Abstract
Introduction
Understanding HIV incidence and risk behaviour among populations being considered for HIV vaccine studies is necessary for the appropriate design of trials.
Methods
Between May 2012 and June 2015, we recruited men aged 18–49 years from urban and peri-urban areas of Rustenburg, a mining town in the North West Province, South Africa. Men who reported HIV-risk behaviour were followed for nine to 12 months to determine HIV incidence and factors associated with condom use.
Results
A total of 400 HIV uninfected men were enrolled; 366 (91.5%) had at least one follow-up visit and were included in the analysis; 47.6% were under 25 years of age. HIV incidence was 1.9 per 100 person-years (95% CI: 0.79–4.56). Among heterosexual men (N = 339), 80.8% reported having vaginal intercourse with multiple partners in the past three months, among whom 74.1% reported inconsistent condom use. Sixty-eight percent reported vaginal intercourse with new female partners, of whom 40.6% reported inconsistent condom use. Over half (55.6%) of men who had sex with men (N = 27) reported anal intercourse with multiple male partners in the past three months, of whom 68.2% reported using condoms inconsistently. Men who had more than two female partners in the last three months (n = 121) were more likely to use condoms inconsistently (aOR 4.31, 95% CI: 1.34–13.8); in contrast, those with more than one new female sex partner (aOR 0.13, 94% CI 0.04–0.44), and whose sexual debut was after 19 years of age (aOR 0.39, 95% CI: 0.15–1.01) were less likely to use condoms inconsistently.
Conclusion
HIV incidence was low and similar to other studies of heterosexual men in South Africa. To identify men at high risk for HIV for enrolment in prevention trials, future researchers may need to focus on those who report early sexual debut and who report having multiple sexual partners. Men in newer relationships appear to use condoms more frequently.
Introduction
South Africa has the highest burden of HIV/AIDS in the world. In 2016, 7.1 million people were estimated to be living with HIV and 270,000 people became newly infected that year [1]. Prevention strategies that have been implemented include promotion of condom use, prevention of maternal-to-child-transmission of HIV, treatment of infected persons with highly active antiretroviral therapy (HAART), voluntary medical male circumcision and pre-exposure prophylaxis (PrEP) [2, 3]. Prospective cohort-based surveys offer precise measurement of HIV incidence and risk factors [4]; however, most estimates of new HIV infections in South Africa have primarily been measured through changes in prevalence over time or laboratory determination of recent infections [5]. In addition, the majority of cohort studies measuring HIV incidence in South Africa have been conducted among women [6–9], with only a few among men who have sex with men (MSM) [10, 11] and heterosexual males [12, 13]. Additional prospective cohort-based information on HIV incidence and risk behaviour among heterosexual men is necessary for the design of HIV vaccine efficacy trials and effective prevention strategies.
Rustenburg is a platinum-mining town in the North West province of South Africa and has a population of approximately 626,522 [14]. HIV prevalence rates are high in this area. In 2012, 20.3% of adult men and women in the province were estimated to be HIV infected [5], and in 2013, 31.5% of antenatal women in and around Rustenburg were estimated to be HIV sero-positive [15]. In two longitudinal cohorts using different recruitment strategies and eligibility criteria, HIV incidence among women in the area was found to be 3.0 per 100 person-years of observation (p100pyo) (95% CI: 0.4–10.8) in 2008–2009 [7], and 8.9 p100pyo (95% CI: 5.7–14.0) in 2010–2011 [10]. Based on a longitudinal cohort study, HIV incidence among MSM, also from the Rustenburg area, was found to be 9.5 p100pyo (95% CI: 2.4–38.0) [10]. However, information on heterosexual men in the Rustenburg area is limited. Therefore, we recruited a cohort of primarily heterosexual men from the same area, and followed them over time to determine HIV incidence and to characterize risk behaviour.
Methods
Study subjects
Between May 2012 and June 2015, we recruited men 18–49 years of age from urban and peri-urban areas of Rustenburg for enrolment into a longitudinal observational cohort study. Research staff approached men in the central business district, townships, primary health care clinics, shopping centres, taxi stands, taverns, and car wash stations, as well as during general community meetings and HIV awareness campaigns. Some men heard about the study by word of mouth. Staff briefly explained that the study was recruiting men for a 9-to-12 month follow-up study that would include repeat HIV testing. Men who were interested were asked to come to the research site for evaluation and enrolment. Starting in March 2013, we also began recruiting men by snow-ball sampling in which enrolled participants were each given five coupons to give to their friends or acquaintances, encouraging them to come to the study site for more information and screening. We did not collect information on how enrolled participants were recruited. Participants received mobile phone airtime worth R 30 (2.50 USD) for each referral who was screened.
