Abstract
Two experiments were designed to explore the effects of coated zinc (Zn) oxide nanoparticles (NZO) on the diarrhea ratio, antioxidant capacity, intestinal morphology, and zinc excretion in growing pigs. In Exp.1, 270 growing pigs (21.88 ± 0.8 kg initial BW) were allocated to three treatments, each for 30 d: (i) control group (CG), basal diet containing Zn-free premix + 100 mg Zn/kg from ZnSO4; (ii) high Zn (HZN), basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; (iii) coated nano ZnO (CNZO), basal diet containing Zn-free premix + 100 mg Zn/kg from coated NZO. In Exp.2, 21 crossbred growing pigs (17.04 ± 0.01 kg initial BW) were allocated to three treatments, each for 28 d: (i) HZN, basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; (ii) low concentration of nano ZnO (LNZO), basal diet containing Zn-free premix + 100 mg Zn/kg from 5% coated NZO material; (iii) high concentration of nano ZnO (HNZO), basal diet containing Zn-free premix + 100 mg Zn/kg from 10% coated NZO material. In Exp. 1, compared with the CG diet, CNZO significantly reduced the diarrhea rate (P < 0.05) and increased the activities of glutathione peroxidase and superoxide dismutase (P < 0.05). Compared with HZN, CNZO decreased the activities of serum alanine aminotransferase, and alkaline phosphatase, as well as the fecal zinc concentration (P < 0.05). In Exp. 2, pigs fed LNZO or HNZO had an increased final BW, average daily weigh and diarrhea rate, and a decreased level of Zn in the plasma, liver, and feces on day 14 compared with the HZN group (P < 0.05). The villous height and villous height/crypt depth ratio of duodenum were higher (P < 0.05) in the HZN group than the HNZO group, whereas the higher villous height of jejunum was observed in the LNZO group compared with that in the HNZO group (P < 0.05). We found that CNZO (100 mg/kg Zn) could improve the antioxidant capacity and reduce fecal Zn emission. However, the diarrhea rate was not effectively suppressed when compared with the HNZO supplementation. Furthermore, coated NZO material of 5% concentration is more effective in improving the morphology of intestinal villus.
Keywords: growing pigs, intestinal morphology, zinc excretion, zinc oxide
INTRODUCTION
The diarrhea in growing pigs has been increased in incidence of outbreaks worldwide. Diarrhea results in a reduction of the normal growth rate by 27% to 50% in pigs (Campbell et al., 2013). An effective inhibitor for the occurrence of diarrhea is in high demand from the global swine industry.
Dietary zinc oxide (ZnO) at therapeutic concentrations from 2,000 to 4,000 mg/kg could effectively prevent and treat postweaning diarrhea (Shelton et al., 2011). A large dose of ZnO could increase pigs’ growth performance and reduce the colonization and population of microbes in the gastrointestinal tract (Fairbrother et al., 2005; Cho et al., 2015). However, only 10% to 25% of dietary ZnO can be absorbed by pigs, which may lead to the excretion of mass heavy metals into the environment, resulting in environmental pollution. The maximum dose of Zn/kg in a pig’s diet has been restricted to 150 mg in Europe (European Communities, 2003), which is far below ZnO therapeutic levels. The Ministry of Agriculture in China limited the supplementation of ZnO at 110 mg/kg for the diet of growing pigs. Thus, restricting the supplemented ZnO in piglet diets and strengthening the biological effect of ZnO have both attracted extensive attention from researchers.
Improvements in the production process, such as envelope and nanotechnology, may feature advantages for the efficiency of nutrients and additives (Bauer et al., 2004). Nanoparticles decrease the particle size (1 to 100 nm) of nutrients, which can then more readily cross gut barriers and have greater absorption and permeability rates (Florence et al., 1995; Buzea et al., 2017; Cho et al., 2013). Compared with ZnO, nano ZnO (NZO) has a higher bioavailability, significantly promotes animal growth and survival, and enhances immunity (Croteau et al., 2011; Sirelkhatim et al., 2015). Coating technology would be able to protect effective components from being degraded by stomach juices and released slowly into the intestine. However, scarce information is known about the effects of coated NZO on the growth performance of pigs. The present study was performed to validate the hypothesis that coated NZO, a new coating product, at a low supplementation level can substitute the high dose zinc (Zn) used for reducing the diarrhea rate and fecal Zn excretion and increasing the antioxidant capacity of growing pigs.
Different concentrations of coated NZO materials may affect the biological efficiency of supplementation. We conjectured that a higher concentration of effective coated NZO might cause an increase in the adsorptive behavior of surface ions, which directly results in negative effects on intestinal morphology and growth performance of pigs. Thus, this study also investigated the effects of different concentrations of coated NZO materials on the diarrhea rate and intestinal morphology of growing pigs.
MATERIALS AND METHODS
The Chinese Academy of Science Institutional Animal Care and Use Committee reviewed and approved the design and procedures in this experiment (Kong et al., 2007). Pigs in both experiments were housed in a finishing building equipped with a controlled environment. Dry feed and clean water were freely accessible.
Experiment 1
A total of 270 crossbred growing pigs (Duroc × Landrace × Yorkshire, castrated males), initially 21.88 ± 0.8 kg BW, were blocked by weight and randomly assigned into three dietary treatments. Each treatment contained 90 piglets arranged in six replicates of 15 piglets. Dietary treatments were: (i) control group (CG), basal diet containing Zn-free premix + 100 mg Zn/kg from ZnSO4; (ii) HZN, basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; (iii) CNZO, basal diet containing Zn-free premix + 100 mg Zn/kg from coated NZO. All diets were formulated to meet or exceed the NRC (2012) requirements for growing pigs (Table 1). Coated NZO, containing 5% zinc oxide, was supplied by Hangzhou King Techina Technology Co., Ltd (Hangzhou, China). The experiment lasted for 30 d.
Table 1.
Diet composition for Exp. 1
| Diets1 | |||
|---|---|---|---|
| Item | CG | HZN | CNZO |
| Ingredient, kg/metric ton | |||
| Corn | 650.0 | 650.0 | 650.0 |
| Soybean meal | 250.0 | 250.0 | 250.0 |
| Wheat bran | 60.0 | 60.0 | 60.0 |
| bran powder | 7.83 | 5.03 | 7.995 |
| Vitamin premix2 | 0.40 | 0.40 | 0.40 |
| Mineral premix, Zn free2 | 1.24 | 1.24 | 1.24 |
| Salt | 3.60 | 3.60 | 3.60 |
| Monocalcium phosphate | 12.0 | 12.0 | 12.0 |
| Phytase | 0.40 | 0.40 | 0.40 |
| Lysine | 4.00 | 4.00 | 4.00 |
| Limestone | 9.60 | 9.60 | 9.60 |
| Choline chloride | 0.60 | 0.60 | 0.60 |
| Antioxidants | 0.04 | 0.04 | 0.04 |
| ZnSo4 H2O | 0.29 | 0.29 | |
| ZnO | 2.96 | ||
| NZO | 0.125 | ||
| Total | 1,000 | 1,000 | 1,000 |
| Calculated analysis | |||
| DE, kcal/kg | 3,161.45 | 3,152.51 | 3,154.80 |
| CP, % | 17.36 | 17.33 | 17.34 |
| Ca, % | 0.71 | 0.71 | 0.71 |
| Total P, % | 0.59 | 0.59 | 0.59 |
| Available P, % | 0.33 | 0.33 | 0.33 |
| Lys, % | 1.14 | 1.14 | 1.14 |
| Met, % | 0.28 | 0.28 | 0.28 |
| Met + Cys, % | 0.60 | 0.60 | 0.60 |
| Zn, mg/kg | 220 | 2470 | 220 |
1CG = control group, basal diet containing Zn-free premix + 100 mg Zn/kg from ZnSO4; HZN = basal diet containing Zn-free premix + 2250 mg Zn/kg from ZnO; CNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from coated nano zinc oxide.
