Abstract
Eight crossbred steers (BW 719.0 ± 65.0 kg) with ruminal and duodenal cannulae were used to examine the effect of trace mineral (TM) source on digestibility; ruminal and duodenal solubility of Cu, Zn, and Mn; and in vitro release of Cu, Zn, and Mn from the solid fraction of ruminal digesta. Experiment 1 determined the effect of TM source on DM and NDF digestibility in steers fed a corn silage and steam-flaked corn-based diet. Treatments consisted of 10 mg Cu, 20 mg Mn, and 30 mg Zn/kg DM from either sulfate TM (STM) or hydroxy TM (HTM) sources. Following a 14-d adaptation period, total fecal output was collected for 5 d. Dry matter digestibility was not affected by treatment, but NDF digestibility tended (P < 0.09) to be greater in HTM vs. STM supplemented steers. In Exp. 2, steers were fed a diet without supplemental Cu, Zn, or Mn for 19 d. Steers were then administrated a pulse dose of STM or HTM (2× the National Research Council requirements for Cu, Mn, and Zn) via the rumen fistula. Ruminal and duodenal samples were obtained at 2-h intervals starting at −4 and ending at 24 h relative to dosing. Ruminal soluble Cu and Zn concentrations were affected by treatment, time, and treatment × time. Soluble concentrations and percent soluble Cu and Zn in ruminal digesta increased (P < 0.05) above 0-h values for 10 h following dosing with STM, but not HTM. Concentrations of Cu and Zn in ruminal solid digesta were also affected by treatment, time, and treatment × time. Steers dosed with STM had greater (P < 0.05) solid digesta Cu concentrations at 2 and 4 h but lesser (P < 0.05) concentrations from 6 to 20 h post-dosing than those receiving HTM. Ruminal solid digesta Zn concentrations were greater (P < 0.05) in HTM vs. STM-dosed steers from 6 through 24 h post-dosing. Distribution of Mn in ruminal digesta was affected by TM source, but to a lesser extent than Zn and Cu. Duodenal soluble TM concentrations were variable and not affected by treatment. Binding strength of TM to ruminal solid digesta was estimated at 0, 6, and 12 h post-dosing using dialysis against chelating agents. The percentage of Cu and Zn released from ruminal solid digesta by dialysis against Tris–EDTA was greater (P < 0.05) at 12 h post-dosing from steers receiving HTM vs. STM. Results indicate that Cu and Zn from HTM have low solubility in the rumen and appear to be less tightly bound to ruminal solid digesta than Cu and Zn from STM.
Keywords: digestibility, solubility, steers, trace minerals
INTRODUCTION
Trace mineral (TM) sources vary in water solubility, and this may affect their reactivity with antagonists and microorganisms in the rumen. Copper from Cu hydroxychloride (CuOHCl) was relatively insoluble (0.6%) in water (pH 7.0) and highly soluble (81.4%) at a low pH (2.2), whereas Cu from CuSO4 was almost completely soluble in both water and at a low pH (Spears et al., 2004). Relative bioavailability of Cu from CuOHCl was greater than CuSO4 based on plasma Cu, plasma ceruloplasmin, and liver Cu concentrations in steers fed a corn silage-based diet supplemented with the Cu antagonists Mo and S (Spears et al., 2004). Spears et al. (2004) suggested low solubility of CuOHCl under slightly acidic pH conditions may have reduced interactions of Cu with antagonists in the rumen. Zinc hydroxychloride (ZnOHCl) has also been reported to have low solubility in water (Cao et al., 2000) and was less soluble in the rumen of cattle when compared with Zn from ZnSO4 (Shaeffer, 2006). Moreover, cattle receiving ZnOHCl had greater apparent Zn absorption and retention than cattle supplemented with ZnSO4 following a 14-d depletion period (Shaeffer et al., 2017).
Greater soluble concentrations of Cu and Zn in the ruminal environment can decrease fiber digestion in the rumen (Durand and Kawashima, 1980). Faulkner and Weiss (2017) recently reported that dairy cows supplemented with hydroxy TM (HTM) had greater NDF digestibility than those supplemented with sulfate TM (STM) sources. Therefore, our working hypothesis was that utilizing HTM would result in lower soluble TM concentrations in the rumen, thus affecting fermentation and mineral interactions differently from sulfate sources. The objectives of the current experiments were to examine the influence of TM source on 1) apparent DM and NDF digestibility in steers; 2) ruminal and duodenal solubility of Cu, Zn, and Mn; and 3) in vitro release of Cu, Zn, and Mn from the solid portion of ruminal digesta using dialysis against chelating agents.
MATERIALS AND METHODS
Prior to the initiation of this experiment, all animal care, handling, and procedures described herein were approved by the Colorado State University Animal Care and Use Committee (IACUC approval #13-4437A).
Experiment 1
Eight crossbred steers (BW 719.0 ± 65.0 kg) fitted with ruminal (10.2-cm rolled rumen cannula) and duodenal (2.54-cm-diameter closed T-cannula) cannulae (Bar Diamond, Inc. Parma, ID) were utilized to examine DM and NDF digestibility. Steers were blocked by BW and randomly assigned to 1 of 2 TM source treatments: 1) STM (10 mg Cu/kg DM from CuSO4; 20 mg Mn/kg DM from MnSO4; 30 mg Zn/kg DM from ZnSO4) and 2) HTM (10 mg Cu/kg DM from CuOHCl, 20 mg Mn/kg DM from MnOHCl, 30 mg Zn/kg DM from ZnOHCl; IntelliBond C, IntelliBond M, and IntelliBond Z, respectively; Micronutrients LLC, Indianapolis, IN). All steers were fed a diet containing 49% corn silage, 49% steam-flaked corn, and 2% supplement on a DM basis (Table 1). Diets were formulated to meet or exceed NRC (2000) requirements. Steers were housed in 2 pens (n = 4 steers per pen) and fed a diet with the appropriate TM treatments for 7 d. At the initiation of week 2, steers were housed in individual pens within the metabolism building (2.5 × 2.5 m pens equipped with automatic waters, individual plastic feeders, rubber matted floors, and a drain) for 2 d to acclimate to the building environment. Steers were then moved into individual metabolism stalls (3.0 × 1.1 m pens equipped with automatic waters, individual plastic feeders, and rubber-matted floors) for a 5-d acclimation period. During the acclimation period, DMI for each steer was determined. At the end of the acclimation period, steers from each treatment (STM and HTM) were paired based on their mean DMI over the 5-d period. The criteria for pairing was that 2 paired animals had to differ less than 5% in DMI. Once animals were appropriately paired by DMI, each individual steer within a pair was fed the same amount of feed. Feed delivered to each steer within a pair was 90% of the DMI of the steer within the pair with the lowest average DMI during the acclimation period. This ensured equal amounts of mineral intake for individual steers within a pair (block) during the 5-d total fecal collection period. The next 5 d served as the sample collection period.
