Abstract
Background
Hypertension and diabetes represent the first and third highest contributors to global disability. While mobile health (mHealth) messaging programs have rapidly increased in low- and middle-income countries (LMIC), adaptations for specific patient health needs is a new approach to manage chronic conditions.
Objective
The primary aim of this study is to develop and test an mHealth communication intervention using electronic data capture (by tablet) and voice messaging to improve hypertension and diabetes self-management in Cambodia. The secondary aim is to share results with the Cambodian Ministry of Health and development partners to inform health policy and develop strategies for hypertension and diabetes control.
Methods
The study design is a cluster randomized controlled clinical trial randomizing each of 75 Community peer educators (PEs), trained and coordinated by MoPoTsyo Patient Information Center in Phnom Penh, into one of 3 groups of 25 (approximately 60 patients each) to receive either tablet+messages, tablet only, or no intervention (control). The total sample within each group includes 25 clusters and approximately 1500 patients located in 7 Operational Districts in rural regions or urban slums in Cambodia. The interventions (groups 1 and 2) were compared with usual PE monitoring without the tablet or mHealth messaging interventions. Focus groups and informant interviews were conducted to develop messages according to specific themes—medications adherence, laboratory testing, physician visits, obesity, smoking, and general lifestyle issues. Using the data received at monthly PE monitoring meetings, patients will receive specific messages based on their individual health challenges. Following the intervention completion, clinical and process outcomes will be compared with baseline metrics between groups.
Results
PEs were randomized in July 2017, and the intervention was implemented in September 2017 through June 2018. Analyses are underway.
Conclusions
This project is unique in its combination of electronic data transfer, which can be accessed immediately, with voice messages most relevant to individual patients’ needs. Positive results will indicate the value of using targeted messaging in patient-specific, self-management issues to improve hypertension and diabetes control.
International Registered Report Identifier (IRRID)
DERR1-10.2196/11614
Keywords: Cambodia, diabetes, hypertension, mHealth, mobile phone
Introduction
The incidence and prevalence of cardiovascular disease and its risk factors, including hypertension and diabetes mellitus, is increasing rapidly in low- and middle-income countries (LMIC) [1-3]. After the turn of the century, epidemiological trends emerged that were characterized by a major shift in the size and relative magnitude of many risk factors for mortality and disability. High systolic blood pressure and high fasting plasma glucose increased steadily in impact on the global burden of disease [4]. Between 1990 and 2015, systolic blood pressure increased across the globe with associated increases in both mortality and disability [5]. In 2015, the top 3 greatest contributors to global disability-adjusted life-years among level 3 risks were high systolic blood pressure, smoking, and high fasting plasma glucose [6]; these increases are noteworthy as their sequelae, heart disease and stroke, are now the leading cause of death in these nations [7]. In Cambodia, it has been estimated that more than half (52.0%) of the total diabetic population is untreated, and despite treatment, only 24% of all diabetes is adequately controlled [8]. Poverty, inequality, lack of education, along with the nature of the nutritional transition, are root causes of the problem while limited resources mean that noncommunicable diseases (NCDs) must compete for political attention and financial investment. The global trend of increasing risk factors for cardiovascular diseases will continue unless effective methods are implemented to not only identify high-risk individuals but also implement effective and sustainable lifestyle and pharmacological interventions [9]. Tools and interventions to improve the self-management of cardiovascular disease risk factors, such as hypertension and diabetes, are greatly needed in community settings where health care resources may be limited.
Mobile health (mHealth) messaging programs have rapidly increased in LMIC and provide a means to support self-management programs especially needed for NCDs. A number of studies have focused on short message service (SMS) text messaging to improve outcomes for hypertension [10,11] and diabetes [11,12-14]. While these studies have generally shown messaging to be an acceptable format to participants, results have been mixed, with several showing small changes in some disease outcomes [10,12-14] or behavioral change [11,15]. However, no consistent patterns between intervention and control groups have been found. While mHealth messaging systems in the literature have varied in terms of features provided, the evaluation of targeting patients with voice messages to address individualized problems has been limited.
This study aims to leverage the activities of a Cambodian nongovernmental organization, MoPoTsyo Patient Information Center, to test if improvement of data transfer using electronic data capture combined with sending targeted voice messages to patients, would improve outcomes for hypertension and diabetes. As peer educators (PEs) were already established in the MoPoTsyo system to monitor hypertension and diabetes across the country, it provided us with the opportunity to both increase the time from patient measurement to data utilization (through e-tablets) and to use these data to individualize messages targeting specific issues revealed from the data (voice messages).
The primary aim of this study is to develop and test an mHealth communication intervention using electronic data capture and voice messaging to improve hypertension and diabetes self-management in Cambodia by implementing a combined electronic health (eHealth) and mHealth intervention. The secondary aim is to share results with the Cambodian Ministry of Health and development partners to inform health policy and develop strategies for hypertension and diabetes control.
Methods
Human Subjects Approval
This is a randomized controlled clinical trial to test an intervention comprising faster data capture plus targeted voice messages compared with PE monthly monitoring in a community-based sample in Cambodia for improvement of hypertension and diabetes outcomes. This study received Institutional Review Board approval from the University of Washington Division of Human Subjects and the National Ethics Committee for Health Research in Cambodia. All PEs, regardless of study allocation, and participants assigned to the telephone message intervention provided written informed consent. Informed consent from community participants who did not receive mHealth messages were waived by the Institutional Review Boards as routine monitoring provided study outcome data for these groups. This study was determined to not qualify as an “Applicable Clinical Trial” according to the National Institutes of Health definition [16] at the time it was initiated and, thus, was not registered in clinicaltrials.gov. As the risk of harm is minimal in this study, no criteria for discontinuation were developed. An Advisory Committee comprising Cambodian Ministry of Health officials, health care providers, and technology experts was convened to provide oversight and guidance to the study.
