Abstract
This review summarizes studies we conducted to test the hypothesis that size of the ovarian reserve (number of healthy follicles and oocytes in ovaries) positively impacts ovarian function and fertility in cattle. Key results, primarily in Bos taurus dairy cattle, show that antral follicle count (AFC) during follicular waves is highly variable between individuals, but very highly repeatable within individuals. Cycling heifers with low (≤15 follicles ≥3 mm, ~20% of a herd) vs. a high AFC (≥25, ~20% of a herd) have a smaller ovarian reserve, higher FSH but lower anti-Müllerian hormone (AMH), androstenedione, estradiol, and progesterone concentrations. Moreover, cattle with low AFC have a thinner endometrium, decreased response of granulosal, thecal, or luteal cells to FSH or LH and a poorer response to superovulation compared to cattle with high AFC. Interestingly, cows with a very high AFC as heifers have reduced fertility, fewer lactations, and shorter herd longevity, whereas cows with a low vs. intermediate AFC have reduced fertility, fewer lactations, and shorter herd longevity. Anti-Müllerian hormone concentrations are static within individuals but highly positively correlated with AFC, but fertility is not correlated with circulating AMH concentration in heifers and dairy cows with low vs. a higher AMH as heifers have reduced fertility and a shorter herd longevity. Anti-Müllerian hormone concentrations in dairy heifers are a moderately heritable trait (36%), and negatively impacted by inadequate maternal nutrition during early pregnancy or high maternal somatic cell count. We conclude that genetic or environmental manipulations of AMH could enhance size of the ovarian reserve and ovarian function, thereby improving fertility, response to superovulation, and longevity in dairy cows.
Keywords: anti-Müllerian hormone, dairy cows, ovarian reserve
INTRODUCTION
Cattle are born with a highly variable number of morphologically healthy follicles and oocytes, the ovarian reserve, which is determined during gestation, decreases with age, and is not replenished after birth (Erickson, 1966). The potential association between the size of the ovarian reserve and fertility in female cattle is very intriguing and has recently gained more interest among scientists and veterinarians thanks to the validation of 2 reliable markers of the size of the ovarian reserve in cattle: the number of follicles recruited during waves of follicular development and peripheral concentrations of anti-Müllerian hormone (AMH) reviewed in (Ireland et al., 2011; Mossa et al., 2017). This review presents an overview of the findings concerning the association between the ovarian reserve and several measures of fertility in Bos taurus cattle and the potential use of AMH as a biomarker predictive of reproductive performance in cattle.
ANTRAL FOLLICLE COUNT REPEATABILITY AND VARIABILITY
During the bovine estrous cycle, antral follicle growth occurs in FSH-induced waves at 7 to 10 d intervals (Adams et al., 1992). Daily ovarian ultrasonography was used to determine number of follicles ≥3 mm in diameter growing during waves and to examine its variation both among and within individual cattle (Burns et al., 2005). The peak number of follicles recruited per wave (antral follicle count, AFC) was highly variable among individuals (range 8 to 54 follicles), but highly repeatable (0.95, 1 = perfect) in the same or consecutive estrous cycles within each animal (Burns et al., 2005). These initial findings were confirmed in subsequent studies conducted on larger number of animals (188 follicular waves for 69 animals; Ireland et al., 2007) and it was also demonstrated that AFC repeatability was not influenced by 1) cattle age or breed; 2) season or stage of lactation; and 3) span of time between AFC measurements in the same individual (Burns et al., 2005; Ireland et al., 2007, 2008, 2009; Jimenez-Krassel et al., 2009; Mossa et al., 2012). Thus, we conclude that cattle can be reliably phenotyped based on AFC.
ENDOCRINE AND ANATOMICAL PHENOTYPES ASSOCIATED WITH AFC
The frequency distribution of AFC was similar among the different herds we studied: the majority of individuals had an AFC of 16 to 24 follicles per wave (arbitrarily classified as Intermediate), whereas AFC was ≤15 (low) in ~20% of a herd and ≥25 (high) in ~20% of animals (Ireland et al., 2007; Mossa et al., 2012). Hence, this classification was used to test the hypothesis that AFC is positively associated with the size of the ovarian reserve in cattle. The total number of morphologically healthy follicles and oocytes was ~80% lower in young adult beef heifers with low vs. high AFC (Table 1) and was very highly positively associated with AFC (r, correlation coefficient; r = 0.89, P < 0.001), demonstrating that the size of the ovarian reserve is dramatically smaller in age-matched cattle with low vs. high AFC and can be reliably estimated with AFC (Ireland et al., 2008).
Table 1.
Average peak antral follicle count (AFC), ovary size, and total number of follicles in ovaries of cattle with consistently low (≤15 follicles ≥3 mm in diameter) vs. high (≥25 follicles) peak AFC during follicular waves (Ireland et al., 2008)
| Follicle parameters | Low AFC | High AFC1 |
|---|---|---|
| Peak AFC per wave | 11.95 ± 1.2 | 39.61 ± 2.3*** |
| Ovary wet weight (g) | 3.05 ± 0.33 | 7.11 ± 0.41*** |
| Ovary height (mm) | 12.3 ± 1.1 | 15.5 ± 0.8* |
| Ovary length (mm) | 23.3 ± 1.4 | 28.3 ± 1.3* |
| Total no. of follicles in ovaries | ||
| Healthy + atretic | 88,960 ± 27,315 | 829,185 ± 248,327** |
| Healthy | 6,016 ± 1,685 | 29,056 ± 4,564** |
| Percentage healthy | 8.44 ± 2.25 | 4.37 ± 1.05 |
| Healthy per gram ovary | 2,110 ± 371 | 3,869 ± 535* |
| Polyovular | 352 ± 217 | 66,080 ± 53,965** |
| Percentage polyovular | 0.32 ± 0.22 | 5.43 ± 3.05 |
Statistical significance within row =
P < 0.05;
P < 0.01;
P < 0.001.
In the same study, peripheral FSH concentrations were also inversely associated with the number of follicles (Fig. 1), as reported for dairy (Haughian et al., 2004) and beef heifers (Ireland et al., 2007), lactating beef cows (Singh et al., 2004), and nonlactating dairy cows (Mossa et al., 2010b). Moreover, young beef heifers with low AFC had higher basal and episodic LH secretion on day 11 of the estrous cycle compared with age-matched animals with high AFC (Jimenez-Krassel et al., 2009). These findings indicate that gonadotropin secretion is inversely associated with the size of the ovarian reserve in cattle. Nevertheless, the causes of the different peripheral FSH and LH levels between cattle with high vs. low numbers of follicles are yet to be identified.