Table 1.
Ingredient composition of basal diets (% DM)
| Ingredient | Experiment 1 | Experiment 2 |
|---|---|---|
| Corn silage | 49.0 | 50.0 |
| Steam-flaked corn | 49.0 | 50.0 |
| Ground corn | 2.0 | — |
| Mineral premix1 | + | + |
| Chemical composition | ||
| DM, % | 46.3 | 45.2 |
| CP, % | 10.5 | 10.4 |
| NEm, Mcal/kg | 1.97 | 1.96 |
| NEg, Mcal/kg | 1.32 | 1.31 |
| Fat, % | 3.6 | 3.5 |
| ADF, % | 14.7 | 14.9 |
| NDF, % | 28.1 | 28.4 |
| Calcium, % | 0.19 | 0.20 |
| Phosphorus, % | 0.23 | 0.22 |
| Sulfur, % | 0.12 | 0.13 |
| Copper, mg/kg | 2.1 | 2.2 |
| Manganese, mg/kg | 19.0 | 19.2 |
| Iron, mg/kg | 78.5 | 79.0 |
| Zinc, mg/kg | 15.1 | 15.0 |
1Provided per kilogram of total diet: 0.20% salt, 0.5 mg of I from ethylenediamine dihydroiodide, 0.10 mg of Co from CoCO3, and 0.10 mg of Se from NaSeO3.
Feeding and fecal collection.
Diets were fed twice daily (60% of the ration in the morning and 40% of the ration on the afternoon). Appropriate TM treatment supplements were manufactured prior to the initiation of the experiment. Ground corn was used as the carrier. Immediately after feeding the basal diet, the appropriate TM supplement amounts (60% of the ration in the morning and 40% of the ration on the afternoon) were top-dressed and mixed thoroughly by hand for each feeding within a day. Total fecal output was measured daily for individual steers during the 5-d collection period. The fecal bags used for fecal collection were similar to those described by Tolleson and Erlinger (1989) and Border et al. (1963). Feces collected each day (over a 24-h period) were weighed, thoroughly mixed, and sampled (10.0% of wet weight). Duplicate, individual fecal samples were sealed in plastic bags, labeled, and stored at −20 °C. Prior to DM and NDF analysis, samples were proportionally composited across all collection days for each animal. Dry matter analysis was determined by placing a known mass of wet material in a forced-air drying oven for 48 h at 100 °C. After drying, samples were allowed to cool in a desiccator and then weighed. Percent DM was calculated using the formula: DM % = (1.0 − [(wet weight − dry weight)/wet weight]) ×100. Neutral detergent fiber was analyzed using an Ankom 200 Fiber analyzer (Ankom Technology Corp., Macedon, NY) with a heat stable α-amylase (Van Soest et al., 1991).
Experiment 2
The same 8 crossbred steers utilized in Exp. 1 were used in Exp. 2. Immediately after the termination of Exp. 1, all steers were housed outdoors in a group pen and fed a corn silage-based diet for 90 d. On day 91, steers were transitioned over a 14-d period to a diet containing 50% corn silage and 50% steam-flaked corn on a DM basis (Table 1). No supplemental Cu, Zn, or Mn was added to the diet. On day 15, steers were housed in the metabolism barn in individual pens as described above. Steers were allowed to acclimate to their new environment for 5 d. At the end of the 5-d acclimation phase, steers were paired as described in Exp. 1. Experimental treatments consisted of 1) STM (20 mg Cu/kg DM from CuSO4, 40 mg Mn/kg DM from MnSO4, 60 mg Zn/kg DM from ZnSO4) and 2) HTM (20 mg Cu/kg DM from CuOHCl, 40 mg Mn/kg DM from MnOHCl, 60 mg Zn/kg DM from ZnOHCl). Trace mineral treatments were weighed in amounts to provide 2 times NRC (2000) values for Cu, Zn, and Mn. A 10 kg of DMI was used to calculate the total amount of TM added to the pulse-dose TM supplement. Individual TM treatments were thoroughly mixed with 0.23 kg of ground corn and administered as a single pulse-dose via the rumen fistula. Immediately post-administration, the supplement was thoroughly mixed with the rumen contents by hand. Ruminal and duodenal samples were obtained at 2-h intervals starting at −4 and ending at 24 h relative to dosing. Before each sampling time, the rumen contents were thoroughly mixed by hand and pH was determined by inserting a portable pH meter (EcoTestr pH 2+; Oakton Instruments, Vernon Hills, IL) into the geometric center of the rumen. The pH meter was standardized prior to each collection time point. Immediately after pH determination, a grab sample of ruminal contents was obtained from the same location (approximately 250 g). Duodenal samples were obtained by allowing duodenal contents (approximately 50.0 mL) to run directly into collection tubes. During the collection period, steers had access to feed (Table 1) and water.
Ruminal and duodenal samples were centrifuged at 28,000 × g in graduated centrifuge tubes shortly after being collected. Once centrifuged, the volume of supernatant was determined and frozen at −20 °C until TM analysis could be performed. The Cu, Zn, and Mn concentrations of the supernatant and pellet fractions were considered to be the soluble and solid (insoluble) fractions of these elements, respectively. To express the soluble fraction of each element as a percentage of total element in the rumen sample (supernatant + pellet), the total mg of each mineral (Cu, Zn, and Mn) were calculated for the supernatant fraction (using the measured volume in milliliter after centrifugation) and the pellet fraction (using the weight of the pellet after centrifugation). The amount (in mg) of each element within the 2 fractions was then summed and the supernatant element (in milligram) was divided by the total amount of the element (supernatant + pellet in milligrams) and multiplied by 100 to obtain the percentage of each element that was soluble. Ruminal solid digesta samples from collection times 0, 6, and 12 h were also exposed to dialysis as described below. All TM were quantified via inductively coupled plasma-mass spectrometry (PerkinElmer; NexION 2000 B).
Dialysis of ruminal insoluble digesta.
Rumen digesta pellets from the 0-, 6-, and 12-h sampling times were dried at 60 °C for 24 h and finely ground using a mortar and pestle. A subsample of each pellet was analyzed for TM, and the remaining samples were divided into equal quantities and dialyzed against one of the following chelating buffers: 1) 0.01 M ethylenediaminetetraacetate in 0.05 M tris(hydroxymethyl)aminomethane (Tris–EDTA) and 2) 0.01 M l-histidine hydrochloride in 0.05 M Tris (Tris–His).