2Provided per kilogram of diet: vitamin A, 1 750 IU; vitamin D3, 200 IU; vitamin E, 11 IU; vitamin K, 0.5 mg; vitamin B1 1.00 mg; vitamin B2, 3.00 mg; vitamin B6, 3.00 mg; biotin, 0.05 mg; folic acid, 0.30 mg; niacin acid, 30.00 mg; pantothenic acid, 300 mg; Cu (CuSO4 ·5H2O), 5.00 mg; Fe (FeSO4 ·7H2O), 100.00 mg; Mn (MnSO4 ·H2O), 3.00 mg; Se, 0.30 mg; I, 0.14 mg; Co, 0.12 mg.
BW and feed intake were weighed at 1 and 30 d to determine the ADG, ADFI, and F/G ratio. During the experimental process, the fecal consistency was observed and recorded twice daily beginning on the first day. Meanwhile, the anal swelling and nature of the excrement were checked at least once a day for determining the diarrhea score of each pig. The fecal score was recorded, as described by Marquardt et al. (2013), with a score from 0 to 3 (0: normal feces, 1: moist feces, 2: mild diarrhea, and 3: severe diarrhea). The daily diarrhea rate was calculated by counting pigs with a fecal score of 2 or higher. The diarrhea rate was calculated as the number of piglets with diarrhea/(total numbers of piglets × days) × 100. The collection of fecal samples from each pen was initiated on day 28 and ended on day 30, and stored at −20 °C for zinc-level analysis using a Varian ICP-OES (Model IRIS Intrepid II, Thermal Jarrell Ash, Waltham, MA).
On day 30, a randomly selected piglet from each pen was chosen. Blood samples were drawn from a precaval vein into vacuum tubes without EDTA. Serum was collected after centrifugation at 3,500 × g for 15 min at 4 °C, and the supernatant was stored at −20°C until analysis for biochemical indices. Serum levels of total protein (TP), albumin, aspartate aminotransferase, alanine aminotransferase (ALT), alkaline phosphatase (ALP), creatine phosphokinase, and urea nitrogen were detected by an automatic biochemical analyzer (Cobas311, F. Hoffmann-La Roche Ltd, Basel, Switzerland) and commercial kits (Roche, Basel, Switzerland) according to the manufacturers’ instructions.
The level of globulin (GLB) in the serum was assayed by a specific sandwich ELISA kit (ELISA Ready-SET-GO, eBioscience, CA). The GLB index was standardized to the protein concentration in each sample.
Additionally, the serum antioxidant enzyme activities were analyzed with commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and determined using a multimode microplate reader (Infinite M200 PRO, Tecan, Switzerland) (Ren et al., 2012). The activities of the total-antioxidant capacity, glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and catalase (CAT), as well as the content of malondialdehyde (MDA) were determined according to the manufacturer’s instructions.
Experiment 2
Assessment of initial content of coated zinc oxide. The ZnO content in the NZO materials was identified by titration according to the method of Shim et al. (Hunter, 1965). Briefly, 0.2 g of coated NZO materials was transferred to 250 mL conical flasks and mixed with 5 mL of 23.4% hydrochloric acid. The mixture was heated at 80 ℃ until completely dissolved, followed by the addition of 25 mL distilled water, one drip of 0.025% methyl red ethanol solution (0.025 g methyl red in 100 mL ethanol), and 40% ammonia solution until the dissolution became microscopic yellow. Then, the mixture was added to 25 mL distilled water, 10 mL ammonia–ammonium chloride buffer (pH 10.0), and 0.1 g eriochrome black T indicator. The content of ZnO was titrated with EDTA (0.05 mol/L) until the dissolution color change from purple to pure blue. One milliliter of EDTA (1.0 mol/L) is equivalent to 81.39 mg ZnO.
The calculation formula is as follows:
where X is the content of zinc oxide, V is the volume of EDTA titrant consumed by sample, mL, C is the concentration of EDTA titrant, mol/L, 0.08139 is the quality of zinc oxide that equivalent to 1 mL EDTA standard solution [C (EDTA) = 1.0 mol/L], g, m is the weight of sample, g.
Animal Experimental Design
A total of 21 Duroc × Landrace × Yorkshire growing pigs (castrated males), initially 17.04 ± 0.01 kg BW, were blocked by weight and randomly assigned into three dietary treatments. Each treatment contained seven piglets, arranged in seven replicates. Pigs were housed in individual pens for 28 d. Dietary treatments were: (i) HZN, basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; (ii) LNZO, basal diet containing Zn-free premix + 100 mg Zn/kg from 5% coated NZO material; (iii) HNZO, basal diet containing Zn-free premix + 100 mg Zn/kg from 10% coated NZO material. All diets were formulated to meet or exceed the NRC (2012) requirements for growing pigs (Table 2). The coated NZO materials were supplied by Hangzhou King Techina Technology Co., Ltd (Hangzhou, China).
Table 2.
Diet composition for Exp. 2
| Diets1 | |||
|---|---|---|---|
| Item | HZN | LNZO | HNZO |
| Ingredient, kg/metric ton | |||
| Corn | 680.00 | 680.00 | 680.00 |
| Soybean meal | 280.00 | 280.00 | 280.00 |
| bran powder | 4.00 | 4.00 | 4.0625 |
| Phytase | 0.20 | 0.20 | 0.20 |
| Vitamin premix2 | 0.30 | 0.30 | 0.30 |
| Mineral premix, Zn free2 | 1.00 | 1.00 | 1.00 |
| Limestone | 12.00 | 12.00 | 12.00 |
| Dicalcium phosphate | 4.00 | 4.00 | 4.00 |
| Choline | 0.40 | 0.40 | 0.40 |
| Salt | 4.00 | 4.00 | 4.00 |
| Lysine | 2.00 | 2.00 | 2.00 |
| Methionine | 0.20 | 0.20 | 0.20 |
| Others | 0.98 | 0.98 | 0.98 |
| Zeolite powder | 8.67 | 10.8 | 10.8 |
| ZnO | 2.25 | — | — |
| 5% NZO | — | 0.125 | — |
| 10% NZO | — | — | 0.0625 |
| Total | 1,000 | 1,000 | 1,000 |
| Calculated analysis | |||
| DE, kcal/kg | 3,213.78 | 3,213.78 | 3,214.92 |
| CP, % | 18.02 | 18.02 | 18.66 |
| Ca, % | 0.62 | 0.62 | 0.62 |
| Total P, % | 0.43 | 0.43 | 0.43 |
| Available P, % | 0.20 | 0.20 | 0.20 |
| Lys, % | 0.99 | 0.99 | 0.99 |
| Met, % | 0.32 | 0.32 | 0.32 |
| Met + Cys, % | 0.64 | 0.64 | 0.64 |
1HZN = basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; LNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 5% coated NZO material; HNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 10% coated NZO material.
