Abstract
Pigs exposed to heat stress (HS) reduce feed intake and consequently the consumption of AA. Adding extra protein-bound or free AA to the diet may correct the reduced AA intake of HS pigs. However, extra protein-bound AA may further increase the body heat load, whereas extra free AA does not affect the heat load of HS pigs. Two experiments were conducted. In Exp. 1, the performance depression because of HS, compared with thermal neutrality, was determined with 30 pigs (31.1 ± 1.2 kg BW) fed diets with AA only as protein or as a mix of protein and free AA. Heat stress pigs consumed 18 to 25% less Lys and Thr than thermal neutral. In Exp. 2, the effect of extra dietary protein-bound or free AA on performance and serum concentration of AA in 25 HS pigs (33.6 ± 0.65 kg BW) was evaluated. Treatments were as follows: CON, wheat-soybean meal-free Lys-Thr-Met diet; xP diet, 26% more protein than the CON diet; xAA diet, 24% or more of each AA than the recommended level. Pigs were fed ad libitum. Blood samples were collected between 1600 and 1700 h, when pigs were exposed to the highest ambient temperature (around 41.3 °C). Body temperature ranged daily from 39.9 to 41.1 °C. The performance data were reported already. Pigs fed the xP diet consumed more of all indispensable AA and dispensable AA than the CON pigs (P < 0.05), and more Arg, Ile, Asp, Glu, Gly, and Ser (P < 0.05) than the xAA pigs. Except for Arg, xAA pigs consumed more indispensable AA than the CON pigs (P < 0.05). Serum Arg, His, Lys, Phe, Thr, Trp, and Val, was higher (P < 0.05) in xP than in CON pigs. Except for Ile serum, indispensable AA were higher in xAA than in CON pigs (P < 0.05). Serum Ile, Leu, Thr, and Val were higher (P < 0.05), and Met tended to be higher (P < 0.10) in xAA than in xP pigs. The difference of Ile, Leu, Met, Thr, and Val between the CON and the xAA pigs was larger than that between the CON and the xP pigs (P < 0.05). Serum Asn and Tyr were higher, and Cys and Glu were lower (P < 0.05) in xP than in CON pigs. Serum Cys tended to be lower in xAA than in CON pigs (P < 0.10). Asp and Glu were higher (P < 0.05) in xAA pigs than in xP pigs. In conclusion, these serum AA results combined with the reported performance data indicate that extra free AA in diets for HS pigs may help to correct the reduced AA availability and performance of HS pigs, although higher levels of specific AA such as Ile and Met might be needed.
Keywords: extra supplemental amino acids, heat stress, pigs, serum amino acids
INTRODUCTION
Pigs exposed to heat stress (HS) decrease their voluntary feed intake. Compared with thermal neutral pigs, HS pigs reduce 20% to 40% the feed intake (Quiniou et al., 2001; Morales et al., 2014) as a means to decrease body heat production and to maintain body thermal balance (Collin et al., 2001). The reduced feed intake of HS pigs results in decreased intake of AA and other nutrients. Moreover, damaged intestinal mucosa (Pearce et al., 2013) and diminished abundance of intestinal AA transporters (Morales et al., 2015) in HS pigs are expected to reduce the absorption of AA. Indeed, HS pigs compared with thermal neutral pigs reduce up to 30% the absorption of AA (Morales et al., 2016b). Hence, a reduced AA intake combined with a depressed AA absorption inevitably reduces the AA availability for growth of HS pigs (Morales et al., 2016b).
Increasing the AA level, by elevating the content of protein-bound or free AA in the diet may help to overcoming the low AA availability in HS pigs. Feeding high protein diets, however, increases the thermal effect of feeding resulting in higher heat production (Noblet et al., 2001; Kerr et al., 2003), which may further reduce the intake and absorption of AA. In contrast, dietary free AA may not generate additional body heat. Nevertheless, the AA availability in HS pigs fed diets added with extra AA either in protein-bound or in free form has not been reported. The postprandial concentration of free AA in serum is an indicator of AA availability for growth in pigs (Reverter et al., 2000; Yen et al., 2004). Thus, we hypothesized that adding extra protein-bound or free AA to the diet would differently affect the serum concentration of free AA and growth performance of HS pigs. Two studies were conducted to analyze the effect of supplying extra protein-bound or free AA in the diet on performance and serum concentration of free AA in HS pigs; the serum AA data are included in this report.
MATERIALS AND METHODS
General Experimental Procedure
Two experiments were conducted with 55 crossbred (Landrace × Hampshire × Duroc) during the summer of year 2016. The pigs were individually housed in metabolism pens with elevated iron-mesh floor and equipped with a stainless-steel self-feeder and a nipple water drinker. The ambient temperature inside the experimental rooms was recorded with the aid of a Higrothermograph (Thermotracker HIGRO; iButtonLink LLC, Whitewater, WI) set to record those values every 15 min during the whole study. The pigs used in the present experiments were cared for in accordance with the guidelines established in the Official Mexican Regulations on Animal Care (NOM-062-ZOO-1999, 2001).
Experiment 1 was conducted with 30 pigs (31.1 ± 1.2 kg initial BW) to determine the magnitude of the feed and AA intake reduction and its associated growth rate depression provoked by their exposure to HS, in comparison with thermal neutral pigs. The treatments were as follows: thermal neutral pigs fed a 22% CP control diet (TN-C); HS pigs fed the control diet (HS-C); HS pigs fed a 14% CP, AA-supplemented diet (HS-AA). The control and low-protein diets (Table 1) were formulated to meet the standardized ileal digestible (SID) requirement of the first (Lys) and the first 3 (Lys, Thr, and Met) limiting AA, respectively, for pigs within the range of 25 to 50 kg (NRC, 2012). The TN-C pigs were housed inside an air-conditioned room where ambient temperature was maintained relatively constant (19.5 to 23.5 °C). The HS pigs were housed inside a room kept under natural climate conditions where ambient temperature consistently varied every day from 24.5 to 42.6 °C during the whole study. As reported previously (Morales et al., 2018), HS pigs reduced the intake of Lys, Thr, and Met in comparison with the control thermal neutral pigs. These AA intake reductions were associated with a similar BW gain decrease of HS pigs.
Table 1.