Initial HIV testing prior to screening and enrolment
At the study site, staff described the study, and individuals who were interested were asked if they were aware of their HIV status; those who said they were HIV seropositive were excluded. The remaining men underwent risk reduction counselling and were tested for HIV using the Alere DetermineTM HIV1-2 rapid test (Alere, USA) on finger prick blood samples; those with a positive result were retested using Uni-goldTM (Trinity Biotech, Ireland). Discordant results were resolved by using an HIV-1 p24Ag ELISA test (HIV Combi PT, Roche Switzerland) on a venous blood sample; the test was performed at Lancet Laboratories, Rustenburg. Men who were HIV seropositive on both rapid tests or by ELISA were referred for care and were not eligible for screening and enrolment. The remaining men were invited to participate in the study and provided signed informed consent in the language of their choice (English, Setswana or Xhosa).
Screening and enrolment
Screening and enrolment visits were conducted on the same or different days; if the enrolment visit took place more than 28-days after the screening visit, then HIV testing and screening for behavioural risk factors were repeated to ensure continued eligibility.
Screening
Participants completed an interviewer-administered questionnaire on socio-demographics and HIV-risk behaviour) during the previous three months (S1–S6 Files) that asked about the following: total number of male and female partners, total number of new male and female partners, condom use with all partners and with new partners (never, sometimes, frequently, always), and self-reported history of symptoms of sexually transmitted infection (STI) (penile discharge, painful urination, dyspareunia or penile sores).We classified inconsistent condom use as never, frequently or sometimes using condoms with partners in the last three months, and consistent condom use as always using a condom with all partners [16, 17]. Clinicians conducted a genital exam; STIs were diagnosed syndromically and treated according to national guidelines. Uncircumcised men were referred to local health care clinics for voluntary medical male circumcision (VMMC). We did not collect information on how many uncircumcised men subsequently underwent VMMC. As part of the study screening procedures, venous blood samples were collected for repeat HIV rapid testing using the same algorithm described above, and those who were identified as HIV infected were referred for care and removed from the study.
Enrolment
Eligibility for enrolment included being confirmed as HIV uninfected and reporting high risk behaviour. High risk behaviour was defined as being diagnosed with an STI on exam, or reporting at least one of the following in the last three months: 1) being treated for, diagnosed with, or reporting symptoms of an STI, 2) having vaginal or anal intercourse with more than one sexual partner, 3) having vaginal or anal intercourse with a new sexual partner, or 4) having sex with a partner known to be HIV infected. MSM were defined as men who described their sexual orientation as homosexual or bisexual, and/or who reported having insertive or receptive anal intercourse with another man in the last three months. Men who reported male-male sex and who also reported having sex with women were classified as MSM.
Follow-up
Detailed contact information was collected at enrolment; participants were asked to return three months later, and again between nine to 12 months after baseline. Men were contacted by phone at six months to update their address and other contact details. At each follow-up visit, men underwent HIV testing of venous blood samples, risk reduction counselling, and a clinical exam for the presence of STIs with treatment as needed. Risk assessment questionnaires were not administered during follow-up visits. Attempts were made to contact men who missed follow-up appointments by phone or through a home visit.
The study was reviewed and approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal, and the Research Committee of the Northwest Provincial Department of Health. Men were reimbursed R50 (4.20 USD) at each visit for their time and transport costs.
Analyses
Data were analysed using STATA V14.0 (Stata Corporation, College Station, Texas, USA). Socio-demographic and behavioural characteristics of heterosexual men (N = 339) were compared to MSM (N = 27) using descriptive statistics. Pearson’s Chi square (χ2) and fisher’s exact tests were used to compare proportions (%) for categorical variables and Mann-Whitney U-test or Student’s t-test was used to compare means and or medians for continuous variables. HIV seroconversion was estimated to have occurred halfway between the date of the last HIV negative test, and the date when HIV antibodies were detected. HIV incidence was calculated as the number of seroconversions divided by the total number of person-years of observation, and presented as p100pyo with 95% confidence intervals (CI). Evaluation of factors associated with HIV incidence was not conducted due to the small number of men who seroconverted.
Logistic regression analysis was used to determine the relationship of socio-demographic and risk-behaviour variables with inconsistent condom use with all types of partners in the last three months (compared to consistent use). We performed univariate logistic regression to evaluate the association of all baseline variables with inconsistent condom use; factors associated with inconsistent condom use at p< = 0.20 were included in a backward stepwise multivariable logistic regression model. Variables with a p-value of <0.05 were retained in the final model; age was included as a potential confounder. All statistical tests were two-sided and p-values <0.05 were considered statistically significant.
Results
A total of 1394 men presented to the study site for screening, of whom nine (0.6%) reported that they were HIV infected, and 37 (2.6%) decided they were not interested in the study (Fig 1). The remaining 1348 were offered HIV testing and counselling, of whom 123 (9.1%) were identified as being HIV seropositive on testing; 48 were excluded because they preferred a consent language not available or they were not within the target age group; an additional 354 decided not to participate due to work commitments or because they said they were not interested. Staff explained the informed consent to the remaining 823 men, of whom 92 failed an assessment of understanding, and 22 decided not to enrol. The remaining 709 men were screened for eligibility based on risk behaviour, and underwent repeat HIV testing. Of these, 304/709 (42.8%) were ineligible because they did not meet the criteria for being high risk, and five (0.7%) were found to be HIV-infected. The HIV prevalence among the 1394 men who initially presented to the study was 9.8%, including those who reported that they were HIV infected as well as those who were identified as HIV seropositive on testing.
