Abstract
The objective of this study was to evaluate the effect of cinnamaldehyde, on feed intake, rumen fermentation, nutrient digestibility, milk yield, and components in lactating dairy cows. Six lactating Holstein dairy cows (3 ruminally cannulated and 3 noncannulated) averaging 263 ± 41 d in milk (DIM) and 754 ± 45 kg of BW at the beginning of the study were used. Cows were randomly assigned to 1 of 3 treatments in a replicated 3 × 3 Latin square design with 19 d periods (14 d for diet adaptation and 5 d for sample collection). Treatments were 0, 2, or 4 mg/kg of BW of cinnamaldehyde. Cinnamaldehyde was mixed with 40 g of corn meal and top-dressed onto the total mixed ration (TMR). Diet was fed as a TMR and contained 37% corn silage, 18.5% mixed-mostly grass silage, 24.5% energy supplement, 16.5% protein supplement, and 3.5% vitamin and mineral mix on a DM basis. The dietary nutrient composition averaged 15.1% CP, 37.8% NDF, and 24.7% ADF. Cows were fed and milked twice daily. No differences were observed for DMI (mean = 24.6 kg/d), milk yield (mean = 28.4 kg/d), 3.5% fat-corrected milk (FCM; mean = 30.6 kg/d), and 3.5% energy-corrected milk (ECM; mean = 30.7 kg/d). The dose of cinnamaldehyde did not have any effect on milk components, rumen fermentation, or pH. There were no differences in nutrient digestibility, but there was a trend for a quadratic effect for DM digestibility (P = 0.09): 74.4%, 76.3%, and 73.7% for treatments 0, 2, and 4 mg/kg of BW of cinnamaldehyde, respectively. A linear effect (P = 0.02) and a quadratic effect (P < 0.02) observed for urinary urea N and a quadratic effect (P = 0.03) for allantoin and total purine derivatives with the 2 mg/kg treatment being the lesser value. These data suggest that cinnamaldehyde at these dosages may have an antimicrobial effect in the rumen as suggested by a lesser concentration of urinary total purine derivatives. Overall, supplementing lactating dairy cows with cinnamaldehyde had no effect on feed intake, milk yield, or milk components. However, it appears that cinnamaldehyde has a negative effect on rumen microbial protein synthesis as suggested by the reduced concentration of urinary purine derivatives.
Keywords: cinnamaldehyde, digestibility, cow, essential oil, urinary purine derivative
INTRODUCTION
Plants produce many varieties of natural organic compounds that are derived from secondary metabolism that appear to have no direct function in the plant’s growth or development (Gershenzon and Croteau, 1991; Cowan, 1999). These compounds are difficult to classify since their synthesis, properties, and mechanisms of actions are often overlapped; however, they can be generally structured into 3 groups: saponins, tannins, and essential oils (EO) (Calsamiglia et al., 2007). The plant compounds saponins and tannins have been extensively researched, however, information on the effects of EO on rumen microbial fermentation and animal performance is still relatively limited. Essential oils are naturally occurring secondary metabolites acquired from the plant volatile fraction most commonly by steam distillation, or they can be acquired by chemical extraction (Gershenzon and Croteau, 1991). Essential oils have been studied since the beginning of the 20th century and the most important activity of these compounds are antiseptic and antimicrobial (Cowan, 1999; Burt, 2004). They are considered safe for human and animal consumption and are categorized as generally recognized as safe (GRAS; US FDA, 2004). Essential oils can interact with microbial cell membranes and inhibit the growth of some Gram-positive and Gram-negative bacteria resulting in an inhibition of deamination and methanogenesis, yielding lesser ammonia N, methane, and acetate and in greater propionate and butyrate concentrations (Calsamiglia et al., 2007). With greater protein concentrations in animal diets, there are increased concerns about environmental N contamination. Essential oils may affect rumen fermentation and decrease ammonia production in the rumen; thus, decreasing N excretion (Calsamiglia et al., 2007). Essential oils have also been shown to manipulate bacterial populations involved in ruminal biohydrogenation to modify the fatty acid composition of milk. Cinnamaldehyde is an EO that appears to be a natural alternative to antibiotics and functions similarly to ionophores (Calsamiglia et al., 2007). It is found in the bark of cinnamon trees and is the active component of cinnamon oil (Cinnamomum cassia) accounting for 75% of its composition (Calsamiglia et al., 2007). It is a phenylpropanoid with antimicrobial activity. Cinnamaldehyde has been studied in poultry for effects on microbial communities (Hume et al., 2006; Venkitanarayanan et al., 2013); feedlot cattle for feed efficiency effects (Yang et al., 2010b; Vakili et al., 2013); and lactating dairy cows for effects on DMI, milk yield and components (Tassoul and Shaver, 2009; Tekippe et al., 2013; Wall et al., 2014). It has been shown to be effective against many mastitis causing organisms in vitro (Ananda Baskaran et al., 2009). Differences in DMI response and performance have been observed; however, there are numerous inconsistencies in animal responses, which could be a result of the source of EO, active ingredient composition, application rates, the mixture of EO being fed, and experimental conditions. With antibiotic growth promotors for animal diets having been banned in the European Union since 2006, EO may be an alternative to favorably alter ruminal metabolism to improve feed efficiency and animal productivity. Supplementing cinnamaldehyde could improve rumen health and feed utilization, thereby, improving milk quality and yield and lowering feed costs.
The objective of this study was to evaluate the effects of cinnamaldehyde, at 3 different feeding rates to determine the optimal dose to improve apparent total tract nutrient digestibility and estimate its effect on rumen microbial protein synthesis.
MATERIALS AND METHODS
The experiment was conducted at the University of New Hampshire Fairchild Dairy Teaching and Research Center (Durham, NH) from March 30th to May 28th, 2016. Care and handling of the animals were approved in accordance with the University of New Hampshire Institutional Animal Care and Use Committee guidelines (Protocol #160203).
