Abstract
The objective of this experiment was to evaluate the impact of complexed trace mineral supplementation on ovum pick-up (OPU) and in vitro embryo production in lactating beef cows. Thirty days prior to fixed-time artificial insemination (FTAI; day −30), 68 postpartum cows were stratified by BW, BCS, and parity before being randomly assigned to 10 pens of either a treatment (TRT; n = 5) or a control (CNT; n = 5) group. Each group received a weekly mineral supplement allotment of 1.16 kg × week−1 × cow–calf pair−1 for 14 wk. Cows assigned to the TRT group received a mineral supplement that contained amino acid complexes of zinc, copper, and manganese, as well as cobalt glucoheptonate (Availa Plus; Zinpro Corp., Eden Prairie, MN, USA), while cows assigned to the CNT group received a mineral supplement that was formulated to contain similar concentrations of these trace minerals from inorganic sources. All cows were submitted to a 7 d CO-Synch + CIDR protocol on day −10 and bred using FTAI on day 0. Pregnancy diagnosis was performed on day 28 and nonpregnant cows were removed. All pregnant cows were subjected to ovum pick-up (OPU) on day 52 and 67 of gestation. Cumulus-oocyte complexes (COCs) were evaluated and graded prior to in vitro fertilization (IVF). Analysis of variance was conducted to determine effects of treatment on response variables, and pen was considered the experimental unit. Supplement consumption did not differ (P = 0.48) between treatments (1.16 ± 0.12 vs. 1.07 ± 0.15 kg of DM × week−1 × cow–calf pair−1 for TRT and CNT, respectively). Total COC recovery was greater (P = 0.03) from TRT when compared with CNT cows (22.4 ± 2.0 vs. 16.4 ± 1.4 COCs × pen−1, respectively) and the number of COCs meeting maturation criteria was increased in TRT cows (P = 0.05) when compared with CNT cows (15.9 ± 1.6 vs. 11.8 ± 1.0 COCs × pen−1, respectively). Production of transferable embryos tended to be greater (P = 0.06) for TRT than CNT cows (4.7 ± 0.6 vs. 2.7 ± 0.7 embryos × pen−1, respectively). Furthermore, when expressed as a ratio, the number of recovered COCs meeting maturation criteria that were required to produce a transferable embryo tended to be lower for TRT than CNT cows (3.10 ± 0.93 vs. 7.02 ± 1.60; P = 0.06). In summary, complete replacement with complexed trace mineral improved COC recovery and in vitro embryo production when compared with inorganic forms of these trace minerals in beef cows.
Keywords: beef cattle, complexed, embryo transfer, in vitro fertilization, reproduction, trace mineral
INTRODUCTION
Global adoption of reproductive technologies in beef herds has improved production efficiency. Production and transfer of in vitro-fertilized (IVF) embryos has more than tripled over the last 15 yr (Perry, 2016). While in vivo-derived embryo collection still slightly outpaces IVF embryo production in North America, South America transferred 6 times as many IVF embryos as it did in vivo-derived embryos in 2016. Research must now explore methods to maximize the number of viable embryos that can be produced by a single donor. While advancements in collection procedures and hormonal manipulations have been made, there has been limited focus on nutritional management of donors to increase IVF embryo production.
Trace minerals play an essential role in proper physiological function. More specifically, copper (Cu), iodine (I), manganese (Mn), selenium (Se), and Zinc (Zn) all participate in reproductive processes and are crucial for early embryonic development (Hostetler et al., 2003). Supplementation with Zn, Mn, and Cu improves cumulus-oocyte complex (COC) maturation, reduces apoptosis of cumulus cells, and increases the success of early embryonic development through the blastocyst stage in multiple species including the mouse, cow, pig, and human, when incorporated into in vitro embryo production systems (Gao et al., 2007; Anchordoquy et al., 2011, 2014a, 2014b; Ménézo et al., 2011; Picco et al., 2012; Jeon et al., 2014; Geravandi et al., 2017). Trace minerals have been shown to play a role in the formation of metalloenzymes, which are important in several metabolic processes, including lipid metabolism, glucose utilization, DNA synthesis and transport, and free radical metabolism (Cunnane et al., 1993; De Haan et al., 1994; Townsend et al., 1994; Jovanovic-Peterson and Peterson, 1996; Hostetler et al., 2003). Additionally, Zn and Se have been associated with improved DNA integrity of cumulus cells and spermatozoa (Picco et al., 2010; Shi et al., 2010).
Previous studies have reported increases in liver and/or serum trace mineral concentrations when animals were fed organic rather than inorganic forms of trace minerals, due to potential differences in absorption or utilization (Kincaid et al., 1986; Nocek et al., 2006; Marques et al., 2016). Organic forms are comprised of a metal ion bound with a carbon-based molecule, potentially providing an alternative route of absorption and/or limiting their interaction with other nutrients in the digestive tract. Conversely, inorganic forms of trace minerals are subject to interactions that limit bioavailability; more commonly referred to as antagonisms (Spears, 1996). The effects of trace mineral forms on bioavailability have been inconsistent in the literature, with other authors reporting conflicting results (Mahan et al., 1999; Olson et al., 1999; Wolter et al., 1999). Despite varying bioavailability responses, multiple studies have reported improved reproductive outcomes in dairy cows supplemented with organic forms of trace minerals, ranging from increased estrus expression to decreased percentage of cows open at 150 d in milk (Campbell et al., 1999; Uchida et al., 2001; Ballantine et al., 2002; Nocek et al., 2006; Griffiths et al., 2007; Rabiee et al., 2010). Nonetheless, previous authors have reported mixed results in beef cattle (Olson et al., 1999; Muehlenbein et al., 2001; Ahola et al., 2004; Lamb et al., 2008; Hackbart et al., 2010). The majority of these reports, however, have evaluated partial rather than complete replacement of trace minerals from inorganic sources with various organic forms, which is considered to be the current industry standard. Therefore, the objective of this study was to determine the effect of supplemental trace mineral form (100% inorganic vs. 100% complexed) on COC recovery and IVF embryo production in suckled beef cows. We hypothesized that complete replacement of supplemental inorganic sources of trace minerals with a complexed source of trace minerals would increase COC recovery and in vitro embryo yield from suckled beef cows.
MATERIALS AND METHODS
All procedures reported herein were approved by the University of Tennessee Institutional Animal Care and Use Committee and adhered to the criteria outlined by the ADSA-ASAS-PSA Guide for Care and Use of Agricultural Animals in Research and Teaching.
Cattle, Management, and Experimental Design
This study was conducted at the East Tennessee AgResearch and Education Center (ETREC) Blount Farm located in Alcoa, TN. Cattle utilized in this experiment were maintained on mixed pastures that contained endophyte-infected Festuca arundinacea (tall fescue), Trifolium repens (white clover), and Trifolium pratense (red clover) forages, and provided with ad libitum access to water and shade. Prior to the initiation of the experiment (day −120 through −30), all cows were provided ad libitum access to a common complete mineral supplement that contained only inorganic forms of trace minerals for a 90-d washout period. Thirty days prior to fixed-time artificial insemination (FTAI; day −30), 68 postpartum Angus cows were stratified by BW, BCS, ultrasound-estimated rump fat, ultrasound-estimated rib fat, days postpartum, and age before being equally and randomly assigned to either a treatment (TRT) or a control (CNT) group in 5 different pens. Each group received a weekly complete mineral supplement allotment in order to provide 1.16 kg of DM × week−1 × cow–calf pair−1 for 14 wk. Mineral supplement consumption was measured weekly and calculated by difference between offerings and refusals, upon which time all remaining mineral supplement was removed and replaced with the next week’s allotment. Weekly samples of mineral supplement offerings and refusals were collected, frozen, and stored at −20 °C until further analysis. Cow–calf pairs assigned to the TRT group received a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained amino acid complexes of Zn, Cu, and Mn, along with cobalt glucoheptonate (AvailaPlus; Zinpro Corp., Eden Prairie, MN 55344; Table 1), while pairs assigned to the CNT group received a similar custom-formulated and contractually-manufactured mineral supplement formulated to contain the same concentrations of these trace minerals, but from inorganic sources (Table 1).