Fig 1. Screening and enrolment of adult men into a longitudinal cohort study, Rustenburg, South Africa, 2012–2015.
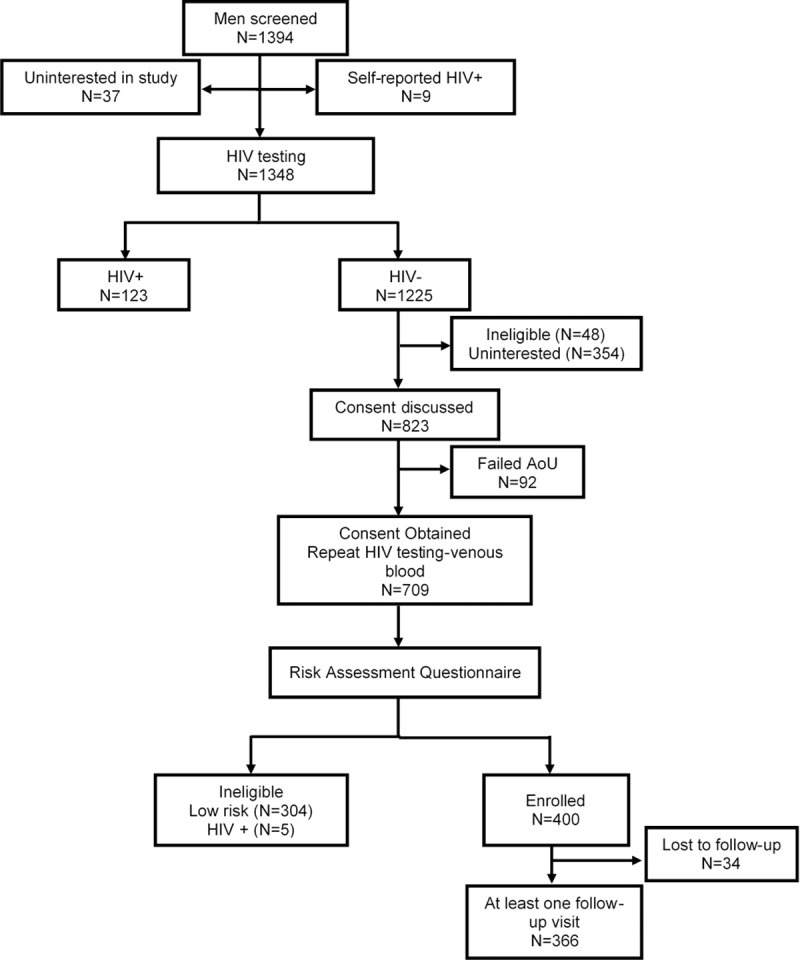
(AoU)-Assessment of Understanding of the informed consent.
A total of 400 men were enrolled into the cohort and completed baseline data collection of whom 34 (8.5%) did not return for any follow-up visits and were excluded from analysis; thus, 366 men were included in the final cohort. We did not find any significant differences in socio-demographic characteristics and HIV-risk behaviour between men who were retained in the study and those who did not return for follow-up (S1 Table).
Baseline characteristics
Among the 366 men in the cohort, 27 were classified as being MSM (Table 1). The mean age of all men was 26 (SD 5.5) years; nearly half (47.6%) were less than 25 years of age; 48.9% were unemployed and 53.8% had some post-secondary education. A total of 58 (15.8%) reported being less than 15 years old at the time of their sexual debut; the majority (65.6%) reported becoming sexually active between 15 and 18 years of age. About a third (34.4%) were circumcised. Although 25.1% of men reported having a symptom of an STI in the past three months, only 6.1% were classified as having an STI based on syndromic diagnosis at enrolment. Among men who reported only having heterosexual sex (N = 339), 80.8% had two or more partners in the previous three months, of whom 203/274 (74.1%) reported inconsistent condom use. Nearly three-quarters (68.1%) of heterosexual men reported vaginal intercourse with a new female sexual partner in the past three months, of whom 93/229 (40.6%) reported using condoms inconsistently. Only a few heterosexual men (4.1%) reported sexual intercourse with partners known to be HIV infected; five of these 14 men reported using condoms inconsistently with those partners.
