the severity of anemia. Both TNF-α and IFN-γ cytokines were significantly increased in the bone marrow of dogs with moderate anemia. Dogs with severe anemia exhibited alterations exclusively in IFN-γ expression compared with healthy dogs (Figure 4). Both cytokines were not correlated with IGF-I levels in the bone marrow/serum in the evaluated groups.
Figure 4.
IFN-γ and TNF-α expression in the canine bone marrow. IFN-γ expression in the bone marrow of infected dogs according to the severity of anemia and in healthy dogs (A). TNF-α expression in the bone marrow of infected dogs according to the severity of anemia and in healthy dogs (B). *P < 0.05 (Kruskal–Wallis and Dunn’s tests).
DISCUSSION
For the present study, we raised the hypothesis that during active phase of VL, infection may modulate IGF-I production and may consequently contribute to the development of anemia under inflammatory cytokine environment. We initially examined IGF-I and its binding protein IGFBP3 levels in samples from individuals living in a VL-endemic area, including individuals with either active VL or cured VL, asymptomatic Leishmania-infected individuals, and noninfected controls. We observed that IGF-I and IGFBP3 levels were decreased as well as hemoglobin concentrations and hematocrit values in active VL. Notably, the lower IGF-I and IGFPB3 serum concentrations were positively correlated with lower hemoglobin levels. There was also no correlation of low IGF-I level and hypoalbuminemia, a value more related to severity of disease in human VL, reinforcing our hypothesis. We thus extended our analysis to canine VL because it is considered a good model to study the pathological changes during the progression of VL.23 Anemia is also an important alteration in canine VL and was similar to human VL in that it was normocytic and normochromic to variable degrees of severity.24,25 In the analysis of IGF-I expression in the bone marrow, we observed a correlation between low IGF-I expression in the bone marrow of infected dogs and severe anemia and low hematocrit levels, suggesting the possible involvement of this growth factor in the impairment of erythropoiesis at this stage of the disease. By contrast, serum IGF-I levels were reduced in dogs with severe but not with moderate anemia, but the reduction was not correlated with hematological parameters. Because in human active VL, a positive correlation between hemoglobin and serum IGF-I protein levels was noted, we hypothesize that the differences in the role of IGF-I might be attributed to the animal species. Canine VL data are consistent with the findings in experimental models in which local IGF-I production is related to the cellularity of hematopoietic cells in the bone marrow but not to low serum IGF-I levels.26 Altogether, the data suggest that IGF-I exhibits changes in expression levels in the bone marrow during disease progression, even when the disease is progressing with only moderate alterations in peripheral blood hematological parameters. These effects were observed in VL dogs with moderate anemia that has not developed into severe anemia. Then in the final stage, in human active VL and in dogs with severe anemia, IGF-I expression/production becomes decreased.
Reinforcing our hypothesis and the findings, this link between low IGF-I level and anemia was also reported in literature. Previous studies have shown that IGF-I seems to influence the hematopoietic system because IGF-I receptor has been found in hematopoietic cells, for example, erythrocytes, and also IGF-I administration was able to stimulate in vitro and in vivo erythropoiesis in normal or pathological conditions.27 A study of adult, nondiabetic subjects revealed a correlation between low IGF-I and hemoglobin levels and showed that IGF-I levels were associated with an increased risk of anemia after adjusting for several confounders.28 It was also suggested that the decrease in IGF-I can contribute to the impairment of erythropoiesis and anemia because IGF-I directly stimulates erythropoiesis in vitro and in vivo.29,30 Furthermore, low IGF-I levels may potentiate an increase in inflammatory molecules, which may inhibit erythroid precursor proliferation.31,32 In adolescents with iron deficiency anemia, a significant decrease in serum IGF-I concentrations correlated with free erythrocyte protoporphyrin in mature red blood cells, suggesting a role for IGF-I in iron metabolism and protoporphyrin synthesis.31 Low IGFBP3 levels observed in active VL patients may have an IGF-I–independent effect on the development of anemia based on a recent study that demonstrated complex formation of IGFBP3, with transferrin influencing iron supply.32 Low IGFBP3 levels would lead to iron deficiency anemia. Nonetheless, the anemia observed in VL in the present study was normocytic and normochromic, thereby suggesting that the participation of IGFBP3 in the pathogenesis of the anemia was less likely in patients enrolled in the present study.