2Provided per kilogram of diet: vitamin A, 1 750 IU; vitamin D3, 200 IU; vitamin E, 11 IU; vitamin K, 0.5 mg; vitamin B1 1.00 mg; vitamin B2, 3.00 mg; vitamin B6, 3.00 mg; biotin, 0.05 mg; folic acid, 0.30 mg; niacin acid, 30.00 mg; pantothenic acid, 300 mg; Cu (CuSO4 ·5H2O), 5.00 mg; Fe (FeSO4 ·7H2O), 100.00 mg; Mn (MnSO4 ·H2O), 3.00 mg; Se, 0.30 mg; I, 0.14 mg; Co, 0.12 mg.
The pigs’ weight and feed intake were measured at 0, 14, and 28 d for calculating the ADG, ADFI, and F/G ratio. The diarrhea rate was monitored throughout the experiment. After 28 d, pigs were fasted overnight for 12 h before slaughter. Samples of plasma on 14 and 28 d, and feces on 28 d were collected as described for Exp. 1. After euthanasia, the abdominal cavity was opened quickly, and the duodenum, jejunum, and ileum were sheared into 2 cm segments, then fixed in phosphate-buffered paraformaldehyde (4%, pH 7.6) for histological measurements (Xiao et al., 2013a). The small intestinal samples were trimmed to a thickness of 5 to 6 μm. They were dehydrated in alcohol, embedded in paraffin, and cut into 4 μm sections for staining with hematoxylin and eosin as described previously (Xiao et al., 2013b). Using light microscopy (Leica DMI3000 B, China), 10 microscopic fields were randomly selected from every animal and examined at 100× magnification for measuring the villus height and crypt depth (Tan et al., 2009). Additionally, the samples of serum (14 and 28 d), liver (28 d), and feces (28 d) were collected and analyzed for zinc levels according to the previous research (Zhang et al., 2017). Serum (5 mL), liver, and feces samples (5.00 ± 0.10 g) were weighed in triplicate and subjected to acid digestion using a mixture of nitric and perchloric acids following heating (80 °C for 60 min, 120 °C for 30 min, and 180 °C for 30 min). The samples were dried at 260 °C and redissolved in 5 mL of 1% HNO3. Samples were then transferred to a 100-mL volumetric flask and diluted with 1% HNO3. Subsequently, filtered solution of samples was subjected to ICP-OES for determining zinc levels.
Statistical Analysis
Each pen of pigs was the experimental unit for Exp. 1, and individual pig was the experimental unit for Exp. 2. Data were presented as means ± SEM. Results were analyzed statistically using one-way ANOVA of SPSS 23.0 (SPSS Inc., Chicago, IL). The normality of the diarrhea rate data was tested for by Kolmogorov–Smirnov’s test. Differences among groups were examined using Duncan’s multiple-range test, which were considered significant or a trend if the P-value was P < 0.05 or 0.05 < P ≤ 0.10.
RESULTS
Experiment 1
Growth performance and diarrhea rate. The effects of dietary coated NZO on the growth performance and diarrhea rate of growing pigs are shown in Table 3. Compared with the CG group, HZN tended to increase the ADFI (0.05 < P ≤ 0.10). The diarrhea rate showed a non-normal distribution (K-S test). Both HZN and CNZO treatments significantly reduced the diarrhea rate of pigs by 52.91% (P < 0.05) and 21.27% (P < 0.05), respectively, compared with the CG group.
Table 3.
Effects of dietary coated nano ZnO on the growth performance and diarrhea rate of growing pigs (Exp. 1)
| Diets1 | ||||
|---|---|---|---|---|
| Item | CG | HZN | CNZO | P-value |
| Initial BW, kg | 21.90 ± 1.65 | 21.62 ± 0.91 | 21.52 ± 0.60 | 0.971 |
| Final BW, kg | 34.83 ± 1.44 | 37.07 ± 1.16 | 33.76 ± 1.27 | 0.245 |
| ADG, g/d | 438.55 ± 22.91 | 506.91 ± 34.19 | 431.96 ± 17.20 | 0.105 |
| ADFI, kg/d | 1.12 ± 0.06 | 1.30 ± 0.03 | 1.15 ± 0.05 | 0.066 |
| F/G ratio | 2.29 ± 0.15 | 2.44 ± 0.18 | 2.55 ± 0.24 | 0.631 |
| Diarrhea rate, % | 83.39 ± 3.37c | 39.27 ± 3.04a | 65.65 ± 4.17b | <0.01 |
Data are expressed as means ± SEM (n = 6). Means within a row with different superscripts are significantly different (P < 0.05).
1CG = control group, basal diet containing Zn-free premix + 100 mg Zn/kg from ZnSO4; HZN = basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; CNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from coated nano zinc oxide.
Serum biochemical parameters and antioxidant indexes. Pigs fed the HZN diet had a higher serum ALP (P < 0.05) compared with the CG and CNZO diets (Table 4). Compared with CNZO, HZN had a significant trend of an increased serum ALT level (0.05 < P ≤ 0.10).
Table 4.
Effects of dietary coated nano ZnO on serum biochemical parameters and antioxidant indexes in growing pigs (Exp. 1)
| Diets1 | ||||
|---|---|---|---|---|
| Item2 | CG | HZN | CNZO | P-value |
| Biochemical parameters | ||||
| TP, g/L | 62.50 ± 1.91 | 62.86 ± 1.38 | 61.02 ± 1.92 | 0.75 |
| Glucose, mmol/L | 3.21 ± 0.48 | 2.59 ± 0.64 | 2.88 ± 0.43 | 0.707 |
| ALT, U/L | 49.92 ± 5.22 | 59.52 ± 2.94 | 46.80 ± 1.43 | 0.076 |
| GLB, g/L | 35.67 ± 2.10 | 35.00 ± 1.56 | 32.33 ± 1.98 | 0.345 |
| ALP, U/L | 164.43 ± 12.07a | 284.18 ± 21.01b | 206.58 ± 30.16a | 0.008 |
| AST, U/L | 62.53 ± 12.10 | 76.72 ± 9.76 | 78.73 ± 10.77 | 0.533 |
| ALB, g/L | 26.90 ± 0.83 | 27.82 ± 0.76 | 28.53 ± 1.32 | 0.531 |
| CK, U/L | 540.84 ± 52.62 | 1288.46 ± 361.43 | 1120.98 ± 272.15 | 0.151 |
| Urea, mmol/L | 3.88 ± 0.37 | 5.20 ± 0.51 | 4.75 ± 0.59 | 0.206 |
| Antioxidant indexes | ||||
| T-AOC, U/mL | 0.02 ± 0.001 | 0.03 ± 0.004 | 0.03 ± 0.002 | 0.133 |
| CAT, U/mL | 67.45 ± 1.80 | 123.59 ± 1.11 | 79.78 ± 1.57 | 0.076 |
| SOD, U/mL | 22.71 ± 0.34a | 23.05 ± 0.30a | 25.77 ± 0.37b | 0.022 |
| GSH-Px, U/mL | 431.78 ± 9.78a | 498.00 ± 5.76c | 469.92 ± 2.46b | <0.001 |
| MDA, nmol/mL | 12.12 ± 1.42 | 11.16 ± 1.38 | 5.88 ± 0.55 | 0.546 |
Data are expressed as means ± SEM (n = 6). Means within a row with different superscripts are significantly different (P < 0.05).