Study Setting
In Cambodia, where greater life expectancy is causing rapid increases in NCDs [17], nongovernmental organizations have stepped in when government programs have not been able to address disease locally. MoPoTsyo Patient Information Center is a Cambodian nongovernmental organization for people with chronic disease in Cambodia [18]. It was established in 2004 to provide an institutional and practical response to the information and care needed by patients with hypertension and diabetes. Their model recruits and involves patients as volunteers and trains them to provide counseling on NCDs and monitor key health indicators over time. PEs see patients on a monthly basis to reinforce training and monitor key metrics, including blood pressure, glucose, weight, and adherence to medications. Patients keep paper logs of their health information that are updated at each PE visit. Currently, logs summaries are transferred to the MoPoTsyo Patient Information Center for manual entry and to update patient histories. Since 2005, MoPoTsyo has trained >200 PEs who have registered >31,000 patients in 7 rural provinces and poor urban slum areas of Phnom Penh. Patient and pharmacy records have been used to document the success of the program showing dramatic improvement in chronic disease management [19].
Seven Operational Districts (ODs) representing rural geographic regions or urban slums were selected for inclusion in the project; these included 4 ODs in Kampong Speu province, 2 ODs in Kampong Thom province, 1 OD in Kampong Cham province, and the municipality of Phnom Penh. This area includes 87 PEs who were included for simple randomization (by computer-generated random numbers) into the 3 arms of the study. All patients registered into the MoPoTsyo Patient Information Center system at the time of PE randomization were included as the community sample to receive mHealth messages with no exclusion criteria. Owing to the nature of this intervention, it was not possible to blind participants to study allocation. However, as patients of PEs were clustered geographically, there was a minimum chance of patients interacting across clusters to discuss the study.
Approach
In this study, we are collaborating with MoPoTsyo Patient Information Center to enhance their Peer Educator Network model through the application of mHealth-tailored phone messaging and improved eHealth communication throughout the system. A focus on hypertension, justified by the extremely low medication adherence of hypertensive patients compared with diabetic patients, shifts the intervention to an area of great need. Two interventions will be tested, one at the PE level and one at the patient level. The first includes providing electronic tablets to PEs for data collection and transfer to the MoPoTsyo database in Phnom Penh. The tablets are intended to speed up data collection and entry, reduce paper, increase accuracy, and eliminate lengthy distances that must be traveled to bring paperwork to MoPoTsyo. In addition, the tablets will allow PEs to scan a patient’s record “handbook” (log kept with the patient) to be verified by MoPoTsyo quality control staff should data appear suspicious. At the patient level, voice messages developed to address specific patient problems (eg, uncontrolled blood pressure or glucose, medications not picked up at the pharmacy, weight gain, etc) will be sent to patients based on the data received from the monthly visits to the PE. This study has randomly selected 75 PEs for assignment to 1 of 3 intervention groups—tablet+messaging, tablet only, or control (no tablet or voice messages). Figure 1 shows the study design. As we estimate that each of the 25 PEs in each group monitors about 60 patients, each arm of the study will include about 1500 participants for a total community sample of about 4500.
Figure 1.
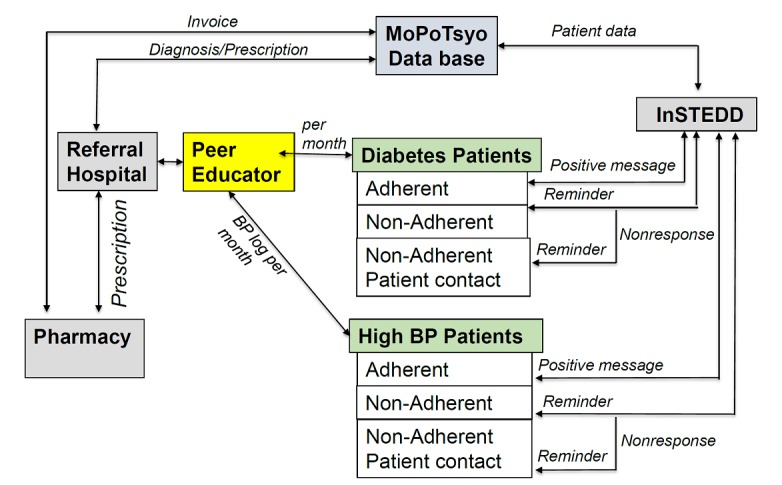
Study design of the project. BP: blood pressure; InSTEDD: Innovative Support to Emergencies, Diseases and Disasters.
Electronic Data Capture
Fifty PEs (25 in the tablet+messaging group and 25 in the tablet only group) received a 12-inch Chuwi Hi12 Tablet with touchscreen compatible for Windows 10-Android 5.1 to record patient information each month. Data are entered online or offline during the patient visit with backup recorded in the patient handbook (log). Data are transferred to the MoPoTsyo database stored on a Dell PowerEdge T630 Server, RAM 32GB (Gigabytes), HDD 4TB, (hard disk 4 Terabytes), speed 2.4 GHz, Cache 20MB. The operating system is Windows Server 2012R2 with database application Microsoft SQL server 2014. Initial training has been provided by MoPoTsyo staff to assure PEs are comfortable with using the tablets for their monthly monitoring visits. It has become clear, perhaps because of their older age and lack of experience, that many of the PEs are challenged by the technical aspects of this new tool. We have begun weekly Web-conferences (broadcast on the tablets) to help address problems and increase PE skills in tablet use. In addition, pharmacies have received tablets to input prescriptions and provide information to MoPoTsyo regarding invoices when medications are purchased. We do not anticipate significant dropout of PEs based on historical experience. PEs seldom leave MoPoTsyo unless illness or death intervenes. Retention efforts for community participants include the free health monitoring provided by PEs and the social interactions that result in this popular program. PEs in all groups are coached to be vigilant in responding to patient questions and needs as well as to contact the MoPoTsyo Center with any issues that arise.