Figure 1.
Peak number of follicles, diameter of dominant (solid line) and largest subordinate (dotted line) follicle, and serum concentrations of FSH and estradiol for beef heifers that had low (≤15 follicles, n = 11 animals) vs. high peak numbers of follicles (≥25 follicles, n = 8) ≥3 mm in diameter during the first nonovulatory follicular wave. Symbols represent the average ± SEM for heifers with low (open symbols) or high (solid symbols) numbers of follicles during waves. Data were aligned relative to the peak of the first postovulatory increase in serum FSH concentrations (Ireland et al., 2007).
The hypothesis that the difference in FSH concentrations in cattle with chronically high vs. low numbers of follicles during follicular waves is due to a greater capacity of the pituitary gland to produce gonadotropins was tested (Mossa et al., 2010b). We measured FSH and LH secretion basally and in response to gonadotropin-releasing hormone (GnRH) or follicular fluid (rich in factors with negative feedback effect on gonadotropin secretion) following bilateral ovariectomy of cows. Before ovariectomy, these cows had chronically high vs. low circulating FSH concentrations during follicular waves. Gonadotropin concentrations after ovariectomy, GnRH injection, or follicular fluid challenge were similar between groups, indicating that inherent capacity of the pituitary gland to secrete gonadotropins does not differ between cattle with high vs. low numbers of follicles during follicular waves (Mossa et al., 2010b). Thus, we speculated that the chronically enhanced FSH secretion in individuals with reduced ovarian reserves may be controlled by a lower total production of ovarian feedback factors in cattle with low AFC. However, circulating concentrations of estradiol (Fig. 1) and inhibin-A, the main FSH negative feedback hormones in cattle (Bleach et al., 2001), were similar between cattle with chronically high vs. low FSH concentrations during follicular waves (Burns et al., 2005; Ireland et al., 2007; Mossa et al., 2010b). Moreover, diameter of dominant and largest subordinate follicle did not differ in cattle with low vs. high peak numbers of follicles (Fig. 1; Ireland et al., 2007). Therefore, the causes of the high FSH and LH gonadotropin concentrations in cattle with a reduced ovarian reserve remain unclear.
We subsequently tested the hypothesis that the heightened gonadotropin secretion observed in cattle with low AFC may alter ovarian function. A study was designed to determine if progesterone concentrations, differentiation and function of the corpus luteum (CL), and endometrial thickness differed during estrous cycles of young adult heifers with low vs. high AFC (Jimenez-Krassel et al., 2009). Evidence from young adult beef heifers and late-lactation dairy cows indicated that progesterone concentrations between day 3 and 14 of the estrous cycle were 30% to 50% lower in cattle with low vs. high AFC (Fig. 2) and that such differences were repeatable from one estrous cycle to the next (Jimenez-Krassel et al., 2009). In addition, endometrial thickness was lower in cattle with low vs. high AFC (P < 0.01) and decreased from day 0 to 6 after ovulation (P < 0.01) in cattle with high AFC, whereas it remained constant across time in animals with low AFC (Jimenez-Krassel et al., 2009). Because low serum progesterone concentrations and poor endometrial development are associated with embryo mortality in cattle (Diskin and Morris, 2008) and infertility in women (Basir et al., 2002), respectively, embryo mortality may be more frequent in cattle with a small compared with larger ovarian reserve.
Figure 2.
Alterations in serum concentrations of progesterone during the bovine estrous cycle (day 0 = estrus, day 1 = ovulation). Blood samples were obtained daily during different days of the estrous cycle for animals in the low vs. high group in study 1 (3- to 5-yr-old Holstein dairy cows, n = 3 animals or estrous cycles per group), study 2 (crossbred beef heifers, 19.8 ± 0.7 mo old, n = 11 or 14 animals or estrous cycles per group), and study 4 (crossbred beef heifers, 10- to 12-mo-old, n = 4 animals or estrous cycles per group). Each symbol represents the daily mean (±SEM) progesterone value for animals with a consistently low (≤15 follicles per wave, n = 32 estrous cycles for 25 animals) vs. high (≥25 follicles per wave, n = 30 cycles for 22 animals) AFC during follicular waves. Asterisks indicate significant differences (**P < 0.01) between groups. ANOVA indicated an overall significant effect of group (low vs. high, P < 0.02), day of cycle (P < 0.001), and group by day interaction (P < 0.001; Jimenez-Krassel et al., 2009).
To further investigate the potential association between size of the ovarian reserve and ovarian function, we tested the hypothesis that androgen production differs between cattle with high vs. low AFC. Serum testosterone concentrations were lower overall during estrous cycles of young adult beef cattle and older dairy cows with low vs. high AFC. Further, the abundance of thecal CYP17A1 mRNA (codes for a cytochrome P450 enzyme involved in androgen synthesis), LH-induced androstenedione (a potent androgen) production by thecal cells (Fig. 3), and follicular fluid concentrations of androstenedione in ovulatory follicles were diminished compared with those in cattle with high AFC (Scheetz et al., 2012). Thus, we concluded that ovarian androgen production is lower in cattle with low vs. high numbers of antral follicles growing during follicular waves, possibly due to a reduced responsiveness of thecal cells to LH.
Figure 3.
Alterations in basal and LH-induced thecal production of androstenedione in cattle with low vs. high AFC. Androstenedione production by theca interna isolated from first-wave dominant follicles of ovaries from animals with low (n = 3) or high (n = 3) AFC. Theca interna (2 pieces per well) from each follicle were cultured in duplicate for 24 h with or without 4 ng/mL LH. Androstenedione production was normalized to total protein contained in thecal tissue after culture. Each bar represents the mean ± SEM for each follicle number group. Asterisk indicates significant (P < 0.05) difference between means (Mossa et al., 2010a).
