Abstract
Tall fescue [Lolium arundinaceum (Scheyreb.) Darbysh] is the primary cool season forage grass in the Southeastern United States. Most tall fescue contains an endophytic fungus (Epichloë coenophiala) that produces ergot alkaloids and upon ingestion induces fescue toxicosis. The objective of this study was to assess how exposure to endophyte-infected (E+; 1.77 mg hd−1 d−1 ergovaline and ergovalinine) or endophyte-free (E-; 0 mg hd−1 d−1 ergovaline and ergovalinine) tall fescue seed fed during 2 stages of gestation (MID, days 35–85/LATE, days 86–133) alters placental development. Thirty-six, fescue naïve Suffolk ewes were randomly assigned to 1 of 4 fescue treatments: E−/E−, E−/E+, E+/E−, or E+/E+. Ewes were individually fed the same amount of E+ or E− seed mixed into total mixed ration during MID and LATE gestation. Terminal surgeries were conducted on day 133 of gestation. Ewes fed E+ fescue seed had elevated (P < 0.001) ergot alkaloid excretion and reduced (P < 0.001) prolactin levels during the periods when fed E+ seed. Ewes switched on day 86 from E− to E+ seed had a 4% reduction (P = 0.005) in DMI during LATE gestation, which translated to a 2% reduction (P = 0.07) in DMI overall. Average daily gain was also reduced (P = 0.049) by 64% for E−/E+ ewes during LATE gestation and tended to be reduced (P = 0.06) by 33% overall. Ewes fed E+ seed during LATE gestation exhibited a 14% and 23% reduction in uterine (P = 0.03) and placentome (P = 0.004) weights, respectively. Caruncle weights were also reduced by 28% (P = 0.003) for E−/E+ ewes compared with E−/E− and E+/E−. Ewes fed E+ seed during both MID and LATE gestation exhibited a 32% reduction in cotyledon (P = 0.01) weights, whereas ewes fed E+ seed only during MID gestation (E+/E−) had improved (P = 0.01) cotyledon weights. The percentage of type A placentomes tended to be greater (P = 0.08) for E+/E+ ewes compared with other treatments. Other placentome types (B, C, or D) did not differ (P > 0.05). Total fetal weight per ewe was reduced (P = 0.01) for ewes fed E+ seed during LATE gestation compared with E−; however, feeding E+ seed during MID gestation did not alter (P = 0.70) total fetal weight per ewe. These results suggest that exposure to ergot alkaloids during LATE (days 86–133) gestation has the greatest impact on placental development by reducing uterine and placentome weights. This, in turn, reduced total fetal weight per ewe by 15% in ewes fed E+ seed during LATE gestation (E−/E+ and E+/E+).
Keywords: ergot alkaloids, fescue toxicosis, growth restriction, intrauterine placenta, sheep
INTRODUCTION
Tall fescue [Lolium arundinaceum (Schreb.) Darbysh] is the primary cool season forage in the Southeastern United States due to its drought tolerance, disease resistance, superior growth, and overall hardiness. Such characteristics are attributed to an endophyte (Epichloë coenophiala; previously Neotyphodium coenophialum) found within the plant that produces ergot alkaloids (Young et al., 2014). Ingestion of ergot alkaloids induces fescue toxicosis, which reduces weight gain and causes reproductive problems (Schmidt et al., 1982; Hoveland et al., 1984; Porter and Thompson, 1992). Ergot alkaloids are structurally similar to biogenic amines, serotonin and dopamine, and bind to their receptors resulting in decreased serum prolactin concentrations and vasoconstriction (Aiken et al., 2007; Klotz et al., 2009; Strickland et al., 2011).
Ergovaline and ergovalinine are the predominant (84%–97%) ergot alkaloids in endophyte-infected (E+) tall fescue seed and are likely responsible for the vasoconstrictive events observed in fescue toxicosis (Lyons et al., 1986; Strickland et al., 2011; Foote et al., 2012). Ergovaline is a potent vasoconstrictor in the bovine umbilical and uterine arteries potentially reducing blood flow to developing placental tissues and fetuses (Dyer, 1993). Placental weight is highly correlated with fetal birth weight in cases of induced placental dysfunction such as hyperthermia, maternal undernutrition, and utero-placental embolism (Wallace, 1948; Alexander and Williams, 1971; Lang et al., 2000). Reduced birth weights have also been reported in offspring born to dams exposed to endophyte-infected tall fescue during gestation (Watson et al., 2004; Duckett et al., 2014a). Little research has examined how timing of ergot alkaloid exposure alters placental function and development during gestation. The objective of this study was to assess how feeding tall fescue seed containing ergot alkaloids during mid and/or late gestation alters maternal parameters and placental development.
MATERIALS AND METHODS
All animal experimental procedures were reviewed and approved by the Clemson University Institutional Animal Care and Use Committee (AUP 2014-081). This study was conducted at the Clemson University Small Ruminant Facility, Pendleton, SC.
Experimental Design
Mature Suffolk ewes, naïve to endophyte-infected tall fescue, were purchased in Northeast Iowa and transported to Clemson University 90 d prior to the start of the experiment. At the start of the experiment, ewes (n = 57; BW = 78.24 ± 9.5 kg) were weighed and evaluated for BCS (1–5; 1 = thin to 5 = fat; Sheep Prod. Industry Dev. Program 2016). Ewes were blocked by BCS and divided into 10 groups (n = 5–7/group) for estrous synchronization to facilitate a surgery schedule. Each week, one group of ewes was synchronized using an intravaginal controlled internal drug release (CIDR) insert (Eazi-Breed CIDR, Zoetis Animal Health) for 7 d. Upon CIDR removal, ewes were given prostaglandin F2α (12.5 mg i.m.; Lutalyse; Pfizer, New York, NY) and turned in with a purebred Suffolk ram. The ram was fitted with a marking harness and crayon that was changed weekly. Ewes were checked twice daily for marks to estimate breeding date and confirmed pregnant by transrectal ultrasonography on day 30 of gestation. Group 10 did not result in enough pregnant ewes for assignment to all 4 treatments and therefore only 9 groups were used for this study. Ewes (n =36; BW = 83.26 + 8.14 kg; 4/group; 9 groups) that were confirmed pregnant were randomly assigned to 1 of 4 treatments: E−/E−, E−/E+, E+/E−, or E+/E+ within group. Ewes were individually penned into stalls (1.8 × 0.5 × 0.91 m) after 0700 h and individually fed their respective treatment diet for 90 min. After individual feeding, ewes were removed from stalls and kept in a large pen (10–12 hd/pen) with ad libitum access to water and minerals (Purina Sheep Mineral, Land O’Lakes Inc., Arden Hills, MN), and with access to inside and outside areas devoid of forage or hay.
Endophyte-infected (E+; Black Magic turf-type tall fescue seed) and endophyte-free (E−; Bull turf-type tall fescue seed) seeds were grown in Oregon and obtained from Caudill Seed Warehouse (Louisville, KY). Seed samples were collected and analyzed for ergot alkaloid content according to Aiken et al. (2009). Black Magic (E+) tall fescue seed contained 4.14 μg/g DM of ergovaline and ergovalinine (Table 1). Both varieties of fescue seed tested negative for the presence of ergotamine. Tall fescue seed was fed individually to supply 1.77 mg hd−1 d−1 of ergovaline and ergovalinine for E+ and the same weight of E− tall fescue seed was fed to supply 0 mg hd−1 d−1 of ergovaline and ergovalinine. The dose level of ergovaline/ergovalinine fed daily was based on previous research in which seed was fed at a concentration of 0.8 mg/kg DM in the total mixed ration (TMR) and daily intake per ewe per day was estimated (Aiken et al., 2009; Duckett et al., 2014a). The TMR composition was formulated to minimize sorting of the seed when mixed in the TMR and incorporated 25% cottonseed hulls as a source of roughage (McCann et al., 1990). Samples of seed and TMR were subjected to nutrient analyses and rations were developed to meet NRC requirements for pregnant ewes with twins during early and late gestation (NRC, 2007). Immediately prior to feeding, fescue seed (E+ or E−) was added to individual TMR rations, mixed thoroughly, and fed according to treatment assignment. Ewes within each group were fed equal amounts of TMR and seed daily to maintain similar feed intake across all treatments. Four ewes had complications during late pregnancy and were removed from the study: one for pregnancy toxemia (E−/E−), one for preterm abortion (E−/E+), and two that presented with dead lambs at surgery (E+/E−; E+/E+).
Table 1.
Nutrient composition of the diet including total mixed ration (TMR) and endophyte-infected or endophyte-free tall fescue seed fed during MID and LATE gestation
| TMR composition | % of ration, DM basis | |
|---|---|---|
| Corn grain, cracked | 35.0 | |
| Cottonseed hulls | 25.0 | |
| Soybean hulls | 20.5 | |
| Molasses | 14.0 | |
| Soybean meal | 4.5 | |
| Limestone | 1.0 | |
| Tall fescue seed | kg hd−1 d−1 DM | Ergovaline + Ergovalinine content, mg hd−1 d−1 DM |
| Endophyte-infected (E+) seed, Tall Fescue Black Magic | 0.44 | 1.77 |
| Endophyte-free (E-) seed, Tall Fescue Bull | 0.44 | 0.00 |
| Total nutrient composition | kg hd−1 d−1 DM | |
| MID gestation, days 35–85 | ||
| TDN | 0.98 | |
| Crude protein | 0.16 | |
| LATE gestation, days 86–133 | ||
| TDN | 1.42 | |
| Crude protein | 0.22 |
Maternal Sample Collection
Blood, urine, and rectal temperature were collected at days 29, 50, 85, 105, and 133 at 0800 h. Whole blood samples were collected into serum and EDTA-coated tubes by jugular venipuncture. Plasma was obtained by centrifuging at 537 × g for 20 min at 4 °C immediately following collection and stored at −20 °C. Whole blood was allowed to clot for 30 min at room temperature and then at 4 °C overnight. Serum was collected by centrifuging for 20 min at 537 × g at 4 °C and stored at −20 °C. Urine samples were collected via transient aponea (Benech et al., 2015) and stored at −20 °C for ergot alkaloid analysis. Rectal temperatures were obtained using a rectal thermometer after sample collection.
Surgical Collection
On day 133 of gestation, ewes were transported to Godley-Snell Research Facility (14.3 km) for terminal surgery. Each ewe was given an intravenous injection of Ketamine (10 mg/kg) and Diazepam (0.25 mg/kg) upon arrival for sedation and intubation. Ewes were intubated with a 10-mm endotracheal tube and placed on 4% to 5% isoflurane with 1 to 2 L/min of O2 for induction. Upon successful anesthetization, ewes were maintained at 3% to 5% isoflurane with 1–2 L/min of O2 and placed on a ventilator at 15 to 20 breaths per minute. Corneal reflex and heart rate were monitored to confirm adequate levels of anesthesia. The abdominal area was shaved, scrubbed with chlorhexidine, and ewes were subjected to a mid-ventral laparotomy (Rueda et al., 1995). Once the uterus was exposed, blood samples from the maternal uterine artery were collected. A small incision was made in the uterine wall for the collection of amniotic fluid and to expose the umbilical cord. Blood samples were collected from the fetal umbilical vein prior to removal of each fetus. Once removed, fetal lambs were euthanized with a 3-mL intracardiac injection of Beuthanasia-D Special (Merck Animal Health, Madison, NJ) prior to sample collection. Each fetus was towel dried and fetal weight was collected. Once all fetuses were removed, ewes were euthanized with an intravenous injection of 20-mL Beuthanasia-D Special. The uterus was excised proximal to the cervix, drained of fluid, and weighed. Ewes were eviscerated for collection of organ (liver, kidney, spleen, and heart) and adipose weights. Samples of the liver were removed, frozen in liquid nitrogen, and stored at −80 °C for subsequent RNA extraction. Ewes were then weighed for collection of an eviscerated body weight (EvBW) with pelt still on.
