Abstract
A study was conducted using 3 groups of gestating gilts and sows (n = 98) to determine the effects of Pichia guilliermondii (Pg), a whole cell–inactivated yeast product (CitriStim; ADM Alliance Nutrition), on performance and immune parameters of dams and litters. Within 24 h of breeding, gilts and sows were allotted to 1 of 3 treatments consisting of a control (SC) diet or SC diet supplemented with 0.1 (S1) or 0.2% (S2) Pg. Dietary treatments were maintained through lactation. Colostrum and milk (day 14) samples were collected for IgA, IgG, and IgM analysis. Blood samples were collected from sows on day 110 of gestation (group 3 only), while at weaning for all 3 groups, and from piglets at 14 d of age for peripheral white blood cell counts and serum IgA, IgG, and IgM analysis. Inclusion of Pg resulted in an increase in number born alive as the level of Pg increased (12.49, 13.33, and 13.43 born alive per litter for SC, S1, and S2, respectively; linear effect [LS], P = 0.003). Additionally, the percentage of piglets weighing less than 0.9 kg at birth was reduced in sows provided Pg at 0.1% or 0.2% compared with control (LS, P = 0.006). Sows receiving Pg during gestation and lactation also weaned a greater number of piglets (10.31, 10.55, and 10.60 weaned per litter in control, 0.1% and 0.2% Pg, respectively; LS, P = 0.02). However, percent preweaning mortality was 17.58%, 19.38%, and 19.61% for control, 0.1%, and 0.2% Pg, respectively (LS, P = 0.02). There were no differences in gestation BW gain, farrowing (days 110 to 48 h postfarrowing) or lactation (day 110 to weaning) BW loss, number of mummies or stillborn, or piglets’ individual birth or weaning weight. On day 110 of gestation, the neutrophil concentration (quadratic effect [QS], P = 0.03) and neutrophil:lymphocyte ratio (QS, P = 0.04) in peripheral blood were greater in S1 than SC, with S2 being intermediate. At weaning there was a linear increase in neutrophil concentration (P = 0.03), neutrophil:lymphocyte ratio (P = 0.01), and percentage of neutrophils in the leukocyte population (P = 0.01) as level of Pg increased in sow diets. In conclusion, Pg inclusion in sow diets linearly increased total number born alive and weaned, with no change in average birth or weaning weight, and decreased the number of lightweight pigs at birth. However, inclusion of Pg had no effect on immune parameters measured in milk, colostrum, or day 14 piglet serum, but increased the peripheral blood neutrophil concentration of gilts and sows.
Keywords: immune function, pig, sow, yeast product
INTRODUCTION
Yeast products have been shown to enhance swine performance as both a probiotic (living, viable microorganism, such as active dry yeast) or prebiotic (yeast cell culture or cell wall components). However, the effect of yeast products varies. No performance differences were reported when yeast culture was supplemented to sows (Veum et al., 1995). However, improvements in number born alive and weaned, as well as piglet body weight at birth, days 14, and 28 of lactation were observed when a yeast cell wall product was fed (Czech et al., 2010). Additionally, improvements in litter weaning weight and piglet ADG were reported in pigs from sows fed a yeast cell culture product (Shen et al., 2011). Supplementation with yeast culture has been demonstrated to improve milk production and feed intake in ruminants (Robinson and Garrett, 1999; Dann et al., 2000), as well as reproductive performance in pigs (Kim et al., 2008, 2010).
Mannan oligosaccharides and β-glucans are components of the yeast cell wall that confer beneficial effects on immune parameters in sows (Shen et al., 2011). Several potential mechanisms have been proposed as to how these benefits are conveyed, including direct binding of pathogenic bacteria in the intestinal lumen (Spring et al., 2000), thereby flushing them from the digestive tract, as well as immunomodulation (Davis et al., 2004). An inactivated, whole yeast cell product (CitriStim, ADM Animal Nutrition; Quincy, IL) which contains the remaining Pichia guilliermondii (Pg) cells and cell contents from the production of citric acid was reported to modulate localized immunomodulatory effects in broilers in response to Pg with no enhancement in performance (Shanmugasundaram and Selvaraj, 2012). The objective of this study was to determine the efficacy of Pg in enhancing dam and litter performance and modulating immune function.
MATERIALS AND METHODS
Animals and Experimental Diets
Animal management and experimental procedures conducted during this study were approved by the University of Arkansas Institutional Animal Care and Use Committee.
A total of 98 Dekalb-Monsanto Line GPK 35 gilts and sows were allotted to 1 of 3 dietary treatments based on parity, and body weight, at breeding. The 3 dietary treatments (Table 1) were a gestation control diet (SC), or the control diet supplemented with 0.1% (S1), or 0.2% Pg (S2). Average parity was 1.72 ± 0.34, 1.73 ± 0.34, and 2.06 ± 0.34 for SC, S1, and S2, respectively. Gilts and sows were housed in individual gestation stalls (0.61 × 2.13 m) and provided approximately 2.26 kg of feed per day and had free access to water throughout the gestation period. On day 110 of gestation, gilts and sows were individually weighed and moved to the farrowing facility where they were housed in individual farrowing crates (1.22 × 2.13 m). Upon farrowing, sows were fed ad libitum, maintaining gestation treatments through the lactation period. Both gestation and lactation diets (Table 1) were formulated to meet or exceed NRC (1998) requirements for gestating and lactating sows, respectively. Cross-fostering occurred within treatment groups and occurred within 24 h of farrowing.