Composition of the experimental diets (%, as fed basis)1
| Ingredient | Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|---|
| Control | LP-AA | CON | xP | xAA | ||
| Wheat | 64.90 | 91.46 | 86.64 | 76.14 | 85.50 | |
| Soybean meal | 30.30 | 4.00 | 10.00 | 20.50 | 10.00 | |
| L-Lys.HCl | — | 0.80 | 0.56 | 0.56 | 0.88 | |
| L-Thr | — | 0.27 | 0.14 | 0.14 | 0.29 | |
| DL-Met | — | 0.11 | 0.06 | 0.06 | 0.18 | |
| L-Trp | — | 0.04 | — | — | 0.03 | |
| L-Phe | — | 0.09 | — | — | 0.08 | |
| L-Leu | — | 0.25 | — | — | 0.18 | |
| L-Ile | — | 0.13 | — | — | 0.07 | |
| L-Val | — | 0.17 | — | — | 0.14 | |
| L-His | — | 0.08 | — | — | 0.05 | |
| Calcium carbonate | 1.25 | 1.40 | 1.40 | 1.40 | 1.40 | |
| Dicalcium phosphate | 1.00 | 0.65 | 0.65 | 0.65 | 0.65 | |
| Iodized salt | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | |
| Vitamin and mineral premix2 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | |
| Canola oil | 2.00 | — | — | — | — | |
| Calculated content | ||||||
| NE, MJ/kg 3 | 9.88 | 10.21 | 10.02 | 9.88 | 10.10 | |
| CP, % | 21.72 | 13.91 | 15.10 | 18.98 | 16.05 | |
Diets: Control, only protein-bound AA diet; LP-AA, low-protein diet added with free AA; CON, control diet; xP, CON + 30% protein as soybean meal; xAA, CON + 26% free AA.
Supplied per kg of diet: Vitamin A, 4,800 IU; vitamin D3, 800 IU; vitamin E, 4.8 IU; vitamin K3, 1.6 mg; riboflavin, 4 mg; D-pantothenic acid, 7.2 mg; niacin, 16 mg; vitamin B12, 12.8 mg; Zn, 64 mg; Fe, 64 mg; Cu, 4 mg; Mn, 4 mg; I, 0.36 mg; Se, 0.13 mg. The premix was supplied by Nutrionix, S.A., Hermosillo, México.
Based on the ingredients NE values (MJ/kg): Wheat, 10.50; soybean meal, 8.12 (Sauvant et al., 2004).
In Exp. 2, we attempted to correct the reduced AA intake observed in HS pigs compared with the thermal neutral pigs of Exp. 1 by increasing its dietary content either in protein-bound or in free form (Table 2). Twenty-one pigs (33.6 ± 0.65 kg initial BW) housed inside a room kept under natural ambient temperature conditions were used. The pigs were randomly allotted to 1 of 3 treatments: 1) control, 15.0% CP diet formulated with wheat, soybean meal, and supplemental free Lys, Thr, and Met, to meet the SID requirements of all indispensable AA (NRC, 2012) plus vitamins and minerals (CON); 2) extra protein diet (xP), and 3) extra free AA diet (xAA). The xP diet contained 26% more CP than the CON diet, by adjusting the wheat and soybean meal levels, without modifying the levels of free Lys, Thr, and Met. The xAA diet contained the same levels of wheat and soybean meal as in the CON diet, but was supplemented with free Lys, Thr, Met, Trp, Phe, Leu, Ile, His, and Val at levels that supplied at least 24% more of each AA (Fig. 1), compared with the NRC (2012) requirements. The analyzed AA content and the SID coefficients for wheat and soybean meal (Stein et al., 2001) were used to calculate the SID contents of AA in the diets. The NE content in the CON and xAA diets was 10.02 and 10.10 MJ of per kg, whereas in the xP diet, it was 9.88 MJ of per kg. All pigs were fed ad libitum and had free access to purified water all the time during the 21-d study. In addition, intestinal temperature was measured in 6 ileal-cannulated pigs of similar BW and housed in the same HS room and at the same time. Additional information regarding housing and experimental procedures is described in detail by Morales et al. (2018).
Table 2.
Analyzed AA composition of the diets in Exp. 2 (%, as fed basis)1
| Diet | Diet | ||||||
|---|---|---|---|---|---|---|---|
| Indispensable | CON | xP | xAA | Dispensable | CON | xP | xAA |
| Arg | 0.80 | 0.98 | 0.83 | Ala | 0.58 | 0.69 | 0.57 |
| His | 0.36 | 0.44 | 0.42 | Asp | 1.06 | 1.44 | 1.09 |
| Ile | 0.61 | 0.78 | 0.69 | Glu | 3.52 | 4.06 | 3.60 |
| Leu | 1.04 | 1.28 | 1.25 | Pro | 1.22 | 1.31 | 1.22 |
| Lys | 1.09 | 1.38 | 1.41 | Gly | 0.62 | 0.73 | 0.60 |
| Met | 0.30 | 0.45 | 0.42 | Ser | 0.60 | 0.74 | 0.61 |
| Phe | 0.70 | 0.86 | 0.79 | Tyr | 0.40 | 0.43 | 0.41 |
| Thr | 0.62 | 0.70 | 0.76 | ||||
| Val | 0.70 | 0.85 | 0.85 | ||||
Figure 1.
Percentage of the requirement of each standardized ileal digestible AA of 25- to 50-kg pigs (NRC, 2012) met by each one of the experimental diets.
Blood Sample Collection and Chemical Analyses
On day 20 of Exp. 2, blood samples (approximately 7 mL) were collected by venipuncture of the jugular vein from all pigs to analyze the serum concentrations of AA and some AA metabolites. The sampling of blood was performed between 1600 and 1700 h, when the ambient temperature reached its highest value (around 41.3 °C) in the day. Blood samples were kept on ice during the whole collection procedure. Immediately after collection, the blood samples were centrifuged at 1,500 × g, 4 °C for 10 min to separate serum from blood cells. Serum samples were freeze-dried and stored at −20 °C until analysis. The serum concentration of free AA was analyzed (method 982.30E; AOAC, 2006). Wheat, soybean meal, and experimental diets were analyzed for AA content (method 982.30; AOAC, 2006) after acid hydrolysis with 6 N HCl, but tryptophan was not analyzed. The analyses of AA were performed by HPLC with postcolumn ninhydrin derivatization, and the use of a fluorescence detector.
Calculations and Statistical Analyses
The relative differences in serum concentrations of all AA between pigs fed the CON diet and the xP or the xAA diet (Exp. 2) were calculated. Analyses of data for the serum concentration of each AA, and the differences between treatments were performed according to a completely randomized design. The individual pig was considered as the experimental unit. The model considered the fixed effects of diet and the random effects of pig and ambient temperature. Three contrasts were constructed to evaluate the effects of extra protein-bound AA, and extra free AA compared with CON as follows: C1, CON vs. xP; C2, CON vs. xAA; C3, xP vs. xAA). In addition, multiple correlation analyses between intake and serum concentration of each AA, and between AA were performed. Because of differences in the AA digestibilities among the xP and xAA diets, 2 groups of correlation analyses were performed separately: one group included data of the CON and xP pigs, and the other group included data of the CON and xAA pigs. Probability levels of P ≤ 0.05 and 0.05 < P ≤ 0.10 were defined as significant differences and tendencies, respectively.