Table 1. Baseline socio-demographic and behavioural characteristics of adult HIV negative men enrolled in a longitudinal cohort study between 2012–2015, Rustenburg, South Africa.
| Total (N = 366) |
Heterosexual (N = 339) |
MSM (N = 27) |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | n | (%) | n | (%) | n | (%) | ||
| Age, yrs. | ||||||||
| Mean (SD) | 26.2±5.5 | 26.5±5.5 | 21.8±2.5 | |||||
| Education | ||||||||
| Primary school | 4 | 1.1 | 4 | 1.2 | 0 | 0.0 | ||
| Some Secondary school | 165 | 45.1 | 149 | 44.2 | 16 | 59.3 | ||
| Some-Post Secondary school | 197 | 53.8 | 186 | 55.2 | 11 | 40.7 | ||
| Employment | ||||||||
| Unemployed | 179 | 48.9 | 167 | 49.2 | 12 | 44.4 | ||
| Student | 34 | 9.3 | 26 | 7.7 | 8 | 29.6 | ||
| Employed <40 hrs./wk. | 40 | 10.9 | 37 | 10.9 | 3 | 11.1 | ||
| Employed ≥40 hrs./wk. | 113 | 30.9 | 109 | 32.2 | 4 | 14.8 | ||
| Marital status | ||||||||
| Single | 347 | 94.8 | 321 | 94.7 | 26 | 96.3 | ||
| Married | 16 | 4.4 | 16 | 4.7 | 0 | 0.0 | ||
| Divorced/Separated | 2 | 0.5 | 2 | 0.6 | 0 | 0.0 | ||
| Age at sexual debut, yrs. | ||||||||
| <15 | 58 | 15.8 | 51 | 15.1 | 7 | 25.9 | ||
| 15–18 | 240 | 65.6 | 224 | 66.1 | 16 | 59.2 | ||
| ≥19 | 68 | 18.5 | 64 | 18.8 | 4 | 14.8 | ||
| Circumcision | ||||||||
| Uncircumcised | 240 | 65.5 | 223 | 65.7 | 17 | 62.9 | ||
| Medical circumcision | 86 | 23.5 | 79 | 23.3 | 7 | 25.9 | ||
| Cultural circumcision | 40 | 10.9 | 37 | 10.9 | 3 | 11.1 | ||
| Self-report of any STI symptoms, last 3 mo. | 92 | 25.1 | 89 | 25.3 | 3 | 11.1 | ||
| STI by syndromic diagnosis | 22 | 6.1 | 22 | 6.5 | 0 | 0.00 | ||
| No. female sex partners, last 3 mo. | ||||||||
| 0 | 24 | 6.6 | 3 | 0.9 | 21 | 77.8 | ||
| 1 | 65 | 17.8 | 62 | 18.3 | 3 | 11.1 | ||
| 2 | 153 | 41.8 | 153 | 45.1 | 0 | 0.0 | ||
| ≥3 | 124 | 33.9 | 121 | 35.7 | 3 | 11.1 | ||
| Condom use with female sex partners, last 3 mo. (n = 342) | ||||||||
| n = 336 | n = 5 | |||||||
| Inconsistent | 256 | 74.8 | 251 | 74.7 | 5 | 83.3 | ||
| Consistent | 86 | 25.1 | 85 | 25.3 | 1 | 16.7 | ||
| No. new female sex partners, last 3 mo.a (n = 352) | ||||||||
| n = 339 | n = 13 | |||||||
| 0 | 118 | 33.5 | 108 | 31.9 | 10 | 76.9 | ||
| 1 | 151 | 42.9 | 150 | 44.2 | 1 | 7.6 | ||
| 2 | 46 | 13.1 | 46 | 13.6 | 0 | 0.0 | ||
| ≥3 | 37 | 10.5 | 35 | 10.3 | 2 | 15.4 | ||
| Condom use with new female sex partners, last 3 mo.a (n = 232) | ||||||||
| n = 229 | n = 3 | |||||||
| Inconsistent | 95 | 40.9 | 93 | 40.6 | 2 | 66.7 | ||
| Consistent | 137 | 59.1 | 136 | 59.4 | 1 | 33.3 | ||
| No. male sex partners, last 3 mo. | ||||||||
| 0 | 344 | 18.5 | 339 | 100.0 | 5 | 18.5 | ||
| 1 | 7 | 1.9 | 0 | 0.0 | 7 | 25.9 | ||
| 2 | 7 | 1.9 | 0 | 0.0 | 7 | 25.9 | ||
| ≥3 | 8 | 2.2 | 0 | 0.0 | 8 | 29.6 | ||
| Condom use with male sex partners, last 3 mo. (n = 22) | ||||||||
| Inconsistent | 15 | 68.2 | - | - | 15 | 68.2 | ||
| Consistent | 7 | 31.8 | - | - | 7 | 31.8 | ||
| Had known HIV infected sex partners, last 3 mo.1 | 15 | 4.2 | 14 | 4.1 | 1 | 4.0 | ||
| Condom use with HIV infected partners, last 3 mo.a (n = 12) | ||||||||
| n = 12 | n = 11 | n = 1 | ||||||
| Inconsistent | 6 | 50.0 | 5 | 45.5 | 1 | 100.0 | ||
| Consistent | 6 | 50.0 | 6 | 54.5 | 0 | 0.0 | ||
a Differences in total numbers among categories are due to missing values.