Table 1.
Composition (as-fed) of gestation and lactation diets1
| Item | Gestation | Lactation |
|---|---|---|
| Ingredients (%) | ||
| Corn | 54.52 | 54.805 |
| Soybean meal, 48% | 9.50 | 28.00 |
| Dried distillers grains with solubles | 30.00 | 10.00 |
| Fat (yellow grease) | 1.00 | 2.00 |
| Dicalcium phosphate | 1.875 | 2.40 |
| Limestone | 1.175 | 0.75 |
| Salt | 0.45 | 0.50 |
| l-Lysine | 0.15 | 0.175 |
| l-Threonine | 0.00 | 0.04 |
| Potassium magnesium sulfate2 | 0.65 | 0.65 |
| Sow add pack3 | 0.25 | 0.25 |
| Vitamin premix4 | 0.25 | 0.25 |
| Mineral premix5 | 0.15 | 0.15 |
| Ethoxyquin6 | 0.03 | 0.03 |
| Calculated composition7 | ||
| ME, kcal/kg | 3296 | 3328 |
| CP, % | 17.07 | 20.67 |
| SID Lys, % | 0.652 | 1.065 |
| Available P, % | 0.544 | 0.547 |
| Ca, % | 0.952 | 0.956 |
| SID Met+Cys:Lys | 81 | 56 |
| SID Thr:Lys | 73 | 64 |
| SID Trp:Lys | 19 | 19 |
| SID Ile:Lys | 80 | 69 |
| SID Val:Lys | 100 | 78 |
1Control diets for gestation and lactation. For the S1 (0.1%) and S2 (0.2%) diets P. guilliermondii was added at the expense of corn.
2Dynamate, Mosaic Feed Ingredients, Plymouth, MN.
3The sow add pack provided the following per kg of complete diet: 22.05 IU of vitamin E, 551.15 mg of choline, 1.65 mg of folic acid, 4.96 mg of vitamin B6, 0.22 mg of biotin, and 0.20 mg of chromium.
4The vitamin premix provided the following per kg of complete diet: 397.5 mg of Ca, 11,022.9 IU of vitamin A, 1,377.9 IU of vitamin D3, 44.09 IU of vitamin E, 0.0386 mg vitamin B12, 4.41 mg of menadione, 8.27 mg of riboflavin, 27.56 mg of D-pantothenic acid, and 49.6 mg of niacin.
5The mineral premix provided the following per kg of complete diet: 84 mg of Ca, 165 mg of Fe, 165 mg of Zn, 39.6 mg of Mn, 16.5 mg of Cu, 0.3 mg of I, and 0.3 mg of Se.
6Quinguard, Novus International, Inc., St. Louis, MO.
7ME = metabolizable energy; CP = crude protein; SID = standard ileal digestible.
Individual body weight was recorded for gilts and sows at breeding, 110 d of gestation, 48 h postfarrowing, and at weaning, which occurred approximately 21 d postfarrowing. Individual daily feed intake of each sow was also recorded during lactation. At farrowing, and again at weaning, the total number of live piglets was counted, and individual piglet body weight was recorded. Additionally, the number of stillborn and mummies were recorded.
Within 24 h of parturition, colostrum samples were collected, and approximately 14 d later sows were administered oxytocin to stimulate milk letdown, and milk samples were collected to determine concentrations of IgA, IgG, and IgM.
Blood samples were obtained via jugular venipuncture from sows on day 110 (third farrowing group only), and at weaning (all 3 groups) and from 14-d-old pigs (one piglet per litter, n = 27 [13 or 14 of each sex] per treatment). Blood for serum analysis was collected into evacuated tubes containing clot activator (BD Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ). Blood samples were centrifuged at 1,300 × g for 20 min; serum was transferred into 5.0-ml polypropylene sample tubes and stored at −20°C until analyzed for IgA, IgG, and IgM. An additional sample was obtained at each time point in K2-EDTA tubes (BD Vacutainer) for determination of leukocyte differentials using a blood hematology system (Hemavet 950 FS, Drew Scientific, Waterbury, CT).
Chemical Analysis
Colostrum, milk, and serum samples were analyzed for IgA, IgG, and IgM with commercially available ELISA kits per manufacturer’s instructions (Pig ELISA Quantitation Set, Bethyl Laboratories, Inc., Montgomery, TX). All samples were diluted (50 mM Tris, 0.14 M NaCl, 1% BSA, 0.05% Tween 20) as necessary to fall within the standard curve which ranged from 15.6 to 1,000 ng/mL for IgA and IgM and 7.8 to 1,000 ng/mL for IgG. The resulting data were multiplied by the dilution factor to obtain the reported concentrations for each immunoglobulin. The intra- and interassay coefficients of variation were 5.3% and 10.7%, 3.3% and 5.9%, and 6.0% and 8.6% for IgA, IgG, and IgM, respectively.