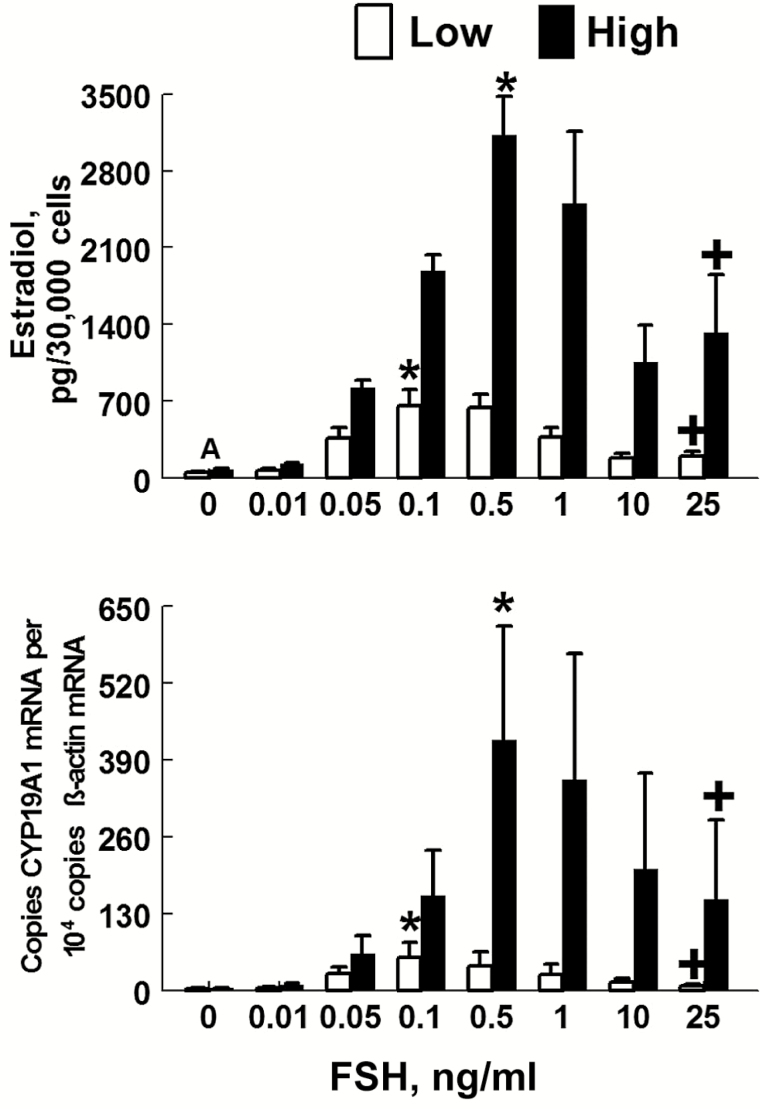
This hypothesis was confirmed by the finding that the capacity of luteinized granulosal cells isolated from dominant follicles and of luteal cells isolated from CL on day 12 of the estrous cycle to produce progesterone basally and in response to LH is lower for cattle with low vs. high AFC (Jimenez-Krassel et al., 2009). Moreover, using an in vitro model, we determined whether FSH action on granulosal cells differed between cattle with low vs. high AFC. The basal and FSH-induced capacity to produce estradiol (Fig. 4) and AMH, as well as the expression of mRNAs for CYP19A1 (aromatase, Fig. 4), FSH receptor, and AMH were lower in nonluteinized and luteinized granulosal cells collected from low compared to high AFC groups of cattle (Scheetz et al., 2012). These findings indicate that granulosal cells from the low AFC responded minimally to FSH. Because cattle with low AFC have chronically heightened gonadotropin peripheral concentrations (Burns et al., 2005; Ireland et al., 2007; Jimenez-Krassel et al., 2009; Mossa et al., 2010b), FSH receptors in granulosal cells may be desensitized and, consequently, granulosal cells may be refractory to FSH. The minimal response of granulosal cells to FSH could be among the causes of the poor response to gonadotropin stimulation in cattle with a reduced ovarian reserve (Table 2).
Figure 4.
Effect of FSH on estradiol production and abundance of CYP19A1 mRNA in granulosa cells from cattle with low vs. a high AFC (Scheetz et al., 2012). Granulosa cells were treated with various doses of FSH for 6 d. Estradiol production (top panel) and abundance of CYP19A1 mRNA (bottom panel) were determined on day 6 of culture in the High and Low AFC group. For the top panel, bars represent the overall mean ± SEM for the mean estradiol concentrations in media for 3 pools of granulosa cells from 3 to 5 cows per pool. The mean estradiol value for each pool was generated after measurement of 3 replicate media samples per pool. Each replicate sample contained media from 2 culture wells combined. For the bottom panel, bars represent the mean ± SEM for abundance of CYP19A1 mRNA in granulosa cells in 3 pools of granulosa cells from 3 to 5 cows per pool. Each pool contained mRNA isolated from granulosa cells from 6 culture wells combined. Results of ANOVA indicated that overall concentration of estradiol (P < 0.001) and abundance of CYP19A1 (P < 0.001) mRNA for all doses of FSH (0 to 25 ng/mL) combined were higher for granulosa cells from the High vs. the Low AFC group. The A above bars at the 0 FSH dose indicates a significant (P < 0.01) difference between means. The asterisks above bars indicate that estradiol production or abundance of CYP19A1 mRNA increased (P < 0.001) linearly in response to FSH doses from 0 to 0.1 ng/mL or 0 to 0.5 ng/mL in the low or high AFC group, respectively. The plus symbol above bars indicates that estradiol production or abundance of CYP19A1 mRNA decreased (P < 0.05) linearly in response to FSH doses >0.1 ng/mL or >0.5 ng/mL for the low or high AFC group, respectively (Scheetz et al., 2012).
Table 2.
