Abstract
Yeast products may serve as functional ingredients due to their benefits on host health but vary greatly in source, composition, and functionality, justifying research in host species of interest. In this study, a Saccharomyces cerevisiae fermentation product (SCFP) was investigated as a dietary supplement for adult dogs. Adult female beagles (n = 12; mean age = 3.3 ± 0.8 yr; mean BW = 10.3 ± 0.68 kg) were fed the same diet, but supplemented with three levels of SCFP (125, 250, and 500 mg/d) or a placebo (sucrose) via gelatin capsules in a replicated 4 × 4 Latin square design. Fecal samples for nutrient digestibility, fecal characteristics and microbial populations as well as blood samples for immune indices were collected after a 21-d adaptation phase in each period. A separate palatability test was conducted to examine palatability of an SCFP-containing diet (0.2% of diet). All data, except for palatability data, were analyzed by Mixed Models procedure of SAS (version 9.4). A paired t-test was conducted to analyze data from the palatability test. Supplementation of SCFP did not affect total tract apparent macronutrient and energy digestibilities or fecal characteristics. Fecal phenol and total phenol + indole concentrations decreased linearly with SCFP dosage (P < 0.05). Relative abundance of Bifidobacterium was greater (P < 0.05), while Fusobacterium was lower (P < 0.05) in SCFP-supplemented dogs. Total white blood cell counts were decreased by SCFP (P < 0.05). The percentage of natural killer cells and antigen-presenting cells were not altered by SCFP. However, when comparing control vs. all SCFP treatments, SCFP-supplemented dogs had greater (P < 0.05) major histocompatibility complex class II presenting B cell and monocyte populations than control dogs. IFN-γ secreting helper and cytotoxic T cells increased linearly with SCFP consumption (P < 0.05). Immune cells derived from SCFP-supplemented dogs produced less (P < 0.05) TNF-α than those from control dogs when cells were stimulated with agonists of toll-like receptors 2, 3, 4, and 7/8. A linear increase (P < 0.05) in serum IgE with SCFP dosage was noted. In the palatability test, a 1.9:1 consumption ratio was observed for the SCFP-containing diet vs. control diet, demonstrating a preference (P < 0.05) for SCFP. Results of this study suggest that SCFP supplementation may be beneficial to adult dogs by positively altering gut microbiota, enhancing immune capacity and reducing inflammation.
Keywords: canine, immunity, microbiota, Saccharomyces cerevisiae fermentation product
INTRODUCTION
With the recent trend of pet humanization, the demand for functional ingredients that provide health benefits beyond basic nutrition has been increasing in the pet food industry. Saccharomyces cerevisiae fermentation product (SCFP) is a dry product produced via S. cerevisiae fermentation and includes residual yeast cells, yeast cell wall fragments, fermentation metabolites, and media used during fermentation, and may serve as a functional ingredient due to its benefits to animals. Research in various animal species has demonstrated the effectiveness of SCFP to improve health and performance. In humans, supplementation of SCFP has been shown to improve immune responses to flu and allergy (Moyad et al., 2008, 2009, 2010) as well as modulate gut microbiota (Pinheiro et al., 2017). SCFP has been reported to increase ADG in growing piglets (Shen et al., 2009) and enhance reproductive performance in sows (Kim and Brandherm, 2008; Kim et al., 2010; Shen et al., 2011). In broiler chickens, SCFP has been shown to improve ADG (Gao et al., 2008) and intestinal morphology, including greater villus height to crypt ratio (Gao et al., 2008) as well as modulated T-cell populations (Gao et al., 2009). Supplementation of SCFP in weaning calves led to less Salmonella intestinal colonization and enhanced rumen development (Magalhães et al., 2008; Brewer et al., 2014). In addition, SCFP was shown to reduce inflammatory response in dairy cows with subacute ruminal acidosis (Li et al., 2016).
While there is no research specifically studying the effects of SCFP in dogs, there have been studies investigating yeast and yeast-related products in dogs. Results of those studies have demonstrated beneficial effects of supplementation. Middelbos et al. (2007) and Stercova et al. (2016) supplemented dogs with S. cerevisiae yeast cell walls and live S. cerevisiae, respectively. Both studies reported reduced fecal Escherichia coli counts in yeast-supplemented dogs. Effects of yeast-derived mannanoligosaccharides (MOS) have also been studied in dogs (Swanson et al., 2002a; Swanson et al., 2002b; Grieshop et al., 2004; Pawar et al., 2017). Those studies suggested that MOS could increase fecal Bifidobacterium and Lactobacillus, elevate ileal IgA concentrations, and modulate white blood cell (WBC) populations. Even though some of those studies have evaluated immune responses to yeast products in dogs, the measured indices were limited. For example, only serum and ileal Ig were measured due to the lack of available techniques at the time. Blood cell counts were also studied using a hematology analyzer or flow cytometry. However, data of some immune cells such as B cell and natural killer (NK) cell are not available. In addition, to our knowledge, functional tests for immune cell responsiveness have not been conducted in dogs fed yeast products.
Therefore, considering the lack of information available, the objective of the present study was to investigate the effects of a SCFP (Diamond V Mills, Inc., Cedar Rapids, IA) in adult dogs. The effects of SCFP on fecal characteristics, apparent total tract macronutrient digestibility (ATTD), fecal fermentative end-products, fecal microbiota, immune responses, and diet palatability were tested. We hypothesized that SCFP would positively shift fecal microbiota and improve immune responses in dogs as well as increase diet palatability. Finally, we hypothesized that there would be no adverse effects on fecal characteristics, ATTD, and fecal fermentative end-products. Due to the lack of SCFP data in companion animals, the current study was conducted with dogs so that the results could be applied to pet foods.
MATERIALS AND METHODS
Animals, Diets, and Treatments
Twelve healthy adult female beagles (age: 3.3 ± 0.8 yr, BW: 10.3 ± 0.68 kg) were used in this study. All animal care and experimental procedures were approved by the University of Illinois Institutional Animal Care and Use committee before experimentation (protocol no. 16092). All dogs were evaluated and shown to be healthy based on the serum chemistry results and a physical examination by a veterinarian before and after the study. Dogs were housed individually in pens (1.22 m wide × 1.85 m long) in an environmentally controlled room on a 12 h light: 12 h dark cycle. Dogs had access to fresh water ad libitum at all times and were fed once a day to maintain BW throughout the study. Dogs were weighed and body condition scores were assessed using a nine-point scale (Laflamme, 1997) weekly. Dogs consumed the same premium-style extruded kibble diet formulated to meet all Association of American Feed Control Officials (AAFCO, 2015) nutrient recommendations for adult dogs at maintenance (Table 1).
Table 1.
Ingredient and chemical composition of the experimental diet
| Ingredient | Amount |
|---|---|
| %, as-is | |
| Brewer’s rice | 45.26 |
| Chicken byproduct meal | 32.00 |
| Chicken fat | 9.00 |
| Corn | 6.00 |
| Cellulose | 6.00 |
| Salt | 0.50 |
| Potassium chloride | 0.45 |
| Taurine | 0.30 |
| Mineral premix1 | 0.18 |
| Vitamin premix2 | 0.18 |
| Choline chloride | 0.13 |
| Analyzed composition | |
| DM, % | 93.3 |
| %, DM | |
| Ash | 5.1 |
| CP | 26.9 |
| Acid hydrolyzed fat | 14.6 |
| Total dietary fiber | 10.3 |
| ME, kcal/g3 | 3.7 |
1Provided per kilogram diet: Mn (as MnSO4), 66.0 mg; Fe (as FeSO4), 120.0 mg; Cu (as CuSO4), 18.0 mg; Co (as CoSO4), 1.20 mg; Zn (as ZnSO4), 240.0 mg; I (as KI), 1.80 mg; Se as (Na2SeO3), 0.24 mg.
2Provided per kilogram diet: vitamin A, 5.28 mg; vitamin D3, 0.04 mg; vitamin E, 120.0 mg; vitamin K, 0.88 mg; thiamine, 4.40 mg; riboflavin, 5.72 mg; pantothenic acid, 22.0 mg; niacin, 39.6 mg; pyridoxine, 3.52 mg; biotin, 0.13 mg; folic acid, 0.44 mg; vitamin B12; 0.11 mg.
3Metabolizable energy (ME, kcal/g) = (3.5 kcal/g × CP %) + (8.5 kcal/g × acid hydrolyzed fat %) + (3.5 kcal/g × nitrogen-free extract %); nitrogen-free extract (%) = 100% − (CP % + acid hydrolyzed fat % + total dietary fiber % + ash %).
The SCFP treatment is a dried product produced via S. cerevisiae fermentation that contains fermentation metabolites and some residual yeast cells. Nutrient analysis of the SCFP revealed that it contained 94.6% DM, 20.0% ash, 27.7% CP, 4.2% crude fat, and 20.3% total dietary fiber. Treatments were given via gelatin capsules daily prior to the diet, and included a placebo (125 mg sucrose/d), 125 mg SCFP/d, 250 mg SCFP/d, and 500 mg SCFP/d. A replicated 4 × 4 Latin square design was used. Each 28-d period consisted of an adaptation phase (days 1 to 21), a fecal collection phase (days 22 to 26), and a blood collection phase (days 27 to 28).