1CG = control group, basal diet containing Zn-free premix + 100 mg Zn/kg from ZnSO4; HZN = basal diet containing Zn-free premix + 2250 mg Zn/kg from ZnO; CNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from coated nano zinc oxide.
2TP = total protein; ALB = albumin; AST = aspartate aminotransferase; ALT = alanine aminotransferase; ALP = alkaline phosphatase; CK = creatine phosphokinase; GLB = globulin; T-AOC = total antioxidant capacity; CAT = catalase; SOD = superoxide dismutase; GSH-Px = glutathione peroxidase; MDA = malondialdehyde.
Compared with CG, HZN showed a growing tendency for the high activity of CAT in serum (0.05 < P ≤ 0.10). The activity of GSH-Px increased (P < 0.05) in HZN and CNZO-fed pigs compared with the CG group. Pigs fed CNZO had a higher activity of serum SOD (P < 0.05) compared with the CG and HZN groups.
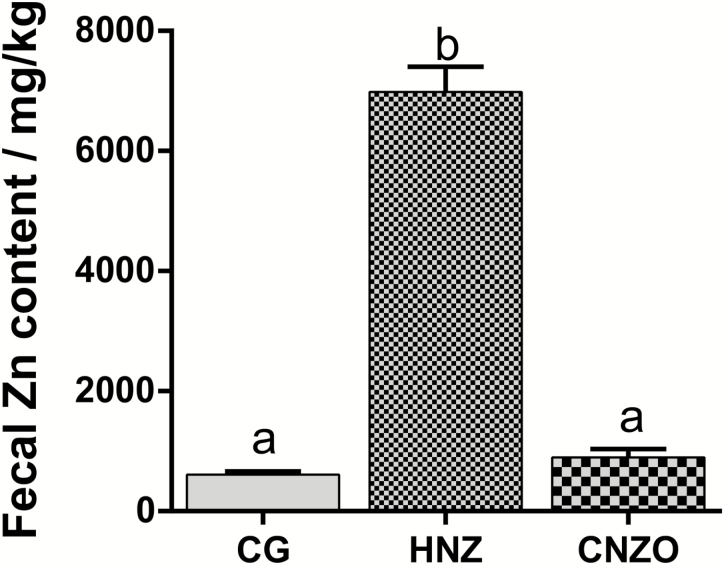
Fecal zinc level. The fecal zinc level is presented in Figure 1. Compared with the CG and CNZO groups, the HZN group showed a remarkable increase in the excretion of Zn (P < 0.05).
Figure 1.
Effect of dietary coated nano ZnO on fecal zinc levels in growing pigs. CG = control group, basal diet containing Zn-free premix + 100 mg Zn/kg from ZnSO4; HZN = basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; CNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from coated nano zinc oxide. Data are expressed as means ± SEM (n = 6). Means within a row with different superscripts are significantly different (P < 0.05).
Experiment 2
Coated zinc oxide content. Two different concentrations of coated NZO materials were analyzed in this study. Assay results showed that the contents of ZnO in 5% coated NZO and 10% coated NZO materials were 5.13 ± 0.000% and 10.09±0.001%, respectively. These materials were confirmed to be in accordance with the experimental requirements.
Growth performance. Table 5 shows the effects of dietary coated NZO materials from different ZnO concentrations on the growth performance and diarrhea rate. Pigs fed LNZO or HNZO had a higher (P < 0.05) BW on day 28 compared with those fed an HZN diet. From days 1 to 14, pigs fed LNZO or HNZO had higher (P < 0.05) ADG and diarrhea rates, whereas the F/G ratio was decreased (P < 0.05) compared with the HZN diet. From days 14 to 28, pigs fed HZN had a higher ADFI than the HNZO group (P < 0.05). Compared with the HZN diet, both LNZO and HNZO had significantly increased (P < 0.05) diarrhea rates. Overall (days 1 to 28), pigs fed LNZO and HNZO had a better ADG (P < 0.05), a higher diarrhea rate (P < 0.05), and a lower F/G ratio (P < 0.05) compared with the HZN diet.
Table 5.
Effects of different concentrations of coated nano ZnO materials on the growth performance and diarrhea rate in growing pigs (Exp. 2)
| Diets1 | ||||
|---|---|---|---|---|
| Item | HZN | LNZO | HNZO | P-value |
| BW, kg | ||||
| Day 1 | 17.05 ± 0.49 | 17.04 ± 0.62 | 17.04 ± 0.45 | 1 |
| Day 14 | 21.13 ± 0.25 | 22.53 ± 0.78 | 22.81 ± 0.50 | 0.133 |
| Day 28 | 25.23 ± 0.89a | 28.86 ± 0.97b | 28.09 ± 0.45b | 0.015 |
| Days 1 to 14 | ||||
| ADG, g | 338.46 ± 29.75a | 437.18 ± 17.40b | 429.49 ± 20.47b | 0.001 |
| ADFI, kg | 1.04 ± 0.000 | 1.04 ± 0.000 | 1.04 ± 0.000 | 1 |
| F/G ratio | 3.11 ± 0.001b | 2.38 ± 0.001a | 2.44 ± 0.001a | 0.001 |
| Diarrhea rate, % | 5.95 ± 1.94a | 29.93 ± 2.65b | 23.43 ± 1.89b | <0.01 |
| Days 14 to 28 | ||||
| ADG, g | 497.22 ± 73.12 | 581.94 ± 73.90 | 439.28 ± 38.52 | 0.132 |
| ADFI, kg | 1.24 ± 0.06b | 1.19 ± 0.02ab | 1.13 ± 0.01a | 0.037 |
| F/G ratio | 2.54 ± 0.74 | 2.13 ± 0.52 | 2.69 ± 0.22 | 0.192 |
| Diarrhea rate, % | 11.90 ± 0.74a | 53.06 ± 3.24b | 52.03 ± 2.99b | <0.01 |
| Days 1 to 28 | ||||
| ADG, g | 354.40 ± 40.77a | 472.57 ± 31.29b | 441.71 ± 14.99b | 0.032 |
| ADFI, kg | 1.08 ± 0.03 | 1.11 ± 0.01 | 1.08 ± 0.009 | 0.589 |
| F/G ratio | 3.18 ± 0.45b | 2.40 ± 0.15a | 2.45 ± 0.08a | 0.022 |
| Diarrhea rate, % | 8.89 ± 0.98a | 41.50 ± 2.56b | 37.73 ± 3.33b | <0.01 |
Data are expressed as means ± SEM (n = 7). Means within a row with different superscripts are significantly different (P < 0.05).