Table 1.
Formulated and analyzed composition1 of complete free-choice mineral supplements provided to cow–calf pairs from day −30 to day 67
| TRT2 | CNT3 | ||||
|---|---|---|---|---|---|
| Nutrient | Formulated | Analyzed | Formulated | Analyzed | Units, of DM |
| Ca | 15.25 | 16.65 | 15.25 | 16.65 | % |
| Na | 6.14 | 9.4 | 6.18 | 9.8 | % |
| P | 6.00 | 4.95 | 6.02 | 4.95 | % |
| Mg | 1.51 | 1.65 | 1.49 | 1.7 | % |
| S | 0.23 | 0.92 | 0.85 | 1.02 | % |
| K | 0.05 | 0.1 | 0.09 | 0.2 | % |
| Zn | 7,528 | 7,361 | 7,524 | 6,800 | mg/kg |
| Mn | 2,500 | 2,746.5 | 2,503 | 3,306.5 | mg/kg |
| Cu | 1,250 | 1,357 | 1,247 | 1,387 | mg/kg |
| Fe | 1,018 | 3,790.5 | 1,039 | 3,872.5 | mg/kg |
| Co | 125 | 163.5 | 127 | 135.5 | mg/kg |
| Se | 30 | 38.0 | 30 | 43.0 | mg/kg |
| Vitamin A | 442 | -- | 442 | -- | kIU/kg |
| Vitamin D | 44.3 | -- | 44.3 | -- | kIU/kg |
| Vitamin E | 2204 | -- | 2204 | -- | IU/kg |
Mineral composition determined using Inductively Coupled Plasma – Mass Spectrometry (ICP – MS).
TRT = treatment; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained amino acid complexes of Zn, Cu, and Mn, as well as Co glucoheptonate.
CNT = control; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained a similar level of Zn, Cu, Mn, and Co from inorganic sources.
All cows were submitted to a 7-d CO-Synch + CIDR protocol on day −10 and FTAI on day 0 to a single sire by 2 technicians. Pregnancy diagnosis was performed via transrectal ultrasound on day 28, and pregnancy was determined by the presence of an embryonic/fetal heartbeat. Cows diagnosed as nonpregnant were removed from the experiment and not included in the ovum pick-up (OPU) or IVF protocol. Immediately following pregnancy diagnosis, all nonpregnant cows were removed leaving 10 pens of 3 or 4 cow–calf pairs per pen [5 TRT pens (n = 5; 20 total cows) and 5 CNT pens (n = 5; 18 total cows)]. Cow–calf pairs remained on their original TRT or CNT designation within the same pen. All pregnant cows were subjected to OPU and COCs were processed for in vitro embryo production twice; once on day 52 and again on day 67.
Liver Biopsies
Liver biopsies were collected from all cows on day −30 and 67 by a single technician using procedures previously described by Chapman et al. (1963). In brief, the site of incision was shaved, and 10 mL of 2% lidocaine was administered subcutaneously between the 11th and 12th ribs on the animal’s right side prior to biopsy collection to provide local anesthesia. Once anesthetized, the biopsy site was disinfected, and a stab incision was made using a scalpel. A biopsy needle (Tru-Cut biopsy needle; Care Fusion Corporation, San Diego, CA) was placed in the incision and progressed cranially and ventrally toward the opposite side until the needle had advanced into the liver. Three biopsy samples per time point were collected per cow, immediately placed on ice, and stored at −20 °C until further analysis.
OPU Procedure, and In Vitro Embryo Production
Ovum pick-up was performed on all cows by a single operator that was blinded to the treatments on day −30, and on all pregnant cows on day 52 and 67, as previously described by Seneda et al. (2001). In brief, 5 mL of 2% lidocaine was administered via epidural prior to OPU. An ultrasound-guided OPU device (Aloka 500V, Hitachi Aloka Medical America, Wallingford, CT 06492), equipped with a 5-MHz curved probe coupling and an 18-gauge needle was used. The intravaginal OPU device was connected to a 50-mL conical tube, loaded with 20 mL of collection medium (Tissue Culture Medium 199 with Hank’s Salts supplemented with 15 mM HEPES, 4.2 mM NaHCO3, 2% fetal bovine serum, 2 mM L-glutamine, 50 U/mL penicillin, 50 µg/mL streptomycin, and 10,000 U/L heparin), and connected to a vacuum pump set at 90 to 100 mmHg. All follicles greater than 5 mm were aspirated to collect COCs. Media tubes containing COCs were then transported in incubators and held at 38.5 °C until further processing.
Cumulus-oocyte complexes were washed extensively to remove blood cells and debris before being evaluated and graded (A to D) by a single technician blinded to the treatments using the criteria previously described by De Loos et al. (1989). In brief, category A COCs had an oocyte with homogenous, evenly granulated cytoplasm and numerous layers of compact, nonexpanded cumulus cells. Category B COCs were similar to A, but with fewer layers of cumulus cells. Category C COCs had ooplasmic irregularities, very few cumulus cells, and/or expanding cumulus cells. Category D COCs were atretic with expanded and dark cumulus vestment, denuded, lysed, and/or otherwise morphologically abnormal. All COCs graded A through C were pooled within pen and underwent in vitro maturation, fertilization, and embryo culture as previously described by Rispoli et al. (2011). All grade D COCs were discarded.
After initial processing, oocytes were placed in maturation media containing M-199 with Earle’s salts, 10% (v/v) FBS, 50 μg/mL gentamicin, 5 μg/mL Folltropin-V, 0.2 mM sodium pyruvate and 2 mM L-glutamine for 24 h at 38.5 °C. Fertilization was performed using a pool of semen from 2 Angus sires, and all oocytes from cows within the same pen were fertilized together as a single group. After fertilization, zygotes were cultured at 38.5 °C for 8 d in 500 μL of mKSOM with 5.5% CO2, 7.0% O2, and 87.5% N. Presumptive zygotes were denuded 15 ± 2 h following the addition of semen. Cleavage rate was assessed 3 d following fertilization, and the number of 1-, 2-, 4-, and 8- to 16-cell embryos was recorded. Eight days after performing IVF, the quality of embryos progressing to the blastocyst stage of development was assessed (1 to 4) using the procedures outlined in the IETS guidelines (Robertson and Nelson, 1999).
Mineral Analyses
Weekly samples of mineral supplement offerings and refusals were dried overnight at 60 °C in a forced-air oven before being analyzed to determine DM content, which was then used to determine mineral supplement intake (DM-basis). Elemental composition of mineral supplement samples and liver biopsies were analyzed using Inductively Coupled Plasma – Mass Spectrometry (ICP – MS) by an independent contractual analysis laboratory (Michigan State University Veterinary Diagnostic Laboratory, Lansing, MI 48910) using the methods previously described by Wahlen et al. (2005). Prior to analysis, biopsy samples were sectioned. When a sufficient quantity of sample was available, a section of each sample was dried for 12 h at 75 °C to determine the DM fraction and calculate the dried sample mass, while a separate section was digested for 12 h at 95 °C in trace element grade nitric acid at a ratio of approximately 1 part dry tissue mass to 10 parts nitric acid. If there was not enough sample to determine the DM fraction separately, the section of sample used for the mineral analysis was dried prior to nitric acid digestion. Digested samples were then diluted with deionized water to a 100:1 ratio of water to dried tissue mass. Only biopsy samples from cows that were confirmed pregnant at day 28 of gestation underwent liver mineral analysis. A calibration standard was added to a fraction of each digested and diluted tissue sample, and the solution was diluted 20:1 with 0.5% EDTA and Triton X-100, 1% ammonium hydroxide, and 2% propanol, and 5 ppb of scandium and 7.5 ppb of germanium, rhodium, indium, and bismuth were used as internal standards. The ICP – MS (Agilent 7900; Agilent Technologies Inc., Santa Clara, CA 95051) was tuned to yield a minimum of 7,500 cps sensitivity for 1 ppb yttrium (mass 89), less than 1.0% oxide level as determined by the 156/140 mass ratio, and less than 2.0% double charged ions as determined by the 70/140 mass ratio. Elemental concentrations were calibrated using a 5-point linear curve in an analyte-internal standard response ratio. Commercial standards were used (Inorganic Ventures, Christiansburg, VA 24073) and National Institute of Standards and Technology (NIST, Gaithersburg, MD 20899) bovine liver and muscle standards were used as controls.