Fecal Sample Collection and Fecal Characteristics
During the fecal collection phase of each period, all feces were collected from the pen floor, weighed, scored and frozen at −20 °C until analyses. Fecal samples were scored according to a five-point scale with 1 = hard, dry pellets; small hard mass; 2 = hard formed, dry stool; remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool; assumes shape of container; 5 = watery, liquid that can be poured. One fresh fecal sample from each dog in each period was collected within 15 min of defecation for measurement of pH, DM, fermentative end-product concentrations [short-chain fatty acids (SCFA), branched-chain fatty acids (BCFA), ammonia, phenol, and indole] and microbiota composition. Fecal pH was measured immediately using a pH meter (Denver Instrument, Bohemia, NY) equipped with an electrode (Beckman Instruments Inc., Fullerton, CA). Four aliquots were collected for further analyses: (i) for DM determination, the aliquot was dried at 105 °C for 2 d; (ii) for SCFA, BCFA, and ammonia measurements, the aliquot was mixed with 2 N hydrochloric acid in a 1:1 (weight:weight) ratio and stored at −20 °C until analyses; (iii) for phenol and indole measurements, the aliquot was stored at −20 °C until analyses; and (iv) for microbial analyses, the aliquot was frozen at −80 °C until analyses.
Apparent Total Tract Macronutrient and Energy Digestibility
Diet subsamples were collected. Total fecal samples were composited and dried at 57 °C for a week. Both diet and fecal samples were ground through a 2-mm screen using a Wiley Mill (model 4, Thomas Scientific, Swedesboro, NJ). DM and OM contents were analyzed according to the Association of Official Analytical Chemists (AOAC, 2006; DM: method 934.01; OM: method 942.05). Fat content was measured using acid hydrolysis methods of the American Association of Cereal Chemists (AACC, 1983) and Budde (1952). CP content was calculated from Leco total nitrogen values (TruMac N, Leco Corporation, St. Joseph, MI; AOAC, 2006). Gross energy was measured using an oxygen bomb calorimeter (model 1261, Parr Instruments, Moline, IL). Total dietary fiber content was determined for diet samples according to (Prosky et al., 1985). ATTD of nutrients and energy was calculated using the following equation: digestibility (%) = [nutrient intake (g/d) – fecal output (g/d)]/nutrient intake (g/d) × 100%.
Fecal Fermentative End-Products
Fecal SCFA (acetate, propionate, and butyrate) and BCFA (valerate, isovalerate, and isobutyrate) concentrations were determined by gas chromatography according to Erwin et al. (1961). During analyses, a gas chromatograph (Hewlett-Packard 5890A series II, Palo Alto, CA) and a glass column (180 cm × 4 mm i.d.) packed with 10% SP to 1200/1% H3PO4 on 80/100 mesh Chromosorb WAW (Supelco Inc., Bellefonte, PA) were used. Nitrogen was the carrier gas with a flow rate of 75 mL/min. Temperatures of the oven, detector, and injector were 125, 175, and 180 °C, respectively. Fecal ammonia concentrations were determined according to the method of Chaney and Marbach (1962). Fecal phenol and indole concentrations were evaluated by gas chromatography according to Flickinger et al. (2003).
Fecal Microbiota
Fecal DNA extraction, amplification, and sequencing.
Total DNA from fresh fecal samples was extracted using Mo-Bio PowerSoil Kits (MO BIO Laboratories, Inc., Carlsbad, CA), followed by quantification of extracted DNA using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY). Quality of extracted DNA was assessed by electrophoresis using agarose gels (E-Gel EX Gel 1%; Invitrogen, Carlsbad, CA). Bacterial 16S rRNA gene amplicons of 252 bp from the V4 region were generated using a Fluidigm Access Array (Fluidigm Corporation, South San Francisco, CA) with Roche High Fidelity Fast Start Kit (Roche, Indianapolis, IN). The primers 515 F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) that target the 252 bp-fragment of V4 region were used for amplification (primers synthesized by IDT Corporation, Coralville, IA; Caporaso et al., 2012). Quality of the amplicons was assessed using a Fragment Analyzer (Advanced Analytics, Ames, IA) followed by amplicon size selection using electrophoresis and a Qiagen gel purification kit (Qiagen, Valencia, CA). The appropriate profile and average size of purified amplicons were then confirmed using a Bioanalyzer (Agilent Technologies, Santa Clara, CA). Amplicons were sequenced using Illumina sequencing on MiSeq using v3 reagents (Illumina Inc., San Diego, CA) at the W. M. Keck Center for Biotechnology at the University of Illinois.
Bioinformatics.
Quantitative Insights Into Microbial Ecology (QIIME 1.9.1; Caporaso et al., 2010) was used to process the sequence data. Sequence data with quality value ≥20 derived from the sequencing process were demultiplexed. Sequences were clustered into operational taxonomic units (OTUs) using UCLUST (Edgar, 2010) through an open-reference OTU picking strategy against the Greengenes 13_8 reference database (DeSantis et al., 2006) with a 97% similarity threshold. Singletons and OTUs that had <0.01% of the total observation were discarded. A total of 1,608,461 16S rRNA-based amplicon sequences were obtained, with an average of 34,222 reads per sample. An even sampling depth of 20,935 sequences per sample was used for assessing α- and β-diversity measures. α-diversity was estimated using chao1, observed OTU, phylogenetic diversity whole tree, Shannon and Simpson metrices. β-diversity was calculated using weighted and unweighted UniFrac (Lozupone and Knight, 2005) distance measures and presented with principal coordinates analysis plots. Statistical analysis was conducted via Statistical Analyses of Metagenomic Profiles software 2.1.3 (Parks et al., 2014) using ANOVA and Tukey–Kramer multiple comparison tests. All tests were corrected for multiple inferences using the Benjamini–Hochberg method (Benjamini and Hochberg, 1995) to control for false discovery rate. Statistical significance was set at P < 0.05.
Blood Sample Collection
During the blood collection phase of each period, a total of 30 mL of blood was collected via jugular puncture. Samples were immediately transferred to appropriate vacutainer tubes: (i) 20 mL in plasma tubes containing heparin (no. 366480, Becton Dickinson, Franklin Lakes, NJ) for peripheral blood mononuclear cell (PBMC) collection; (ii) 2 mL in whole blood tubes containing K2EDTA additive (no. 367842, Becton Dickinson) for complete blood count analyses; and (iii) 8 mL in serum tubes containing a clot activator and gel for serum separation (no. 367974, Becton Dickinson) for serum chemistry profile, Ig, and oxidative stress marker analyses. Serum was isolated by centrifuging at 1,300 × g at 4 °C for 10 min (Beckman CS-6R centrifuge; Beckman Coulter Inc., Brea, CA).
Immune Function
Serum chemistry and complete blood count.
Serum chemistry profile and complete blood count were analyzed using a Hitachi 911 clinical chemistry analyzer (Roche Diagnostics, Indianapolis, IN) at the University of Illinois Veterinary Medicine Diagnostics Laboratory.
Serum Igs and oxidative stress markers.
The concentrations of serum IgA, IgG, IgM, and IgE and oxidative stress markers [superoxide dismutase (SOD) and malondialdehyde (MDA)] were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Igs: Immunology Consultants Laboratory, Inc., Portland, OR; SOD: US Biological, Salem, MA; MDA: MyBioSource, San Diego, CA).
Immune cell populations.
Ficoll (Sigma, St. Louis, MO) was added to blood in a 1:1 volume ratio and centrifuged at 300 × g at 4 °C for 30 min to separate PBMC from blood. The percentage of T cells, NK cells, and antigen-presenting (AP) cells was evaluated by flow cytometry. For T-cell populations, PBMC was distributed into two tubes (1 × 106 cells/tube) with one tube as control and the other stimulated with a cell stimulation cocktail (phorbol 12-myristate 13-acetate, ionomycin, brefeldin A, and monensin; eBioscience, San Diego, CA). Both tubes were incubated at 37 °C in 5% CO2 for 4 h followed by surface marker labeling with antibodies including anti-CD3-fluorescein isothiocyanate (FITC), anti-CD4-allophycocyanin (APC), and anti-CD8-pacific blue (Serotec, Raleigh, NC). After labeling, cells were fixed and permeabilized with fixation buffer and permeabilization buffer (eBioscience). Intracellular marker staining was then performed with anti-IFN-γ-phycoerythrin (PE) (Serotec). For NK cell populations, one aliquot of cells (1×106 cells/tube) was labeled with anti-CD5-APC antibody (Serotec). For AP cells, the cells of interest included B cells and monocytes presenting major histocompatibility complex class II (MHC-II) on the cell surface. One aliquot of PBMC (1 × 106 cells/tube) was stained with anti-CD14-APC, anti-CD21-PE, and anti-MHC-II-FITC antibodies (Serotec). Populations of T cells, NK cells, and AP cells were then determined by a BD LSR flow cytometer (Becton Dickinson). Flow cytometry data were analyzed using FCS Express 6 Flow Cytometry Software (De Novo Software, Glendale, CA). Gating strategy used to determine immune cell populations are shown in Supplementary Figure 1. For NK cells, the population was determined according to Huang et al. (2008).