RESULTS
The daily ambient temperature recorded inside the HS room during the 10-d period before blood sampling varied within the same day (27.7 to 37.7 °C; Fig. 2). Body temperature of HS pigs ranged daily from 39.9 to 41.1 °C, following a pattern similar to that of ambient temperature. Despite the exposure of all pigs to these experimental conditions, no effect was observed in their health during the whole study. The performance and carcass traits results were reported already (Morales et al., 2018). Briefly, in Exp. 1, HS pigs reduced 18% to 25% the intake of Lys, Thr, and Met in comparison with the control thermal neutral pigs. The BW gain of HS pigs (770 g/d) also decreased compared with the controls (938 g/d). In Exp. 2, no effect was observed on feed intake due to the source of AA (free vs. protein-bound). However, the daily weight gain of pigs fed the xAA diet (761 g/d) was 18% higher than that of the CON pigs (644 g/d), although it did not differ between pigs fed the CON or the xP diet (714 g/d).
Figure 2.
Daily variations of ambient temperature measured at 15-min intervals with the aid of a thermograph during the 10-d period before blood sampling. Each line represents a single day, and each data point represents the hourly average of 4 temperature recordings.
The daily intake (g) of indispensable AA by all pigs, calculated using the daily feed intake data and the SID content of AA previously reported by Morales et al. (2018), is presented in Table 3. The consumption of all indispensable AA by pigs fed the xP diet was higher than that of pigs fed the CON diet (P < 0.01); also, although with the exception of Arg, pigs fed the xAA diet consumed more of each indispensable AA than pigs fed the CON diet (P < 0.05). Pigs fed the xP diet consumed more Arg and Ile (P < 0.05) and tended to consume more Phe (P < 0.10) than those fed the xAA diet, but the consumption of His, Leu, Lys, Met, Thr, and Val did not differ (P > 0.10) among these 2 groups of pigs. As for the dispensable AA (Table 4), pigs fed the xP diet consumed more of all dispensable AA than pigs fed the CON diet (P < 0.05); xP pigs also consumed more Asp, Glu, Gly, and Ser (P < 0.05), and tended to consume more Ala (P < 0.10) than pigs fed the xAA diet. There were no differences in the dispensable AA intake between the CON or the xAA pigs.
Table 3.
Daily intake (g) of indispensable AA by pigs exposed to heat stress conditions and fed a standard diet (CON) or this diet with extra AA supplied either in protein-bound (xP) or in free form (xAA)
| Treatment1 | Contrasts, P2 | ||||||
|---|---|---|---|---|---|---|---|
| AA | CON | xP | xAA | SEM | C1 | C2 | C3 |
| Arginine | 12.63 | 16.14 | 13.53 | 0.53 | <0.001 | 0.246 | 0.003 |
| Histidine | 5.68 | 7.25 | 6.65 | 0.24 | <0.001 | 0.004 | 0.266 |
| Isoleucine | 9.63 | 12.85 | 11.25 | 0.42 | <0.001 | 0.015 | 0.016 |
| Leucine | 16.42 | 21.06 | 20.39 | 0.71 | <0.001 | 0.001 | 0.498 |
| Lysine | 17.21 | 22.73 | 23.00 | 0.73 | <0.001 | <0.001 | 0.807 |
| Methionine | 4.73 | 7.41 | 6.85 | 0.23 | <0.001 | <0.001 | 0.110 |
| Phenylalanine | 11.05 | 14.17 | 12.88 | 0.47 | <0.001 | 0.014 | 0.072 |
| Threonine | 9.79 | 11.53 | 12.40 | 0.41 | 0.009 | <0.001 | 0.155 |
| Valine | 11.05 | 14.00 | 13.87 | 0.47 | <0.001 | <0.001 | 0.841 |
Treatment: CON, heat stress pigs fed a standard diet; xP, heat stress pigs fed excess protein-bound AA; xAA, heat stress pigs fed excess free AA.
Contrasts: C1, CON vs. xP; C2, CON vs. xAA; C3, xP vs. xAA.
Table 4.
Daily intake (g) of dispensable AA by pigs exposed to heat stress conditions and fed a standard diet (CON) or this diet with extra AA supplied either in protein-bound (xP) or in free form (xAA)
| Treatment1 | Contrasts, P2 | ||||||
|---|---|---|---|---|---|---|---|
| AA | CON | xP | xAA | SEM | C1 | C2 | C3 |
| Alanine | 9.16 | 11.36 | 9.97 | 0.51 | 0.008 | 0.278 | 0.074 |
| Aspartate | 16.73 | 23.72 | 17.78 | 0.73 | <0.001 | 0.331 | <0.001 |
| Glutamate | 55.57 | 66.88 | 58.72 | 2.26 | 0.003 | 0.339 | 0.022 |
| Glycine | 9.79 | 12.02 | 9.79 | 0.40 | 0.001 | 1.000 | 0.001 |
| Proline | 19.26 | 21.58 | 19.26 | 0.76 | 0.047 | 0.558 | 0.138 |
| Serine | 9.48 | 12.19 | 9.95 | 0.40 | <0.001 | 0.411 | 0.001 |
| Tyrosine | 6.31 | 7.08 | 6.69 | 0.25 | 0.046 | 0.309 | 0.279 |
Treatment: CON, heat stress pigs fed a standard diet; xP, heat stress pigs fed excess protein-bound AA; xAA, heat stress pigs fed excess free AA.
Contrasts: C1, CON vs. xP; C2, CON vs. xAA; C3, xP vs. xAA.
The serum concentration of free indispensable AA is presented in Table 5. Compared with pigs fed the CON diet, serum Arg, His, Lys, Phe, Thr, Trp, and Val were higher (P < 0.05), and Leu tended to be higher (P < 0.10) in pigs fed the xP diet, but serum Ile and Met were not different. Except for Ile, the serum concentrations of free indispensable AA were higher in pigs fed the xAA diet compared with the CON pigs (P < 0.05). Serum Ile, Leu, Thr, and Val were higher (P < 0.05), and Met tended to be higher (P < 0.10) in pigs fed the xAA diet than in those with the xP diet; serum Arg, His, Lys, Phe, and Trp did not differ among these 2 groups of pigs. The difference in serum Ile, Leu, Met, Thr, and Val between the CON and xAA pigs was larger (P < 0.05) than that between the CON and xP pigs (Fig. 3). Regarding the serum concentration of free dispensable AA (Table 6), Asn and Tyr were higher (P < 0.05), Cys and Glu were lower (P < 0.05), and Ala and Gln tended to be lower (P ≤ 0.10) in the xP than in the CON pigs; serum Asp, Gly, Pro, and Ser did not differ among these pigs. Except for Cys that tended to be lower in pigs fed the xAA diet (P < 0.10), serum dispensable AA did not differ between the CON and xAA pigs. Compared with pigs fed the xP diet, serum Asp and Glu were higher (P < 0.05) and Asn and Tyr tended to be lower (P < 0.10) in pigs fed the xAA diet.