Statistical Analyses
One-way ANOVA was conducted using the GLIMMIX procedure of SAS (SAS v.9.4, SAS Inst. Inc., Cary, NC) to quantify differences among cow- and calf-related quantitative and binary data. Main effects of treatment on COC recovery, cleavage rate, and transferable embryo production were determined using pen as the experimental unit and corrected for n/pen. Main effects of treatment on calf-related data were determined using pen as the experimental unit, and the model included sex and the interaction between treatment and sex as blocking factors. Results are reported as least square means ± SE of the mean after being adjusted to calf gender and separated using the PDIFF function. Liver mineral concentrations on day −30 were used as a covariate for determining the effect of treatment on liver mineral concentrations at day 67. In addition, the relationship between liver trace mineral concentrations and the number of COCs recovered per pen that met maturation criteria, independent of treatment, was determined using the PROC GENMOD procedure, corrected for over-dispersion. The PROC GENMOD procedure was also used to conduct an odds ratio analysis with a model that included a fixed effect of treatment, where COC recovery and transferable embryo production were considered the response variables. Significance was set at P ≤ 0.05, and tendencies were determined when 0.05 < P ≤ 0.10. Unless otherwise stated, means are reported LSMeans ± SEM per cow within pen.
RESULTS
Cow and Calf Performance
At the beginning of the experiment (day −30), no differences in BW (P = 0.75), BCS (P = 0.94), ultrasound-estimated rib fat (P = 0.71) or rump fat (P = 0.34), and age (P = 0.54) or days postpartum (P = 0.60) were observed between TRT and CNT cows (Table 2). Pregnancy rate was 64.71 and 52.90 % between TRT and CNT cows at day 28. Measurements of cow and calf performance (Table 3) reflect data collected from the cow–calf pairs in which the cow was diagnosed as pregnant on day 28 and the pair remained on the study for its entire duration. Throughout the experiment, cow and calf performance did not differ (P ≥ 0.12) as a result of trace mineral form for any response variables.
Table 2.
Body composition, reproductive parameters, and age of cows at study initiation (day −30) and pregnancy rate at day 28 of gestation
| Item | TRT1 | CNT2 | SEM | P-value |
|---|---|---|---|---|
| N | 34 | 34 | -- | -- |
| BW, kg | 622.18 | 628.91 | 16.29 | 0.75 |
| BCS | 5.85 | 5.83 | 0.16 | 0.94 |
| Rib fat3, cm | 0.98 | 0.93 | 0.05 | 0.71 |
| Rump fat3, cm | 1.49 | 1.32 | 0.13 | 0.34 |
| Day postpartum | 93.97 | 94.73 | 1.02 | 0.60 |
| Age, years | 4.47 | 4.88 | 0.47 | 0.54 |
| Pregnancy rate, % | 64.71 (22/34) | 52.9 (18/34) | -- | -- |
TRT = treatment; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained amino acid complexes of Zn, Cu, and Mn, as well as Co glucoheptonate beginning on day −30.
CNT = control; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained a similar level of Zn, Cu, Mn, and Co from inorganic sources beginning on day −30.
Rib fat and rump fat were estimated via transdermal ultrasonography.
Table 3.
Body weight (BW) growth performance and body composition of pregnant cow–calf pairs submitted to ovum pick-up
| Parameter1 | TRT2 | CNT3 | P-value | SEM |
|---|---|---|---|---|
| Cow | ||||
| n | 20 | 18 | -- | -- |
| Initial BW, kg | 613.64 | 638.91 | 0.40 | 21.02 |
| Final BW, kg | 628.48 | 643.50 | 0.54 | 16.98 |
| Total BW gain, kg | 14.83 | 4.90 | 0.46 | 7.95 |
| Initial BCS | 5.75 | 5.80 | 0.83 | 0.19 |
| Final BCS | 6.00 | 6.12 | 0.71 | 0.28 |
| Initial rib fat4, cm | 1.00 | 0.95 | 0.61 | 0.07 |
| Final rib fat4, cm | 0.72 | 0.75 | 0.67 | 0.05 |
| Initial rump fat4, cm | 1.50 | 1.52 | 0.94 | 0.19 |
| Final rump fat4, cm | 1.55 | 1.64 | 0.68 | 0.16 |
| Calf | ||||
| Overall | ||||
| n | 20 | 18 | -- | -- |
| Initial BW, kg | 99.22 | 100.50 | 0.80 | 3.93 |
| Final BW, kg | 217.16 | 217.03 | 0.99 | 7.23 |
| Total BW gain, kg | 117.93 | 117.11 | 0.91 | 4.05 |
| Bull | ||||
| n | 12 | 10 | -- | -- |
| Initial BW, kg | 97.26 | 107.18 | 0.12 | 5.05 |
| Final BW, kg | 216.42 | 231.92 | 0.15 | 9.42 |
| Total BW gain, kg | 121.38 | 126.22 | 0.54 | 5.53 |
| Heifer | ||||
| n | 8 | 8 | -- | -- |
| Initial BW, kg | 97.26 | 92.14 | 0.17 | 5.34 |
| Final BW, kg | 218.26 | 198.54 | 0.13 | 7.09 |
| Total BW gain, kg | 112.75 | 105.71 | 0.41 | 3.57 |
Initial measurements were collected on day −30 and final measurements were collected on day 67.
TRT = treatment; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained amino acid complexes of Zn, Cu, and Mn, as well as Co glucoheptonate.
CNT = control; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained a similar level of Zn, Cu, Mn, and Co from inorganic sources.
Measurements were collected via transdermal ultrasonography.
Supplement Mineral Composition, Intake, and Liver Mineral Composition
Formulated and analyzed nutrient composition of the complete free-choice mineral supplements offered between day −30 and 67 are described in Table 1. Dry matter and moisture content of TRT and CNT mineral supplements averaged 98.0 and 2.0%, respectively. Mineral supplement consumption did not differ (P = 0.48) between TRT and CNT cow–calf pairs (1.12 ± 0.07 vs. 1.06 ± 0.07 kg of DM × week−1 × cow–calf pair−1).
Liver mineral concentrations are reported in Table 4. Baseline liver mineral concentrations at day −30 did not differ between TRT and CNT cows for Cu (P = 0.30), Zn (P = 0.49), Mn (P = 0.37), and Co (P = 0.99). At day 67, liver concentrations of Co were higher in TRT than CNT cows (0.45 ± 0.02 vs. 0.31 ± 0.01 µg × g dry weight−1 for TRT and CNT, respectively; P < 0.0001), but no differences were observed for Cu (P = 0.43), Zn (P = 0.15) or Mn (P = 0.35).
Table 4.
Liver mineral concentrations1 on day 67 for cows submitted to ovum pick-up
| TRT2 | CNT3 | P-value | SEM | |
|---|---|---|---|---|
| Cu | 179.66 | 185.53 | 0.43 | 17.97 |
| Zn | 103.13 | 93.66 | 0.15 | 9.04 |
| Mn | 10.26 | 8.85 | 0.35 | 1.37 |
| Co | 0.45a | 0.31b | <0.0001 | 0.02 |
Within a row, means without a common superscript differ significantly (P < 0.05).
Within a row, means without a common superscript differ significantly (P < 0.05).
Liver concentrations are reported in µg × g−1 of dry weight.
TRT = treatment; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained amino acid complexes of Zn, Cu, and Mn, as well as Co glucoheptonate.
