Abstract
Fescue toxicosis is a multifaceted syndrome common in cattle grazing endophyte-infected tall fescue. The objective of this study was to evaluate the impact of the slick hair trait on physiological and reproductive parameters in heifers experiencing fescue toxicosis. Angus × Senepol heifers (n = 31) were blocked by weight (393.5 ± 17.3 kg) and phenotype relative to hair coat at birth, and randomly fed novel endophyte fescue (EN) or endophyte-infected fescue (EI) haylage in a total mixed ration for 91 d. Weekly measurements were collected to monitor heifer growth and response during ergot alkaloids exposure. Following 28 d of treatment, estrus was synchronized and heifers were inseminated. Ovary mapping and AI pregnancy rate were examined via transrectal ultrasonography. Blood samples were taken for genotyping: slick (S) or wildtype (W). Data were analyzed using repeated measures in PROC MIXED of SAS including fescue treatment (EN vs. EI), genotype (S vs. W), and sample collection time as main effects. Body condition scores were decreased for W heifers compared with S heifers (5.48 vs. 5.66, respectively; P < 0.0001). Surface temperature was greater for EI-W heifers (37.2 °C) compared with other groups (36.4, 36.6, 36.7 °C for EN-S, EN-W, EI-S, respectively; P < 0.05). Serum PRL concentrations were reduced for EI heifers compared with EN heifers (133.5 vs. 163.1 ng/mL, respectively; P < 0.05). The average number of 2 to 4 mm follicles were greater in EI-W heifers (13.8 follicles) compared with other groups (12.2, 10.6, and 11.1 for EN-S, EN-W, and EI-S, respectively; P < 0.0001). However, the average number of preovulatory follicles (≥9 mm) were reduced in EI-W heifers (0.52 follicles) compared with other heifer groups (0.94, 0.88, and 0.85 ± 0.04 for EN-S, EN-W, and EI-S, respectively; P < 0.05). Ovulatory follicle size was smaller in EI-W heifers compared with EN-W heifers (9.14 vs. 11.57 mm, respectively; P = 0.05). Corpus luteum area was reduced in EI-W heifers (235.1 mm2) compared with other heifer groups (297.2, 272.7, and 276.8 mm2 for EN-S, EN-W, and EI-S, respectively; P < 0.05). Concentrations of P4 were greater for EN heifers compared with EI heifers (2.7 vs. 1.8 ng/mL, respectively; P < 0.05). Pregnancy was not established in EI-W heifers (0%) compared with other heifer groups (37.5%, 57.1%, and 62.5% for EN-S, EN-W, and EI-S, respectively; P < 0.05). Overall, the slick hair mutation appears to aid in offsetting the physiological symptoms associated with fescue toxicosis and helps to improve reproductive performance.
Keywords: fescue toxicosis, slick hair trait, beef heifer, ovary
INTRODUCTION
Tall fescue (Lolium arundinaceum [Schreb.] Darbysh.) is a cool-season perennial forage utilized by cow-calf producers in the Southern regions of the United States (Ball et al., 2007). Fescue toxicosis is a result of cattle grazing endophyte (Epichloë coenophiala)-infected tall fescue, specifically consuming ergot alkaloids produced by the endophytic fungus. This disease contributes to an estimated $2 billion annually in economic loss predominantly due to a reduction in conception rates, which are vital to the success of a cow–calf operation (Kallenbach, 2015).
Cattle grazing endophyte-infected tall fescue have impaired reproductive function, including altered ovarian follicle development (McKenzie and Erickson, 1991; Burke and Rorie, 2002) and reduced circulating steroid hormone concentrations (Paterson et al., 1995). A reduction in serum prolactin (PRL) concentrations is a hallmark of fescue toxicosis and is largely regulated by the neurotransmitter, dopamine. The structural similarities between dopamine and the ergoline ring common to all ergot alkaloids enable the agonistic effects on the dopamine D2 receptor (Elsasser and Bolt, 1987; Larson et al., 1994), therefore reducing PRL secretion.
In recent years, a genetic trait has been identified in Senepol and other Criollo (Bos taurus) cattle breeds that is associated with high heat tolerance and a slick hair coat (Olson et al., 2003; Mariasegaram et al., 2007; Porto-Neto et al., 2018). A mutation occurs in the prolactin receptor (PRLR) resulting in a slick haired cow (Littlejohn et al., 2014). While the effects of endophyte-infected tall fescue on reproduction have been well documented, the impact of the slick trait has not been investigated. Therefore, the aim of this study is to evaluate the effect of the slick trait on cattle exposed to ergot alkaloids, specifically describing changes in ovarian function, reproductive hormone secretion, and pregnancy rates.
MATERIALS AND METHODS
The study was conducted at the Butner Beef Cattle Field Laboratory (BBCFL) in Bahama, NC, and was approved by the Institutional Animal Care and Use Committee at North Carolina State University (13-093-A).
Animals and Treatment
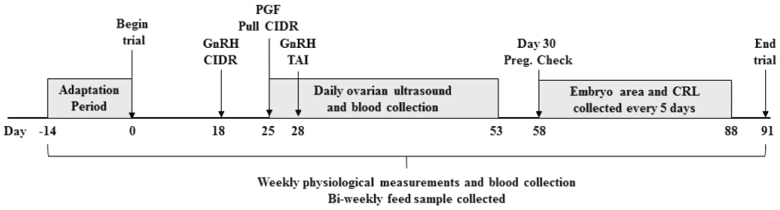
Cattle performance and forage data were collected late May to late August 2015 (see experimental timeline in Figure 1). Angus × Senepol heifers (n = 31), with no prior exposure to endophyte-infected fescue, were loaned from CEFS (Cherry Research Farm at the Center for Environmental Farming System in Goldsboro, NC) and delivered to BBCFL in April of 2015. At birth, a phenotype (slick or wildtype) was assigned to each heifer based on technique described by Olson et al. (2003). Upon arrival, heifers were blocked by weight and phenotype then randomly assigned to receive either “toxic” endophyte-infected fescue (EI; 421 g ergovaline/kg of BW) or novel “nontoxic” endophyte fescue (EN; control, 36 g ergovaline/kg of BW) haylage in a total mixed ration. Towards the end of the experiment, animals were genotyped (Recombinetics, Inc. St. Paul, MN): slick (S, mutation in PRLR) or wildtype (W). For analysis, individual treatments were designated as follows: novel endophyte slick (EN-S; n = 8), novel endophyte wildtype (EN-W; n = 7), endophyte-infected slick (EI-S; n = 9), and endophyte-infected wildtype (EI-W; n = 6). One heifer from EI-S was removed from the study due to the presence of cystic follicles. Animals were placed in a slotted floor barn with individual feeding gates (American Calan, Northwood, New Hampshire) for individual feed intake regulation.
Figure 1.
Experimental timeline used for beef heifers consuming either novel endophyte (EN) or endophyte-infected (EI) fescue with slick hair mutation (S) or wild-type (W) from late-May to late-August 2015.