Placentome Collection and Evaluation
Immediately following removal of the first fetus, 2 placentomes of the type B morphology (as defined below) were selected adjacent to the initial incision. These placentomes were weighed and a sample was collected for preservation in optimal cutting temperature (O.C.T) compound for later histology. The 2 placentomes were then separated into fetal cotyledon and maternal caruncle before flash freezing in liquid nitrogen for later RNA isolation. If true type B placentomes were unavailable adjacent to the initial incision, 2 placentomes closest in morphology to type B were selected instead. After removal of the uterus, the remaining placentomes were excised and any remaining endometrial and fetal membranes were removed. Classification according to morphological type (A–D) was based on procedures of Vatnick et al. (1991). Placentomes were considered to be of a type A morphology if they were round with the maternal caruncle containing the fetal cotyledon. If the fetal cotyledon had started growing up and over the top of the maternal caruncle, they were classified as type B. Type C placentomes were those that appeared more flat or discoid with the fetal cotyledon covering the top of the placentome. Placentomes were considered to be of type D morphology if the fetal cotyledon had overtaken the maternal caruncle, often causing them to invert. In cases of an intermediate type, the placentomes were categorized as the more advanced type. The classification of placetomes according to morphology was conducted by the same person each time to ensure consistency. After placetomes were typed and counted, the cotyledon and caruncle were carefully separated. Total cotyledon and caruncle weights were collected by type.
Ergot Alkaloid Excretion and Prolactin
Urinary alkaloid excretion analysis was conducted by Agrinostics Limited, Co. (Watkinsville, GA) as described previously (Hill et al., 2000). Serum prolactin levels were analyzed on all ewes over time using the RIA procedures of Bernard et al. (1993). The intra-assay variance for prolactin was 5.99% and the interassay variance was 4.47%.
Hormonal Analyses and qPCR
A subset (n = 4 per treatment; n = 16 total) of ewes closest to the mean for placental and fetal weight were selected from each treatment. Serum was analyzed for cortisol and thyroxine (T4) at days 85 and 133 using ovine-specific ELISA kits (ABNOVA, Taipai, Taiwan). The cortisol ELISA had an intra-assay variance 6.09% and a limit of detection at 0.1 ng/mL. The T4 ELISA had an intra-assay variance 9.9% and a limit of detection at 2.0 ng/mL. Triiodothyronine (T3) serum concentrations at days 85 and 133 were analyzed using a bovine-specific T3 ELISA kit (ABNOVA, Taipai, Taiwan). The T3 ELISA had an intra-assay variance 8.8% and a limit of detection at 0.2 ng/mL. The cortisol, T3, and T4 ELISA assays were validated compared with other assays (Young et al., 2003; Rumsey et al., 1999; Robbins, 1973) according to the manufacturer. Serum insulin concentrations at days 85 and 133 were analyzed using an ovine insulin ELISA (Mercodia, NC). The insulin ELISA had an intra-assay variance of 5.22% and a limit of detection at 0.025 µg/L. Plasma glucose levels at days 85 and 133 were analyzed using a colorimetric assay with Glucose Hexokinase Reagent (Pointe Scientific, Canton, MI). The glucose assay had an intra-assay variance of 8.12% and a limit of detection of 0.6 mg/dL. Glucose and insulin assays were validated for use in sheep (Volpi-Lagreca and Duckett, 2017). The concentration of insulin-like growth factor-1 (IGF-1) in serum at days 85 and 133 was analyzed using a human-based ELISA kit (Enzo Life Sciences, Farmingdale, NY). The IGF-1 ELISA had an intra-assay variance of 5.06% and a limit of detection of 50 pg/mL. The human IGF-1 ELISA assay has been validated for use in small ruminants (Castagnino et al., 2015).
Gene expression analysis was conducted using quantitative real-time RT-PCR methods according to Duckett et al (2009, 2014b). Briefly, total RNA was collected from snap-frozen tissue samples using Trizol reagent and the PureLink Mini RNA purification kit (Invitrogen, Thermo Fisher Scientific, Waltham, MA) according to manufacturer specifications. RNA yield and quality was determined using the NanoDrop 1000 spectrophotometer (Thermo Scientific, Thermo Fisher, Waltham, MA). RNA was converted to cDNA using qScript cDNA SuperMix (Quanta Bio, Beverly, MA) according to the manufacturer instructions. Real-time PCR was performed using an Eppendorf Realplex Mastercycler (Eppendorf AG, Hamburg, Germany) and Perfecta (Quanta Bio, Beverly, MA) SYBR green according to the manufacturer’s specifications. An initial hold of 2 min at 95 °C was followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s.
Primers for vascular endothelial growth factor A (VEGFA), serotonin 2A receptor (5HT2A), estrogen-related receptor gamma type 1 (ERRG1), cytochrome P450 3A4 (CYP3A4), adiponectin (ADIPOQ), glucose transporter 3 (GLUT3), tumor necrosis factor-α (TNFα), interleukin 6 (IL6), and interleukin 1-β (IL10), insulin-like growth factor 1 (IGF1), insulin-like growth factor 2 (IGF2), glucose transporter 2 (GLUT2), cytochrome P450 2E1 (CYP2E1), and CYP3A4 were designed to span exon boundaries using Primer 3 software (Table 2). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-actin (bACT), thymus cell antigen 1 (THY1), tubulin (TUB), and cyclophilin (CYC) were tested for stability in each tissue using RefFinder (Xie et al., 2012) for the selection of the most stable housekeeping gene. The most stable housekeeping gene for each tissue (GAPDH for cotyledon and CYC for liver) was used for normalization. Results are expressed as relative abundance from the control (E−/E−).
Table 2.
Primer sequences
| Gene | Forward | Reverse | Efficiency |
|---|---|---|---|
| VEGFA | tcaccaaagccagcacatag | cctcggcttgtcacattttt | 0.94 |
| 5HT2A | gcagaatgccaccaactattt | accggtacccatagaggatg | 0.88 |
| ERRG1 | agatccccagaccaagtgtg | cctcctgaagaatgccttgc | 0.92 |
| CYP3A4 | cagcctggtgctcctctatc | caaacaccctttcggtagga | 1.04 |
| CYP2E1 | gggaatcttttgcaactgga | tcctctgccagaaaactcgt | 1.09 |
| ADIPOQ | caggagtcccaggcagaaag | aagtagtacagcccggggat | 0.90 |
| GLUT3 | ctctactgctgggcttcacc | ctttgccttctcctcctcct | 1.06 |
| GLUT2 | ggttcatggtggctgagttt | tccgcaatgtactggaaaca | 0.99 |
| TNFa | gaggtgctctccaacaaagc | tggccagagactcacctctt | 1.00 |
| IL6 | cgtcgacaaaatctctgcaa | gcatccatctttttcctcca | 0.97 |
| IL1b | cagccgtgcagtcagtaaaa | gaagctcatgcagaacacca | 1.03 |
| IGF1 | ttgcacttcagaagcaatgg | ggaggatgtgactggcatct | 0.98 |
| IGF2 | accctccagtttgtctgtgg | acacatccctctcggacttg | 0.99 |
| GAPDH | gggtcatcatctctgcacct | ggtcataagtccctccacga | 1.01 |
Statistical Analysis
The univariate procedure of SAS was used to test all variables for normality. Serum prolactin concentrations were not normally distributed (Shapiro–Wilk test, P < 0.0001) and therefore were log-transformed to preserve normality. Data were analyzed in a completely randomized block design using Mixed procedure of SAS (SAS 9.3, SAS Institute Inc., Cary, NC) with tall fescue treatment (E−/E−, E−/E+, E+/E−, E+/E+) in the model. Group (block) was included as a random variable in the model. Ewe was the experimental unit for all analyses. For hormonal and excretion data, repeated measures analysis with autoregressive covariance structure was used to evaluate fescue treatment, day of collection, and interaction. Fetal lamb number (single, twin, and triplet) was included as a covariate when significant (P < 0.05). Fetal lamb sex was evaluated as a covariate but was not significant (P > 0.05) for any variable and therefore not included in the final model. Least square means were generated and separated using a protected least significant difference test. Significance was determined at P < 0.05 with trends at P < 0.10.
RESULTS AND DISCUSSION
Ergovaline Intake
Tall fescue seed was individually fed to ewes to provide a daily dose of 1.77 mg hd−1 d−1 of ergovaline and ergovalinine for E+ treatments throughout the study. The seed head of tall fescue contains the highest concentrations of ergot alkaloids in the endophyte-infected tall fescue plant and therefore was used to deliver a constant daily dose of ergovaline and ergovalinine to the ewes in this study (Rottinghaus et al., 1991). This dose level of ergovaline and ergovalinine was based on previous research in which seed was fed at a concentration of 0.8 mg/kg DM in the TMR and daily intake per ewe was estimated (Aiken et al., 2009; Duckett et al., 2014a). The same dose level of ergovaline and ergovalinine fed to E+ was maintained throughout gestation even though nutrient requirements change with stage of gestation (NRC, 2007). This translated to a concentration of 0.93 mg/kg DM in MID gestation and 0.73 mg/kg DM in LATE gestation for this study. Aiken et al. (2009) found that concentrations of 0.85 mg of ergovaline/kg DM fed to heifers reduced blood flow at the caudal artery and that concentrations as low as 0.39 mg ergovaline/kg DM would still induce a vasoconstrictive response (Aiken et al., 2007, 2009). Concentrations of 0.65 and 0.75 mg ergovaline/kg DM are also known to elicit toxicosis symptoms in steers and sheep, respectively (Hill et al., 1994; Looper et al., 2007). Moreover, concentrations of 0.24 (chemical seed head suppression) and 0.56 mg/kg DM ergovaline both induced vasoconstriction of biopsied lateral saphenous vein in steers (Klotz et al., 2007, 2018). In sheep, a threshold level of 500 to 800 ppb (0.5–0.8 mg/kg DM) was reported for the induction of symptoms associated with fescue toxicosis (Craig et al., 2014). Therefore, the levels of ergovaline fed during this study (0.93 mg/kg in MID or 0.73 mg/kg in LATE) are within the range (0.39–0.85) where fescue toxicosis would be observed. If calculated on a BW basis, the intake levels ranged from 17.9 to 21.2 μg ergovaline and ergovalinine/kg BW in E+ ewes at the start and end of the study, respectively.
Maternal characteristics of ewes by fescue treatment are presented in Table 3. There were no differences (P > 0.18) in ewe age, BCS, and day of gestation at surgery due to fescue treatment. Fetal number and fetal sex also did not differ (P > 0.44) between fescue treatments. Overall lambing rate was 180% in this experiment, which is within the normal range for mature Suffolk ewes (Notter, 2000). Maternal BW throughout gestation was not affected (P > 0.40) by fescue seed treatment. Dry matter intake from days 29 to 85 did not differ (P = 0.32) by fescue seed treatment or stage of gestation. Ewes that were switched from E− to E+ treatment (E−/E+) at day 86 had decreased (P = 0.005) DMI from days 86 to 133 compared with other treatments (E−/E−, E−/E+, or E+/E+). This translated to a trend (P = 0.07) for reduced overall DMI from days 29 to 133 for E−/E+ fescue treatment compared with E−/E− or E+/E−. Average daily gain (ADG) did not differ (P = 0.29) by fescue treatment from days 29 to 85. Ewes fed E−/E+ seed exhibited less (P = 0.049) ADG during LATE gestation compared with ewes fed E−/E− or E+/E−. Overall ADG tended (P = 0.06) to be reduced for E−/E+ fed ewes compared with E+/E− ewes. Reductions in DMI, ADG, and overall animal performance are known to correspond with exposure to endophyte-infected tall fescue (Paterson et al., 1995; Matthews et al., 2005). Lambs fed E+ tall fescue seed exhibited reductions in DMI compared with their E− counterparts, but DMI improved when sainfoin, a tannin-rich legume, was supplemented to E+ lambs (Villalba et al., 2016). Additionally, lactating ewes fed endophyte-infected tall fescue hay had reduced DMI and lighter BW compared with ewes fed endophyte-free tall fescue hay (Zbib et al., 2014). It is important to note that ewes were individually fed the same amount of TMR and seed daily throughout this study in order to maintain similar nutrient intakes. Therefore, the 4% reduction in DMI for ewes switched from E− to E+ during LATE gestation (E−/E+) was a result of greater feed refusal.