Animals, Experimental Design, and Diets
Six multiparous Holstein dairy cows (3 ruminally cannulated) averaging 263 ± 41 d in milk (DIM) and 754 ± 45 kg of BW at the beginning of the study were used. Cows were randomly assigned to 1 of 3 treatments in a replicated 3 × 3 Latin square design. Treatments were 0, 2, or 4 mg/kg of BW cinnamaldehyde (≥95%, Sigma-Aldrich Corp., St. Louis, MO) based on research conducted by Chapman et al. (2016, 2017). Cinnamaldehyde was mixed with 40 g of corn meal and top-dressed onto the total mixed ration (TMR). Monensin was not included in the diet. Each experimental period lasted 19 d, with 14 d for diet adaptation and 5 d for data and sample collection. Initial BW was taken for 2 consecutive days (Cardinal, Northeast Scale Co. Inc., Hooksett, NH) before the experiment to determine the dose of cinnamaldehyde and then taken the last 2 d of each period. Feeding rate of cinnamaldehyde was adjusted based on BW for each period. Cows were housed in a tie-stall barn, and the stalls had mattresses bedded with kiln-dried sawdust. Cows had access to water at all times via automated water bowls (DeLaval, Tumba, Sweden). Each cow had an individual wooden feed tub (90 × 90 × 90 cm) to allow for measurements of daily feed intake. Diets were fed as TMR and were prepared and fed twice daily, at 0630 and 1630 h, using a Super Data Ranger mixer (American Calan Inc., Northwood, NH). The experimental diets are given in Table 1.
Table 1.
Ingredient composition of the diet
| Ingredient | DM,% |
|---|---|
| Corn silage | 37.0 |
| Grass silage | 18.5 |
| Protein supplement1 | 16.6 |
| Energy supplement2 | 24.5 |
| Vitamin/Mineral supplement3 | 3.4 |
1Protein supplement contained 69.4% soybean meal, 22% canola meal, 6.9% distillers dried grains, and 1.7% urea.
2Energy supplement contained 45.8% corn meal, 34% beet pulp, 15.2% steamed flaked corn, and 5.0% molasses.
3Vitamin/mineral supplement provided: 14.2% Ca, 14.21% NaCl, 13.92% Na, 8.67% Cl, 6.10% Mg, 1.81% P, 0.26% S, 0.23% Fe, 0.13% Zn, 0.08% Mn, 271 mg/kg Cu, 28.7 mg/kg Co, 6.5 mg/kg I, 9.3 mg/kg Se, 199,403 IU/kg vitamin A, 46,016 IU/kg vitamin D3, 1503 IU/kg vitamin E.
Feed Sampling and Analysis
Total mixed ration amounts fed and refused were measured daily before the afternoon feeding to determine DMI. Samples of TMR and orts were collected before the afternoon feeding during the 5 d sample period and frozen (−20 °C) for later analysis. Frozen samples were thawed and dried in a forced hot-air convection oven at 55 °C for 48 h to determine DM (1380FMS; VWR Scientific, Radnor, PA). Orts samples were composited by cow by period, and TMR samples were composited by period. Samples were ground through a 1-mm screen using a Wiley mill (Thomas Scientific, Swedesboro, NJ). Samples were shipped to a commercial laboratory for nutritional analyses (Dairy One Forage Laboratory, Ithaca, NY). The following analyses were conducted on the feed samples: absolute DM (method 930.15; AOAC International, 2006), total N (methods 990.03 and 992.23 967.07; AOAC International, 2006), NDF (method 6 in an Ankom Fiber Analyzer A2000 with α-amylase and sodium sulfite; Ankom Technology, Fairpoint, NY; solutions as in Van Soest et al. 1991), ADF (method 5 in an Ankom Fiber Analyzer A2000; Ankom Technology; method 973.18, AOAC International, 1998), ether extract [extraction by a Soxtec HT6 System (Foss North America, Eden Prairie, MN) using anhydrous diethyl ether (method 2003.05, AOAC International, 2006), and ash (method 942.05; AOAC International, 2006). Nonfiber carbohydrate was calculated as 100% − (% CP + % NDF + % EE + % ash).
Milk Sampling and Analyses
Cows were milked twice a day, at 0530 and 1600 h, with milk yield recorded throughout the experiment. Milk samples were collected for 4 consecutive milking sessions day 15 and 16 of each period, preserved in tubes containing 2-bromo-2-nitropropan-1,3 diol, pooled by cow according to morning and afternoon milk weights, and refrigerated at 4 °C until shipped to Dairy One Cooperative Inc. for determination of fat, true protein, lactose, and milk urea nitrogen by Fourier transform mid-infrared spectroscopy using a MilkoScan model FT+ or 6000 (Foss Inc., Hillerød, Denmark) and somatic cell score (SCC) by flow cytometry in a Fossomatic FC or 5000 (Foss Inc.). Concentrations and yields of milk components were calculated as the average between the duplicate samples. Calculation of energy-corrected milk (ECM) was done based on Tyrrell and Reid (1965), whereas 4% fat-corrected milk (FCM) was determined according to Gaines and Davidson (1923).
