Abstract
Two studies were conducted to investigate the effect of live yeast (LY) on the in vitro fermentation characteristics of wheat, barley, corn, soybean meal (SBM), canola meal, and distillers dried grains with solubles (DDGS). In Study 1, LY yeast was added directly to in vitro fermentations inoculated with feces from lactating sows, whereas as in study 2, feces collected from lactating sows fed LY as a daily supplement was used. Selected feedstuffs were digested and the residue added to separate replicated (n = 3) fermentation reactions. Study 1 was conducted in two blocks, whereas study 2 was conducted using feces collected after a period of 3 (Exp. 1) or 4 wk (Exp. 2) of LY supplementation. Accumulated gas produced over 72 h was modeled for each substrate and the kinetics parameters compared between LY and control groups. The molar ratio of the volatile fatty acids (VFAs) produced in vitro were also compared at 12 and 72 h of incubation. In study 1, in vitro addition of yeast increased (P < 0.001) the rate of gas production (Rmax). However, a yeast × substrate effect (P < 0.05) observed for total gas accumulated (A), time to half asymptote (B), and time required to reach maximum rate of fermentation (Tmax) suggested that yeast-mediated increases in extent and rate of fermentation varied by substrate. Greater total gas production was observed only for corn and SBM, associated with greater B and Tmax. Supplementation with LY appeared to increase A and Rmax although with variation between experiments and substrates. In Exp. 1, LY decreased (P < 0.05) B and Tmax. However, a yeast × substrate effect (P < 0.05) was observed for only A (for wheat, barley, corn, and corn DDGS) and Rmax (wheat, barley, corn, and wheat DDGS). In Exp. 2, LY increased (P < 0.0001) A and decreased B. However, an interaction (P < 0.05) with substrates was observed for Rmax (except SBM) and Tmax. With exception of the DDGS samples, LY supplementation increased (P < 0.05) VFA production at 12 and 72 h of incubation. Yeast increased (P < 0.05) the molar ratios of acetic acid and branch-chain fatty acids at 12 h of incubation; however, this response was more variable by substrate at 72 h. In conclusion, LY supplementation increased the rate and extent of in vitro fermentation of a variety of substrates prepared from common feedstuffs. Greater effects were observed when LY was fed to sows than added directly in vitro, suggesting effects on fermentation were not mediated directly.
Keywords: in vitro fermentation, live yeast, pig, supplementation, volatile fatty acid production
INTRODUCTION
Live yeast (LY) probiotics based on Saccharomyces cerevisiae have been reported to improve fiber fermentation, gut health, and performance in several animal species (Broadway et al., 2015; Montes de Oca et al., 2016). However, reports of positive responses to LY supplementation in monogastric animals are limited (Mathew et al., 1998; Paryad and Mahmoudi, 2008), and sometimes, contradictory (Kornegay et al., 1995; Jurgens et al., 1997). These contrasting results could be due to the differing amounts/strains of LY used or differences in dietary composition (Oeztuerk et al., 2005). However, the exact mode of action of LY is still not clear. Thus, it has not been established whether LY supplementation exerts positives effect by influencing gut immunity (Roselli et al., 2017) or indirectly through modification in gut microbiota composition to promote fiber fermentation (Liao and Nyachoti 2017) to generate metabolites including short-chain fatty acids (SCFA) that can promote gut health (Bindelle et al., 2008) and contribute to energy supply (Varel and Yen, 1997, Dierick et al., 1989). In ruminants, LY enhanced fiber fermentation and SCFA production thereby stabilizing rumen pH (Wiedmeier et al., 1987, Fonty and Chaucheyras-Durand, 2006, Desnoyers et al., 2009). These effects of LY were greater in a fibrous diet than a high concentrate diet further confirming the role of LY in enhancing fermentation of fibrous feedstuffs (Opsi et al., 2012). The present study investigated LY effect on fiber fermentation using an in vitro model that simulated the distal gut of swine. The objective was to evaluate the effect of LY added directly to substrates in vitro or fed to lactating sows as a daily supplement on the in vitro fermentation characteristics of selected feed ingredients used for pig feed.
MATERIALS AND METHODS
The experiment was conducted at the Prairie Swine Centre, Inc., Saskatoon, Saskatchewan, Canada. The experimental protocol and procedures involving animals were approved by the University of Saskatchewan’s Animal Research Ethics Board and adhered to the Canadian Council on Animal Care guidelines for humane animal use (CCAC, 2009). All the pigs were PIC genetics (Camborough Plus × C337; PIC Canada Ltd.).
Experimental Design and Animals
Two separate studies were conducted to achieve the objectives of this research. For study 1, an experiment with six replicates conducted in two blocks (three replicates per block) was conducted using fresh feces collected from lactating sows from the herd at Prairie Swine Centre Inc. fed a standard wheat-based diet (Supplementary Table 1). For study 2, the fresh fecal samples used for the in vitro fermentation experiments were obtained from 16 sows belonging to the same herd fed wheat-based diet (Supplementary Table 1) and supplemented with (n = 8) or without (n = 8) a LY probiotic (Actisaf Sc 47 HR+; CNCMI-4407, Phileo Lesaffre Animal Care, France) by top dressing with 1 × 1010 cfu of LY probiotics per kilogram of diet (yeast). Feces were collected directly from the rectum into air-tight 50 mL test tubes after 3 (Exp. 1) or 4 (Exp. 2) wk of yeast supplementation. After collection, fecal samples were transported to the lab under an anaerobic condition using anaerobic jars placed in a cooler box containing warm water (39 °C). A portion of the fecal samples was used to determine LY count by plating serially diluted fecal samples on YGC agar plates.
Substrates and In Vitro Enzymatic Digestion
Seven feed ingredients were used as substrates for the in vitro digestion and fermentation experiments: barley, corn, wheat, canola meal, soybean meal (SBM), distillers dried grains with solubles (DDGS) derived from corn (corn DDGS), and wheat (wheat DDGS). Grass silage was included as a positive control for high fiber ingredients. The samples were subjected to in vitro chemical and enzymatic digestion using porcine pepsin and pancreatic enzymes following a previously described protocol (Boisen and Fernandez, 1997). Briefly, 4 g of each of these ingredients, ground in a laboratory mill (Restch ZM1, Newton, PA) to pass through 1 mm screen were dried and weighed into 1 L conical flasks in triplicates. Subsequently, 200 mL of phosphate buffer (0.1 M, pH 6.0) and 80 mL 0.2 M HCl were added to each flask. After adjusting the pH to 2 using 1 M HCl or NaOH, 4 mL chloramphenicol (Sigma C-0378) solution (0.5 g 100 mL−1 ethanol) and 8 mL of freshly prepared pepsin solution (25 g L−1, porcine pepsin: 2000 FIP-U g−1, Merck no. 7190 in 0.2M HCl) were added to each flask. The flasks were covered with tin foil and placed in a water bath at 39 °C for 2 h under gentle agitation (50 rpm).