CNT = control; cow–calf pairs were provided with ad libitum access to a weekly 1.16 kg of DM × cow–calf pair−1-allotment of a custom-formulated and contractually-manufactured complete free-choice mineral supplement that contained a similar level of Zn, Cu, Mn, and Co from inorganic sources.
COC Recovery
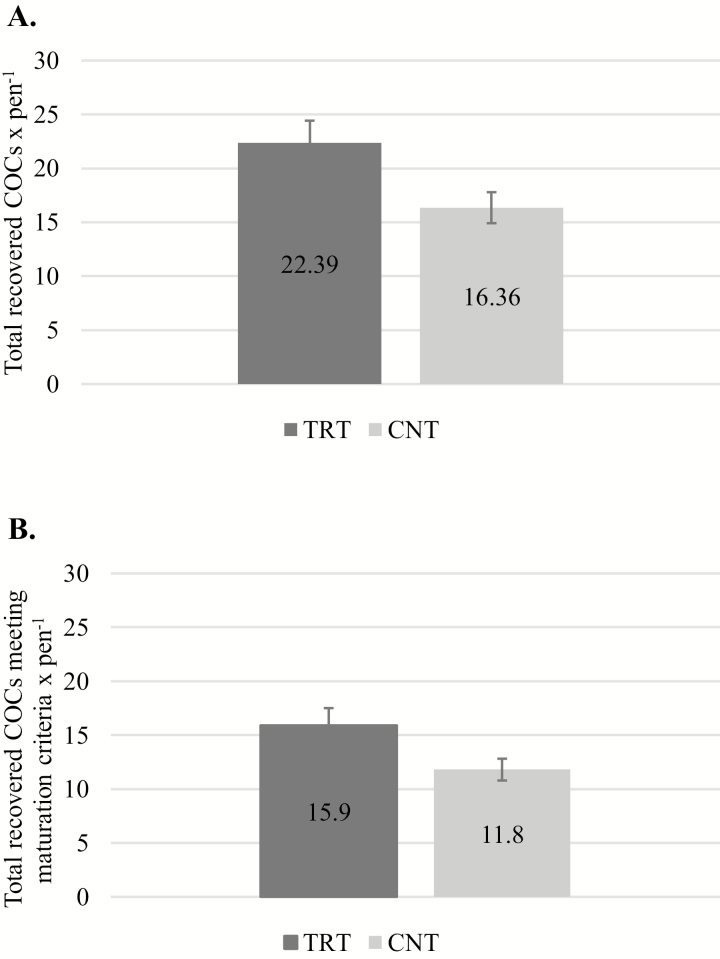
No differences were observed between TRT and CNT cows at day −30 for total COCs recovered (3.90 ± 0.94 vs. 4.06 ± 0.87 COCs × cow−1, respectively; P = 0.90) or COCs meeting maturation criteria (2.40 ± 0.45 vs. 3.17 ± 0.75 COCs classified A through C × cow−1, respectively; P = 0.41). At days 52 and 67, a greater number of total COCs were recovered (Fig. 1A) from TRT than CNT cows (22.39 ± 2.04 vs. 16.36 ± 1.44 COCs classified A through D × pen−1, respectively; P = 0.03). Moreover, the number of COCs meeting maturation criteria (classified A through C; Fig. 1B) was greater from cows receiving complexed trace minerals than cows receiving inorganic trace minerals (15.94 ± 1.60 vs. 11.80 ± 1.01 COCs × pen−1 for TRT vs. CNT, respectively; P = 0.05). Additionally, odds ratio analysis demonstrated a 53% greater likelihood of recovering a COC (P = 0.003) and 43% greater likelihood of recovering a COC that meets maturation criteria (P = 0.03) from TRT than CNT cows.
Figure 1.
(A) Total recovered COCs. Pens supplemented with a complexed form of trace minerals had a greater total number of recovered COCs than pens supplemented with a similar level of trace minerals from inorganic sources (P = 0.03). (B) Total recovered COCs meeting maturation criteria. Pens supplemented with a complexed form of trace minerals had a greater total number of recovered COCs that met maturation criteria (P = 0.05).
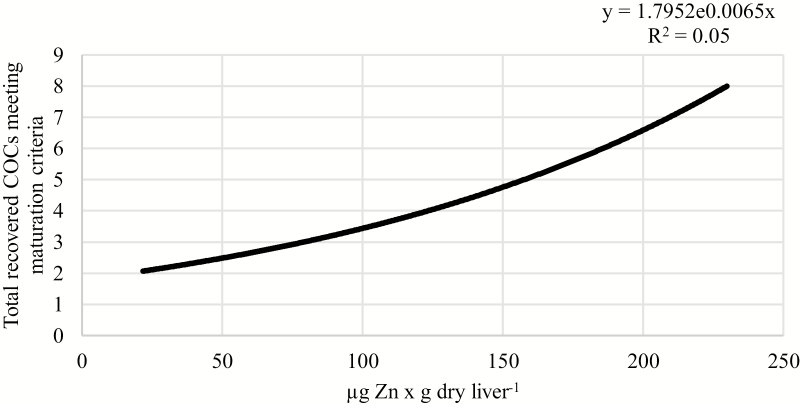
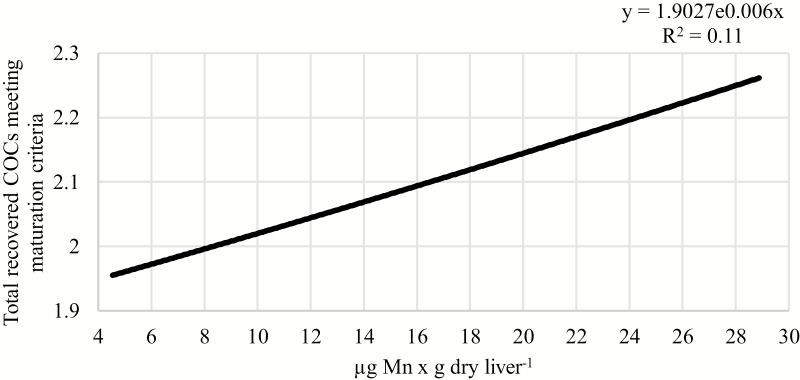
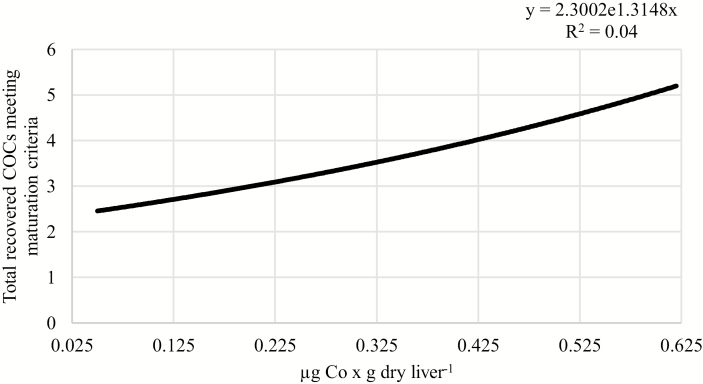
Positive relationships were observed between liver concentrations of Zn or Mn and the number of recovered COCs that met maturation criteria. As liver concentration of Zn increased from 20 to 230 µg × g dry weight−1, the number of COCs meeting maturation criteria increased from 2.07 to 8.00 per cow (P = 0.04; R2 = 0.05; Fig. 2). Similarly, as liver concentration of Mn increased from 4.5 to 29 µg × g dry weight−1, the number of recovered COCs meeting maturation criteria increased from 1.96 to 2.26 per cow (P = 0.003; R2 = 0.11; Fig. 3). Furthermore, Co concentration tended to be positively associated with the number of recovered COCs meeting maturation criteria. As liver concentration of Co increased from 0.05 to 0.62 µg × g dry weight−1, the number of recovered oocytes meeting maturation criteria tended to increase from 2.46 to 5.20 per cow (P = 0.07; R2 = 0.04; Fig. 4).