Rumen, Urinary, and Fecal Sampling and Analyses
Ruminal samples were taken from the 3 cannulated cows (square 1) fitted with ruminal cannulas (10 cm i.d.; Bar Diamond Inc., Parma, ID) on day 15 of each period at the following times after feeding at 0630 h: 0, 1, 2, 3, 5, 7 and 9 h. Samples were taken using a 40-cm long, 2.5-cm diameter polyvinyl chloride tube hooked to a volumetric flask attached to an 85-mL vacuum bulb for suctioning (VWR International, Radnor, PA). The PVC tube was inserted in the rumen via an orifice in the cannula cap and the site of sampling was verified by hand via the ruminal cannula before a sample was taken. Samples were collected from the cranial, ventral, and caudal sacs at various depths, yielding a final volume of approximately 400 mL per sampling. Ruminal fluid was immediately transported to the laboratory, filtered through 4 layers of cheesecloth, and measured for pH using a portable pH meter (model SP20; VWR International, Bridgeport, NJ). The area under the curve for pH over time was calculated using the trapezoidal rule (Phillips and Taylor, 1973) which is analogous to the method of Mackie and Gilchrist (1979) for analysis of rumen pH curves. Following pH readings, a subsample of 42 mL was acidified with 1.2 mL of 6 N HCl into a centrifuge tube and frozen (−20°C) for later analysis of NH3. Samples were thawed at room temperature, vortexed, and centrifuged at 3,125 × g for 20 min at 22 °C. Next, 10 mL of supernatant was added to a beaker containing 1 mL of pH ionic strength adjuster (Orion 951211; Thermo Fisher Scientific, Chelmsford, MA), and gaseous NH3 released was measured using an ion-selective electrode meter (Orion Star A214 Benchtop pH/ISE Meter; Thermo Scientific, Waltham, MA) and finally converted to NH3-N. The area under the curve (AUC) for NH3-N is calculated using the trapezoidal rule as described previously for rumen fluid pH. A ruminal fluid subsample (8 mL) was added to a cryovial containing 0.2 mL of 50% H2SO4 (vol/vol) and stored at −20 °C for later VFA analyses using a gas chromatograph equipped with a flame ionization detector (model 3300; Varian Inc., Palo Alto, CA) and a 2 m × 2 mm glass column packed with 10% stationary phase 1200/1 H3PO4 on 80/100 Chromosorb W-AW media (Supelco Inc., Bellefonte, PA) at the West Virginia University Rumen Fermentation Profiling Laboratory (Morgantown, WV). Methane (mmol/100 mol VFA) was calculated according to the equation of Moss et al. (2000).
Spot urine samples were collected for 4 consecutive days (day 16 to 19 in each period) by stimulation of the pudendal nerve massaging the area below the vulva to account for diurnal variation in the excretion of urinary metabolites (total of 8 spot samples). On day 16, cows were sampled at 0330 and 1530 h, day 17 at 0630 and 1830 h, day 18 at 0930 and 2130 h, and day 19 at 0030 and 1230 h. Subsamples (1.05 mL per sampling point) were pooled over the 4 d, yielding a total of 8.4 mL of urine, which was placed in 50-mL centrifuge tubes containing 33.6 mL of 0.072 N H2SO4 and stored (−20 °C) for later analyses of allantoin, uric acid, urea N, and total N. Subsamples of 5.25 mL of urine per sampling point were also added to 50-mL centrifuge tubes containing 1.2 mL of 6 N HCl and pooled over the 3 sampling days for later analysis of NH3 using the methodology described for the ruminal samples. After thawing at room temperature, urine samples were analyzed colorimetrically for concentrations of allantoin (Chen et al., 1992), uric acid (assay kit no. 1045–225; Stanbio Laboratory, Boerne, TX), and creatinine (assay kit no. 500701; Cayman Chemical Co., Ann Arbor, MI), and total N (micro-Kjeldahl analysis, AOAC 1990; Dairy One Forage Laboratory). Creatinine was assayed using a chromate microplate reader set at a wavelength of 492 nm (Awareness Technology Inc., Palm City, FL). Daily urine volume was estimated from the urinary concentration of creatinine assuming a constant creatinine rate of 29 mg/kg of BW (Valadares et al., 1999). Urinary urea N, allantoin, and uric acid were read at wavelengths of 540, 522, and 520 nm, respectively, on a UV/visible spectrophotometer (Beckman Coulter Inc., Brea CA). Urinary excretion of total purine derivatives (PD) was calculated by adding allantoin plus uric acid. Determining urinary PD can be used to estimate microbial protein synthesis as most of the nucleic acids leaving the rumen are of microbial origin and most feeds have low purine content and are fermented in the rumen (Chen and Gomes, 1992).
Fecal grab samples were collected directly from the rectum concurrently with the collection of urine. On day 16, cows were sampled at 0330 and 1530 h, day 17 at 0630 and 1830 h, day 18 at 0930 and 2130 h, and day 19 at 0030 and 1230 h. Samples were pooled by cow over 4 d to obtain a single composite and stored at −20 °C in plastic bags. At the end of each sampling period, pooled fecal samples were thawed, placed in aluminum trays, and kept inside a forced-air oven at 55 °C until completely dried (approximately 72 h). Dried samples were ground to pass through a 1-mm screen (Wiley mill) and analyzed for DM, ash, total N, NDF, and NFC as described for feed. Samples were also analyzed for AIA by Dairy One Forage Laboratory, Ithaca, NY for determination of apparent total tract digestibility of nutrients.
Statistical Analyses
Data were analyzed as a replicated 3 × 3 Latin square for all variables except rumen VFA and pH which were analyzed as a single 3 × 3 Latin square using the Mixed Procedure (SAS, version 9.4, Cary, NC). Rumen fluid pH was converted to H ion concentration prior to analyzing as recommended by Murphy (1982) using the general linear model procedure of SAS (version 9.4). Standard error for pH data is expressed as H ions. Treatment × square interactions were determined for the replicated squares. If treatment × square interaction was not significant it was removed from the model. Cow within square was considered random. For repeated measures, three covariance structures were tested: autoregressive 1, compound symmetry, and Toeplitz and the structure that resulted in the lowest Bayesian information criteria was used. Linear and quadratic orthogonal comparisons were made to determine optimum feeding rate. Significance was at P ≤ 0.05 and trends were 0.10 ≤ P >0.05.