For dialysis, regenerated cellulose dialysis tubing (31.8 mm diameter, 30 µm wall thickness, molecular weight cutoff 6,000 to 8,000; Fisher Scientific, Pittsburgh, PA) was cut into 10.0-cm segments and treated to remove metal contamination by the method described by the Ohio Agriculture Research and Development Center (https://stockingerlab.osu.edu/sites/stockinger/files/imce/PDFs/Protocols/DialysisTubing.pdf). Dialysis tubing was stored in 50% ethanol, 50% deionized water, and 1 mM EDTA at 4 °C prior to use. The diluted buffers were prepared immediately prior to use and the pH adjusted to 6.8 with either 6.0 N HCl (Tris–EDTA) or sodium hydroxide (Tris–His). Samples were placed into 10.0 mL of the appropriate buffer, placed into dialysis tubing pre-wet with deionized water, the tubing was sealed with clips, and the samples dialyzed against 1.0 L of the same buffer for 16 h at 4 °C with continuous stirring. The buffer was then changed, and dialysis continued for another 6 h. Samples were removed from dialysis tubing, placed into pre-weighed acid-washed crucibles, and dried overnight at 60 °C. After drying, samples were weighed, ashed, resuspended in 1.2 N HCl, and then all metals were quantified as previously described.
Statistics
Dry matter and NDF apparent digestibility (Exp. 1) were analyzed using a mixed effects model (PROC MIXED, SAS Inst. Inc., Cary, NC) for a completely randomized block design. Ruminal and duodenal Cu, Zn, and Mn concentrations (Exp. 2) were analyzed using a mixed effects model repeated measures analysis (PROC MIXED) for a completely randomized block design. In both experiments, treatment, time, and the interaction of treatment and time were considered fixed effects, and individual animal was considered the experimental unit. The specified term used in the repeated statements was time, and the subject was animal (treatment). Multiple covariance structures were compared using AIC to determine the most appropriate covariance structure for data analysis. The AR(1) covariance structure was the most appropriate for the repeated measures analysis. For all response variables, significance was determined at P ≤ 0.05, and tendencies were determined at P ≤ 0.10. In the event of a significant treatment × time interaction, treatment means were separated using the PDIFF option of the LSMEANS statement of SAS.
RESULTS
Experiment 1
Dry matter intake and apparent DM digestibility (P = 0.18) were not affected by treatment (Table 2). Apparent digestibility of NDF tended (P = 0.09) to be greater in steers fed HTM than in those supplemented with STM.
Table 2.
Influence of trace mineral source on DM and NDF digestibility (Exp 1)
| Item | Treatment | SEM | P | |
|---|---|---|---|---|
| HTM1 | STM2 | |||
| DM intake, kg/d | 9.89 | 9.92 | 0.96 | <0.98 |
| DM digestibility, % | 70.7 | 65.6 | 2.4 | <0.18 |
| NDF digestibility, % | 41.2 | 37.8 | 1.7 | <0.09 |
1HTM = hydroxy trace minerals: 30 mg of Zn/kg DM from ZnOHCl; 10 mg of Cu/kg DM from CuOHCl; 20 mg of Mn/kg DM from MnOHCl.
2STM = sulfate trace minerals: 30 mg of Zn/kg DM from ZnSO4; 10 mg of Cu/kg DM from CuSO4; 20 mg of Mn/kg DM from MnSO4.
Experiment 2
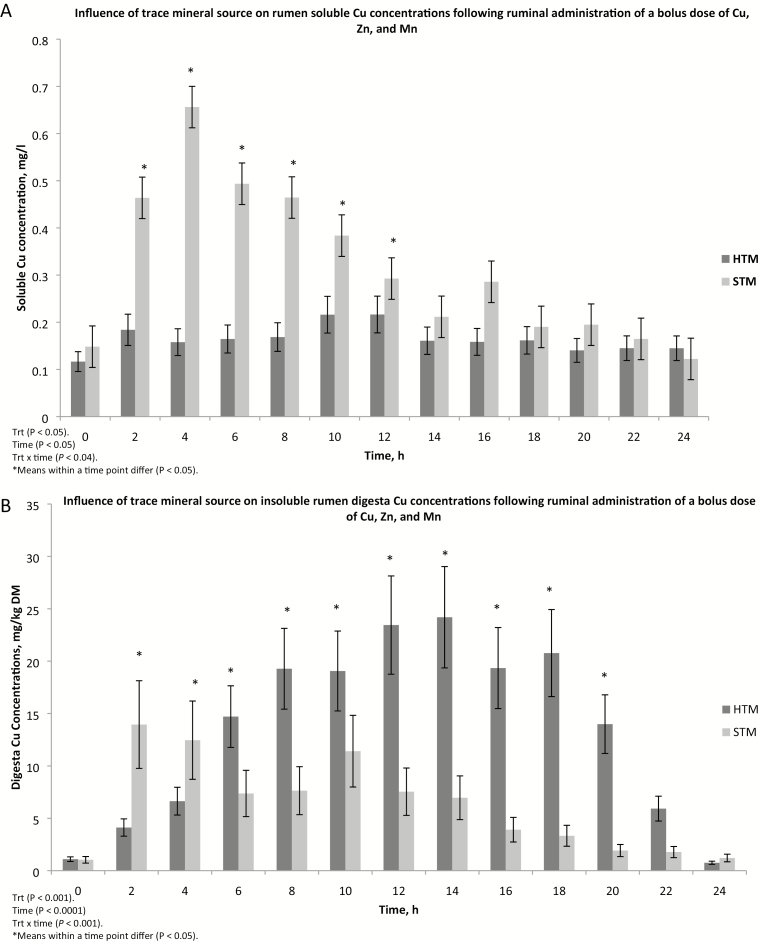
Rumen pH averaged 6.23 across all sampling times and was not affected by TM source (P = 0.24), time (P = 0.52), or treatment × time (P = 0.85; data not shown). Ruminal soluble Cu concentrations were affected by treatment (P < 0.05), time (P < 0.001), and a treatment × time interaction (P < 0.04; Fig. 1A). Compared with pre-dosing concentrations, ruminal soluble Cu concentrations were greater (P < 0.05) at 2, 4, 6, 8, 10, 12, and 16 h post-dosing in steers given STM. Ruminal soluble Cu concentrations did not increase (P > 0.36) over time following dosing in steers receiving HTM. Soluble Cu concentrations were greater (P < 0.05) in steers receiving STM compared with those given HTM at 2, 4, 6, 8, 10, and 12 h post-dosing. Ruminal soluble Cu, expressed as percent of total Cu in the rumen sample, was also affected by treatment (P < 0.05), time (P < 0.04), and treatment × time (P < 0.04; Fig. 1C). Percent soluble Cu in ruminal fluid was greater (P < 0.05) from 2 to 10 h post-dosing in STM steers than in HTM steers. Percent ruminal soluble Cu did not increase following the pulse dose in HTM steers. Compared with 0-h values, percentage ruminal soluble Cu was greater (P < 0.05) at 2, 4, 6, 8, 10, 12, and 16 h post-dosing in steers receiving STM. Ruminal solid digesta Cu concentrations were affected by treatment (P < 0.001), time (P < 0.001), and treatment × time (P < 0.0001; Fig. 1B). In steers dosed with HTM, ruminal solid digesta Cu concentrations were greater (P < 0.05) from 4 to 20 h post-dosing relative to pre-dosing (time 0) values. Between 2 and 14 h post-dosing, ruminal solid Cu concentrations were greater (P < 0.05) than pre-dosing concentrations in steers receiving STM. Steers given STM had greater (P < 0.05) ruminal solid digesta Cu concentrations at 2 and 4 h but lesser (P < 0.05) solid Cu concentrations from 6 to 20 h post-dosing than those receiving HTM.