Following pepsin hydrolysis, 80 mL of a phosphate buffer solution (0.2 M, pH 6.8) and 40 mL of 0.6 M NaOH solution were added to the solution. The pH was adjusted to 6.8 with 1 M HCl or NaOH and 8 mL of freshly prepared pancreatin solution (100 g pancreatin (Sigma P-1750) L−1 in 0.6 M NaOH) were added to each flask. Thereafter, the flasks were covered with tin foil and hydrolysis was continued for 4 h under the same conditions.
After hydrolysis, the residues were collected by filtration through a nylon bag (42 μm), washed with ethanol (2 × 25 mL 95% ethanol) and acetone (2 × 25 mL 99.5% acetone), dried for 24 h at 60 °C ± 1, and weighed. The dried digested residues from multiple batches were later pooled for in vitro fermentation. The in vitro digestibility of DM (IVDMD) of the substrates was calculated as follows:
Inoculum Preparation and In Vitro Fermentation
To investigate the effect of yeast on in vitro gas production, in vitro fermentation experiments were conducted using sow feces as a source of inoculum. In study 1, yeast was added directly to the fermentation bottles together with the substrates. In study 2, yeast was fed directly to sows and fecal samples taken from sows (control and yeast groups) on weeks 3 and 4 were used for the in vitro fermentation.
The in vitro fermentation procedure followed the protocol previously described for ruminants (Menke and Steingass, 1988) as adapted to the pig (Bindelle et al., 2007a). Briefly, fecal samples, collected and transported as described above, were opened inside an anaerobic chamber and 20 g feces from each of the eight sows in a group were mixed together in a big container. Fecal slurry (10%) was prepared inside the anaerobic chamber by mixing 100 g of the pooled feces with 900 mL of buffer solution composed of macro and micro minerals, carbonate buffer, and reducing solution (Menke and Steingass, 1988), stirred continuously to homogenize and filtered through a double-layered cheese cloth. For the experiment where LY was supplemented in vitro, on two separate occasions, fecal slurries from untreated sows were either left unsupplemented or inoculated with LY to achieve 5 × 106 cfu yeast per milliliter. The amount of LY added to the slurry was determined based on the number of yeast cultured from a gram of cecal content from pigs supplemented with 1010 cfu of LY per kilogram of diet based on our own unpublished data. The in vitro fermentation was conducted in triplicate by adding 30 mL of the prepared fecal slurry into 140 mL serum vials containing 200 mg of the digested substrates for each ingredient. The serum vials were sealed with butyl rubber stoppers and aluminum caps to contain gas pressure produced due to fermentation and incubated at 39 °C incubator inside the anaerobic chamber for 72 h. The experimental scheme was as follows: seven ingredients × two treatment groups × three replicates + three blanks (containing only the inoculum).
Measuring Gas Production and Calculation of Gas Production Kinetics
The head-space gas pressure released due to fermentation of substrates was recorded at 0, 2, 4, 8, 12, 16, 24, 36, 48, and 72 h of incubation by measuring inner pressure of the bottles using a pressure transducer. Gas production (G), measured in pound-force per square inch (psi), was converted into milliliter per gram DM for each time point based on common gas laws. These values were then fitted into a previously described model equation (Groot et al., 1996) shown below to develop gas accumulation curves for each substrate during the 72 h of incubation and to estimate fermentation kinetics parameters.
When t > 0, G (mL g−1 DM) denotes the gas accumulation to a given time; A (mL g−1 DM) represents the maximum gas volume for t = ∞, and B in hours is the time to half asymptote when G = A/2, while C represents a constant determining the slope of the inflexion point of the profile. Two additional parameters were calculated from this equation: Rmax, the maximum rate of gas production (mL g−1 DM × h), when the microbial population no longer limits the fermentation, and Tmax, the time at which Rmax is reached. These kinetics parameters were statistically analyzed to compare the rate of fermentation between different ingredients and treatment groups.
Measuring Volatile Fatty Acids Produced During In Vitro Fermentation
Only samples from fermentation broths obtained from study 2, when sows where fed LY for 3 wk, were used for volatile fatty acid (VFA) determination. One milliliter sample of the fermentation broth taken at 12 and 72 h was mixed with 25% phosphoric acid in 4:1 sample-to-acid ratio and stored in −80 °C until required for analysis. SCFA and branch chain fatty acid (BCFA) were analyzed by gas chromatography (Agilent 6890 series GC system; Agilent Technologies, Santa Clara, CA) fitted with a flame ionization detector and a fused-silica capillary column (ZB-FFAP capillary column; 30 m length × 0.32 mm × 0.25 μm film thickness; ZEBRON, Phenomenex, Torrance, CA) as described by Jha et al. (2011). The amount of each SCFA produced during the in vitro fermentation was expressed as milligram per gram DM by taking into consideration all the dilution factors used during the in vitro fermentation and during gas chromatography analysis.
Statistical Analyses
All statistical analyses were performed using the SAS 9.3 software (SAS Inc., Cary, NC). The DM content and IVDMD of each ingredient were analyzed by one-way ANOVA using the mixed procedure for complete randomized design. For study 1 (yeast added directly to substrates in vitro), the data were combined to obtain six replicates per sample with block as a random effect. For study 2 (sows fed with or without yeast), data for the two experiments were analyzed separately (n = 3) to avoid confounding effects of 3 or 4 wk LY supplementation. Data for the gas production kinetics during in vitro fermentation were analyzed using Proc mixed for factorial treatment structure with yeast and substrate as classification criteria. Similarly, VFA production levels and the molar ratio of the various SCFA were analyzed as a factorial treatment design with time point (12 and 72 h), yeast, and substrate as classification criteria. Data for DM content and IVDMD were reported as means with standard deviation, whereas data for fermentation kinetics and VFA were reported as least square means and pooled SEM. Significance was defined as P < 0.05, and treatments means were separated using the LSD option of SAS.
RESULTS
DM and In Vitro DM Digestibility of Ingredients
The IVDMD varied (P < 0.0001) among the ingredients (Table 1). Wheat and SBM had the highest (P < 0.05) IVDMD, followed by barley and wheat DDGS, whereas corn DDGS had the lowest (P < 0.05) IVDMD as expected.
Table 1.