RESULTS
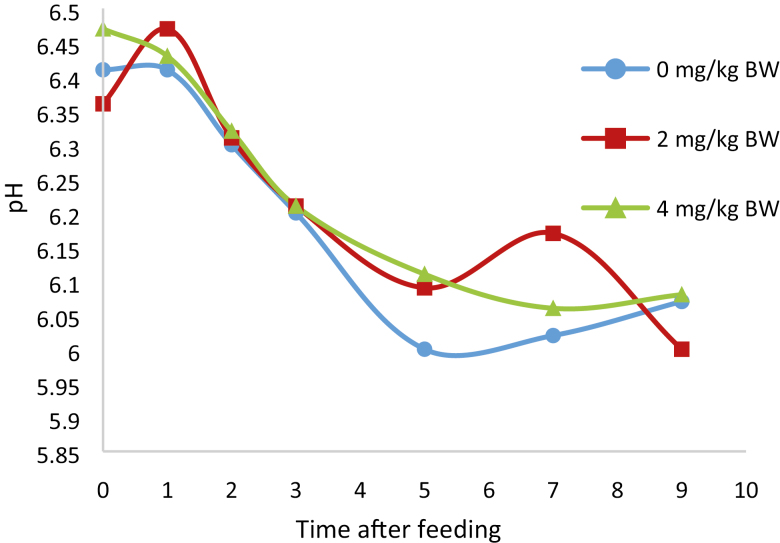
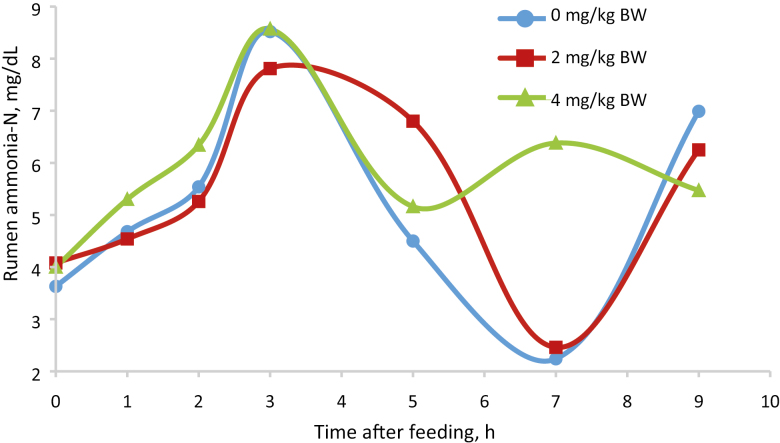
Nutrient analyses appear in Table 2. Treatment × square interactions were not significant for any variable and removed from the model. There was no treatment by hour interactions for rumen ammonia concentrations, pH, VFA, or methane. There were no effects of treatment on DMI, milk yield or composition, or efficiency of production (Table 3). There was a trend for a quadratic response for apparent total tract DM (P = 0.09), and OM (P = 0.10) digestibilities with the 2 mg/kg treatment resulting in the greatest digestibility values (Table 4). Cinnamaldehyde supplementation did not affect digestibility of the other nutrients measured (Table 4). Nitrogen intake, urinary excretion, N in milk, feces, and urine were similar among treatments (Table 5). Purine derivatives were affected by cinnamaldehyde supplementation (Table 5). Urinary-urea N production decreased both linearly (P = 0.02) and quadratically (P < 0.02) with the lesser production with the 2 mg/kg cinnamaldehyde treatment. Allantoin production resulted in a quadratic response (P = 0.03) with the lesser amount being the 2 mg/kg treatment. Total PD followed the same pattern as allantoin (P = 0.03). Purine derivatives and allantoin tended (P = 0.06) to decrease as cinnamaldehyde supplementation increased. These data suggest that cinnamaldehyde supplementation reduced ruminal microbial protein synthesis. Ruminal pH AUC, NH3-N measurements, VFA, acetic acid + butyric acid: propionic acid and methane concentration were similar across treatments (Table 6, Figs. 1 and 2).
Table 2.
Nutrient analyses of diet
| Nutrient | DM ± SD, % |
|---|---|
| Crude protein | 15.1 ± 0.08 |
| NDF | 37.8 ± 2.0 |
| ADF | 24.7 ± 1.4 |
| NFC1 | 34.7 ± 2.2 |
| Fat | 3.9 ± 0.3 |
| Ash | 8.4 ± 0.09 |
| Ca | 0.86 ± 0.025 |
| P | 0.52 ± 0.004 |
| Mg | 0.28 ± 0.004 |
| Na | 0.17 ± 0.01 |
| K | 1.40 ± 0.06 |
| S | 0.28 |
1NFC = Nonfiber carbohydrates = 100 – (crude protein + NDF +Fat +Ash).
Table 3.