To monitor physiological responses to ergot alkaloids, BW, BCS (scale of 1 to 9; adapted from Whitman, 1975), rectal temperature, surface temperature, heart rate, caudal blood pressure, respiration rate, caudal artery and vein diameter, and hematocrit were measured weekly as previously described by Eisemann et al. (2014). Surface temperature was accessed via thermal imaging camera (Fluke Ti45FT IR Flexcam, Fluke Corporation, Everett, WA). The heifers had an 18 × 20 cm square clipped behind the left shoulder using a #10 blade. Each week, images were taken within the square and the highest, lowest, and average temperature were recorded (SmartView 3.5 Thermal Imager Software; Eisemann et al., 2014). Heart rate and caudal blood pressure were measured three times for each heifer weekly using a 16 to 24 cm blood pressure cuff (LifeSource A&D Engineering Inc., San Jose, CA). The tail was held steady during these measurements to minimize variation. Caudal artery and vein diameter were measured using Doppler ultrasonography (M-Turbo, SonoSite Inc., Bothell, WA). Hair coat scores (HCS; adapted from Olsen et al., 2003) and hair coat shedding scores (HSS; scale of 1 [slick summer coat] to 5 [full winter coat]; adapted from Gray et al., 2011) were collected by two trained technicians weekly and composited.
For a subset of heifers (n = 20; EN-S: n = 5, EN-W: n = 5, EI-S: n = 4, and EI-W: n = 6), a calibrated iButton temperature data logger was affixed to the bottom side of a progesterone-free controlled internal drug release (CIDR; Zoetis, Parsippany, NJ) device and inserted into the vagina. The data loggers were set to record internal temperature every 10 min for two 7-d intervals during the treatment period (technique adapted from Niu et al., 2014): period 1 being days 14 to 21 and period 2 being days 56 to 63. Data were blocked into 2 h intervals for analysis.
Temperature and Temperature Humidity Index
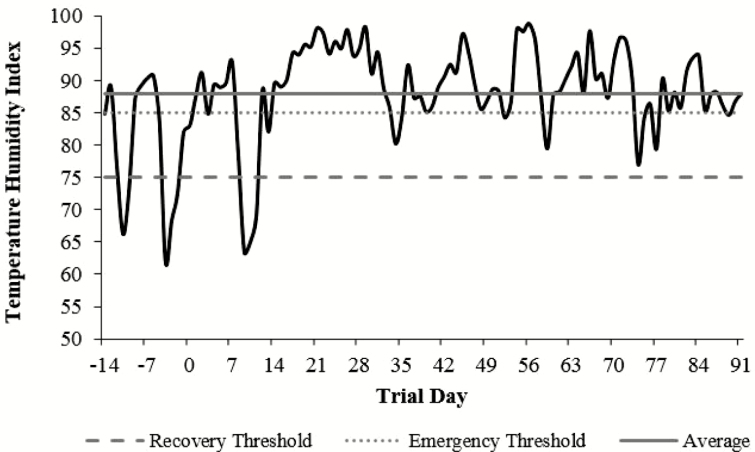
Ambient temperature and humidity were collected weekly during data collection. Additionally, records were obtained from the National Weather Service Henderson Oxford Airport station, approximately 40 km from BBCFL (Figure 2). Temperature humidity index (Buffington et al., 1981) was calculated using the formula:
Figure 2.
Environmental temperature humidity index (THI) retrieved daily at noon from late May to late August 2015. THI = Tdb – [0.55 – (0.55 × RH/100) × (Tdb – 58)]; Tdb = dry bulb temperature (°F), and RH = relative humidity. Recovery (non-life threatening, 75) and emergency (high-risk, 85) thresholds as described by Hahn (1999). Average represents the overall average THI (88) throughout the duration of the trial.
where Tdb represents dry bulb temperature (°F) and RH represents relative humidity.
Diet
Total mixed rations (TMRs) were fed and were based on either Kentucky-31 Tall Fescue (EI, 851 µg/kg total ergot alkaloid and 175 µg/kg ergovaline) or nontoxic-infected tall fescue (MaxQII Texoma, EN, 66 µg/kg total ergot alkaloid and 60 µg/kg ergovaline) haylage. Haylage was harvested by swathing, wilting, and chopping, and was preserved in AG bags in the fall of 2014. The TMRs were formulated according to National Research Council (1996) requirements for 0.9 kg/d ADG when limited to a DMI of 2% of BW (71% TDN and 14% CP). DMI was adjusted every 2 wk as the heifers gained weight to maintain the desired rate of feed intake and gain as described previously (Poole et al., 2018).
The EI TMR was initially 75% haylage, 8% corn, 1.3% soybean meal, 3.1% of a commodity pellet, and 11.9% ground-infected fescue seed (Piedmont Tall Fescue, Southern States Cooperative, Richmond, VA). Midway through the experiment (day 31), a new lot of EI seed was obtained that had a reduced concentration of total ergot alkaloid, so the diet was reformulated to contain 75% EI haylage, 19% ground infected fescue seed, 4% corn, 1% soybean meal, and 0% commodity pellets. The EN TMR contained 63% haylage, 12% corn, 5% soybean meal, and 19% commodity pellets. Both TMRs contained limestone, salt, and a vitamin and trace mineral premix formulated to meet requirements of the heifers. All heifers had ad libitum access to water throughout the experiment.
Infected tall fescue seed was added into the EI TMR to provide a dietary level of 1,000 µg/kg of total ergot alkaloid, while the EN TMR contained 40 µg/kg total ergot alkaloid (Agrinostics, Ltd., Watkinsville, GA). Subsequent analysis of the seed and haylage for ergovaline (MU Veterinary Medical Diagnostic Lab; Rottinghaus, 1993) showed that the EI TMRs contained 421 µg/kg ergovaline, the EN TMR contained 36 µg/kg ergovaline. No ergot alkaloids other than ergovaline were found.
Estrus Synchronization and Reproductive Measurements
Eighteen days after dietary treatment was initiated, all heifers were subjected to the 7-d CO-Synch + CIDR synchronization protocol. For this protocol, a 100 µg dose of gonadotropin releasing hormone (GnRH, 2 mL Factrel; Zoetis, Parsippany, NJ) was given at the time of CIDR device (1.38 g of progesterone [P4]; Zoetis) insertion on day 18. At CIDR removal on day 25, a 25 mg dose of prostaglandin F2α (PGF2α, 5 mL Lutalyse; Zoetis, Parsippany, NJ) was given followed by a 100 µg dose of GnRH and timed artificial insemination (TAI) on day 28. Animals were synchronized to display estrus on day 28 of the feeding period (see experimental timeline in Figure 1). All heifers were inseminated to a bull of known fertility and a single inseminator. Ovary mapping was performed daily from days 25 to 53 and follicles and corpus luteum (CL) presence were recorded. Follicles were classified based on size: preselected follicles 2 to 4 mm, selected follicles 5 to 8 mm, and preovulatory follicles ≥9 mm. Thirty days post-TAI (day 58), pregnancy status was determined by transrectal ultrasonography with a SonoSite M-Turbo ultrasound system equipped with a 10 to 5 MHz transducer (FUJIFILM SonoSite, Inc., Bothell, WA). Embryo area and crown-to-rump length (CRL) was measured every 5 d from days 58 to 88.