DM content (%) and in vitro DM digestibility (%) of ingredients used for in vitro fermentation1
| Ingredient | DM | SD | CV | IVDMD | SD | CV |
|---|---|---|---|---|---|---|
| Wheat | 92.28d | 0.25 | 0.27 | 82.83a | 2.15 | 2.60 |
| Barley | 92.53d | 0.24 | 0.26 | 76.76b | 1.50 | 1.96 |
| Corn | 91.56d | 1.02 | 1.12 | 74.77c | 1.21 | 1.62 |
| Soybean meal | 94.58bc | 0.18 | 0.19 | 81.84a | 0.81 | 0.99 |
| Canola | 93.05cd | 0.30 | 0.33 | 63.08d | 0.98 | 1.56 |
| Corn DDGS | 94.36bc | 0.07 | 0.07 | 55.13f | 0.63 | 1.135 |
| Wheat DDGS | 95.22ab | 2.61 | 2.74 | 77.03b | 0.69 | 0.90 |
| Grass | 96.75a | 0.19 | 0.19 | 57.11e | 0.96 | 1.68 |
| SEM | 0.53 | 0.41 | ||||
| P-value | <.0001 | <.0001 |
a–fWithin a variable, means with different superscripts differ (P < 0.05).
CV (%) = percent coefficient of variation; IVDMD=in vitro DM digestibility; DDGS = distillers dried grains with solubles.
Yeast Count in Sow Feces
Unpublished data from our laboratory suggest that environmental yeast shedding in nonyeast supplemented pigs can reach 103 cfu g−1 of feces. Therefore, we investigated the number of yeast shedding in sows fed diets supplemented without or with LY. Fecal yeast shedding (log cfu/g of feces) did not differ between the two groups of sows at the start of LY supplementation (1.53 vs. 1.76; control vs. supplemented). However, by week 3 of supplementation, fecal yeast shedding increased (P < 0.05) by more than 100 fold in the LY-supplemented sows (3.77) but not for the control sows (1.43).
In Vitro Gas Production
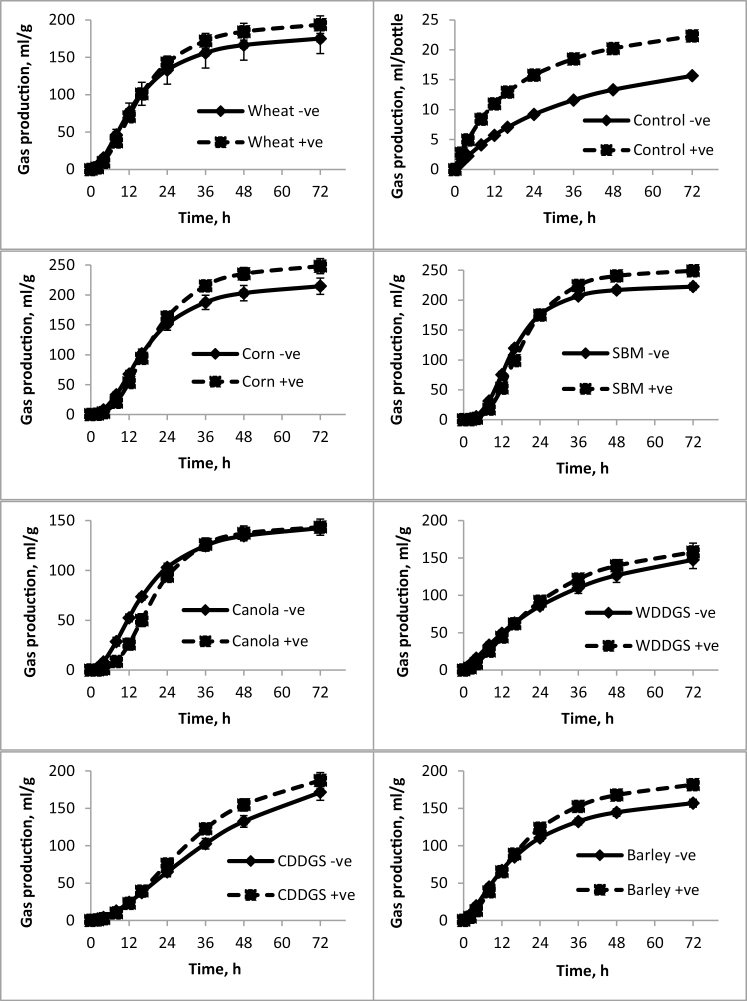
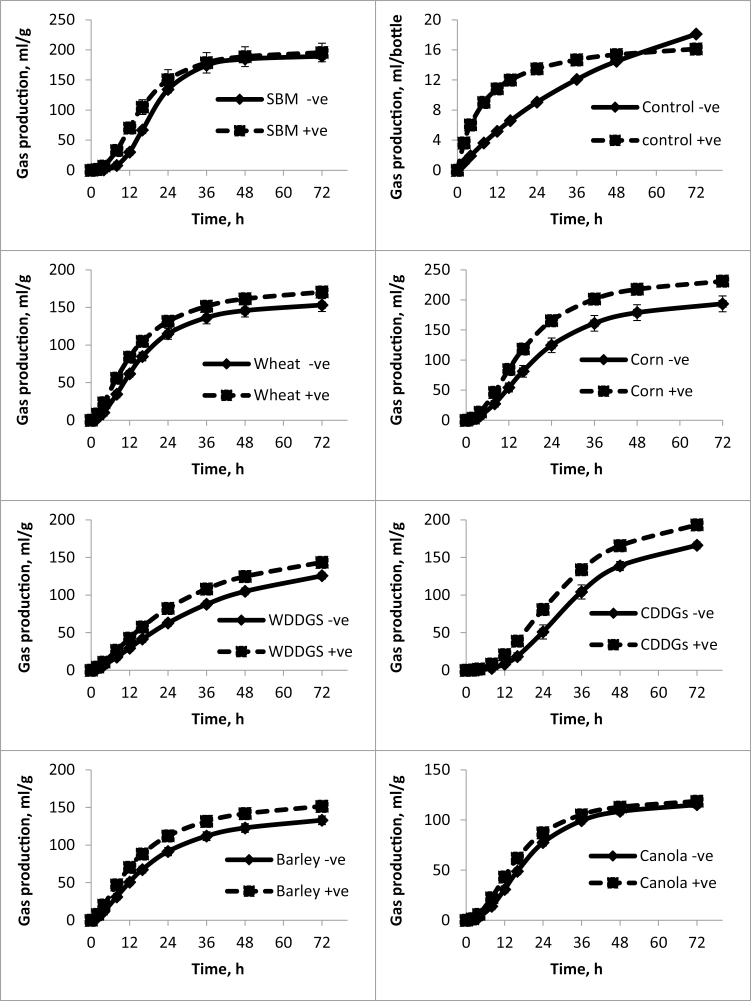
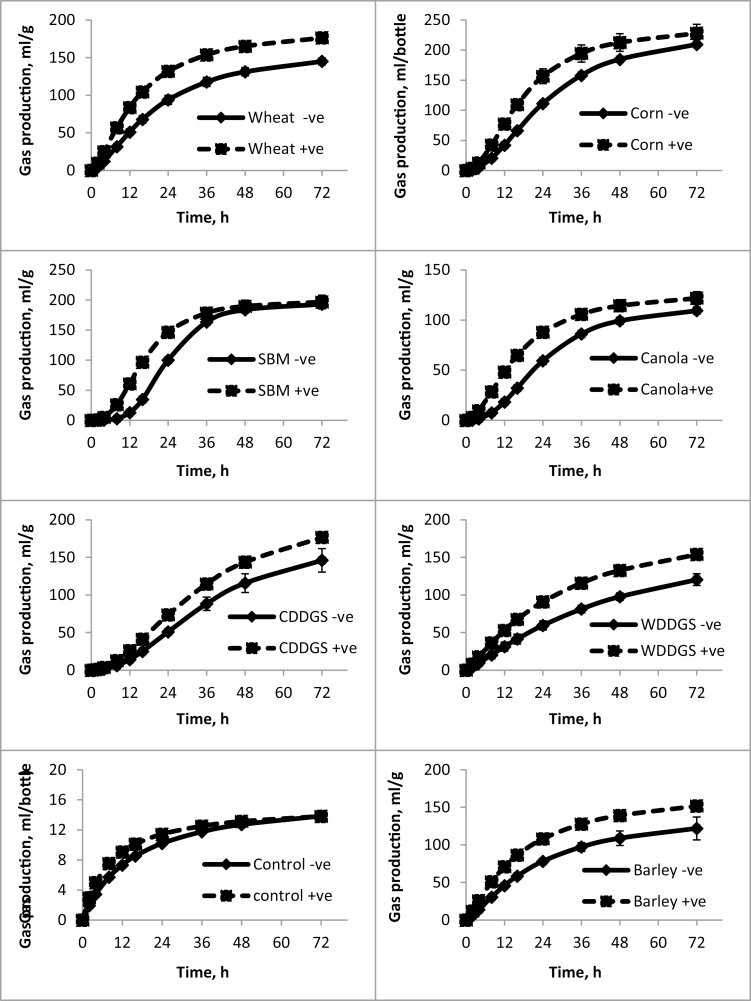
The rate and extent of fermentation (Tables 2–4) of selected substrate residues (i.e., following in vitro digestion and filtration) among all experiments conducted were similar such that the highest gas volumes were observed for corn, SBM, and corn DDGS, whereas the lowest gas volumes were observed for canola. Maximum fermentation rate was highest for corn and SBM and lowest for the DDGS (corn and wheat). Interestingly, fitted gas accumulation curves (Figures 1–3) illustrated that gas production for corn and wheat DDGS did not plateau during the 72 h incubation period and may therefore not have been complete.
Table 2.
Gas production kinetics parameters for the different substrates following addition of yeast in vitro1,2
| Item3 | A | B | R max | T max | |
|---|---|---|---|---|---|
| Yeast | No | 200.96 | 22.05 | 7.00b | 9.94b |
| Yes | 208.84 | 21.14 | 7.60a | 13.47a | |
| SEM | 3.34 | 0.33 | 0.03 | 0.22 | |
| Substrate | Wheat | 195.45b | 15.53d | 8.25c | 8.70c |
| Barley | 186.59b | 17.39c | 6.62d | 6.93d | |
| Corn | 239.56a | 18.38c | 9.62b | 13.04b | |
| Soybean meal | 239.69a | 16.91cd | 11.76a | 13.28b | |
| Canola | 147.97c | 17.91c | 6.24d | 12.64b | |
| Wheat DDGS | 191.87b | 27.09b | 4.59e | 7.28d | |
| Corn DDGS | 233.15a | 37.95a | 4.03e | 20.09a | |
| SEM | 6.26 | 0.62 | 0.20 | 0.41 | |
| Wheat | No | 190.16f | 15.18g | 7.92 | 7.34f |
| Yes | 200.75cdef | 15.88fg | 8.57 | 10.07cde | |