Dry matter intake, milk yield, and composition of cows fed cinnamaldehyde
| Treatment1 | Contrast2 (P =) | |||||
|---|---|---|---|---|---|---|
| Item | Con | 2 | 4 | SEM | Lin | Quad |
| DMI, kg/d | 24.1 | 25.0 | 24.8 | 0.36 | 0.68 | 0.75 |
| BW, kg | 794 | 800 | 800 | 23.3 | 0.35 | 0.60 |
| Milk fat, % | 3.95 | 3.99 | 4.00 | 0.12 | 0.41 | 0.89 |
| Milk fat, kg/d | 1.11 | 1.13 | 1.15 | 0.05 | 0.36 | 0.90 |
| Milk protein, % | 3.47 | 3.49 | 3.48 | 0.10 | 0.63 | 0.52 |
| Milk protein, kg/d | 0.98 | 0.98 | 1.00 | 0.05 | 0.61 | 0.70 |
| Milk lactose, % | 4.73 | 4.75 | 4.77 | 0.06 | 0.11 | 0.91 |
| Milk lactose, kg/d | 1.33 | 1.34 | 1.37 | 0.05 | 0.51 | 0.82 |
| Milk TS3, % | 13.0 | 13.1 | 13.1 | 0.14 | 0.26 | 0.89 |
| MUN4, mg/dL | 11.8 | 12.0 | 12.2 | 0.76 | 0.60 | 0.98 |
| SCC score5 | 3.73 | 3.49 | 2.95 | 1.91 | 0.11 | 0.78 |
| FCM6, kg/d | 30.3 | 30.5 | 31.0 | 1.23 | 0.46 | 0.82 |
| ECM7, kg/d | 30.8 | 30.9 | 31.4 | 1.21 | 0.49 | 0.79 |
| ECM/DMI8 | 1.27 | 1.24 | 1.27 | 0.05 | 0.95 | 0.59 |
1Treatment: Con = 0 cinnamaldehyde, 2 = 2 mg/kg BW cinnamaldehyde, and 4 = 4 mg/kg BW cinnamldehyde.
2Contrast: Lin = linear, Quad = quadratic.
3Milk TS = milk total solids.
4MUN = milk urea nitrogen.
5SCC score= somatic cell score.
6FCM = 4% fat-corrected milk.
7ECM = energy-corrected milk.
8ECM/DMI = efficiency of ECM production (ECM, kg/DMI, kg).
Table 4.
Apparent total tract nutrient digestibility of cows fed varying amounts of cinnamaldehyde
| Treatment1 | Contrast2 (P =) | |||||
|---|---|---|---|---|---|---|
| Item | Con | 2 | 4 | SEM | Lin | Quad |
| DM,% | 74.4 | 76.3 | 73.7 | 1.01 | 0.63 | 0.09 |
| OM,% | 76.1 | 78.0 | 75.4 | 1.05 | 0.63 | 0.10 |
| CP,% | 69.7 | 71.5 | 68.4 | 1.22 | 0.45 | 0.12 |
| NDF,% | 61.5 | 64.5 | 60.3 | 1.82 | 0.64 | 0.14 |
| NFC3,% | 93.1 | 94.1 | 93.4 | 0.97 | 0.83 | 0.48 |
| Fat, % | 85.8 | 85.4 | 85.1 | 0.63 | 0.46 | 0.98 |
1Treatment: Con = 0 cinnamaldehyde, 2 = 2 mg/kg BW cinnamaldehyde, and 4 = 4 mg/kg BW cinnamaldehyde.
2Contrast: Lin = linear, Quad = quadratic.
3NFC = nonfiber carbrohydrate.
Table 5.
N balance and purine derivatives of cows fed varying amounts of cinnamaldehyde
| Treatment1 | Contrast2(P=) | |||||
|---|---|---|---|---|---|---|
| CON | 2 | 4 | SEM | Lin | Quad | |
| N intake, g/d | 596 | 611 | 609 | 16.7 | 0.29 | 0.40 |
| Creatinine, mg/L | 1245.9 | 1191.7 | 1175.8 | 63.5 | 0.36 | 0.77 |
| Urinary excretion, L/d | 18.6 | 19.7 | 20.2 | 0.93 | 0.18 | 0.77 |
| N in milk, g/d | 153.3 | 153.0 | 156.1 | 7.51 | 0.61 | 0.71 |
| N in feces, g/d | 180 | 174 | 194 | 9.68 | 0.34 | 0.28 |
| N in urine, g/d | 183 | 185 | 190 | 8.80 | 0.55 | 0.94 |
| N retention, g/d | 79.4 | 99.0 | 69.2 | 11.8 | 0.54 | 0.12 |
| Urinary Urea N, g/d | 45.7 | 30.0 | 34.4 | 3.30 | 0.02 | <0.02 |
| Allantoin, mmol/d | 563.6 | 461.3 | 495.6 | 22.1 | 0.06 | 0.03 |
| Uric acid, mmol/d | 26.5 | 26.2 | 27.2 | 1.48 | 0.74 | 0.71 |
| Total PD3, mmol/d | 590.1 | 487.5 | 522.8 | 22.1 | 0.06 | 0.03 |
1Treatment: Con = 0 cinnamaldehyde, 2 = 2 mg/kg BW cinnamaldehyde, and 4 = 4 mg/kg BW cinnamaldehyde.
2Contrast: Lin = linear, Quad = quadratic.
3Total PD = total purine derivatives.
Table 6.
Rumen pH area under the curve, rumen ammonia, volatile fatty acid concentrations, and methane production of cows fed varying amounts of cinnamaldehyde
| Treatment1 | Contrast2 (P=) | ||||||
|---|---|---|---|---|---|---|---|
| Item | Con | 2 | 4 | SEM3 | Lin | Quad | Trt × h4 |
| Rumen pH | 6.18 | 6.20 | 6.21 | 3.63×10–8 | 0.60 | 0.86 | 0.99 |
| Rumen pH, AUC5 | 55.7 | 56.0 | 56.2 | 0.99 | 0.72 | 0.93 | |
| Rumen NH3-N, mg/dL | 5.16 | 5.31 | 5.89 | 0.59 | 0.38 | 0.77 | 0.91 |
| Rumen NH3-N, AUC6 | 45.3 | 48.3 | 55.1 | 3.91 | 0.15 | 0.72 | |
| Total VFA, µmol/mL7 | 103.9 | 101.9 | 100.7 | 5.88 | 0.72 | 0.96 | 0.45 |
| Acetic acid, % | 65.8 | 65.6 | 66.2 | 1.15 | 0.82 | 0.79 | 0.96 |
| Propionic acid, % | 20.4 | 20.8 | 19.1 | 1.04 | 0.41 | 0.45 | 0.97 |
| Butyric acid, % | 11.0 | 11.3 | 12.0 | 0.45 | 0.20 | 0.70 | 0.68 |
| Isobutyric acid, % | 0.87 | 0.89 | 0.86 | 0.04 | 0.35 | 0.14 | 0.35 |
| Valeric acid, % | 1.21 | 1.12 | 1.25 | 0.07 | 0.66 | 0.24 | 0.22 |
| Isovaleric acid, % | 0.52 | 0.29 | 0.52 | 0.11 | 0.97 | 0.16 | 0.11 |
| (A+B)/P8 | 3.78 | 3.74 | 4.12 | 0.26 | 0.42 | 0.55 | 0.98 |
| CH4, (mmol/100 mol VFA)9 | 28.4 | 28.3 | 29.4 | 0.79 | 0.45 | 0.60 | 0.96 |
1Treatment: Con = 0 cinnamaldehyde, 2 = 2 mg/kg BW cinnamaldehyde, and 4 = 4 mg/kg BW cinnamldehyde.