Figure 2.
Relationship between liver Zn concentration and the total number of recovered COCs meeting maturation criteria, independent of supplemental trace mineral source. As liver concentration of Zn increased, the total number of recovered COCs that met maturation criteria increased (P = 0.04; R2 = 0.05).
Figure 3.
Relationship between liver Mn concentration and the total number of recovered COCs meeting maturation criteria, independent of supplemental trace mineral source. As liver concentration of Mn increased, the total number of recovered COCs that met maturation criteria increased (P = 0.003; R2 = 0.11).
Figure 4.
Relationship between liver Co concentration and the total number of recovered COCs meeting maturation criteria, independent of supplemental trace mineral source. As liver concentration of Co increased, the total number of recovered COCs that met maturation criteria tended to increase (P = 0.07; R2 = 0.04).
In Vitro Embryo Production
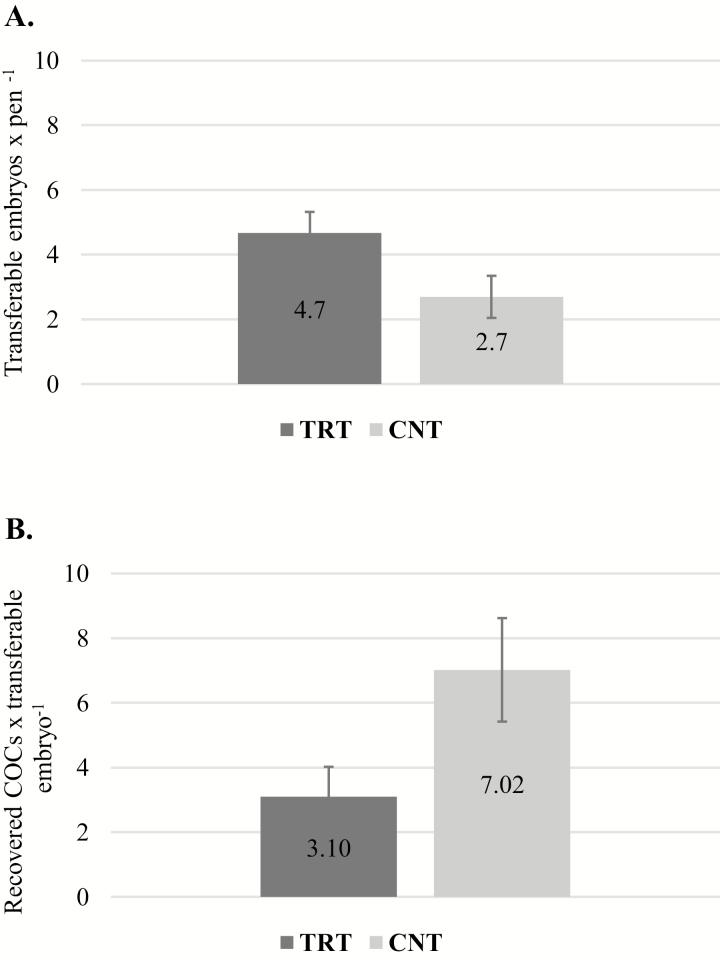
Cleavage rate (79.74 ± 3.63% vs. 79.95 ± 5.36% for TRT and CNT, respectively; P = 0.97) and the percentage of embryos that progressed to the 8- to 16-cell stage (56.14 ± 5.48% vs. 64.94 ± 6.10% for TRT and CNT, respectively; P = 0.44) were unaffected by trace mineral form. Percentage of 8 to 16 cell embryos that progressed to the blastocyst stage, however, tended to increase when embryos were derived from TRT when compared to CNT COCs (66.57 ± 11.50% vs. 40.83 ± 5.54% for TRT and CNT, respectively; P = 0.08). Additionally, transferable embryo yield (embryos with quality grades 1 to 3) tended to be greater for pens that received complexed trace minerals than their contemporaries that received a similar level of inorganic trace minerals (4.70 ± 0.64 vs. 2.70 ± 0.65 embryos × pen−1 for TRT and CNT, respectively; P = 0.06; Fig. 5A). Freezable embryos (embryos with quality grades 1 and 2) also tended to increase from pens receiving complexed trace minerals compared with those receiving an inorganic form of trace minerals (3.37 ± 0.46 vs. 1.75 ± 0.46 embryos × pen−1 for TRT and CNT, respectively; P = 0.06). Furthermore, when expressed as a ratio, the number of recovered COCs meeting maturation criteria that were required to produce a transferable embryo tended to be lower for TRT than CNT cows (3.10 ± 0.93 vs. 7.02 ± 1.60; P = 0.06; Fig. 5B), which is a measure of efficiency in an in vitro embryo production system. Moreover, odds ratio analysis demonstrated that COCs from TRT cows were 75% (P = 0.02) more likely to produce a transferable embryo.
Figure 5.
(A) In-vitro-fertilized embryo production of suckled cows supplemented with a complexed form of trace minerals (TRT) or a similar level of trace minerals from inorganic sources (CNT). Production of embryos tended to be greater for TRT than CNT pens (P = 0.06). (B) Ratio of recovered COCs to transferable embryo produced tended to be lower (signifying an increasing in efficiency) for TRT than CNT pens (P = 0.06).
DISCUSSION
Trace mineral deficiencies affect several physiological processes which compromise immune function, growth and development, and reproductive performance. Common reproductive symptoms of a trace mineral deficiency include increased number of services per conception, prolonged anestrus interval, decreased ovulation rates, decreased pregnancy rates, and increased embryonic mortality and pregnancy loss (Hostetler et al., 2003; Ahola et al., 2004; Mohebbi-Fani et al., 2010). When compared to inorganic sources, complexed trace minerals have been reported to increase the bioavailability of mineral elements in livestock (Underwood, 1977; Kincaid et al., 1986; Rojas et al., 1995; Olson et al., 1999; Nocek et al., 2006; Marques et al., 2016). However, the impact of mineral source on reproductive performance has been variable between studies (Muehlenbein et al., 2001; Ahola et al., 2004; Lamb et al., 2008). In the current study, there were clear increases in reproductive performance, as indicated by increased COC recovery, freezable and transferable embryo yield, as well as a numerical increase in pregnancy rate despite limited differences in liver mineral concentrations in cows supplemented with complexed trace minerals. Additionally, cows supplemented with complexed trace minerals were 43% more likely to have a COC meeting maturation criteria and were 75% more likely to produce a transferable embryo when compared with the cows supplemented with an inorganic source of trace minerals. Similarly, Griffiths et al. (2007) reported differences in reproductive performance of grazing dairy cows supplemented with inorganic trace mineral forms or the same complexed trace minerals used in the current study; differences included earlier estrus expression postpartum and increased pregnancy rate 48 d following the initiation of the breeding season, despite no differences in liver Zn or Mn concentrations (Griffiths et al., 2007). Using a chelated source of organic trace minerals, Lamb et al. (2008) found no difference in number of total or transferable embryos recovered from superovulated heifers fed with either organic trace mineral, inorganic trace minerals (positive control), or heifers not receiving trace minerals (negative control; Lamb et al., 2008). However, the heifers that received organic trace minerals yielded fewer unfertilized oocytes when compared to positive or negative controls. Additionally, Hackbart et al. (2010) saw no improvement in embryo quality from Holstein donors undergoing in vivo embryo collection and supplemented with the same organic trace mineral complexes that were fed in the current study (Hackbart et al., 2010). One of the uniqe aspects of the current experiment is that 100% of the added trace mineral content of the TRT mineral supplement was from a complexed source, rather than a portion from an organic source, and the remainder from inorganic sources, and likely explains the differences in results between this and previous experiments. Ahola et al. (2004) saw no difference in reproductive performance in grazing beef cows, but utilized organic trace minerals in proteinate form (Ahola et al., 2004), which differs from the complexed trace mineral forms (amino acid complexes and cobalt glucoheptonate) used in the present study and animals were fed in rather large replicates potentially increasing variation in mineral intake. Similarly, the former experiment replaced 50% of trace minerals from inorganic sources with an inorganic form, which is a common industry practice. The role of trace mineral source on oocyte quality, embryo development, and reproductive performance is complex and incompletely defined. In the present study, supplementation with 100% of trace minerals from a complexed source clearly improved metrics of reproductive performance. Although most data exists for spermatozoa, positive results have been observed in germ cell development from animals and humans supplemented with trace minerals, including increased antioxidant enzyme activity and decreased production of reactive oxygen species (Özkaya and Nazıroğlu, 2010; Shi et al., 2010; Narasimhaiah et al., 2018). Trace mineral availability may contribute to increased internal protection by the developing embryo from ROS produced via its own metabolism, through this increased enzymatic activity (Guerin et al., 2001).