Voice Message Development
We used an exploratory qualitative study design to hear patient and PE perspectives on NCD management and mHealth. We used the 32-item checklist Consolidated Criteria for Reporting Qualitative Research [20] to guide our study design, data collection, analysis, and reporting of our research study. The Information-Motivation-Behavior theoretical framework was used to guide mHealth message development [21] based on providing accurate information, personal motivation, and social motivation to impact behavioral change in chronic disease self-management. We conducted focus groups (N=59) in Khmer with MoPoTsyo patients at 6 ODs within the study territory (Kampong Speu, Chamkar Leu, Baray-Santuk, Stoong, Kong Pisey, and Phnom Penh) to better understand the content of messages that would be most effective and provide guidance on logistics for sending them. Focus groups were held at community Health Centers and lasted for approximately 1.5 hours each. Focus group discussions were audiorecorded with participant permission. For the message content, the focus groups discussed current activities for managing NCDs (eg, medication use, doctor’s visits, lab tests and monitoring, PE groups, and lifestyle changes in diet, physical activity, smoking, and alcohol use), as well as facilitators and barriers to these activities. For the message format, questions included the frequency and duration of messages, preference for text or voicemail format, access to cell phones, the best time of day to send, and ability to respond to a text. At each district, 2 PEs (convenience sample) were also interviewed to evaluate features of the tablet for tracking their patients, to provide outreach and assure usability.
The taxonomy of behavioral change communication deemed most appropriate for our intervention can be best described by the Behaviour Change Wheel [22] using the following: sources of behavior of psychological capability, reflective motivation, and automatic motivation; intervention functions of education, persuasion, incentivization, and enablement; and policies driven by communication, marketing, and service provision.
Voice Message Implementation
We are contracting with experts at Innovative Support to Emergencies, Diseases and Disasters (InSTEDD) Southeast Asia to develop and implement the mHealth app for sending targeted phone messages to MoPoTsyo patients. The app “Verboice” is being used to access the information available in the MoPoTsyo database to tailor messages according to the specific characteristics of patients’ disease. Verboice is a free and open-source tool developed by InSTEDD that runs apps through voice, allowing users to listen and record messages in their own language. At the time of informed consent, patients provided 2 current cell phone numbers, their own plus a backup belonging to a family member. Following the development of specific content for message themes identified in the focus groups, electronic capture of data provided by the PEs will allow us to match the specific issues and needs related to hypertension and diabetes management and send relevant messages to individual patients.
Outcomes
Process outcomes will follow guidelines of the RE-AIM Model [23] to include Reach (how representative is the sample to the targeted population?), Effectiveness (clinical and intermediate outcomes), Adoption (uptake, utilization, and initial implementation), Implementation (fidelity including adherence and delivery), Maintenance (sustainability), as well as acceptability (satisfaction with the intervention). Key clinical outcomes will include control of blood pressure and glucose, medication adherence, use of medical services (laboratory and physician office visits), and improvement in lifestyle factors such as smoking, body mass index, diet, and exercise. Comparisons will be made by the study intervention group at the end of the trial and by changes in clinical outcomes pre- and postintervention period. Furthermore, comparisons will be made for message effectiveness to determine whether certain message topics led to better clinical outcomes.
Data Management
Data collected by PEs during the pilot study will be entered into the tablet at their homes and will be transferred electronically to the newly enhanced Patient Information System database at MoPoTsyo headquarters. The collected data will be stored on a relational database management system and will be securely backed up to an offsite data center affiliated with MoPoTsyo. All data, however, will become a part of the patients’ medical record for longitudinal tracking and access to clinical staff. These data will be available for inclusion in individual, as well as combined, data reports that are a part of the proposed information system.
All data will be monitored by a trained information techinology staff funded under the program dedicated to overseeing data. He will check for missingness and consistency using preprogrammed algorithms to identify problems. After cleaning, data to be analyzed for outcomes will be deidentified and formatted into analytic files for use by coinvestigators and analysts for evaluation.
Data Analysis
Qualitative Analysis
Audiorecordings from the focus group discussions will be transcribed and translated into English. A subset of transcripts will be back-translated to assure accuracy. The transcripts will be analyzed thematically according to the grounded theory approach. Analysis and interviewing will proceed concurrently so that participant responses and emerging themes can shape future focus group discussions and interviews. A coding scheme will be developed on the basis of the topic guide and emerging themes from the transcripts. Two researchers will independently code all transcripts and compare results. Any discrepancies in coding will be discussed and resolved by the research team. Results will be used to inform researchers of the content of messages within the mHealth app and improve the protocol for the introduction of eHealth communications within this project.