More than three-quarters (81.5%) of MSM reported having either receptive or insertive anal intercourse with at least one male sexual partner during the past three months, of whom 15/22 (68.2%) reported inconsistent condom use. Over half (55.6%) of MSM reported anal intercourse with two or more male partners in the past three months, of whom 11/15 (73.3%) reported using condoms inconsistently. Less than a quarter of MSM (22.2%) reported having vaginal intercourse with a female in the past three months. Among the 22 MSM who reported recent male-male sexual behaviour, six (27.2%) reported having insertive anal intercourse, of whom three (50.0%) reported inconsistent condom use; 18 (81.8%) reported having receptive anal intercourse, of whom 12 (66.6%) reported inconsistent condom use with these partners (S2 Table).
HIV incidence and retention
Among the 366 men retained in the study 54 (14.7%) returned for only one follow up visit, and 312 (85.3%) completed both follow-up visits. The median follow-up time was 8.2 months (range: 2.3–23.8), with 263.4 total pyo. Five men became HIV-infected, resulting in an HIV incidence of 1.9 p100pyo (95% CI: 0.8–4.6). Three of the five HIV seroconversions were identified at the final nine or 12 month visit, and the median follow-up time among the five HIV infected men was 9.3 months. All five participants who seroconverted were heterosexual, became sexually active before they were 18 years of age, and had two or more sexual partners in the last three months; only two of the five reported using condoms consistently. None reported having intercourse with a known HIV-infected person. If we exclude the MSM, the total follow-up time among heterosexual men in this study was 243.2 years with an HIV incidence of 2.1 p100pyo (95% CI: 0.84–4.9).
Inconsistent condom use and socio-demographic factors
Bivariate and multivariable analysis of risk factors associated with inconsistent condom use are shown in Table 2. There was no association between age, being employed and circumcision status and inconsistent use. Men who reported having more than two female sexual partners in the past three months were more likely to inconsistently use condoms (aOR: 4.31; 95% CI: 1.34–13.8). In contrast, men with one or more new female sex partners (aOR: 0.13; 95% CI: 0.04–0.44) were more likely to use condoms. Although not statistically significant, men who had their sexual debut at age ≥ 19 years (aOR: 0.39; 95% CI: 0.15–1.01, p = 0.05) were also more likely to use condoms.
Table 2. Association of factors with using condoms inconsistently with all partners in last 3 months, among heterosexual men.
| Characteristic | Total (N = 336) |
Condom use | Univariate analysis | Multivariable analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consistent (n = 85) |
Inconsistent (n = 251) |
|||||||||||
| N | n | (%) | n | (%) | Unadjusted OR | 95% CI | p-value | Adjusted OR | 95% CI | p-value | ||
| Age, yrs. | ||||||||||||
| 18–24 | 151 | 40 | 26.5 | 111 | 73.5 | Ref | ||||||
| 25–30 | 120 | 31 | 25.8 | 89 | 74.2 | 1.03 | 0.59–1.78 | 0.90 | 1.31 | 0.71–2.35 | 0.37 | |
| >30 | 65 | 14 | 21.5 | 51 | 78.5 | 1.31 | 0.66–2.63 | 0.44 | 1.72 | 0.81–3.63 | 0.16 | |
| Age at sexual debut, yrs. | ||||||||||||
| <15 | 51 | 9 | 17.6 | 42 | 82.4 | Ref | ||||||
| 15–18 | 222 | 56 | 25.3 | 166 | 74.7 | 0.64 | 0.29–1.39 | 0.26 | 0.57 | 0.26–1.28 | 0.18 | |
| ≥19 | 63 | 20 | 31.7 | 43 | 68.3 | 0.46 | 0.19–1.13 | 0.09 | 0.39 | 0.15–1.01 | 0.05 | |
| Employment Status | ||||||||||||
| Unemployed | 166 | 44 | 26.5 | 122 | 73.5 | Ref | ||||||
| Student | 25 | 6 | 24.0 | 19 | 76.0 | 1.14 | 0.43–3.04 | 0.79 | - | - | - | |
| Employed Part-Time | 37 | 10 | 27.1 | 27 | 72.9 | 0.97 | 0.43–2.17 | 0.94 | - | - | - | |
| Employed Full time | 108 | 25 | 23.2 | 83 | 76.8 | 1.19 | 0.68–2.10 | 0.53 | - | - | - | |
| Circumcised | ||||||||||||
| No | 258 | 63 | 24.4 | 195 | 75.6 | Ref | ||||||
| Yes | 78 | 22 | 28.2 | 56 | 71.7 | 0.82 | 0.46–1.45 | 0.51 | - | - | - | |
| Self-report of any STI symptoms, last 3 mo. | ||||||||||||
| No | 248 | 68 | 27.4 | 180 | 72.3 | Ref | ||||||
| Yes | 88 | 17 | 19.3 | 71 | 80.7 | 1.57 | 0.86–2.86 | 0.14 | - | - | - | |
| No. of female sex partners, last 3 mo. | ||||||||||||
| 1 | 62 | 14 | 22.6 | 48 | 77.4 | Ref | ||||||
| 2 | 153 | 46 | 30.1 | 107 | 69.9 | 0.68 | 0.34–1.35 | 0.27 | 0.89 | 0.42–1.92 | 0.76 | |
| >2 | 121 | 25 | 20.6 | 96 | 79.4 | 1.12 | 0.53–2.35 | 0.76 | 4.31 | 1.34–13.8 | 0.01 | |
| No. of new female sex partners, last 3 mo. | ||||||||||||
| 0 | 105 | 20 | 19.1 | 85 | 80.9 | Ref | ||||||
| 1 | 150 | 40 | 26.7 | 110 | 73.3 | 0.65 | 0.35–1.18 | 0.16 | 0.56 | 0.28–1.11 | 0.09 | |
| >1 | 81 | 25 | 30.8 | 56 | 69.2 | 0.53 | 0.27–1.04 | 0.06 | 0.13 | 0.04–0.44 | 0.001 | |
Odds Ratio (OR) refers to the odds of inconsistently using a condom compared to consistent condom use.