1HZN = basal diet containing Zn-free premix + 2250 mg Zn/kg from ZnO; LNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 5% coated NZO material; HNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 10% coated NZO material.
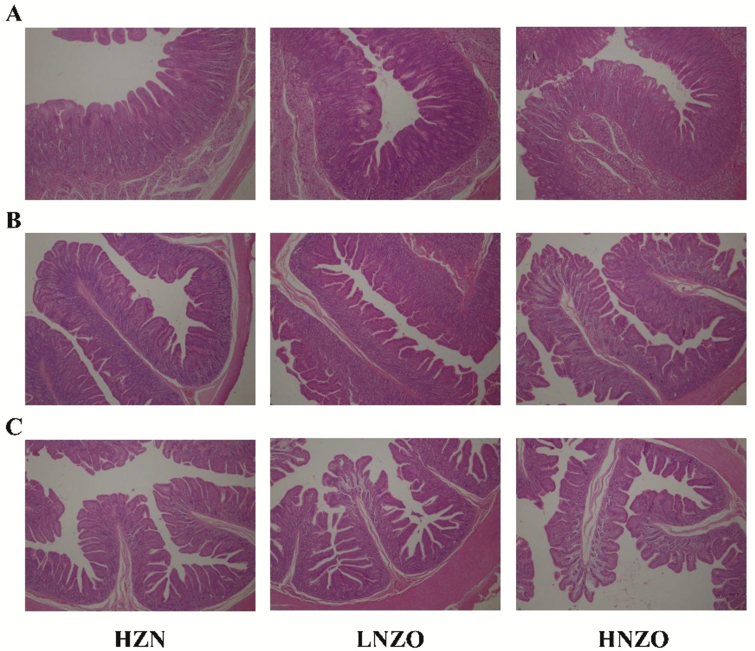
Morphology of the small intestine. The villous was arranged more closely and tightly in duodenum, jejunum, and ileum in the pigs fed with the HZN diet compared with the NZO diet, as shown in Figure 2. In the HNZO group, damage to all of the small intestine (duodenum, jejunum, and ileum) structure appeared, which reduced with digestion time. However, the villous was higher and the extent of injury was relatively lower when pigs were fed an LNZO diet as opposed to the HNZO diet.
Figure 2.
Hematoxylin and eosin stained sections of the intestine (200 nm). (A) Duodenum, (B) Jejunum, (C) Ileum. HZN = basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; LNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 5% coated NZO material; HNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 10% coated NZO material.
The measured data of villous height, crypt depth, and villous height/crypt depth of the small intestine are shown in Table 6. The HZN diet increased the villous height (P < 0.05) of the duodenum in pigs, compared with the LNZO and HNZO groups. There was a significant trend (0.05 < P ≤ 0.10) on the villous height/crypt of the duodenum and villous heights of the jejunum depth. No significant effect on the ileum among the three treatments.
Table 6.
Effects of different concentrations of coated nano ZnO materials on the morphology of the small intestine of growing pigs (Exp. 2)
| Diets1 | ||||
|---|---|---|---|---|
| Item | HZN | LNZO | HNZO | P-value |
| Duodenum | ||||
| Villous height, μm | 418.89 ± 15.90b | 397.96 ± 17.60ab | 342.53 ± 21.00a | 0.031 |
| Crypt depth, μm | 515.00 ± 29.07 | 525.38 ± 44.02 | 509.24 ± 27.37 | 0.942 |
| Villous height/Crypt depth | 0.86 ± 0.04 | 0.80 ± 0.06 | 0.69 ± 0.04 | 0.065 |
| Jejunum | ||||
| Villous height, μm | 379.75 ± 13.84 | 411.58 ± 11.28 | 363.72 ± 16.63 | 0.064 |
| Crypt depth, μm | 301.05 ± 24.46 | 288.31 ± 8.24 | 290.69 ± 19.37 | 0.865 |
| Villous height/Crypt depth | 1.34 ± 0.12 | 1.50 ± 0.05 | 1.31 ± 0.09 | 0.237 |
| Ileum | ||||
| Villous height, μm | 406.56 ± 20.72 | 433.98 ± 16.42 | 380.10 ± 26.60 | 0.252 |
| Crypt depth, μm | 224.17 ± 16.16 | 241.51 ± 17.42 | 195.71 ± 24.73 | 0.293 |
| Villous height/crypt depth | 1.96 ± 0.17 | 1.99 ± 0.20 | 1.97 ± 0.11 | 0.994 |
Data are expressed as means ± SEM (n = 7). Means within a row with different superscripts are significantly different (P < 0.05).
1 HZN = basal diet containing Zn-free premix + 2250 mg Zn/kg from ZnO; LNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 5% coated NZO material; HNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 10% coated NZO material.
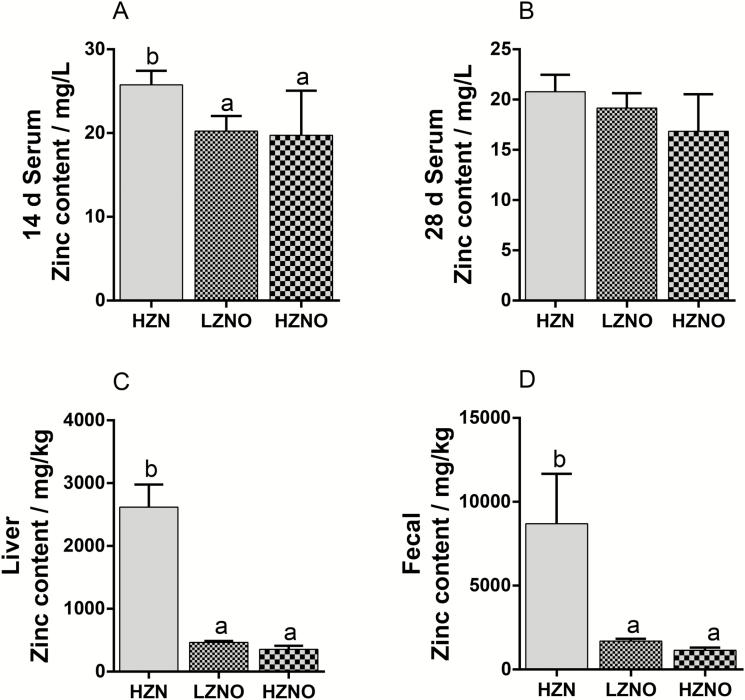
Tissues and fecal zinc levels. The content of zinc in the serum, liver, and feces of growing pigs fed dietary coated NZO materials from different ZnO concentrations is shown in Figure 3. Compared with the LNZO and HNZO treatments, dietary supplementation with HZN remarkably increased the Zn levels in the plasma on day 14 (P < 0.05), and improved Zn contents in liver and feces on day 28 (P < 0.05).