Responsiveness of leukocytes to toll-like receptor (TLR) agonists.
PBMC (1 × 105 cells/well) was seeded into 96-well plates. Agonists of TLR2 (100 μg/mL zymosan; Invivogen, San Diego, CA), TLR3 (50 μg/mL polyinosinic–polycytidylic acid sodium salt, poly(I:C); Sigma), TLR4 (100 ng/mL LPS; Sigma), and TLR7/8 (5 μg/mL resiquimod, Invivogen) were added into assigned wells separately. After 24 h of incubation, supernatants were collected for measurements of TNF-α concentration using a commercial ELISA kit (R&D systems, Minneapolis, MN).
Palatability Test
A standard 2-d palatability test was conducted at Kennelwood Inc. (Champaign, IL) to compare the control diet containing no SCFP and a diet containing 0.2% SCFP, which represented the highest dose tested in the first study. Twenty beagle dogs (BW: 13.9 ± 3.9) were used. Two bowls, each containing 500 g of diet, were offered once daily for 2 d. Bowl placement was reversed daily to prevent left–right bias and both bowls were presented for 30 min. If one diet was completely consumed prior to the end of the 30 min, both bowls were removed. Food consumption and first choice preference were recorded for each dog.
Statistical Analyses
All data, except that for the palatability test, were analyzed using the Mixed Models procedure of SAS (version 9.4; SAS Institute, Cary, NC) with treatment as a fixed effect and dog as a random effect. Linear, quadratic, and control vs. yeast effects were tested using contrasts. A paired t-test was conducted to analyze data from the palatability test. Data are reported as means ± pooled SEMs with statistical significance set as P < 0.05 and trend set as P < 0.10.
RESULTS
Food Intake, BW, and Apparent Total Tract Macronutrient and Energy Digestibility
Average daily food intake was 176.6 g/d and was not affected by SCFP supplementation (Table 2). Dog BW (average: 10.7 kg) and body condition scores (average: 5.3 on nine-point scale) were not different among treatment groups over the course of the study. Supplementation of SCFP did not influence ATTD of DM (86.9%), OM (88.9%), CP (87.6%), fat (96.7%), or energy (89.2%).
Table 2.
Daily intake, BW, body condition score, and apparent total tract macronutrient and energy digestibility of dogs supplemented with a yeast fermentation product
| Saccharomyces cerevisiae fermentation product, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 125 | 250 | 500 | SEM | Linear | Quadratic | 0 vs. yeast |
| Daily intake, g/day | 175.2 | 176.0 | 176.6 | 178.4 | 7.11 | 0.40 | 0.86 | 0.56 |
| BW, kg | 10.7 | 10.7 | 10.7 | 10.7 | 0.38 | 0.37 | 0.28 | 0.80 |
| Body condition score1 | 5.4 | 5.4 | 5.3 | 5.3 | 0.36 | 0.46 | 0.93 | 0.65 |
| Digestibility, % | ||||||||
| DM | 87.4 | 87.0 | 86.5 | 86.5 | 0.02 | 0.43 | 0.83 | 0.48 |
| OM | 89.3 | 89.0 | 88.7 | 88.6 | 0.01 | 0.43 | 0.87 | 0.50 |
| CP | 88.1 | 87.7 | 87.7 | 86.8 | 0.02 | 0.31 | 0.80 | 0.45 |
| Crude fat | 96.8 | 96.8 | 96.5 | 96.6 | 0.00 | 0.33 | 0.72 | 0.42 |
| Energy | 89.6 | 89.3 | 88.9 | 88.9 | 0.01 | 0.40 | 0.82 | 0.45 |
1A nine-point scale body condition system was used (Laflamme, 1997).
Fecal Characteristics and Fecal Fermentative End-Products
Fecal characteristics, including pH, fecal score, fecal DM, and wet fecal output, were not affected by treatment when each was analyzed against one another individually (Table 3). Fecal score, however, was linearly increased (P < 0.05) with increasing SCFP consumption. When comparing control vs. all SCFP treatments, fecal score was greater (P < 0.05) in SCFP-supplemented dogs. With the exception of phenol and total phenols + indoles, fecal fermentative end-products were not altered by treatment (Table 3). Dogs supplemented with 500 mg SCFP had lower (P < 0.05) fecal phenol concentrations than dogs in the control group. When comparing the control vs. all SCFP treatments, fecal phenol concentration was lower (P < 0.05) in SCFP-supplemented dogs. A significant linear effect was also observed for fecal phenol and total fecal phenol + indole concentrations, with higher SCFP dosages leading to lower (P < 0.05) fecal phenol and total phenol + indole concentrations.
Table 3.
Fecal characteristics and fermentative end-products of dogs supplemented with a yeast fermentation product
| Saccharomyces cerevisiae fermentation product, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 125 | 250 | 500 | SEM | Linear | Quadratic | 0 vs. yeast |
| Fecal characteristics | ||||||||
| pH | 7.06 | 6.95 | 7.30 | 7.02 | 0.31 | 0.72 | 0.50 | 0.89 |
| Fecal score1 | 2.73 | 2.80 | 2.83 | 2.83 | 0.08 | 0.04 | 0.38 | 0.04 |
| Fecal DM, % | 40.72 | 41.20 | 41.68 | 42.05 | 3.01 | 0.37 | 0.90 | 0.46 |
| Fecal output (days 22 to 26), g | 59.78 | 60.81 | 59.64 | 59.37 | 6.06 | 0.89 | 0.70 | 0.89 |
| Fecal metabolites, µmol/g DM | ||||||||
| Acetate | 160.51 | 164.71 | 162.19 | 151.76 | 22.29 | 0.37 | 0.51 | 0.81 |
| Propionate | 73.28 | 79.10 | 78.43 | 72.23 | 12.24 | 0.79 | 0.23 | 0.57 |
| Butyrate | 38.61 | 48.22 | 35.59 | 42.83 | 17.44 | 0.98 | 0.80 | 0.63 |
| Total SCFA2 | 272.40 | 292.03 | 276.21 | 266.82 | 39.45 | 0.47 | 0.44 | 0.84 |
| Isobutyrate | 6.59 | 6.79 | 6.54 | 6.20 | 1.09 | 0.41 | 0.66 | 0.79 |
| Isovalerate | 9.93 | 9.79 | 9.36 | 8.90 | 1.67 | 0.19 | 0.95 | 0.37 |
| Valerate | 0.58 | 0.83 | 0.64 | 0.73 | 0.32 | 0.55 | 0.63 | 0.24 |
| Total BCFA2 | 17.10 | 17.41 | 16.54 | 15.83 | 2.87 | 0.29 | 0.80 | 0.60 |
| Phenol | 0.82a | 0.59ab | 0.47ab | 0.38b | 0.50 | <0.01 | 0.29 | <0.01 |
| Indole | 1.98 | 2.17 | 2.09 | 1.83 | 0.40 | 0.38 | 0.10 | 0.74 |
| Total P/I | 2.80 | 2.76 | 2.56 | 2.21 | 0.76 | 0.03 | 0.49 | 0.18 |
| Ammonia | 133.04 | 143.37 | 127.47 | 128.39 | 20.61 | 0.38 | 0.65 | 0.94 |
1Fecal scores: 1 = hard, dry pellets; small hard mass; 2 = hard formed, dry stool; remains firm and soft; 3 = soft, formed and moist stool, retains shape; 4 = soft, unformed stool; assumes shape of container; 5 = watery, liquid that can be poured.
2Total short-chain fatty acids (SCFA) = acetate + propionate + butyrate; total branched-chain fatty acids (BCFA) = valerate + isovalerate + isobutyrate; Total P/I = phenol + indole
a,b Mean values within a row with unlike superscript letters differ (P < 0.05).