Although liver concentrations are often used as the standard for assessing mineral status in cattle, previous reports suggest that a large portion of some trace minerals may be stored more significantly in other body tissues, such as Zn and Mn in the kidneys and bone (Underwood, 1977; Rojas et al., 1995). In the present study, with exception to Co, differences in liver mineral concentrations in animals fed a complexed form of trace minerals were not detected when compared with animals fed a similar level of trace minerals from an inorganic source. These results are similar to those reported by Marques et al. (2016). The authors reported limited differences in liver mineral concentrations between cows supplemented with organic or inorganic sources of trace minerals during late gestation, however reported robust differences in offspring performance and physiological response. Interestingly, Olson et al. supplemented cows above required nutrient values with complexed or inorganic trace minerals and observed no differences in liver mineral concentration; however, reported differences in reproductive performance (Olson et al., 1999). Thus, the lack of differences in liver mineral content between TRT and CON groups in the present study is consistent with previous reports in the field. Interestingly in the current study, when analyzed using poisson or logistic regression, liver concentrations of Zn, Mn and Co, regardless of supplemental trace mineral form, were associated with increases in number of oocytes meeting maturation criteria, suggesting that liver mineral status is associated with increased reproductive parameters.
The mechanism by which complexed trace minerals led to increases in COC recovery and increased IVF efficiency remain unknown. Overall, the data is limited for direct mechanisms explaining these increases. In the current study, Co was the only mineral that was elevated in the liver of cows supplemented with complexed trace minerals. Additionally, the number of recovered COCs meeting maturation criteria more than doubled when Co concentration increased from 0.05 to 0.62 µg × g dry weight−1. In ruminants, vitamin B12 synthesis by rumen microorganisms requires Co as a necessary substrate. Previous work has shown that supplementation of cows with Co results in higher liver concentrations of vitamin B12 (Judson et al., 1997; Stangl et al., 2007), and higher pregnancy rates when compared with nonsupplemented cows (Judson et al., 1997). One possible explanation for the observed increase in reproductive performance in the present study is that increased levels of dietary Co improve nutritional status, which potentially leads to a more favorable environment for follicular and COC development (Leroy et al., 2008a, 2008b). There are reports that supplementation of drinking water with Co glucoheptonate and amino acid complexes of Zn, Mn, and Cu increase Cu and vitamin B12 stores, lactation performance, and fertility of grazing lactating dairy cows (Griffiths et al., 2007). Nonetheless, few reports exist regarding local mechanisms or effects on follicular development, COC recovery, or embryo quality. Cobalt supplementation, however, has been shown to decrease morphological abnormalities of embryos and trophectoderm cell number in the ewe (Mitchell et al., 2007), while feeding Co-deficient diets increased trophectoderm number (Kakar et al. (2005). Additionally, concentrations of Co in the follicular fluid of ovulatory-sized follicles are significantly greater than in follicles less than 5 mm in size in lactating dairy cattle (Kor et al., 2013).
Although the differences in liver Zn concentrations were not statistically significant between TRT and CNT cows in the present study, the liver Zn concentration of cows supplemented with a complexed form of trace minerals was numerically greater. Similarly, a positive relationship between liver Zn concentration and number of COCs recovered was observed and is consistent with previous findings (Tian and Diaz, 2013). In the Zn-deficient mourse, Tian and Diaz (2013) observed epigenetic defects in oocytes that resulted in decreased in vitro fertilization efficiency, but no effects when fertilization was performed in vivo. Rat dams fed Zn-deficient diets also produced abnormal or incompetent embryos (Hurley and Shrader, 1975). Additionally, the in vitro Zn supplementation of embryos collected from Zn-deficient dams did not reverse the negative effects (Record et al., 1985). Thus, the authors concluded that Zn was critical for normal in vivo oocyte maturation and embryo development. Additionally, Spears (1996) also reported an increase in reproductive performance during the first 21 d of the breeding season when beef cows were fed amino acid-complexed forms of Zn and Mn.
CONCLUSIONS
Although differences in liver trace mineral concentrations were not observed, with exception of Co, the results of this experiment support the hypothesis that complete replacement of supplemental trace minerals from inorganic sources with a complexed form of trace minerals increases IVF embryo production efficiency, and that trace mineral status is associated with IVF embryo production success in suckled beef cows. Furthermore, when compared to previous findings, the results of this experiment suggest that complete replacement of inorganic with a complexed source of trace minerals may be necessary in order to achieve reproductive benefit. Further research is necessary to elucidate the specific mechanisms by which complexed trace minerals improve reproductive efficiency, and to determine if these effects transpose to increased success of embryo transfer, artificial insemination, and natural service matings.
Conflict of interest statement. None declared.
Footnotes
This project was supported in part by Zinpro Corporation (Eden Prairie, MN) and USDA-NIFA Hatch/Multistate Project W3112-TEN00506: Reproductive performance in domestic animals.
The authors would like to acknowledge the East Tennessee Research and Education Center for providing cattle used in this experiment, and Mr. Brandon Beavers for his assistance with their management.