Statistical Analysis
All response variables were initially analyzed using the GLM Procedure of SAS (Cary, NC) with a model that included only the fixed effects of treatment and parity group. All response variables with P < 0.20 were considered as dependent variables. The STEPWISE procedure of SAS was run separately for each response variable to determine which variables could be used as covariates in the subsequent mixed model analysis. Independent variables with P < 0.15 were retained in PROC STEPWISE. For the sow and litter data, the covariates identified using the STEPWISE procedure were included in the PROC MIXED analysis with replicate and parity group (0, 1, 2+) as random effects. Parity group was considered a random effect due to a high number of 2+ parity sows enrolled on the second replicate. Immunoglobulin levels in sow serum, milk, and colostrum were analyzed using the PROC MIXED of SAS with treatment as the fixed effect, whereas replicate and parity group were random effects. Peripheral blood leukocyte data were analyzed using the GLM Procedure of SAS with the fixed effects of treatment, replicate, and parity group. Orthogonal contrasts were used to determine linear and quadratic responses to increasing levels of Pg. The experimental unit was the sow/farrowing crate. Differences were considered significant at P < 0.05.
RESULTS
There were no significant differences observed in gestation weight gain, total weight loss after farrowing, overall average daily feed intake, birth weight, number of stillborn or mummies, weaning weight, or average daily gain (Table 2). However, there was a tendency (quadratic effect, P = 0.07) for increased average daily feed intake during week 3 in S1 gilts and sows (Table 2).
Table 2.
Effect of supplementation of inactivated P. guilliermondii (Pg) whole yeast cell product during gestation and lactation on sow reproductive performance
| Item1 | SC2n = 32 | S1 n = 33 | S2 n = 33 | SEM | Linear P-value | Quadratic P-value |
|---|---|---|---|---|---|---|
| 110 d sow BW, kg | 261.97 | 264.53 | 263.22 | 2.52 | 0.52 | 0.24 |
| Sow farrowing BW, kg | 250.67 | 250.63 | 250.44 | 3.36 | 0.92 | 0.97 |
| Sow weaning BW, kg | 239.10 | 239.81 | 240.03 | 2.37 | 0.72 | 0.91 |
| Sow gestation BW gain, kg | 51.26 | 53.82 | 52.50 | 2.52 | 0.52 | 0.24 |
| Sow farrowing BW loss, kg | −11.56 | −14.12 | −12.81 | 2.52 | 0.52 | 0.24 |
| Sow lactation BW loss, kg | −12.50 | −7.89 | −10.05 | 4.61 | 0.32 | 0.11 |
| Sow total BW loss, kg | −23.85 | −23.34 | −22.53 | 2.89 | 0.62 | 0.95 |
| Week 1 lactation ADFI, kg | 4.64 | 4.51 | 4.63 | 0.20 | 0.95 | 0.44 |
| Week 2 lactation ADFI, kg | 7.00 | 6.87 | 6.96 | 0.15 | 0.84 | 0.52 |
| Week 3 lactation ADFI, kg | 7.19 | 7.53 | 7.26 | 0.14 | 0.73 | 0.07 |
| Overall lactation ADFI, kg | 6.17 | 6.39 | 6.24 | 0.39 | 0.78 | 0.40 |
| Total birth weight, kg | 17.73 | 16.97 | 17.42 | 0.54 | 0.67 | 0.32 |
| Average birth weight, kg | 1.35 | 1.34 | 1.34 | 0.01 | 0.52 | 0.80 |
| Number of stillborn | 0.86 | 0.82 | 1.17 | 0.22 | 0.28 | 0.42 |
| Number of mummies | 0.44 | 0.22 | 0.39 | 0.11 | 0.73 | 0.14 |
| Total weaning weight, kg | 63.14 | 61.95 | 61.65 | 1.62 | 0.47 | 0.80 |
| Average weaning weight, kg | 6.03 | 5.91 | 5.83 | 0.16 | 0.33 | 0.90 |
| Piglet average daily gain, kg/d | 0.22 | 0.22 | 0.22 | 0.001 | 0.24 | 0.45 |
| Percent weaned ≤ 3.18 kg | 3.60 | 5.11 | 6.28 | 1.32 | 0.16 | 0.91 |
| Preweaning mortality, % | 17.58 | 19.38 | 19.61 | 0.56 | 0.02 | 0.25 |
A total of 98 sows (Dekalb-Monsanto) were allotted to 1 of 3 dietary treatments based on parity, and body weight at breeding. Data were analyzed using Mixed procedure of SAS as randomized complete block design with treatment as fixed effect, whereas replicate and parity group (0, 1, 2+) were random effects. Orthogonal contrasts were conducted to determine the linear and quadratic response to increasing level of Pg.