Blood Sampling and Assays
Blood samples were collected from the jugular vein into 10-mL sterile vacutainer serum blood collection tubes without additive (Vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ). Blood samples were immediately placed on ice and later centrifuged at 1,500 × g for 20 min at 4 °C. The serum was transferred into 5 mL polystyrene vials (BD Falcon, Franklin Lakes, NJ) and stored at −80 °C for analysis.
Serum concentrations of PRL were analyzed on days 0, 14, 28, 42, and 56. Sample concentrations were determined by a commercially available Bovine Prolactin ELISA kit (MyBioSource; San Diego, CA) and previously validated (Poole et al., 2018). A sample control was included in each assay replicate. The inter-assay coefficient of variation based on the duplicate sample controls was 14.45%, and the intra-assay coefficient of variation was 9.93%.
Serum concentrations of estradiol (E2) were analyzed on days 28, 31, 34, 37, 40, 43, 46, and 49 to encompass follicular wave activity throughout the 21 d estrous cycle. Serum E2 was extracted using the procedures of Hendricks et al. (1971). Briefly, 2 mL of ether (Anhydrous Baker Analyzed A.C.S. Reagent, J.T.Baker; Thermo Fisher Scientific Inc., Waltham, MA) was added to 500 μL of serum in a 16 × 100 mm borosilicate glass tube (VWR, Radnor, PA). Samples were capped and shaken for 10 min on an Eberbach Lateral Shaker and frozen at approximately −20 °F for 2 to 3 h until serum is solidly frozen. Ether supernatant was poured from the frozen serum into a 12 × 75 mm culture tube and placed in a Thermolyne Dry-Bath to evaporate ether from tubes at 45 °C used filtered air, followed by adding 50 μL of Steroid Diluent Buffer (MP BioMedicals, Santa Ana, CA) to all tubes. Extracted samples were assayed using the Double Antibody 17β-Estradiol 125I RIA kit (MP BioMedicals) and were counted for 1 min using the Micromedic 4/600 Automatic Gamma Counter. The intra- and interassay coefficient of variation was 8.63% and 11.39%, respectively.
Serum concentrations of P4 were analyzed on days 28, 33, 38, and 43. Concentrations were determined using Immuchem Coated Tube Progesterone 125I RIA assay (ICN Parmaceuticals, Inc., Costa Mesa, CA) and were counted for 1 min using the Cobra II Auto Gamma Counter (Packard Instrument Company, Meriden, CT) as previously described by Lyons et al. (2016). The intra- and interassay coefficient of variation was 6.12% and 10.42%, respectively.
Serum concentrations of pregnancy-specific protein B (PSPB) were analyzed 28 d postbreeding (day 56) using the commercially available BioPRYN ELISA assay (Biotracking LLC; Moscow ID) as previously described by Mercadante et al. (2016). All samples were run in a single assay and the intra-assay coefficient of variation was 3.88%.
Statistical Analysis
Data were analyzed using the MIXED procedure of SAS 9.3 (SAS Inst. Inc., 1996) with repeated measures and individual animal as the experimental unit. The model for performance data, ovarian measurements, hormone concentrations, pregnancy rates, and embryo measurements included fescue treatment (EN vs. EI), genotype (S vs. W), sample collection time, and interactions. Results were recorded as least squares means ± SEM. Terms with a significance value of P > 0.20 were removed from the complete model in a stepwise manner to derive the final reduced model for each variable. A statistical significance was reported at P ≤ 0.05. A tendency was reported at P > 0.05 and ≤0.10.
RESULTS
Animal Performance
All animal performance data are summarized in Table 1. At the start of the trial, there were no differences in average initial BW, final BW, DMI, or ADG. Body condition scores were different by genotype with BCS of W heifers being less than BCS of S heifers (5.48 vs. 5.66 ± 0.03, respectively; P < 0.0001). As expected, HCS were reduced in S heifers when compared with W heifers (1.94 vs. 3.22 ± 0.09, respectively; P < 0.0001). Additionally, HSS were decreased in S heifers when compared with W heifers (1.90 vs. 2.84 ± 0.09, respectively; P < 0.0001). As for fescue treatment effects, EN heifers had decreased HCS when compared with EI heifers (2.37 vs. 2.79 ± 0.11, respectively; P < 0.0001). Similarly, HSS were reduced for EN heifers when compared with EI heifers (2.14 vs. 2.60 ± 0.08, respectively; P < 0.0001).
Table 1.
Physiological parameters for beef heifers consuming either novel endophyte (EN) or endophyte-infected (EI) fescue with slick hair mutation (S) or wildtype (W) from late May to late August 2015
| Treatment1,2 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | EN-S | EN-W | EI-S | EI-W | SEM | Fescue | Genotype | Interaction |
| Initial BW, kg | 393.2 | 388.3 | 396.9 | 395.1 | 17.3 | 0.762 | 0.848 | 0.929 |
| Final BW, kg | 422.0 | 411.2 | 426.7 | 417.9 | 19.0 | 0.767 | 0.611 | 0.960 |
| DMI, kg/d | 7.64 | 7.36 | 7.49 | 7.02 | 0.32 | 0.447 | 0.249 | 0.765 |
| ADG, kg/d | 0.46 | 0.37 | 0.47 | 0.36 | 0.07 | 0.933 | 0.156 | 0.892 |
| BCS3 | 5.70a | 5.50b | 5.62a | 5.47b | 0.04 | 0.146 | <0.0001* | 0.432 |
| HCS4 | 1.64a | 3.09b | 2.23c | 3.35b | 0.09 | <0.0001* | <0.0001* | 0.073† |
| HSS5 | 1.59a | 2.69b | 2.20c | 2.99b | 0.11 | <0.0001* | <0.0001* | 0.149 |
| Rectal temperature, °C | 39.72 | 39.72 | 39.65 | 39.69 | 0.04 | 0.148 | 0.592 | 0.534 |
| Surface temperature, °C | 36.44a | 36.64a | 36.65a | 37.19b | 0.10 | 0.002* | 0.004* | 0.090† |
| Heart rate | 82.9a | 81.4a | 75.5b | 81.0a | 1.5 | 0.010* | 0.192 | 0.022* |
| Respiration rate | 52.6 | 52.7 | 51.3 | 52.8 | 1.0 | 0.541 | 0.433 | 0.441 |
| Artery diameter, mm | 3.22 | 3.18 | 3.06 | 3.17 | 0.5 | 0.115 | 0.526 | 0.127 |
| Vein diameter, mm | 4.51ab | 4.47ab | 4.37a | 4.65b | 0.8 | 0.769 | 0.115 | 0.041* |
| Systolic BP6, mmHg | 147.2a | 143.5a | 136.9b | 138.1b | 1.5 | <0.0001* | 0.399 | 0.105 |
| Diastolic BP, mmHg | 85.8a | 84.7a | 75.9c | 80.7b | 1.4 | <0.0001* | 0.187 | 0.031* |
| Hematocrit7 | 36.3a | 35.2b | 35.6b | 33.7c | 0.3 | <0.0001* | <0.0001* | 0.133 |
| Pregnancy rate, % | 37.5ab | 57.1a | 62.5a | 0.0b | 16.8 | 0.347 | 0.213 | 0.021* |
Within row, means without a common superscript significantly differ (P ≤ 0.05).