Figure 1.
The influence of trace mineral source on copper distribution within the ruminal contents of steers receiving a pulse dose of either sulfate trace minerals (STM; 20 mg Cu/kg DM from CuSO4; 40 mg Mn/kg DM from MnSO4; 60 mg Zn/kg DM from ZnSO4) or hydroxy trace minerals (HTM; 20 mg Cu/kg DM from CuOHCl; 40 mg Mn/kg DM from MnOHCl; 60 mg Zn/kg DM from ZnOHCl). The x-axis denotes sampling time in hours; the y-axis denotes (A) rumen soluble copper concentrations in mg/L; (B) solid rumen digesta copper concentrations in mg/kg DM; and (C) the percent soluble copper in ruminal digesta. Error bars represent SE (Exp. 2).
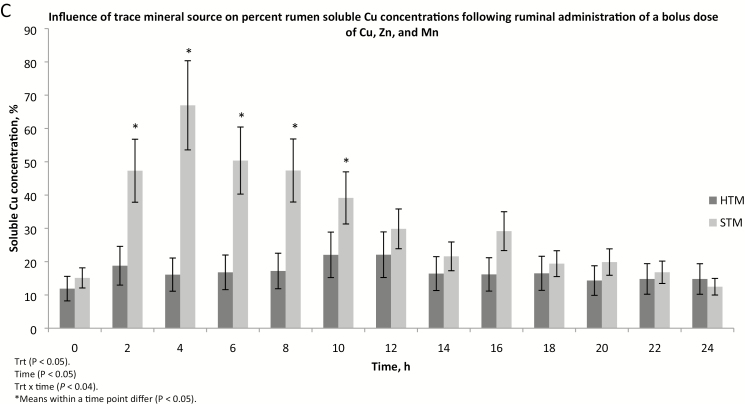
Ruminal soluble Zn was affected by treatment (P < 0.04), time (P < 0.01), and treatment × time (P < 0.05; Fig. 2A). Steers dosed with STM had greater (P < 0.05) soluble Zn concentrations than those given HTM at 2, 4, 6, 8, and 10 h following the pulse dose. Soluble Zn concentrations did not increase (P > 0.50) following pulse dosing in steers given HTM. Compared with pre-dosing concentrations, ruminal soluble Zn concentrations were greater at 2, 4, 6, 8, 10, and 14 h post-dosing in steers receiving STM. Percent ruminal soluble Zn did not increase (P > 0.10) post-dosing in steers dosed with HTM (Fig. 2C). In steers dosed with STM, percent ruminal soluble Zn was greater (P < 0.05) at 2, 4, 6, and 16 h following dosing compared with pre-dosing concentrations. Percent ruminal soluble Zn was greater (P < 0.05) in STM compared with HTM-dosed steers 2 through 20 h post-dosing. Digesta Zn concentrations in the ruminal solid or insoluble fraction were affected by treatment (P < 0.02), time (P < 0.001), and treatment × time (P < 0.02; Fig. 2B). Ruminal solid digesta Zn concentrations did not increase (P > 0.42) following the pulse dose in steers receiving STM. Compared with 0-h values, solid digesta Zn concentrations in HTM-dosed steers were greater (P < 0.05) at 8, 10, 12, 14, 16, 18, 20, and 24 h post-dosing. Steers receiving HTM had greater (P < 0.05) solid digesta Zn concentrations than STM-dosed steers from 6 through 24 h post-dosing.
Figure 2.
The influence of trace mineral source on zinc distribution within the ruminal contents of steers receiving a pulse dose of either sulfate trace minerals (STM; 20 mg Cu/kg DM from CuSO4; 40 mg Mn/kg DM from MnSO4; 60 mg Zn/kg DM from ZnSO4) or hydroxy trace minerals (HTM; 20 mg Cu/kg DM from CuOHCl; 40 mg Mn/kg DM from MnOHCl; 60 mg Zn/kg DM from ZnOHCl). The x-axis denotes sampling time in hours; the y-axis denotes (A) rumen soluble zinc concentrations in mg/L; (B) solid rumen digesta zinc concentrations in mg/kg DM; and (C) the percent soluble zinc in ruminal digesta. Error bars represent SE (Exp. 2).