Statistical Analyses
Statistical analysis will be performed collaboratively between Cambodian collaborators and the University of Washington to provide experience to them in this activity. All outcomes, both process and clinical, will be described as N (%) or mean (SD) for categorical and continuous data, respectively. Changes in primary study outcomes, including blood pressure, fasting glucose, and medication adherence, will be calculated as the difference from the baseline to 12-month follow-up. We will evaluate study results 3 ways—first as an intention-to-treat comparison of study outcomes associated with patients in the intervention group versus the control group compared by a change in PE overall clinical outcomes for their patients (mean blood pressure, fasting glucose, and medication adherence). In addition, we will conduct an efficiency analysis using linear regression for the study outcomes evaluating group assignment, as well as other variables, calculated at the PE level (ie, % women, mean education, etc). Finally, we will analyze data at the patient level using Generalize Estimating Equations for each outcome evaluating group assignment and clustering data on PE. Primary dependent variables will include changes in the 3 pre and post continuous clinical outcomes—systolic and diastolic blood pressure, fasting blood glucose, and medication adherence as an intermediate outcome. Covariates will include demographics, baseline clinical values, and urban or rural residency of patients. In these analyses, we will use complier-average causal effects (CACE) to look at the efficacy and effectiveness of the mHealth intervention, which takes the clustering effect of PEs, as well as response of patients into consideration. In these patient-level models, we will conduct an intention-to-treat analysis to evaluate the total causal effect of assigning a PE to the mHealth intervention, regardless of compliance. CACE will also allow us to estimate the mHealth intervention’s effectiveness on patient compliers, considering phone messages received and listened to. In CACE, actual treatment received (compliance to the mHealth treatment) becomes a postrandomized variable and effectiveness based on process outcomes (reach and compliance) are addressed. Analyses will be performed using STATA (StataCorp College Station). No interim analyses are planned.
Power
We will conduct the primary analysis evaluating mean differences of change in study outcomes by PE group assignment. With a sample of 50 (PE pairwise comparisons between the 2 intervention groups and controls) assuming an alpha of.05 and improvements of controls at a rate similar to 12-month change previously seen, we have 94% power to assess a 50% greater improvement in systolic blood pressure in the intervention group, and 79% power to assess a 40% greater improvement. We will have >99% power to detect differences in efficacy analyses. We believe that these are realistic goals based on results of other rigorous mHealth studies in LMIC [24]. Power calculations were completed using STATA (StataCorp College Station).
Results
Results of the qualitative research were used to develop the telephone messages according to 6 themes—medications adherence, laboratory testing, physician visits (either annual or for alert determined at PE visit), obesity and weight gain, smoking, and general lifestyle issues (diet, exercise, salt intake, etc). Based on the identified facilitators and barriers, the messages are designed to provide education, motivation, and reminders to help support self-management and address obstacles. Specific scripts were tested with patients and recorded in Khmer by a professional recording studio (Women’s Media Center of Cambodia) using music and voice intonations suggested by advisors.
An algorithm was developed to identify patients needing specific content messages based on the most recent data that were sent electronically to the MoPoTsyo database by the PEs. The delivery of messages was decided according to focus group suggestions, including the use of voicemails rather than text, sending messages at dinnertime or shortly afterward so that a person is at home and can share with family members, limiting frequency to 2-3 messages per week, and eliminating interactive requirements owing to limited access to smartphones and lower cell phone literacy in many rural communities. Results of Verboice tracking allows us to determine which messages successfully reached patients (for later efficacy analyses) and allows the system to send messages additional times when not initially completed.
The focus groups and informant interviews used to develop the mHealth messages were completed during the Spring of 2017. Results of this qualitative work were used to develop the mHealth messages, which were then tested over the summer. Following several revisions of content wording, music, and messenger voice, messages were finalized in July 2017 after which InSTEDD provided channels for delivery of specific themes of message to patients identified by PE data. While the implementation was originally scheduled for 6 months from September 2017 to March 2018, the period was extended 4 additional months because of budgetary efficiencies. Data are currently being cleaned and analyzed. We project that we will have results by Spring 2019.
We plan to disseminate results of this study through workshops and presentations to our Advisory Committee, the Cambodian Ministry of Health (Department of Preventive Medicine), and other health partners (eg, National Institute of Public Health in Phnom Penh) and technology experts (private and public). Furthermore, results will be available in report and presentation format on the website of MoPoTsyo Patient Information Center and the University of Washington Department of Family Medicine. We will use the results of the study to provide evidence-based recommendations to the Ministry of Health as they prepare pilot and other programs and policy for addressing NCDs across Cambodia.
Discussion
This protocol provides information on a new intervention to develop and test mHealth and eHealth interventions to utilize an existing peer support network for improving hypertension and diabetes management in Cambodia. Our formative work, to develop and pilot-test the messages and distribute the tablets, suggests that his approach may be feasible in other LMIC.
The value of peer support in chronic disease management has been well documented and is a viable alternative when health care infrastructure is inadequate to meet needs [25]. While mHealth messaging programs have rapidly increased in LMIC, evaluations of these programs remain limited and provide inconsistent results [26]. The variety of different features and measured outcomes included in such studies also makes comparisons difficult. While the majority of studies in the literature utilize SMS text messaging, we chose voice messages in our intervention owing to the low literacy rate of our target population. In addition, other mHealth studies in low-resource settings have documented a preference for voice messages by targeted populations. For example, 99% of mothers participating in the Mobile Midwife program in Ghana preferred voice messages over text [27]. Similarly, a study of 488 mobile phone users in India found that 89% preferred to receive medication reminders by voice calls over SMS text messages [28]. Several other studies selecting a voice approach have reported positive results [29-31].