| Barley | No | 175.61f | 16.76efg | 6.41 | 5.29g |
| Yes | 197.57ef | 18.03def | 6.83 | 8.57ef | |
| Corn | No | 223.07bcde | 17.30defg | 8.95 | 11.43c |
| Yes | 256.06a | 19.44d | 10.29 | 14.64b | |
| Soybean meal | No | 226.24bc | 15.37g | 11.72 | 11.72c |
| Yes | 253.14a | 18.45de | 11.80 | 14.84b | |
| Canola | No | 149.23g | 16.23efg | 6.09 | 9.60de |
| Yes | 146.71g | 19.60d | 6.40 | 15.68b | |
| Wheat DDGS | No | 199.36def | 30.26b | 4.43 | 4.29g |
| Yes | 184.39f | 23.93c | 4.75 | 10.27cd | |
| Corn DDGS | No | 243.05ab | 43.24a | 3.48 | 19.94a |
| Yes | 223.25bcd | 32.65b | 4.58 | 20.24a | |
| SEM | 8.84 | 0.87 | 0.29 | 0.58 | |
| P-values | |||||
| Yeast | 0.107 | 0.0614 | 0.001 | <.0001 | |
| Substrate | <.0001 | <.0001 | <.0001 | <.0001 | |
| Yeast × substrate | 0.031 | <.0001 | 0.311 | 0.0004 |
a–gWithin a variable and fixed effect, means with different superscripts differ (P < 0.05).
1Data are from Study 1 whereby the yeast probiotic was added directly in vitro. No = yeast not added; Yes = yeast added.
2 A = total gas production (mL/g DM); B = time to half asymptote (h); Rmax = rate of gas production (mL g-1 DM h-1); and Tmax = time required to reach maximum rate of fermentation (h).
3DDGS = distillers dried grains with solubles.
Figure 1.
Fitted gas accumulation curves modeled using a previously described mathematical formula (Groot et al., 1996) that show the amount of gas produced in milliliter per gram of DM and accumulated during the indicated time period in Study 1 when yeast was added in vitro. Values represent mean (n = 6) and SD of the mean. +ve means yeast was added and –ve means no yeast added.
Figure 2.
Fitted gas accumulation curves modeled using a previously described mathematical formula (Groot et al., 1996) which show the amount of gas produced in milliliter per gram of DM and accumulated during the indicated time. Data for Study 2, Exp. 1 when yeast was fed to sows for 3 wk. Values represent mean (n = 3) and SD of the mean. +ve means yeast was added and –ve means no yeast added.
Figure 3.
Fitted gas accumulation curves modeled using a previously described mathematical formula (Groot et al., 1996) which show the amount of gas produced in milliliter per gram of DM and accumulated during the indicated time. Data for Study 2, Exp. 2 when yeast was fed to sows for 4 wk. Values represent mean (n = 3) and SD of the mean. +ve means yeast was added and –ve means no yeast added.
In study 1, when yeast was added directly to the in vitro incubation medium containing fecal slurry from nontreated sows and without addition of substrate, the rate and extent of gas fermentation was increased (Figure 1, upper left panel). This posed a challenge as fermentation parameters determined without addition substrates are subtracted from fermentation parameters determined when substrate is added. Thus, results reported in Table 2 represent the net effect of yeast when substrate was present accounting for the large increase in fermentation observed without additional substrate.