Table 3.
Maternal body weight, average daily gain, and DMI of ewes fed endophyte-infected (E+) or endophyte-free (E−) seed during MID (days 38–85)/LATE (days 86–133) gestation
| Fescue treatment | E−/E− | E+/E− | E−/E+ | E+/E+ | SEM |
|---|---|---|---|---|---|
| Ewe (n) | 8 | 8 | 8 | 8 | |
| Ewe age, yr | 4.5 | 4.9 | 5.2 | 4.8 | 0.30 |
| Ewe BCS1 | 2.8 | 3.2 | 2.8 | 2.99 | 0.26 |
| Day of gestation | 132.2 | 132.4 | 133.3 | 133.3 | 0.45 |
| Fetal number/ewe | 1.75 | 1.88 | 1.75 | 2.00 | 0.20 |
| Fetal sex (1 = male) | 1.46 | 1.40 | 1.54 | 1.64 | 0.13 |
| Ewe BW, kg | |||||
| Day 29 | 85.62 | 81.53 | 81.70 | 80.94 | 3.59 |
| Day 50 | 81.22 | 78.69 | 79.35 | 78.72 | 3.30 |
| Day 85 | 91.25 | 90.56 | 89.94 | 94.35 | 4.43 |
| Day 105 | 96.50 | 96.73 | 91.68 | 91.31 | 3.92 |
| Day 133 | 100.23 | 101.96 | 93.61 | 95.34 | 4.04 |
| DMI, kg/d | |||||
| Days 29–85 | 1.91 | 1.91 | 1.91 | 1.88 | 0.013 |
| Days 86–133 | 2.46a | 2.46a | 2.34b | 2.43a | 0.025 |
| Overall, days 29–133 | 2.18c | 2.17c | 2.13d | 2.15cd | 0.014 |
| ADG, kg/d | |||||
| Days 29–85 | 0.100 | 0.161 | 0.147 | 0.228 | 0.044 |
| Days 86–133 | 0.187a | 0.237a | 0.076b | 0.175ab | 0.040 |
| Overall, days 29–133 | 0.140d | 0.196c | 0.114d | 0.159cd | 0.021 |
1BCS scores (1–5; 5 = heaviest condition; 1 = thinnest condition).
abMeans in the same row with uncommon superscripts differ (P < 0.05).
cdMeans in the same row with uncommon superscripts differ (P < 0.10).
Urinary Ergot Alkaloid Excretion
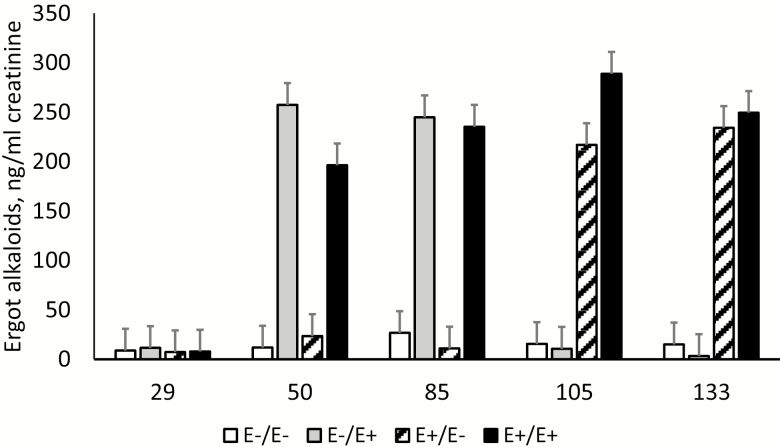
The interaction between tall fescue seed treatment by day of gestation on urinary ergot alkaloid excretion was significant (P < 0.001; Figure 1). On day 29 (pretreatment), there were no differences (P > 0.05) between fescue treatments in ergot alkaloid excretion. Ewes consuming E+ tall fescue seed (E+/E− and E+/E+) during MID (days 35–85) gestation had elevated (P < 0.001) urinary ergot alkaloid excretion compared with ewes fed E− tall fescue seed (E−/E+ and E−/E−). On days 105 and 133, ewes fed E+ tall fescue seed (E−/E+ and E+/E+) during LATE (days 86–133) gestation had elevated (P < 0.001) ergot alkaloid excretion compared with those fed E− tall fescue seed (E+/E− and E−/E−) during that time. Urine, bile, and feces are the primary routes for the elimination of ergot alkaloids in livestock (Stuedemann et al., 1998; Schultz et al., 2006). Stuedemann et al. (1998) found approximately 96% of ergopeptine alkaloids consumed by cattle grazing pastures contaminated with endophyte-infected tall fescue are excreted in the urine. It was also determined that urinary excretion of ergot alkaloids can be used as a measurement of exposure to endophyte-infected tall fescue and to assess fescue toxicosis (Hill et al., 2000). In steers, the excretion of alkaloids in the urine has been shown to occur within 12 h of introducing endophyte-infected tall fescue into the diet (Stuedemann et al., 1998). The results presented here support previous conclusions that urinary ergot alkaloid excretion is an effective and dynamic way to monitor ergot alkaloid consumption and to affirm treatment efficacy.
Figure 1.
Urinary ergot alkaloid excretion over time in ewes fed endophyte-infected (E+) or endophyte-free (E−) seed during days 35 to 85 (MID)/days 86 to 133 (LATE) of gestation.
Rectal Temperature
Rectal temperature was not affected (P > 0.05) by fescue seed treatment. Research has drawn mixed conclusions about the impact ergot alkaloids have on the ability to regulate body temperature. Ergot alkaloid consumption can interfere with the regulation of body temperature if the resulting vasoconstriction limits the release of heat superficially at the skin’s surface during hot summer months (Rhodes et al., 1991; Aldrich et al., 1993a). The current study was conducted during the winter and early spring months (November to March) in South Carolina when daytime temperatures were below 26 °C and heat stress was not present in the ewes.
Serum Hormone Concentrations
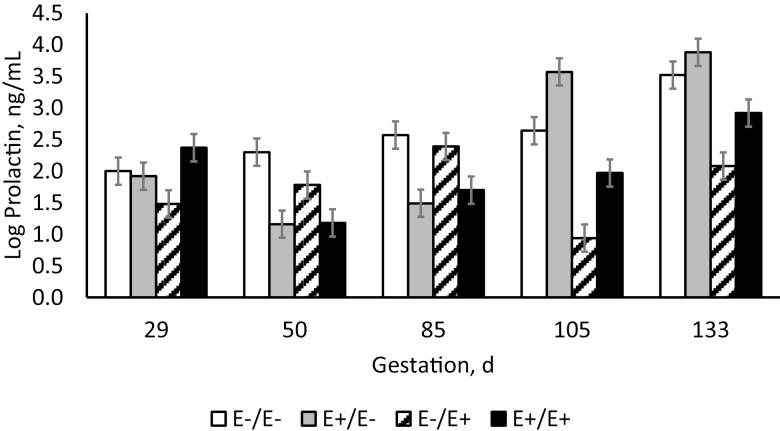
The interaction between tall fescue seed treatment by day of gestation on log-transformed serum prolactin concentration differed (P < 0.001; Figure 2). On day 29, prior to the start of treatment, prolactin concentrations were reduced (P = 0.004) for E−/E+ ewes compared with E+/E+ ewes. On days 50 and 85, ewes fed E+ tall fescue seed during that time period (E+/E− and E+/E+) had reduced (P < 0.001) serum prolactin levels compared with ewes fed E− tall fescue seed (E−/E− and E−/E+). On days 105 and 133, ewes fed E+ tall fescue seed during that time period (E−/E+ and E+/E+) had reduced (P < 0.001) serum prolactin levels compared with ewes fed E− tall fescue seed (E−/E− and E+/E−). For ewes fed E− seed during LATE gestation, prolactin concentrations were elevated (P < 0.04) at days 105 and 133 compared with concentrations at day 29. In contrast, prolactin concentrations for ewes fed E+ seed during LATE gestation did not differ (P > 0.05) on days 105 and 133 when compared with concentrations at day 29. Ewes switched from E+ to E− seed at day 86 had prolactin levels that were elevated (P = 0.004) at day 105 compared with E−/E− ewes indicating a compensatory response after the removal of E+ fescue seed. Prolactin concentrations are known to gradually climb throughout gestation with the highest levels closest to parturition (Chamley et al., 1973). Reductions in serum prolactin concentrations are a classic response to ergot alkaloid exposure and have been consistently reported in cattle, horses, and sheep (McCann et al., 1992; Emile et al., 2000; Parish et al., 2003; Koontz et al., 2012; Stowe et al., 2013). This response is due to the ability of ergot alkaloids to interact with D2-dopamine receptors and suppress prolactin production (Sibley and Creese, 1983; Klotz, 2015). Prolactin is crucial in mammary development, milk production, and milk letdown during late gestation and after parturition (Hooley et al., 1978). Exposure to E+ fescue seed depressed prolactin levels which may interfere with mammary development and milk production if levels do not recover prior to parturition (Akers et al., 1981). In the current study, prolactin depression at day 130 appears greatest for ewes fed E+ fescue seed only during LATE gestation (E−/E+) when compared with ewes fed E+ throughout both MID and LATE gestation (E+/E+).
Figure 2.
Log-transformed serum prolactin concentrations over time in ewes fed endophyte-infected (E+) or endophyte-free (E−) seed during days 35 to 85 (MID)/days 86 to 133 (LATE) gestation.
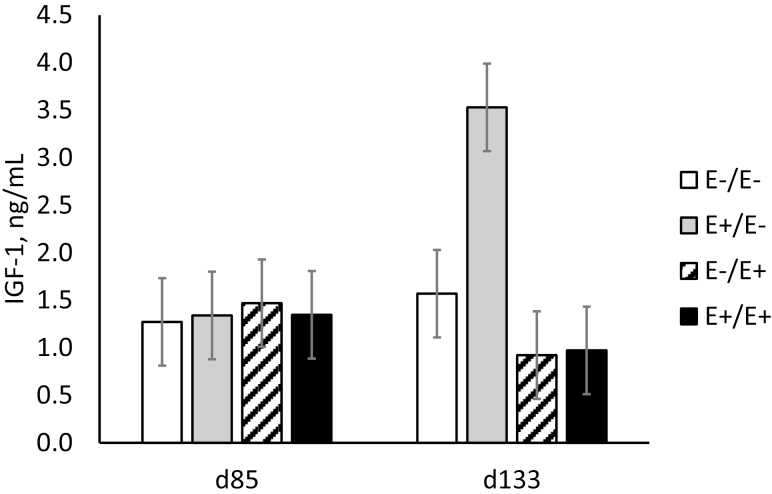
There were no differences (P > 0.05) in cortisol, triiodothyronine (T3), thyroxine (T4), insulin, or glucose concentration by fescue seed treatment at the end of MID gestation (day 85) or the end of LATE gestation (day 133; data not shown). Similarly, plasma cortisol, T3, and T4 concentrations were not altered when E+ fescue seed was fed to heifers (Aldrich et al., 1993b). Insulin-like growth factor 1 concentrations did differ (P = 0.02) between fescue seed treatments at day 133 (Figure 3). Ewes that switched from E+ to E− fescue seed on day 86 (E+/E−) had elevated IGF1 concentrations at day 133 compared with all other treatments. Reductions in IGF1 concentrations are associated with glucose insufficiency in ewes, which results in a shift in nutrient partitioning (Tygesen et al., 2008). Although intact IGF1 does not cross the placenta, increases in IGF1 concentrations may stimulate placental growth through IGF1 receptors (Browne and Thorburn, 1989; Reynolds et al., 1997). Such a compensatory response in IGF1 levels after switching from E+ to E− treatment may be an attempt to stimulate placental development and rescue fetal growth.
Figure 3.