Within row, means without a common superscript significantly differ (P ≤ 0.05).
Within row, means without a common superscript significantly differ (P ≤ 0.05).
Values are reported as least square means for the experiment.
EN-S = novel endophyte fescue with slick hair coat; EN-W = novel endophyte fescue with wildtype hair coat; EI-S = endophyte-infected fescue and slick hair coat; EI-W = endophyte-infected fescue with wildtype hair coat.
BCS (1 to 9 scale).
HCS = hair coat score (1 to 5 scale).
HSS = hair shedding score (1 to 5 scale).
BP = blood pressure.
Hematocrit is represented by the percentage of packed red cells in blood.
P-values < 0.05 determined significant.
P-values 0.05 > P ≤ 0.10 determined a statistical tendency.
Rectal temperatures were not different among heifer groups (P > 0.05). Surface temperature was greater in EI-W heifers when compared with other heifer groups (P < 0.05). Respiratory rates were similar among heifer groups (P > 0.05). Caudal artery diameter is not different among heifer groups (P > 0.05). There was an interaction with caudal vein diameter being greater in EI-W heifers when compared with EI-S heifers (P < 0.05); however, there were no difference when compared with EN-S or EN-W heifers (P > 0.05). There was an interaction with EI-S heifers having a decreased heart rate when compared with other heifer groups (P < 0.05). There was a fescue treatment effect with decreased systolic blood pressure in EI heifers compared with EN heifers (137.5 vs. 145.3 ± 1.1 mmHg, respectively, P < 0.0001). Similarly, there was an interaction with diastolic blood pressure being reduced in EI-S heifers as opposed to other heifer groups (P < 0.05). Hematocrit was decreased in EI-W heifers compared with other heifer groups (P < 0.05).
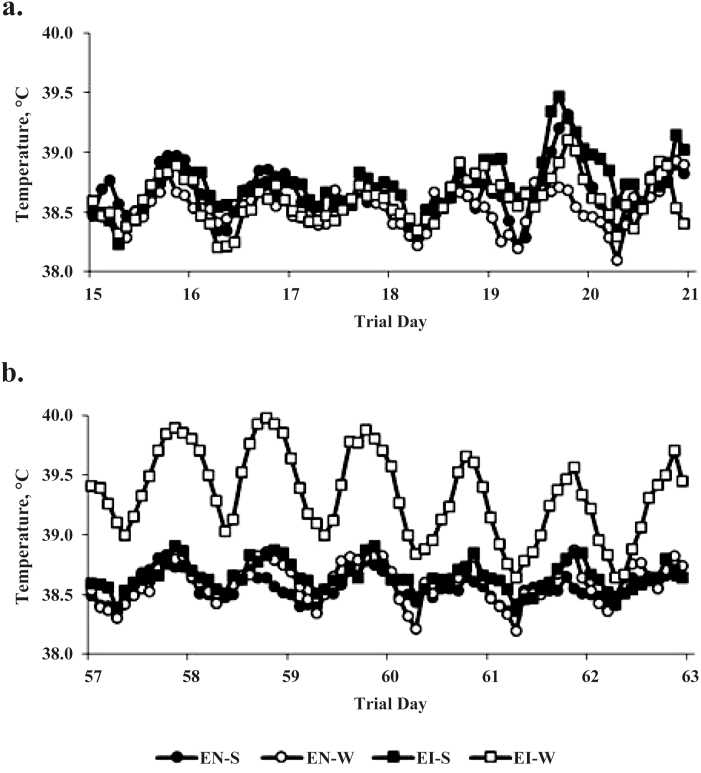
There was no difference in daily vaginal temperatures in period 1 (38.7, 38.5, 38.7, and 38.6 ± 0.1 °C for EN-S, EN-W, EI-S, and EI-W, respectively; Figure 3a). However, there were differences observed in daily vaginal temperatures with EI-W heifers (39.3 ± 0.2 °C) having greater body temperature compared with other heifer groups in period 2 (38.6, 38.6, and 38.6 ± 0.2 °C for EN-S, EN-W, and EI-S, respectively; P < 0.0001; Figure 3b).
Figure 3.
For a subset of heifers consuming novel endophyte fescue with the slick hair trait (black circles, EN-S; n = 5) or the wildtype hair coat (open circles, EN-W; n = 5) and endophyte-infected fescue with the slick hair trait (black squares, EI-S; n = 4) or the wildtype hair coat (open squares, EI-W; n = 6), an iButton temperature data logger recorded vaginal temperature every 2 h for two 7-d periods: (a) period 1 being days 14 to 21 and (b) period 2 being days 56 to 63.
Reproductive Measurements
All reproductive measurement data are summarized in Table 2. The average number of preselected follicles (2 to 4 mm) were greater in EI-W heifers compared with other heifer groups (P < 0.0001). No differences (P > 0.05) were detected in the average number of selected follicles (5 to 8 mm). The average number of preovulatory follicles (≥9 mm) were reduced in EI-W heifers compared with other heifer groups (P < 0.05). Additionally, the size of the ovulatory follicle was smaller in EI-W heifers as opposed to EN-W heifers (P = 0.05). Likewise, CL area was smaller in EI-W heifers compared with other heifer groups (P < 0.05). Ultimately, there was an interaction observed with heifers in the EI-W group was unable to establish pregnancy as opposed to other heifer groups (P < 0.05; Table 1). No differences were observed in embryo area or CRL between EN-S, EN-W, and EI-S heifer groups (P < 0.05).
Table 2.
Reproductive parameters in beef heifers consuming either novel endophyte (EN) or endophyte-infected (EI) fescue with slick hair mutation (S) or wildtype (W) from late May to late August 2015
| Treatment12 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | EN-S | EN-W | EI-S | EI-W | SEM | Fescue | Genotype | Interaction |
| Follicle number3 | ||||||||
| Preselected follicles4 | 12.18a | 10.60b | 11.07ab | 13.77c | 0.48 | 0.034* | 0.257 | <0.0001* |
| Selected follicles5 | 3.09 | 3.30 | 3.05 | 2.96 | 0.13 | 0.146 | 0.663 | 0.273 |
| Preovulatory follicles6 | 0.94a | 0.88a | 0.85a | 0.52b | 0.04 | <0.0001* | <0.0001* | 0.002* |
| Ovarian structure sizes | ||||||||
| Ovulatory follicle, mm | 9.88ab | 11.57a | 10.25ab | 9.14b | 0.69 | 0.150 | 0.674 | 0.053† |
| Luteal area, mm2 | 297.2a | 272.7a | 276.8a | 235.1b | 11.7 | 0.015* | 0.005* | 0.467 |
| Reproductive hormones | ||||||||
| Estradiol, pg/mL | 6.57a | 4.97b | 7.78a | 4.58b | 1.82 | 0.447 | 0.0001* | 0.191 |
| Progesterone, ng/mL | 2.38ab | 3.01a | 1.97ab | 1.53b | 0.46 | 0.035* | 0.837 | 0.243 |
| Prolactin, ng/mL | 140.9ab | 186.3a | 124.9b | 137.2ab | 31.2 | 0.050* | 0.057† | 0.873 |
| PSPB7, ng/mL | 3.36 | 3.05 | 3.13 | N/A | 0.28 | 0.515 | 0.430 | 0.848 |
Within row, means without a common superscript significantly differ (P ≤ 0.05).