Fecal Microbiota
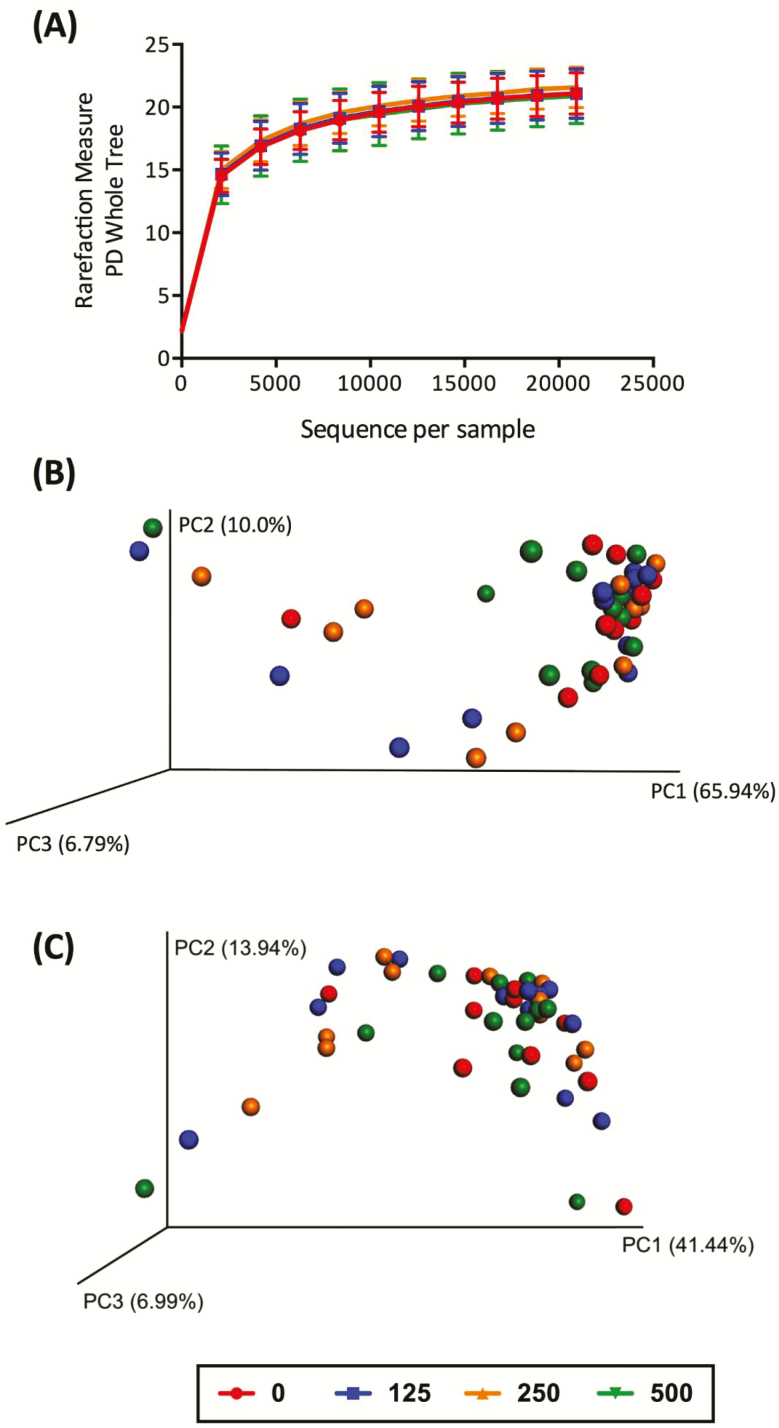
α diversity of the fecal microbial community, which represents species richness and evenness within a sample, was not altered by SCFP supplementation according to the rarefaction curves and values obtained from different diversity metrics (Figure 1A). β-diversity, which represents species richness among samples, was not different among treatment groups in both unweighted and weighted UniFrac distance measures (Figures 1B and C). For all dogs, the predominant bacterial phyla were Fusobacteria (31.31% to 38.37%), Firmicutes (25.33% to 32.59%), Bacteroidetes (23.74% to 26.30%), Proteobacteria (8.26% to 8.71%), and Actinobacteria (1.59% to 4.79%). The predominant bacterial genera were Fusobacterium (29.6% to 38.5%), Bacteroides (14.9% to 15.9%), and Allobaculum (4.8% to 11.7%) (Table 4). At the phyla level, relative abundance of Firmicutes tended to increase linearly (P < 0.10) with SCFP supplementation. When comparing control vs. all SCFP treatments, relative abundance of Actinobacteria was higher (P < 0.05) and Firmicutes tended to be higher (P < 0.10) in SCFP-supplemented dogs. A linear effect (P < 0.05) was observed in Fusobacteria, with higher SCFP doses leading to lower relative abundance of Fusobacteria. In addition, relative abundance of Fusobacteria overall were lower (P < 0.05) in SCFP-supplemented dogs when comparing control vs. all SCFP treatments. At the genera level, linear increases (P < 0.05) were noted in relative abundance of Bifidobacterium and Prevotella as SCFP increased. When comparing control vs. all SCFP treatments, relative abundance of Bifidobacterium was higher (P < 0.05) and Prevotella tended to be higher (P < 0.10) in SCFP-supplemented dogs. Relative abundance of Fusobacterium decreased linearly (P < 0.05) with SCFP supplementation. When comparing control vs. all SCFP treatments, relative abundance of Fusobacterium was lower (P < 0.05) in SCFP-supplemented dogs.
Figure 1.
Fecal microbiota communities of dogs supplemented with 0, 125, 250, and 500 mg yeast fermentation product/d. (A) Alpha diversity measures, including phylogenetic diversity (PD) whole tree (shown), suggested that species richness within a sample was not affected by treatment (P > 0.05). Principal coordinates analysis plots of unweighted (B) and weighted (C) UniFrac distances of fecal microbial communities were not altered by treatment (P > 0.05).
Table 4.
Predominant bacterial phyla and genera (% of total sequences) in the feces of dogs supplemented with a yeast fermentation product
| Saccharomyces cerevisiae fermentation product, mg/d | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phylum | Genus1 | 0 | 125 | 250 | 500 | SEM | Linear | Quadratic | 0 vs. yeast |
| Actinobacteria | 1.59 | 4.79 | 3.24 | 2.99 | 3.87 | 0.16 | 0.16 | 0.03 | |
| Bifidobacterium | 1.33 | 4.54 | 2.96 | 2.64 | 3.92 | 0.05 | 0.08 | 0.01 | |
| Bacteroidetes | 26.30 | 23.74 | 23.91 | 24.75 | 5.12 | 0.60 | 0.43 | 0.33 | |
| Bacteroides | 15.83 | 14.45 | 14.92 | 15.21 | 3.53 | 0.78 | 0.56 | 0.52 | |
| Prevotella | 0.69 | 1.57 | 0.99 | 0.95 | 0.98 | 0.04 | 0.73 | 0.06 | |
| Firmicutes | 25.33 | 30.93 | 32.19 | 32.59 | 9.31 | 0.08 | 0.40 | 0.06 | |
| Allobaculum | 4.49 | 10.26 | 9.22 | 10.93 | 9.58 | 0.13 | 0.69 | 0.10 | |
| Blautia | 1.56 | 1.63 | 1.80 | 1.85 | 0.60 | 0.41 | 0.98 | 0.54 | |
| Clostridium | 5.94 | 4.98 | 5.52 | 5.50 | 1.80 | 0.83 | 0.58 | 0.54 | |
| Faecalibacterium | 1.63 | 1.23 | 1.03 | 1.53 | 0.72 | 0.63 | 0.43 | 0.38 | |
| Lactobacillus | 2.80 | 3.73 | 4.08 | 3.06 | 3.98 | 0.67 | 0.54 | 0.80 | |
| Phascolarctobacterium | 0.60 | 0.58 | 0.74 | 0.68 | 0.18 | 0.21 | 0.70 | 0.41 | |
| Fusobacteria | 38.37 | 31.84 | 31.86 | 31.31 | 7.54 | 0.03 | 0.18 | 0.01 | |
| Fusobacterium | 38.32 | 31.81 | 31.82 | 31.28 | 7.53 | 0.03 | 0.18 | 0.01 | |
| Proteobacteria | 8.31 | 8.58 | 8.71 | 8.26 | 2.14 | 0.91 | 0.72 | 0.96 | |
| Anaerobiospirillum | 1.55 | 1.88 | 1.91 | 2.25 | 0.98 | 0.65 | 0.33 | 0.34 | |
| Sutterella | 6.40 | 6.57 | 6.18 | 5.91 | 1.52 | 0.57 | 0.76 | 0.83 |
1All genera with relative abundance >0.5% of total sequences are presented.
Immune Function
Serum chemistry profile and blood cell counts.
Serum metabolites were within reference ranges for all dogs, except for globulin, and albumin: globulin ratio (Supplementary Table 1). Globulin was slightly lower and albumin: globulin ratio was slightly higher than the reference range for dogs in all treatment groups. None of the serum metabolites were altered by treatment when analyzed individually. A quadratic effect (P < 0.05) was noted for total bilirubin and a linear increase (P < 0.05) with increasing SCFP was observed for corticosteroid-induced alkaline phosphatase. Most of the blood cell counts were within reference ranges for dogs supplemented with all treatments (Supplementary Table 2). Dogs treated with 250 and 500 mg SCFP had slightly lower WBC counts than the reference range. Significant linear and control vs. all SCFP treatment effects were observed for WBC counts, with dogs having lower (P < 0.05) total WBC with increasing SCFP dose. Also, dogs supplemented with 250 mg SCFP had lower (P < 0.05) WBC counts than dogs in the control group.
Serum oxidative stress markers and Igs.
Serum oxidative stress markers (SOD and MDA) as well as IgA, IgE, IgG, and IgM were not altered by treatment when analyzed individually (Table 5). For IgE, a linear effect was observed, with higher SCFP dosages leading to higher (P < 0.05) circulating IgE concentrations.
Table 5.