Evidence that the size of the ovarian reserve, as assessed by antral follicle count (AFC) and/or circulating concentrations of anti-Müllerian hormone (AMH), is positively associated with gonadotropin stimulation in cattle
| Breed and number | Biomarker | Positive correlation with response to hormonal stimulation | Reference |
|---|---|---|---|
| Beef heifers (25) | AFC before treatment | Number of CLs, embryos/oocytes recovered (P < 0.05) | Ireland et al. (2007) |
| Holstein cows (18) | AFC and AMH | AFC and number of large follicles (r = 0.75, P < 0.001)AMH and number of CLs (r = 0.64, P < 0.01) | Rico et al. (2009) |
| Holstein cows (52) | AMH | Number of large follicles (r = 0.46, P < 0.001)Number of CLs (r = 0.43, P < 0.01) | Rico et al. (2012) |
| Japanese Black (12) | AMH | Number of total follicles (r = 0.646, P < 0.001)Number of ova/embryos (r = 0.734, P < 0.001) | Hirayama et al. (2012) |
| Taurus indicus Braford (40) | AFC at 9 to 20 mo | Number of embryos/oocytes recovered at 24 mo of age (P < 0.05) | Silva-Santos et al. (2014) |
| Lactating Holstein cows (72) | AMH | Number of CLs (r = 0.65, P < 0.01)Number of total structures collected (r = 0.50, P < 0.01)Number of total transferable embryos (r = 0.28, P < 0.02) | Souza et al. (2015) |
| Japanese Black (6) | AMH | Number of embryos/oocytes (r = 0.637, P = 0.005)Number of transferable embryos (r = 0.640, P = 0.004) | Nabenishi et al. (2017) |
ASSOCIATION BETWEEN VERY HIGH AFC AND POOR FERTILITY AND PRODUCTIVITY
The hypothesis that heifers with a very high AFC are subfertile and, therefore, removed from a herd for poor reproductive performance at a greater rate and have a shorter productive herd life compared with age-matched herdmates with a lower AFC was tested. A single ultrasound measurement of follicle numbers (rather than daily ovarian ultrasonography throughout consecutive follicular waves; Burns et al., 2005) was conducted on cycling Holstein heifers (n = 440, 11 to 15 mo old). Heifers were ranked into a high- (≥25 follicles), mid- (16 to 24), or low-range (≤15) follicle number group (FNG). All heifers not removed from the herd before first calving (n = 408) had the opportunity to start their fifth or sixth lactation after birth of their first calf. Dairy cows classified into the high-range FNG as heifers had fewer (P < 0.02) lactations started and completed compared with the cows in the low-range FNG. Cows in the high-range FNG also had, on average, a 180-d shorter (P < 0.01) productive herd life compared with cows in the low-range FNG. The probability of being culled after birth of the first calf was 33% (hazard ratio = 1.33, P = 0.05) greater for cows in the high- compared with low- but not mid-range FNG. During the first lactation, days open were greater (P ≤ 0.05), whereas total percent pregnant was lower (P ≤ 0.05) for cows in the high- compared with mid- or low-range FNG. During the fourth lactation, services per conception tended to be greater in the high- compared with low-range FNG (P < 0.09). However, no differences were detected for any reproductive performance measure for heifers or for cows when all lactations were combined (Jimenez-Krassel et al., 2017). Taken together, these findings indicate that dairy heifers with ≥25 follicles ≥3 mm in diameter have suboptimal fertility and a shorter productive herd life compared with herdmates with fewer follicles. The reason too many follicles, like too few follicles, is also linked to suboptimal fertility and shorter herd longevity is unknown. Nevertheless, a similar association between high follicle numbers and impaired fertility is reported in women with polycystic ovarian syndrome (PCOS; Wiser et al., 2013; Walters et al., 2018). Reduced rate of follicular loss by atresia and anomalous dynamics of follicular formation and growth have been proposed as potential causes of the increase in follicle numbers in women with PCOS, but evidence is still limited (Webber et al., 2003). It is plausible that cattle with a very high AFC have an intrinsic ovarian abnormality, similar to women with PCOS, that impairs their fertility (Jimenez-Krassel et al., 2017). This speculation is indirectly supported by the finding that cattle with an AFC ≥ 25 were ~20% of animals of the herd in different studies (Ireland et al., 2007; Mossa et al., 2012), suggesting that farmers may be removing from the herd cattle with high follicle numbers because of their subfertility.
DIFFERENCES IN FERTILITY AND PRODUCTIVITY BETWEEN COWS WITH LOW VS. INTERMEDIATE AFC
The hypothesis that low AFC could be associated with reduced fertility was tested in dairy cows (Holstein-Friesian, n = 306; Mossa et al., 2012). All follicles ≥3 mm were counted for 2 consecutive days during the first wave of the estrous cycle. Based on AFC, cows were classified into 3 groups: low (≤15 follicles), intermediate (16 to 24 follicles), and high (≥25 follicles). Pregnancy rates at first service were 35.2% in the low, 47.1% in the intermediate, and 34.5% in the high group and the odds ratio (OR) of a successful pregnancy at first service was lower (P < 0.05) in animals with low vs. intermediate AFC, whereas no difference existed between animals in the high vs. the low group. Pregnancy rates at the end of the breeding season were 83.2%, 86.7%, and 91.2% in the low, intermediate, and high groups, respectively. Cows with a high AFC had 3.34 times greater odds of being pregnant at the end of the breeding season compared with animals with low AFC (P < 0.05), whereas no difference was detected between the intermediate and high or between the intermediate and low groups. Cows with a low AFC received more services during the overall breeding season compared with animals with an intermediate AFC (2.7 vs. 2.3; P < 0.05), but no difference was detected in the number of inseminations received by cows in the high vs. low group (Mossa et al., 2012). Further, cows with a low AFC also had fewer lactations (1.9 vs. 2.6) compared with their herdmates with the highest AFC. Thus, we concluded that dairy cows with a low number of ovarian follicles (≤15) have lower reproductive performance compared with cows with higher numbers of follicles growing during follicular waves. Taken together, these findings (Mossa et al., 2012; Jimenez-Krassel et al., 2017) imply that the relationship between AFC, and consequently the size of the ovarian reserve, and fertility and longevity may not be linear in cattle.
AMH REPEATABILITY AND ASSOCIATION WITH AFC
Growing evidence indicates that AMH concentrations vary minimally during estrous cycles in cattle. A single AMH measurement in young adult beef heifers was highly correlated (r = 0.97) with the average for multiple AMH measurements during different days of the same or multiple estrous cycles (Ireland et al., 2011). In dairy cows AMH concentrations were static during the same estrous cycle (Rico et al., 2009; Souza et al., 2015), on different days of 2 estrous cycles (Rico et al., 2009), and within the same individual during natural and synchronized estrous cycles (Pfeiffer et al., 2014). These findings imply that AMH concentrations can be reliably determined with a single blood sample on a random day of the cycle in adult cattle.