Regarding the mechanisms promoting decreased levels of IGF-I and IGFBP3 in active VL patients, these levels may be caused by decreased expression by cytokine-dependent downregulation rather than increased consumption. Given that this reduction may result from cytokine effects, we assessed cytokine levels. Increased IFN-γ, IL-6, IL-10, IL-12 p70, and GM-CSF and decreased IL-7 levels were observed in active VL. However, changes in these cytokines levels and IGF-I/ IGFBP3 levels did not correlate with the samples from active VL patients, suggesting a cytokine-independent mechanism for decreased IGF-I levels in active VL. Because our previous data are suggestive of a post-transcriptional blockade of IGF-I expression in the liver,33 we hypothesize that alterations in microRNA expression are induced by Leishmania infection.34 Indeed, recent studies have shown that rs35767 polymorphism near IGF1 could be associated with hemoglobin concentration and increase the risk of anemia, without influencing confounding factors such as gender, age, smoking status, glomerular filtration rate, diagnosis of type 2 diabetes, and others.35
Inflammatory cytokines may also influence erythropoiesis, and especially IFN-γ36,37 could lead to anemia in active VL by an IGF-I–independent mechanism.38 Despite the fact that increased cytokines were observed in active VL, there was a correlation between IFN-γ and hemoglobin levels but not with any other cytokines which could suggest a role of this cytokine in the anemia of VL. Furthermore, it was recently shown in murine VL that the defect of the erythropoiesis was related to the presence of IFN-γ–producing CD4+ T cells.37 However, this cytokine has also been reported to stimulate the growth of hematopoietic cells by reducing apoptosis through a mechanism other than modulating Fas and one related to the expression of Bcl-x that is dependent on the stage of maturation of the hematopoietic progenitors.39
When we evaluated bone marrow alterations in dogs with VL, important erythroid cell changes were seen in infected dogs. A lower erythroblast count was related to lower erythrocyte counts and hematocrit and hemoglobin concentrations in the peripheral blood, suggesting that the infection may cause impairment of erythropoiesis.24 Decreased nucleated erythroid precursor cells and considerably reduced nucleated erythroid mature cells were also observed in our infected dogs, suggesting some disturbance in the maturation process where inflammatory cytokines may play any role.
Considering TNF-α and IFN-γ in relation to IGF-I expression in canine samples, our findings do not support the role of these cytokines in controlling IGF-I expression in canine VL, which is consistent with that observed in active VL in humans. The canine data also demonstrated that IGF-I was altered in infected dogs with severe anemia, but no correlation with TNF-α and IFN-γ was observed. Furthermore, both cytokines are implicated in anemia in chronic diseases, exhibiting direct inhibitory effects on hematopoiesis in vivo and in vitro.36,38,40 However, other controversial experimental data indicate that anemia is similar in TNF-α and/or IFN-γ receptor–deficient mice and wild-type mice,41,42 thus leaving this question unresolved.
By analyzing the role of IGF-I in human active VL disease and demonstrating that canine VL exhibits varying severities of anemia, we suggest that IGF-I expression or production is progressively altered. IGF-I levels increase during certain phases of VL evolution, and these levels subsequently decrease in severe cases. The increased production in certain phases may correlate with hematopoiesis that may become exhausted in the final phase of the disease. In addition, inflammatory cytokines as IFN-γ are known to inhibit erythroid cell proliferation, downregulating stem cell factor and erythropoietin receptors but not IGF-I receptor in erythroid colony-forming cells from human blood.39 Thus, the present data on VL suggest a role for IGF-I in the pathogenesis of anemia, which appears to be independent of cytokine alterations.