Figure 3.
Effects of dietary coated nano zinc oxide materials with different concentrations on the zinc contents in growing pigs. (A) the content of zinc in the serum on day 14, (B) the content of zinc in the serum on day 28, (C) the content of zinc in the liver, (D) the content of zinc in the feces. HZN = basal diet containing Zn-free premix + 2,250 mg Zn/kg from ZnO; LNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 5% coated NZO material; HNZO = basal diet containing Zn-free premix + 100 mg Zn/kg from 10% coated NZO material. Data are expressed as means ± SEM (n = 7). Means within a row with different superscripts are significantly different (P < 0.05).
DISCUSSION
Experiment 1
Many factors, including the different growth stages of the pigs, administration periods, housing conditions, and climatic factors, may influence the effects of ZnO supplementation (Miller et al., 2009). Recently, NZO is becoming widely applicated in feed additives because of its high stability and antibacterial activity. Milani et al. (2017) proved that dietary supplementation of 60 mg/kg of zinc oxide nanoparticles could improve the immunity and growth performance of piglets. A diet with 800 mg/kg of nano ZnO increased the ADG and decreased the diarrhea rate in weaned piglets (Wang et al., 2018). Compared with the high dose of ZnO, dietary NZO also had a good digestive physiology and strong antibacterial activity against Escherichia coli K88 (Trckova et al., 2015). These results suggest that NZO at a low supplementation level could potentially substitute the pharmacological dose of ZnO for improving the growth performance and decreasing the incidence of diarrhea. Compared with previous reports, we found that dietary supplementation of coated nano ZnO (100 mg/kg Zn) could decrease the diarrhea rate by 21%, but the effect was not as strong as in the high-ZnO diet. Furthermore, we sought to explore its possible mechanism in controlling diarrhea in the growing pig model.
Biochemical indicators of serum are an important index for indicating biological processes. We found that pigs fed the high dose of ZnO (HZN diet) had higher ALP and ALT activities than those fed a coated NZO diet. This finding was consistent with Cho et al. (2015), who reported that increasing the absorption of Zn could increase the ALP activity in serum. Zinc, as a cofactor, had direct stimulatory effects on ALP and bone mineralization (Sebahat et al., 2005; Érika Dantas de Medeiros et al., 2015). However, the ALT level, as a critical indictor of liver disease, escapes from injured hepatic cells into the plasma. Thus, the increase in the ALT and ALP activities in the serum may explain the damaging influence on liver cells of high-Zn-treated pigs. Additionally, the adverse effects due to the toxicity of high Zn diets have been reported by Starke et al. (2014). On the contrary, a low dietary dose of coated NZO may be more beneficial to physical health.
Furthermore, the antioxidant capacity of growing pigs is fundamental for maintaining the normal metabolic state to protect a pig’s health, we hypothesized that the effects of dietary NZO could promote growth by indirectly regulating the antioxidant capacity of pigs. It has been confirmed that zinc contributes to regulating the redox status and maintaining the integrity of cell membranes (Prasad, 2008). High doses of ZnO (3000 mg/kg) supplementation reduced the serum MDA concentration and increased the T-SOD activity in piglets (Zhu et al., 2017). Our findings also show that pigs fed a high dose of ZnO had an improved antioxidant capacity (CAT and GSH-Px activities). Meanwhile, a low dose of coated NZO could increase the activities of SOD and GSH-Px in the serum. Generally, the SOD, CAT, and GSH-Px are key parameters reflecting the status of the antioxidant capacity in plasma. Zinc acts as an essential component in Cu-Zn-SOD, which was positively correlated with the dietary Zn level (Fathi et al., 2016). Here, a low supplementation level of coated NZO had a positive effect on Cu-Zn-SOD activity, while a high dose of Zn had no effect on Cu-Zn-SOD activity. It indicates that high dose of Zn may play an antagonism role in the absorption of Cu and enzyme synthesis such as Cu-Zn-SOD. As Zhao et al. (2014) reported, only the appropriate level of NZO could increase Cu-Zn-SOD activity, thereby improving the antioxidant capacity. Dietary supplementation with lipid matrix-coated ZnO could improve the antioxidant capacity of weaning piglets (Upadhaya et al., 2018). As a result, we conclude that lower doses of coated NZO (100 mg Zn/kg) could be used in substitution for the high doses of ZnO, to enhance the antioxidant capacity of growing pigs.
Although a pharmacological dose of ZnO is widely used to decrease the incidence of diarrhea and improve the growth performance in piglets, more attention should paid to the environmental pollution caused by excessive Zn residues. Fecal excretion of Zn was positively related to the concentrations of supplemented Zn in the diet (Carlson et al., 2004). As observed in the present study, the decreased fecal Zn indicated that replacing high ZnO in the diet with coated NZO was beneficial to reduce the environmental pollution. This is consistent with Upadhaya et al.’s (2018) findings, which showed that using lower doses of lipid matrix-coated ZnO (<1,000 mg/kg) could reduce the excretion of Zn compared with the conventional high dose of Zn. The accumulation of Zn has been documented as being closely related to tissue damage (Wang et al., 2017). As suggested by the present study, compared with the high zinc diet, a low concentration of coated NZO (100 mg Zn/kg) in the diet is more suitable for alleviating diarrhea and reducing fecal zinc emissions of growing pigs.
Experiment 2
ZnO in nanosize presents strong bioactivity at low concentrations due to their high surface area to volume ratio and unique chemical and physical properties (Rai et al., 2009). The present study verified the effects of coated NZO materials with different concentrations on growing pigs. In this study, both low (5%) and high (10%) concentrations of coated NZO materials improved the final BW, ADG, and feed conversion rate for the whole trial period. However, the supplementation of coated NZO materials with different concentrations is not as good as a high concentration of Zn, in reducing the occurrence of diarrhea. This is explained due to an increase in the concentration nanoparticles, which result in the increased surface area. A lager surface area will result in a greater antimicrobial activity (Espitia et al., 2012). Padmavathy and Vijayaraghavan (2008) also suggest that the abrasive surface of ZnO nanoparticles in lager concentration could cause mechanical damage to the cell membrane. Different concentrations of coating techniques, applied in the feeding additives, might resulted in the variations of stability, absorption rates, and antimicrobial activity. Thus, more related studies are need to support this hypothesis.