1BW = body weight; ADFI = average daily feed intake.
2SC = Sow control diet; S1 = Control diet supplemented with 0.1% Pg; S2 = Control diet supplemented with 0.2% Pg.
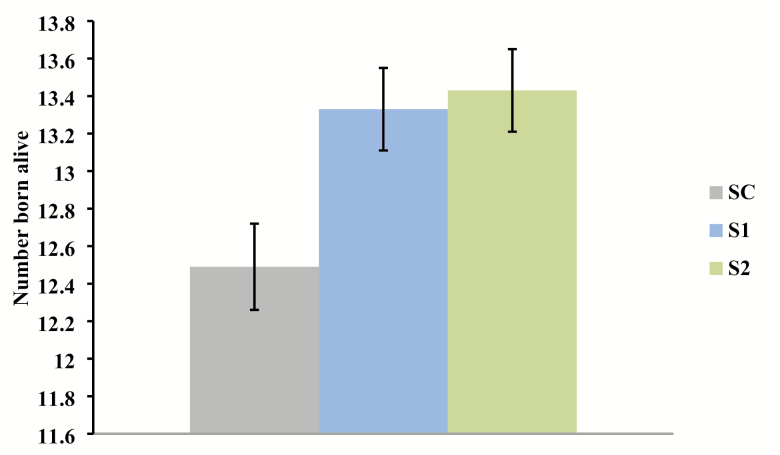
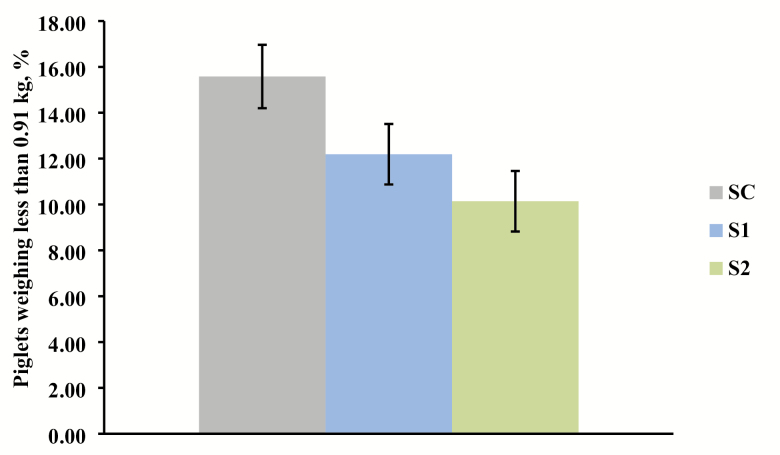
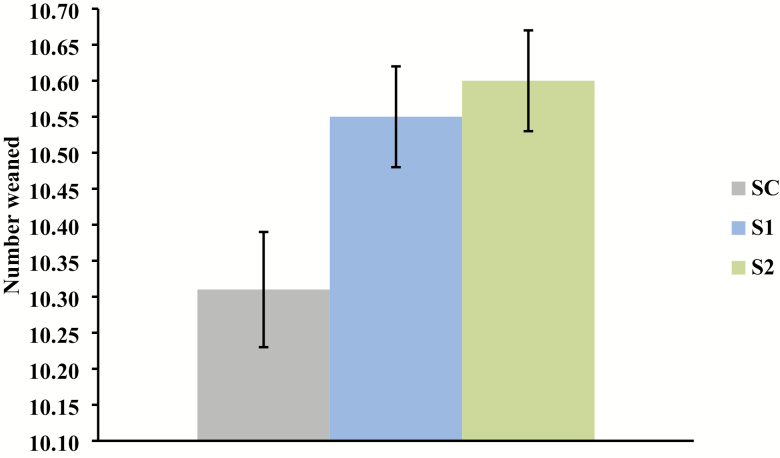
Sows supplemented with Pg gave birth to more pigs born alive per litter than those receiving the control diet (linear effect, P = 0.003; Figure 1); however, average piglet birth weight did not differ (Table 2). Thus, pig body weight was consistent though litter size increased in sows provided diets supplemented with Pg. Additionally, there was a linear decrease in the percentage of pigs weighing less than 0.9 kg at birth as the level of Pg provided to sows increased (linear effect, P = 0.006; Figure 2). The number of pigs weaned was significantly increased in sows provided Pg during gestation and lactation (linear effect, P = 0.01; Figure 3). However, there was no difference among groups in average piglet weaning weight. There was a small increase in preweaning mortality for sows provided Pg (linear effect, P = 0.01; Table 2).
Figure 1.
Effect of increasing level of inactivated P. guilliermondii (Pg) whole yeast cell product supplementation in the diet on number of pigs born alive (linear effect, P = 0.003). SC = Sow control diet (n = 32); S1 = Control + 0.1% Pg (n = 33); S2 = Control + 0.2% Pg (n = 33).
