Abstract
In Colombia Aedes (Stegomyia) aegypti is the main vector of urban arboviruses such as dengue, chikungunya and Zika. This urban mosquito has a well-established capacity to develop insecticide resistance to different types of insecticides (pyrethroids, organochlorides, organophosphates), using multiple resistance mechanisms. An understanding of ongoing resistance mechanisms is critical to determining the activities of vector control programs.
In order to identify the biochemical and molecular mechanisms associated with pyrethroid resistance in Colombia, three laboratory-selected strains resistant to DDT, Propoxur and lambdacyhalothrin, and 7 field-collected strains were evaluated. CDC bioassays were performed to measure the susceptibility status to pyrethroid type I (permethrin) and II (deltamethrin and lambdacyhalothrin), and potential cross-resistance to different types of insecticides; organochlorine (DDT), carbamates (propoxur) and organophosphates (malathion). The enzymatic activity of esterases, glutathione S-transferases (GST) and P450 monooxygenases were biochemically determined. Frequencies of kdr mutations Val1016Ile and Phe1534cys were determined through real-time PCR. The Rockefeller strain of Aedes (Stegomyia) aegypti was used as the susceptible control.
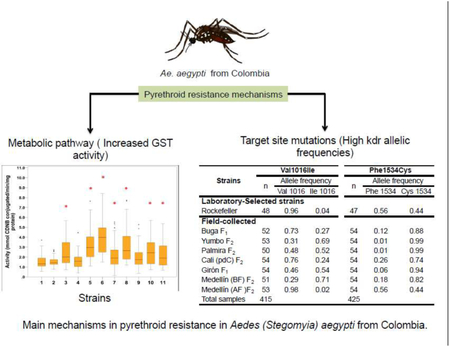
The laboratory-selected strains “propoxur” and “lambdacyhalothrin” and one field population (Medellín (BF) F2 were resistant to all evaluated pyrethroids. Six of the seven field populations as well as the laboratory- selected “DDT” strain were resistant to permethrin. All the evaluated strains were resistant to DDT. Cross-resistance between lambdacyhalothrin and propoxur was observed in the laboratory-selected strains; however, all field-collected strains were susceptible to propoxur and no evidence of malathion resistance was found. The main biochemical mechanism for resistance observed in the field-collected strains was related to the enzyme GST. Further, the frequencies of kdr mutations alleles associated with insecticide resistance were high and ranged from 0.02 to 0.72 for Ile1016 and from 0.44 to 0.99 for Cys1534. Strains with high frequencies of both kdr mutations were resistant to both type I and II pyrethroids. These results suggest that Ae. aegypti from Colombia have developed multiple resistance mechanisms associated with pyrethroid resistance; therefore a resistance management strategy against these field populations of Ae. Aegypti, incorporating these findings is strongly recommended.
Keywords: Aedes aegypti, pyrethroids, resistance, insecticides, glutathion S-transferases, kdr mutations
Graphical Abstract
1. Introduction
Mosquitoes of Aedes genus are vectors of at least 20 arboviruses that currently affect human health (Chouin-Carneiro et al., 2016; Conway et al., 2014; Gubler, 2002). Two species, Aedes (Stegomyia) aegypti (Ae. aegypti) and Ae. (Stegomyia) albopictus are considered the main vectors of dengue virus (DENV), chikungunya (CHIKV) and Zika virus (ZIKV) in urban areas (Karunamoorthi and Sabesan, 2013). Worldwide, Dengue virus is the most important arbovirus, causing an estimated 390 infections annually, and at least 12,500 deaths, most of these in children (Bhatt et al., 2012).
In Colombia, Ae. aegypti is considered the main vector of DENV, CHIKV and ZIKV (Mattar et al., 2015; Padilla, J.C.; Rojas, D.P; Saenz, 2012; Parra et al., 2016). Control of Ae. aegypti, is executed by the Vector-Control programs of the local public health departments. For larval control, chemical insecticides such as temephos, growth regulators, and bioinsecticides derived from bacteria are used as larvicide. For the adult control mainly the organophosphorus (OP) malathion and the pyrethroids (PYRs) deltamethrin and lambdacyhalothrin insecticides are employed in ultra low volume spraying (ULV) in public spaces (Grisales et al., 2013; Maestre-Serrano et al., 2014; Roldan S Ardila, L Santacoloma, 2013).