Confidence Interval (CI).
Discussion
Only a few cohort studies have reported the incidence of HIV infection among heterosexual men in South Africa [12, 13] or in other southern African countries [18, 19]. Here, we report the incidence of HIV infection among men from in and around Rustenburg, a mining town in the North West Province of South Africa. We found the overall incidence to be 1.9 p100pyo, which is similar to national estimates of recent infection among adult males (1.21 p100py; 95% CI: 0.97–1.45) based on use of the limiting-antigen avidity (LAg) assay on HIV-infected samples obtained during a 2012 national population-based survey [5]. Mathematical modelling of data from the 2012 survey resulted in a similar estimate of HIV incidence among men (1.6 p100py [95%CI: 0.6–2.7]) [5].
Our data support the results of other studies in southern Africa that found that heterosexual men have a lower HIV incidence compared to women [5, 12, 13, 19, 20]. Several factors may have biased our incidence estimate, however. Because almost half our cohort were under the age of 25 years, our estimates may not be representative of the overall male population in South Africa; national estimates of HIV prevalence are higher among men aged 25–49 years [5]. At each study visit, participants received risk reduction counselling which may have reduced their subsequent risk behaviour; however, we did not collect data on behaviour at follow up visits to see if they changed over time. On the other hand, some studies suggest that impact of counselling on STI and/or HIV acquisition may be limited [21]. The short median follow-up time in our study (8.2 months) may not have been long enough to identify many new HIV infections, although HIV incidence is often highest early in cohort participation [10]. As not all men were retained, it is possible that men at higher risk of acquiring HIV dropped out. However, we found no significant differences in the demographics and HIV-risk behaviour between men who were retained and those who were not. Because of stigma associated with HIV, it is possible that high risk men may have under-reported their HIV-risk behaviour at screening and therefore were considered ineligible to participate in our study. Treatment of STIs and male circumcision may also have contributed to reduced risk of HIV acquisition. Finally, since the research clinic was open only during working hours, men who were employed and could have been at higher risk may have declined enrolment; nearly 50% of men in our sample were unemployed.
To better understand characteristics of men at high risk for HIV infection, we examined the relationship between socio-demographic and behavioural factors, and inconsistent condom use. Similar to previous studies, we observed that men who had multiple female sexual partners were least likely to use condoms [22], although those who had new female sexual partners were more likely to use condoms. Previous studies have shown that men in new/casual relationships are more likely to use condoms, but as relationships become more stable, condom use declines [23–25]. We also observed that those who had earlier sexual debut were less likely to use condoms consistently. Previous studies have shown that males who had their first sexual debut before the age 15 age were less likely to engage in unprotected sex compared to those who had early sexual debut [26]. Based on these observations, future researchers may need to focus on men who report early sexual debut, who have multiple sexual partners and/or men in who are in longer term relationships to identify men at high risk for HIV infection.
Our study has several limitations in addition to those described above. Identification of men based on self-report of risk behaviour may have been subject to recall bias or mis-reporting due to stigma. Studies among adult men and women in Uganda, and among women in South Africa found low HIV incidence rates even though individuals reported engaging in high HIV risk behaviour [7, 27, 28]. Additional studies are needed to determine how best to recruit high risk heterosexual men; possibilities include identifying men from hot-spots or based on their social and sexual network characteristics [29, 30]. We did not collect data on whether uncircumcised men underwent circumcision after being referred by study staff. Because our recruitment strategy targeted men who thought they were HIV-uninfected, we may have inadvertently pre-selected men who were at lower risk. Even though we attempted to recruit men at high risk, 9.8% of those who presented at the clinic were identified as being HIV infected, which is lower than the prevalence of 14.5% among the general adult male population (15–49 years of age) in South Africa in 2012 [5]
This study adds important information to the limited body of knowledge regarding the incidence of HIV infection among heterosexual men in South Africa. Our findings suggests that vaccine efficacy trials that aim to include heterosexual South African men will require more specific recruitment strategies and better characterization of behavioural risk profiles to recruit men at risk of HIV. Results from our study suggest enrolling those with multiple partners and those whose sexual debut occurred when young. In addition, the eligibility criteria for enrolment may need to include whether condoms were used with their sexual partners.