For experiments examining the in vitro supplementation of LY (study 1), the effect of yeast on the final gas volume (A) was not consistent among the different feed ingredients tested (Table 2 and Figure 1) as indicated by a yeast × substrate effect (P = 0.031). Yeast increased (P < 0.05) A for corn and SBM and tended to increase (P < 0.090) for barley. Yeast also increased (P = 0.001) the rate of gas production (Rmax) for all the ingredients tested. However, a yeast × substrate effect (P < 0.0001) on the time to half asymptote (B) was observed such that yeast shortened B for wheat DDGS (from 43.2 to 32.7) and corn DDGS (from 30.3 to 23.9 h) but increased B for SBM (from 15.4 to 18.5 h) and canola meal (from 16.2 to 19.6 h) (Table 2). Finally, a yeast × substrate effect (P < 0.001) was observed for time to reach maximum fermentation rate (Tmax) such that Tmax was increased for all substrates with the exception of corn DDGS.
In study 2, fecal slurries incubated without substrate from yeast-fed sows tended to produce a higher volume of gas than from the control sows (Figures 2 and 3). Generally, LY supplementation increased gas accumulation for all added substrates even after correction for gas accumulation at each time point without added substrate (Figures 2 and 3). Furthermore, results were similar for fecal slurries harvested after 3 (Table 3) or 4 (Table 4) wk of in vivo LY supplementation. The greatest responses observed for LY supplementation occurred for the fermentation of corn, followed by corn DDGS in Exp. 1 (Figure 2), and wheat followed by wheat DDGs in Exp. 2 (Figure 3). Overall, statistical analyses on the fermentation kinetics parameters for Exps. 1 (Table 3) and 2 (Table 4) revealed a positive effect of yeast on increasing A and Rmax (P < 0.0001). Yeast also decreased (P < 0.0001) B and shortened (P < 0.0001) Tmax. Combined, yeast, on average, increased the A from 171.7 to 193 mL g−1 DM and Rmax from 5.4 to 6.9 mL g−1 DM h−1, while decreasing the Tmax from 13.6 to 9.1 h and shortening B from 25.3 to 19.6 h.
Table 3.
Gas production kinetics parameters for the different substrates when yeast was directly fed to sows for 3 wk1,2
| Item3 | A | B | R max | T max | |
|---|---|---|---|---|---|
| Yeast | No | 167.15b | 22.45a | 5.91b | 13.60a |
| Yes | 186.46a | 18.81b | 7.03a | 10.00b | |
| SEM | 2.02 | 0.46 | 0.12 | 0.25 | |
| Substrate | Wheat | 170.48c | 14.14c | 7.67b | 7.13e |
| Barley | 155.31d | 16.12bc | 5.98c | 6.39e | |
| Corn | 224.29a | 18.09b | 8.30b | 10.82cd | |
| Soybean meal | 195.49b | 17.19b | 9.77a | 13.60b | |
| Canola | 121.86e | 17.40b | 4.92d | 11.53c | |
| Wheat DDGS | 172.44c | 30.48a | 3.58e | 9.67d | |
| Corn DDGS | 197.79b | 31.00a | 5.08d | 23.51a | |
| SEM | 3.78 | 0.86 | 0.22 | 0.49 | |
| Wheat | No | 159.45gh | 15.07 | 6.93c | 8.88 |
| Yes | 181.52de | 13.21 | 8.40b | 5.38 | |
| Barley | No | 145.47h | 17.54 | 5.09d | 7.64 |
| Yes | 165.14fg | 14.70 | 6.87c | 5.15 | |
| Corn | No | 205.3bc | 19.71 | 6.96c | 12.09 |
| Yes | 243.28a | 16.47 | 9.63a | 9.54 | |
| Soybean meal | No | 191.04cd | 18.99 | 9.93a | 16.28 |
| Yes | 199.94bc | 15.39 | 9.61a | 10.92 | |
| Canola | No | 120.89i | 18.89 | 4.59de | 12.92 |
| Yes | 122.83i | 15.91 | 5.26d | 10.14 | |
| Wheat DDGS | No | 167.36efg | 34.35 | 3.07f | 11.12 |
| Yes | 177.53def | 26.61 | 4.09e | 8.22 | |
| Corn DDGS | No | 180.55def | 32.61 | 4.79de | 26.27 |
| Yes | 215.02b | 29.38 | 5.36d | 20.75 | |
| SEM | 5.34 | 1.22 | 0.31 | 0.66 | |
| P-values | |||||
| Yeast | <.0001 | <.0001 | <.0001 | <.0001 | |
| Substrate | <.0001 | <.0001 | <.0001 | <.0001 | |
| Yeast × substrate | 0.017 | 0.335 | 0.002 | 0.108 |
a–iWithin a variable and fixed effect, means with different superscripts differ (P < 0.05).
1No = yeast not added; Yes = yeast added.
2 A = total gas production (mL/g DM); B = time to half asymptote (h); Rmax = rate of gas production (mL g−1 DM h−1); and Tmax = time required to reach maximum rate of fermentation (h).
3DDGS = distillers dried grains with solubles.
Table 4.
Gas production kinetics parameters for the different substrates when yeast was directly fed to sows for 4 wk1,2
| Item3 | A | B | R max | T max | |
|---|---|---|---|---|---|
| Yeast | No | 176.23b | 28.23a | 4.84b | 13.68a |
| Yes | 199.54a | 20.36b | 6.84a | 8.17b | |
| SEM | 3.29 | 0.78 | 0.09 | 0.29 | |
| Substrate | Wheat | 178.35c | 17.20b | 6.62c | 6.26d |
| Barley | 162.59c | 19.49b | 5.63d | 4.00e | |
| Corn | 242.57a | 21.37b | 7.57b | 12.07c | |
| Soybean meal | 204.87b | 19.99b | 8.91a | 15.82b | |
| Canola | 123.47d | 19.80b | 4.35e | 12.37c | |
| Wheat DDGS | 197.15b | 36.81a | 3.84f | 5.66d | |
| Corn DDGS | 206.2b | 35.41a | 3.94ef | 20.28a | |
| SEM | 6.16 | 1.45 | 0.17 | 0.55 | |
| Wheat | No | 164.9 | 20.18 | 5.01e | 7.63f |
| Yes | 191.8 | 14.22 | 8.22b | 4.89 gh | |
| Barley | No | 149.0 | 22.4 | 4.28fg | 5.23 g |
| Yes | 176.1 | 16.53 | 6.99c | 2.77 h | |
| Corn | No | 233.89 | 25.18 | 6.11d | 14.97 d |
| Yes | 251.26 | 17.56 | 9.03a | 9.17 ef | |
| Soybean meal | No | 196.32 | 23.67 | 8.60ab | 20.64 b |
| Yes | 213.42 | 16.31 | 9.23a | 10.99 e | |
| Canola | No | 117.19 | 23.58 | 3.62gh | 16.56 cd |
| Yes | 129.75 | 16.02 | 5.08e | 8.18 f | |
| Wheat DDGS | No | 186.92 | 44.07 | 2.86i | 7.53 f |
| Yes | 207.39 | 29.55 | 4.82ef | 3.79 gh | |
| Corn DDGS | No | 185.3 | 38.50 | 3.37hi | 23.18 a |
| Yes | 227.1 | 32.33 | 4.51ef | 17.38 c | |
| SEM | 8.71 | 2.05 | 0.24 | 0.77 | |
| P-values | |||||
| Yeast | <.0001 | <.0001 | <.0001 | <.0001 | |
| Substrate | <.0001 | <.0001 | <.0001 | <.0001 | |
| Yeast × substrate | 0.711 | 0.393 | <.0001 | 0.0003 |
a–iWithin a variable and fixed effect, means with different superscripts differ (P < 0.05).