Figure 2.
Effect of increasing level of inactivated P. guilliermondii (Pg) whole yeast cell product supplementation in gestation and lactation diets on the percentage of lightweight (≤ 0.91 kg) piglets born alive (linear effect, P = 0.006). SC = Sow control diet (n = 32); S1 = Control + 0.1% Pg (n = 33); S2 = Control + 0.2% Pg (n = 33).
Figure 3.
Effect of increasing level of inactivated P. guilliermondii (Pg) whole yeast cell product supplementation in gestation and lactation diets on total number of pigs weaned (linear effect, P = 0.01). SC = Sow control diet (n = 32); S1 = Control + 0.1% Pg (n = 33); S2 = Control + 0.2% Pg (n = 33).
No differences were observed in IgA, IgG, or IgM among treatments in colostrum or milk, or serum from sows (Table 3). However, the neutrophil concentration (quadratic effect, P = 0.03) and the neutrophil:lymphocyte ratio (quadratic effect, P = 0.04) were greater on day 110 of gestation in S1 than SC, with S2 intermediate (Table 4). Additionally, lymphocytes comprised a greater (quadratic effect, P = 0.03) percentage of leukocytes in SC than S1, with S2 intermediate. At weaning, a linear increase in neutrophil concentration (5.07, 5.76, and 6.28 K/μL; P = 0.03), neutrophil:lymphocyte ratio (1.66, 1.96, and 2.34; P = 0.01), and the percentage of neutrophils (43.11%, 47.04%, and 50.08%; P = 0.01) in the leukocyte population was observed in sow fed SC, S1, and S2, respectively (Table 4). No differences were observed in the serum concentration of IgA, IgG, or IgM among treatments or concentration and proportions among peripheral blood leukocytes of 14-d-old suckling pigs (Supplementary Table 1).
Table 3.
Effects of inactivated P. guilliermondii (Pg) whole yeast cell product supplementation during gestation and lactation on sow serum, colostrum, and milk IgA, IgG, and IgM concentration
| Item | SC1n = 32 | S1 n = 33 | S2 n = 33 | SEM | Linear P-value | Quadratic P-value |
|---|---|---|---|---|---|---|
| Serum | ||||||
| IgA, mg/mL | ||||||
| day 110 | 0.89 | 1.03 | 0.80 | 0.17 | 0.71 | 0.36 |
| Weaning | 1.04 | 1.19 | 1.20 | 0.11 | 0.30 | 0.59 |
| IgG, mg/mL | ||||||
| day 110 | 15.13 | 13.62 | 13.03 | 2.46 | 0.32 | 0.79 |
| Weaning | 15.39 | 15.50 | 14.94 | 2.07 | 0.67 | 0.72 |
| IgM, mg/mL | ||||||
| day 110 | 4.83 | 6.19 | 5.24 | 2.68 | 0.68 | 0.17 |
| Weaning | 2.87 | 3.29 | 3.08 | 0.21 | 0.48 | 0.23 |
| Colostrum and Milk2 | ||||||
| IgA, mg/mL3 | ||||||
| Colostrum | 10.06 | 11.24 | 10.27 | 0.92 | 0.81 | 0.17 |
| Milk | 5.14 | 5.34 | 5.05 | 0.43 | 0.80 | 0.43 |
| IgG, mg/mL3 | ||||||
| Colostrum | 65.65 | 68.47 | 70.23 | 5.45 | 0.31 | 0.89 |
| Milk | 0.38 | 0.38 | 0.37 | 0.05 | 0.91 | 0.70 |
| IgM, mg/mL3 | ||||||
| Colostrum | 5.50 | 5.71 | 5.51 | 0.47 | 0.98 | 0.65 |
| Milk | 2.43 | 2.54 | 2.63 | 0.29 | 0.42 | 0.95 |
Colostrum, milk, and serum samples were analyzed for IgA, IgG, and IgM with commercially available ELISA kits per manufacturer’s instructions (Pig ELISA Quantitation Set, Bethyl Laboratories, Inc., Montgomery, TX).
1SC = Sow control diet; S1= Control diet supplemented with 0.1% Pg; S2 = Control diet supplemented with 0.2% Pg.
2Colostrum collected within 24 h postfarrowing. Milk collected at approximately day 14 postfarrowing.
3Concentration in colostrum differs from milk (P < 0.01).
Table 4.