LITERATURE CITED
- Ahola J. K., Baker D. S., Burns P. D., Mortimer R. G., Enns R. M., Whittier J. C., Geary T. W., and Engle T. E.. 2004. Effect of copper, zinc, and manganese supplementation and source on reproduction, mineral status, and performance in grazing beef cattle over a two-year period. J. Anim. Sci. 82:2375–2383. doi:10.2527/2004.8282375x [DOI] [PubMed] [Google Scholar]
- Anchordoquy J. P., Anchordoquy J. M., Picco S. J., Sirini M. A., Errecalde A. L., and Furnus C. C.. 2014b. Influence of manganese on apoptosis and glutathione content of cumulus cells during in vitro maturation in bovine oocytes. Cell Biol. Int. 38:246–253. doi:10.1002/cbin.10195 [DOI] [PubMed] [Google Scholar]
- Anchordoquy J. M., Anchordoquy J. P., Sirini M. A., Picco S. J., Peral-García P., and Furnus C. C.. 2014a. The importance of having zinc during in vitro maturation of cattle cumulus-oocyte complex: Role of cumulus cells. Reprod. Domest. Anim. 49:865–874. doi:10.1111/rda.12385 [DOI] [PubMed] [Google Scholar]
- Anchordoquy J. M., Picco S. J., Seoane A., Anchordoquy J. P., Ponzinibbio M. V., Mattioli G. A., Peral García P., and Furnus C. C.. 2011. Analysis of apoptosis and DNA damage in bovine cumulus cells after exposure in vitro to different zinc concentrations. Cell Biol. Int. 35:593–597. doi:10.1042/CBI20100507 [DOI] [PubMed] [Google Scholar]
- Ballantine H., Socha M., Tomlinson D. A. D., Johnson A., Fielding A., Shearer J., and Van Amstel S.. 2002. Effects of feeding complexed zinc, manganese, copper, and cobalt to late gestation and lactating dairy cows on claw integrity, reproduction, and lactation performance. The Professional Animal Scientist. 18:211–218. [Google Scholar]
- Campbell M. H., Miller J. K., and Schrick F. N.. 1999. Effect of additional cobalt, copper, manganese, and zinc on reproduction and milk yield of lactating dairy cows receiving bovine somatotropin. J. Dairy Sci. 82:1019–1025. doi:10.3168/jds.S0022-0302(99)75322-1 [DOI] [PubMed] [Google Scholar]
- Chapman Jr H., Cox D., Haines C., and Davis G.. 1963. Evaluation of the liver biopsy technique for mineral nutrition studies with beef cattle. J. Anim. Sci. 22:733–737. doi:10.2527/jas1963.223733x [Google Scholar]
- Cunnane S. C., Yang J., and Chen Z. Y.. 1993. Low zinc intake increases apparent oxidation of linoleic and alpha-linolenic acids in the pregnant rat. Can. J. Physiol. Pharmacol. 71:205–210. doi:10.1139/y93-032 [DOI] [PubMed] [Google Scholar]
- De Haan J. B., Tymms M. J., Cristiano F., and Kola I.. 1994. Expression of copper/zinc superoxide dismutase and glutathione peroxidase in organs of developing mouse embryos, fetuses, and neonates. Pediatr. Res. 35:188–196. doi:10.1203/00006450-199402000-00013 [DOI] [PubMed] [Google Scholar]
- De Loos F., van Vliet C., van Maurik P., and Kruip T. A.. 1989. Morphology of immature bovine oocytes. Gamete Res. 24:197–204. doi:10.1002/mrd.1120240207 [DOI] [PubMed] [Google Scholar]
- Gao G., Yi J., Zhang M., Xiong J., Geng L., Mu C., and Yang L.. 2007. Effects of iron and copper in culture medium on bovine oocyte maturation, preimplantation embryo development, and apoptosis of blastocysts in vitro. J. Reprod. Dev. 53:777–784. doi:10.1262/jrd.18109 [DOI] [PubMed] [Google Scholar]
- Geravandi S., Azadbakht M., Pourmoradi M., and Nowrouzi F.. 2017. Zinc supplementation of vitrification medium improves in vitro maturation and fertilization of oocytes derived from vitrified-warmed mouse ovaries. Cryobiology 74:31–35. doi:10.1016/j.cryobiol.2016.12.007 [DOI] [PubMed] [Google Scholar]
- Griffiths L., Loeffler S., Socha M., Tomlinson D., and Johnson A.. 2007. Effects of supplementing complexed zinc, manganese, copper and cobalt on lactation and reproductive performance of intensively grazed lactating dairy cattle on the South Island of New Zealand. Anim. Feed Sci. Technol. 137:69–83. doi:10.1016/j.anifeedsci.2006.10.006 [Google Scholar]
- Guerin P., El Mouatassim S., and Ménézo Y.. 2001. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 7:175–189. doi:10.1093/humupd/7.2.175 [DOI] [PubMed] [Google Scholar]
- Hackbart K. S., Ferreira R. M., Dietsche A. A., Socha M. T., Shaver R. D., Wiltbank M. C., and Fricke P. M.. 2010. Effect of dietary organic zinc, manganese, copper, and cobalt supplementation on milk production, follicular growth, embryo quality, and tissue mineral concentrations in dairy cows. J. Anim. Sci. 88:3856–3870. doi:10.2527/jas.2010-3055 [DOI] [PubMed] [Google Scholar]
- Hostetler C. E., Kincaid R. L., and Mirando M. A.. 2003. The role of essential trace elements in embryonic and fetal development in livestock. Vet. J. 166:125–139. doi:10.1016/S1090-0233(02)00310-6 [DOI] [PubMed] [Google Scholar]
- Hurley L. S., and Shrader R. E.. 1975. Abnormal development of preimplantation rat eggs after three days of maternal dietary zinc deficiency. Nature 254:427–429. [DOI] [PubMed] [Google Scholar]
- Jeon Y., Yoon J. D., Cai L., Hwang S. U., Kim E., Zheng Z., Lee E., Kim D. Y., and Hyun S. H.. 2014. Supplementation of zinc on oocyte in vitro maturation improves preimplatation embryonic development in pigs. Theriogenology 82:866–874. doi:10.1016/j.theriogenology.2014.06.021 [DOI] [PubMed] [Google Scholar]
- Jovanovic-Peterson L., and Peterson C. M.. 1996. Vitamin and mineral deficiencies which may predispose to glucose intolerance of pregnancy. J. Am. Coll. Nutr. 15:14–20. doi:10.1080/07315724.1996.10718560 [DOI] [PubMed] [Google Scholar]
- Judson G. J., McFarlane J. D., Mitsioulis A., and Zviedrans P.. 1997. Vitamin B12 responses to cobalt pellets in beef cows. Aust. Vet. J. 75:660–662. doi:10.1111/j.1751-0813.1997.tb15365.x [DOI] [PubMed] [Google Scholar]
- Kakar M. A., Maddocks S., Lorimer M. F., Kleemann D. O., Rudiger S. R., Hartwich K. M., and Walker S. K.. 2005. The effect of peri-conception nutrition on embryo quality in the superovulated ewe. Theriogenology 64:1090–1103. doi:10.1016/j.theriogenology.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Kincaid R., Blauwiekel R., and Cronrath J.. 1986. Supplementation of copper as copper sulfate or copper proteinate for growing calves fed forages containing molybdenum1. J. Dairy Sci. 69:160–163. doi:10.3168/jds.S0022-0302(86)80381-2 [Google Scholar]
- Kor N. M., Khanghah K. M., and Veisi A.. 2013. Follicular fluid concentrations of biochemical metabolites and trace minerals in relation to ovarian follicle size in dairy cows. Annu. Rev. Res. Biol. 3:397–404. [Google Scholar]
- Lamb G. C., Brown D. R., Larson J. E., Dahlen C. R., Dilorenzo N., Arthington J. D., and Dicostanzo A.. 2008. Effect of organic or inorganic trace mineral supplementation on follicular response, ovulation, and embryo production in superovulated angus heifers. Anim. Reprod. Sci. 106:221–231. doi:10.1016/j.anireprosci.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Opsomer G., Van Soom A., Goovaerts I. G., and Bols P. E.. 2008a. Reduced fertility in high-yielding dairy cows: are the oocyte and embryo in danger? Part I. The importance of negative energy balance and altered corpus luteum function to the reduction of oocyte and embryo quality in high-yielding dairy cows. Reprod. Domest. Anim. 43:612–622. doi:10.1111/j.1439-0531.2007.00960.x [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Van Soom A., Opsomer G., Goovaerts I. G., and Bols P. E.. 2008b. Reduced fertility in high-yielding dairy cows: are the oocyte and embryo in danger? Part II. Mechanisms linking nutrition and reduced oocyte and embryo quality in high-yielding dairy cows. Reprod. Domest. Anim. 43:623–632. doi:10.1111/j.1439-0531.2007.00961.x [DOI] [PubMed] [Google Scholar]