However the use of malathion has decreased, due to the public's rejection of this insecticide for its strong odor, corrosive effect and possible associations with cancer risk in humans (Bonner et al., 2010; Dusfour et al., 2011; Sritharan et al., 2017). Currently, PYRs are the insecticides that are more frequently used. Pyrethroids are the only recommended by WHO for the treatment of mosquito nets, walls and different materials (WHO, 2006), because they are safe for close contact due to their lower toxicity to mammals. Additionally, they have a fast and persistent effect on mosquitos in low doses, and are odorless, making them ideal for use in ultra low volume fumigations (ULV) in public spaces (WHO/CDS/WHOPES/GCDPP, 2005).
However, pyrethroid resistance has been progressively documented in several countries, among them Colombia, hindering the actions of vector control in those regions where it has been detected (Moyes et al., 2017; Ranson et al., 2008). The enzymatic mechanisms involved in pyrethroid resistance include three of the major groups of detoxification enzymes, which are esterases, P450 monooxygenases and glutathione S-transferases (GST) (Enayati et al., 2005; Liu, 2015; Ranson et al., 2008).
Another pyrethroid resistance mechanism is the alteration of the target sites of the insecticide known as kdr "knockdown resistance". This mechanism of resistance is caused by mutations in the gene that encodes the voltage-dependent sodium channel of mosquitoes, and reduces the binding of the PYRs to its target site in the protein (Brengues et al., 2003; Saavedra-Rodriguez et al., 2007). In Latin America the Val1016Ile and Phe1534Cys mutations have been associated with pyrethroid resistance in Ae. aegypti (Aguirre-Obando et al., 2017; Alvarez et al., 2015; Brito et al., 2018; Francis et al., 2017; Maestre-Serrano et al., 2014; Saavedra-rodriguez et al., 2018; Saavedra-Rodriguez et al., 2007; Vera-Maloof et al., 2015).
In Colombia, the emergence of resistance to PYRs has been reported in several populations of Ae. aegypti (Instituto Nacional de Salud, 2014). Previous studies of pyrethroid resistance mechanisms in Colombia have evaluated either biochemical or molecular tests, but not both. The enzymatic mechanisms described include alteration in levels of esterases, P450 monooxygenases (Ocampo et al., 2011; Santacoloma et al., 2012; Santacoloma Varón et al., 2010) and glutathione S-transferases (Fonseca-González et al., 2011). Additionally, the presence of both kdr mutations (Val1016IIe and Phe1534Cys) has been reported in Colombia in the Caribbean region (Atencia et al., 2016; Maestre-Serrano et al., 2014). Recently a new mutation, Val1419Ile, was reported which was observed in similar frequencies with Val1016Ile; the presence of both mutations was associated with lambdacyhalothrin resistance in three populations of mosquitoes studied in the country (Granada et al., 2018).
This project, is the first reported study where all known enzymatic and molecular resistant mechanisms associated to pyrethroids in Ae. aegypti from Colombia were evaluated, in order to identify the main resistance mechanisms present in the country. As reference, we used laboratory-selected strains derived from field-collected mosquitoes from Colombia to identify the selected mechanism after continuous pressure with a particular insecticide. Additionally, we evaluated the existence of cross-resistance among different classes of insecticides. These results may help to develop resistance management strategies to decrease the emergence of PYRs resistance in Ae. aegypti from Colombia.