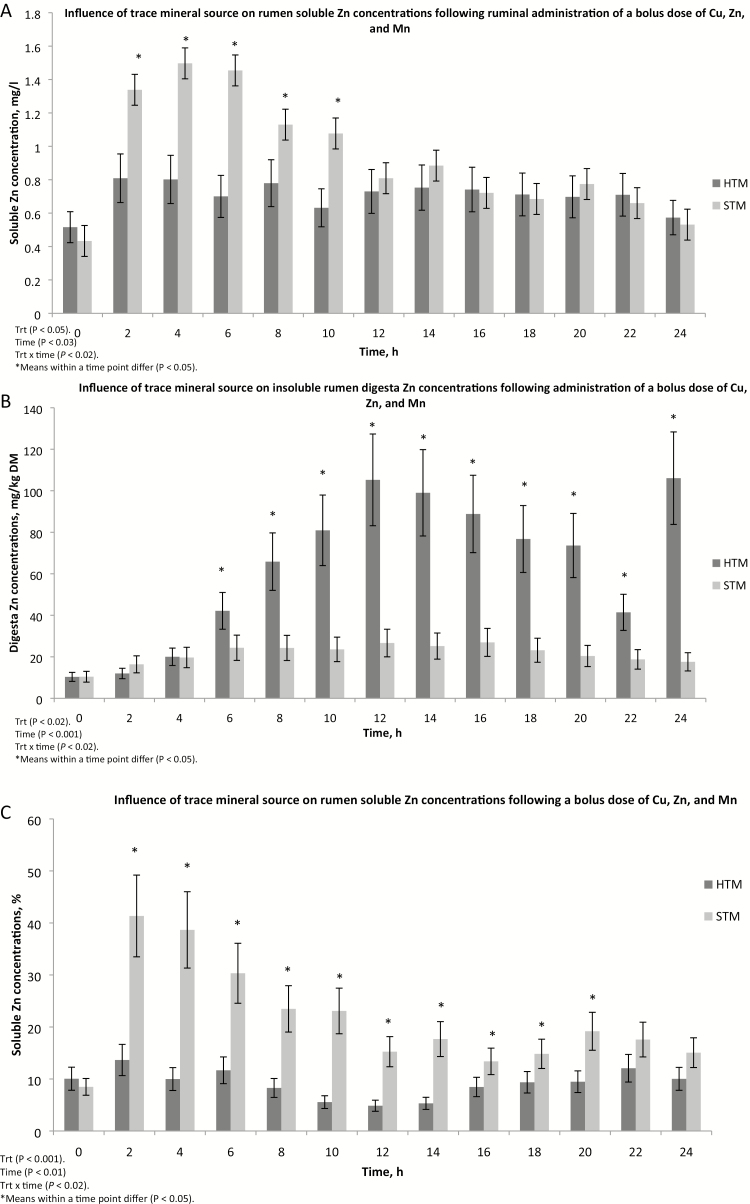
Soluble Mn concentrations in ruminal fluid were affected by time (P < 0.001) and treatment × time (P < 0.01; Fig. 3A). Ruminal soluble Mn concentrations were greater (P < 0.05) in STM-dosed steers at 4 and 8 h and tended (P < 0.10) to be greater at 10 and 12 h post-dosing compared with steers given HTM. Steers receiving HTM had greater (P < 0.05) ruminal soluble Mn concentrations at 24 h and tended (P < 0.10) to have greater concentrations at 2 and 18 h post-dosing than STM-dosed steers. Ruminal soluble Mn concentrations were lesser (P < 0.05) at 0 h than at all times post-dosing in HTM steers. Compared with 0-h values, STM-dosed steers had greater (P < 0.05) ruminal soluble Mn concentrations at all times post-dosing except at 2 and 24 h. Ruminal soluble Mn, expressed as a percent of total Mn in rumen samples, was also affected by time (P < 0.001) and treatment × time (P < 0.001; Fig. 3C). Percent soluble Mn in steers receiving STM was greater (P < 0.05) from 2 through 20 h post-dosing compared with pre-dosing concentrations. Compared with time 0, percent ruminal soluble Mn was greater (P < 0.05) at 18, 20, 22, and 24 h and tended (P < 0.10) to be greater at 2, 4, and 6 h post-dosing in steers given HTM. Steers dosed with STM had greater (P < 0.05) percent ruminal soluble Mn at 4 and 8 h post-dosing than steers given HTM. However, at 24 h post-dosing, percent soluble Mn was greater (P < 0.05) in HTM steers compared with STM steers. Ruminal solid digesta Mn concentrations were affected by treatment (P < 0.04), time (P < 0.001), and treatment × time (P < 0.001; Fig. 3B). Manganese concentrations in ruminal solid digesta were increased (P < 0.05) above pre-dosing concentrations from 2 through 22 h post-dosing in HTM steers. In steers receiving STM, ruminal solid Mn concentrations were increased (P < 0.05) compared with pre-dosing concentrations from 6 through 24 h post-dosing. Steers dosed with HTM had greater (P < 0.05) ruminal soluble digesta Mn concentrations than STM-dosed steers at 2, 4, 8, 10, 14, 16, and 20 h post-dosing.
Figure 3.
The influence of trace mineral source on manganese distribution within the ruminal contents of steers receiving a pulse dose of either sulfate trace minerals (STM; 20 mg Cu/kg DM from CuSO4; 40 mg Mn/kg DM from MnSO4; 60 mg Zn/kg DM from ZnSO4) or hydroxy trace minerals (HTM; 20 mg Cu/kg DM from CuOHCl; 40 mg Mn/kg DM from MnOHCl; 60 mg Zn/kg DM from ZnOHCl). The x-axis denotes sampling time in hours; the y-axis denotes (A) rumen soluble manganese concentrations in mg/L; (B) solid rumen digesta manganese concentrations in mg/kg DM; and (C) the percent soluble manganese in ruminal digesta. Error bars represent SE (Exp. 2).
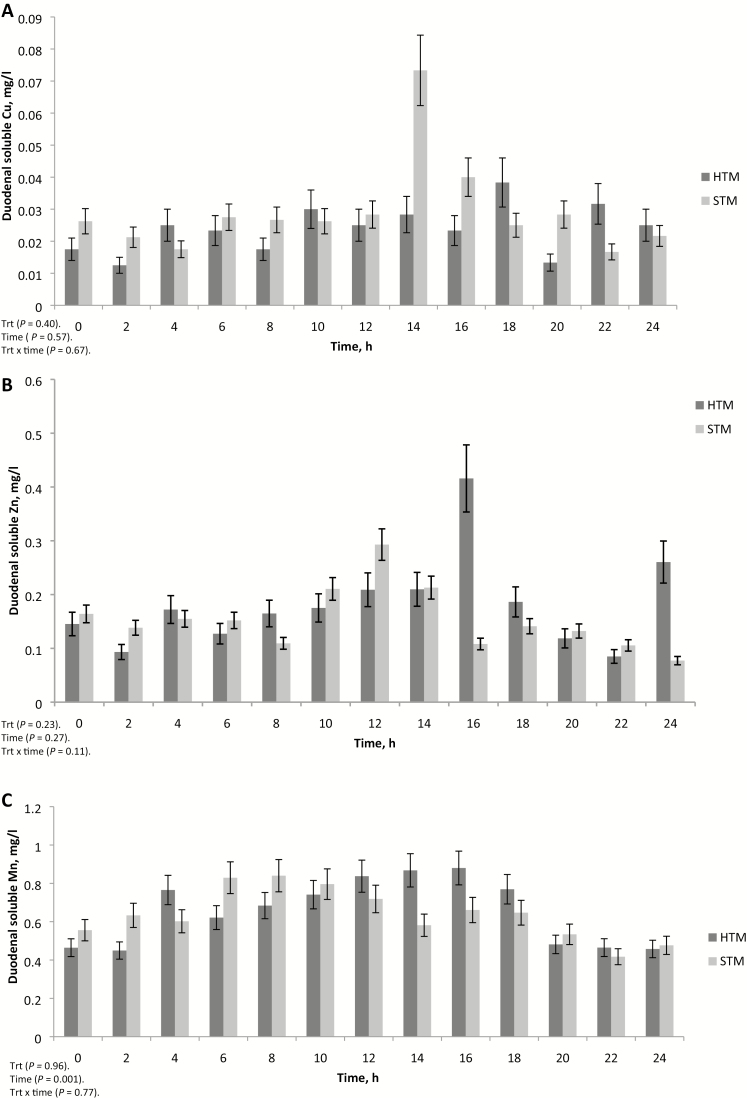
Duodenal soluble TM concentrations were variable and not affected by treatment or treatment × time (Fig. 4A–C). In general, no noticeable patterns were observed for soluble Cu and Zn concentrations in duodenal samples over the 24-h sampling period. However, a noticeable spike in soluble Cu concentrations was observed at 14 h for steers receiving STM, and similar spikes were observed for soluble Zn concentrations at 16 and 24 h in steers receiving HTM. It is unclear why these large increases in soluble Cu and Zn were observed at these specific time points. Manganese duodenal soluble concentrations were affected by time (P < 0.001; Fig. 4C). Compared with 0-h values, duodenal soluble Mn concentrations were greater (P < 0.05) at 6, 8, and 10 h post-dosing. Duodenal soluble Mn concentrations were also greater (P < 0.05) at 4, 6, 8, 10, 12, 14, and 16 h than at 20, 22, and 24 h post-dosing.