The intestinal morphology plays an important role in the uptake of nutrients and the occurrence of diarrhea (Lee et al., 2013). Changes in the intestinal morphology, including villus atrophy and crypt hyperplasia, are associated with the malabsorption and growth inhibition of pigs (Xiong et al., 2016). Previous researchers have shown that a high dietary zinc treatment could improve the morphology of the small intestine (Li et al., 2006). In agreement with these studies, the present study demonstrates that pigs supplemented with a high level of ZnO had an improved intestinal morphology. Thus, the mechanism for high ZnO inhibiting diarrhea is partly due to the improvement of the damage to the intestinal morphology of pigs. The supplementation with coated NZO at 5% concentration showed a lower intestinal injury and higher villus height than that at 10%. This might be related with the coating materials used for the high concentration supplementation, which can easily cause compaction, thereby damaging the gastrointestinal tract. The mechanisms of coated NZO on the intestinal barrier and integrity need to be further explored.
Also, pigs fed on a diet with 5% NZO or 10% NZO (100 mg Zn/kg) had markedly reduced concentrations of Zn in the plasma, liver, and feces on d 14 compared with the high-Zn group. The possible explanation for the reduction in fecal excretion of Zn had no relation to the coating technology or source, which was directly affected by the low dose of Zn (Shen et al., 2014). However, the mechanisms for the intestinal transport and absorption of zinc should be further investigated.
In summary, the present research indicates that in growing pigs, dietary-coated NZO (100 mg/kg Zn) supplementation improves the antioxidant capacity and reduces the fecal Zn emission without a negative effect on the growth performance. Additionally, 5% coated NZO material caused less intestinal injury compared with 10% coated NZO material. Reducing ZnO using coated NZO improved the growth performance, but the diarrhea rate was not as effectively suppressed compared with the high ZnO supplementation. In addition to up to 100 mg/kg of coated NZO, the control of diarrhea in piglets also requires the assistance of functional additives.
Conflict of interest statement. No conflicts of interest to this work.
Footnotes
The present work was jointly supported by National Key Research and Development Program of China (2018YFD0501101 and 2016YFD0501201), Natural Science Foundation of Hunan Province of China (2018JJ3579), Key Programs of frontier scientific research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), Youth Talent Program of Hunan Province (2018RS3110), National Natural Science Foundation of China (31501964), Youth Innovation Team Project of ISA, CAS (2017QNCXTD_TBE), and China Agriculture Research System (CARS-35). We thank Hangzhou King Techina Technology Company Academician Expert Workstation and Shandong Newhope-Liuhe Group Company Academician Expert Workstation for providing technical assistance.
LITERATURE CITED
- Bauer L. A., Birenbaum N. S., and Meyer G. J.. 2004. Biological applications of high aspect ratio nanoparticles. J. Mater. Chem. 14:517–526. doi:10.1039/B312655B [Google Scholar]
- Buzea C., and Pacheco I.. 2017. Nanomaterial and nanoparticle: origin and activity[M]// nanoscience and plant–soil systems. Berlin, Germany: Springer International Publishing. doi:10.1007/978-3-319-46835-8_3 [Google Scholar]
- Campbell J. M., Crenshaw J. D., and Polo J.. 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:19. doi:10.1186/2049-1891-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. S., Boren C. A., Wu C., Huntington C. E., Bollinger D. W., and Veum T. L.. 2004. Evaluation of various inclusion rates of organic zinc either as polysaccharide or proteinate complex on the growth performance, plasma, and excretion of nursery pigs. J. Anim. Sci. 82:1359–1366. doi:10.2527/2004.8251359x [DOI] [PubMed] [Google Scholar]
- Cho W. S., Kang B. C., Lee J. K., Jeong J., Che J. H., and Seok S. H.. 2013. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 10:9. doi:10.1186/1743-8977-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J. H., Upadhaya S. D., and Kim I. H.. 2015. Effects of dietary supplementation of modified zinc oxide on growth performance, nutrient digestibility, blood profiles, fecal microbial shedding and fecal score in weanling pigs. Anim. Sci. J. 86:617–623. doi:10.1111/asj.12329 [DOI] [PubMed] [Google Scholar]
- Croteau M. N., Dybowska A. D., Luoma S. N., and Valsami-Jones E.. 2011. A novel approach reveals that zinc oxide nanoparticles are bioavailable and toxic after dietary exposures. Nanotoxicology 5:79–90. doi:10.3109/17435390.2010.501914 [DOI] [PubMed] [Google Scholar]
- Érika Dantas de Medeiros R., de Brito N. J., Dantas M. M., De A. S. A., Das G. A. M., and Brandão-Neto J.. 2015. Effect of zinc supplementation on GH, IGF1, IGFBP3, OCN, and ALP in non-zinc-deficient children. J. Am. Coll. Nutr. 34:290–299. doi:10.1080/07315724.2014.929511 [DOI] [PubMed] [Google Scholar]
- Espitia P. J. P., Soares N. de F. F., Coimbra J. S. dos R., Andrade N. J., Cruz R. S., and Medeiros E. A. A.. 2012. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food. Bioprocess Technol. 5:1447–1464. doi:10.1007/s11947-012-0797-6 [Google Scholar]
- European Communities 2003. Commission regulation (EC) no. 1334/2003 of 25 July 2003. Amending the conditions for authorization of a number of additives in feeding stuffs belonging to the group of trace elements. Off. J. Eur. Union. 6:1–1. [Google Scholar]
- Fairbrother J. M., Nadeau E., and Gyles C. L.. 2005. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6:17–39. doi:10.1079/AHR2005105 [DOI] [PubMed] [Google Scholar]
- Fathi M., Haydari M., and Tanha T.. 2016. Effects of zinc oxide nanoparticles on antioxidant status, serum enzymes activities, biochemical parameters and performance in broiler chickens. J. Livest. Sci. Technol. 4: 7–13. doi:10.22103/JLST.2016.1509 [Google Scholar]
- Florence A. T., Hillery A. M., Hussain N., and Jani P. U.. 1995. Factors affecting the oral uptake and translocation of polystyrene nanoparticles: histological and analytical evidence. J. Drug Target. 3:65–70. doi:10.3109/10611869509015936 [DOI] [PubMed] [Google Scholar]
- Hunter T. L. 1965. Determination of zinc in rubber products via EDTA titration. Anal. Chem. 37:1436–1437. doi:10.1021/ac60230a040 [Google Scholar]
- Kong X. F., Wu G. Y., Liao Y. P., Hou Z. P., Liu H. J., and Yin F. G.. 2007. Dietary supplementation with Chinese herbal ultra-fine powder enhances cellular and humoral immunity in early-weaned piglets. Livest. Sci. 108:94–98. doi:10.1016/j.livsci.2007.01.002 [Google Scholar]