Serum oxidative stress markers and Igs of dogs supplemented with a yeast fermentation product
| Saccharomyces cerevisiae fermentation product, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 125 | 250 | 500 | SEM | Linear | Quadratic | 0 vs. yeast |
| Oxidative stress markers | ||||||||
| Superoxide dismutase, µg/mL | 1.26 | 1.46 | 1.85 | 1.84 | 0.88 | 0.17 | 0.45 | 0.16 |
| Malondialdehyde, nmol/mL | 6.52 | 6.62 | 6.88 | 7.61 | 4.12 | 0.57 | 0.93 | 0.58 |
| Igs | ||||||||
| IgA, mg/mL | 3.41 | 3.77 | 3.10 | 4.27 | 1.50 | 0.66 | 0.56 | 0.65 |
| IgG, mg/mL | 21.47 | 21.85 | 21.39 | 22.09 | 2.82 | 0.61 | 0.75 | 0.60 |
| IgM, mg/mL | 2.67 | 2.78 | 2.82 | 2.80 | 8.41 | 0.83 | 0.98 | 0.98 |
| IgE, µg/mL | 19.39 | 18.49 | 23.19 | 23.36 | 0.67 | 0.05 | 0.49 | 0.34 |
Immune cell populations.
NK cell and AP cell (B-cell and monocyte) populations were not altered when individual treatments were compared (Table 6). However, an overall effect was observed when comparing controls vs. all SCFP treatments for the populations of B cells and monocytes presenting MHC-II, with dogs supplemented with SCFP having greater (P < 0.05) MHC-II+ B cell and monocyte populations than control dogs. T-cell populations were not affected by SCFP when analyzed individually (Table 7). When cells were stimulated, a linear effect was observed in IFNγ-secreting helper T cells and IFNγ-secreting cytotoxic T cells, with higher SCFP dosages leading to higher (P < 0.05) IFNγ-secreting cell populations.
Table 6.
Natural killer cell and antigen-presenting cell populations of dogs supplemented with a yeast fermentation product
| Saccharomyces cerevisiae fermentation product, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 125 | 250 | 500 | SEM | Linear | Quadratic | 0 vs. yeast |
| Natural killer cell, % of lymphocyte | 14.34 | 10.52 | 10.27 | 12.55 | 3.07 | 0.39 | 0.12 | 0.11 |
| Antigen-presenting cells | ||||||||
| B cell, % of lymphocyte | 8.08 | 7.35 | 9.37 | 7.96 | 2.51 | 0.44 | 0.86 | 0.85 |
| B cell, MHC II+, % of B cell1 | 96.26 | 97.20 | 97.44 | 97.25 | 1.56 | 0.12 | 0.29 | 0.05 |
| Monocyte, % of white blood cell | 4.16 | 4.29 | 4.77 | 3.99 | 2.37 | 0.81 | 0.95 | 0.73 |
| Monocyte, MHC II+, % of monocyte1 | 55.90 | 51.18 | 59.04 | 61.33 | 7.63 | 0.86 | 0.68 | 0.05 |
1B cells or monocytes that present major histocompatibility complex class II (MHC II).
Table 7.
T-cell populations of dogs supplemented with a yeast fermentation product
| Saccharomyces cerevisiae fermentation product, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 125 | 250 | 500 | SEM | Linear | Quadratic | 0 vs. yeast |
| Control | ||||||||
| Lymphocyte, % of PBMC1 | 21.28 | 16.63 | 18.92 | 18.84 | 11.45 | 0.76 | 0.37 | 0.23 |
| T cell, % of lymphocyte | 83.32 | 80.11 | 78.77 | 77.54 | 5.10 | 0.07 | 0.74 | 0.42 |
| Helper T cell, % of lymphocyte | 37.84 | 36.97 | 34.54 | 34.59 | 4.58 | 0.07 | 0.76 | 0.28 |
| Cytotoxic T cell, % of lymphocyte | 32.23 | 31.80 | 32.74 | 31.34 | 4.55 | 0.62 | 0.63 | 0.80 |
| Helper: cytotoxic T-cell ratio | 1.31 | 1.23 | 1.13 | 1.16 | 0.26 | 0.29 | 0.40 | 0.23 |
| IFN-γ secreting T cell, % of lymphocyte | 0.06 | 0.11 | 0.08 | 0.11 | 0.02 | 0.73 | 0.83 | 0.99 |
| IFN-γ secreting helper cell, % of lymphocyte | 0.01 | 0.01 | 0.01 | 0.02 | 0.00 | 0.38 | 0.13 | 0.95 |
| IFN-γ secreting cytotoxic cell, % of lymphocyte | 0.01 | 0.03 | 0.03 | 0.04 | 0.01 | 0.02 | 0.67 | 0.03 |
| Stimulation2 | ||||||||
| Lymphocyte, % of PBMC1 | 63.71 | 60.78 | 65.08 | 61.57 | 7.73 | 0.90 | 0.64 | 0.81 |
| T cell, % of lymphocyte | 76.05 | 76.74 | 75.16 | 71.82 | 7.43 | 0.12 | 0.89 | 0.57 |
| Helper T cell, % of lymphocyte | 40.12 | 42.9 | 37.46 | 38.25 | 3.91 | 0.21 | 0.29 | 0.86 |
| Cytotoxic T cell, % of lymphocyte | 22.58 | 23.9 | 24.09 | 23.75 | 3.84 | 0.65 | 0.50 | 0.74 |
| Helper: cytotoxic T-cell ratio | 1.90 | 1.93 | 1.70 | 1.72 | 0.35 | 0.72 | 0.39 | 0.56 |
| IFN-γ secreting T cell, % of lymphocyte | 7.56 | 7.20 | 8.76 | 9.29 | 1.11 | 0.22 | 0.41 | 0.72 |
| IFN-γ secreting helper cell, % of lymphocyte | 3.18 | 3.53 | 4.62 | 4.64 | 0.47 | 0.02 | 0.81 | 0.14 |
| IFN-γ secreting cytotoxic cell, % of lymphocyte | 2.75 | 2.72 | 3.43 | 3.24 | 0.50 | 0.05 | 0.45 | 0.33 |
1PBMC = peripheral blood mononuclear cells.
2Cells were stimulated with cell stimulation cocktail (phorbol 12-myristate 13-acetate, ionomycin, brefeldin A, and monensin) for 4 h.
Responsiveness of toll-like receptors.
The responsiveness of TLR to agonists was evaluated by measurement of TNF-α concentrations (Table 8). TNF-α concentrations of control wells were not different among treatment groups. In cells stimulated with TLR2, TLR3, TLR4, and TLR7/8 agonists, an overall effect of controls vs. all SCFP treatments was observed, with cells obtained from dogs supplemented with SCFP having a lower (P < 0.05) TNF-α secretion than cells collected from control dogs. For cells stimulated with TLR2 and TLR7/8 agonists, dogs supplemented with 125 or 500 mg SCFP had lower (P < 0.05) TNF-α concentrations than control dogs. A linear effect was observed for cells stimulated with TLR2 and TLR7/8 agonists, with TNF-α concentrations being reduced with increasing SCFP dosage. For cells stimulated with TLR3 agonist, SCFP did not affect TNF-α concentrations when treatment groups were compared individually. For cells stimulated with TLR4 agonist, dogs supplemented with 125 mg SCFP had lower (P < 0.05) TNF-α concentrations than control dogs. A quadratic effect (P < 0.05) was also observed for cells stimulated with TLR7/8 agonist. Control dogs had the highest TNF-α concentrations, which was greatly reduced in dogs supplemented with 125 mg SCFP, and increased to an intermediate concentration for those supplemented with 250 and 500 mg.
Table 8.
TNF-α concentrations (pg/mL) in cell culture supernatant of dogs supplemented with a yeast fermentation product
| Saccharomyces cerevisiae fermentation product, mg/d | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 125 | 250 | 500 | SEM | Linear | Quadratic | 0 vs. yeast |
| Control | 396.64 | 279.23 | 408.20 | 323.31 | 150.30 | 0.50 | 0.47 | 0.64 |
| Zymosan (TLR2 agonist) | 705.15a | 492.72b | 591.74ab | 518.74b | 179.72 | 0.04 | 0.46 | <0.01 |
| Poly (I:C)1 (TLR3 agonist) | 455.75 | 353.67 | 414.94 | 360.36 | 104.82 | 0.16 | 0.43 | 0.05 |
| Lipopolysaccharide (TLR4 agonist) | 540.78a | 372.15b | 454.12ab | 416.18ab | 128.96 | 0.08 | 0.09 | <0.01 |
| R848 (resiquimod) (TLR7/8 agonist) | 2267.06a | 1432.79b | 1628.43ab | 1581.30b | 484.06 | 0.01 | 0.04 | <0.01 |
1Poly (I:C): polyinosinic:polycytidylic acid.
a,bMean values within a row with unlike superscript letters differ (P < 0.05).
Palatability
In the palatability test, a 1.93:1 total consumption ratio was observed for the SCFP-containing diet vs. control diet, demonstrating a preference (P < 0.05) for the diet containing yeast. Using data from both days, the yeast-containing diet was consumed first on 27/40 occasions (vs. 13/40 for control diet).