Within row, means without a common superscript significantly differ (P ≤ 0.05).
Within row, means without a common superscript significantly differ (P ≤ 0.05).
Values are reported as least square means for the experiment.
EN-S = novel endophyte fescue with slick hair coat; EN-W = novel endophyte fescue with wildtype hair coat; EI-S = endophyte-infected fescue and slick hair coat; EI-W = endophyte-infected fescue with wildtype hair coat.
Follicle number is defined as a follicle observed during ovary mapping via transrectal ultrasonography.
Follicles were classified based on size: preselected follicles 2 to 4 mm, selected follicles 5 to 8 mm, and preovulatory follicles ≥9 mm.
Follicles were classified based on size: preselected follicles 2 to 4 mm, selected follicles 5 to 8 mm, and preovulatory follicles ≥9 mm.
Follicles were classified based on size: preselected follicles 2 to 4 mm, selected follicles 5 to 8 mm, and preovulatory follicles ≥9 mm.
PSPB = pregnancy-specific protein B.
P-values < 0.05 determined significant.
P-values 0.05 > P ≤ 0.10 determined a statistical tendency.
Hormone Profiles
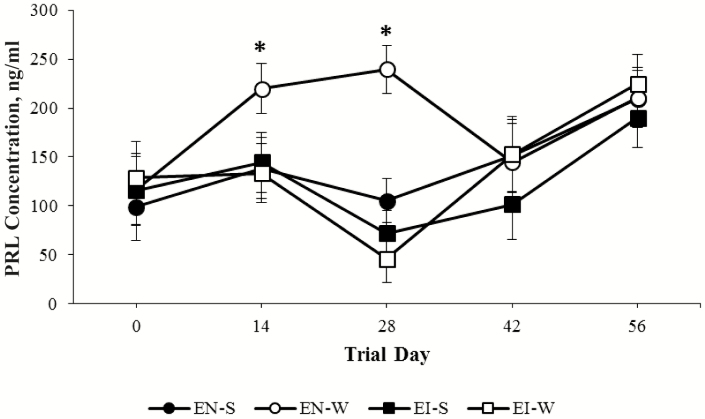
All hormone profile data are summarized in Table 2. Overall serum PRL concentrations were reduced for EI heifers compared with EN heifers (133.5 vs. 163.1 ± 10.7 ng/mL, respectively, P = 0.050). Interestingly, a tendency was observed with S heifers having a reduction in overall serum PRL concentrations when compared with W heifers (133.8 ± vs. 162.8 ± 10.7 ng/mL, respectively, P = 0.06). Moreover, serum PRL concentrations fluctuated throughout the duration of the trial (P < 0.001; Figure 4). Although there were no fescue treatment differences in overall serum E2 concentrations (5.8 vs. 6.2 ± 0.4 pg/mL for EN and EI, respectively, P = 0.45), overall serum E2 concentrations were elevated for S heifers compared with W heifers (7.1 vs. 4.9 ± 0.4 pg/mL, respectively, P < 0.001). Overall serum P4 concentrations were elevated for EN heifers compared with EI heifers (2.7 vs. 1.8 ± 0.3 ng/mL, respectively, P < 0.05). No differences were observed in the overall P4 concentrations between S and W heifers. Finally, no differences were detected in serum concentrations of PSPB on day 28 of gestation between EN-S, EN-W, and EI-S heifer groups.
Figure 4.
Prolactin (PRL) concentrations in heifers consuming novel endophyte fescue with the slick hair trait (black circles, EN-S; n = 8) or the wildtype hair coat (open circles, EN-W; n = 7) and endophyte-infected fescue with the slick hair trait (black squares, EI-S; n = 9) or the wildtype hair coat (open squares, EI-W; n = 6). *P-value < 0.05 determined significant.
DISCUSSION
The poor performance and reproductive outcomes experienced by EI-W heifers in the present study were remarkably similar to heat-stressed animals. Typically, Bos indicus-influenced cattle, such as Brahman, are common in areas with high environmental temperatures (i.e., tropical environment), because of their heat tolerance (Cartwright, 1980; Turner, 1980). However, there are numerous negative features of B. indicus-influenced cattle such as obstinate temperament, late maturing, increased calving interval, and poor carcass quality. Conversely, the Senepol is a Bos taurus breed that display similar heat tolerant capabilities yet docile temperament, unlike B. indicus-influenced cattle (Hammond et al., 1996). The heat tolerance capability of Senepol cattle was associated with a slick hair coat which is a dominant, heritable genetic trait (Olson et al., 2003). Specifically, this trait in Senepol cattle is due in part to a frame shift mutation in exon 10 of the prolactin receptor (PRLR) on bovine chromosome 20 (Mariasegaram et al., 2007; Littlejohn et al., 2014). Because of this, it is believed that the Senepol breed can be utilized as a genetic resource that could reduce problems associated with fescue toxicosis. An experiment conducted by Browning (2004), compared physiological parameters associated with heat tolerance between purebred Hereford and Senepol steers consuming endophyte-infected tall fescue hay. Senepol steers had improved gains, decreased respiration rates, reduced shade use, and reduced skin surface temperatures when compared with Hereford steers, demonstrating the heat tolerance capability of Senepol cattle (Browning, 2004). Similarly in the present study, physiological responses differed between treatment groups. Numerous reports have indicated that cattle consuming ergot alkaloids have a retained and rough hair coat (reviewed by Strickland et al., 2011). While the mechanism of this physiological effect is not well understood, it is known that the retained winter coat exacerbates heat stress during the summer months, and this phenomenon was observed in the EI-W heifers as indicated by the HCS and HSS. Additionally, it has been shown that exposure to ergovaline can result in bioaccumulation within the vasculature, therefore prolonging the effects of ergot alkaloid induced vasoconstriction (Klotz et al., 2009). The combination of a retained winter coat and bioaccumulation of ergovaline most likely contributes to the increased skin surface temperatures experienced by EI-W heifers. While rectal temperature and respiration rate (common responses to heat stress) did not differ between groups, hematocrit values were reduced in EI-W heifers. Hematocrit is the proportion, by volume, of the blood that consists of red blood cells and it is an indicator of dehydration (Nordenson, 2006). Therefore, with EI-W heifers having a decreased hematocrit value, this would indicate that the heifers were overhydrating and attempting to cool down. Moreover, previous studies have shown that cattle consuming endophyte-infected tall fescue have elevated heart rate (Bond et al., 1984), and that additional heat stress exacerbates heart rate (Walls and Jacobsen, 1970). Interestingly, EI-S heifers displayed a decreased heart rate and blood pressure compared with other heifer groups. A decrease in heart rate typically would result in decreased blood pressure if other variables that affect pressure are unchanged (Melbin and Detweiler, 1993). Therefore, while the EI-S heifers were consuming ergot alkaloids, the heifers displayed a heat tolerance capability which is associated with having a slick hair genotype.