Figure 4.
Duodenal soluble copper (A), manganese (B), and zinc (C) concentrations of steers receiving a pulse dose of sulfate trace minerals (STM; 20 mg Cu/kg DM from CuSO4; 40 mg Mn/kg DM from MnSO4; 60 mg Zn/kg DM from ZnSO4) or hydroxy trace minerals (HTM; 20 mg Cu/kg DM from CuOHCl; 40 mg Mn/kg DM from MnOHCl; 60 mg Zn/kg DM from ZnOHCl). The y-axis denotes soluble mineral concentrations in mg/L, and the error bars represent SE (Exp. 2).
Copper, Zn, and Mn concentrations in ruminal solid digesta and the percentage of each TM released on dialysis against Tris–EDTA or Tris–His prior (0 h) to TM dosing were similar across treatments (Table 3). At 6 h post-dosing, TM concentrations in solid digesta were not affected by treatment (P > 0.10). By 12 h following dosing, steers given HTM had greater Cu (P < 0.05) and Zn (P < 0.001) concentration in ruminal soluble digesta than steers dosed with STM. The percentage of Zn released from solid digesta by dialysis against Tris–EDTA was greater at 6 (P < 0.05) and 12 h (P < 0.04) post-dosing from steers given HTM compared with those receiving STM. Ruminal digesta from steers dosed with HTM released a greater (P < 0.05) percentage of Cu during dialysis against Tris–EDTA at 12 h and a numerical increase (P = 0.11) in the release of Cu from digesta at 6 h following dosing in comparison with STM steers. Percentage of Zn and Cu released during dialysis against the weaker chelator, Tris–His, was low and not affected by TM source. Manganese concentrations in solid digesta and percentage released with dialysis against Tris–EDTA and Tris–His were not affected by TM source.
Table 3.
Influence of dialysis on copper, manganese, and zinc release from rumen solid digesta 0, 6, and 12 h after receiving a pulse dose of 20 mg copper, 40 mg manganese, and 60 mg zinc/kg DM from either HTM or STM sources
| Item | Treatment1 | SEM | P | |
|---|---|---|---|---|
| HTM | STM | |||
| Initial mineral concentration of digesta, mg/kg DM | ||||
| 0 h | ||||
| Copper | 1.1 | 1.3 | 0.41 | <0.94 |
| Manganese | 8.2 | 9.0 | 1.2 | <0.88 |
| Zinc | 10.9 | 11.2 | 1.1 | <0.83 |
| 6 h | ||||
| Copper | 15.2 | 7.2 | 3.9 | <0.11 |
| Manganese | 22.3 | 21.0 | 4.2 | <0.79 |
| Zinc | 42.1 | 24.3 | 8.3 | <0.17 |
| 12 h | ||||
| Copper | 28.7 | 6.9 | 6.8 | <0.05 |
| Manganese | 31.7 | 30.0 | 5.2 | <0.91 |
| Zinc | 109.4 | 26.4 | 9.6 | <0.001 |
| Tris–EDTA2, % mineral released | ||||
| 0 h | ||||
| Copper | 43.2 | 47.3 | 2.3 | <0.72 |
| Manganese | 53.2 | 51.8 | 3.6 | <0.81 |
| Zinc | 61.2 | 64.3 | 4.5 | <0.91 |
| 6 h | ||||
| Copper | 54.2 | 37.2 | 8.2 | <0.11 |
| Manganese | 82.3 | 84.5 | 24.3 | <0.93 |
| Zinc | 89.3 | 41.2 | 14.8 | <0.05 |
| 12 h | ||||
| Copper | 78.2 | 30.2 | 20.3 | <0.05 |
| Manganese | 89.9 | 88.4 | 27.3 | <0.86 |
| Zinc | 77.8 | 31.2 | 18.3 | <0.04 |
| Tris–His3, % mineral released | ||||
| 0 h | ||||
| Copper | 3.2 | 4.1 | 1.2 | <0.84 |
| Manganese | 1.9 | 2.1 | 0.5 | <0.91 |
| Zinc | 5.2 | 4.6 | 0.7 | <0.85 |
| 6 h | ||||
| Copper | 10.2 | 9.4 | 1.8 | <0.84 |
| Manganese | 14.2 | 16.3 | 3.2 | <0.86 |
| Zinc | 31.2 | 24.6 | 6.3 | <0.78 |
| 12 h | ||||
| Copper | 7.1 | 1.9 | 4.3 | <0.65 |
| Manganese | 2.7 | 2.3 | 1.02 | <0.86 |
| Zinc | 21.4 | 6.1 | 8.7 | <0.78 |
1HTM = hydroxy trace minerals; STM = sulfate trace minerals.
20.01 M ethylenediaminetetraacetate in 0.05 M tris(hydroxymethyl)aminomethane.
30.01 M l-histidine hydrochloride in 0.05 M Tris.
DISCUSSION
Trace minerals in the ruminal environment can exist in a soluble form (either in the ionic form or as a soluble complex) or in an insoluble form (Bremner, 1970). Minerals that become soluble in the rumen may bind to various feed constituents, ruminal metabolites, or microbial matter to form insoluble complexes. Previous studies (Bremner, 1970; Ivan and Veira, 1981; Waghorn et al., 1990) have examined the distribution of TM in the rumen of sheep fed forage diets. In these studies, dietary TM were derived completely (Bremner, 1970; Waghorn et al., 1990) or primarily (Ivan and Veira, 1981) from forages. In the present study, TM in ruminal fractions would have been derived from feed ingredients as well as the pulse dose of TM.