Supplementary Files
Acknowledgments:
We would like to acknowledge F. A. L. Costa (in memoriam) who established the basis for research in veterinary pathology, particularly canine VL, at the Universidade Federal do Piaui.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Saporito L, Giammanco GM, De Grazia S, Colomba C, 2013. Visceral leishmaniasis: host-parasite interactions and clinical presentation in the immunocompetent and in the immunocompromised host. Int J Infect Dis 17: 572–576. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho MDT, Alonso DP, Vendrame CMV, Costa DL, Costa CHN, Werneck GL, Ribolla PEM, Goto H, 2014. Lipoprotein lipase and PPAR alpha gene polymorphisms, increased very-low-density lipoprotein levels, and decreased high-density lipoprotein levels as risk markers for the development of visceral leishmaniasis by Leishmania infantum. Mediators Inflamm 2014: 230129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto Y, Cheng J, Omachi S, Morimoto A, 2017. Prevalence, severity, and pathogeneses of anemia in visceral leishmaniasis. Parasitol Res 116: 457–464. [DOI] [PubMed] [Google Scholar]
- 4.Costa CHN, Werneck GL, Costa DL, Holanda TA, Aguiar GB, Carvalho AS, Cavalcanti JC, Santos LS, 2010. Is severe visceral leishmaniasis a systemic inflammatory response syndrome? A case control study. Rev Soc Bras Med Trop. 43: 386–392. [DOI] [PubMed] [Google Scholar]
- 5.Lafuse WP, Story R, Mahylis J, Gupta G, Varikuti S, Steinkamp H, Oghumu S, Satoskar AR, 2013. Leishmania donovani infection induces anemia in hamsters by differentially altering erythropoiesis in bone marrow and spleen. PLoS One 8: e59509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotterell SEJ, Engwerda CR, Kaye PM, 2000. Enhanced hematopoietic activity accompanies parasite expansion in the spleen and bone marrow of mice infected with Leishmania donovani. Infect Immun 68: 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto AI, Brown N, Preham O, Doehl JSP, Ashwin H, Kaye PM, 2017. TNF signalling drives expansion of bone marrow CD4+ T cells responsible for HSC exhaustion in experimental visceral leishmaniasis. PLoS Pathog 13: e1006465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zumkeller W, 2002. The insulin-like growth factor system in hematopoietic cells. Leuk Lymphoma 43: 487–491. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW, 2008. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 252: 91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos-Oliveira JR, Regis EG, LealCá CRB, Cunha RV, BozzaPatrí PT, Da-Cruz AM, 2011. Evidence that lipopolisaccharide may contribute to the cytokine storm and cellular activation in patients with visceral leishmaniasis. PLoS Negl Trop Dis 5: e1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.dos Santos PL, et al. 2016. The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLoS Negl Trop Dis 10: e0004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arkins S, Rebeiz N, Brunke-Reese DL, Biragyn A, Kelley KW, 1995. Interferon-gamma inhibits macrophage insulin-like growth factor-I synthesis at the transcriptional level. Mol Endocrinol 9: 350–360. [DOI] [PubMed] [Google Scholar]
- 13.Fan J, Char D, 1995. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by tumor necrosis factor. Am J Physiol 269: R1204–R1212. [DOI] [PubMed] [Google Scholar]
- 14.Askenasy N, 2015. Interferon and tumor necrosis factor as humoral mechanisms coupling hematopoietic activity to inflammation and injury. Blood Rev 29: 11–15. [DOI] [PubMed] [Google Scholar]
- 15.Braz RFS, Nascimento ET, Martins DRA, Wilson ME, Pearson RD, Reed SG, Jeronimo SMB, 2002. The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. Am J Trop Med Hyg 67: 344–348. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa MY, Kohayagawa A, Brandão LP, Morgulis MSFa, Hagiwara MK, 2005. Evaluation of neutrophil oxidative metabolism in canine monocytic ehrlichiosis. Vet Clin Pathol 34: 213–217. [DOI] [PubMed] [Google Scholar]
- 17.Messick J, 2010. Erithrocytes. Schalm’s Veterinary Hematology. Ames, IA: Wiley-Blackwell. [Google Scholar]
- 18.Harvey JW, Stevens A, Lowe JS, Scott I, 2012. Veterinary Hematology. St. Louis, MO: Elsevier Inc. [Google Scholar]
- 19.Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 20.Elmlinger MW, Kühnel W, Weber MM, Ranke MB, 2004. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3). Clin Chem Lab Med 42: 654–664. [DOI] [PubMed] [Google Scholar]
- 21.Lopes AC, 2006. Tratado de Clínica médica, Vol. 3 São Paulo, Roca. [Google Scholar]
- 22.Daneshbod Y, Dehghani SJ, Daneshbod K, 2010. Bone marrow aspiration findings in kala-azar. Acta Cytol 54: 12–24. [DOI] [PubMed] [Google Scholar]