Insulin-like growth factor 1 (IGF1) serum concentrations for ewes fed endophyte-infected (E+) or endophyte-free (E−) seed during days 35 to 85 (MID)/days 86 to 133 (LATE) of gestation.
Placental Measures
Empty uterine, placentome, cotyledon, and caruncle weights are presented in Table 4. Empty uterine and total placentome weights were lighter (P < 0.02) in ewes fed fescue seed during late gestation (E−/E+ and E+/E+) compared with ewes fed E− fescue seed (E−/E−, E+/E−). Total caruncle weights were increased (P < 0.04) for ewes fed E+/E− compared with ewes fed E+ during late gestation (E−/E+ and E+/E+). Caruncle weights were also increased (P = 0.003) for E−/E− ewes compared with E−/E+. Total cotyledon weights were reduced (P = 0.02) for E+/E+ ewes compared with E−/E− ewes. Total placentome number did not differ (P = 0.52) between fescue seed treatments. Caruncle and cotyledon weights by placentome type did not differ (P > 0.11) among fescue seed treatments. Total number of each placentome type also did not differ (P > 0.12) among fescue seed treatments. The percentage of A placentomes tended (P = 0.09) to be greater for E+/E+ ewes compared with all other treatments (E−/E−, E−/E+, or E+/E−). The percentage of other placentome types (B, C, or D) did not differ (P > 0.40) by fescue seed treatment. The development of the sheep placenta occurs rapidly from approximately days 40 to 80 of gestation with a shift from growth to placental remodeling past day 80 (Ehrhardt and Bell, 1995). The remodeling phase focuses on increasing vascularity and overall functional capacity of the placenta during the last half of gestation to support rapid fetal growth as parturition approaches (Borowicz et al., 2007). It has been hypothesized that the progression from the less advanced morphological type A placentome to the more advanced types B, C, and D may be driven by compromised intrauterine conditions, especially during late gestation, and may be a compensatory response to increase vascularity and nutrient exchange in an attempt to rescue the growing fetus (Hoet and Hanson, 1999; Vonnahme et al., 2006). Morphology progression has also been seen in cases of undernutrition, hypoxia, and carunclectomy (Penninga and Longo, 1998; Hoet and Hanson, 1999; Osgerby et al., 2004; Zhang et al., 2016). However, even in verified cases of adverse intrauterine conditions, advancement in morphology is not always observed. In some cases, placentome size appears to be a better indicator of vascular function compared with morphology alone; however, it is important to note that progression in placentome type is positively correlated with placentome size and weight (Vonnahme et al., 2008). Ewes fed E+/E+ fescue seed tended to have a greater percentage of type A placentomes compared with the other fescue treatments. This, along with the overall reductions in total placental mass, suggests that exposure to E+ fescue impairs vascular development of the placenta, especially during late gestation when placental remodeling is occurring.
Table 4.
Effect of feeding E+ or E− tall fescue seed on uterine weight, placentome weight, and placentome numbers during MID (days 35–85)/LATE (days 86–133) gestation
| Fescue treatment | E−/E− | E+/E− | E−/E+ | E+/E+ | SEM |
|---|---|---|---|---|---|
| Empty uterine weight, g | 2748.9a | 2726.6a | 2306.6b | 2386.8b | 118.3 |
| Total placentome weight, g | 844.32a | 908.55a | 684.34b | 657.21b | 49.45 |
| Total caruncle weight, g | 207.11ab | 218.47a | 157.76c | 186.92bc | 10.46 |
| Total cotyledon weight, g | 636.66ab | 690.27a | 526.02bc | 471.21c | 46.68 |
| Total placentome number | 98.09 | 106.47 | 92.22 | 97.97 | 6.70 |
| Type A | |||||
| Cotyledon wt, g | 97.43 | 83.19 | 87.84 | 174.79 | 37.34 |
| Caruncle wt, g | 45.49 | 35.24 | 38.67 | 77.32 | 16.74 |
| Number | 20.6 | 14.9 | 20.4 | 39.0 | 7.28 |
| Number, % of total | 19.7d | 15.74d | 17.7d | 40.0c | 7.71 |
| Type B | |||||
| Cotyledon wt, g | 350.36 | 305.97 | 189.87 | 210.56 | 51.57 |
| Caruncle wt, g | 116.27 | 104.49 | 66.44 | 79.47 | 16.16 |
| Number | 56.1 | 55.4 | 35.9 | 45.2 | 8.88 |
| Number, % of total | 55.7 | 44.5 | 38.0 | 43.0 | 8.26 |
| Type C | |||||
| Cotyledon wt,g | 107.37 | 202.01 | 188.67 | 85.29 | 67.68 |
| Caruncle wt, g | 29.48 | 58.85 | 43.37 | 24.50 | 17.20 |
| Number | 14.4 | 28.1 | 27.8 | 11.8 | 9.40 |
| Number, % of total | 14.4 | 27.2 | 34.1 | 15.0 | 9.59 |
| Type D | |||||
| Cotyledon wt, g | 61.59 | 105.78 | 39.74 | 33.73 | 43.64 |
| Caruncle wt, g | 15.86 | 19.89 | 9.29 | 5.64 | 9.51 |
| Number | 5.4 | 8.6 | 6.5 | 4.6 | 5.02 |
| Number, % of total | 8.2 | 13.2 | 8.3 | 5.3 | 6.47 |
abMeans in the same row with uncommon superscripts differ (P < 0.05).
cdMeans in the same row with uncommon superscripts differ (P < 0.10).
Although research examining the impact of ergot alkaloids on placental development is limited, altered placental development and fetal growth have been well documented in cases of hyperthermia which results in severe fetal growth restriction (Bell et al., 1987; Bell et al., 1989; Thureen et al., 1992; Arroyo et al., 2006). Growth restriction reported in cases of hyperthermia is similar in severity to that previously associated with the consumption of fescue seed during gestation (Duckett et al., 2014a). Reductions in uterine blood flow often accompany hyperthermia and have been reported in sheep and cows (Reynolds et al., 1985; Bell et al., 1987; Dreiling et al., 1991). It is hypothesized that a vasopressin-mediated response to increased core body temperature may work to redirect blood flow away from the uterus, thus resulting in reduced placental growth and development (Dreiling et al., 1991). Exposure to ergot alkaloids may result in similar reductions in blood flow to the placenta during development due to their vasoconstrictive nature. Doppler ultrasonography has shown reduced caudal artery area and blood flow rates in heifers consuming endophyte-infected tall fescue seed (Aiken et al., 2007; Aiken et al., 2013). The vasoconstrictive effects were not alleviated after 30 d on an endophyte-free diet. Additionally, ergot alkaloids have been known to cause contractile responses in a variety of vessels and species such as the lateral saphenous veins in cattle, the distal palmar artery of horses, the carotid and auricular arteries in goats, tail arteries in rats, and iliac arteries of guinea pigs (Schoning et al., 2001; Klot et al., 2007; McDowell et al., 2013; Aiken and Flythe, 2014). It was also documented that heifers exposed to E+ fescue exhibited reductions in the uterine vein and artery (and ovarian vein and artery) during the later stages of the estrous cycle (Poole et al., 2018). This further supported the conclusion made by Dyer (1993) that ergovaline, specifically, is a potent vasoconstrictor in the bovine umbilical and uterine arteries and has the potential to directly impair blood flow to the uterus and developing placenta during gestation. Restrictions in uterine blood flow can reduce oxygen content within the placenta, disrupt normal vascular progression, and stunt overall development (Yates et al., 2011). The current data suggests that exposure to E+ fescue seed during LATE gestation stunts development of the placenta as indicated by a 23% reduction in overall placental mass compared with E− treatment. It is hypothesized that this stunted development is likely due to the vasoconstrictive effects that ergot alkaloids exert on uterine and placental blood flow. The effect on placental growth and development seems especially crucial during the second half of gestation when placental remodeling occurs.
Placental Efficiency
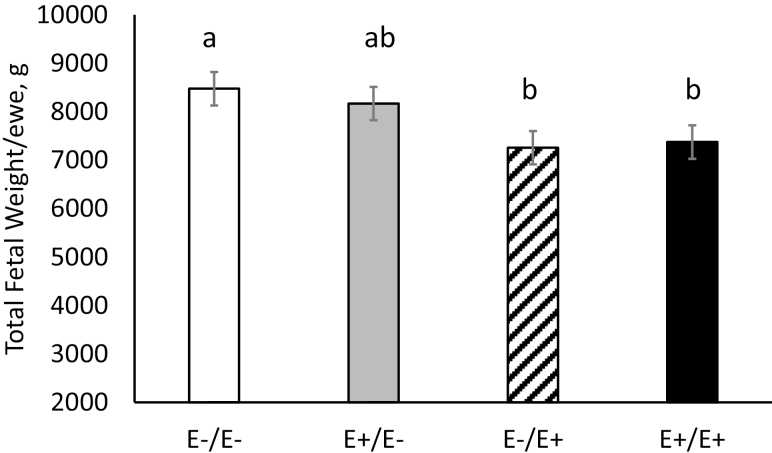
Total fetal weight per ewe by fescue seed treatment is shown in Figure 4. Feeding E+ fescue seed during LATE gestation (days 86–133) reduced (P = 0.01) total fetal weight per ewe by 15% compared with E− fescue during the same stage of gestation. Similarly, Duckett et al. (2014a) reported a reduction in birth weight of lambs at parturition when ewes were fed E+ tall fescue seed; however, the magnitude of the reduction was greater (36%) in the previous study. There were several differences between the studies including ewe breed type (Southdown vs. Suffolk), feeding system (pen vs. individual), and gestational age when fetal weight was obtained (birth vs. day 133 of gestation). Calves born to cows that grazed E+ tall fescue during gestation had reduced birth and weaning weights (Watson et al., 2004). In contrast, 2 other studies did not find differences in calf birth weight when cows grazed E+ tall fescue during gestation compared with calves born to cows grazing nontoxic, novel endophyte-infected tall fescue pastures (Caldwell et al., 2013; Shoup et al., 2016). Over 80% of fetal growth occurs in twin-bearing ewes during the last trimester of gestation which may explain why ergot alkaloid exposure during late gestation appears to have the greatest impact on fetal growth (Rattray et al., 1974). The previous study conducted by Duckett et al. (2014a) also revealed a difference in gestation length based on fescue seed treatment. Therefore, the present study was conducted to compare fetal weight and composition based on fescue treatment at the same gestational age.
Figure 4.
Total fetal weight (g) produced per ewe at day 133 of gestation for ewes fed endophyte-infected (E+) or endophyte-free (E−) seed during MID (days 35–85)/LATE (days 86–133) gestation. abMeans differ (P < 0.05) by fescue treatment.