Table 5.
Concentrations (µg/mL) of indispensable AA in the serum of pigs exposed to heat stress conditions and fed a standard diet (CON) or this diet with excess AA either in protein-bound (xP) or in free form (xAA)
| Treatment1 | Contrasts, P2 | ||||||
|---|---|---|---|---|---|---|---|
| AA | CON | xP | xAA | SEM | C1 | C2 | C3 |
| Arginine | 24.50 | 29.38 | 31.20 | 1.34 | 0.021 | 0.003 | 0.354 |
| Histidine | 6.92 | 9.96 | 9.19 | 0.56 | 0.002 | 0.012 | 0.347 |
| Isoleucine | 13.12 | 12.33 | 14.32 | 0.60 | 0.367 | 0.179 | 0.033 |
| Leucine | 16.77 | 19.01 | 22.30 | 0.86 | 0.086 | <0.001 | 0.016 |
| Lysine | 16.92 | 26.56 | 28.40 | 1.13 | <0.001 | <0.001 | 0.266 |
| Methionine | 3.16 | 3.23 | 4.26 | 0.37 | 0.887 | 0.050 | 0.065 |
| Phenylalanine | 9.28 | 11.51 | 11.28 | 0.40 | 0.001 | 0.003 | 0.691 |
| Threonine | 10.70 | 15.03 | 20.71 | 1.40 | 0.046 | <0.001 | 0.012 |
| Tryptophan | 8.38 | 10.96 | 10.50 | 0.55 | 0.005 | 0.015 | 0.561 |
| Valine | 25.26 | 29.89 | 33.92 | 1.01 | 0.005 | <0.001 | 0.013 |
Treatment: CON, heat stress pigs fed a standard diet; xP, heat stress pigs fed excess protein-bound AA; xAA, heat stress pigs fed excess free AA.
Contrasts: C1, CON vs. xP; C2, CON vs. xAA; C3, xP vs. xAA.
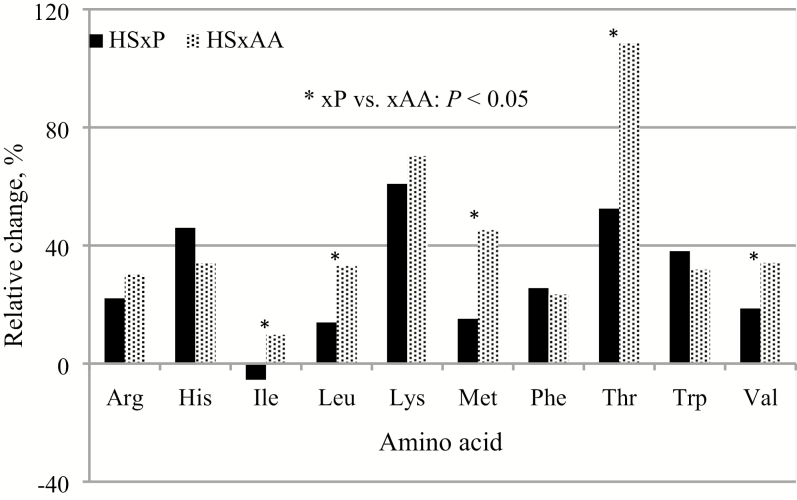
Figure 3.
Relative change in the serum concentrations of indispensable AA in pigs fed the diet with excess AA either in protein-bound (xP) or in free form (xAA), compared with pigs fed the control diet.
Table 6.
Concentrations (µg/mL) of dispensable AA in the serum of pigs exposed to heat stress conditions and fed a standard diet (CON) or this diet with excess AA either in protein-bound (xP) or in free form (xAA)
| Treatment1 | Contrasts, P2 | ||||||
|---|---|---|---|---|---|---|---|
| AA | CON | xP | xAA | SEM | C1 | C2 | C3 |
| Alanine | 45.26 | 36.38 | 41.62 | 3.69 | 0.100 | 0.496 | 0.331 |
| Asparagine | 5.09 | 6.44 | 5.21 | 0.41 | 0.037 | 0.843 | 0.054 |
| Aspartate | 3.61 | 3.24 | 4.03 | 0.19 | 0.195 | 0.145 | 0.011 |
| Cysteine | 1.29 | 0.32 | 0.57 | 0.28 | 0.027 | 0.090 | 0.527 |
| Glutamate | 20.59 | 14.61 | 20.31 | 1.46 | 0.011 | 0.893 | 0.015 |
| Glutamine | 49.37 | 43.59 | 44.78 | 2.07 | 0.067 | 0.138 | 0.689 |
| Glycine | 55.27 | 53.81 | 58.78 | 2.84 | 0.722 | 0.396 | 0.235 |
| Proline | 30.72 | 29.90 | 29.47 | 1.99 | 0.775 | 0.661 | 0.878 |
| Serine | 14.08 | 16.63 | 13.42 | 1.47 | 0.239 | 0.755 | 0.144 |
| Tyrosine | 12.73 | 16.85 | 13.31 | 1.21 | 0.030 | 0.741 | 0.057 |
Treatment: CON, heat stress pigs fed a standard diet; xP, heat stress pigs fed excess protein-bound AA; xAA, heat stress pigs fed excess free AA.
Contrasts: C1, CON vs. xP; C2, CON vs. xAA; C3, xP vs. xAA.
The serum concentrations of AA metabolites in pigs fed the CON, xP, or xAA diet are presented in Table 7. Orn, Tau, α-amino-adipic acid, and urea were higher (P < 0.50), and homocysteine tended to be higher (P < 0.10) in pigs fed the xP diet than in those fed the CON diet. However, serum Tau and α-amino-adipic acid only tended to be higher in pigs fed the xAA diet, compared with the CON pigs. Serum cysta/allothionine and sarcosine were higher, urea was lower (P < 0.50), and Orn tended to be lower (P < 0.10) in pigs fed the xAA diet than in those fed the xP diet.
Table 7.