Interactive systems, allowing the recipient to respond to a message on their phone pad, have suggested benefits, although we chose to exclude such a feature as our focus groups informed us that this was confusing to patients. In terms of outcomes, many studies have reported benefits in proxy measures, such as medication adherence, clinic attendance, or behavioral change [30,32-36]. Although improvements in clinical outcomes may be of greatest value in disease control, studies reporting these outcomes are unfortunately less common [13,29,37,38]. Finally, while it is established that mHealth messaging systems involving personalized content are generally more successful [39], personalized interventions are also less common in LMIC, and accessing patient data that are both appropriate and timely may not be possible in many of these settings. As mHealth solutions for electronic health recording and data capture increase, tools for monitoring and accessing timely patient data can provide community health workers, PEs, and providers the information they need to offer relevant and personalized care to patients. Such an approach may be especially useful for managing patients with chronic conditions. This protocol was designed to address these barriers.
While this protocol has many strengths, including the stable infrastructure and PE training already in place by MoPoTsyo, there are a number of limitations, which primarily involve the ability of the targeted messages to reach participants. Problems may involve cell phone network coverage and reliability, cell phone number changes, use of phones across family members, and lack of interest in listening to the messages. Other problems may include the reluctance of PEs to use the tablets in a timely manner, loss of tablets, and factors outside of our control (ie, lack of money to cover medications, etc). While strategies have been developed to minimize these issues, they may still impact the effect of the intervention.
The protocol described here represents an effort to integrate improved data technology for collecting data (eHealth) with individualized voice messaging (mHealth) to encourage self-management of hypertension and diabetes in a low-resource setting. We hypothesize that targeting specific health issues of relevance to patients will be more effective in encouraging good behavior than generic messaging often used in mHealth studies. We hope to show that the promotion of greater communication across providers and patients is feasible in LMIC and will result in better clinical outcomes for their patients.
Acknowledgments
We acknowledge the peer educators and patients of MoPoTsyo Patient Information Center for their enthusiasm in participating in this project.
This research is supported by the National Institutes of Health Fogarty International Center grant 1R21TW010160-01. The funding source had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Abbreviations
- CACE
complier-average causal effects
- eHealth
electronic health
- InSTEDD
Innovative Support to Emergencies, Diseases and Disasters
- LMIC
low- and middle-income countries
- mHealth
mobile health
- NCD
noncommunicable disease
- OD
Operational District
- PE
peer educator
- SMS
short message service
Peer-reviewer report from the National Institutes of Health.
Footnotes
Authors' Contributions: The overall study direction and monitoring was provided by ALF and MvP; study design by ALF, JPL, MvP, and CC; operational activities, including data collection, by HH and LS; data management by HH; data analysis by ALF, LS, and HH; study monitoring and advisement by JPL and CC; and writing by ALF, LS, and NI.
Conflicts of Interest: None declared.
References
- 1.Hunter DJ, Reddy KS. Noncommunicable diseases. N Engl J Med. 2013 Oct 03;369(14):1336–43. doi: 10.1056/NEJMra1109345. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin AA, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de VKC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De LD, Degenhardt L, Delossantos A, Denenberg J, Des JDC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo J, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De LFR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh P, Yip P, Zabetian A, Zheng Z, Lopez AD, Murray CJL, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0.S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danaei G, Singh GM, Paciorek CJ, Lin JK, Cowan MJ, Finucane MM, Farzadfar F, Stevens GA, Riley LM, Lu Y, Rao M, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 2013 Apr 09;127(14):1493–502, 1502e1. doi: 10.1161/CIRCULATIONAHA.113.001470. http://europepmc.org/abstract/MED/23481623 .CIRCULATIONAHA.113.001470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A, Delwiche K, Estep K, Frostad JJ, Astha KC, Kyu HH, Moradi-Lakeh M, Ng M, Slepak EL, Thomas BA, Wagner J, Aasvang GM, Abbafati C, Abbasoglu OA, Abd-Allah F, Abera SF, Aboyans V, Abraham B, Abraham JP, Abubakar I, Abu-Rmeileh NME, Aburto TC, Achoki T, Adelekan A, Adofo K, Adou AK, Adsuar JC, Afshin A, Agardh EE, Al KMJ, Al LFH, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Ali MK, Alla F, Allebeck P, Allen PJ, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Ameh EA, Ameli O, Amini H, Ammar W, Anderson BO, Antonio CAT, Anwari P, Argeseanu CS, Arnlöv J, Arsenijevic VSA, Artaman A, Asghar RJ, Assadi R, Atkins LS, Atkinson C, Avila MA, Awuah B, Badawi A, Bahit MC, Bakfalouni T, Balakrishnan K, Balalla S, Balu RK, Banerjee A, Barber RM, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Barrientos-Gutierrez T, Basto-Abreu AC, Basu A, Basu S, Basulaiman MO, Batis RC, Beardsley J, Bedi N, Bekele T, Bell ML, Benjet C, Bennett DA, Benzian H, Bernabé E, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Bikbov B, Bin AAA, Blore JD, Blyth FM, Bohensky MA, Bora BB, Borges G, Bornstein NM, Bose D, Boufous S, Bourne