2Contrast: Lin = linear, Quad = quadratic.
3For pH, the SEM is the H ion concentration.
4Treatment by hour interaction.
5Rumen pH area under the curve, pH × hours.
6Rumen NH3-N area under the curve, mg/dL × h.
7TotalVFA = total volatile fatty acids.
8(A+B)/P = (acetic acid + butyric acid)/Propionic acid.
9Methane concentration calculated according to the method of Moss et al., (2000).
Figure 1.
pH after the feeding of cows supplemented with cinnamaldehyde (SE 0.14).
Figure 2.
Rumen NH3-N (mg/dL) after feeding cows supplemented with cinnamaldehyde (SE 1.56 mg/dL).
DISCUSSION
Cinnamaldehyde has been studied in feedlot cattle (Yang et al., 2010a, 2010b; Vakili et al., 2013). Yang et al. (2010a) observed that DMI responded quadratically with steers supplemented with cinnamaldehyde (0, 400, 800, or 1,600 mg/d) with the greatest response being observed at 400 mg/d. These researchers observed a similar response in feedlot cattle over the first 28 d in a different experiment, but the response diminished after 28 d (Yang et al. 2010b). Vakili et al. (2013) observed no differences in DMI in calves fed 5 g/d of thyme and cinnamaldehyde. Benchaar et al. (2008) observed no differences in DMI in a lactating cow study where cows were fed either 0 or 1 g cinnamaldehyde/cow/d. These results were similar to the DMI in the current study where cows consumed either 0, 2, or 4 mg/kg of cinnamaldehyde of BW. Based on the BW of cows in our study, cinnamaldehyde intakes averaged 0, 1.6, and 3.2 g/d. Khiaosa-ard and Zebeli (2013) performed a meta-analysis of ruminants fed EO and found no effect on DMI in lactating cows with an intercept of 21.39 kg. The data of Benchaar et al. (2008) and the model of Khiaosa-ard and Zebeli (2013) concur with the results of the present study (Table 3). It appears that DMI responses to cinnamaldehyde supplementation occur in diets with a greater proportion of concentrate. Essential oils appear to be more effective when supplemented in low rumen pH environments such as when feeding high concentrate diets, commonly fed in feedlots and suggest that their mechanism of action is the inhibition of methanogenesis and deamination (Calsamiglia et al., 2007)
There were no differences in milk composition or yield in the present study (Table 3) which supports the work of Benchaar (2016) who observed no effects of 50 mg cinnamaldehyde/kg DM (approximately 1.09 g) on DMI on milk yield, milk composition, or milk component yield. Benchaar et al. (2008) observed trends for decreased lactose content and increased SCC score in milk from cows supplemented with EO. However, Khiaosa-ard and Zebeli (2013) found that higher doses of EO (>150 mg/kg DMI) increased milk protein variables (percent and yield) with the greatest response at 430 mg/kg DMI. However, cows in the present study consumed approximately 64 and 128 mg/kg DMI resulting in no differences in protein variables. These results and those of Khiaosa-ard and Zebeli (2013) suggest that greater doses of EO than those fed in the current study may increase milk protein variables. There are few experiments evaluating cinnamaldehyde on milk production and composition, but there appears to be little to no effect of cinnamaldehyde except at greater doses. However, there have been no lactation studies utilizing cinnamaldehyde. Other lactating cow studies studying EO have evaluated combinations. This precludes the ability to determine which, (if any), EO caused the outcome.
Apparent total tract digestibility of DM and OM (Table 4) tended to respond quadratically to cinnamaldehyde supplementation (P ≤ 0.10) with the 2 mg/kg treatment resulted in greater digestibilities. However, no other differences in nutrient digestibility were observed. Silva et al. (2018) observed a trend for improved DM digestibility when either monensin, EO, or EO plus amylase were fed to lactating cows compared with cows not fed these additives.
Allantoin, and therefore, total PD responded in a quadratic fashion with the lesser concentration being the 2 mg/kg dose (P = 0.03; Table 5). These data suggested that the rumen microbial protein synthesis would be reduced in the 2 mg/kg treatment (Table 6). These data along with the increased DM and OM digestibility at the 2 mg/kg dose suggest that these nutrients were digested postruminally. Hart et al. (2008) suggested that EO may reduce the population of hyper-ammonia-producing bacteria involved in amino acid deamination, but stated that this response might be dependent on the chemical makeup of the EO. In this experiment, cinnamaldehyde at these doses did not affect rumen ammonia concentrations or ruminal ammonia AUC, suggesting that it did not reduce the hyper-ammonia-producing bacteria.