The experiments presented here indicate that Cu and Zn from HTM have low solubility in the ruminal environment at a pH of 6.23. The lack of a time effect on rumen pH may be due to hand mixing of the rumen contents prior to pH measurement. Soluble concentrations and percent soluble Cu and Zn did not increase following a pulse dose of 600 mg Zn, 200 mg Cu, and 400 mg Mn from HTM. These amounts would equate to daily supplemental concentration of approximately 60 mg Zn, 20 mg Cu, and 40 mg Mn/kg DM. In contrast, the same dose of STM resulted in a large increase in soluble concentrations and percentage of the total Cu and Zn in ruminal fluid being soluble. Soluble concentrations of Zn and Cu were increased above 0-h values for 10 h post-dosing, whereas percent soluble Cu and Zn was greater for 12 and 6 h, respectively, post-dosing in STM-dosed steers. Ruminal soluble Cu concentrations were also greater in steers fed diets supplemented with CuSO4 compared with CuOHCl (Genther and Hansen, 2015). Steers supplemented with ZnSO4 had greater ruminal soluble Zn concentrations at 2 h post-feeding than those receiving ZnOHCl (Shaeffer, 2006). In contrast, Genther and Hansen (2015) reported that steers supplemented with ZnOHCl had greater ruminal soluble Zn concentrations than those supplemented with ZnSO4. The discrepancy between results obtained in these studies may relate to how ruminal samples were processed after collection. In the present study and the Shaeffer’s (2006) experiment, ruminal samples were centrifuged shortly after being collected and the supernatant was collected before storing at −20 °C for later determination of ruminal soluble Zn. In the study by Genther and Hansen (2015), ruminal samples were frozen prior to centrifugation and separation of the soluble and insoluble. Freezing and thawing may have altered the distribution of Zn between the soluble and insoluble fraction in this study.
Ruminal solid digesta concentrations of Cu and Zn were also affected by TM source. With STM supplementation, concentrations of Zn in the solid ruminal fraction did not increase while Cu concentrations peaked at 2 to 4 h post-dosing. Concentrations of Zn and Cu in ruminal solid digesta increased with time up to 12 h post-dosing in steers given HTM. Digestion and disappearance of feed carbohydrate with time after feeding can likely explain the increased concentrations of Zn and Cu in ruminal solid digesta with time after dosing. The fact that Zn and Cu concentrations in ruminal solid digesta were much greater in steers receiving HTM compared with those dosed with Zn and Cu STM from 6 to at least 20 h post-dosing suggest that STM may have exited the rumen at a faster rate than HTM. In a similar experiment, Kennedy et al. (1993) pulse-dosed fistulated Holstein steers with 0 or 800 mg of Zn from either Zn polysaccharide or ZnO and sampled ruminal contents at 0, 1, 2, 4, 6, and 8 h post-dosing. The majority of Zn was associated with the insoluble ruminal digesta fraction compared with the rumen fluid fraction through 8 h post-dosing. Furthermore, the polysaccharide Zn source resulted in greater Zn concentrations in the solid ruminal digesta fraction when compared with Zn from ZnO.
Both Mn sources appeared to behave differently in the rumen than Zn and Cu HTM or STM sources. Steers dosed with MnOHCl had greater soluble Mn concentrations relative to 0-h values at all times post-dosing. It is unclear if Mn released from MnOHCl in the ruminal soluble fraction was present in the ionic form or as a soluble complex. Compared with baseline 0-h values, ruminal soluble Mn concentrations in steers dosed with STM did not increase until 4 h but remained greater through 22 h post-dosing. In contrast, ruminal soluble Zn and Cu concentrations were greater by 2 h but returned to pre-dosing values by 10 to 16 h post-dosing in steers given STM. Differences among TM sources in ruminal distribution were also smaller for Mn than those observed for Zn and Cu. Compared with HTM-dosed steers, those receiving STM only had greater ruminal soluble Mn concentrations at 4 and 8 h post-dosing, while solid digesta Mn was lesser from 2 through 16 h post-dosing. Genther and Hansen (2015) reported that steers supplemented with MnSO4 had greater ruminal soluble Mn concentrations than those fed diets supplemented with MnOHCl.
The percentage of total Cu and Zn in soluble ruminal contents was affected by time after dosing in steers pulse dosed with STM. However, at most sampling times, the majority of Zn, Cu, and Mn in ruminal contents were in the solid fraction for both TM sources. Over the entire 24-h period, the percent in the solid ruminal fraction of Cu, Zn, and Mn was 84.3%, 90.8%, and 90.7%, respectively, for HTM supplemented steers and 70.0%, 89.4%, and 77.2%, respectively, for STM supplemented steers. These data are in agreement with previous research. In sheep fed dried grass, 85%, 76%, and 93% of Zn, Mn, and Cu, respectively, in ruminal contents were present in an insoluble form (Bremner, 1970). Waghorn et al. (1990) reported that 89% to 95% of Zn, 85% to 98% of Mn, and 87% to 91% of Cu in ruminal contents were in the solid fraction in sheep fed different forage species. In addition to forage or diet type, chelating agents may affect ruminal distribution of Zn. Ruminal administration of the chelator nitrilotriacetic acid 30 min prior to feeding increased ruminal soluble concentrations of Zn, but not Cu and Mn in sheep (Ivan et al., 1979).
The tendency for NDF digestibility to be greater in steers fed HTM compared with those fed STM may relate to the lower ruminal soluble TM concentrations observed in steers dosed with HTM. In vitro studies have indicated that Zn and Cu can adversely affect fiber digestion (Durand and Kawashima, 1980). Lactating dairy cows supplemented with HTM had greater (51.2% vs. 49.8%) NDF digestibility than cows supplemented with similar concentrations of STM (Faulkner and Weiss, 2017). In the current experiment, an a priori power calculation was conducted to determine the number of animals need for this experiment. However, the variation obtained in this experiment was slightly greater than expected. This indicates that Exp. 1 may have been underpowered to determine the influence of treatment on NDF digestibility.