DISCUSSION
The demand for functional ingredients in the pet food industry continues to grow as pet owners are more focused on pet health and wellness. Yeast products may serve as functional ingredients in pet foods, with interest in them due to the health benefits shown in other animals. In the present study, we investigated the effects of SCFP on ATTD, fecal characteristics, fecal fermentative end-products, fecal microbiota, and immune indices in adult beagles. Overall, ATTD and fecal characteristics were not affected by SCFP. Supplementation of SCFP decreased fecal phenol concentrations and altered fecal microbial composition. Our data also suggested that supplementation of SCFP could modulate immune cell populations, decrease TLR responsiveness and increase serum IgE concentrations. Finally, diet palatability was enhanced with inclusion of 0.2% SCFP.
All dogs remained healthy throughout the study. Some parameters from serum chemistry profile (globulin; albumin: globulin ratio) and WBC counts were slightly out of the reference range. However, no clinical signs were noted during the study. A quadratic effect was observed for total bilirubin and a linear increase was noted for corticosteroid-induced alkaline phosphatase. Because both changes were small and within reference ranges, they are likely not physiologically relevant.
As expected, supplementation of SCFP did not influence food intake, BW, and ATTD. Similar results were reported in dogs with various kinds of yeast products (Swanson et al., 2002a; Grieshop et al., 2004; Pawar et al., 2017). Another study, however, observed a lower ATTD of DM and CP in yeast-supplemented dogs (Middelbos et al., 2007). The lower digestibility may have been due to increased bacterial growth and excretion due to increased fermentation. In that scenario, undigested protein in the colon can be utilized by bacteria for synthesis of bacterial proteins, which results in an underestimation of CP digestibility (Cummings et al., 1979). The unchanged ATTD in our study could be explained by the highly digestible diet.
Most of the fecal characteristics were not affected by SCFP, which is in line with other dog studies (Swanson et al., 2002a; Pawar et al., 2017). Only fecal score was increased with SCFP dosages. Although higher fecal scores (wetter feces) were observed, all feces remained in the ideal fecal score range (2.5 to 3 on five-point scale). Supplementation of SCFP did not alter fecal SCFA concentrations. Because SCFA are rapidly absorbed by colonocytes, it is often difficult to detect alterations in fecal SFCA (Von Engelhardt et al., 1989). These results agree with other dog studies that have reported unchanged fecal SCFA concentrations (Swanson et al., 2002a; Stercova et al., 2016; Pawar et al., 2017). Because SCFP is a fermentation product, the components of SCFP may not be fermented, but utilized by the bacteria or host directly. Similar with Swanson et al. (2002a), dogs supplemented with SCFP in the present study had decreased concentrations of fecal phenol and total fecal phenol + indole. The SCFP contains ~44% carbohydrates and the decreased fecal phenol concentrations may in part be due to an increased intake of undigested carbohydrate in the SCFP-treated dogs. However, it is questionable because it would translate to only 220 mg of carbohydrate in the highest supplemented group (500 mg/d) and decreased concentrations of fecal protein fermentative end-products by supplementation of oligosaccharides have only been reported in dogs given higher dosages (Terada et al., 1992; Zentek et al., 2002). Bacteria utilize undigested nutrients for bacterial protein synthesis in the colon. Increased carbohydrates from SCFP may serve as an energy source for colonic bacteria, which results in lower utilization of proteins and therefore decreased protein catabolites. However, we did not observe decreased BCFA and ammonia.
In agreement with other dog studies investigating yeast products (Grieshop et al., 2004; Middelbos et al., 2007; Stercova et al., 2016), SCFP slightly affected fecal microbiota. Although species richness and evenness were not different among treatment groups, relative abundances of a few taxa were altered by SCFP. The predominant phyla observed in this study were similar to that reported previously (Suchodolski et al., 2008; Handl et al., 2011; Swanson et al., 2011; Deng and Swanson, 2015). However, the relative abundances were different from other studies. For example, Fusobacteria (31.31% to 38.37%) were higher and Firmicutes (25.33% to 32.59%) were lower compared with those in other studies (Fusobacteria: 0.3% to 25.0%; Firmicutes: 35.0% to 93.4%.) (Handl et al., 2011; Suchodolski, 2011; Swanson et al., 2011). These differences could be due to many factors such as diet composition, dog breed or sex, DNA extraction and amplification methods or sequencing methods (Deng and Swanson, 2015). Firmicutes tended to increase while Fusobacteria decreased with higher SCFP doses. Similar results were reported in dogs fed a low level of dietary fiber (Middelbos et al., 2010). However, limited data are available to speculate whether this is beneficial to dogs. Clinical studies have shown lower Fusobacteria in dogs with inflammatory bowel diseases (IBD) (Vázquez-Baeza et al., 2016). The diets were not measured or controlled in those studies, however. Therefore, it is difficult to determine whether lower abundances of Fusobacteria contributed to or were caused by IBD. Bifidobacterium increased in SCFP-supplemented dogs, which is in agreement with previous dog studies testing yeast products (Swanson et al., 2002b; Grieshop et al., 2004). Increased abundance of Bifidobacterium is often associated with a healthy gut in terms of reduced pathogenic bacteria (Araya-Kojima et al., 1995; Fujiwara et al., 2001) and enhanced immune function (Chiang et al., 2000; Wu et al., 2009). The increased Bifidobacterium in the current study may be due to fermentability of β-glucan or MOS present in SCFP (Swanson et al., 2002b; Zhao and Cheung, 2011). By utilizing such compounds, Bifidobacterium may have an advantage and grow at a greater rate but exact reason is unknown.
Several studies have demonstrated that yeast cell wall or yeast-derived MOS could affect immune cell numbers in dogs (Swanson et al., 2002a; Grieshop et al., 2004; Middelbos et al., 2007; Pawar et al., 2017). In the present study, we observed lower total WBC counts in SCFP-supplemented dogs. This is consistent with other studies in dogs and pigs (Middelbos et al., 2007; Shen et al., 2011; Sanchez et al., 2018), where supplementation of yeast cell wall or yeast fermentation product led to lower WBC counts. Another study from Shen et al. (2009) reported that pigs supplemented with yeast culture had enhanced T helper-1 (Th1) responses in the gut. Increased mucosal immunity, which serves as the first barrier, may prevent pathogens from migrating into the mucosa, thereby mitigating the need to generate an immune response. However, this hypothesis cannot be confirmed in the current study because mucosal immunity was not evaluated. It is also possible that the immune capacity is enhanced and thus fewer immune cells are needed. This hypothesis is partially supported by our immune cell population data. In our study, the populations of AP cells, including B cells and monocytes expressing surface MHC class II were increased in SCFP-supplemented dogs. AP cells process antigens and present antigen peptides to helper T cells in an MHC class II-dependent manner. As such, MHC class II expression is needed to stimulate helper T cells. In addition, the populations of activated Th1 (IFN-γ-secreting helper T cells) were increased by SCFP. The enhanced Th1 response could be caused by β-glucan, one of the yeast cell wall components that has been shown to trigger Th1 responses (Suzuki et al., 2001; Plat and Mensink, 2005). Our findings suggest that SCFP may enhance the capacity of immune cells, especially Th1 responses, to react to antigens entering the body potentially requiring fewer immune cells to maintain health.
Secretion of TNF-α, a pro-inflammatory cytokine, from PMBC was decreased by SCFP, which suggests a potential anti-inflammatory effect of SCFP. This could be possibly explained by β-glucan. Soltys and Quinn (1999) observed a decreased production of TNF-α and IL-6 in monocytes and lymphocytes from mice treated with β-glucan. Li et al. (2006) also reported that supplementation of S. cerevisiae β-glucan resulted in lower concentrations of plasma TNF-α and IL-6 during LPS challenge. An alternative hypothesis is that the cells are activated by SCFP and are refractory to additional TLR stimulation. This hypothesis is in agreement with our data demonstrating increased percentages of AP expressing MHC II. Finally, gut microbiota may also play a role in the anti-inflammatory effects (Cerf-Bensussan and Gaboriau-Routhiau, 2010; Hooper et al., 2012). In our study, we observed increased relative abundance of Bifidobacterium in SCFP-supplemented dogs. Bifidobacterium has been shown to decrease the expression of TLRs, NFκB, and cytokines in vivo and in vitro (Riedel et al., 2006; O’Mahony et al., 2008; Heuvelin et al., 2009; Okada et al., 2009).
Supplementation of SCFP did not alter serum Ig concentrations, with the exception of IgE. Similar results of serum IgA, IgG, IgM were observed in other studies where dogs were supplemented with MOS or yeast cell wall (Swanson et al., 2002a; Grieshop et al., 2004; Middelbos et al., 2007). Serum IgE concentration was not reported in other dog studies. In our study, serum IgE concentrations elevated linearly with increasing SCFP dosage, which may have been due to enhanced Th2 responses to SCFP. This finding warrants further investigation into the effects of SCFP on other T cell subsets such as Th2, Th17, and regulatory T cells. In addition, there were no allergic symptoms noticed during the study. More data about SCFP-specific IgE need to be collected to understand this outcome.
Yeast products have been used as palatability enhancers in pet food industry for many years (Swanson and Fahey, 2006). In the current study, inclusion of 0.2% SCFP in the diet increased palatability as shown using a standard 2-d palatability test. Similar results were reported when dogs were offered 7.5% sugarcane yeast (Martins et al., 2014). However, Pawar et al. (2017) did not observe increased palatability when dogs were fed 1.5% MOS. Although not fully researched, many factors in yeast products such as the umami taste from nucleotides (Ugawa and Kurihara, 1994) as well as protein and fat content may contribute to enhanced palatability.