Effect of inactivated P. guilliermondii (Pg) whole yeast cell product supplementation during gestation and lactation on concentration and proportions among peripheral blood leukocytes of sows at gestation day 110 and weaning
| Item | SC1n = 32 | S1 n = 33 | S2 n = 33 | SEM | Linear P-value | Quadratic P-value |
|---|---|---|---|---|---|---|
| Day 110 gestation | ||||||
| WBC, K/µL2 | 9.99 | 11.76 | 10.58 | 0.67 | 0.54 | 0.09 |
| NE, K/µL | 4.17 | 6.44 | 5.60 | 0.53 | 0.07 | 0.03 |
| LY, K/µL | 3.31 | 2.82 | 2.91 | 0.26 | 0.30 | 0.39 |
| MO, K/µL | 0.22 | 0.22 | 0.21 | 0.03 | 0.76 | 0.89 |
| EO, K/µL | 2.15 | 2.12 | 1.78 | 0.28 | 0.42 | 0.70 |
| BA, K/µL | 0.14 | 0.15 | 0.07 | 0.03 | 0.20 | 0.35 |
| NE:LY | 1.30 | 2.59 | 2.12 | 0.34 | 0.10 | 0.04 |
| NE, % | 42.54 | 54.79 | 52.32 | 3.91 | 0.10 | 0.14 |
| LY, % | 33.16 | 24.60 | 27.89 | 2.14 | 0.10 | 0.03 |
| MO, % | 2.23 | 1.88 | 2.05 | 0.24 | 0.61 | 0.38 |
| EO, % | 20.74 | 17.48 | 17.00 | 2.45 | 0.30 | 0.65 |
| BA, % | 1.33 | 1.24 | 0.73 | 0.30 | 0.18 | 0.57 |
| Weaning | ||||||
| WBC, K/µL | 12.21 | 12.25 | 12.39 | 0.64 | 0.84 | 0.95 |
| NE, K/µL | 5.07 | 5.76 | 6.28 | 0.38 | 0.03 | 0.85 |
| LY, K/µL | 3.19 | 2.99 | 2.94 | 0.14 | 0.23 | 0.68 |
| MO, K/µL | 0.33 | 0.39 | 0.33 | 0.03 | 0.87 | 0.08 |
| EO, K/µL | 3.32 | 2.88 | 2.64 | 0.36 | 0.17 | 0.82 |
| BA, K/µL | 0.30 | 0.21 | 0.19 | 0.06 | 0.17 | 0.64 |
| NE:LY | 1.66 | 1.96 | 2.34 | 0.18 | 0.01 | 0.86 |
| NE, % | 43.11 | 47.04 | 50.08 | 2.00 | 0.01 | 0.86 |
| LY, % | 26.84 | 25.11 | 24.62 | 1.12 | 0.16 | 0.65 |
| MO, % | 2.66 | 3.20 | 2.76 | 0.21 | 0.72 | 0.06 |
| EO, % | 25.32 | 22.97 | 21.02 | 1.69 | 0.07 | 0.92 |
| BA, % | 2.07 | 1.67 | 1.52 | 0.25 | 0.11 | 0.69 |
Whole blood was obtained from each sow at day 110 of gestation (group 3 only), and weaning into K2-EDTA tubes (BD Vacutainer) for determination of leukocyte differentials using a blood hematology system (Hemavet 950 FS, Drew Scientific, Waterbury, CT).
1SC = Sow control diet; S1 = Control diet supplemented with 0.1% Pg; S2 = Control diet supplemented with 0.2% Pg.
2WBC = White blood cells; NE = Neutrophils; LY = Lymphocytes; MO = Monocytes; EO = Eosinophils; BA = Basophils; NE:LY = Neutrophil:lymphocyte ratio; (K/µL) = 1,000 cells/µL; (%) = percentage of total WBC.
DISCUSSION
Many yeast culture products are derived from Saccharomyces cerevisiae; however, other yeast products exist that have not been as extensively studied. Data from the current study indicate a potential benefit to dietary supplementation during gestation and lactation with a product derived from P. guilliermondii.
Supplementing gestation diets with 0.1% or 0.2% Pg did not improve gestation body weight gain when compared with animals receiving control diets. No difference in gestation body weight gain was observed when yeast culture was supplemented throughout gestation (Shen et al., 2011), or beginning at day 60 of gestation (Veum et al., 1995). Additionally, dietary treatment was carried through lactation in both studies with no difference observed in lactation body weight loss.
Daily feed intake was similar among dietary treatment groups during the first 2 wk of lactation. However, during week 3 there was a tendency for increased ADFI in sows that received lactation diets supplemented with 0.1% Pg. Others have reported no difference in ADFI between sows fed control diets or yeast culture supplemented diets over the lactation period (Veum et al., 1995; Kim et al., 2008; Shen et al., 2011) or even an increase in ADFI for primiparous gilts fed control diets compared with those fed yeast culture-supplemented diets (Kim et al., 2010).