RR, Brainin M, Brazinova A, Breitborde NJ, Brenner H, Briggs ADM, Broday DM, Brooks PM, Bruce NG, Brugha TS, Brunekreef B, Buchbinder R, Bui LN, Bukhman G, Bulloch AG, Burch M, Burney PGJ, Campos-Nonato IR, Campuzano JC, Cantoral AJ, Caravanos J, Cárdenas R, Cardis E, Carpenter DO, Caso V, Castañeda-Orjuela CA, Castro RE, Catalá-López F, Cavalleri F, Çavlin A, Chadha VK, Chang J, Charlson FJ, Chen H, Chen W, Chen Z, Chiang PP, Chimed-Ochir O, Chowdhury R, Christophi CA, Chuang T, Chugh SS, Cirillo M, Claßen TKD, Colistro V, Colomar M, Colquhoun SM, Contreras AG, Cooper C, Cooperrider K, Cooper LT, Coresh J, Courville KJ, Criqui MH, Cuevas-Nasu L, Damsere-Derry J, Danawi H, Dandona L, Dandona R, Dargan PI, Davis A, Davitoiu DV, Dayama A, de CEF, De LCV, De LD, de LG, Degenhardt L, del PB, Dellavalle RP, Deribe K, Derrett S, Des JDC, Dessalegn M, deVeber GA, Devries KM, Dharmaratne SD, Dherani MK, Dicker D, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duan L, Durrani AM, Ebel BE, Ellenbogen RG, Elshrek YM, Endres M, Ermakov SP, Erskine HE, Eshrati B, Esteghamati A, Fahimi S, Faraon EJA, Farzadfar F, Fay DFJ, Feigin VL, Feigl AB, Fereshtehnejad S, Ferrari AJ, Ferri CP, Flaxman AD, Fleming TD, Foigt N, Foreman KJ, Paleo UF, Franklin RC, Gabbe B, Gaffikin L, Gakidou E, Gamkrelidze A, Gankpé FG, Gansevoort RT, García-Guerra FA, Gasana E, Geleijnse JM, Gessner BD, Gething P, Gibney KB, Gillum RF, Ginawi IAM, Giroud M, Giussani G, Goenka S, Goginashvili K, Gomez DH, Gona P, Gonzalez DCT, González-Castell D, Gotay CC, Goto A, Gouda HN, Guerrant RL, Gugnani HC, Guillemin F, Gunnell D, Gupta R, Gupta R, Gutiérrez RA, Hafezi-Nejad N, Hagan H, Hagstromer M, Halasa YA, Hamadeh RR, Hammami M, Hankey GJ, Hao Y, Harb HL, Haregu TN, Haro JM, Havmoeller R, Hay SI, Hedayati MT, Heredia-Pi IB, Hernandez L, Heuton KR, Heydarpour P, Hijar M, Hoek HW, Hoffman HJ, Hornberger JC, Hosgood HD, Hoy DG, Hsairi M, Hu G, Hu H, Huang C, Huang JJ, Hubbell BJ, Huiart L, Husseini A, Iannarone ML, Iburg KM, Idrisov BT, Ikeda N, Innos K, Inoue M, Islami F, Ismayilova S, Jacobsen KH, Jansen HA, Jarvis DL, Jassal SK, Jauregui A, Jayaraman S, Jeemon P, Jensen PN, Jha V, Jiang F, Jiang G, Jiang Y, Jonas JB, Juel K, Kan H, Kany RSS, Karam NE, Karch A, Karema CK, Karthikeyan G, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Keren A, Khader YS, Khalifa SEAH, Khan EA, Khang Y, Khatibzadeh S, Khonelidze I, Kieling C, Kim D, Kim S, Kim Y, Kimokoti RW, Kinfu Y, Kinge JM, Kissela BM, Kivipelto M, Knibbs LD, Knudsen AK, Kokubo Y, Kose MR, Kosen S, Kraemer A, Kravchenko M, Krishnaswami S, Kromhout H, Ku T, Kuate DB, Kucuk BB, Kuipers EJ, Kulkarni C, Kulkarni VS, Kumar GA, Kwan GF, Lai T, Lakshmana BA, Lalloo R, Lallukka T, Lam H, Lan Q, Lansingh VC, Larson HJ, Larsson A, Laryea DO, Lavados PM, Lawrynowicz AE, Leasher JL, Lee J, Leigh J, Leung R, Levi M, Li Y, Li Y, Liang J, Liang X, Lim SS, Lindsay MP, Lipshultz SE, Liu S, Liu Y, Lloyd BK, Logroscino G, London SJ, Lopez N, Lortet-Tieulent J, Lotufo PA, Lozano R, Lunevicius R, Ma J, Ma S, Machado VMP, MacIntyre MF, Magis-Rodriguez C, Mahdi AA, Majdan M, Malekzadeh R, Mangalam S, Mapoma CC, Marape M, Marcenes W, Margolis DJ, Margono C, Marks GB, Martin RV, Marzan MB, Mashal MT, Masiye F, Mason-Jones AJ, Matsushita K, Matzopoulos R, Mayosi BM, Mazorodze TT, McKay AC, McKee M, McLain A, Meaney PA, Medina C, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mendoza W, Mensah GA, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Misganaw A, Mishra S, Mohamed IN, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montañez HJC, Montico M, Moore AR, Morawska L, Mori R, Moschandreas J, Moturi WN, Mozaffarian D, Mueller UO, Mukaigawara M, Mullany EC, Murthy KS, Naghavi M, Nahas Z, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KMV, Nash D, Neal B, Nejjari C, Neupane SP, Newton CR, Ngalesoni FN, Ngirabega JDD, Nguyen G, Nguyen NT, Nieuwenhuijsen MJ, Nisar MI, Nogueira JR, Nolla JM, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh I, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orozco R, Pagcatipunan RS, Pain AW, Pandian JD, Panelo CIA, Papachristou C, Park E, Parry CD, Paternina CAJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pedraza LS, GBD 2013 Risk Factors Collaborators Lancet. 2015 Dec 5;386(10010):2287–323. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen AK, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Bärnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catalá-López F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang Y, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki MES, Murray CJL. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015. JAMA. 2017 Dec 10;317(2):165–182. doi: 10.1001/jama.2016.19043.2596292 [DOI] [PubMed] [Google Scholar]
- 6.GBD 2015 Risk Factors Collaborators Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016 Oct 08;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(16)31679-8 .S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood) 2007;26(1):13–24. doi: 10.1377/hlthaff.26.1.13. http://europepmc.org/abstract/MED/17211010 .26/1/13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otgontuya D, Oum S, Palam E, Rani M, Buckley BS. Individual-based primary prevention of cardiovascular disease in Cambodia and Mongolia: early identification and management of hypertension and diabetes mellitus. BMC Public Health. 2012 Apr 02;12:254. doi: 10.1186/1471-2458-12-254. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-12-254 .1471-2458-12-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom D, Chisholm D, Jane-Llopis E, Prettner K, Stein A, Feigl A. World Health Organization and World Economic Forum. [2017-09-30]. From Burden to “Best Buys”: Reducing the Economic Impact of Non-Communicable Diseases in Low- and Middle-Income Countries http://www.who.int/nmh/publications/best_buys_summary.pdf?ua=1 .