1No = yeast not added; Yes = yeast added.
2 A = total gas production (mL/g DM); B = time to half asymptote (h); Rmax = rate of gas production (mL g−1 DM h−1); and Tmax = time required to reach maximum rate of fermentation (h).
3DDGS = distillers dried grains with solubles.
For study 2, there was a yeast × substrate effect (P < 0.05) on A in Exp. 1, such that yeast increased A for corn, corn DDGS, barley, and wheat only. However, there was no yeast × substrate effect on the A in Exp. 2 (Table 4). Further, there was a yeast × substrate effect (P < 0.05) on Rmax in Exp. 1 (P < 0.01) and Exp. 2 (P < 0.0001), the highest effect being on corn. In Exp. 1, yeast decreased (P < 0.05) the B and shortened Tmax for all substrates tested (Table 3). Similarly, yeast decreased (P < 0.05) the B and Tmax for all substrates tested in Exp. 2, but a yeast × substrate effect (P < 0.001) for Tmax indicated that the yeast-mediated decrease was greater for some substrates (Table 4).
Volatile Fatty Acid Production
Data on VFA production were reported for only study 2 when sows were fed yeast for 3 wk. There were a number of significant yeast × substrate and yeast × substrate × time effects (P < 0.05) observed for total VFA production and molar ratios (Table 5). Generally, yeast supplementation appeared to increase the total VFA production with the greatest effects observed for wheat and SBM at 12 h, and for corn at 72 h of incubation. The molar ratios of acetic acid and the BCFA appeared to increase when yeast was supplemented at 12 h but this effect was less apparent at 72 h of incubation. In the case of corn, the molar ratio of acetic acid increased with yeast supplementation at both 12 and 72 h, whereas the molar ratio of propionic acid decreased with yeast supplementation at both 12 and 72 h. For SBM, there were no differences for the molar ratios of acetic and propionic acids at 12 h. However, the molar ratio of acetic increased, whereas that of propionic decreased at 72 h with yeast supplementation. Taken together, the highest effect of yeast supplementation was observed for corn fermentation; total VFA production increased from 183.7 to 223.6 mg g−1 DM and from 358 to 446.3 mg g−1 DM at 12 and 72 h of incubation, respectively. Yeast also increased the molar ratios of acetic acid from 58.3% to 60.2% and from 61.8% to 64.6% at 12 and 72 h, respectively.
Table 5.
Total volatile fatty acid (VFA) production (mg/g DM) and molar ratio (%) of the three main VFA and branch chain fatty acid (BCFA) after 12 and 72 h of in vitro fermentation of different ingredients with fecal material collected from control sows or yeast fed sows1,2
| Total VFA | Acetic | Propionic | Butyric | BCFA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Substrate | Time, h | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes |
| Wheat | 12 | 197.6 | 284.1 | 62.5 | 67.6 | 22.4 | 19.4 | 14.1 | 10.7 | 1.0 | 2.3 |
| Barley | 185.7 | 215.6 | 62.3 | 67.0 | 24.0 | 20.6 | 12.2 | 10.0 | 1.5 | 2.4 | |
| Corn | 183.7 | 223.6 | 58.3 | 60.2 | 20.6 | 19.5 | 19.6 | 18.8 | 1.6 | 1.6 | |
| Soybean meal | 176.0 | 233.3 | 64.0 | 63.4 | 27.8 | 27.8 | 6.2 | 6.7 | 2.0 | 2.2 | |
| Canola | 150.2 | 159.4 | 71.4 | 73.1 | 22.2 | 20.3 | 5.3 | 4.6 | 1.1 | 2.0 | |
| Wheat DDGS | 133.1 | 134.7 | 67.0 | 69.5 | 23.2 | 21.4 | 9.3 | 8.1 | 0.5 | 1.1 | |
| Corn DDGS | 124.4 | 110.1 | 67.1 | 66.9 | 21.2 | 21.4 | 6.8 | 6.7 | 4.9 | 4.9 | |
| Wheat | 72 | 280.6 | 296.9 | 65.5 | 63.9 | 19.9 | 21.6 | 9.7 | 11.1 | 4.9 | 3.4 |
| Barley | 272.9 | 309.9 | 68.3 | 65.0 | 18.3 | 20.7 | 8.1 | 9.7 | 5.3 | 4.6 | |
| Corn | 358.0 | 446.3 | 61.8 | 64.6 | 21.6 | 19.0 | 12.5 | 13.0 | 4.2 | 3.5 | |
| Soybean meal | 394.6 | 405.9 | 64.4 | 67.4 | 23.5 | 21.0 | 6.7 | 6.5 | 5.4 | 5.2 | |
| Canola | 247.1 | 272.8 | 74.4 | 71.6 | 15.1 | 16.7 | 5.5 | 6.7 | 5.0 | 5.1 | |
| Wheat DDGS | 289.7 | 325.7 | 77.8 | 75.8 | 12.5 | 13.9 | 6.0 | 6.4 | 3.6 | 3.9 | |
| Corn DDGS | 436.5 | 434.6 | 79.6 | 75.7 | 10.4 | 12.7 | 5.8 | 7.0 | 4.2 | 4.5 | |
| SEM | 15.562 | 0.415 | 0.333 | 0.162 | 0.227 | ||||||
| P-values | |||||||||||
| Yeast | <.0001 | 0.002 | 0.002 | 0.035 | 0.261 | ||||||
| Substrate | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | ||||||
| Time | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | ||||||
| Yeast × substrate | 0.044 | <.0001 | <.0001 | <.0001 | 0.129 | ||||||
| Yeast × time | 0.975 | <.0001 | <.0001 | <.0001 | <.0001 | ||||||
| Substrate × time | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | ||||||
| Yeast × subs × time | 0.097 | <.0001 | <.0001 | <.0001 | 0.0003 |
1Data is from Study 2 when sows were fed diets with or without probiotic supplementation for 3 wk. No = yeast not added; Yes = yeast added.