- Lee H., Park J. H., Park D. I., Kim H. J., Cho Y. K., Sohn C. I., Jeon W. K., Kim B. I., and Chae S. W.. 2013. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J. Neurogastroenterol. Motil. 19:244–250. doi:10.5056/jnm.2013.19.2.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. L., Yin J. D., Li D. F., Chen X. J., Zang J. J., and Zhou X.. 2006. Dietary supplementation with zinc oxide increases IGF-I and IGF-I receptor gene expression in the small intestine of weanling piglets. J. Nutr. 136:1786–1791. doi:10.1093/jn/136.7.1786 [DOI] [PubMed] [Google Scholar]
- Marquardt R. R., Jin L. Z., Kim J. W., Fang L., Frohlich A. A., and Baidoo S. K.. 2013. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immunol. Med. Microbiol. 23:283–288. doi:10.1111/j.1574-695X.1999.tb01249.x [DOI] [PubMed] [Google Scholar]
- Milani N. C., Sbardella M., Ikeda N. Y., Arno A., Mascarenhas B. C., and Miyada V. S.. 2017. Dietary zinc oxide nanoparticles as growth promoter for weanling pigs. Anim. Feed Sci. Tech. 227: 13–23. doi:10.1016/j.anifeedsci.2017.03.001 [Google Scholar]
- Miller H. M., Toplis P., and Slade R. D.. 2009. Can outdoor rearing and increased weaning age compensate for the removal of in-feed antibiotic growth promoters and zinc oxide? Livest. Sci. 125:121–131. doi:10.1016/j.livsci.2009.03.014} [Google Scholar]
- NRC 2012. Nutrient requirements of swine. Washington DC:National Academy Press. [Google Scholar]
- Padmavathy N., and Vijayaraghavan R.. 2008. Enhanced bioactivity of Zno nanoparticles—an antimicrobial study. Sci. Technol. Adv. Mater. 9:035004. doi:10.1088/1468-6996/9/3/035004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A. S. 2008. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 43:370–377. doi:10.1016/j.exger.2007.10.013 [DOI] [PubMed] [Google Scholar]
- Rai M., Yadav A., and Gade A.. 2009. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27:76–83. doi:10.1016/j.biotechadv.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Ren W., Yin Y., Liu G., Yu X., Li Y., Yang G., Li T., and Wu G.. 2012. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42:2089–2094. doi:10.1007/s00726-011-0942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebahat I. L., Abdullah B., Serap T., Yavuz T., and Goncagül H.. 2005. Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3, somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J. Pediatr. Endocrinol Metab. 18:69–74. doi:10.1515/JPEM.2005.18.1.69 [DOI] [PubMed] [Google Scholar]
- Shelton N. W., Tokach M. D., Nelssen J. L., Goodband R. D., Dritz S. S., and Derouchey J. M.. 2011. Effects of copper sulfate, tri-basic copper chloride, and zinc oxide on weanling pig performance. J. Anim. Sci. 89:2440–2451. doi:10.4148/2378–5977.7027 [DOI] [PubMed] [Google Scholar]
- Shen J. H., Chen Y., Wang Z. S., Zhou A. G., He M., Mao L., Zou H. W., Peng Q. H., Xue B., Wang L. Z., et al. 2014. Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. Br. J. Nutr. 111:2123–2134. doi:10.1017/S0007114514000300 [DOI] [PubMed] [Google Scholar]
- Sirelkhatim A., Mahmud S., Seeni A., Kaus N. H. M., Ann L. C., Bakhori S. K. M., Hasan H., and Mohamad D.. 2015. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 7:219–242. doi:10.1007/s40820-015-0040-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke I. C., Pieper R., Neumann K., Zentek J., and Vahjen W.. 2014. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 87:416–427. doi:10.1111/1574-6941.12233 [DOI] [PubMed] [Google Scholar]
- Tan B. E., Yin Y. L., Liu Z. Q., Li X. G., Xu H. J., Kong X. F., Huang R. L., and Tang W. J.. 2009. Dietary l-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. 37:169–175. doi:10.1007/s00726-008-0148-0 [DOI] [PubMed] [Google Scholar]
- Trckova M., Lorencova A., Hazova K., and Zajacova Z. S.. 2015. Prophylaxis of post-weaning diarrhoea in piglets by zinc oxide and sodium humate. Vet. Med. 60:351–360. doi:10.17221/8382-VETMED [Google Scholar]
- Upadhaya S. D., Ki Y. M., Lee K. Y., and Kim I. H.. 2018. Use of protected zinc oxide in lower doses in weaned pigs in substitution for the conventional high dose zinc oxide. Anim. Feed Sci. Technol. 240:1–10. doi:10.1016/j.anifeedsci.2018.03.012 [Google Scholar]
- Wang C., Cheng K., Zhou L., He J., Zheng X., Zhang L., Zhong X., and Wang T.. 2017. Evaluation of long-term toxicity of oral zinc oxide nanoparticles and zinc sulfate in mice. Biol. Trace Elem. Res. 178:276–282. doi:10.1007/s12011-017-0934-1 [DOI] [PubMed] [Google Scholar]
- Wang C., Zhang L., Ying Z., He J., Zhou L., Zhang L., Zhong X., and Wang T.. 2018. Effects of dietary zinc oxide nanoparticles on growth, diarrhea, mineral deposition, intestinal morphology, and barrier of weaned piglets. Biol. Trace Elem. Res. 185:364–374. doi:10.1007/s12011-018-1266-5 [DOI] [PubMed] [Google Scholar]
- Xiao H., Tan B. E., Wu M. M., Yin Y. L., Li T. J., Yuan D. X., and Li L.. 2013a. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function. J. Anim. Sci. 91:4750–4756. doi:10.2527/jas.2013-6427 [DOI] [PubMed] [Google Scholar]
- Xiao D. F., Tang Z. R., Yin Y. L., Zhang B., Hu X. G., Feng Z. M., and Wang J. Q.. 2013b. Effects of dietary administering chitosan on growth performance, jejunal morphology, jejunal mucosal sIgA, occluding, claudin-1 and TLR4 expression in weaned piglets challenged by enterotoxigenic Escherichia coli. Int. Immunopharmacol. 17:670–676. doi:10.1016/j.intimp [DOI] [PubMed] [Google Scholar]
- Xiong X., Yang H., Hu X., Wang X., Li B., Long L., Li T., Wang J., Hou Y., Wu G., et al. 2016. Differential proteome analysis along jejunal crypt-villus axis in piglets. Front. Biosci. (Landmark Ed.). 21:343–363. doi:10.2741/4392 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wan D., Zhou X., Long C., Wu X., Li L., He L., Huang P., Chen S., Tan B., et al. 2017. Diurnal variations in iron concentrations and expression of genes involved in iron absorption and metabolism in pigs. Biochem. Biophys. Res. Commun. 490:1210–1214. doi:10.1016/j.bbrc.2017.06.187 [DOI] [PubMed] [Google Scholar]
- Zhao C. Y., Tan S. X., Xiao X. Y., Qiu X. S., Pan J. Q., and Tang Z. X.. 2014. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 160:361–367. doi:10.1007/s12011-014-0052-2 [DOI] [PubMed] [Google Scholar]
- Zhu C., Lv H., Chen Z., Wang L., Wu X., Chen Z., Zhang W., Liang R., and Jiang Z.. 2017. Dietary zinc oxide modulates antioxidant capacity, small intestine development, and jejunal gene expression in weaned piglets. Biol. Trace Elem. Res. 175:331–338. doi:10.1007/s12011-016-0767-3 [DOI] [PubMed] [Google Scholar]