Induction of fescue toxicosis starts to occur ~21 d post-exposure to ergot alkaloids. This is indicated by a reduction in prolactin secretion, urinary excretion of ergot alkaloids, and vasoconstrictive activity (Hill et al., 2000; Klotz et al., 2016). Therefore, while EI heifers had reduced prolactin concentrations 14 d post initiation of the trial, there were no differences among heifer groups in internal data logger temperature (i.e., period 1). However, as the trial progressed to 56 d, prolactin concentrations normalized between groups, yet EI-W heifers still had a retained, rough hair coat. Thus, only EI-W heifers had significantly increased internal data logger temperature in period 2. Ultimately as ambient temperatures increased, heifers that possessed the slick hair trait while consuming the endophyte-infected fescue haylage (EI-S) were able to better dissipate heat and have improved overall performance.
In addition to poor performance displayed by cattle suffering from fescue toxicosis, it has been reported that they experience altered follicular dynamics, impaired luteal function, and a reduction in cholesterol and steroid hormone concentrations. Burke et al. (2001) investigated the interaction between heat stress and consumption of endophyte-infected fescue on follicular dynamics. The combination of heifers consuming the endophyte-infected seed in heat stress conditions resulted in a smaller preovulatory follicle diameter and that consumption of endophyte-infected seed alone resulted in a decrease in the number of large follicles (Burke et al., 2001). Similarly in the present study, it was observed that EI-W heifers had a reduction in ovulatory follicle size. Additionally, EI-W heifers had an increase in the number of preselected follicles (2 to 4 mm), however, no change in the number of selected follicles (5 to 8 mm), yet a decrease in the number of preovulatory follicles (>9 mm). This is indicative of a dysregulation from the selection to preovulatory phase of folliculogenesis. Interestingly, EI-S heifers had similar follicular dynamics to heifers consuming endophyte-free haylage. This would signify that the increase in body temperature due to having a wildtype hair coat, in combination with consumption of endophyte-infected fescue (EI-W), contributes to heat stress and potentially alters the efficiency of ovarian follicular selection and dominance. The concept of heat stress altering folliculogenesis is not novel. A study by Badinga et al. (1993), demonstrated that cows exposed to heat stress conditions did not have a suppression of medium sized (6 to 9 mm) follicles, however did observe smaller preovulatory follicles (Badinga et al., 1993). Additionally, a study completed by Wolfenson et al. (1997) observed that cows subjected to heat stress conditions had decreased 17β-estradiol production by the granulosa cells in the follicular fluid (Wolfenson et al., 1997). Moreover, in the current study, EI-S heifers had elevated serum estradiol concentrations, thus signifying that the ovarian follicles were properly functioning even while consuming endophyte-infected fescue. Together, this indicates that the dysregulation of folliculogenesis and the reduction in steroid hormone concentrations, specifically estradiol, observed in cattle consuming endophyte-infected tall fescue may be solely attributed to the heat stress component of fescue toxicosis.
There have been varying reports regarding the impact of fescue toxicosis on luteal formation and function. Numerous findings report that endophyte-infected fescue reduces CL size and progesterone secretion (Estienne et al., 1990; Mahmood et al., 1994; Burke et al., 2001; Jones et al., 2003), while others observe no differences (Fanning et al., 1992, Seals et al., 2005). This variation in responses could be due to the fact that circulatory ovarian steroid concentrations, specifically progesterone, are not only dependent on the rate of secretion but also on the metabolism in the liver and on the incidence of vasodilation or vasoconstriction. The bovine CL is a highly vascular tissue that possesses numerous angiogenic factors; however, it has been proposed that vascular endothelial growth factor (VEGF) is responsible for the initial stages of angiogenesis. During the estrous cycle, VEGF concentrations are greatest early in the cycle when luteal vascularization is occurring (Berisha et al., 2000). Interestingly, a hypoxic environment is important for the initiation of VEGF-induced angiogenesis; however, hypoxia will inhibit progesterone secretion mid-cycle (Nishimura and Okuda, 2010). With angiogenic activity occurring in the growing CL, adrenergic receptors will localize in the vasculature and mediate vasoconstriction. Oliver et al. (1998) demonstrated that adrenergic receptors, specifically α2-adrenergic receptors, have a high affinity for endophyte-infected tall fescue produced ergot alkaloid peptides (Oliver et al., 1998). Therefore, the vasoconstrictive activity of ergot alkaloids may potentially be influencing blood flow to the female reproductive tract, specifically the ovary. In the current study, it was observed that EI-W heifers had a reduction in CL area, which would be a direct result of the observed smaller ovulatory follicle size. It has been shown smaller ovulatory follicles will subsequently develop into smaller CL, and secrete less progesterone when compared with larger ovulatory follicles (Vasconcelos et al., 2001). Follicular granulosa cells differentiate into large luteal cells (Smith et al., 1994), and ~80% of progesterone secreted is by large luteal cells (Niswender et al., 1985). Therefore, it has been suggested that development of a normal CL depends on the preovulatory follicle having an adequate number of granulosa cells capable of synthesizing sufficient progesterone after luteinization (McNatty et al., 1979). Furthermore, it was observed that EI heifers, regardless of genotype, had reduced serum progesterone concentrations. Together, this signifies the importance of ovarian blood flow, and that the vasoconstrictive activity observed in cattle consuming endophyte-infected tall fescue may be hindering proper ovarian function (Poole et al., 2018). Finally, pregnancy was not established in EI-W heifers. The authors acknowledge the limited dataset and that results may differ with more animals per treatment. However, it is important to note that decreased pregnancy rates is a common result of fescue toxicosis and heat stress (reviewed by Porter and Thompson, 1992; Hansen, 2009).
In conclusion, the slick hair mutation observed in Senepol cattle appears to alleviate many of the heat stress symptoms associated with fescue toxicosis and therefore helps to improve reproductive performance. Further research is necessary to elucidate the mechanistic actions responsible for reduced fertility in cattle suffering from fescue toxicosis, specifically evaluating the impact on oocyte competence, fertilization, and early embryonic development.
ACKNOWLEDGMENTS
The authors thank Dean Askew, Greg Shaeffer, and the rest of the Butner crew (Butner Beef Cattle Field Laboratory, Bahama, NC) for their consistent help with animal care and Andrew Meier at CEFS and the North Carolina Department of Agriculture and Consumer Services Cherry Research Farm, in Goldsboro, NC for the use of these heifers. We thank J. Chris Mackey, Sarah M. Long, and Jacob Loyd for all their help with data collection and laboratory analysis.
Conflict of interest statement. None declared.
Footnotes
This study was funded by North Carolina Cattlemen’s Association, North Carolina Agricultural Foundation, Inc., and Hatch Project 02420.