Supporting information
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank the study participants and the clinical and laboratory staff at The Aurum Institute Rustenburg Clinical Research Site for expert patient care and handling of specimen. We also thank Nisha Naidoo for coordinating the study, and Ireen Mosweu for coordinating the recruitment and data collection activities. This work was supported by International AIDS Vaccine Initiative (IAVI). IAVI is funded by many donors, including the generous support of the American people through the United States Agency for International Development (USAID). A full list of IAVI donors may be found at www.iavi.org. These data have been presented in part at HIV Research for Prevention 2016 in Chicago, United States of America, 7–21 October 2016. The contents of this manuscript are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government.
We wish to acknowledge the support from the University of California, San Francisco’s International Traineeships in AIDS Prevention Studies (ITAPS), U.S. NIMH, R25MH064712 and the International AIDS Vaccine Initiative.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was funded by the US Agency for International Development (USAID) under Cooperative Agreement No. GPO-A-00-05-00022-00 and the Contraceptive and Reproductive Health Technologies Research and Utilisation (CRTU) Program. The funders were involved in the study design of the protocols that were implemented at the site. The Aurum Institute conceptualized the idea for the manuscript and the funders provided technical guidance. We wish to acknowledge the support from the University of California, San Francisco’s International Traineeships in AIDS Prevention Studies (ITAPS), U.S. NIMH, R25MH064712 and the International AIDS Vaccine Initiative.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS DATA 2017. Available from: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf. [PubMed]
- 2.World Health Organization. Guideline on when to start antiretroviral therapy and on preexposure prophylaxis for HIV 2015. Available from: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. [PubMed]
- 3.UNAIDS. Prevention Gap Report 2016. Available from: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf.
- 4.McDougal JS, Pilcher CD, Parekh BS, Gershy-Damet G, Branson BM, Marsh K, et al. Surveillance for HIV-1 incidence using tests for recent infection in resource-constrained countries. AIDS. 2005;19 Suppl 2:S25–30. Epub 2005/06/03. . [DOI] [PubMed] [Google Scholar]
- 5.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. Cape Town: 2014. [DOI] [PubMed] [Google Scholar]
- 6.Abdool Karim Q, Kharsany AB, Frohlich JA, Werner L, Mlotshwa M, Madlala BT, et al. HIV incidence in young girls in KwaZulu-Natal, South Africa—public health imperative for their inclusion in HIV biomedical intervention trials. AIDS Behav. 2012;16(7):1870–6. 10.1007/s10461-012-0209-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldblum PJ, Latka MH, Lombaard J, Chetty C, Chen PL, Sexton C, et al. HIV incidence and prevalence among cohorts of women with higher risk behaviour in Bloemfontein and Rustenburg, South Africa: a prospective study. BMJ Open. 2012;2(1):e000626 10.1136/bmjopen-2011-000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramjee G, Wand H, Whitaker C, McCormack S, Padian N, Kelly C, et al. HIV incidence among non-pregnant women living in selected rural, semi-rural and urban areas in KwaZulu-Natal, South Africa. AIDS Behav. 2012;16(7):2062–71. 10.1007/s10461-011-0043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramjee G, Moonsamy S, Abbai NS, Wand H. Individual and Population Level Impact of Key HIV Risk Factors on HIV Incidence Rates in Durban, South Africa. PLoS One. 2016;11(4):e0153969 10.1371/journal.pone.0153969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamali A, Price MA, Lakhi S, Karita E, Inambao M, Sanders EJ, et al. Creating an African HIV clinical research and prevention trials network: HIV prevalence, incidence and transmission. PLoS One. 2015;10(1):e0116100 10.1371/journal.pone.0116100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane T, Osmand T, Marr A, Struthers H, McIntyre JA, Shade SB. Brief Report: High HIV Incidence in a South African Community of Men Who Have Sex With Men: Results From the Mpumalanga Men's Study, 2012–2015. J Acquir Immune Defic Syndr. 2016;73(5):609–11. 10.1097/QAI.0000000000001162 . [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves JR, Bonell CP, Morison LA, Kim JC, Phetla G, Porter JD, et al. Explaining continued high HIV prevalence in South Africa: socioeconomic factors, HIV incidence and sexual behaviour change among a rural cohort, 2001–2004. AIDS. 2007;21 Suppl 7:S39–48. 10.1097/01.aids.0000300534.97601.d6 . [DOI] [PubMed] [Google Scholar]
- 13.Barnighausen T, Tanser F, Gqwede Z, Mbizana C, Herbst K, Newell ML. High HIV incidence in a community with high HIV prevalence in rural South Africa: findings from a prospective population-based study. AIDS. 2008;22(1):139–44. 10.1097/QAD.0b013e3282f2ef43 . [DOI] [PubMed] [Google Scholar]
- 14.Bojanala Platinum District Municipality. Final Integrated Development Plan 2017/2022. 2017.