Rumen VFA concentrations and estimated methane concentrations were not affected by cinnamaldehyde, which is supported by other studies feeding cinnamaldehyde to dairy cows (Benchaar et al., 2008; Benchaar, 2016). Yang et al. (2010) observed no effects on ruminal VFA concentrations in feedlot steers. There are a lot of inconsistencies in animal responses to EO, which could be a result of the source of EO and active ingredient composition, application rates, and experimental conditions (Benchaar et al., 2008; Benchaar, 2016). However, most EO modified rumen fermentation by changing VFA production and/or protein metabolism when fed at high doses; therefore, confirming their antimicrobial activities and ability to manipulate rumen fermentation (Calsamiglia et al., 2007). They appear to be more effective when supplemented in low rumen pH environments such as when feeding high concentrate diets and suggest that there mechanism of action is the inhibition of methanogenesis and deamination (Calsamiglia et al., 2007). Differences in results may be from feeding the EO for only short periods; however, they appear to diminish over time suggesting rumen bacteria may become acclimated to EO supplementation at low doses (Cardozo et al., 2004).
In conclusion, results from this study suggest that DM and OM tend to be enhanced at the 2 mg/kg of BW dose, but also resulted in the lowest yield of PD suggesting that microbial protein synthesis is reduced at this dose. We do not have an explanation as to why this result did not occur with the 4 mg/kg treatment. These results suggest that possibly nutrients were digested postruminally. Oh et al. (2018) suggested that EO can bypass the rumen. Franz et al. (2010) indicated that some EO can withstand degradation in ruminal fluid for up to 24 h. The overall effects of different EO on rumen microbial fermentation may be the result of different sensitivities of specific microbial populations to these compounds. Because EO are diet and pH dependent, the selection of the appropriate EO will depend on specific goals and performances of the animal. More research with longer duration of dosing cinnamaldehyde needs to be conducted with lactating dairy cows. Based on urinary PD, these results suggest that rumen microbial protein synthesis is altered by cinnamaldehyde. However, a direct method should be used when evaluating the effect of cinnamaldehyde on rumen microbial protein synthesis.
Conflict of interest statement. None declared.
Footnotes
The authors thank George Walker Fund and the New Hampshire Agricultural Experiment Station. Partial funding was provided by the New Hampshire Agricultural Experiment Station. This is Scientific Contribution Number 2790. This work was supported by the USDA National Institute of Food and Agriculture Project (Hatch Multistate NC2042; accession number 1001283). The authors also thank the Fairchild Teaching and Research Center (University of New Hampshire) staff for use of the animals and assistance throughout the study.
LITERATURE CITED
- Ananda Baskaran S., Kazmer G. W., Hinckley L., Andrew S. M., and Venkitanarayanan K.. 2009. Antibacterial effect of plant-derived antimicrobials on major bacterial mastitis pathogens in vitro. J. Dairy Sci. 92:1423–1429. doi:10.3168/jds.2008-1384 [DOI] [PubMed] [Google Scholar]
- AOAC International. 1998. Official methods of analysis. 16th ed. AOAC International, Arlington, VA. [Google Scholar]
- AOAC International. 2006. Official methods of analysis. 18th ed. AOAC International, Gaithersburg, MD. [Google Scholar]
- Benchaar C. 2016. Diet supplementation with cinnamon oil, cinnamaldehyde, or monensin does not reduce enteric methane production of dairy cows. Animal. 10:418–425. doi:10.1017/S175173111500230X [DOI] [PubMed] [Google Scholar]
- Benchaar C., McAllister T. A., and Chouinard P. Y.. 2008. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts. J. Dairy Sci. 91:4765–4777. doi:10.3168/jds.2008-1338 [DOI] [PubMed] [Google Scholar]
- Burt S. 2004. Essential oils: Their antibacterial properties and potential applications in foods–a review. Int. J. Food Microbiol. 94:223–253. doi:10.1016/j.ijfoodmicro.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Calsamiglia S, Busquet M., Cardozo P. W., Castillejos L., and Ferret A.. 2007. Essential oils as modifiers of rumen microbial fermentation: A review. J Dairy Sci. 90:2580–2595. doi:10.3168/jds.2006–644 [DOI] [PubMed] [Google Scholar]
- Cardozo P. W., Calsamiglia S., Ferret A., and Kamel C.. 2004. Effects of natural plant extracts on ruminal protein degradation and fermentation profiles in continuous culture. J. Anim. Sci. 82:3230–3236. doi:10.2527/2004.82113230x [DOI] [PubMed] [Google Scholar]
- Chapman C. E., Cabral R. G., Aragona K. M., and Erickson P. S.. 2016. Short communication: Cinnamaldehyde taste preferences of weaned dairy heifers. J. Dairy Sci. 99:3607–3611. doi:10.3168/jds.2015-10582 [DOI] [PubMed] [Google Scholar]
- Chapman C. E., Chester-Jones H., Ziegler D., Clapper J. A. and Erickson P. S.. 2017. Effects of cinnamaldehyde or monensin on performance of weaned Holstein dairy heifers. J. Dairy Sci. 100:1712–1719. doi:10.3168/jds.2016–11893 [DOI] [PubMed] [Google Scholar]
- Chen X. B., and Gomes M. J.. 1992. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives- An overview of the technical details. Int. Feed Resources Unit, Rowett Research Institute, Aberdeen, UK. [Google Scholar]