2. Materials and methods
2.1. Study area
Field collected mosquitoes were obtained from different cities in Colombia: Giron (Department (State) of Santander), Buga, Palmira, Yumbo and Calineighbourhood “Paso del Comercio” (Valle del Cauca) and Medellin (Antioquia) during 2014-2015 (Figure 1). Mosquito samples from Medellin were collected in two areas, one with high history of ULV spraying with malathion (denominated in this study as Medellin AF) and the second from an area of low history of insecticide application (Medellin BF). We decided to divide them under the criterion of “ ULV spraying frequency” because we wanted to see within the same city how the populations might vary in response to the frequency of the vector control interventions carried out by the vector control program of Medellin. All locations were selected because they had history of insecticide resistance and in each case, the samples were provided by the local secretary of health.
Figure 1.
Map of Colombia. Collection sites of Aedes (Stegomyia) aegypti used in the present study.
2.2. Experimental design
The laboratory experiments were carried out at CIDEIM (Centro Internacional de Entrenamiento e Investigaciones Médicas) located in Cali- Colombia, and under the approval of the Institutional Ethics Committee of Animal Research in Experimentation CIEIA/CIDEIM.
For the study, F1-F2 generation of field collected Ae. aegypti mosquitoes from seven locations in Colombia and three laboratory selected resistant strains that had been continuously selected (as described below) by treatment with DDT (F30), Lamdacyhalothrin (F29) and Propoxur (F29) were evaluated. The laboratory resistant strains were used as biological models that allow us to understand the evolution of the resistance phenomenon when they are exposed continuously to a same insecticide.
The selection process was performed using the methodology described by Ocampo et al. (2011). The selection process was carried out using the CDC bottle bioassay and exposing the adults to the insecticide concentrations, as described as follow. Each strain had repeated exposures to lambdacyhalothrin (5 μg/ml × 10 minutes), DDT (150 μg/ml × 30 minutes), and propoxur (15 μg/ml × 40 minutes). These times/concentrations were selected at the beginning of the selection process as they resulted in 50% survival with these strains and were used throughout the selection process. Then, after the exposure, live mosquitoes were separated for egg production. Selections were carried out over two or three generations to allow the population to recover from any fitness limitations.
Aedes aegypti eggs and larvae from the field collection sites were transported to the CIDEIM laboratory and the colonies maintained under standard laboratory conditions (Ocampo et al., 2011). Adult female mosquitoes between 1-3 days post emergence were independently evaluated with CDC bottle bioassays, biochemical assays and kdr mutation analysis.
2.3. Insecticide bioassays
2.3.1. CDC bottle bioassays
The susceptibility status to pyrethroid and cross-resistance to other insecticides was evaluated by bioassays using the standardized CDC methodology (Brogdon, et al., 2010). The technical grade insecticides were obtained from: SIGMA: deltamethrin (Lot SZ13C059XV), and from Chem Service: permethrin (Lot 3373000, N-12848-250MG), lambdacyhalothrin (Lot 3123900, N-12307-100MG), DDT mixture (p-p’ and o,p’ isomers), (Lot 3871600, N-11567-250MG) malathion (Lot 3494600, N-1246-100MG), propoxur (Lot 3050100, N-11128-250MG) and temephos (Lot 31716CO, N-10996-100MG). Concentration and diagnostic time (DT) previously reported for Ae. aegypti were re-evaluated with the new products using a Rockefeller strain (Fonseca-González et al., 2011; Ocampo et al., 2011; Santacoloma et al., 2012). The following concentrations and DT (resistance thresholds) were established for this study: Permethrin (21.5 μg/mL-45 min), deltamethrin (6.25 μg/mL-40 min), lambdacyhalothrin (6.25 μg/mL-40 min), DDT (2000 μg/mL-60 min), malathion (100 μg/mL-45 min), and propoxur (12.5 μg/mL-45 min). Most of the concentrations and DT corresponds with what was previously published except for DDT. The new lot of the DDT product required increasing the concentration from 150ug/mL to 2000ug/mL to kill the susceptible Rockefeller strain. We used this higher concentration to run de bioassays with all the study strains. Results were expressed as the percent of mortality at the concentration and diagnosis time (DT) established. The resistance threshold was identified using the susceptible strain (Rockefeller) as control.