LITERATURE CITED
- Badinga L., Thatcher W. W., Diaz T., Drost M., and Wolfenson D.. 1993. Effect of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology. 39:797–810. [DOI] [PubMed] [Google Scholar]
- Ball D. M., Hoveland C. S., and Lacefield G. D... 2007. Overview of Southern forages. In: Southern forages: modern concepts for forage crop management. 4th ed. Norcross, GA: IPNI; p. 20–25. [Google Scholar]
- Berisha B., Schams D., Kosmann M., Amselgruber W., and Einspanier R.. 2000. Expression and tissue concentration of vascular endothelial growth factor, its receptors, and localization in the bovine corpus luteum during estrous cycle and pregnancy. Biol. Reprod. 63:1106–1114. doi:10.1095/biolreprod63.4.1106 [DOI] [PubMed] [Google Scholar]
- Bond J., Powell J. B., Undersander D. J., Moe P. W., Tyrrell H. F., and Oltjen R. R... 1984. Forage composition and growth and physiological characteristics of cattle grazing several varieties of tall fescue during summer conditions. J. Anim. Sci. 59:584–593. [Google Scholar]
- Browning R., Jr 2004. Effects of endophyte-infected tall fescue on indicators of thermal status and growth in hereford and senepol steers. J. Anim. Sci. 82:634–643. doi:10.2527/2004.822634x [DOI] [PubMed] [Google Scholar]
- Buffington D.E., Collazo-arocho A, Canton G. H, Pitt D. 1981. Black globe-humidity index (BGHI) as a comfort equation for dairy cows. T. Am. Soc. Assoc. Exe. 24:711–714. [Google Scholar]
- Burke J. M., Rorie R. W.. 2002. Changes in ovarian function in mature beef cows grazing endophyte infected tall fescue. Theriogenology. 57(6):1733–1742. PMID: 12035982. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Spiers D. E., Kojima F. N., Perry G. A., Salfen B. E., Wood S. L., Patterson D. J., Smith M. F., Lucy M. C., Jackson W. G., et al. 2001. Interaction of endophyte-infected fescue and heat stress on ovarian function in the beef heifer. Biol. Reprod. 65:260–268. doi:10.1095/biolreprod65.1.260 [DOI] [PubMed] [Google Scholar]
- Cartwright T. C. 1980. Prognosis of Zebu cattle: research and application. J. Anim. Sci. 50:1221–1226. [Google Scholar]
- Eisemann J. H., Huntington G. B., Williamson M., Hanna M., and Poore M.. 2014. Physiological responses to known intake of ergot alkaloids by steers at environmental temperatures within or greater than their thermoneutral zone. Front. Chem. 2:96. doi:10.3389/fchem.2014.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser T. H. and Bolt D. J... 1987. Dopaminergic-like activity in toxic fescue alters prolactin but not growth hormone or thyroid stimulating hormone in ewes. Domest. Anim. Endocrinol. 4:259–269. [DOI] [PubMed] [Google Scholar]
- Estienne M. J., Schillo K. K., Fitzgerald B. P., Hielman S. M., Bradley N. W., and Boling J. A... 1990. Effects of endophyte-infected fescue on puberty onset and luteal activity in beef heifers. J. Anim. Sci. 68(Suppl. 1):468. [Google Scholar]
- Fanning M. D., Spitzer J. C., Cross D. L., and Thompson F. N.. 1992. A preliminary study of growth, serum prolactin and reproductive performance of beef heifers grazing acremonium coenophialum-infected tall fescue. Theriogenology. 38:375–384. [DOI] [PubMed] [Google Scholar]
- Gray K. A., Smith T., Maltecca C., Overton P., Parish J. A., and Cassady J. P... 2011. Differences in hairy coat shedding, and effects on calf weaning weight and BCS among Angus dams. Livest. Sci. 140:68–71. doi:10.1016/j.livesci.2011.02.009 [Google Scholar]
- Hahn G. L. 1999. Dynamic responses of cattle to thermal heat loads. J. Anim. Sci. 77(Suppl. 2):10–20. [DOI] [PubMed] [Google Scholar]
- Hammond A. C., Olson T. A., Chase C. C. Jr, Bowers E. J., Randel R. D., Murphy C. N., Vogt D. W., and Tewolde A.. 1996. Heat tolerance in two tropically adapted Bos taurus breeds, senepol and romosinuano, compared with brahman, angus, and hereford cattle in florida. J. Anim. Sci. 74:295–303. [DOI] [PubMed] [Google Scholar]
- Hansen P. J. 2009. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364:3341–3350. doi:10.1098/rstb.2009.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill N. S., Thompson F. N., Stuedemann J. A., Dawe D. L., and Hiatt E. E. 3rd. 2000. Urinary alkaloid excretion as a diagnostic tool for fescue toxicosis in cattle. J. Vet. Diagn. Invest. 12:210–217. doi:10.1177/104063870001200303 [DOI] [PubMed] [Google Scholar]
- Jones K. L., King S. S., Griswold K. E., Cazac D., and Cross D. L.. 2003. Domperidone can ameliorate deleterious reproductive effects and reduced weight gain associated with fescue toxicosis in heifers. J. Anim. Sci. 81:2568–2574. doi:10.2527/2003.81102568x [DOI] [PubMed] [Google Scholar]
- Kallenbach R. L. 2015. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: coping with tall fescue toxicosis: solutions and realities. J. Anim. Sci. 93:5487–5495. doi:10.2527/jas.2015-9229 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Bussard J. R., Foote A. P., Harmon D. L., Goff B. M., Schrick F. N., and Strickland J. R... 2016. Vasoactivity and vasoconstriction changes in cattle related to time off toxic endophyte-infect tall fescue. Toxins. 8:271. doi: 10.3390/toxins8100271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. 2009. Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro. J. Anim. Sci. 87:2437–2447. doi:10.2527/jas.2008-1692 [DOI] [PubMed] [Google Scholar]
- Larson B. T., Sullivan D. M., Samford M. D., Kerley M. S., Paterson J. A., and Turner J. T.. 1994. D2 dopamine receptor response to endophyte-infected tall fescue and an antagonist in the rat. J. Anim. Sci. 72:2905–2910. [DOI] [PubMed] [Google Scholar]
- Littlejohn M. D., Henty K. M., Tiplady K., Johnson T., Harland C., Lopdell T., Sherlock R. G., Li W., Lukefahr S. D., Shanks B. C., et al. 2014. Functionally reciprocal mutations of the prolactin signalling pathway define hairy and slick cattle. Nat. Commun. 5:5861. doi:10.1038/ncomms6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S. E., Shaeffer A. D., Drewnoski M. E., Poore M. H., and Poole D. H.. 2016. Effect of protein supplementation and forage allowance on the growth and reproduction of beef heifers grazing stockpiled tall fescue. J. Anim. Sci. 94:1677–1688. doi:10.2527/jas.2015-9969 [DOI] [PubMed] [Google Scholar]