A high positive correlation (r > 0.90) was assessed between the variation in AFC, AMH, and histological determination of total number of morphologically healthy (primordial, transitory, primary, secondary, and antral) follicles and oocytes in ovaries of young adult cattle (Ireland et al., 2008). Also, the overall average AMH concentration during ovulatory follicular waves per animal was highly correlated (r = 0.92) with average peak AFC during the 2 or 3 waves of an estrous cycle (Ireland et al., 2008). A positive association was also detected between the number of follicles and AMH in dairy and zebu cattle (Rico et al., 2009; Baldrighi et al., 2014), proving the reliability of both AFC and AMH as biomarkers predictive of the size of the ovarian reserve in age-matched cattle.
LACK OF CORRELATION BETWEEN AMH AND FERTILITY IN HEIFERS
The AMH concentrations were determined in young adult Holstein heifers (n = 281, 11 to 15 mo of age). Animals were partitioned into quartiles based on their AMH concentrations (mean pg/mL; Q1 = 19; Q2 = 41.8; Q3 = 68.9; and Q4 = 153.2) and several measures of reproductive performance before and after calving in the same individuals were analyzed until the start of their third lactation (Jimenez-Krassel et al., 2015a). Conception rates after the first AI (as heifers) averaged 44.5% (n = 240 animals), but did not differ among quartiles. Also, number of times subjected to AI per conception and total percentage pregnant as heifers were similar among quartiles (Jimenez-Krassel et al., 2015a). The lack of difference in these reproductive parameters among heifers with different AMH concentrations was unexpected, because heifers with reduced ovarian reserve (assessed with AFC) have diminished ovarian function (Jimenez-Krassel et al., 2009; Mossa et al., 2010b), endometrial development (Jimenez-Krassel et al., 2009), oocyte quality (Ireland et al., 2009) compared with heifers with higher AFC. It is plausible that the reduction in ovarian function in Q1 heifers was not marked enough to decrease the various reproductive parameters we analyzed (conception rates, number of services per conception, total pregnancy rates) compared with heifers in Q2, Q3, and Q4. Another potential explanation is that unidentified factors may have negatively impacted on reproductive parameters in this study, since conception rates at first AI were rather low in the herd. Alternatively, size of the ovarian reserve and the associated alterations in ovarian function may not impact fertility in young adult heifers.
REDUCED FERTILITY AND LONGEVITY IN COWS WITH LOW AMH AS HEIFERS
However, in the same cohort of cattle discussed above (Jimenez-Krassel et al., 2015a), total percent of cows pregnant for all lactations combined was lower (P < 0.01) for the quartile of cows with the lowest AMH concentrations (Q1) as heifers compared with Q2 or Q3, but was not different from Q4. Also, culling rates for poor reproductive performance were highest for Q1 cows during the first lactation (Jimenez-Krassel et al., 2015a). Nevertheless, other reproductive parameters (conception rates, number of times subjected to AI per conception, days open, and calving intervals) were similar among quartiles during each lactation and overall (Jimenez-Krassel et al., 2015a). Others report that dairy cows with high AMH had greater pregnancy rates and lower incidence of pregnancy loss between day 30 and 65 of gestation (Ribeiro et al., 2014). Thus, combined results support the hypothesis that heifers with reduced AMH have suboptimal fertility after the birth of their calf, but the potential use of AMH in heifers to predict fertility throughout their productive life warrants further research.
We also tested the hypothesis that AMH in heifers was positively linked to productive herd life (time in herd after calving) (Jimenez-Krassel et al., 2015a). Results showed that Q1 cows with the lowest AMH concentrations as heifers completed fewer lactations compared to Q3 cows and had a 180-d average shorter productive herd life compared with Q2 and Q3 cows (Jimenez-Krassel et al., 2015a). By the end of the study 24%, 37%, 43%, and 32% of the cows in Q1, Q2, Q3, and Q4, respectively, remained in the herd, but the probability of being culled after birth of the first calf was higher for the Q1 compared with Q2, Q3, and Q4 cows. Results indicate that a single determination of AMH concentrations in young adult Holstein heifers is predictive of their future herd longevity.
HERITABILITY AND DEVELOPMENT PROGRAMMING OF AMH CONCENTRATIONS
The majority of female reproductive traits in dairy and beef cattle tend to be lowly heritable (Berry et al., 2014). Nevertheless, the identification of a biomarker highly correlated with fertility and moderate to highly heritable may significantly contribute to the genetic improvement of reproductive performance in dairy cattle. To estimate the AMH heritability, circulating AMH concentrations were measured in 2,905 dairy Holstein heifers (11 to 15 mo of age) that were genotyped for SNP markers and their 4-generation pedigree information were collected (Nawaz et al., 2018). The genomic heritability (proportion of variance of a trait that can be explained in the population by a linear regression on a set of markers; de Los Campos et al., 2015) of AMH was 0.36 ± 0.03 and a similar estimation was reported in a study from another research group on 198 Canadian Holstein cows (0.46 ± 0.31; Gobikrushanth et al., 2018; Table 3). Pedigree-based heritability of AMH was also estimated (heritability estimate 0.43 ± 0.07; Nawaz et al., 2018) and such estimates, both genomic and pedigree based, are the highest for any trait associated with reproduction in female cattle (Berry et al., 2014). We previously investigated the heritability of AFC in dairy cows (0.31 ± 0.14) and heifers (0.25 ± 0.13) and concluded that AFC is a moderately heritable genetic trait (Walsh et al., 2014; Table 3). In addition, using genome-wide association analysis, a relevant overlap was detected between the genes that influence AMH concentrations and those that affect superovulatory traits in cattle (Nawaz et al., 2018). For example AMH was associated with the prostaglandin-endoperoxide synthase 1 (PTSGS1; Nawaz et al., 2018), a gene that has in turn been associated with the number of collectible and viable embryos in cattle (Jaton et al., 2018). Taken together, these findings (Walsh et al., 2014; Gobikrushanth et al., 2018; Nawaz et al., 2018) indicate that genetic selection on size of the ovarian reserve (as assessed by AMH and AFC) may enhance fertility in cattle (Table 3). Nevertheless, the potential positive genetic correlation with economically relevant production traits needs to be unraveled. For example, level of milk production was not correlated with AMH (Jimenez-Krassel et al., 2015b) and AFC was negatively associated with genetic merit for milk fat concentration (Walsh et al., 2014).