- Cowan M. M. 1999. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12:564–582. doi: 10.1128/CMR.12.4.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C., Baser K. H. C., and Windisch W.. 2010. Essential oils and aromatic plants in animal feeding-A European perspective. A review. Flavour Fragrance J. 25:327–340. doi:10.1002/ffj.1967 [Google Scholar]
- Gershenzon J., and Croteau R.. 1991. Terpenoids. In: Rosenthal G. A., and Berenbaum M. R., editors. Herbivores: their interactions with secondary plant metabolites, Vol 1 Academic Press, San Diego, CA: p. 165–219. [Google Scholar]
- Hart K. J., Yáñez-Ruiz D. R., Duval S. M., McEwan N. R., and Newbold C. J.. 2008. Plant extracts to manipulate rumen fermentation. Anim. Feed Sci. Technol. 147:8–35. doi:10.1016/j.anifeedsci.2007.09.007 [Google Scholar]
- Hume M. E., Clemente-Hernández S., and Oviedo-Rondón E. O.. 2006. Effects of feed additives and mixed Eimeria species infection on intestinal microbial ecology of broilers. Poult. Sci. 85:2106–2111. doi:10.1093/ps/85.12.2106 [DOI] [PubMed] [Google Scholar]
- Khiaosa-ard R., and Zebeli Q.. 2013. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J. Anim. Sci. 91:1819–1830. doi:10.2527/jas.2012-5691 [DOI] [PubMed] [Google Scholar]
- Mackie R. I., and Gilchrist F. M.. 1979. Changes in lactate-producing and lactate-utilizing bacteria in relation to pH in the rumen of sheep during stepwise adaptation to a high-concentrate diet. Appl. Environ. Microbiol. 38:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A. R., Jouany J. P., and Newbold J.. 2000. Methane production by ruminants: Its contribution to global warming. Ann. Zootechnol. 49:231–253. doi:10.1051/animres:2000119 [Google Scholar]
- Murphy M. R. 1982. Analyzing and presenting pH data. J. Dairy Sci. 65:161–163. doi:10.3168/jds.S0022-0302(82)82165–6 [Google Scholar]
- Oh J., Harper M., Lang C. H., Wall E. H., and Hristov A. N.. 2018. Effects of phytonutrients alone or in combination with monensin on productivity in lactating dairy cows. J. Dairy Sci. 101:7190–7198. doi:10.3168/jds.2018-14439 [DOI] [PubMed] [Google Scholar]
- Phillips G. M., and Taylor P. J.. 1973. Theory and applications of numerical analysis. Academic Press, New York. [Google Scholar]
- Silva G. G., Takiya C. S., Del Valle T. A., de Jesus E. F., Grigoletto N. T. S., Nakadonari B., Cortinhas C. S., Acedo T. S., and Rennó F. P.. 2018. Nutrient digestibility, ruminal fermentation, and milk yield in dairy cows fed a blend of essential oils and amylase. J. Dairy Sci. 101:9815–9826. doi:10.3168/jds.2018-14789 [DOI] [PubMed] [Google Scholar]
- Tassoul M. D., and Shaver R. D.. 2009. Effect of a mixture of supplemental dietary plant essential oils on performance of periparturient and early lactation dairy cows. J. Dairy Sci. 92:1734–1740. doi:10.3168/jds.2008-1760 [DOI] [PubMed] [Google Scholar]
- Tekippe J. A., Tacoma R., Hristov A. N., Lee C., Oh J., Heyler K. S., Cassidy T. W., Varga G. A., and Bravo D.. 2013. Effect of essential oils on ruminal fermentation and lactation performance of dairy cows. J. Dairy Sci. 96:7892–7903. doi:10.3168/jds.2013-7128 [DOI] [PubMed] [Google Scholar]
- Tyrrell H. F., and Reid J. T.. 1965. Prediction of the energy value of cow’s milk. J. Dairy Sci. 48:1215–1223. doi:10.3168/jds.S0022-0302(65)88430-2 [DOI] [PubMed] [Google Scholar]
- US FDA (US Food and Drug Administration). 2004. 21CFR184. Direct food substances affirmed as generally recognized as safe https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=184&showFR=1. Accessed February 17, 2016.
- Vakili A. R., Khorrami B., Mesgaran M. D., and Parand E.. 2013. The effects of thyme and cinnamon essential oils on performance, rumen fermentation and blood metabolites in holstein calves consuming high concentrate diet. Asian-Australas. J. Anim. Sci. 26:935–944. doi:10.5713/ajas.2012.12636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadares R. F. D., Broderick G. A., Valadares Filho S. C., and Clayton M. K.. 1999. Effect of replacing alfalfa silage with high moisture corn on ruminal protein synthesis estimated from excretion of total purine derivatives. J. Dairy Sci. 82:2686–2696. doi:10.3168/jds.S0022-0302(99)75525–6 [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Venkitanarayanan K., Kollanoor-Johny A., Darre M. J., Donoghue A. M., and Donoghue D. J.. 2013. Use of plant-derived antimicrobials for improving the safety of poultry products. Poult. Sci. 92:493–501. doi:10.3382/ps.2012-02764 [DOI] [PubMed] [Google Scholar]
- Wall E. H., Doane P. H., Donkin S. S., and Bravo D.. 2014. The effects of supplementation with a blend of cinnamaldehyde and eugenol on feed intake and milk production of dairy cows. J. Dairy Sci. 97:5709–5717. doi:10.3168/jds.2014-7896 [DOI] [PubMed] [Google Scholar]
- Yang W. Z., Ametaj B. N., Benchaar C., and Beauchemin K. A.. 2010a. Dose response to cinnamaldehyde supplementation in growing beef heifers: Ruminal and intestinal digestion. J. Anim. Sci. 88:680–688. doi:10.2527/jas.2008-1652 [DOI] [PubMed] [Google Scholar]
- Yang W. Z., Ametaj B. N., Benchaar C., He M. L., and Beauchemin K. A.. 2010b. Cinnamaldehyde in feedlot cattle diets: Intake, growth performance, carcass characteristics, and blood metabolites. J. Anim. Sci. 88:1082–1092. doi:10.2527/jas.2008-1608 [DOI] [PubMed] [Google Scholar]