- Mahmood T., Ott R. S., Foley G. L., Zinn G. M., Schaeffer D. J., and Kesler D. J.. 1994. Growth and ovarian function of weanling and yearling beef heifers grazing endophyte-infected tall fescue pastures. Theriogenology. 42:1149–1158. [DOI] [PubMed] [Google Scholar]
- Mariasegaram M., Chase C. C. Jr, Chaparro J. X., Olson T. A., Brenneman R. A., and Niedz R. P.. 2007. The slick hair coat locus maps to chromosome 20 in senepol-derived cattle. Anim. Genet. 38:54–59. doi:10.1111/j.1365-2052.2007.01560.x [DOI] [PubMed] [Google Scholar]
- McKenzie P. P., and Erickson B. H... 1991. Effects of fungal-infested fescue on gonadotrophin secretion and folliculogenesis in beef heifers. J. Anim. Sci. 69(Suppl.):387.2005032 [Google Scholar]
- McNatty K. P., Smith D. M., Makris A., Osathanondh R., and Ryan K. J.. 1979. The microenvironment of the human antral follicle: interrelationships among the steroid levels in antral fluid, the population of granulosa cells, and the status of the oocyte in vivo and in vitro. J. Clin. Endocrinol. Metab. 49:851–860. doi:10.1210/jcem-49-6-851 [DOI] [PubMed] [Google Scholar]
- Melbin J., and Detweiler D. K... 1993. The cardiovascular system and blood flow. In: Swenson M. J., Reece W. O., editors, Dukes’ physiology of domestic animals. Ithaca, NY: Cornell University Press; pp: 64–89. [Google Scholar]
- Mercadante V. R., Fontes P. L., Ciriaco F. M., Henry D. D., Moriel P., Ealy A. D., Johnson S. E., DiLorenzo N., and Lamb G. C.. 2016. Effects of recombinant bovine somatotropin administration at breeding on cow, conceptus, and subsequent offspring performance of beef cattle. J. Anim. Sci. 94:2128–2138. doi:10.2527/jas.2015-0217 [DOI] [PubMed] [Google Scholar]
- National Research Council 1996. Nutrient requirements of beef cattle, 7th Rev. edn. Washington, DC: National Academy Press. [Google Scholar]
- Nishimura R. and Okuda K... 2010. Hypoxia is important for establishing vascularization during corpus luteum formation in cattle. J. Reprod. Dev. 56:110–116. doi:10.1262/jrd.09-162E [DOI] [PubMed] [Google Scholar]
- Niswender G. D., Schwall R. H., Fitz T. A., Farin C. E., and Sawyer H. R.. 1985. Regulation of luteal function in domestic ruminants: new concepts. Recent Prog. Horm. Res. 41:101–151. [DOI] [PubMed] [Google Scholar]
- Niu M., Ying Y., Bartell P. A., and Harvatine K. J.. 2014. The effects of feeding time on milk production, total-tract digestibility, and daily rhythms of feeding behavior and plasma metabolites and hormones in dairy cows. J. Dairy Sci. 97:7764–7776. doi:10.3168/jds.2014-8261 [DOI] [PubMed] [Google Scholar]
- Nordenson N. 2006. Gale encyclopedia of medicine. 3rd ed. Detroit, MI:Gale Group. [Google Scholar]
- Oliver J. W., Strickland J. R., Waller J. C., Fribourg H. A., Linnabary R. D., and Abney L. K.. 1998. Endophytic fungal toxin effect on adrenergic receptors in lateral saphenous veins (cranial branch) of cattle grazing tall fescue. J. Anim. Sci. 76:2853–2856. [DOI] [PubMed] [Google Scholar]
- Olson T. A., Lucena C., Chase C. C. Jr, and Hammond A. C.. 2003. Evidence of a major gene influencing hair length and heat tolerance in Bos taurus cattle. J. Anim. Sci. 81:80–90. doi:10.2527/2003.81180x [DOI] [PubMed] [Google Scholar]
- Paterson J., Forcherio C., Larson B., Samford M., and Kerley M.. 1995. The effects of fescue toxicosis on beef cattle productivity. J. Anim. Sci. 73:889–898. [DOI] [PubMed] [Google Scholar]
- Poole D. H., Lyons S. E., Poole R. K., and Poore M. H.. 2018. Ergot alkaloids induce vasoconstriction of bovine uterine and ovarian blood vessels. J. Anim. Sci. 96:4812–4822. doi:10.1093/jas/sky328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. K. and Thompson F. N. Jr. 1992. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 70:1594–1603. [DOI] [PubMed] [Google Scholar]
- Porto-Neto L. R., Bickhart D. M., Landaeta-Hernandez A. J., Utsunomiya Y. T., Pagan M., Jimenez E., Hansen P. J., Dikmen S., Schroeder S. G., Kim E. S., et al. 2018. Convergent evolution of slick coat in cattle through truncation mutations in the prolactin receptor. Front. Genet. 9:57. doi:10.3389/fgene.2018.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottinghaus G. E., Schultz L. M., Ross P. F., and Hill N. S.. 1993. An HPLC method for the detection of ergot in ground and pelleted feeds. J. Vet. Diagn. Invest. 5:242–247. doi:10.1177/104063879300500216 [DOI] [PubMed] [Google Scholar]
- SAS SAS system for mixed models. Cary, NC: SAS Institute Inc., 1996. [Google Scholar]
- Seals R. C., Schuenemann G. M., Lemaster J. W., Saxton A. M., Waller J. C., and Schrick F. N... 2005. Follicular dynamics in beef heifers consuming ergotamine tartrate as a model of endophyte-infected tall fescue consumption. JAVA. 4:97–102. [Google Scholar]
- Smith M. F., McIntush E. W., and Smith G. W.. 1994. Mechanisms associated with corpus luteum development. J. Anim. Sci. 72:1857–1872. [DOI] [PubMed] [Google Scholar]
- Strickland J. R., Looper M. L., Matthews J. C., Rosenkrans C. F. Jr., Flythe M. D., and Brown K. R.. 2011. Board-invited review: St. Anthony’s fire in livestock: causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi:10.2527/jas.2010-3478 [DOI] [PubMed] [Google Scholar]
- Turner J. W. 1980. Genetic and biological aspects of zebu adaptability. J. Anim. Sci. 50:1201–1205. [DOI] [PubMed] [Google Scholar]
- Vasconcelos J. L., Sartori R., Oliveira H. N., Guenther J. G., and Wiltbank M. C.. 2001. Reduction in size of the ovulatory follicle reduces subsequent luteal size and pregnancy rate. Theriogenology. 56:307–314. doi: 10.1016/S0093-691X(01)00565-9 [DOI] [PubMed] [Google Scholar]
- Walls J. R. and Jacobson D. R... 1970. Skin temperature and blood flow in the tail of dairy heifers administered extracts of toxic tall fescue. J. Anim. Sci. 30:420–423. [DOI] [PubMed] [Google Scholar]
- Whitman R. W. 1975. Weight change, body condition and beef-cow reproduction [PhD dissertation] Fort Collins:Colorado State University. [Google Scholar]
- Wolfenson D., Lew B. J., Thatcher W. W., Graber Y., and Meidan R.. 1997. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 47:9–19. [DOI] [PubMed] [Google Scholar]