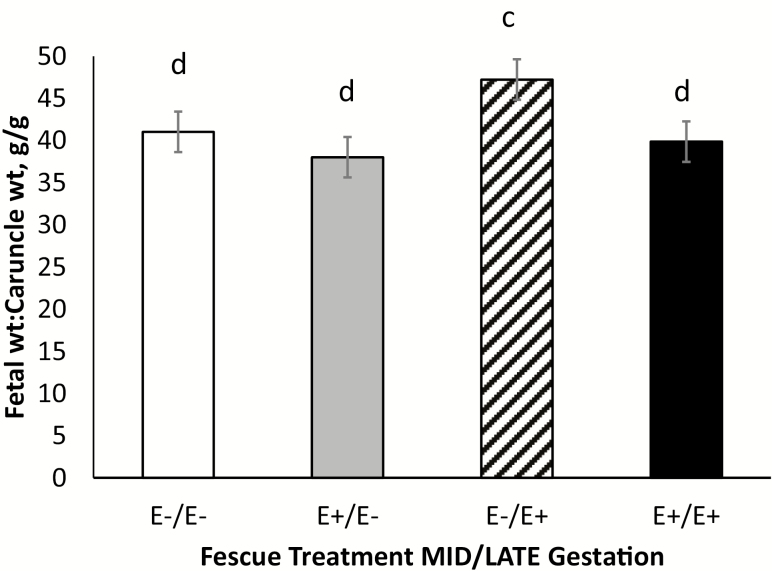
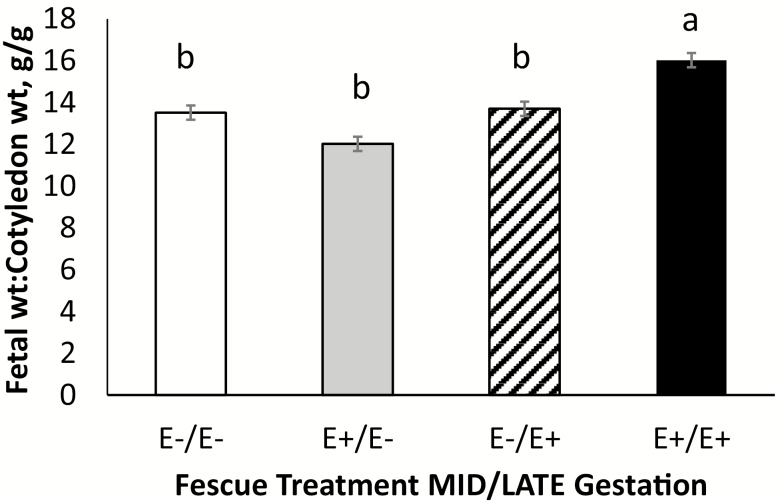
The ratio of total fetal weight to caruncle weight and total fetal weight to cotyledon weight is presented in Figures 5 and 6, respectively. Ewes fed E−/E+ fescue seed tended to have greater (P = 0.07) total fetal weight to caruncle weight compared with those fed E+ fescue seed during MID gestation (E+/E− and E+/E+). Ewes fed E+/E+ fescue seed had greater (P = 0.01) total fetal weight to cotyledon weight compared with the other fescue treatments. Because placental size directly affects nutrient transport potential, placental mass is directly related to fetal growth in utero. Placental efficiency is defined as the birthweight:placental weight ratio and is indicative of how well development and function of the placenta adapted to meet the nutrient demands of the fetus (Wilson and Ford, 2001; Fowden et al., 2009). It has been established that the sheep placenta undergoes an exponential increase in overall weight until approximately day 90 with the most rapid growth occurring between days 50 and 60 (Ehrhardt and Bell, 1995). After this, the weight of the placenta actually declines overtime (Alexander, 1964). Vascular growth in the caruncle appears to be exponential until approximately day 40 when it levels off (Stegeman, 1972). In contrast, the vascular growth in the cotyledon remains consistent until mid-gestation before rapidly increasing thereafter. When fetal weight production is normalized to cotyledon weight, ewes on E+/E+ treatment exhibited the greatest fetal:cotyledon weight ratio. This was due to a reduction in both fetal weight and cotyledon weights for E+/E+ ewes. This indicates that ewes fed E+ fescue seed during MID and LATE gestation may have resulted in placental adaptation to regulate nutrient transfer efficiency in order to compensate for reductions in overall placental mass (Hayward et al., 2016). An increase in the fetal:placental weight ratio has previously been indicative of fetal and placental adaptation that supports and maintains fetal growth during adverse intrauterine conditions (Fowden et al., 2008).
Figure 5.
Total fetal weight (g) to caruncle weight (g) ratio for ewes fed endophyte-infected (E+) fescue seed or endophyte-free (E−) fescue seed treatment during MID (days 35–85)/LATE (days 86–133) gestation. cdMeans differ (P < 0.10) by fescue treatment.
Figure 6.
Total fetal weight (g) to cotyledon weight (g) ratio for ewes fed endophyte-infected (E+) seed or endophyte-free (E−) seed treatment during MID (days 35–85) and LATE (days 86–133) gestation. abMeans differ (P < 0.05) by fescue treatment.
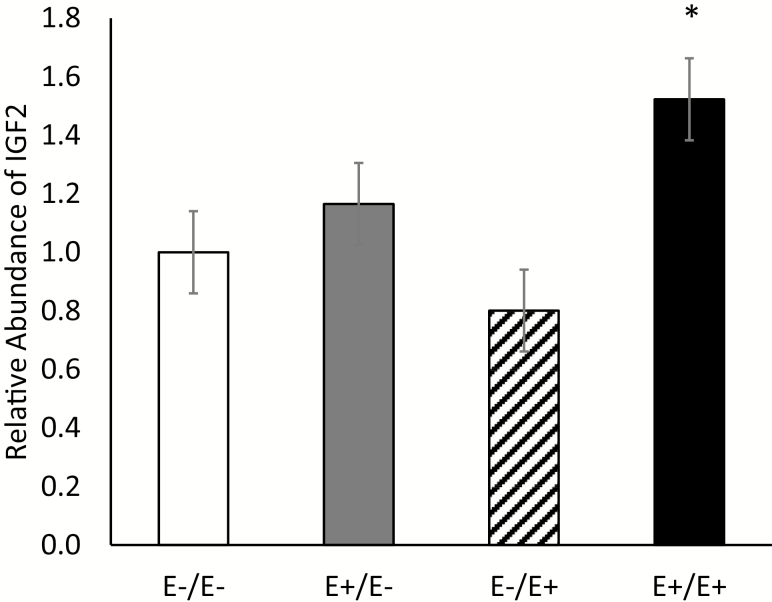
Several genes involved in vascular development, growth regulation, and energy metabolism were analyzed to determine differences due to fescue seed treatment. VEGFA, a growth factor involved in angiogenesis and vascular development, is well documented in placental development (Carr et al., 2016). Ergovaline is a known agonist of the 5HT2A G protein-coupled receptor in the serotonin receptor family (Klotz et al., 2012). The ERRG1 receptor plays a role in energy metabolism of the placenta which is impaired in causes of IUGR (Poidatz et al., 2012). CYP3A4 is a cytochrome P450 that has been identified in the xenobiotic metabolism of several ergot alkaloids (Strickland et al., 2011). ADIPOQ is an adipocytokine associated with glucose regulation and fatty acid breakdown and is secreted in the human placenta (Chen et al., 2006). GLUT3 is a glucose transporter that is altered in cases of human IUGR (Janzen et al., 2013). IGF1 and IGF2 are known to regulate fetal and placental growth during gestation and have been correlated with birth weight in several species (Lee et al., 1993; Kind et al., 1995; Thakur et al., 2000). IGF2 abundance differed (P = 0.02) based on fescue seed treatment with ewes fed E+ fescue seed during LATE gestation exhibiting relative abundance levels greater than that of E− ewes (Figure 7). Fescue seed treatment did not alter (P > 0.05) gene expression for any other genes tested in cotyledon tissue (data not shown). IGFs are crucial growth factors for fetal development and are synthesized in both the placenta and the fetus (Borowicz et al., 2007; Hiden et al., 2009). Previous research has shown that both IGF1 and IGF2 are present in placental tissues but IGF2 appears to be the predominant IGF present in the fetal mesoderm (Wathes et al., 1998; Han and Carter, 2000). The same is true for cotyledon tissue in the present study wherein IGF2 was found to be in greater abundance than IGF1 at day 133 of gestation. IGF2 may work as a sensor to drive changes in the placenta during adverse conditions such as reduced uteroplacental blood flow or heat stress (Sferruzzi-Perri, 2017). Zhang et al. (2016) reported similar findings as those discussed here after the removal of maternal caruncles in ewes prior to pregnancy. Placental IGF2 abundance was increased (along with placental capillary volume and surface area) which indicates a level of placental adaptation, though placental mass and fetal weight were still reduced (Zhang et al., 2016). Exposure to E+ fescue seed treatment during both MID and LATE gestation (E+/E+) resulted in greater cotyledon IGF2 abundance which may indicate an attempt at placental adaptation even though overall placental mass and fetal weights were still reduced compared with E−/E− ewes.
Figure 7.
Insulin-like growth factor 2 (IGF2) mRNA abundance levels in cotyledon tissue for ewes fed endophyte-infected (E+) or endophyte-free (E−) seed during MID (days 35–85)/LATE (days 86–133) of gestation. *Denotes treatment differs from E−/E− (P < 0.04).
Ewe Organ and Tissue Weights
Ewe organs and tissue weights are presented in Table 5. There were no differences (P > 0.05) in BW, eviscerated BW, 12th rib fat thickness, 12th rib longissimus muscle depth, or percent kidney fat between fescue seed treatments. Organ weights also did not differ (P > 0.05) between fescue seed treatments. The liver is the primary site of toxicant metabolism and previous research suggests that it plays a role in the metabolism of ergot alkaloids (Peyronneau et al., 1994; Moubarak and Rosenkrans, 2000). In the case of poultry, feeding high levels of ergot alkaloids increased liver weights and resulted in hepatotoxic effects (Danicke, 2015, 2016, 2017). Gene expression for CYP3A4, CYP2E1, IL-6, and GLUT2 was examined in ewe liver tissue. The CYP3A subfamily, specifically CYP3A4, has been reported in previous literature to be involved in the metabolism of ergot alkaloids (Moubarak and Rosenkrans, 2000). CYP2E1, another cytochrome P450 member, is known to regulate toxins in the maternal liver that can induce IUGR (Wang et al., 2009). An up-regulation of inflammatory cytokines, like IL-6, has been reported to cause the down regulation of CYP P450 enzymes (Yang et al., 2012). The GLUT2 transporter is responsible for the bidirectional movement of glucose by hepatocyte cells and differences in the expression of GLUT2 could indicate abnormalities in hepatic glucose transport (Bell et al., 1990). Exposure to E+ fescue seed during MID and/or LATE gestation did not alter (P > 0.05) abundance of genes tested in the liver but CYP2E1 was found to be in a greater relative abundance than CYP3A4. The CYP3A family of is suspected to be the primary group of xenobiotic enzymes responsible for metabolism of ergot alkaloids. However, Szotakova et al. (2004) showed that there is a significant species to species variation in cytochrome p450 enzymes when it comes to processing xenobiotic compounds. Previously, cows grazing endophyte-infected tall fescue pastures after heavy nitrogen fertilization presented with necrotic lesions in the perirenal, pelvic, abdominal, and abomasal fat tissues at slaughter (Rumsey et al., 1979; Studemann et al., 1985). There was no evidence of necrotic fat lesions in the perirenal or mesenteric fat depots excised from any of the ewes in the present study.
Table 5.
Ewe tissue and organ weights at day 133 of gestation by endophyte-infected (E+) or endophyte-free (E−) treatment during days 86 to 133 (LATE) of gestation
| Fescue treatment | E−/E− | E+/E− | E−/E+ | E+/E+ | SEM |
|---|---|---|---|---|---|
| BW at surgery, kg | 97.61 | 101.96 | 92.93 | 96.25 | 3.49 |
| Eviscerated BW, kg | 60.06 | 60.69 | 57.84 | 55.35 | 3.36 |
| 12th rib fat thickness, cm | 0.51 | 0.65 | 0.36 | 0.45 | 0.10 |
| Longissimus muscle depth, cm | 3.63 | 3.85 | 3.74 | 3.37 | 0.26 |
| KPH fat, g | 892.1 | 848.4 | 700.6 | 667.9 | 133.0 |
| KPH, % BW | 0.91 | 0.82 | 0.73 | 0.65 | 0.11 |
| Organ Weights, g | |||||
| Liver | 1174.7 | 1169.0 | 1047.0 | 1112.3 | 50.4 |
| Kidneys | 178.47 | 161.58 | 164.59 | 156.90 | 6.85 |
| Heart | 366.55 | 356.68 | 384.71 | 375.01 | 12.57 |
| Spleen | 468.87 | 432.62 | 373.76 | 459.57 | 64.66 |
| Organ Weights, % of BW | |||||
| Liver | 1.20 | 1.14 | 1.13 | 1.17 | 0.049 |
| Kidneys | 0.18 | 0.16 | 0.18 | 0.17 | 0.010 |
| Heart | 0.38cd | 0.35d | 0.42c | 0.40cd | 0.018 |
| Spleen | 0.47 | 0.42 | 0.40 | 0.47 | 0.060 |
As a percentage of BW, heart weight tended (P = 0.08) to be heavier for ewes on E+ treatment during LATE gestation. Several drugs with an agonist action on 5HT2 receptors, including several derived from ergot alkaloids, are known to increase valvular heart disease (Andrejak and Tribouilloy, 2013). Valvular heart disease and other chronic diseases of the heart have been known to increase overall heart weight (Kumar et al., 2014). Additionally, ergot alkaloids have been known to cause hypertension through vascular smooth muscle constriction that may lead to myocardial infarctions in extreme cases (Joyce and Gubbay, 1982). Intravenous infusions of ergotamine tartrate or ergonovine maleate in heifers resulted in increased diastolic and mean arterial pressures (Browning and Leite-Browning, 1997). Ergotamine tartrate also increased systolic pressure and lowered heart rate. Aiken et al. (2007) reported decreased systolic pressure, diastolic pressure, and heart rate in heifers consuming E+ fescue. Steers on E+ fescue seed experienced reduced heart rate and systolic pressures while also experiencing increased diastolic pressures and respiration rates (Eisemann et al., 2014). They also reported a reduced heart rate and systolic pressures, and greater diastolic pressures and respiration rates. Weiner (1980) suggested that blood pressure increase would be related to the peripheral vasoconstriction.