- 10.Bobrow K, Farmer AJ, Springer D, Shanyinde M, Yu L, Brennan T, Rayner B, Namane M, Steyn K, Tarassenko L, Levitt N. Mobile Phone Text Messages to Support Treatment Adherence in Adults With High Blood Pressure (SMS-Text Adherence Support [StAR]): A Single-Blind, Randomized Trial. Circulation. 2016 Feb 09;133(6):592–600. doi: 10.1161/CIRCULATIONAHA.115.017530. http://europepmc.org/abstract/MED/26769742 .CIRCULATIONAHA.115.017530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacking D, Haricharan HJ, Brittain K, Lau YK, Cassidy T, Heap M. Hypertension Health Promotion via Text Messaging at a Community Health Center in South Africa: A Mixed Methods Study. JMIR Mhealth Uhealth. 2016 Mar 10;4(1):e22. doi: 10.2196/mhealth.4569. http://mhealth.jmir.org/2016/1/e22/ v4i1e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty AS, Chamukuttan S, Nanditha A, Raj RKC, Ramachandran A. Reinforcement of adherence to prescription recommendations in Asian Indian diabetes patients using short message service (SMS)--a pilot study. J Assoc Physicians India. 2011 Nov;59:711–4. [PubMed] [Google Scholar]
- 13.Ramachandran A, Snehalatha C, Ram J, Selvam S, Simon M, Nanditha A, Shetty AS, Godsland IF, Chaturvedi N, Majeed A, Oliver N, Toumazou C, Alberti KG, Johnston DG. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013 Nov;1(3):191–8. doi: 10.1016/S2213-8587(13)70067-6.S2213-8587(13)70067-6 [DOI] [PubMed] [Google Scholar]
- 14.Shariful ISM, Niessen LW, Ferrari U, Ali L, Seissler J, Lechner A. Effects of Mobile Phone SMS to Improve Glycemic Control Among Patients With Type 2 Diabetes in Bangladesh: A Prospective, Parallel-Group, Randomized Controlled Trial. Diabetes Care. 2015 Aug;38(8):e112–3. doi: 10.2337/dc15-0505.38/8/e112 [DOI] [PubMed] [Google Scholar]
- 15.Rubinstein A, Miranda JJ, Beratarrechea A, Diez-Canseco F, Kanter R, Gutierrez L, Bernabé-Ortiz A, Irazola V, Fernandez A, Letona P, Martínez H, Ramirez-Zea M. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 2016 Jan;4(1):52–63. doi: 10.1016/S2213-8587(15)00381-2.S2213-8587(15)00381-2 [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health. [2018-12-17]. Identifying an “Applicable Clinical Trial” under FDAAA https://grants.nih.gov/ClinicalTrials_fdaaa/docs/Flow_chart-ACT_only.pdf .
- 17.World Bank Country Data: Cambodia. [2017-09-29]. https://data.worldbank.org/country/cambodia .
- 18.van Olmen Josefien, Eggermont N, van Pelt Maurits, Hen H, de Man Jeroen, Schellevis F, Peters DH, Bigdeli M. Patient-centred innovation to ensure access to diabetes care in Cambodia: the case of MoPoTsyo. J Pharm Policy Pract. 2016 Jan 21;9:1. doi: 10.1186/s40545-016-0050-1. https://joppp.biomedcentral.com/articles/10.1186/s40545-016-0050-1 .50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi D, LoGerfo J, van Pelt M, Mielcarek B, Huster K, Haider M, Thomas B. Evaluation of a multi-faceted diabetes care program including community-based peer educators in Takeo province, Cambodia, 2007-2013. PLoS ONE. 2017 Sep 25;12(9):e0181582. doi: 10.1371/journal.pone.0181582. http://dx.plos.org/10.1371/journal.pone.0181582 .PONE-D-17-12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007 Dec;19(6):349–57. doi: 10.1093/intqhc/mzm042. http://intqhc.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=17872937 .mzm042 [DOI] [PubMed] [Google Scholar]
- 21.Osborn CY, Egede LE. Validation of an Information-Motivation-Behavioral Skills model of diabetes self-care (IMB-DSC) Patient Educ Couns. 2010 Apr;79(1):49–54. doi: 10.1016/j.pec.2009.07.016. http://europepmc.org/abstract/MED/19699601 .S0738-3991(09)00319-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michie S, van Stralen Maartje M, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011 Apr 23;6:42. doi: 10.1186/1748-5908-6-42. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-6-42 .1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999 Sep;89(9):1322–7. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anstey WJ, Goudge J, Gómez-Olivé FX, Huxley C, Dodd K, Griffiths F. mHealth text and voice communication for monitoring people with chronic diseases in low-resource settings: a realist review. BMJ Glob Health. 2018;3(2):e000543. doi: 10.1136/bmjgh-2017-000543. http://europepmc.org/abstract/MED/29527356 .bmjgh-2017-000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher EB, Boothroyd RI, Elstad EA, Hays L, Henes A, Maslow GR, Velicer C. Peer support of complex health behaviors in prevention and disease management with special reference to diabetes: systematic reviews. Clin Diabetes Endocrinol. 2017;3:4. doi: 10.1186/s40842-017-0042-3. https://clindiabetesendo.biomedcentral.com/articles/10.1186/s40842-017-0042-3 .42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chib A, van Velthoven Michelle Helena, Car J. mHealth adoption in low-resource environments: a review of the use of mobile healthcare in developing countries. J Health Commun. 2015;20(1):4–34. doi: 10.1080/10810730.2013.864735. [DOI] [PubMed] [Google Scholar]
- 27.Grameen Foundation. MOTECH Mobile technology for community health in Ghana. 2011. Mar, http://www.cs.washington.edu/education/courses/cse490d/12sp/docs/MOTECH.pdf .