The bottles were prepared following the protocol described by Brogdon, et al. (2010) using ethanol as solvent. Briefly, twenty female mosquitoes were introduced into a 250mL treated bottles with the corresponding concentration of insecticide. Each test consisted of four insecticide treatment bottles and one control bottle with ethanol only. Three replicates were conducted for each insecticide and mosquito strain. The number of dead or live mosquitoes was recorded at 5 min intervals over a 1hour period. Then all mosquitoes were transferred to plastic cup free of insecticide and containing a cotton ball soaked in a sugar solution; mortality was determined after 24 hours.
2.3.2. Larval bioassays
Larval bioassays were performed following WHO recommendations (WHO, 2005) using the concentration of temephos 0.012ppm (Instituto Nacional de Salud, 2014; Ocampo et al., 2011). Late third- and early fourth- instar larvae were used for each mosquito population. Four replicates per concentration were tested with 20 larvae per replicate. The mortality was evaluated 24 hours postexposure and the results were recorded as percentage mortality.
2.1. Biochemical assays
The activities of α- and β-esterases, glutathione S-transferase (GST) and P450 monooxygenases were measured using biochemical assays modified as previously described (Penilla et al., 1998) and the protein concentrations were calculated according to Bradford (Bradford, 1976). Batches of 72 one-day-old mosquito females were used in the assay and were individually homogenized on ice in 200 μl of distilled water in a flat-bottomed titer microplate (Penilla et al., 1998).
Esterase activities were quantified using two distinct substrates: α- and β- naphthyl acetate. GST activity was measured using the chlorodinitrobenzene (CDNB) substrate and P450 monooxygenases activity was determined using a heme-peroxidase reaction. Absorbances at the reaction end point were determined using a Multiskan® spectrum spectrophotometer microplate reader. Enzyme activity/mg protein was calculated for all field and laboratory samples (strains); normality tests, descriptive statistics and ANOVA analyses were performed.
The median of enzyme activity for each strain were compared with the susceptible Rockefeller strain by Kruskal–Wallis non- parametric test (P < 0.05) and Dunnet’s tests were used to compare the median against those activities of the reference strain (α 0.05) using SPSS 21 (IBM Corporation Armonk, NY, USA). The results of α- and β- naphthyl acetate and GST activity were reported as μmoles product formed / min / mg protein; the activity of P450 monooxygenases was reported as pmoles of cytochrome P450 / mg protein.
2.2. Detection of kdr mutations
2.2.1. Genotyping assays for Val1016Ile and F1534C mutations- DNA extraction
The presence and frequency of kdr mutations were determined using the methodology previously described (Saavedra-Rodriguez et al., 2007). The DNA of 54 randomly selected adult female mosquitoes from each field population and laboratory strain was extracted and resuspended in 60 μl of 1× TE buffer (Tris-EDTA pH 8.0). The DNA was quantified in a NanoDrop 2000® spectrophotometer and stored at −20 °C.
2.2.2. Real-time PCR assay for the Val1016Ile and Phe1534Cys mutations
Detection of the Val1016Ile mutation was performed using the primers Val1016f (GCGGGCAGGGCGGCGGGGGCGGGGCCACAAATTGTTTCCCACCCGCACCGG) or Ile1016f (GCGGGCACAAATTGTTTCCCACCCGCACTGA) and Ile1016r (TGATGAACCSGAATTGGACAAAAGC) as described by Saavedra-Rodriguez et al., (2007). DNA (2 μl) was amplified in a reaction volume of 20 μl. Each reaction contained 10 μl of SYBR® Green PCR Master Mix (Applied Biosystems 4309155), 0.2 μl of each primer (in ddH2O to a final concentration of 100 picomoles/μl) and 7.6 μl of double distilled water (ddH2O).