CONCLUSION
Feeding endophyte-infected tall fescue seed during MID and LATE gestation induces fescue toxicosis in pregnant ewes as indicated by suppressed prolactin levels and elevated excretion of urinary ergot alkaloids. Our results indicate that exposure to ergot alkaloids during LATE (day 86–133) gestation has the greatest impact on placental development by lowering uterine and placentome weights, which reduced total fetal weight per ewe by 15% in ewes fed E+ seed during LATE gestation (E−/E+ and E+/E+). Exposure to E+ fescue seed during LATE gestation appears to stunt development of the placenta, likely due to the vasoconstrictive effects of ergot alkaloids, during an especially crucial stage of gestation when placental remodeling and the majority of fetal growth occurs. Additional research is needed to assess specific changes in placental structures or nutrient transporters that may be involved in the negative effects of ergot alkaloids on placental development and fetal growth during late gestation.
Footnotes
Technical Contribution No. 6579 of the Clemson University Experiment Station. This research was supported by USDA Agriculture and Food Research Initiative Competitive Grant no. 2015-67015-23218. Appreciation is expressed to M. C. Miller for animal handling, R. L. Smith for laboratory assistance, R. V. Anthony for surgical procedures, and the AVS 4220 Fetal Fescue Research Team for assistance.
LITERATURE CITED
- Aiken G. E., and Flythe M. D.. 2014. Vasoconstrictive responses by the carotid and auricular arteries in goats to ergot alkaloid exposure. Front. Chem. 2:101. doi:10.3389/fchem.2014.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken G. E., Kirch B. H., Strickland J. R., Bush L. P., Looper M. L., and Schrick F. N.. 2007. Hemodynamic responses of the caudal artery to toxic tall fescue in beef heifers. J. Anim. Sci. 85:2337–2345. doi:10.2527/jas.2006-821 [DOI] [PubMed] [Google Scholar]
- Aiken G. E., Klotz J. L., Johnson J. M., Strickland J. R., and Schrick F. N.. 2013. Postgraze assessment of toxicosis symptoms for steers grazed on toxic endophyte-infected tall fescue pasture. J. Anim. Sci. 91:5878–5884. doi:10.2527/jas.2012-5964 [DOI] [PubMed] [Google Scholar]
- Aiken G. E., Strickland J. R., Looper M. L., Bush L. P., and Schrick F. N.. 2009. Hemodynamics are altered in the caudal artery of beef heifers fed different ergot alkaloid concentrations. J. Anim. Sci. 87:2142–2150. doi:10.2527/jas.2008-1562 [DOI] [PubMed] [Google Scholar]
- Akers R. M., Bauman D. E., Capuco A. V., Goodman G. T., and Tucker H. A.. 1981. Prolactin regulation of milk secretion and biochemical differentiation of mammary epithelial cells in periparturient cows. Endocrinology. 109(1):23–30. doi: 10.1210/endo-109-1-23 [DOI] [PubMed] [Google Scholar]
- Aldrich C. G., Paterson J. A., Tate J. L., and Kerley M. S.. 1993a. The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. J. Anim. Sci. 71:164–170. [DOI] [PubMed] [Google Scholar]
- Aldrich C. G., Rhodes M. T., Miner J. L., Kerley M. S., and Paterson J. A.. 1993b. The effects of endophyte-infected tall fescue consumption and use of a dopamine antagonist on intake, digestibility, body temperature, and blood constituents in sheep. J. Anim. Sci. 71:158–163. [DOI] [PubMed] [Google Scholar]
- Alexander G. 1964. Studies on the placenta of the sheep (ovis aries l.). Effect of surgical reduction in the number of caruncles. J. Reprod. Fertil. 7:307–322. [DOI] [PubMed] [Google Scholar]
- Alexander G., and Williams D.. 1971. Heat stress and development of the conceptus in domestic sheep. J Agric Sci. 76(1):53–72. [Google Scholar]
- Andrejak M., and Tribouilloy C.. 2013. Drug-induced valvular heart disease: an update. Arch. Cardiovasc. Dis. 106:333–339. doi:10.1016/j.acvd.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Arroyo J. A., Anthony R. V., Parker T. A., and Galan H. L.. 2006. Differential expression of placental and vascular endothelial nitric oxide synthase in an ovine model of fetal growth restriction. Am. J. Obstet. Gynecol. 195:771–777. doi:10.1016/j.ajog.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., and Seino S.. 1990. Molecular biology of mammalian glucose transporters. Diabetes Care. 13:198–208. [DOI] [PubMed] [Google Scholar]
- Bell A. W., McBride B. W., Slepetis R., Early R. J., and Currie W. B.. 1989. Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J. Anim. Sci. 67:3289–3299. [DOI] [PubMed] [Google Scholar]
- Bell A. W., Wilkening R. B., and Meschia G.. 1987. Some aspects of placental function in chronically heat-stressed ewes. J. Dev. Physiol. 9:17–29. [PubMed] [Google Scholar]
- Benech A., Cal-Pereyra L., Da Silva S., Acosta-Dibarrat J., and Gonzalez-Montana J. R.. 2015. Transient apnoea in sheep: an alternative method for serial urine sample collection. Veterinarski Arhiv. 85(3):293–307. [Google Scholar]
- Bernard J. K., Chestnut A. B., Erickson B. H., and Kelly F. M.. 1993. Effects of prepartum consumption of endophyte-infested tall fescue on serum prolactin and subsequent milk-production of holstein cows. J Dairy Sci. 76(7):1928–1933. [Google Scholar]
- Borowicz P. P., Arnold D. R., Johnson M. L., Grazul-Bilska A. T., Redmer D. A., and Reynolds L. P.. 2007. Placental growth throughout the last two thirds of pregnancy in sheep: vascular development and angiogenic factor expression. Biol. Reprod. 76:259–267. doi:10.1095/biolreprod.106.054684 [DOI] [PubMed] [Google Scholar]
- Browne C. A., and Thorburn G. D.. 1989. Endocrine control of fetal growth. Biol. Neonate. 55:331–346. doi:10.1159/000242937 [DOI] [PubMed] [Google Scholar]
- Browning R., Jr, and Leite-Browning M. L.. 1997. Effect of ergotamine and ergonovine on thermal regulation and cardiovascular function in cattle. J. Anim. Sci. 75:176–181. [DOI] [PubMed] [Google Scholar]
- Caldwell J. D., Coffey K. P., Jennings J. A., Philipp D., Young A. N., Tucker J. D., Hubbell D. S. 3rd, Hess T., Looper M. L., West C. P., et al. 2013. Performance by spring and fall-calving cows grazing with full, limited, or no access to toxic neotyphodium coenophialum-infected tall fescue. J. Anim. Sci. 91:465–476. doi:10.2527/jas.2011-4603 [DOI] [PubMed] [Google Scholar]
- Carr D. J., David A. L., Aitken R. P., Milne J. S., Borowicz P. P., Wallace J. M., and Redmer D. A.. 2016. Placental vascularity and markers of angiogenesis in relation to prenatal growth status in overnourished adolescent ewes. Placenta. 46:79–86. doi:10.1016/j.placenta.2016.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnino D., Harter C. J., Rivera A. R., de Lima L. S., de Oliveira Silva H. G., Biagioli B., de Resende K. T., de Almeida Teixeira I. A. M.. 2015. Changes in maternal body composition and metabolism of dairy goats during pregnancy. Revista Brasileira de Zootecnia. 44(3):92–102. doi:10.1590/S1806-92902015000300003 [Google Scholar]
- Chamley W. A., Buckmaster J. M., Cerini M. E., Cumming I. A., Goding J. R., Obst J. M., Williams A., and Winfield C.. 1973. Changes in the levels of progesterone, corticosteroids, estrone, estradiol-17 beta, luteinizing hormone, and prolactin in the peripheral plasma of the ewe during late pregnancy and at parturition. Biol. Reprod. 9:30–35. [DOI] [PubMed] [Google Scholar]
- Chen J., Tan B., Karteris E., Zervou S., Digby J., Hillhouse E. W., Vatish M., and Randeva H. S.. 2006. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 49:1292–1302. doi:10.1007/s00125-006-0194-7 [DOI] [PubMed] [Google Scholar]
- Craig A. M., Blythe L. L., and Duringer J. M.. 2014. The role of the Oregon State University endophyte service laboratory in diagnosing clinical cases of endophyte toxicoses. J. Agric. Food Chem. 62:7376–7381. doi:10.1021/jf5027229 [DOI] [PubMed] [Google Scholar]
- Dänicke S. 2015. Ergot alkaloids in feed for pekin ducks: toxic effects, metabolism and carry over into edible tissues. Toxins (Basel). 7:2006–2023. doi:10.3390/toxins7062006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dänicke S. 2016. Toxic effects, metabolism, and carry-over of ergot alkaloids in laying hens, with a special focus on changes of the alkaloid isomeric ratio in feed caused by hydrothermal treatment. Mycotoxin Res. 32:37–52. doi:10.1007/s12550-016-0238-x [DOI] [PubMed] [Google Scholar]
- Danicke S. 2017. Ergot alkaloids in fattening chickens (broilers): toxic effects and carry over depending on dietary fat proportion and supplementation with non-starch-polysaccharide (NSP) hydrolyzing enzymes. Toxins (Basel). 9(4):118–140. doi: 10.3390/toxins9040118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiling C. E., Carman F. S. 3rd, and Brown D. E.. 1991. Maternal endocrine and fetal metabolic responses to heat stress. J. Dairy Sci. 74:312–327. doi:10.3168/jds.S0022-0302(91)78175-7 [DOI] [PubMed] [Google Scholar]
- Duckett S. K., Andrae J. G., and Pratt S. L.. 2014a. Exposure to ergot alkaloids during gestation reduces fetal growth in sheep. Front. Chem. 2:68. doi:10.3389/fchem.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett S. K., Pratt S. L., and Pavan E.. 2009. Corn oil or corn grain supplementation to steers grazing endophyte-free tall fescue. II. Effects on subcutaneous fatty acid content and lipogenic gene expression. J Anim Sci. 87(3):1120–1128. doi: 10.2527/jas.2008-1420 [DOI] [PubMed] [Google Scholar]
- Duckett S. K., Volpi-Lagreca G., Alende M., and Long N. M.. 2014b. Palmitoleic acid reduces intramuscular lipid and restores insulin sensitivity in obese sheep. Diabetes. Metab. Syndr. Obes. 7:553–563. doi:10.2147/DMSO.S72695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. C. 1993. Evidence that ergovaline acts on serotonin receptors. Life Sci. 53:PL223–PL228. [DOI] [PubMed] [Google Scholar]
- Ehrhardt R. A., and Bell A. W.. 1995. Growth and metabolism of the ovine placenta during mid-gestation. Placenta. 16:727–741. [DOI] [PubMed] [Google Scholar]
- Eisemann J. H., Huntington G. B., Williamson M., Hanna M., and Poore M.. 2014. Physiological responses to known intake of ergot alkaloids by steers at environmental temperatures within or greater than their thermoneutral zone. Front. Chem. 2:96. doi:10.3389/fchem.2014.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emile J. C., Bony S., and Ghesquière M.. 2000. Influence of consumption of endophyte-infested tall fescue hay on performance of heifers and lambs. J. Anim. Sci. 78: 358–364. [DOI] [PubMed] [Google Scholar]