- 23.Loría-Cervera EN, Andrade-Narváez FJ, 2014. Animal models for the study of leishmaniasis immunology. Rev Inst Med Trop Sao Paulo 56: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolato RDC, et al. 2013. Clinical forms of canine visceral leishmaniasis in naturally Leishmania infantum-infected dogs and related myelogram and hemogram changes. PLoS One 8: e82947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varma N, Naseem S, 2010. Hematologic changes in visceral leishmaniasis/kala azar. Indian J Hematol Blood Transfus 26: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welniak LA, Karas M, Yakar S, Anver MR, Murphy WJ, LeRoith D, 2004. Effects of organ-specific loss of insulin-like growth factor-I production on murine hematopoiesis. Biol Blood Marrow Transplant 10: 32–39. [DOI] [PubMed] [Google Scholar]
- 27.Savino W, Smaniotto S, Dardenne M, 2005. Hematopoiesis. Varela-Nieto I, Chowen JA, eds. The Growth Hormone/Insulin-Like Growth Factor Axis During Development. Advances in Experimental Medicine and Biology, Vol. 567 Boston, MA: Springer. [DOI] [PubMed] [Google Scholar]
- 28.Succurro E, Arturi F, Caruso V, Rudi S, Sciacqua A, Andreozzi F, Hribal ML, Perticone F, Sesti G, 2011. Low insulin-like growth factor-1 levels are associated with anaemia in adult non-diabetic subjects. Thromb Haemost 105: 365–370. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak J, Zhang Q, Pertusini E, Wojczyk BS, Wasik MA, Ratajczak MZ, 1998. The role of insulin (INS) and insulin-like growth factor-I (IGF-I) in regulating human erythropoiesis. Studies in vitro under serum-free conditions—comparison to other cytokines and growth factors. Leukemia 12: 371–381. [DOI] [PubMed] [Google Scholar]
- 30.Miniero R, Altomare F, Rubino M, Matarazzo P, Montanari C, Petri A, Raiola G, Bona G, 2012. Effect of recombinant human growth hormone (rhGH) on hemoglobin concentration in children with idiopathic growth hormone deficiency-related anemia. J Pediatr Hematol Oncol 34: 407–411. [DOI] [PubMed] [Google Scholar]
- 31.Choi JW, Kim SK, 2004. Association of serum insulin-like growth factor-I and erythropoiesis in relation to body iron status. Ann Clin Lab Sci 34: 324–328. [PubMed] [Google Scholar]
- 32.Miljuš G, Malenković V, Nedić O, 2013. The importance of metal ions for the formation and isolation of insulin-like growth factor-binding protein 3–transferrin (IGFBP-3–Tf) complexes, and the analysis of their physiological involvement. Metallomics 5: 251. [DOI] [PubMed] [Google Scholar]
- 33.Pinho FA, Magalhães NA, Silva KR, Carvalho AA, Oliveira FLL, Ramos-Sanchez EM, Goto H, Costa FAL, 2013. Divergence between hepatic insulin-like growth factor (IGF)-I mRNA expression and IGF-I serum levels in Leishmania (Leishmania) infantum chagasi-infected dogs. Vet Immunol Immunopathol 151: 163–167. [DOI] [PubMed] [Google Scholar]
- 34.Lemaire J, Mkannez G, Guerfali FZ, Gustin C, Attia H, Sghaier RM, Dellagi K, Laouini D, Renard P, Sysco-Consortium, 2013. MicroRNA expression profile in human macrophages in response to leishmania major infection. PLoS Negl Trop Dis 7: e2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marini MA, Mannino GC, Fiorentino TV, Andreozzi F, Perticone F, Sesti G, 2017. A polymorphism at IGF1 locus is associated with anemia. Oncotarget 8: 32398–32406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koury MJ, 2014. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev 28: 49–66. [DOI] [PubMed] [Google Scholar]
- 37.Preham O, Pinho FA, Pinto AI, Rani GF, Brown N, Hitchcock IS, Goto H, Kaye PM, 2018. CD4+ T cells alter the stromal microenvironment and repress medullary erythropoiesis in murine visceral leishmaniasis. Front Immunol 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Bruin AM, Voermans C, Nolte MA, 2014. Impact of interferon-γ on hematopoiesis. Blood 124: 2479–2486. [DOI] [PubMed] [Google Scholar]
- 39.Choi I, Muta K, Wickrema A, Krantz SB, Nishimura J, Nawata H, 2000. Interferon gamma delays apoptosis of mature erythroid progenitor cells in the absence of erythropoietin. Blood 95: 3742–3749. [PubMed] [Google Scholar]
- 40.Grigorakaki C, Morceau F, Chateauvieux S, Dicato M, Diederich M, 2011. Tumor necrosis factor alpha-mediated inhibition of erythropoiesis involves GATA-1/GATA-2 balance impairment and PU.1 over-expression. Biochem Pharmacol 82: 156–166. [DOI] [PubMed] [Google Scholar]
- 41.Schubert T, Echtenacher B, Hofstädter F, Männel DN, 2003. TNF-independent development of transient anemia of chronic disease in a mouse model of protracted septic peritonitis. Lab Invest 83: 1743–1750. [DOI] [PubMed] [Google Scholar]
- 42.Schubert TE, Echtenacher B, Hofstädter F, Männel DN, 2005. Failure of interferon-gamma and tumor necrosis factor in mediating anemia of chronic disease in a mouse model of protracted septic peritonitis. Int J Mol Med 16: 753–758. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.