Detection of Phe1534Cys mutation was performed using the protocol described by Yanola et al., (2011). The primers Cys 1534f (GCGGGCAGGGCGGCGGGGGCGGGGCCTCTACTTTGTGTTCTTCATCATGTG), Phe 1534f (GCGGGCTCTACTTTGTGTTCTTCATCATATT) and 1534r (TCTGCTCGTTGAAGTTGTCGAT) were used. The reaction mixture (20 μL) contained 2 μl of DNA, 10 μl of SYBR® Green PCR Master Mix (Applied Biosystems 4309155), 0.066 μl of Cys1534f primer and 0.2 μl of Phe 1534f and 1535r (in ddH2O to a final concentration of 50 picomoles/μl) and 7.7 μl of double distilled water (ddH2O) was added.
2.2.3. Allelic frequencies of Val1016Ile and Phe1534Cys
The allelic frequencies for each mutation were calculated according to methods described (Saavedra-Rodriguez et al., 2007). In brief, the Allelic frequency of Val1016 = p= GG + (GA/2), Ile1016 q =AA+ (GA/2), Phe1534 = p= TT + (TG/2) and Cys1534 = q= GG + (TG/2). The Hardy–Weinberg equilibrium equation was carried out using the online calculator: http://www.oege.org/software/hwe-mr-calc.shtml (accessed 13 November 2017).
In order to determine the possible relationship between the allelic frequencies of Ile1016 and Cys1534 and the percentage of mortality for all PYRs and DDT insecticides evaluated, a correlation was computed using the value of the Pearson correlation coefficient (r) using Graphpad software prisma 6.0 (Moher et al., 2009).
2.2.4. Dilocus haplotype frequencies
The frequencies for each dilocus haplotype were calculated from a table 3×3 (Three 1534 genotypes × three 1016 genotypes) constructed with the nine potential genotypes and following the methodology as described (Vera-Maloof et al., 2015). The dilocus genotypes were classified as: Val1016/Phe1534, Val1016/Cys1534, Ile1016/Phe1534, Ile1016/Cys1534, Val1016/Phe-Cys1534, Val-Ile1016/Phe1534, Val-Ile1016/Phe-Cys1534, Ile1016/Phe-Cys1534 and Val-Ile1016/Cys1534. The frequencies for each dilocus haplotype Ile1016/Cys1534 (IC) “ Resistant”, Val1016/Phe1534 (VF) “Susceptible”, Val1016/Cys1534 (VC), and Ile1016/Phe1534 (IF) were determined using the web tool “CubeX” (Gaunt et al., 2007) http://www.oege.org/software/cubex/ (accessed 14 November 2017).
Table 3.
Frequencies of dilocus haplotypes analysis at loci 1016 and 1534.
| Strains | Size of sample |
Haplotypes frequencies | |||
|---|---|---|---|---|---|
| Val1016/ Phe1534 (VF) |
Val1016/ Cys1534 (VC) |
Ile1016/ Phe1534 (IF) |
Ile1016/ Cys1534 (IC) |
||
| Laboratory-Selected strains | |||||
| Rockefeller | 47 | 0.563 | 0.393 | 0.0 | 0.042 |
| DDT F30 | 46 | 0.195 | 0.695 | 0.0 | 0.108 |