- Mahan D. C., Cline T. R., and Richert B.. 1999. Effects of dietary levels of selenium-enriched yeast and sodium selenite as selenium sources fed to growing-finishing pigs on performance, tissue selenium, serum glutathione peroxidase activity, carcass characteristics, and loin quality. J. Anim. Sci. 77:2172–2179. [DOI] [PubMed] [Google Scholar]
- Marques R. S., Cooke R. F., Rodrigues M. C., Cappellozza B. I., Mills R. R., Larson C. K., Moriel P., and Bohnert D. W.. 2016. Effects of organic or inorganic cobalt, copper, manganese, and zinc supplementation to late-gestating beef cows on productive and physiological responses of the offspring. J. Anim. Sci. 94:1215–1226. doi:10.2527/jas.2015-0036 [DOI] [PubMed] [Google Scholar]
- Ménézo Y., Pluntz L., Chouteau J., Gurgan T., Demirol A., Dalleac A., and Benkhalifa M.. 2011. Zinc concentrations in serum and follicular fluid during ovarian stimulation and expression of zn2+ transporters in human oocytes and cumulus cells. Reprod. Biomed. Online 22:647–652. doi:10.1016/j.rbmo.2011.03.015 [DOI] [PubMed] [Google Scholar]
- Mitchell L. M., Robinson J. J., Watt R. G., McEvoy T. G., Ashworth C. J., Rooke J. A., and Dwyer C. M.. 2007. Effects of cobalt/vitamin B12 status in ewes on ovum development and lamb viability at birth. Reprod. Fertil. Dev. 19:553–562. doi:10.1071/RD07012 [DOI] [PubMed] [Google Scholar]
- Mohebbi-Fani M., Nazifi S., Ansari-Lari M., and Namazi F.. 2010. Mixed mineral deficiencies in a dairy herd with subclinical production disorders. Comp. Clin. Path. 19:37–41. [Google Scholar]
- Muehlenbein E. L., Brink D. R., Deutscher G. H., Carlson M. P., and Johnson A. B.. 2001. Effects of inorganic and organic copper supplemented to first-calf cows on cow reproduction and calf health and performance. J. Anim. Sci. 79:1650–1659. doi:10.2527/2001.7971650x [DOI] [PubMed] [Google Scholar]
- Narasimhaiah M., Arunachalam A., Sellappan S., Mayasula V. K., Guvvala P. R., Ghosh S. K., Chandra V., Ghosh J., and Kumar H.. 2018. Organic zinc and copper supplementation on antioxidant protective mechanism and their correlation with sperm functional characteristics in goats. Reprod. Domest. Anim. 53:644–654. doi:10.1111/rda.13154 [DOI] [PubMed] [Google Scholar]
- Nocek J. E., Socha M. T., and Tomlinson D. J.. 2006. The effect of trace mineral fortification level and source on performance of dairy cattle. J. Dairy Sci. 89:2679–2693. doi:10.3168/jds.S0022-0302(06)72344-X [DOI] [PubMed] [Google Scholar]
- Olson P. A., Brink D. R., Hickok D. T., Carlson M. P., Schneider N. R., Deutscher G. H., Adams D. C., Colburn D. J., and Johnson A. B.. 1999. Effects of supplementation of organic and inorganic combinations of copper, cobalt, manganese, and zinc above nutrient requirement levels on postpartum two-year-old cows. J. Anim. Sci. 77:522–532. [DOI] [PubMed] [Google Scholar]
- Özkaya M. O., and Nazıroğlu M.. 2010. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil. Steril. 94:2465–2466. doi:10.1016/j.fertnstert.2010.01.066 [DOI] [PubMed] [Google Scholar]
- Perry G. 2016. 2015 statistics of embryo collection and transfer in domestic farm animals. Embryo Transfer Newsl. 34(4):10–24. [Google Scholar]
- Picco S. J., Anchordoquy J. M., de Matos D. G., Anchordoquy J. P., Seoane A., Mattioli G. A., Errecalde A. L., and Furnus C. C.. 2010. Effect of increasing zinc sulphate concentration during in vitro maturation of bovine oocytes. Theriogenology 74:1141–1148. doi:10.1016/j.theriogenology.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Picco S. J., Rosa D. E., Anchordoquy J. P., Anchordoquy J. M., Seoane A., Mattioli G. A., and Furnus C. C.. 2012. Effects of copper sulphate concentrations during in vitro maturation of bovine oocytes. Theriogenology 77:373–381. doi:10.1016/j.theriogenology.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Rabiee A. R., Lean I. J., Stevenson M. A., and Socha M. T.. 2010. Effects of feeding organic trace minerals on milk production and reproductive performance in lactating dairy cows: A meta-analysis. J. Dairy Sci. 93:4239–4251. doi:10.3168/jds.2010-3058 [DOI] [PubMed] [Google Scholar]
- Record I. R., Tulsi R. S., Dreosti I. E., and Fraser F. J.. 1985. Cellular necrosis in zinc-deficient rat embryos. Teratology 32:397–405. doi:10.1002/tera.1420320310 [DOI] [PubMed] [Google Scholar]
- Rispoli L. A., Lawrence J. L., Payton R. R., Saxton A. M., Schrock G. E., Schrick F. N., Middlebrooks B. W., Dunlap J. R., Parrish J. J., and Edwards J. L.. 2011. Disparate consequences of heat stress exposure during meiotic maturation: Embryo development after chemical activation vs fertilization of bovine oocytes. Reproduction 142:831–843. doi:10.1530/REP-11-0032 [DOI] [PubMed] [Google Scholar]
- Robertson I., Nelson R. E.. 1999. Certificação e identificação de embriões. In: Stringfellow DA, Seidel SM, editors. Manual da Sociedade Internacional de Transferência de Embriões. 3rd ed. International Embryo Transfer Society, Inc., Savoy, IL. p. 109–122. [Google Scholar]
- Rojas L. X., McDowell L. R., Cousins R. J., Martin F. G., Wilkinson N. S., Johnson A. B., and Velasquez J. B.. 1995. Relative bioavailability of two organic and two inorganic zinc sources fed to sheep. J. Anim. Sci. 73:1202–1207. doi:10.2527/1995.7341202x [DOI] [PubMed] [Google Scholar]
- Seneda M. M., Esper C. R., Garcia J. M., Oliveira J. A., and Vantini R.. 2001. Relationship between follicle size and ultrasound-guided transvaginal oocyte recovery. Anim. Reprod. Sci. 67:37–43. doi:10.1016/S0378-4320(01)00113-0 [DOI] [PubMed] [Google Scholar]
- Shi L., Yue W., Zhang C., Ren Y., Zhu X., Wang Q., Shi L., and Lei F.. 2010. Effects of maternal and dietary selenium (se-enriched yeast) on oxidative status in testis and apoptosis of germ cells during spermatogenesis of their offspring in goats. Anim. Reprod. Sci. 119:212–218. doi:10.1016/j.anireprosci.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Spears J. W. 1996. Organic trace minerals in ruminant nutrition. Anim. Feed Sci. Technol. 58:151–163. doi:10.1016/0377-8401(95)00881-0 [Google Scholar]
- Stangl G. I., Schwarz F. J., Müller H., and Kirchgessner M.. 2007. Evaluation of the cobalt requirement of beef cattle based on vitamin B12, folate, homocysteine and methylmalonic acid. Br. J. Nutr. 84:645–653. doi:10.1017/S0007114500001987 [DOI] [PubMed] [Google Scholar]
- Tian X., and Diaz F. J.. 2013. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev. Biol. 376:51–61. doi:10.1016/j.ydbio.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend S. F., Briggs K. K., Krebs N. F., and Hambidge K. M.. 1994. Zinc supplementation selectively decreases fetal hepatocyte DNA synthesis and insulin-like growth factor II gene expression in primary culture. Pediatr. Res. 35(4 Pt 1):404–408. [PubMed] [Google Scholar]
- Uchida K., Mandebvu P., Ballard C., Sniffen C., and Carter M.. 2001. Effect of feeding a combination of zinc, manganese and copper amino acid complexes, and cobalt glucoheptonate on performance of early lactation high producing dairy cows. Anim. Feed Sci. Technol. 93:193–203. doi:10.1016/S0377-8401(01)00279-6 [Google Scholar]
- Underwood E. J. 1977. Trace elements in human and animal nutrition. 4th ed. Academic Press, Inc., New York, USA. [Google Scholar]
- Wahlen R., Evans L., Turner J., and Hearn R.The use of collision/reaction cell ICP-MS for the determination of elements in blood and serum samples. Spectroscopy. Online. 20. . 2005. http://www.spectroscopyonline.com/use- collisionreaction-cell-icp-ms-determination-elements-blood-and-serum-samples http://www.spectroscopyonline.com/use- collisionreaction-cell-icp-ms-determination-elements-blood-and-serum-samples
- Wolter B., Ellis M., McKeith F., Miller K., and Mahan D.. 1999. Influence of dietary selenium source on growth performance, and carcass and meat quality characteristics in pigs. Can. J. Anim. Sci. 79:119–121. doi:10.4141/A98-028 [Google Scholar]