When TM derived from feedstuffs or supplemental sources become soluble in the rumen, they can interact with ruminal metabolites, feed constituents, or microorganisms to form insoluble complexes. Depending on the nature of their binding, TM that become insoluble in the ruminal environment may be less available for absorption in the small intestine than forms that remain soluble or forms that never become soluble in the rumen. In the present experiment, ruminal solid digesta was dialyzed against 2 chelating agents to assess the strength in which TM were bound in digesta. Histidine is a relatively weak chelator, whereas EDTA is a stronger chelating agent. Previous research has shown that the ability of chelators to remove Zn from protein sources during dialysis may be useful in estimating in vivo bioavailability (Jones et al., 1985). The amount of Cu and Zn removed from solid digesta by Tris–histidine was small and not affected by TM source. When ruminal solid digesta collected at 12 h post-dosing was dialyzed against Tris–EDTA, considerably more Zn and Cu were released from digesta from steers given HTM compared with STM. This suggests that Cu and Zn in the ruminal solid fraction from steers dosed with STM were more tightly bound and perhaps less available for absorption in the small intestine. Release of Mn from digesta with dialysis against EDTA was greater at 6 and 12 h post-dosing and was not affected by TM source. Bremner (1970) evaluated the strength of binding of TM to the solid rumen residue in sheep fed dried grass with no supplemental TM. Strength of binding to the solid rumen digesta appeared to be in the order of Cu > Zn > Mn based on the extent of dissolution of the metals on treatment with acid or with EDTA (Bremner, 1970). In the present experiment, binding strength to the solid digesta in steers receiving STM was Cu = Zn > Mn (P < 0.05) when dialyzed against EDTA at 6 and 12 h post-dosing.
Soluble concentrations of TM in the duodenum were not affected by TM source. Trace minerals in the duodenum represented not only those from feed ingredients and supplemental sources but also endogenous TM from intestinal and biliary secretions. The greater ruminal digesta concentrations of Cu and Zn in steers given HTM vs. STM suggest that less of the HTM may have reached the duodenum during the 24-h collection period post-dosing.
Results from this experiment indicate that Cu and Zn from HTM have low solubility in the rumen and appear to be less tightly bound to solid digesta in the rumen than Cu and Zn from STM. The greater solubility of Cu and Zn from STM may alter microbial fermentation in cattle. Faulkner and Weiss (2017) recently reported lower NDF digestibility in lactating dairy cows supplemented with STM compared with those supplemented with HTM. The stronger binding of STM, relative to HTM, to the solid rumen digesta fraction may also reduce their bioavailability in the small intestine of cattle. Future research investigating the flow and passage rate of different TM sources through the abomasum into the duodenum and the duodenal absorption efficiency of Cu and Zn from different TM sources is needed.
Footnotes
Use of trade names in this publication does not imply endorsement by Colorado State University or criticism of similar products not mentioned.
Mention of a proprietary product does not constitute a guarantee or warranty of the products by Colorado State University or the authors and does not imply its approval to the exclusion of other products that may also be suitable.
This research was supported in part by the Colorado State University Agricultural Experiment Station and by Micronutrients, Indianapolis, IN.
LITERATURE CITED
- Border J. R., Harris L. E., and Butcher J. E.. 1963. Apparatus for obtaining sustained quantitative collections of urine from male cattle grazing pasture of range. J. Anim. Sci. 22:521–525. doi:10.2527/jas1963.222521x [Google Scholar]
- Bremner I. 1970. Zinc, copper and manganese in the alimentary tract of sheep. Br. J. Nutr. 24:769–783. doi:10.1079/BJN19700079 [DOI] [PubMed] [Google Scholar]
- Cao J., Henry P. R., Ammerman C. B., Miles R. D., and Littell R. C.. 2000. Relative bioavailability of basic zinc sulfate and basic zinc chloride for chicks. J. Appl. Poult. Res. 9:513–517. doi:10.1093/japr/9.4.513 [Google Scholar]
- Durand M., and Kawashima R.. 1980. Influence of minerals in rumen microbial digestion. In: Ruckebusch Y. and Thivend P., editors, Digestive physiology and metabolism in ruminants. MTP Press Ltd, Lancaster, UK: p. 375–408. doi:10.1007/978-94-011-8067-2_18 [Google Scholar]
- Faulkner M. J., and Weiss W. P.. 2017. Effect of source of trace minerals in either forage- or by-product-based diets fed to dairy cows: 1. Production and macronutrient digestibility. J. Dairy Sci. 100:5358–5367. doi:10.3168/jds.2016-12095 [DOI] [PubMed] [Google Scholar]
- Genther O. N., and Hansen S. L.. 2015. The effect of trace mineral source and concentration on ruminal digestion and mineral solubility. J. Dairy Sci. 98:566–573. doi:10.3168/jds.2014-8624 [DOI] [PubMed] [Google Scholar]
- Ivan M., Jui P., and Hidiroglou. 1979. The effects of nitrilotriacetic acid on solubilities of zinc, copper, manganese, and iron in the stomach of sheep. Can. J. Physiol. Pharmacol. 57:369–374. doi:10.1139/y79-055 [DOI] [PubMed] [Google Scholar]
- Ivan M., and Veira D. M.. 1981. Effect of dietary protein on the solubilities of manganese, copper, zinc, and iron in the rumen and abomasum of sheep. Can. J. Anim. Sci. 61:955–959. doi:10.4141/cjas81-116 [Google Scholar]
- Jones A. O. L., Spivey Fox M. R., and Fry B. E.. 1985. In vitro assessment of zinc binding to protein foods as a potential index of zinc bioavailability. Comparison of in vitro and in vivo data. J. Agric. Food Chem. 33:1123–1128. [Google Scholar]
- Kennedy D. W., Craig W. M., and Southern L. L.. 1993. Ruminal distribution of zinc in steers fed a polysaccharide-zinc complex or zinc oxide. J. Anim. Sci. 71:1281–1287. doi:10.2527/1993.7151281x [DOI] [PubMed] [Google Scholar]
- NRC 2000. Nutrient requirements of beef cattle. 7th rev. ed. Natl. Acad. Press, Washington, DC. doi:10.17226/9791 [Google Scholar]
- Shaeffer G. L. 2006. Evaluation of basic zinc chloride as a zinc source for cattle. MS Thesis. North Carolina State Univ., Raleigh. doi:10.1016/j.anifeedsci.2017.07.013 [Google Scholar]
- Shaeffer G. L., Lloyd K. E., and Spears J. W.. 2017. Bioavailability of zinc hydroxychloride relative to zinc sulfate in growing cattle fed a corn-cottonseed hull-based diet. Anim. Feed Sci. Technol. 232:1–5. doi:10.1016/j.anifeedsci.2017.07.013 [Google Scholar]
- Spears J. W., Kegley E. B., and Mullis L. A.. 2004. Bioavailability of copper from tribasic copper chloride and copper sulfate in growing cattle. Anim. Feed Sci. Technol. 116:1–13. doi:10.1016/j.anifeedsci.2004.06.002 [Google Scholar]
- Tolleson D. R., and Erlinger L. L.. 1989. An improved harness for securing fecal collection bags to grazing cattle. J. Range Manage. 42:396–399. [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Waghorn G. C., Shelton I. D., and Sinclair B. R.. 1990. Distribution of elements between solid and supernatant fractions of digesta in sheep given six diets. New Zealand J. Agric. Res. 33:259–269. doi:10.1080/00288233.1990.10428418 [Google Scholar]