| Propoxur F29 | 54 | 0.027 | 0.648 | 0.0 | 0.324 |
| Lambdacyalothrin F29 | 54 | 0.009 | 0.268 | 0.0 | 0.722 |
| Field-collected strains | |||||
| Buga F1 | 53 | 0.122 | 0.6132 | 0.0 | 0.264 |
| Palmira F1 | 50 | 0.0 | 0.4694 | 0.010 | 0.520 |
| Yumbo F2 | 50 | 0.0 | 0.280 | 0.010 | 0.710 |
| Cali (PdC) F2 | 53 | 0.217 | 0.5377 | 0.047 | 0.198 |
| Girón F2 | 53 | 0.056 | 0.3962 | 0.0 | 0.547 |
| Medellín (BF) F2 | 51 | 0.107 | 0.1863 | 0.058 | 0.647 |
| Medellín (AF) F2 | 52 | 0.567 | 0.4135 | 0.009 | 0.009 |
| Susceptible | Resistent | ||||
3. Results
3.1. Bioassays
The percentages of mortalities to the evaluated insecticides among the different Ae. aegypti strains are shown in Table 1 and Supplementary Material 1 (CDC bioassay figures). Most of the mosquito strains were resistant to permethrin, except mosquitoes from the Medellin (AF) F2 and DDT F30 strains. All laboratory-selected mosquito strains and only one of the field mosquito strain (Medellin (BF) F2) were resistant to deltamethrin. Five strains were resistant to lambdacyhalothrin: two laboratory selected strains (Propoxur and lambdacyhalothrin F29) and three field strains (Yumbo F2; Giron F1 and Medellin (BF) F2). The lambdacyhalothrin F29 strain recorded the lowest mortality rate (17.6%) to lambdacyhalothrin insecticide. Cross- resistance was observed between pyrethroids and carbamates in the selected-laboratory strains. Propoxur F29 was resistant to all PYRs and DDT insecticide, but was 100% susceptible to malathion. Lambdacyhalothrin F29-selected strain were resistant to all PYRs, DDT and propoxur, but was susceptible to the organophosphates malathion and temephos. All analyzed strains were resistant to DDT; in contrast, all strains were susceptible to malathion. Only one field- collected mosquito population (Palmira F2) was resistant to temephos.
Table 1.
Bioassay results of the field collected and laboratory mosquito strains evaluated with seven insecticides.
| Strains | Mean and standard deviations of mortality percentages to the Insecticides tested |
||||||
|---|---|---|---|---|---|---|---|
| Permethrin | Deltamethrin | λ-cyhalothrin | DDT | Propoxur | Malathion | Temephos | |
| Laboratory-Selected strains | |||||||
| Rockefeller | 100 ± 1.2 | 100 ± 1.48 | 100 ± 1.11 | 90 ± 1.16 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| n=130 | n=130 | n=142 | n=148 | n=124 | n=180 | n=80 | |
| DDT F30 | 87 ± 11.4 | 62.28 ± 29.2 | 87.8 ± 8.6 | 80 ± 9.0 | 73.1 ± 37.3 | 90.9 ± 15.7 | 100 ± 0 |