Serum concentrations (µg/mL) of AA metabolites in pigs exposed to heat stress and fed a standard diet (CON) or this diet with excess AA either in protein-bound (xP) or in free form (xAA)
| Treatment1 | Contrasts, P2 | ||||||
|---|---|---|---|---|---|---|---|
| Metabolite | Con | xP | xAA | SEM | C1 | C2 | C3 |
| 1-Met. His | 1.61 | 1.71 | 1.37 | 0.20 | 0.735 | 0.404 | 0.248 |
| 3-Met. His | 0.87 | 0.98 | 0.93 | 0.08 | 0.353 | 0.628 | 0.649 |
| Carnosine | 4.39 | 4.73 | 4.78 | 0.41 | 0.563 | 0.505 | 0.929 |
| Citrulline | 9.21 | 8.38 | 8.67 | 0.65 | 0.381 | 0.561 | 0.762 |
| Cysta/allothionine | 0.29 | 0.23 | 0.32 | 0.03 | 0.133 | 0.451 | 0.032 |
| Homocysteine | 0.10 | 0.18 | 0.15 | 0.03 | 0.062 | 0.275 | 0.392 |
| OH-Lys | 0.84 | 1.28 | 0.85 | 0.32 | 0.245 | 0.977 | 0.360 |
| OH-Pro | 12.55 | 11.63 | 12.71 | 0.72 | 0.378 | 0.872 | 0.301 |
| Ornithine | 8.11 | 10.79 | 8.96 | 0.72 | 0.020 | 0.422 | 0.094 |
| P-Ser | 2.43 | 2.53 | 2.66 | 0.10 | 0.519 | 0.131 | 0.363 |
| Sarcosine | 1.77 | 1.53 | 1.85 | 0.11 | 0.139 | 0.615 | 0.050 |
| Taurine | 12.38 | 16.00 | 15.19 | 1.18 | 0.047 | 0.100 | 0.637 |
| Urea | 207.6 | 279.8 | 221.8 | 15.9 | 0.001 | 0.538 | 0.021 |
| α-amino-adipic acid | 3.69 | 5.04 | 4.91 | 0.47 | 0.050 | 0.086 | 0.844 |
Treatment: CON, heat stress pigs fed a standard diet; xP, heat stress pigs fed excess protein-bound AA; xAA, heat stress pigs fed excess free AA.
Contrasts: C1, CON vs. xP; C2, CON vs. xAA; C3, xP vs. xAA.
The correlation coefficients between intake and serum concentration of free indispensable AA are presented in Table 8. When including in the analysis the serum AA data of CON and xP pigs, there was a positive (r > 0.59) correlation (P < 0.05) between intake and serum Arg, Lys, Phe, Thr, and Val, and a slight tendency for serum Leu and His (P ≤ 0.100). There was no relationship between intake and serum Ile and Met. When including the data of CON and xAA pigs, there was a positive (r > 0.72) correlation (P < 0.05) between intake and serum Lys, Thr, and Val. There was no relationship between intake and serum Arg, His, Ile, Leu, Met, and Phe. Table 9 shows the correlation coefficients between the intake of a specific indispensable AA and the serum concentration of a different but chemically related AA. When the analysis included data of the CON and xP pigs, the intake/serum correlation of cationic AA (Arg/Lys, Lys/Arg, Lys/His) was (P < 0.05) or tended to be (Arg/His, P < 0.10) positive. Regarding the branched-chain AA, the Ile/Val and Leu/Val intake/serum correlations were positive (P < 0.05), but Leu/Ile and Val/Ile were not correlated; Leu intake/serum Lys were also correlated (P < 0.05). In contrast, when data of the CON and xAA pigs were included in the analysis, Arg intake was not correlated with serum His nor Lys, but Lys intake was correlated with serum Arg and His (P < 0.05). As for the branched-chain AA, Leu intake was highly correlated (P < 0.05) with serum Val, but it was not correlated with serum Ile. Also, Leu intake was positively and highly correlated with serum Lys (P < 0.05).
Table 8.
Pearson correlation coefficients between daily intake (I) and serum concentrations (SC) of free indispensable AA calculated using 2 data sets: 1) CON and xP pigs; and 2) CON and xAA pigs
| CON and xP pigs | CON and xAA pigs | |||
|---|---|---|---|---|
| AA | r | P | r | P |
| Arg | 0.668 | 0.017 | 0.415 | 0.180 |
| His | 0.534 | 0.074 | 0.446 | 0.146 |
| Ile | −0.257 | 0.421 | 0.069 | 0.830 |
| Leu | 0.492 | 0.100 | 0.428 | 0.164 |
| Lys | 0.726 | 0.007 | 0.736 | 0.006 |
| Met | 0.034 | 0.915 | 0.374 | 0.230 |
| Phe | 0.593 | 0.042 | 0.308 | 0.329 |
| Thr | 0.470 | 0.023 | 0.799 | 0.002 |
| Val | 0.658 | 0.020 | 0.727 | 0.008 |
Table 9.
Pearson correlation coefficients between intake of a specific indispensable AA and the serum concentration (SC) of a different but related free indispensable AA (I/SC), including 2 data sets: 1) CON and xP pigs; 2) CON and xAA pigs
| CON and xP pigs | CON and xAA pigs | |||
|---|---|---|---|---|
| I/SC | r | P | r | P |
| Arg/His | 0.537 | 0.072 | 0.119 | 0.712 |
| Arg/Lys | 0.692 | 0.013 | 0.235 | 0.462 |
| Lys/Arg | 0.672 | 0.017 | 0.724 | 0.009 |
| Lys/His | 0.571 | 0.050 | 0.567 | 0.050 |
| Ile/Leu | 0.535 | 0.073 | 0.304 | 0.336 |
| Ile/Val | 0.700 | 0.011 | 0.614 | 0.033 |
| Leu/Ile | −0.238 | 0.455 | 0.141 | 0.662 |
| Leu/Val | 0.558 | 0.016 | 0.884 | 0.001 |
| Val/Ile | −0.231 | 0.471 | 0.150 | 0.641 |
| Val/Leu | 0.475 | 0.118 | 0.445 | 0.147 |
| Leu/Lys | 0.697 | 0.012 | 0.656 | 0.020 |
The correlation coefficients between the serum concentration of a specific AA and a different but related AA (SC/SC) or related AA metabolite are presented in Table 10. Considering data from pigs fed the CON or the xP diet, Lys/urea, Arg/urea, Leu/Lys, Leu/Arg, and Leu/Val were correlated (P < 0.05), and Lys/Arg tended to correlate (P < 0.10). However, Leu/Ile and Val/Ile were not correlated. When including data from the CON and xAA pigs in the analysis, Lys/Arg and Ile/Leu were highly (P < 0.01) and Ile/Val tended (P < 0.10) to be correlated, but Lys/urea were not correlated. The Arg/urea, Leu/Lys, Leu/Arg, and Leu/Val were also correlated (P < 0.05).
Table 10.