2BCFA = branch chain fatty acid; DDGS = distillers dried grains with solubles.
DISCUSSION
In ruminants, there is considerable evidence in the literature suggesting that yeast supplementation improves fiber fermentation and stabilizes rumen pH (Desnoyers et al., 2009). Studies reporting on the effect of yeast supplementation in monogastric animals are, however, limited and sometimes contradictory. It has been suggested that the differing yeast amounts/strains used, physiological stage of the animals or differences in the dietary composition is likely the cause (Oeztuerk et al., 2015). Nonetheless, establishing the mode of action of yeast, whether mediated by direct immune stimulation or enhanced fiber fermentation will be critical in understanding conditions under which LY supplementation could result in positive health and performance responses. Here we present data from in vitro fermentation studies designed to determine whether increased rate and or extent of fiber fermentation and SCFA production contributes to performance benefits when LY is supplemented in swine diets.
Despite the overall positive effect of LY on fermentation observed in our studies, LY, when added directly to the substrates in vitro, did not seem to have significant positive effects on the extent and rate of fermentation for most of the feed ingredients tested. However, for corn, the final gas volume and the rate of fermentation significantly increased when LY was added directly in vitro. The LY added in vitro was not expected to modify the fecal microbiota within the short period of incubation to influence the fermentation process, but may act as an additional source of nutrients and vitamins for the microbiota growth and multiplication and hence indirectly enhance fermentation. It is noteworthy that LY alone with no substrate added (yeast-positive blank) produced a significantly higher volume of gas than the LY-negative blanks which was similar in volume to previous reports (Bindelle et al., 2007b). Since the volume of gas produced in the vessels without added substrate (blank) must be subtracted from the volume of gas produced in test vessels, subtraction of the higher gas volumes from yeast-supplemented vessels might have partially masked the positive effect of yeast compared with the subtraction of lower blank values or nonsupplemented test vessels.
In general, the cereal grains (wheat, barley, and corn) seem to be readily fermentable substrates than the corn and wheat DDGS samples, owing to relatively shorter time to half asymptote and enhanced rate of fermentation. This is in agreement with previous findings (Bindelle et al., 2007b, 2011) reporting a relatively slower fermentation rate in feed ingredients with high fiber contents, especially those rich in insoluble fiber. In addition, our data suggest that addition of yeast directly to diet further enhances both the extent and rate of fermentation for the feed ingredients tested compared with the control diet with the highest effect observed on corn and wheat. The possible mode of action is hypothesized to be due to the ability of yeast to reduce the redox potential both in vivo and in vitro (Newbold et al., 1995) thereby promoting the growth of anaerobic bacteria which enhance fiber fermentation. The DDGS contained greater amounts of dietary fiber than the cereal grains, which could be fermented in the presence of yeast to produce more gas. However, the final gas volume produced at the end of 72 h incubation by the DDGS was not different from the gas volume produced by the other ingredients. This could be due partly to the slow fermentation rate observed at the beginning of incubation, especially for corn DDGS, and the fact that fermentation was not yet at its maximum level at 72 h for the DDGS. Indeed, the gas accumulation curves for wheat and corn DDGS did not plateau, suggesting that fermentation and gas production was still going on for those ingredients at 72 h of incubation. Furthermore, the delayed lag phase observed on the DDGS, especially corn DDGS, could be due to the high content of less fermentable fiber in the DDGS compared with the cereal grains.
In vitro fermentation of substrates using feces from LY-supplemented sows also influenced the molar ratio of the individual SCFA. Consistent with previous studies in ruminants (Chaucheyras-Durand and Fonty, 2002), yeast increased the molar ratio of acetic acid, while decreasing that of propionic and butyric acids at 12 h. The type of ingredient and the composition of the fecal microbiota can affect the proportion of each of the SCFA. Fermentation of starch produces a high concentration of butyrate with a relatively low level of acetate; very high concentration of acetate and an almost negligible amount of butyrate was reported from pectin and xylan fermentation (Macfarlane and Macfarlane, 1993). This may explain the relatively higher proportion of acetate observed in the ingredients expected to contain more fiber (canola meal, wheat DDGS, and corn DDGS) compared with the cereal grains (barley, wheat, and corn). On the other hand, the molar ratio of butyric acid was relatively higher in the cereal grains, with the highest proportion observed for corn. This may reflect increased residual starch content following in vitro digestion and filtration of corn and possibly barley and wheat. The molar ratio of acetic acid for the ingredients was higher at 72 h than at 12 h, and the highest difference between the time points was observed for the DDGS samples indicating a slower rate of fermentation in ingredients high fiber content. In contrast, the proportion of butyric acid was lower at 72 h than 12 h, which may be due to decreased fermentable starch content in the later stage of the fermentation process.
An interesting observation was the significant decrease in the molar ratios of BCFA (valeric, isovaleric, and isobutyric) observed for barley, wheat, and corn at 72 h of fermentation with LY compared with the control, suggesting supplementing LY to pigs may reduce protein fermentation in the large intestine by favoring the growth of fiber fermenting bacteria in the hindgut. This may, in turn, suggest that LY supplementation may reduce the protein fermentation and hence the concentration of protein metabolites, such as ammonia, hydrogen sulfide, phenolic compounds, and amines known to negatively affect animal health.
In conclusion, LY increased the fermentation of the feed ingredients tested in the present study and greater effects were observed when LY was fed directly to sows relative to when added directly to the substrates in vitro. Therefore, the present study suggests that supplementing sows daily with an LY probiotic may modify hindgut microbial composition by creating an environment favorable for fiber fermenting bacteria and thereby enhance both the extent and rate of fermentation of various feed ingredients. Further studies are warranted to elucidate the mechanism involved and how this may influence sow productivity. Further, the maximum effect of LY on fermentation kinetics was observed for corn, suggesting that corn-based diets supplemented with LY could have positive effects for pigs. Live yeast supplementation may also decrease protein fermentation in the pig hindgut but this needs to be investigated further.
Conflict of interest statement. None declared.