In conclusion, SCFP may act as a functional ingredient and have positive effects on gut health and immune function in dogs. SCFP slightly modulates fecal microbiota composition and activity by increasing Bifidobacterium, decreasing Fusobacterium, and decreasing phenol and indole concentrations. Supplementation of SCFP enhances Th1 responses, but decreases TLR responses and thus may decrease inflammation. Inclusion of SCFP may also enhance diet palatability. Therefore, SCFP may be used in dog food to improve gut health by shifting gut microbiota positively, elevating immune capacity, and decreasing inflammation. The application of SCFP may be most beneficial to weaning puppies, geriatric dogs, or those with inflammatory conditions because these populations may have unstable or undesirable gut microbiota populations or compromised immune systems.
Conflict of interest statement. None declared.
Supplementary Material
AKNOWLEDGMENT
We sincerely thank Kiley Algya, Toshiro Baba, Chelsea Iennarella, Anne Lee, Juliana Nogueira, Thunyaporn Phungviwatnikul, and Zachary Traughber for their assistance in sample collections. M. R. C. G., K. S. S. and C. M. W. designed the experiment. C.-Y. L., C. A. and A. J. S. performed laboratory analyses. C.-Y. L. performed the animal trials, statistical analysis, and wrote the manuscript. C. M. W. is employed by Diamond V Mills, Inc..
Footnotes
Funding provided by Diamond V Mills, Inc.
LITERATURE CITED
- American Association of Cereal Chemists (AACC) 1983. Approved methods. 8th ed. St Paul, MN:American Association of Cereal Chemists. [Google Scholar]
- Araya-Kojima T., Yaeshima T., Ishibashi N., Shimamura S., and Hayasawa H.. 1995. Inhibitory effects of Bifidobacterium longum BB536 on harmful intestinal bacteria. Bifidobact. Microflora. 14:59–66. doi:10.12938/bifidus1982.14.2_59 [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 2006. Official methods of analysis. 17th ed. Gaithersburg, MD:Association of Official Analytical Chemists. [Google Scholar]
- Association of American Feed Control Officials (AAFCO) 2015. Official publication. Oxford, IN:AAFCO. [Google Scholar]
- Benjamini Y., and Hochberg Y.. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57:289–300. doi:10.2307/2346101 [Google Scholar]
- Brewer M. T., Anderson K. L., Yoon I., Scott M. F., and Carlson S. A.. 2014. Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet. Microbiol. 172:248–255. doi:10.1016/j.vetmic.2014.05.026 [DOI] [PubMed] [Google Scholar]
- Budde E. F. 1952. The determination of fat in baked biscuit type dog foods. J. Assoc. Off. Agric. Chem. 35:799–805. [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7:335–336. doi:10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., Owens S. M., Betley J., Fraser L., Bauer M., et al. 2012. Ultra-high-throughput microbial community analysis on the illumina HiSeq and MiSeq platforms. ISME J. 6:1621–1624. doi:10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N., and Gaboriau-Routhiau V.. 2010. The immune system and the gut microbiota: friends or foes? Nat. Rev. Immunol. 10:735–744. doi:10.1038/nri2850 [DOI] [PubMed] [Google Scholar]
- Chaney A. L., and Marbach E. P.. 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. [PubMed] [Google Scholar]
- Chiang B. L., Sheih Y. H., Wang L. H., Liao C. K., and Gill H. S.. 2000. Enhancing immunity by dietary consumption of a probiotic lactic acid bacterium (Bifidobacterium lactis HN019): optimization and definition of cellular immune responses. Eur. J. Clin. Nutr. 54:849–855. doi:10.1038/sj.ejcn.1601093 [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Hill M. J., Bone E. S., Branch W. J., and Jenkins D. J.. 1979. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 32:2094–2101. doi:10.1093/ajcn/32.10.2094 [DOI] [PubMed] [Google Scholar]
- Deng P., and Swanson K. S.. 2015. Gut microbiota of humans, dogs and cats: current knowledge and future opportunities and challenges. Br. J. Nutr. 113(Suppl.):S6–17. doi:10.1017/S0007114514002943 [DOI] [PubMed] [Google Scholar]
- DeSantis T. Z., Hugenholtz P., Larsen N., Rojas M., Brodie E. L., Keller K., Huber T., Dalevi D., Hu P., and Andersen G. L.. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072. doi:10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi:10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Von Engelhardt W., Rönnau K., Rechkemmer G., and Sakata T.. 1989. Absorption of short-chain fatty acids and their role in the hindgut of monogastric animals. Anim. Feed Sci. Technol. 23:43–53. doi:10.1016/0377-8401(89)90088-6 [Google Scholar]
- Erwin E. S., Marco G. J., and Emery E. M.. 1961. Volatile fatty acid analyses of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi:10.3168/jds.S0022-0302(61)89956-6 [Google Scholar]
- Flickinger E. A., Schreijen E. M., Patil A. R., Hussein H. S., Grieshop C. M., Merchen N. R., and Fahey G. C. Jr. 2003. Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 81:2008–2018. doi:10.2527/2003.8182008x [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Hashiba H., Hirota T., and Forstner J. F.. 2001. Inhibition of the binding of enterotoxigenic escherichia coli Pb176 to human intestinal epithelial cell line HCT-8 by an extracellular protein fraction containing BIF of bifidobacterium longum SBT2928: suggestive evidence of blocking of the binding receptor gangliotetraosylceramide on the cell surface. Int. J. Food Microbiol. 67:97–106. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H. J., Wu S. G., Yu S. H., Yoon I., Moore D., Gao Y. P., Yan H. J., and Qi G. H.. 2009. Effect of Saccharomyces cerevisiae fermentation product on immune functions of broilers challenged with Eimeria tenella. Poult. Sci. 88:2141–2151. doi:10.3382/ps.2009-00151 [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H. J., Yu S. H., Wu S. G., Yoon I., Quigley J., Gao Y. P., and Qi G. H.. 2008. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 87:1377–1384. doi:10.3382/ps.2007-00418 [DOI] [PubMed] [Google Scholar]
- Grieshop C. M., Flickinger E. A., Bruce K. J., Patil A. R., Czarnecki-Maulden G. L., and Fahey G. C. Jr. 2004. Gastrointestinal and immunological responses of senior dogs to chicory and mannan-oligosaccharides. Arch. Anim. Nutr. 58:483–493. doi:10.1080/00039420400019977 [DOI] [PubMed] [Google Scholar]
- Handl S., Dowd S. E., Garcia-Mazcorro J. F., Steiner J. M., and Suchodolski J. S.. 2011. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 76:301–310. doi:10.1111/j.1574-6941.2011.01058.x [DOI] [PubMed] [Google Scholar]
- Heuvelin E., Lebreton C., Grangette C., Pot B., Cerf-Bensussan N., and Heyman M.. 2009. Mechanisms involved in alleviation of intestinal inflammation by bifidobacterium breve soluble factors. PLoS One 4:e5184. doi:10.1371/journal.pone.0005184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L. V., Littman D. R., and Macpherson A. J.. 2012. Interactions between the microbiota and the immune system. Science 336:1268–1273. doi:10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. C., Hung S. W., Jan T. R., Liao K. W., Cheng C. H., Wang Y. S., and Chu R. M.. 2008. CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J. Leukoc. Biol. 84:1501–1510. doi:10.1189/jlb.0408255 [DOI] [PubMed] [Google Scholar]
- Kim S., and Brandherm M.. 2008. Effects of yeast culture supplementation to gestation and lactation diets on growth of nursing piglets. J. Anim. Sci. 21:1011–1014. [Google Scholar]
- Kim S. W., Brandherm M., Newton B., Cook D. R., Yoon I., and Fitzner G.. 2010. Effect of supplementing Saccharomyces cerevisiae fermentation product in sow diets on reproductive performance in a commercial environment. Can. J. Anim. Sci. 90:229–232. [Google Scholar]
- Laflamme D. P. 1997. Development and validation of a body condition score system for dogs: a clinical tool. Canine Pract. 25:10–15. [Google Scholar]
- Li J., Li D., Gong L., Ma Y., He Y., and Zhai H.. 2006. Effects of live yeast on the performance, nutrient digestibility, gastrointestinal microbiota and concentration of volatile fatty acids in weanling pigs. Arch. Anim. Nutr. 60:277–288. doi:10.1080/17450390600785343 [DOI] [PubMed] [Google Scholar]
- Li S., Yoon I., Scott M., Khafipour E., and Plaizier J. C.. 2016. Impact of Saccharomyces cerevisiae fermentation product and subacute ruminal acidosis on production, inflammation, and fermentation in the rumen and hindgut of dairy cows. Anim. Feed Sci. Technol. 211:50–60. doi:10.1016/J.ANIFEEDSCI.2015.10.010 [Google Scholar]