Table 3.
Evidence that the size of the ovarian reserve, as assessed by antral follicle count (AFC) of anti-Müllerian hormone (AMH), is a moderately heritable trait
| Biomarker of ovarian reserve | Animals (n) | Pedigree-based heritability | SNP marker-based genomic heritability | Reference |
|---|---|---|---|---|
| AFC | Dairy heifers (122) and cows (455) | Heifers 0.31 ± 0.14Cows 0.25 ± 0.13 | Walsh et al. (2014) | |
| AMH | Dairy heifers (2,905) | 0.43 ± 0.07 | 0.36 ± 0.03 | Nawaz et al. (2018) |
| AMH | Dairy cows (198) | 0.46 ± 0.07 | Gobikrushanth et al. (2018) |
The ovarian reserve is determined during fetal life (Erickson, 1966). Thus, management of pregnant cattle may influence the environment in which the conceptus develops thereby impacting establishment of the ovarian reserve. The hypothesis that dietary nutritional restriction (to 60% of maternal requirements) during the first trimester of pregnancy (to coincide with the peak in the number of germ cells in fetal ovaries; Erickson, 1966) has permanent effects on the establishment of the ovarian reserve in offspring was tested in beef cattle (Mossa et al., 2013). The ovarian reserve was diminished in heifers born to mothers exposed to nutrient restriction, as assessed by consistently lower circulating AMH concentrations from 4 mo to 1.8 yr of age (Fig. 5), lower AFC from 7 wk to 1.6 yr of age, and increased FSH concentrations (Mossa et al., 2013). In addition, offspring of nutrient-restricted mothers had higher peripheral blood pressure compared to heifers in the control group. These findings imply that imbalanced nutrition can negatively program the development of the ovarian reserve in cattle and can have long-term consequence on their health.
Figure 5.
Circulating AMH concentrations in offspring of cows in the nutrient-restricted (NR, n = 10, white bars) and control (C, n = 13, black bars) groups. NR mothers were individually fed at 0.6 of their maintenance (M) energy requirements, while C mothers were fed 1.2 M from day 11 to day 110 of gestation. Probabilities for the main effects of the repeated-measures ANOVA are given. NS, not significant (Mossa et al., 2013).
In dairy cows, lactation and gestation are concomitant; thus, we tested the hypothesis that cows with a chronic mammary disease produced female calves with an impaired ovarian reserve. Cows with a high number of somatic cell count (SCC) in milk, an index of chronic mammary gland infection (Caraviello et al., 2005), produced daughters with reduced AMH concentrations as young adults (Ireland et al., 2011). These findings suggest that persistent mammary infection in the dam during gestation may impair the establishment of the ovarian reserve of her female offspring and, potentially, their fertility.
CONCLUSIONS
The size of the ovarian reserve is positively associated with ovarian function and with several measures of fertility in Bos taurus cattle (progesterone secretion, endometrial thickness, response to superovulation) (Ireland et al., 2007; Jimenez-Krassel et al., 2009). Anti-Müllerian hormone peripheral concentrations are indicative of the size of the ovarian reserve and a promising biomarker of fertility that could be used to improve breeding schemes for reproductive performance. Nevertheless, the relationship between ovarian reserve, fertility, and longevity needs to be further confirmed in large herds, many breeds, and in cattle farmed under different conditions. On the other hand, the potential impact of managerial factors (i.e., nutrition, disease prevention) of pregnant cattle on the establishment of the ovarian reserve of female calves warrants further research.
ACKNOWLEDGMENTS
Based on presentation given at the Physiology and Endocrinology Symposium: Regulation of the Growing Follicle Pool-Basic and Applied Aspects title “Anto mullerian hormone (AMH): a biomarker for the ovarian reserve, ovarian function and fertility in dairy cows” at the 2018 Annual Meeting of the American Society of Animal Science held in Vancouver, BC, Canada, July 8 to 12, with publication sponsored by the Journal of Animal Science and the American Society of Animal Science. Research support: National Research Initiative Competitive grant no. 2004-35203-14781 and 2007-35203-18178; Animal and Food Research Institute Competitive grant no. 2013-67015-20962 from the USDA National Institute of Food and Agriculture; MSU’s AgBioResearch; and Green Meadow Farms, Inc.
LITERATURE CITED
- Adams G. P., Matteri R. L., Kastelic J. P., Ko J. C., and Ginther O. J.. 1992. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. J. Reprod. Fertil. 94:177–188. doi:10.1530/jrf.0.0940177. [DOI] [PubMed] [Google Scholar]
- Baldrighi J. M., Sá Filho M. F., Batista E. O., Lopes R. N., Visintin J. A., Baruselli P. S., and Assumpção M. E.. 2014. Anti-Mullerian hormone concentration and antral ovarian follicle population in Murrah heifers compared to Holstein and Gyr kept under the same management. Reprod. Domest. Anim. 49:1015–1020. doi:10.1111/rda.12430 [DOI] [PubMed] [Google Scholar]
- Basir G. S., O W. S., So W. W., Ng E. H., and Ho P. C.. 2002. Evaluation of cycle-to-cycle variation of endometrial responsiveness using transvaginal sonography in women undergoing assisted reproduction. Ultrasound Obstet. Gynecol. 19:484–489. doi:10.1046/j.1469-0705.2002.00685.x [DOI] [PubMed] [Google Scholar]
- Berry D. P., Wall E., and Pryce J. E.. 2014. Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 8(Suppl. 1):105–121. doi:10.1017/S1751731114000743 [DOI] [PubMed] [Google Scholar]