Several previous studies have reported no improvement in litter size at birth with Saccharomyces cerevisiae yeast culture supplementation during gestation. Veum et al. (1995), utilizing Yorkshire x Landrace x Duroc genetics, substituted 0%, 0.5%, 1.0%, or 2.0% yeast culture for wheat middlings in sows and gilts fed corn-soybean meal diets containing 25.0% and 12.5% alfalfa meal during gestation and lactation, respectively, whereas Kim et al. (2008) provided 12 g of yeast culture daily by top-dressing diets in a commercial swine facility from days 35 to 109 of gestation and 15 g of the yeast culture from day 109 of pregnancy to day 21 of lactation. Similarly, Shen et al. (2011) provided 12 g of S. cerevisiae fermentation product daily from 5 d before breeding through gestation and 15 g daily through lactation using Camborough-22 genetics fed corn-soybean meal-based diets. Czech et al. (2010) reported that sows (Landrace) provided a yeast cell wall product beginning at 4 wk before farrowing had increased number born alive, increased body weight at birth, and decreased number of stillborn compared with sows provided control wheat, triticale, or barley-soybean meal-based diets. Similarly, we found that supplementation with Pg throughout gestation in sows fed corn-soybean meal diets containing DDGS increased number born alive by almost 1 pig per litter and decreased the percentage of lightweight (≤ 0.91 kg) pigs at birth with increasing dietary inclusion of Pg. Additionally, the number of pigs per litter at weaning was increased in sows that received diets supplemented with Pg during gestation and lactation compared with those that received control diets. The percentage of preweaning mortality was greater for sows provided Pg supplemented compared with the control diet. However, no differences were observed between sows provided the control diet or Pg-supplemented diets in total birth weight, number of stillborn, total or individual weaning weight, or average daily gain. Others have reported improvements in number weaned (Czech et al., 2010) when sows were provided a yeast cell wall product, and litter weight gain (Kim et al., 2008; Shen et al., 2011) in sows provided yeast culture. These authors attributed the improvement at weaning to potential improvements in milk production, milk quality, nutrient digestibility, or immune status provided by yeast culture supplementation. In the current study, increased feed intake in week 3 of lactation did not improve litter weight at weaning, or average daily gain of nursing piglets. These studies suggest that improved reproductive performance was attained with multiple sow genetics in sows fed diets varying in ingredients. These improvements have been observed with yeast cell wall, yeast culture, or a whole yeast product and involved studies with S. cerevisiae- or P. guilliermondii-based products.
To further examine how Pg affected sow and litter immune parameters, we measured peripheral blood white blood cell counts and serum immunoglobulin concentrations from sows on day 110 of gestation and at weaning, as well as suckling pigs at approximately day 14 of age. Additionally, immunoglobulin concentration of colostrum and milk was examined. Supplementation of gestation diets with Pg did not change serum concentrations of IgA, IgG, or IgM on day 110 of gestation, or at weaning, when compared with sows fed the control diet. Similarly, concentrations of IgA, IgG, and IgM in colostrum, or milk, were similar among treatment groups. Based on these data, as expected, there were also no differences among treatments in serum concentrations of IgA, IgG, or IgM of 14-d-old suckling pigs. Shen et al. (2011) also reported no difference in IgG concentration of colostrum or milk of the sow, or serum of 17-d-old suckling pigs, when sows were provided control or yeast culture supplemented gestation and lactation diets. In contrast, Czech et al. (2010) observed that sows provided yeast cell wall from day 84 of gestation through a 28-d lactation period had increased serum IgG on day 110 of gestation and IgA on day 21 of lactation. Additionally, colostrum concentrations of IgG and IgM were increased for sows that were fed diets supplemented with yeast culture compared with those provided control diets. Piglets from these sows had increased serum concentrations of IgG and IgM at birth, and IgG concentrations remained elevated at day 21 of age (Czech et al., 2010). Factors such as diet and immune challenge can affect immunoglobulin levels in serum and colostrum. Nonspecific immunostimulation of sows 4 to 6 wk before parturition caused an increase in serum, as well as colostral, IgG concentration, and improved body weight at 2 and 28 d of age of piglets suckling sows that had been immunostimulated compared with piglets suckling control sows (Krakowski et al., 2002). Thus, stimulation of the immune system near parturition may cause the improvement observed in colostrum and piglet performance.
Sows that were provided a gestation diet supplemented with 0.1% Pg tended to have more leukocytes in the peripheral circulation than those provided the control diet or diet supplemented with 0.2% Pg. Additionally, peripheral blood of sows that received the gestation diet supplemented with 0.1% Pg had a greater neutrophil concentration, a greater percentage of neutrophils in the leukocyte population, and decreased percentage of lymphocytes in the leukocyte population, which increased the neutrophil:lymphocyte ratio. At weaning, a linear increase in neutrophils, the percentage of leukocytes that were neutrophils, and neutrophil:lymphocyte ratio were observed as the level of Pg increased in gestation and lactation diets. These data are contrary to that of Shen et al. (2011), who reported a decrease in peripheral blood neutrophil concentration in response to supplementation of a S. cerevisiae fermentation product. It is interesting to note that these differences were not apparent on day 30 of gestation in the study by Shen, indicating there may be a conditioning period needed to see the effects of the yeast culture product supplementation in blood cell counts. The increase in peripheral blood neutrophil concentration in the current study may indicate that sows provided Pg had an increased inflammatory challenge compared with those receiving the control diet as neutrophils represent the first line of defense in an infection. Additionally, increased neutrophil:lymphocyte ratio is associated with stress in pigs (Stull et al., 1999) and a reduction in neutrophils with concurrent increase in lymphocytes was determined to be an alleviation of inflammation in weaned pigs (Davis et al., 2004), though the opposite was observed in the current study. Unlike their dams, there were no differences in the proportions among leukocytes observed in 14-d-old suckling pigs in the current study. This agrees with Shen, who found modulations in immune cell numbers in sows fed a yeast culture product compared with those receiving a control diet but did not observe differences in the proportions among leukocytes in 17-d-old suckling pigs.