Pearson correlation coefficients between the serum concentration (SC) of a specific AA and the SC of a different but related AA (SC/SC) or an AA metabolite, including 2 data sets: 1) CON and xP pigs; 2) CON and xAA pigs
| CON and xP pigs | CON and xAA pigs | |||
|---|---|---|---|---|
| SC/SC | r | P | r | P |
| Lys/Arg | 0.501 | 0.100 | 0.717 | 0.009 |
| Lys/urea | 0.580 | 0.048 | 0.053 | 0.870 |
| Arg/urea | 0.744 | 0.006 | 0.611 | 0.035 |
| Leu/Lys | 0.800 | 0.002 | 0.916 | <0.001 |
| Leu/Arg | 0.597 | 0.009 | 0.671 | 0.015 |
| Leu/Val | 0.837 | <0.001 | 0.884 | <0.001 |
| Ile/Leu | 0.052 | 0.872 | 0.777 | 0.003 |
| Ile/Val | 0.077 | 0.811 | 0.554 | 0.061 |
DISCUSSION
The ambient temperature recorded during 10 d before the blood sampling indicates that pigs were heat-stressed for at least 10 h every day. Indeed, body temperature (41.1 °C) recorded between 1500 and 1830 h was 1.2 °C higher than the temperature between 0500 and 0700 h. The reduced feed intake of HS pigs in Exp. 1 already reported (Morales et al., 2018) is in agreement with other reports (Renaudeau et al., 2010; Morales et al., 2014) and resulted in an 18% to 25% reduction in the intake of indispensable AA as well as depression in performance. The correction of this reduced AA intake would be expected to correct in turn the performance depression. In fact, as reported before (Morales et al., 2018), the performance and carcass traits of HS pigs (Exp. 2) improved when the diets supplied extra AA in free form, but no effect was observed when HS pigs were fed diets with surplus protein-bound AA. Hence, we hypothesized that differences in AA availability between protein-bound AA and free AA were responsible for that performance difference.
Increasing the AA content in the diet above the NRC (2012) requirement by adding extra protein or extra free AA may help to overcome the reduced AA intake of HS pigs. Excess dietary protein increases the thermic effect of feeding which includes the heat produced during digestion, absorption, and metabolism of nutrients (Noblet et al., 2001; Kerr et al., 2003) that might additionally increase the body temperature of HS pigs thus further reducing their feed intake. However, no effect of feeding either a 22% or an 11% protein diet on the body temperature of thermal neutral pigs was observed (Cervantes et al., 2017). Moreover, increasing the diet protein content by 26% (xP) in Exp. 2 did not affect the feed intake of HS pigs but, as expected, increased the intake of all indispensable AA, ranging from 30% (Thr) to 47% (Met) compared with the CON diet; dispensable AA intake also increased by 23% on average (Morales et al., 2018). These results suggest that HS pigs are capable to dissipate the additional body heat produced by excess protein in the xP diet, which in turn helps to restore their reduced AA intake. On the other hand, because free AA do not affect the digestion-related heat production, extra free AA in the diet are not expected to reduce feed and AA intake. Indeed, except for Arg, pigs fed the xAA diet consumed more of each indispensable AA than the CON pigs (from 17%-Ile-Phe to 45%-Met). Free Arg was not added because it was already about 78% in excess in the CON diet; the other 9 indispensable AA were added in free form at levels that made the xAA diet to contain at least 25% in excess of each AA compared with the requirements for pigs within the BW range of 25 to 50 kg (NRC, 2012). These data indicate that the form (protein-bound or free AA) in which extra AA are fed does not affect the feed intake of HS pigs.
The serum concentration of free AA can be considered indicator of their availability for the growth of animals (Yen et al., 2004). It results mainly from the balance between intestinal absorption and cellular uptake (Reverter et al., 2000), which are affected mostly by the digestible AA intake level, the form (free or protein-bound) AA are included in the diet, the time (absorptive or post-absorptive) when blood samples are collected (Yen et al., 2004; Morales et al., 2016a), and the interactions between AA for absorption (Langer and Fuller, 2000; García et al., 2015; Morales et al., 2015). In feed-restricted pigs, the SC of free AA analyzed during the absorptive phase (2 to 3 h post-prandial) reflects their consumption, digestion, and absorption (Yen et al., 2004), whereas the SC analyzed during the postabsorptive phase (more than 6 h post-prandial) reflects the cellular AA uptake (Reverter et al., 2000; Yen et al., 2004). In agreement, we have consistently observed in feed-restricted (2 or 3 times daily) pigs an increase in the SC of all AA during the absorptive phase, as well as a marked decrease during the postabsorptive phase (Morales et al., 2015, 2016a,b). On the other hand, it is known that free AA are absorbed faster than protein-bound AA and these differences are reflected in their serum concentrations but only when pigs are fed 1 or 2 times a day. In the present study, we were interested in analyzing the SC of AA in pigs under practical feeding conditions; hence, all pigs were fed ad libitum and blood samples were collected when the highest ambient temperature was recorded (between 1600 and 1700 h). From previous trials at this lab (A. Valle, A. Duckens, A. Morales, and M. Cervantes, unpublished data), we observed that HS pigs stop eating from around 1400 to 1900 h. Therefore, we speculate that blood samples were collected at approximately 2 to 3 h post-prandial that probably represented the absorptive phase.
In the present experiment, pigs fed the xP diet were expected to increase the serum concentration of all AA compared with the CON pigs, but it occurred only in 7 of the 10 indispensable AA. Unexpectedly, serum Ile and Met in the xP pigs did not differ from that of the CON pigs; interactions for absorption between AA may be responsible for this lack of response. Branched-chain AA share the same transport system (B0 AT1) for absorption (Bröer, 2008), and excess Leu negatively affects Ile absorption (Langer and Fuller, 2000; García et al., 2015). Also, excess dietary Leu increases activities of branched-chain keto acid dehydrogenase enzymes in liver (Wiltafsky et al., 2010). Because Ile, Leu, and Val share this enzyme complex in their degradative pathways (Langer and Fuller, 2000), excess Leu results in increased catabolism of all branched-chain AA (Block, 1987). The CON diet contained almost twice as much Leu than Ile, and increasing the protein content in the xP made that difference even larger. This may explain why Ile SC did not increase in pigs fed the xP diet compared with the CON pigs. Met, although with lower specificity, is also absorbed through the B0 AT1 system (Bröer, 2008), competing with branched-chain AA, which might also help to explain why serum Met did not increase in pigs fed the xP diet. The serum concentrations of AA metabolites might also reflect differences in the consumption and serum concentrations of AA. Met is a precursor for the synthesis of homocysteine and Cys (Mato et al., 2002); in turn, Cys is utilized to synthesize glutathione, which is an important cellular antioxidant. Serum homocysteine was higher in pigs fed the xP diet than the CON diet, but the opposite happened with Cys. A reduction in serum Cys may indicate an increased synthesis of glutathione because of the exposure of pigs to HS (Litvak et al., 2013). In combination, the higher serum homocysteine and the lower serum Cys may partially explain why serum Met did not increase in pigs fed the xP diet. Moreover, interaction with other AA for absorption or metabolism of Met in the small intestine may also explain that lack of response. On the other hand, serum ornithine was 33% and 21% higher in pigs fed the xP diet in comparison with pigs fed the CON and xAA diet, respectively. Arg is a precursor for the synthesis of ornithine (Wu et al., 2009); thus, the increased serum ornithine is explained by the higher Arg intake by the xP pigs. These results coincide with reports indicating that intake level and form in which AA are included in the diet affect their serum concentration (Yen et al., 2004; Morales et al., 2016a) and explain the positive correlation between intake and serum concentration of most indispensable AA, except for Ile and Met.