- Bleach E. C., Glencross R. G., Feist S. A., Groome N. P., and Knight P. G.. 2001. Plasma inhibin A in heifers: relationship with follicle dynamics, gonadotropins, and steroids during the estrous cycle and after treatment with bovine follicular fluid. Biol. Reprod. 64:743–752. doi:10.1095/biolreprod64.3.743. [DOI] [PubMed] [Google Scholar]
- Burns D. S., Jimenez-Krassel F., Ireland J. L., Knight P. G., and Ireland J. J.. 2005. Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol. Reprod. 73:54–62. doi:10.1095/biolreprod.104.036277 [DOI] [PubMed] [Google Scholar]
- Caraviello D. Z., Weigel K. A., Shook G. E., and Ruegg P. L.. 2005. Assessment of the impact of somatic cell count on functional longevity in Holstein and Jersey cattle using survival analysis methodology. J. Dairy Sci. 88:804–811. doi:10.3168/jds.S0022-0302(05)72745-4 [DOI] [PubMed] [Google Scholar]
- de Los Campos G., Sorensen D., and Gianola D.. 2015. Genomic heritability: what is it? PLoS Genet. 11:e1005048. doi:10.1371/journal.pgen.1005048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin M. G., and Morris D. G.. 2008. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 43(Suppl. 2):260–267. doi:10.1111/j.1439-0531.2008.01171.x [DOI] [PubMed] [Google Scholar]
- Erickson B. H. 1966. Development and senescence of the postnatal bovine ovary. J. Anim. Sci. 25:800–805. doi:10.2527/jas1966.253800x. [DOI] [PubMed] [Google Scholar]
- Gobikrushanth M., Purfield D. C., Colazo M. G., Butler S. T., Wang Z., and Ambrose D. J.. 2018. The relationship between serum anti-Müllerian hormone concentrations and fertility, and genome-wide associations for anti-Müllerian hormone in Holstein cows. J. Dairy Sci. 101:7563–7574. doi:10.3168/jds.2017-13940 [DOI] [PubMed] [Google Scholar]
- Haughian J. M., Ginther O. J., Kot K., and Wiltbank M. C.. 2004. Relationships between FSH patterns and follicular dynamics and the temporal associations among hormones in natural and GnRH-induced gonadotropin surges in heifers. Reproduction 127:23–33. doi:10.1530/rep.1.00030 [DOI] [PubMed] [Google Scholar]
- Hirayama H., Kageyama S., Naito A., Fukuda S., Fujii T., and Minamihashi A.. 2012. Prediction of superovulatory response in Japanese Black cattle using ultrasound, plasma anti-Müllerian hormone concentrations and polymorphism in the ionotropic glutamate receptor AMPA1/GRIA1. J. Reprod. Dev. 58:380–383. doi:10.1262/jrd.11-129S. [DOI] [PubMed] [Google Scholar]
- Ireland J. L., Scheetz D., Jimenez-Krassel F., Themmen A. P., Ward F., Lonergan P., Smith G. W., Perez G. I., Evans A. C., and Ireland J. J.. 2008. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol. Reprod. 79:1219–1225. doi:10.1095/biolreprod.108.071670 [DOI] [PubMed] [Google Scholar]
- Ireland J. J., Smith G. W., Scheetz D., Jimenez-Krassel F., Folger J. K., Ireland J. L., Mossa F., Lonergan P., and Evans A. C.. 2011. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod. Fertil. Dev. 23:1–14. doi:10.1071/RD10226 [DOI] [PubMed] [Google Scholar]
- Ireland J. J., Ward F., Jimenez-Krassel F., Ireland J. L., Smith G. W., Lonergan P., and Evans A. C.. 2007. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum. Reprod. 22:1687–1695. doi:10.1093/humrep/dem071 [DOI] [PubMed] [Google Scholar]
- Ireland J. J., Zielak-Steciwko A. E., Jimenez-Krassel F., Folger J., Bettegowda A., Scheetz D., Walsh S., Mossa F., Knight P. G., Smith G. W., et al. 2009. Variation in the ovarian reserve is linked to alterations in intrafollicular estradiol production and ovarian biomarkers of follicular differentiation and oocyte quality in cattle. Biol. Reprod. 80:954–964. doi:10.1095/biolreprod.108.073791 [DOI] [PubMed] [Google Scholar]
- Jaton C., Schenkel F. S., Sargolzaei M., Cánova A., Malchiodi F., Price C. A., Baes C., and Miglior F.. 2018. Genome-wide association study and in silico functional analysis of the number of embryos produced by Holstein donors. J. Dairy Sci. 101:7248–7257. doi:10.3168/jds.2017-13848 [DOI] [PubMed] [Google Scholar]
- Jimenez-Krassel F., Folger J. K., Ireland J. L., Smith G. W., Hou X., Davis J. S., Lonergan P., Evans A. C., and Ireland J. J.. 2009. Evidence that high variation in ovarian reserves of healthy young adults has a negative impact on the corpus luteum and endometrium during estrous cycles in cattle. Biol. Reprod. 80:1272–1281. doi:10.1095/biolreprod.108.075093 [DOI] [PubMed] [Google Scholar]
- Jimenez-Krassel F., Scheetz D. M., Neuder L. M., Ireland J. L., Pursley J. R., Smith G. W., Tempelman R. J., Ferris T., Roudebush W. E., Mossa F., et al. 2015a. Concentration of anti-Müllerian hormone in dairy heifers is positively associated with productive herd life. J. Dairy Sci. 98:3036–3045. doi:10.3168/jds.2014-8130 [DOI] [PubMed] [Google Scholar]
- Jimenez-Krassel F., Scheetz D. M., Neuder L. M., Ireland J. L., Pursley J. R., Smith G. W., Tempelman R. J., Ferris T., Roudebush W. E., Mossa F., et al. 2015b. Concentration of anti-Müllerian hormone in dairy heifers is positively associated with productive herd life. J. Dairy Sci. 98:3036–3045. doi:10.3168/jds.2014-8130 [DOI] [PubMed] [Google Scholar]
- Jimenez-Krassel F., Scheetz D. M., Neuder L. M., Pursley J. R., and Ireland J. J.. 2017. A single ultrasound determination of ≥25 follicles ≥3 mm in diameter in dairy heifers is predictive of a reduced productive herd life. J. Dairy Sci. 100:5019–5027. doi:10.3168/jds.2016-12277 [DOI] [PubMed] [Google Scholar]
- Mossa F., Carter F., Walsh S. W., Kenny D. A., Smith G. W., Ireland J. L., Hildebrandt T. B., Lonergan P., Ireland J. J., and Evans A. C.. 2013. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 88:92. doi:10.1095/biolreprod.112.107235 [DOI] [PubMed] [Google Scholar]