In summary, supplementation of standard gestation and lactation diets with Pg may be beneficial to sow reproductive performance, as well as the performance of her litter as manifested in an improvement in litter size at birth, as well as at weaning without decreasing pig weight, and a reduction in light weight pigs at birth. Additionally, it appears that supplementation of Pg to gestation and lactation diets alters the immune profile of sow serum, but not colostrum or milk, when provided throughout gestation and lactation. Thus, inactivated P. guilliermondii whole yeast cell product may be a viable alternative to other yeast culture products in its ability to improve reproductive performance and act as an immunomodulator.
Supplementary Material
LITERATURE CITED
- Czech A., Grela E. R., Mokrzycka A., and Pejsak Z.. 2010. Efficacy of mannanoligosaccharides additive to sows diets on colostrum, blood immunoglobulin content and production parameters of piglets. Pol. J. Vet. Sci. 13:525–531. [PubMed] [Google Scholar]
- Dann H. M., Drackley J. K., McCoy G. C., Hutjens M. F., and Garrett J. E.. 2000. Effects of yeast culture (Saccharomyces cerevisiae) on prepartum intake and postpartum intake and milk production of jersey cows. J. Dairy Sci. 83:123–127. doi:10.3168/jds.S0022-0302(00)74863-6 [DOI] [PubMed] [Google Scholar]
- Davis M. E., Maxwell C. V., Erf G. F., Brown D. C., and Wistuba T. J.. 2004. Dietary supplementation with phosphorylated mannans improves growth response and modulates immune function of weanling pigs. J. Anim. Sci. 82:1882–1891. doi:10.2527/2004.8261882x [DOI] [PubMed] [Google Scholar]
- Kim S. W., Brandherm M., Freeland M., Newton B., Cook D., and Yoon I.. 2008. Effects of yeast culture supplementation to gestation and lactation diets on growth of nursing piglets. Asian-Aust. J. Anim. Sci. 21:1011–1014. doi:10.5713/ajas.2008.70438 [Google Scholar]
- Kim S. W., Brandherm M., Newton B., Cook D. R., Yoon I., and Fitzner G.. 2010. Effect of supplementing Saccharomyces cerevisiae fermentation product in sow diets on reproductive performance in a commercial environment. Can. J. Anim. Sci. 90:229–232. doi:10.4141/CJAS09100 [Google Scholar]
- Krakowski L., Krzyzanowski J., Wrona Z., Kostro K., and Siwicki A. K.. 2002. The influence of nonspecific immunostimulation of pregnant sows on the immunological value of colostrum. Vet. Immunol. Immunopathol. 87:89–95. doi:10.1016/S0165-2427(02)00004-1 [DOI] [PubMed] [Google Scholar]
- NRC 1998. Nutrient requirements of swine. 10th rev. ed. Natl. Acad. Press, Washington, DC: p. 111–116. doi:10.17226/6016 [Google Scholar]
- Robinson P. H., and Garrett J. E.. 1999. Effect of yeast culture (Saccharomyces cerevisiae) on adaptation of cows to postpartum diets and on lactational performance. J. Anim. Sci. 77:988–999. doi:10.2527/1999.774988x [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram R., and Selvaraj R. K.. 2012. Effect of killed whole yeast cell prebiotic supplementation on broiler performance and intestinal immune cell parameters. Poult. Sci. 91:107–111. doi:10.3382/ps.2011-01732 [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Carroll J. A., Yoon I., Mateo R. D., and Kim S. W.. 2011. Effects of supplementing saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets. J. Anim. Sci. 89:2462–2471. doi:10.2527/jas.2010-3642 [DOI] [PubMed] [Google Scholar]
- Spring P., Wenk C., Dawson K. A., and Newman K. E.. 2000. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poult. Sci. 79:205–211. doi:10.1093/ps/79.2.205 [DOI] [PubMed] [Google Scholar]
- Stull C. L., Kachulis C. J., Farley J. L., and Koenig G. J.. 1999. The effect of age and teat order on alpha1-acid glycoprotein, neutrophil-to-lymphocyte ratio, cortisol, and average daily gain in commercial growing pigs. J. Anim. Sci. 77:70–74. doi:10.2527/1999.77170x [DOI] [PubMed] [Google Scholar]
- Veum T. L., Reyes J., and Ellersieck M.. 1995. Effect of supplemental yeast culture in sow gestation and lactation diets on apparent nutrient digestibilities and reproductive performance through one reproductive cycle. J. Anim. Sci. 73:1741–1745. doi:10.2527/1995.7361741x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.