- 15.National Department of Health. The 2013 National Antenatal Sentinel HIV Prevalence Survey South Africa. Pretoria: 2013.
- 16.Morris L, Kouya F, Kwalar R, Pilapil M, Saito K, Palmer N, et al. Factors associated with inconsistent condom use in adolescents with negative or unknown HIV status in Northwest Cameroon. AIDS Care. 2014;26(11):1440–5. 10.1080/09540121.2014.920948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muchiri E, Odimegwu C, De Wet N. HIV risk perception and consistency in condom use among adolescents and young adults in urban Cape Town, South Africa: a cumulative risk analysis. Southern African Journal of Infectious Diseases. 2017;32(3):105–10. [Google Scholar]
- 18.Sherr L, Lopman B, Kakowa M, Dube S, Chawira G, Nyamukapa C, et al. Voluntary counselling and testing: uptake, impact on sexual behaviour, and HIV incidence in a rural Zimbabwean cohort. AIDS. 2007;21(7):851–60. 10.1097/QAD.0b013e32805e8711 . [DOI] [PubMed] [Google Scholar]
- 19.Justman J, Reed JB, Bicego G, Donnell D, Li K, Bock N, et al. Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study. Lancet HIV. 2017;4(2):e83–e92. 10.1016/S2352-3018(16)30190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S. Seroprevalence of HIV infection in rural South Africa. AIDS. 1992;6(12):1535–9. . [DOI] [PubMed] [Google Scholar]
- 21.Metsch LR, Feaster DJ, Gooden L, Schackman BR, Matheson T, Das M, et al. Effect of risk-reduction counseling with rapid HIV testing on risk of acquiring sexually transmitted infections: the AWARE randomized clinical trial. JAMA. 2013;310(16):1701–10. 10.1001/jama.2013.280034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shai NJ, Jewkes R, Nduna M, Dunkle K. Masculinities and condom use patterns among young rural South Africa men: a cross-sectional baseline survey. BMC public health. 2012;12:462 10.1186/1471-2458-12-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku L, Sonenstein FL, Pleck JH. The dynamics of young men's condom use during and across relationships. Fam Plann Perspect. 1994;26(6):246–51. Epub 1994/11/01. . [PubMed] [Google Scholar]
- 24.Chamratrithirong A, Kaiser P. The dynamics of condom use with regular and casual partners: analysis of the 2006 National Sexual Behavior Survey of Thailand. PLoS One. 2012;7(7):e42009 Epub 2012/08/04. 10.1371/journal.pone.0042009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbs L, Manning WD, Longmore MA, Giordano PC. Qualities of romantic relationships and consistent condom use among dating young adult Contraceptives: Predictors of Use, Role of Cultural Attitudes & Practices and Levels of Effectiveness. Hauppauge, New York, USA: Nova Science Publishers, Inc.; 2014. p. 157–82. [Google Scholar]
- 26.Baldwin P, Shrestha R, Potrepka J, Copenhaver M. The Age of Initiation of Drug Use and Sexual Behavior May Influence Subsequent HIV Risk Behavior: A Systematic Review. Isrn Aids. 2013;2013:976035 10.1155/2013/976035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruzagira E, Wandiembe S, Abaasa A, Levin J, Bwanika A, Bahemuka U, et al. Prevalence and incidence of HIV in a rural community-based HIV vaccine preparedness cohort in Masaka, Uganda. PLoS One. 2011;6(6):e20684 10.1371/journal.pone.0020684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abaasa A, Asiki G, Price MA, Ruzagira E, Kibengo F, Bahemuka U, et al. Comparison of HIV incidence estimated in clinical trial and observational cohort settings in a high risk fishing population in Uganda: Implications for sample size estimates. Vaccine. 2016;34(15):1778–85. 10.1016/j.vaccine.2016.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metch B, Frank I, Novak R, Swann E, Metzger D, Morgan C, et al. Recruitment of urban US women at risk for HIV infection and willingness to participate in future HIV vaccine trials. AIDS Behav. 2013;17(2):760–72. 10.1007/s10461-012-0351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fearon E, Wiggins RD, Pettifor AE, MacPhail C, Kahn K, Selin A, et al. Associations between friendship characteristics and HIV and HSV-2 status amongst young South African women in HPTN-068. J Int AIDS Soc. 2017;20(4). Epub 2017/12/30. 10.1002/jia2.25029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.