The increased serum concentration of indispensable AA in pigs fed the xAA diet in comparison with the CON diet, including Met, was also expected. The digestion of dietary protein takes place mostly in jejunum and ileum (Leibholz, 1985), but the presence of AA transporters has been documented in duodenum, jejunum, and ileum (Morales et al., 2015); thus, the absorption of free AA can occur along the whole small intestine (Li et al., 2015). On the other hand, competition among AA for transport across the intestine epithelial membrane has been demonstrated for specific groups of AA, including Lys and Arg (Majumder et al., 2009), as well as for Leu, Ile, and Val (Bröer, 2008). Free AA are rapidly absorbed (Morales et al., 2015), whereas protein-bound AA are released later after dietary proteins are digested in jejunum prior to their absorption; hence, we hypothesize that free AA (e.g., Lys, Thr, and Met) do not compete with protein-bound AA for absorption. Thus, absorption of free AA may be more efficient when diets contain a combination of free and protein-bound AA, compared with diets containing only protein-bound AA. This may explain the higher serum concentration of AA in pigs fed the xAA diet. Nonetheless, further studies are needed to demonstrate this hypothesis. The lack of difference in serum Ile between pigs fed the xAA or the CON diet is explained by the elevated content of Leu in both diets. These results are consistent with the lack of correlation between Leu intake and serum Ile.
The form in which extra AA were included (Yen et al., 2004; Morales et al., 2015) as well as the interactions between several AA during absorption and oxidation (Langer and Fuller, 2000; Wiltafsky et al., 2010) may also explain the serum differences between pigs fed the xP or the xAA diet. Serum Ile, Leu, Met, Thr, and Val in pigs fed the xAA diet was 14%, 15%, 24%, 27%, and 12% higher than in pigs fed the xP diet, although their content in both diets was not different or even lower (16%) for Ile in the xAA diet. Total surplus of these AA in the xP diet was bound to protein, but in the xAA diet, 9% (Ile) to 35% (Met) were supplied in free form; these differences may support the hypothesis that absorption of AA in diets containing free AA combined with protein-bound AA are absorbed more efficiently. Regarding interactions among cationic AA, serum Arg did not differ between pigs fed the xP or xAA diet although the xP diet contained 34% more Arg. Moreover, serum Arg in pigs fed the xAA diet was 27% higher than in pigs fed the CON diet although their Arg intake was not different. Arg was supplied only as protein-bound in the CON and xP diets, but the xAA diet contained 56% free Lys. It appears that free Lys was absorbed before Arg was released from dietary proteins; thus, they did not compete each other for absorption in the xAA diet, whereas the opposite might have occurred with protein-bound Arg and Lys in the other 2 diets; this is supported by the positive correlation between Lys intake and serum Arg. The higher intake of free Lys (57%) by pigs fed the xAA diet increased serum Lys by only 7% compared with pigs fed the xP diet. There is no clear explanation for this observation; however, the higher growth rate we reported for the xAA pigs (Morales et al., 2018) suggests that a larger removal of serum Lys might have occurred in pigs fed the xAA diet. Nevertheless, we have to test this hypothesis.
The increased AA intake by pigs fed the xP did not translate into higher growth rate in comparison with the control pigs (Morales et al., 2018). The reduced availability of Ile and Met, as indicated by the unchanged serum concentrations of these AA despite the higher intake of pigs fed the xP diet, may partially explain their lack of growth response. In contrast, the increased intake of free indispensable AA by pigs fed the xAA diet resulted in higher body growth rate and G:F than the CON pigs. Because serum Ile and Met in pigs fed the xAA, in opposition to those fed the xP diet was higher than in pigs fed the CON diet, it seems that these AA limited the growth response of pigs fed the xP diet. In addition, we speculate that pigs fed the xAA diet increased the utilization of serum Lys by augmenting the cellular Lys uptake. Interestingly, the serum concentration of urea was positive and highly correlated with that of Arg and ornithine (urea cycle intermediates) and modestly correlated with serum Lys when the analysis included data from CON and xP pigs. In contrast, when the analysis included data from CON and xAA pigs, only serum Arg was correlated with serum urea, supporting the hypothesis that the xP diet had poor AA balance compared with the xAA diet. Hence, the growth response difference observed between xP and xAA pigs may be explained as follows: 1) xP diet contained 44% excess of total N and approximately 60% of that was dispensable nitrogen, whereas the xAA diet contained 22% excess of total nitrogen and all of that was indispensable; 2) because of the reason mentioned in “1”, the xAA diet was better balanced (Fig. 1) than the xP diet. Those differences might have affected the availability of indispensable AA for growth.
In general, the performance (previously reported) and the serum AA results of the study show that the supplementation of extra AA either in protein-bound or in free form, up to 30% above their required levels, does not affect the voluntary feed intake of HS pigs but differently affect the serum concentration of AA. Extra dietary AA either in free or bound form to intact proteins helps to restore the reduced AA intake observed in the CON pigs. However, extra dietary free AA increase the serum concentration of almost all AA and that increment is larger compared with extra dietary protein-bound AA. Free AA seems to improve the dietary AA balance as indicated by the lower serum urea, which explains the improved growth performance of HS pigs. In conclusion, based on the performance and serum AA data, extra free AA in diets for HS pigs helps to correct the reduced AA availability and growth rate of HS pigs. However, higher levels of specific AA such as Ile and Met might be needed.
Footnotes
The National Science and Technology Council of Méco (CONACYT) is acknowledged for providing scholarships to M. Cház and T. Gó. The authors also thank Evonik Nutrition & Care GmbH for partially funding this project.