- 28.DeSouza SI, Rashmi MR, Vasanthi AP, Joseph SM, Rodrigues R. Mobile phones: the next step towards healthcare delivery in rural India? PLoS One. 2014;9(8):e104895. doi: 10.1371/journal.pone.0104895. http://dx.plos.org/10.1371/journal.pone.0104895 .PONE-D-14-01603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piette JD, List J, Rana GK, Townsend W, Striplin D, Heisler M. Mobile Health Devices as Tools for Worldwide Cardiovascular Risk Reduction and Disease Management. Circulation. 2015 Nov 24;132(21):2012–27. doi: 10.1161/CIRCULATIONAHA.114.008723.CIRCULATIONAHA.114.008723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues R, Shet A, Antony J, Sidney K, Arumugam K, Krishnamurthy S, D'Souza G, DeCosta A. Supporting adherence to antiretroviral therapy with mobile phone reminders: results from a cohort in South India. PLoS One. 2012;7(8):e40723. doi: 10.1371/journal.pone.0040723. http://dx.plos.org/10.1371/journal.pone.0040723 .PONE-D-11-19032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunutsor S, Walley J, Katabira E, Muchuro S, Balidawa H, Namagala E, Ikoona E. Using mobile phones to improve clinic attendance amongst an antiretroviral treatment cohort in rural Uganda: a cross-sectional and prospective study. AIDS Behav. 2010 Dec;14(6):1347–52. doi: 10.1007/s10461-010-9780-2. [DOI] [PubMed] [Google Scholar]
- 32.Hall DMS, Fottrell Edward, Wilkinson Sophia, Byass Peter. Assessing the impact of mHealth interventions in low- and middle-income countries--what has been shown to work? Glob Health Action. 2014;7:25606. doi: 10.3402/gha.v7.25606. http://europepmc.org/abstract/MED/25361730 .25606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, Jack W, Habyarimana J, Sadatsafavi M, Najafzadeh M, Marra CA, Estambale B, Ngugi E, Ball TB, Thabane L, Gelmon LJ, Kimani J, Ackers M, Plummer FA. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010 Nov 27;376(9755):1838–45. doi: 10.1016/S0140-6736(10)61997-6.S0140-6736(10)61997-6 [DOI] [PubMed] [Google Scholar]
- 34.Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque Damien, MacKeen L, Haberer J, Kimaiyo S, Sidle J, Ngare D, Bangsberg DR. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011 Mar 27;25(6):825–34. doi: 10.1097/QAD.0b013e32834380c1. http://europepmc.org/abstract/MED/21252632 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang R, Li X. Electronic messaging support service programs improve adherence to lipid-lowering therapy among outpatients with coronary artery disease: an exploratory randomised control study. J Clin Nurs. 2016 Mar;25(5-6):664–71. doi: 10.1111/jocn.12988. [DOI] [PubMed] [Google Scholar]
- 36.Bigna JJR, Noubiap JJN, Kouanfack C, Plottel CS, Koulla-Shiro S. Effect of mobile phone reminders on follow-up medical care of children exposed to or infected with HIV in Cameroon (MORE CARE): a multicentre, single-blind, factorial, randomised controlled trial. Lancet Infect Dis. 2014 Jul;14(7):600–8. doi: 10.1016/S1473-3099(14)70741-8.S1473-3099(14)70741-8 [DOI] [PubMed] [Google Scholar]
- 37.Peiris D, Praveen D, Johnson C, Mogulluru K. Use of mHealth systems and tools for non-communicable diseases in low- and middle-income countries: a systematic review. J Cardiovasc Transl Res. 2014 Nov;7(8):677–91. doi: 10.1007/s12265-014-9581-5. [DOI] [PubMed] [Google Scholar]
- 38.Kamal AK, Shaikh Q, Pasha O, Azam I, Islam M, Memon AA, Rehman H, Akram MA, Affan M, Nazir S, Aziz S, Jan M, Andani A, Muqeet A, Ahmed B, Khoja S. A randomized controlled behavioral intervention trial to improve medication adherence in adult stroke patients with prescription tailored Short Messaging Service (SMS)-SMS4Stroke study. BMC Neurol. 2015 Oct 21;15:212. doi: 10.1186/s12883-015-0471-5. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-015-0471-5 .10.1186/s12883-015-0471-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan P, Lauver DR. The efficacy of tailored interventions. J Nurs Scholarsh. 2002;34(4):331–7. doi: 10.1111/j.1547-5069.2002.00331.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer-reviewer report from the National Institutes of Health.