- Mossa F., Jimenez-Krassel F., Folger J. K., Ireland J. L., Smith G. W., Lonergan P., Evans A. C., and Ireland J. J.. 2010a. Evidence that high variation in antral follicle count during follicular waves is linked to alterations in ovarian androgen production in cattle. Reproduction 140:713–720. doi:10.1530/REP-10-0214 [DOI] [PubMed] [Google Scholar]
- Mossa F., Jimenez-Krassel F., Scheetz D., Weber-Nielsen M., Evans A. C. O., and Ireland J. J.. 2017. Anti-Müllerian hormone (AMH) and fertility management in agricultural species. Reproduction 154:R1–R11. doi:10.1530/REP-17-0104 [DOI] [PubMed] [Google Scholar]
- Mossa F., Jimenez-Krassel F., Walsh S., Berry D. P., Butler S. T., Folger J., Smith G. W., Ireland J. L., Lonergan P., Ireland J. J., et al. 2010b. Inherent capacity of the pituitary gland to produce gonadotropins is not influenced by the number of ovarian follicles > or = 3 mm in diameter in cattle. Reprod. Fertil. Dev. 22:550–557. doi:10.1071/RD09100 [DOI] [PubMed] [Google Scholar]
- Mossa F., Walsh S. W., Butler S. T., Berry D. P., Carter F., Lonergan P., Smith G. W., Ireland J. J., and Evans A. C.. 2012. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J. Dairy Sci. 95:2355–2361. doi:10.3168/jds.2011-4325 [DOI] [PubMed] [Google Scholar]
- Nabenishi H., Kitahara G., Takagi S., Yamazaki A., and Osawa T.. 2017. Relationship between plasma anti-Müllerian hormone concentrations during the rearing period and subsequent embryo productivity in Japanese Black cattle. Domest. Anim. Endocrinol. 60:19–24. doi:10.1016/j.domaniend.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Nawaz M. Y., Jimenez-Krassel F., Steibel J. P., Lu Y., Baktula A., Vukasinovic N., Neuder L., Ireland J. L. H., Ireland J. J., and Tempelman R. J.. 2018. Genomic heritability and genome-wide association analysis of anti-Müllerian hormone in Holstein dairy heifers. J. Dairy Sci. 101:8063–8075. doi:10.3168/jds.2018-14798 [DOI] [PubMed] [Google Scholar]
- Pfeiffer K. E., Jury L. J., and Larson J. E.. 2014. Determination of anti-Müllerian hormone at estrus during a synchronized and a natural bovine estrous cycle. Domest. Anim. Endocrinol. 46:58–64. doi:10.1016/j.domaniend.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Ribeiro E. S., Bisinotto R. S., Lima F. S., Greco L. F., Morrison A., Kumar A., Thatcher W. W., and Santos J. E.. 2014. Plasma anti-Müllerian hormone in adult dairy cows and associations with fertility. J. Dairy Sci. 97:6888–6900. doi:10.3168/jds.2014-7908 [DOI] [PubMed] [Google Scholar]
- Rico C., Drouilhet L., Salvetti P., Dalbiès-Tran R., Jarrier P., Touzé J. L., Pillet E., Ponsart C., Fabre S., and Monniaux D.. 2012. Determination of anti-Müllerian hormone concentrations in blood as a tool to select Holstein donor cows for embryo production: from the laboratory to the farm. Reprod. Fertil. Dev. 24:932–944. doi:10.1071/RD11290 [DOI] [PubMed] [Google Scholar]
- Rico C., Fabre S., Médigue C., di Clemente N., Clément F., Bontoux M., Touzé J. L., Dupont M., Briant E., Rémy B., et al. 2009. Anti-Mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 80:50–59. doi:10.1095/biolreprod.108.072157 [DOI] [PubMed] [Google Scholar]
- Scheetz D., Folger J. K., Smith G. W., and Ireland J. J.. 2012. Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod. Fertil. Dev. 24:327–336. doi:10.1071/RD11020 [DOI] [PubMed] [Google Scholar]
- Silva-Santos K. C., Santos G. M., Koetz Júnior C., Morotti F., Siloto L. S., Marcantonio T. N., Urbano M. R., Oliveira R. L., Lima D. C., and Seneda M. M.. 2014. Antral follicle populations and embryo production--in vitro and in vivo--of Bos indicus-taurus donors from weaning to yearling ages. Reprod. Domest. Anim. 49:228–232. doi:10.1111/rda.12255 [DOI] [PubMed] [Google Scholar]
- Singh J., Domínguez M., Jaiswal R., and Adams G. P.. 2004. A simple ultrasound test to predict the superstimulatory response in cattle. Theriogenology 62:227–243. doi:10.1016/j.theriogenology.2003.09.020 [DOI] [PubMed] [Google Scholar]
- Souza A. H., Carvalho P. D., Rozner A. E., Vieira L. M., Hackbart K. S., Bender R. W., Dresch A. R., Verstegen J. P., Shaver R. D., and Wiltbank M. C.. 2015. Relationship between circulating anti-Müllerian hormone (AMH) and superovulatory response of high-producing dairy cows. J. Dairy Sci. 98:169–178. doi:10.3168/jds.2014-8182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S. W., Mossa F., Butler S. T., Berry D. P., Scheetz D., Jimenez-Krassel F., Tempelman R. J., Carter F., Lonergan P., Evans A. C., et al. 2014. Heritability and impact of environmental effects during pregnancy on antral follicle count in cattle. J. Dairy Sci. 97:4503–4511. doi:10.3168/jds.2013-7758 [DOI] [PubMed] [Google Scholar]
- Walters K. A., Bertoldo M. J., and Handelsman D. J.. 2018. Evidence from animal models on the pathogenesis of PCOS. Best Pract. Res. Clin. Endocrinol. Metab. 32:271–281. doi:10.1016/j.beem.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Webber L. J., Stubbs S., Stark J., Trew G. H., Margara R., Hardy K., and Franks S.. 2003. Formation and early development of follicles in the polycystic ovary. Lancet 362:1017–1021. doi:10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- Wiser A., Shalom-Paz E., Hyman J. H., Sokal-Arnon T., Bantan N., Holzer H., and Tulandi T.. 2013. Age-related normogram for antral follicle count in women with polycystic ovary syndrome. Reprod. Biomed. Online 27:414–418. doi:10.1016/j.rbmo.2013.06.016 [DOI] [PubMed] [Google Scholar]