- Lozupone C., and Knight R.. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. doi:10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães V. J. A., Susca F., Lima F. S., Branco A. F., Yoon I., and Santos J. E. P.. 2008. Effect of feeding yeast culture on performance, health, and immunocompetence of dairy calves. J. Dairy Sci. 91:1497–1509. doi:10.3168/JDS.2007-0582 [DOI] [PubMed] [Google Scholar]
- Martins M. S., Sakomura N. K., Souza D. F., Filho F. O., Gomes M. O., Vasconcellos R. S., and Carciofi A. C.. 2014. Brewer’s yeast and sugarcane yeast as protein sources for dogs. J. Anim. Physiol. Anim. Nutr. (Berl.) 98:948–957. doi:10.1111/jpn.12145 [DOI] [PubMed] [Google Scholar]
- Middelbos I. S., Godoy M. R., Fastinger N. D., and Fahey G. C.. 2007. A dose-response evaluation of spray-dried yeast cell wall supplementation of diets fed to adult dogs: Effects on nutrient digestibility, immune indices, and fecal microbial populations. J. Anim. Sci. 85:3022–3032. doi:10.2527/jas.2007-0079 [DOI] [PubMed] [Google Scholar]
- Middelbos I. S., Vester Boler B. M., Qu A., White B. A., Swanson K. S., and Fahey G. C. Jr. 2010. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS One 5:e9768. doi:10.1371/journal.pone.0009768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyad M. A., Robinson L. E., Kittelsrud J. M., Reeves S. G., Weaver S. E., Guzman A. I., and Bubak M. E.. 2009. Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: a randomized, double-blind, placebo-controlled trial. Adv. Ther. 26:795–804. doi:10.1007/s12325-009-0057-y [DOI] [PubMed] [Google Scholar]
- Moyad M. A., Robinson L. E., Zawada E. T. Jr, Kittelsrud J. M., Chen D. G., Reeves S. G., and Weaver S. E.. 2008. Effects of a modified yeast supplement on cold/flu symptoms. Urol. Nurs. 28:50–55. [PubMed] [Google Scholar]
- Moyad M. A., Robinson L. E., Zawada E. T., Kittelsrud J., Chen D. G., Reeves S. G., and Weaver S.. 2010. Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. J. Altern. Complement. Med. 16:213–218. doi:10.1089/act.2010.16407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Tsuzuki Y., Hokari R., Komoto S., Kurihara C., Kawaguchi A., Nagao S., and Miura S.. 2009. Anti-inflammatory effects of the genus Bifidobacterium on macrophages by modification of phospho-I kappab and SOCS gene expression. Int. J. Exp. Pathol. 90:131–140. doi:10.1111/j.1365-2613.2008.00632.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony C., Scully P., O’Mahony D., Murphy S., O’Brien F., Lyons A., Sherlock G., MacSharry J., Kiely B., Shanahan F., et al. 2008. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappab activation. PLoS Pathog. 4:e1000112. doi:10.1371/journal.ppat.1000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. H., Tyson G. W., Hugenholtz P., and Beiko R. G.. 2014. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124. doi:10.1093/bioinformatics/btu494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar M. M., Pattanaik A. K., Sinha D. K., Goswami T. K., and Sharma K.. 2017. Effect of dietary mannanoligosaccharide supplementation on nutrient digestibility, hindgut fermentation, immune response and antioxidant indices in dogs. J. Anim. Sci. Technol. 59:11. doi:10.1186/s40781-017-0136-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro I., Robinson L., Verhelst A., Marzorati M., Winkens B., den Abbeele P. V., and Possemiers S.. 2017. A yeast fermentate improves gastrointestinal discomfort and constipation by modulation of the gut microbiome: results from a randomized double-blind placebo-controlled pilot trial. BMC Complement. Altern. Med. 17:441. doi:10.1186/s12906-017-1948-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plat J., and Mensink R. P.. 2005. Food components and immune function. Curr. Opin. Lipidol. 16:31–37. doi:10.1097/00041433-200502000-00007 [DOI] [PubMed] [Google Scholar]
- Prosky L., Asp N. G., Furda I., DeVries J. W., Schweizer T. F., and Harland B. F.. 1985. Determination of total dietary fiber in foods and food products: collaborative study. J. Assoc. Off. Anal. Chem. 68:677–679. [PubMed] [Google Scholar]
- Riedel C. U., Foata F., Philippe D., Adolfsson O., Eikmanns B. J., and Blum S.. 2006. Anti-inflammatory effects of bifidobacteria by inhibition of LPS-induced NF-kappab activation. World J. Gastroenterol. 12:3729–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez N. C. B., Carroll J. A., Broadway P. R., Bass B. E., and Frank J. W.. 2018. Modulation of the acute phase response following a lipopolysaccharide challenge in pigs supplemented with an all-natural Saccharomyces cerevisiae fermentation product. Livest. Sci. 208:1–4. doi:10.1016/j.livsci.2017.11.022 [Google Scholar]
- Shen Y. B., Carroll J. A., Yoon I., Mateo R. D., and Kim S. W.. 2011. Effects of supplementing Saccharomyces cerevisiae fermentation product in sow diets on performance of sows and nursing piglets. J. Anim. Sci. 89:2462–2471. doi:10.2527/jas.2010-3642 [DOI] [PubMed] [Google Scholar]
- Shen Y. B., Piao X. S., Kim S. W., Wang L., Liu P., Yoon I., and Zhen Y. G.. 2009. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 87:2614–2624. doi:10.2527/jas.2008-1512 [DOI] [PubMed] [Google Scholar]
- Soltys J., and Quinn M. T.. 1999. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with β-(1, 6)-branched β-(1, 3)-glucan. Infect. Immun. 67:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stercova E., Kumprechtova D., Auclair E., and Novakova J.. 2016. Effects of live yeast dietary supplementation on nutrient digestibility and fecal microflora in beagle dogs. J. Anim. Sci. 94:2909–2918. doi:10.2527/jas.2016-0584 [DOI] [PubMed] [Google Scholar]
- Suchodolski J. S. 2011. Companion animals symposium: microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 89:1520–1530. doi:10.2527/jas.2010-3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski J. S., Camacho J., and Steiner J. M.. 2008. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 66:567–578. doi:10.1111/j.1574-6941.2008.00521.x [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Adachi Y., Ohno N., and Yadomae T.. 2001. Th1/th2-balancing immunomodulating activity of gel-forming (1–3)-beta-glucans from fungi. Biol. Pharm. Bull. 24:811–819. [DOI] [PubMed] [Google Scholar]
- Swanson K. S., Dowd S. E., Suchodolski J. S., Middelbos I. S., Vester B. M., Barry K. A., Nelson K. E., Torralba M., Henrissat B., Coutinho P. M., et al. 2011. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 5:639–649. doi:10.1038/ismej.2010.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K. S., and Fahey G. C.. 2006. Potential role of yeast and yeast by-products in pet foods. Thrumpton, UK: Nottingham University Press. [Google Scholar]
- Swanson K. S., Grieshop C. M., Flickinger E. A., Bauer L. L., Healy H-P., Dawson K. A., Merchen N. R., and Fahey G. C.. 2002a. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 132:980–989. doi:10.1093/jn/132.5.980 [DOI] [PubMed] [Google Scholar]
- Swanson K. S., Grieshop C. M., Flickinger E. A., Merchen N. R., and Fahey G. C.. 2002b. Effects of supplemental fructooligosaccharides and mannanoligosaccharides on colonic microbial populations, immune function and fecal odor components in the canine. J. Nutr. 132:1717S–1719S. doi:10.1080/0003942021000019126 [DOI] [PubMed] [Google Scholar]
- Terada A., Hara H., Oishi T., Matsui S., Mitsuoka T., Nakajyo S., Fujimori I., and Hara K.. 1992. Effect of dietary lactosucrose on faecal flora and faecal metabolites of dogs. Microb. Ecol. Health Dis. 5:87–92. doi:10.3109/08910609209141294 [Google Scholar]
- Ugawa T., and Kurihara K.. 1994. Enhancement of canine taste responses to umami substances by salts. Am. J. Physiol. 266(3 Pt 2):R944–R949. doi:10.1152/ajpregu.1994.266.3.R944 [DOI] [PubMed] [Google Scholar]
- Vázquez-Baeza Y., Hyde E. R., Suchodolski J. S., and Knight R.. 2016. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 1:16177. doi:10.1038/nmicrobiol.2016.177 [DOI] [PubMed] [Google Scholar]
- Wu S., Rhee K. J., Albesiano E., Rabizadeh S., Wu X., Yen H. R., Huso D. L., Brancati F. L., Wick E., McAllister F., et al. 2009. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15:1016–1022. doi:10.1038/nm.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentek J., Marquart B., and Pietrzak T.. 2002. Intestinal effects of mannanoligosaccharides, transgalactooligosaccharides, lactose and lactulose in dogs. J. Nutr. 132(6 Suppl. 2):1682S–1684S. doi:10.1093/jn/132.6.1682S [DOI] [PubMed] [Google Scholar]
- Zhao J., and Cheung P. C.. 2011. Fermentation of β-glucans derived from different sources by bifidobacteria: evaluation of their bifidogenic effect. J. Agric. Food Chem. 59:5986–5992. doi:10.1021/jf200621y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.