LITERATURE CITED
- AOAC 2006. Official methods of analysis. 18th ed. Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- Block K. P., Aftring R. P., Mehard W. B, and Buse M. G.. 1987. Modulation of rat skeletal muscle branched-chain a-keto acid dehydrogenase in vivo: Effects of dietary protein and meal consumption. J. Clin. Invest. 79:1349–1358. doi:10.1172/JCI112961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S. 2008. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 88:249–286. doi:10.1152/physrev.00018.2006 [DOI] [PubMed] [Google Scholar]
- Cervantes M., Ibarra N., Vásquez N., Reyes F., Avelar E., Espinoza S., and Morales A.. 2017. Serum concentrations of free amino acids in growing pigs exposed to diurnal heat stress fluctuations. J. Therm. Biol. 69:69–75. doi:10.1016/j.jtherbio.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Collin A., van Milgen J., Dubois S., and Noblet J.. 2001. Effect of high temperature and feeding level on energy utilization in piglets. J. Anim. Sci. 79:1849–1857. doi:10.2527/2001.7971849x [DOI] [PubMed] [Google Scholar]
- García H., Morales A., Araiza A., Htoo J. K., and Cervantes M.. 2015. Gene expression, serum amino acid levels, and growth performance of pigs fed dietary leucine and lysine at different ratios. Genet. Mol. Res. 14:1589–1601. doi.org/10.4238/2015.August.19.11 [DOI] [PubMed] [Google Scholar]
- Kerr B. J., Yen J. T., Nienaber J. A., and Easter R. A.. 2003. Influences of dietary protein level, amino acid supplementation and environmental temperature on performance, body composition, organ weights and total heat production of growing pigs. J. Anim. Sci. 81:1998–2007. doi:10.2527/2003.8181998x [DOI] [PubMed] [Google Scholar]
- Langer S., and Fuller M. F.. 2000. Interactions among the branched-chain amino acids and their effects on methionine utilization in growing pigs: Effects on nitrogen retention and amino acid utilization. Br. J. Nutr. 83:43–48. doi:10.1017/S0007114500000076 [PubMed] [Google Scholar]
- Leibholz J. 1985. The digestion of protein in young pigs and the utilization of dietary methionine. Br. J. Nutr. 53:137–47. doi:10.1079/BJN19850018 [DOI] [PubMed] [Google Scholar]
- Li G., Li J., Tan B., Wang J., Kong X., Guan G., Li E., and Yin Y.. 2015. Characterization and regulation of the amino acid transporter SNAT2 in the small intestine of piglets. PLoS ONE 10:e0128207. doi:10.1371/journal.pone.0128207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak N., Rakhshandeh A., Htoo J. K., and de Lange C. F. M.. 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 91:4188–4196. doi:10.2527/jas.2012-6160 [DOI] [PubMed] [Google Scholar]
- Majumder M., Yaman I., Gaccioli F., Zeenko V. V., Wang C., Caprara M. G., Venema R. C., Komar A. A., Snider M. D., and Hatzoglou M.. 2009. The hnRNA-binding proteins hnRNP L and PTB are required for efficient translation of the Cat-1 arginine/lysine transporter mRNA during amino acid starvation. Mol. Cell Biol. 29:2899–2912. doi:10.1128/MCB.01774-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato J. M., Corrales F. J., Lu S. C., and Avila M. A.. 2002. S-Adenosylmethionine: A control switch that regulates liver function. FASEB J. 16:15–26. doi:10.1096/fj.01-0401rev [DOI] [PubMed] [Google Scholar]
- Morales A., Buenabad L., Castillo G., Arce N., Araiza B. A., Htoo J. K., and Cervantes M.. 2015. Low-protein amino acid–supplemented diets for growing pigs: Effect on expression of amino acid transporters, serum concentration, performance, and carcass composition. J. Anim. Sci. 93:2154–2164. doi:10.2527/jas.2014-8834 [DOI] [PubMed] [Google Scholar]
- Morales A., Chávez M., Vásquez N., Htoo J. K., Buenabad L., Espinoza S., and Cervantes M.. 2018. Increased dietary protein or free amino acids supply for heat stress pigs: Effect on performance and carcass traits. J. Anim. Sci. 96:1419–1429. doi:10.1093/jas/sky044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A., Cota S. E. M., Ibarra N. O., Arce N., Htoo J. K., and Cervantes M.. 2016a. Effect of heat stress on the serum concentrations of free amino acids and some of their metabolites in growing pigs. J. Anim. Sci. 94:2835–2842. doi:10.2527/jas.2015-0073 [DOI] [PubMed] [Google Scholar]
- Morales A., Grageola F., García H., Arce N., Araiza B., Yáñez J., and Cervantes M.. 2014. Performance, serum amino acid concentrations and expression of selected genes in pair-fed growing pigs exposed to high ambient temperatures. J. Anim. Physiol. Anim. Nutr. 98:928–935. doi:10.1111/jpn.12161 [DOI] [PubMed] [Google Scholar]
- Morales A., Pérez M., Castro P., Ibarra N., Bernal H., Baumgard L. H., and Cervantes M.. 2016b. Heat stress affects the apparent and standardized ileal digestibilities of amino acids in growing pigs. J. Anim. Sci. 94:3362–3369. doi10.2527/jas.2016-0571 [DOI] [PubMed] [Google Scholar]
- Noblet J., Le Bellego L., Van Migen J., and Dubois S.. 2001. Effects of reduced dietary protein level and fat addition on heat production and nitrogen and energy balance in growing pigs. Anim. Res. 50:227–238. [Google Scholar]
- NOM-062-ZOO-1999 2001. Norma Oficial Mexicana. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Ochoa MLI ed.Diario Oficial de la Federación, México (DF), México. [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. edn. National Academy Press, Washington, DC. [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L.. 2013. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 91:2108–2118. doi:10.2527/jas2012-5738 [DOI] [PubMed] [Google Scholar]
- Quiniou N., Noblet J., van Milgen J., and Dubois S.. 2001. Modelling heat production and energy balance in group-housed growing pigs exposed to low or high ambient temperatures. Br. J. Nutr. 85:97–106. doi:10.1079/BJN2000217 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Anais C., Tel L., and Gourdine J. L.. 2010. Effect of temperature on thermal acclimation in growing pigs estimated using a nonlinear function. J. Anim. Sci. 88:3715–3724. doi:10.2527/jas.2009-2169 [DOI] [PubMed] [Google Scholar]
- Reverter M., Lundh T., Gonda H. L., and Lindberg J. E.. 2000. Portal net appearance of amino acids in growing pigs fed a barley-based diet with inclusion of three different forage meals. Br. J. Nutr. 84:483–494. doi:10.1017/S0007114500001793 [PubMed] [Google Scholar]
- Sauvant D., Perez J. M., and Tran. G. (eds.) 2004. Tables of composition and nutritional value of feed materials: pigs, poultry, cattle, sheep, goats, rabbits, horses, fish.Versailles (France): Wageningen Academic Publishers, Wageningen and INRA Editions. [Google Scholar]
- Stein H. H., Kim S. W., Nielsen T. T., and Easter R. A.. 2001. Standardized ileal protein and amino acid digestibility by growing pigs and sows. J. Anim. Sci. 79:2113–2122. [DOI] [PubMed] [Google Scholar]
- Wiltafsky M. K., Pfaff M. W., and Xaver F.. 2010. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 103:964–976. doi:10.1017/S0007114509992212 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Davis T. A., Kim S. W., Li P., Rhoads J. M., Satterfield M. C., Smith S. B., Spencer T. E., and Yin Y.. 2009. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168. doi:10.1007/s00726-008-0210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J. T., Kerr B. J., Easter R. A., and Parkhurst A. M.. 2004. Difference in rates of net portal absorption between crystalline and protein-bound lysine and threonine in growing pigs fed once daily. J. Anim. Sci. 82:1079–1090. doi:2004.8241079x [DOI] [PubMed] [Google Scholar]