Supplementary Material
LITERATURE CITED
- Bindelle J., Buldgen A., Boudry C., and Leterme P.. 2007a. Effect of inoculum and pepsin-pancreatin hydrolysis on fibre fermentation measured by the gas production technique in pigs. Anim. Feed Sci. Technol. 132:111–122. doi:10.1016/j.anifeedsci.2006.03.009 [Google Scholar]
- Bindelle J., Buldgen A., Lambotte D., Wavreille J., and Leterme P.. 2007b. Effect of pig faecal donor and of pig diet composition on in vitro fermentation of sugar beet pulp. Anim. Feed Sci. Technol. 132:212–226. doi:10.1016/j.anifeedsci.2006.03.010 [Google Scholar]
- Bindelle J., Leterme P., and Buldgen A.. 2008. Nutritional and environmental consequences of dietary fibre in pig nutrition: a review. Biotechnol. Agron. Soc. Environ. 12:69–80. [Google Scholar]
- Bindelle J., Pieper R., Montoya C. A., Van Kessel A. G., and Leterme P.. 2011. Nonstarch polysaccharide-degrading enzymes alter the microbial community and the fermentation patterns of barley cultivars and wheat products in an in vitro model of the porcine gastrointestinal tract. FEMS Microbiol. Ecol. 76:553–563. doi:10.1111/j.1574-6941.2011.01074.x [DOI] [PubMed] [Google Scholar]
- Boisen S. and Fernandez J. A.. 1997. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 68:277–286. [Google Scholar]
- Broadway P. R., Carroll J. A., and Sanchez N. C.. 2015. Live yeast and yeast cell wall supplements enhance immune function and performance in food-producing livestock: a review. Microorganisms. 3:417–427. doi:10.3390/microorganisms3030417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCAC 2009. Guidelines on: The care and use of farm animals in research, teaching and testing. Ottawa, ON, Canada: Canadian Council on Animal Care. [Google Scholar]
- Chaucheyras-Durand F. and Fonty G.. 2002. Influence of a probiotic yeast (Saccharomyces cerevisiae CNCM i-1077) on microbial colonization and fermentations in the rumen of newborn lambs. Microbial. Ecol. Health Dis. 14:30–36. doi:10.1080/089106002760002739 [Google Scholar]
- Desnoyers M., Giger-Reverdin S., Bertin G., Duvaux-Ponter C., and Sauvant D.. 2009. Meta-analysis of the influence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk production of ruminants. J. Dairy Sci. 92:1620–1632. doi:10.3168/jds.2008-1414 [DOI] [PubMed] [Google Scholar]
- Dierick N. A., Vervaeke I. J., Demeyer D. I., and Decuypere J. A.. 1989. Approach to the energetic importance of fiber digestion in pigs .1. Importance of fermentation in the overall energy supply. Anim. Feed Sci. Technol. 23:141–167. doi:10.1016/0377-8401(89)90095-3 [Google Scholar]
- Fonty G. and Chaucheyras-Durand F.. 2006. Effects and modes of action of live yeasts in the rumen. Biologia. 61:741–750. doi:10.2478/s11756-006-0151-4 [Google Scholar]
- Groot J. C. J., Cone J. W., Williams B. A., Debersaques F. M., and Lantinga E. A.. 1996. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 64:77–89. doi:10.1016/S0377-8401(96)01012-7 [Google Scholar]
- Jha R., Bindelle J., Van Kessel A. G., and Leterme P.. 2011. In vitro fibre fermentation of feed ingredients with varying fermentable carbohydrate and protein levels and protein synthesis by colonic bacteria isolated from pigs. Anim. Feed Sci. Technol. 165:191–200. doi:10.1016/j.anifeedsci.2010.10.002 [Google Scholar]
- Jurgens M. H., Rikabi R. A., and Zimmerman D. R.. 1997. The effect of dietary active dry yeast supplement on performance of sows during gestation-lactation and their pigs. J. Anim. Sci. 75:593–597. doi:10.2527/1997.753593x [DOI] [PubMed] [Google Scholar]
- Kornegay E. T., Rhein-Welker D., Lindemann M. D., and Wood C. M.. 1995. Performance and nutrient digestibility in weanling pigs as influenced by yeast culture additions to starter diets containing dried whey or one of two fiber sources. J. Anim. Sci. 73:1381–1389. doi:10.2527/1995.7351381x [DOI] [PubMed] [Google Scholar]
- Liao S. F., and Nyachoti M.. 2017. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 3:331–343. doi:10.1016/j.aninu.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G. T., and Macfarlane S.. 1993. Factors affecting fermentation reactions in the large bowel. Proc. Nutr. Soc. 52:367–373. doi:10.1079/PNS19930072 [DOI] [PubMed] [Google Scholar]
- Mathew A. G., Chattin S. E., Robbins C. M., and Golden D. A.. 1998. Effects of a direct-fed yeast culture on enteric microbial populations, fermentation acids, and performance of weanling pigs. J. Anim. Sci. 76:2138–2145. doi:10.2527/1998.7682138x [DOI] [PubMed] [Google Scholar]
- Menke K. H. and Steingass H.. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28: 7–55. [Google Scholar]
- Montes de Oca R., Salem A. Z. M., Kholif A. E., Fernandez P., Zamora J. L., Monroy H., Perez L. S., and Acosta J.. 2016. Mode of action of yeast in animal nutrition. In: Salem A. Z. M., and Puniya A. K., editors, Yeast additive and animal production, PubBioMed Central Research Publishing Service, India: p. 14–20. [Google Scholar]
- Newbold C. J., Wallace R. J., Chen X. B., and McIntosh F. M.. 1995. Different strains of Saccharomyces cerevisiae differ in their effects on ruminal bacterial numbers in vitro and in sheep. J. Anim. Sci. 73:1811–1818. doi:10.2527/1995.7361811x [DOI] [PubMed] [Google Scholar]
- Oeztuerk H., Schroeder B., Beyerbach M., and Breves G.. 2005. Influence of living and autoclaved yeasts of saccharomyces boulardii on in vitro ruminal microbial metabolism. J. Dairy Sci. 88:2594–2600. doi:10.3168/jds.S0022-0302(05)72935-0 [DOI] [PubMed] [Google Scholar]
- Opsi F., Fortina R., Tassone S., Bodas R., and Lopez S.. 2012. Effects of inactivated and live cells of Saccharomyces cerevisiae on in vitro ruminal fermentation of diets with different forage: concentrate ratio. J. Agric. Sci. 150:271–283. doi:10.1017/S0021859611000578 [Google Scholar]
- Paryad A. and Mahmoudi M.. 2008. Effect of different levels of supplemental yeast (Saccharomyces cerevisiae) on performance, blood constituents and carcass characteristics of broiler chicks. Afr. J. Agric. Res. 3:835–842. [Google Scholar]
- Roselli M., Pieper R., Rogel-Gaillard C., de Vries H., Bailey M., Smidt H., and Lauridsen C.. 2017. Immunomodulating effects of probiotics for microbiota modulation, gut health and disease resistance in pigs. Anim. Feed Sci. Technol. 233:104–119. doi:10.1016/j.anifeedsci.2017.07.011 [Google Scholar]
- Varel V. H., and Yen J. T.. 1997. Microbial perspective on fiber utilization by swine. J. Anim. Sci. 75:2715–2722. doi:10.2527/1997.75102715x [DOI] [PubMed] [Google Scholar]
- Wiedmeier R. D., Arambel M. J., and Walters J. L.. 1987. Effect of yeast culture and aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J. Dairy Sci. 70:2063–2068. doi:10.3168/jds.S0022-0302(87) 80254-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.