- Foote A. P., Harmon D. L., Brown K. R., Strickland J. R., McLeod K. R., Bush L. P., and Klotz J. L.. 2012. Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline. J. Anim. Sci. 90:1603–1609. doi:10.2527/jas.2011-4513 [DOI] [PubMed] [Google Scholar]
- Fowden A. L., Sferruzzi-Perri A. N., Coan P. M., Constancia M., and Burton G. J.. 2009. Placental efficiency and adaptation: endocrine regulation. J. Physiol. 587(Pt 14):3459–3472. doi:10.1113/jphysiol.2009.173013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden A. L., Forhead A. J., Coan P. M., and Burton G.. 2008. The placenta and intrauterine programming. J Neuroendocrinol. 589:7–20. Doi: 10.1111/j.1365-2826.2008.01663.x [DOI] [PubMed] [Google Scholar]
- Han V. K., and Carter A. M.. 2000. Spatial and temporal patterns of expression of messenger RNA for insulin-like growth factors and their binding proteins in the placenta of man and laboratory animals. Placenta. 21:289–305. doi:10.1053/plac.1999.0498 [DOI] [PubMed] [Google Scholar]
- Hayward C. E., Lean S., Sibley C. P., Jones R. L., Wareing M., Greenwood S. L., and Dilworth M. R.. 2016. Placental adaptation: what can we learn from birthweight:placental weight ratio? Front. Physiol. 7:28. doi:10.3389/fphys.2016.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiden U., Glitzner E., Hartmann M., and Desoye G.. 2009. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J. Anat. 215:60–68. doi:10.1111/j.1469-7580.2008.01035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill N. S., Thompson F. N., Dawe D. L., and Stuedemann J. A.. 1994. Antibody binding of circulating ergot alkaloids in cattle grazing tall fescue. Am. J. Vet. Res. 55:419–424. [PubMed] [Google Scholar]
- Hill N. S., Thompson F. N., Stuedemann J. A., Dawe D. L., and Hiatt E. E. 3rd. 2000. Urinary alkaloid excretion as a diagnostic tool for fescue toxicosis in cattle. J. Vet. Diagn. Invest. 12:210–217. doi:10.1177/104063870001200303 [DOI] [PubMed] [Google Scholar]
- Hoet J. J., and Hanson M. A.. 1999. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. J. Physiol. 514 (Pt 3):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley R. D., Campbell J. J., and Findlay J. K.. 1978. The importance of prolactin for lactation in the ewe. J. Endocrinol. 79:301–310. [DOI] [PubMed] [Google Scholar]
- Hoveland C. S., Schmidt S. P., King C. C. Jr, and Clark E. M.. 1984. Summer syndrome on tall fescue. Proc. Tall Fescue Workshop Atlanta, GA 17–18 Mar Univ. Georgia Ext., Athens, GA. [Google Scholar]
- Janzen C., Lei M. Y., Cho J., Sullivan P., Shin B. C., and Devaskar S. U.. 2013. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta. 34:1072–1078. doi:10.1016/j.placenta.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce D. A., and Gubbay S. S.. 1982. Arterial complications of migraine treatment with methysergide and parenteral ergotamine. Br Med J (Clin Res Ed). 285(6337):260–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kind K. L., Owens J. A., Robinson J. S., Quinn K. J., Grant P. A., Walton P. E., Gilmour R. S., and Owens P. C.. 1995. Effect of restriction of placental growth on expression of igfs in fetal sheep: relationship to fetal growth, circulating igfs and binding proteins. J. Endocrinol. 146:23–34. [DOI] [PubMed] [Google Scholar]
- Klotz J. L. 2015. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins (Basel). 7:2801–2821. doi:10.3390/toxins7082801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Egert-McLean A. M., Schrick F. N., Chattopadhyay N., and Harmon D. L.. 2018. Effects of grazing different ergovaline concentrations on vasoactivity of bovine lateral saphenous vein. J. Anim. Sci. 96:3022–3030. doi:10.1093/jas/sky163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Brown K. R., Xue Y., Matthews J. C., Boling J. A., Burris W. R., Bush L. P., and Strickland J. R.. 2012. Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 90:682–693. doi:10.2527/jas.2011-4323 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Bush L. P., Smith D. L., Shafer W. D., Smith L. L., Arrington B. C., and Strickland J. R.. 2007. Ergovaline-induced vasoconstriction in an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 85:2330–2336. doi:10.2527/jas.2006-803 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. 2009. Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro. J. Anim. Sci. 87:2437–2447. doi:10.2527/jas.2008-1692 [DOI] [PubMed] [Google Scholar]
- Koontz A. F., Bush L. P., Klotz J. L., McLeod K. R., Schrick F. N., and Harmon D. L.. 2012. Evaluation of a ruminally dosed tall fescue seed extract as a model for fescue toxicosis in steers. J. Anim. Sci. 90:914–921. doi:10.2527/jas.2011-4292 [DOI] [PubMed] [Google Scholar]
- Kumar N. T., Liestøl K., Løberg E. M., Reims H. M., and Mæhlen J.. 2014. Postmortem heart weight: relation to body size and effects of cardiovascular disease and cancer. Cardiovasc. Pathol. 23:5–11. doi:10.1016/j.carpath.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Lang U., Baker R. S., Khoury J., and Clark K. E.. 2000. Effects of chronic reduction in uterine blood flow on fetal and placental growth in the sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279:R53–R59. doi:10.1152/ajpregu.2000.279.1.R53 [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Chung C. S., and Simmen F. A.. 1993. Ontogeny of the porcine insulin-like growth factor system. Mol. Cell. Endocrinol. 93:71–80. [DOI] [PubMed] [Google Scholar]
- Looper M. L., Edrington T. S., Flores R., Burke J. M., Callaway T. R., Aiken G. E., Schrick F. N., and Rosenkrans C. F. Jr. 2007. Influence of dietary endophyte (neotyphodium coenophialum)-infected tall fescue (festuca arundinacea) seed on fecal shedding of antibiotic resistance-selected escherichia coli O157:H7 in ewes. J. Anim. Sci. 85:1102–1108. doi:10.2527/jas.2006-410 [DOI] [PubMed] [Google Scholar]
- Lyons P. C., Plattner R. D., and Bacon C. W.. 1986. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science. 232:487–489. [DOI] [PubMed] [Google Scholar]
- Matthews A. K., Poore M. H., Huntington G. B., and Green J. T.. 2005. Intake, digestion, and N metabolism in steers fed endophyte-free, ergot alkaloid-producing endophyte-infected, or nonergot alkaloid-producing endophyte-infected fescue hay. J. Anim. Sci. 83:1179–1185. doi:10.2527/2005.8351179x [DOI] [PubMed] [Google Scholar]
- McCann J. S., Caudle A. B., Thompson F. N., Stuedemann J. A., Heusner G. L., and Thompson D. L. Jr. 1992. Influence of endophyte-infected tall fescue on serum prolactin and progesterone in gravid mares. J. Anim. Sci. 70:217–223. [DOI] [PubMed] [Google Scholar]
- McCann M. A., Craddock B. F., Preston R. L., and Ramsey C. B.. 1990. Digestibility of cotton plant by-product diets for sheep at two levels of intake. J. Anim. Sci. 68:285–295. [DOI] [PubMed] [Google Scholar]
- McDowell K. J., Moore E. S., Parks A. G., Bush L. P., Horohov D. W., and Lawrence L. M.. 2013. Vasoconstriction in horses caused by endophyte-infected tall fescue seed is detected with doppler ultrasonography. J. Anim. Sci. 91:1677–1684. doi:10.2527/jas.2012-5852 [DOI] [PubMed] [Google Scholar]
- Moubarak A. S., and Rosenkrans C. F. Jr. 2000. Hepatic metabolism of ergot alkaloids in beef cattle by cytochrome P450. Biochem. Biophys. Res. Commun. 274:746–749. doi:10.1006/bbrc.2000.3210 [DOI] [PubMed] [Google Scholar]
- NRC 2007. Nutrient requirements of sheep tables, p. 244–270. In: Nutrient Requirements of Small Ruminants, Sheep, Goats, Cervids, and New World Camelids. National Research Council of the National Academies, The National Academies Press, Washington, D.C. [Google Scholar]
- Notter D. R. 2000. Effects of ewe age and season of lambing on prolificacy in US targhee, suffolk, and polypay sheep. Small Rumin. Res. 38:1–7. [DOI] [PubMed] [Google Scholar]
- Osgerby J. C., Wathes D. C., Howard D., and Gadd T. S.. 2004. The effect of maternal undernutrition on the placental growth trajectory and the uterine insulin-like growth factor axis in the pregnant ewe. J. Endocrinol. 182:89–103. [DOI] [PubMed] [Google Scholar]
- Parish J. A., McCann M. A., Watson R. H., Paiva N. N., Hoveland C. S., Parks A. H., Upchurch B. L., Hill N. S., and Bouton J. H.. 2003. Use of nonergot alkaloid-producing endophytes for alleviating tall fescue toxicosis in stocker cattle. J. Anim. Sci. 81:2856–2868. doi:10.2527/2003.81112856x [DOI] [PubMed] [Google Scholar]
- Paterson J., Forcherio C., Larson B., Samford M., and Kerley M.. 1995. The effects of fescue toxicosis on beef cattle productivity. J. Anim. Sci. 73:889–898. [DOI] [PubMed] [Google Scholar]
- Penninga L., and Longo L. D.. 1998. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta. 19:187–193. [DOI] [PubMed] [Google Scholar]
- Peyronneau M. A., Delaforge M., Riviere R., Renaud J. P., and Mansuy D.. 1994. High affinity of ergopeptides for cytochromes P450 3A. Importance of their peptide moiety for P450 recognition and hydroxylation of bromocriptine. Eur. J. Biochem. 223:947–956. [DOI] [PubMed] [Google Scholar]
- Poidatz D., Dos Santos E., Brulé A., De Mazancourt P., and Dieudonné M. N.. 2012. Estrogen-related receptor gamma modulates energy metabolism target genes in human trophoblast. Placenta. 33:688–695. doi:10.1016/j.placenta.2012.06.002 [DOI] [PubMed] [Google Scholar]
- Poole D. H., Lyons S. E., Poole R. K., and Poore M. H.. 2018. Ergot alkaloids induce vasoconstriction of bovine uterine and ovarian blood vessels. J. Anim. Sci. 96:4812–4822. doi:10.1093/jas/sky328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. K., and Thompson F. N. Jr. 1992. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 70:1594–1603. [DOI] [PubMed] [Google Scholar]
- Rattray P. V., Garrett W. N., East N. E., and Hinman N.. 1974. Growth, development and composition of the ovine conceptus and mammary gland during pregnancy. J. Anim. Sci. 38:613–626. [DOI] [PubMed] [Google Scholar]
- Reynolds L. P., Ferrell C. L., Nienaber J. A., and Ford S. P.. 1985. Effects of chronic environmental heat-stress on blood-flow and nutrient-uptake of the gravid bovine uterus and fetus. J Agr Sci. 104(Apr):289–297. doi:10.1017/S002185960004394x [Google Scholar]
- Reynolds T. S., Stevenson K. R., and Wathes D. C.. 1997. Pregnancy-specific alterations in the expression of the insulin-like growth factor system during early placental development in the ewe. Endocrinology. 138:886–897. doi:10.1210/endo.138.3.4983 [DOI] [PubMed] [Google Scholar]
- Rhodes M. T., Paterson J. A., Kerley M. S., Garner H. E., and Laughlin M. H.. 1991. Reduced blood flow to peripheral and core body tissues in sheep and cattle induced by endophyte-infected tall fescue. J. Anim. Sci. 69: 2033–2043. [DOI] [PubMed] [Google Scholar]
- Robbins J. 1973. Radioassay and the thyroid gland. Metabolism. 22:1021–1026. [DOI] [PubMed] [Google Scholar]
- Rottinghaus G. E., Garner G. B., Cornell C. N., and Ellis J. L.. 1991. HPLC method for quantitating ergovaline in endophyte-infected tall fescue: seasonal variation of ergovaline levels in stems with leaf sheaths, leaf blades, and seed heads. J Agric Food Chem. 39:112–115. [Google Scholar]
- Rueda B. R., Dunn T. G., Anthony R. V., and Moss G. E.. 1995. Influence of fetal death and fetectomy on gestation and the initiation of parturition in the ewe. Reprod. Fertil. Dev. 7:1221–1225. [DOI] [PubMed] [Google Scholar]
- Rumsey T. S., McLeod K., Elsasser T. H., Kahl S., and Baldwin R. L.. 1999. Effects of oral chlortetracycline and dietary protein level on plasma concentrations of growth hormone and thyroid hormones in beef steers before and after challenge with a combination of thyrotropin-releasing hormone and growth hormone-releasing hormone. J. Anim. Sci. 77:2079–2087. [DOI] [PubMed] [Google Scholar]
- Rumsey T. S., Stuedemann J. A., Wilkinson S. R., and Williams D. J.. 1979. Chemical composition of necrotic fat lesions in beef cows grazing fertilized “kentucky-31” tall fescue. J. Anim. Sci. 48:673–682. [DOI] [PubMed] [Google Scholar]
- Schmidt S. P., Hoveland C. S., Clark E. M., Davis N. D., Smith L. A., Grimes H. W., and Holliman J. L.. 1982. Association of an endophytic fungus with fescue toxicity in steers fed kentucky 31 tall fescue seed or hay. J. Anim. Sci. 55:1259–1263. [DOI] [PubMed] [Google Scholar]
- Schöning C., Flieger M., and Pertz H. H.. 2001. Complex interaction of ergovaline with 5-HT2A, 5-HT1B/1D, and alpha1 receptors in isolated arteries of rat and guinea pig. J. Anim. Sci. 79:2202–2209. [DOI] [PubMed] [Google Scholar]
- Schultz C. L., Lodge-Ivey S. L., Bush L. P., Craig A. M., and Strickland J. R.. 2006. Effects of initial and extended exposure to an endophyte-infected tall fescue seed diet on faecal and urinary excretion of ergovaline and lysergic acid in mature geldings. N. Z. Vet. J. 54:178–184. doi:10.1080/00480169.2006.36692 [DOI] [PubMed] [Google Scholar]
- Sferruzzi-Perri A. N., Sandovici I., Constancia M., and Fowden A. L.. 2017. Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth. J. Physiol. 595:5057–5093. doi:10.1113/JP273330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoup L. M., Miller L. M., Srinivasan M., Ireland F. A., and Shike D. W.. 2016. Effects of cows grazing toxic endophyte-infected tall fescue or novel endophyte-infected tall fescue in late gestation on cow performance, reproduction, and progeny growth performance and carcass characteristics. J. Anim. Sci. 94:5105–5113. doi:10.2527/jas.2016-0819 [DOI] [PubMed] [Google Scholar]
- Sibley D. R., and Creese I.. 1983. Interactions of ergot alkaloids with anterior pituitary D-2 dopamine receptors. Mol. Pharmacol. 23:585–593. [PubMed] [Google Scholar]
- Stegeman H. J. 1972. A study of the maturation of the placenta in sheep. Acta Morphol. Neerl. Scand. 10:400. [PubMed] [Google Scholar]
- Stowe H. M., Miller M., Burns M. G., Calcatera S. M., Andrae J. G., Aiken G. E., Schrick F. N., Cushing T., Bridges W. C., and Pratt S. L.. 2013. Effects of fescue toxicosis on bull growth, semen characteristics, and breeding soundness evaluation. J. Anim. Sci. 91:3686–3692. doi:10.2527/jas.2012-6078 [DOI] [PubMed] [Google Scholar]
- Strickland J. R., Looper M. L., Matthews J. C., Rosenkrans C. F. Jr, Flythe M. D., and Brown K. R.. 2011. Board-invited review: St. Anthony’s fire in livestock: causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi:10.2527/jas.2010-3478 [DOI] [PubMed] [Google Scholar]
- Studemann J. A., Rumsey T. S., Bond J., Wilkinson S. R., Bush L. P., Williams D. J. and Caudle A. B.. 1985. Association of blood cholesterol with occurrence of fat necrosis in cows and tall fescue summer toxicosis in steers. Am. J. Vet. Res. 46(9):1990–1995. [PubMed] [Google Scholar]
- Stuedemann J. A., Hill N. S., Thompson F. N., Fayrer-Hosken R. A., Hay W. P., Dawe D. L., Seman D. H., and Martin S. A.. 1998. Urinary and biliary excretion of ergot alkaloids from steers that grazed endophyte-infected tall fescue. J. Anim. Sci. 76:2146–2154. [DOI] [PubMed] [Google Scholar]
- Szotáková B., Baliharová V., Lamka J., Nozinová E., Wsól V., Velík J., Machala M., Neca J., Soucek P., Susová S., et al. 2004. Comparison of in vitro activities of biotransformation enzymes in pig, cattle, goat and sheep. Res. Vet. Sci. 76:43–51. [DOI] [PubMed] [Google Scholar]
- Thakur A., Sase M., Lee J. J., Thakur V., and Buchmiller T. L.. 2000. Ontogeny of insulin-like growth factor 1 in a rabbit model of growth retardation. J. Surg. Res. 91:135–140. doi:10.1006/jsre.2000.5926 [DOI] [PubMed] [Google Scholar]
- Thureen P. J., Trembler K. A., Meschia G., Makowski E. L., and Wilkening R. B.. 1992. Placental glucose transport in heat-induced fetal growth retardation. Am. J. Physiol. 263(3 Pt 2):R578–R585. doi:10.1152/ajpregu.1992.263.3.R578 [DOI] [PubMed] [Google Scholar]
- Tygesen M. P., Nielsen M. O., Nørgaard P., Ranvig H., Harrison A. P., and Tauson A. H.. 2008. Late gestational nutrient restriction: effects on ewes’ metabolic and homeorhetic adaptation, consequences for lamb birth weight and lactation performance. Arch. Anim. Nutr. 62:44–59. doi:10.1080/17450390701780276 [DOI] [PubMed] [Google Scholar]
- Vatnick I., Schoknecht P. A., Darrigrand R., and Bell A. W.. 1991. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J. Dev. Physiol. 15:351–356. [PubMed] [Google Scholar]
- Villalba J. J., Spackman C., Goff B. M., Klotz J. L., Griggs T., and MacAdam J. W.. 2016. Interaction between a tannin-containing legume and endophyte-infected tall fescue seed on lambs’ feeding behavior and physiology. J. Anim. Sci. 94:845–857. doi:10.2527/jas.2015-9790 [DOI] [PubMed] [Google Scholar]
- Volpi-Lagreca G., and Duckett S. K.. 2017. Supplementation of glycerol or fructose via drinking water to grazing lambs on tissue glycogen level and lipogenesis. J. Anim. Sci. 95:2558–2575. doi:10.2527/jas.2017.1449 [DOI] [PubMed] [Google Scholar]
- Vonnahme K. A., Arndt W. J., Johnson M. L., Borowicz P. P., and Reynolds L. P.. 2008. Effect of morphology on placentome size, vascularity, and vasoreactivity in late pregnant sheep. Biol. Reprod. 79:976–982. doi:10.1095/biolreprod.108.070748 [DOI] [PubMed] [Google Scholar]
- Vonnahme K. A., Hess B. W., Nijland M. J., Nathanielsz P. W., and Ford S. P.. 2006. Placentomal differentiation may compensate for maternal nutrient restriction in ewes adapted to harsh range conditions. J. Anim. Sci. 84:3451–3459. doi:10.2527/jas.2006-132 [DOI] [PubMed] [Google Scholar]
- Wallace L. R. 1948. The growth of lambs before and after birth in relation to the level of nutrition. J Agr Sci. 38(3):243–302. [Google Scholar]
- Wang T., Chen M., Yan Y. E., Xiao F. Q., Pan X. L., and Wang H.. 2009. Growth retardation of fetal rats exposed to nicotine in utero: possible involvement of CYP1A1, CYP2E1, and P-glycoprotein. Environ. Toxicol. 24:33–42. doi:10.1002/tox.20391 [DOI] [PubMed] [Google Scholar]
- Wathes D. C., Reynolds T. S., Robinson R. S., and Stevenson K. R.. 1998. Role of the insulin-like growth factor system in uterine function and placental development in ruminants. J. Dairy Sci. 81:1778–1789. doi:10.3168/jds.S0022-0302(98)75747-9 [DOI] [PubMed] [Google Scholar]
- Watson R. H., McCann M. A., Parish J. A., Hoveland C. S., Thompson F. N., and Bouton J. H.. 2004. Productivity of cow-calf pairs grazing tall fescue pastures infected with either the wild-type endophyte or a nonergot alkaloid-producing endophyte strain, AR542. J. Anim. Sci. 82:3388–3393. doi:10.2527/2004.82113388x [DOI] [PubMed] [Google Scholar]
- Weiner N. 1980. Drugs that inhibit adrenergic nerves and block adrenergic receptors. 6 ed. Macmillian Publishing, New York. [Google Scholar]
- Wilson M. E., and Ford S. P.. 2001. Comparative aspects of placental efficiency. Reprod. Suppl. 58:223–232. [PubMed] [Google Scholar]
- Xie F., Xiao P., Chen D., Xu L., and Zhang B.. 2012. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 80(1):75–84. doi: 10.1007/s11103-012-9885-2 [DOI] [PubMed] [Google Scholar]
- Yang Q., Doshi U., Li N., and Li A. P.. 2012. Effects of culture duration on gene expression of P450 isoforms, uptake and efflux transporters in primary hepatocytes cultured in the absence and presence of interleukin-6: implications for experimental design for the evaluation of downregulatory effects of biotherapeutics. Curr. Drug Metab. 13:938–946. [DOI] [PubMed] [Google Scholar]
- Yates D. T., Green A. S., and Limesand S. W.. 2011. Catecholamines mediate multiple fetal adaptations during placental insufficiency that contribute to intrauterine growth restriction: lessons from hyperthermic sheep. J. Pregnancy. 2011:740408. doi:10.1155/2011/740408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. A., Charlton N. D., Takach J. E., Swoboda G. A., Trammell M. A., Huhman D. V., and Hopkins A. A.. 2014. Characterization of epichloë coenophiala within the US: are all tall fescue endophytes created equal? Front. Chem. 2:95. doi:10.3389/fchem.2014.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. F., Tatter S. B., Valego N. K., Figueroa J. P., Thompson J., and Rose J. C.. 2003. The role of hypothalamic input on corticotroph maturation in fetal sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284:R1621–R1630. doi:10.1152/ajpregu.00572.20t02 [DOI] [PubMed] [Google Scholar]
- Zbib N., Repussard C., Tardieu D., Priymenko N., Domange C., and Guerre P.. 2014. Ergovaline in tall fescue and its effect on health, milk quality, biochemical parameters, oxidative status, and drug metabolizing enzymes of lactating ewes. J. Anim. Sci. 92:5112–5123. doi:10.2527/jas.2014-8106 [DOI] [PubMed] [Google Scholar]
- Zhang S., Barker P., Botting K. J., Roberts C. T., McMillan C. M., McMillen I. C., and Morrison J. L.. 2016. Early restriction of placental growth results in placental structural and gene expression changes in late gestation independent of fetal hypoxemia. Physiol Rep. 4(23) doi: ARTN e1304910.14814/phy2.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]