| n=172 | n=117 | n=143 | n=47 | n=145 | n=81 | n=80 | |
| Propoxur F29 | 57.4 ± 24.6 | 73.8 ± 23.7 | 76.7 ± 6.1 | 58 ± 20 | 66 ± 30 | 100 ± 0 | 100 ± 0 |
| n=352 | n=158 | 141 | 48 | 167 | 83 | 80 | |
| Lamdacyhalothrin F29 | 45.6 ± 20.7 | 66.6 ± 23.4 | 17.2 ± 2.4 | 72.1 ± 5.5 | 59.8 ± 11.5 | 97.7 ± 3.3 | 100 ± 0 |
| n=144 | n=134 | n=176 | n=68 | n=204 | n=180 | n=80 | |
| Buga F1 | 52.9 ± 15.9 | 92.6 ± 12.8 | 87.1 ± 7.4 | 28.1 ± 19.5 | 100 ± 0 | 87.2 ± 12.9 | 100 ± 0 |
| n=217 | n=143 | n=117 | n=56 | n=125 | n=94 | n=80 | |
| Yumbo F2 | 65.7 ± 9.3 | 93.0 ± 9.8 | 79.8 ± 9.8 | 22.2 ± 9.9 | 99.2 ± 1.4 | 100 ± 0 | 100 ± 0 |
| n=141 | n=114 | n=138 | n=56 | n=146 | n=106 | n=80 | |
| Palmira F2 | 76.6 ±3.5 | 94.0 ± 4.8 | 91.9 ± 7.3 | 19.8 ± 14.3 | 100 ± 0 | 88.9 ± 15.7 | 43.7 |
| n=153 | n=297 | n=134 | n=48 | n=133 | n=110 | n=80 | |
| Cali- (PdC) F2 | 71.6 ± 16.4 | 86.8 ± 11.6 | 91.5 ± 9.8 | 9.2 ± 8.3 | 82.5 ± 16.6 | 100 ± 0 | 100 ± 0 |
| n=129 | n=105 | n=285 | n=50 | n=103 | n=115 | n=80 | |
| Girón F1 | 38.3 ± 21.2 | 81.5 ± 14.0 | 62.9 ± 9.6 | 37.6 ± 19.2 | 100 ± 0 | 86.7 ± 18.8 | 100 ± 0 |
| n=190 | n=184 | n=143 | n=45 | n=128 | n=128 | n=80 | |
| Medellín (BF) F2 | 48.9 ± 15.0 | 51.0 ± 7.4 | 38.9 ± 15.2 | 0 ± 0 | 99.1 ± 1.6 | 100 ± 0 | 100 ± 0 |
| n=169 | n=126 | n=139 | n=45 | n=142 | n=126 | n=80 | |
| Medellín (AF) F2 | 98 ± 2.01 | 89.9 ± 6.8 | 81.4 ± 9.5 | 42.3 ± 11.0 | 100 ± 0 | 92.5 ± 10.6 | 100 ± 0 |
| n=143 | n=157 | n=131 | n=59 | n=135 | n=86 | n=80 | |
n= sample size
Mortality percentages in shaded cells indicate that the mosquito population is resistant.
Mortality percentages in bold indicate that the mosquito population is susceptible.
3.2. Biochemical assays
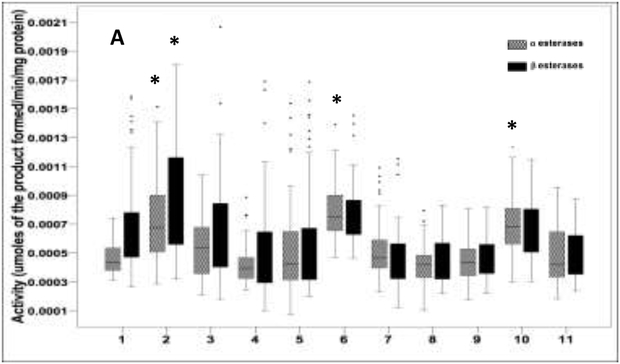
Mean values of enzymatic activity levels for each mosquito strains are shown in in figure 2. The most common enzymatic mechanism of insecticide resistance observed was the increase of the GST enzymes. The glutathione S-transferase activities were significantly elevated in seven out of ten strains evaluated (Dunnett test P < 0.05) (Figure 2). Only mosquitoes from Propoxur F29 strain had high activity of GST in the laboratory-selected strains. Mosquitoes from Yumbo F2 showed the highest average activity of GSTs, followed by Buga F1.
Figure 2.
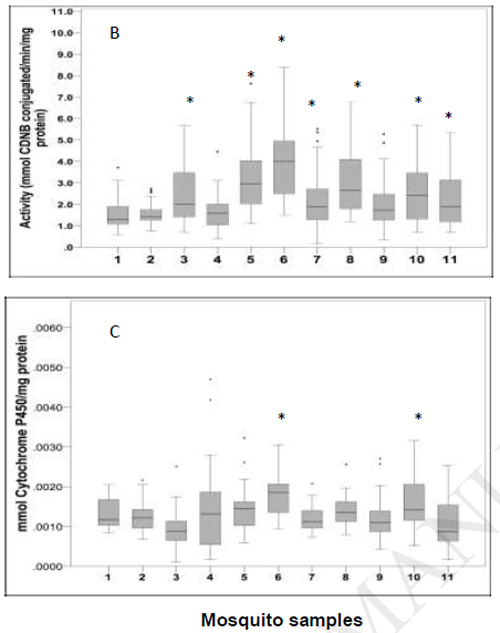
Box-plots of the median enzymes activity: A. α and β esterases assay. B. GST assay. C. P450 monooxygenases assay. The horizontal bar in the box showed the median activity. The lines in the box denotes the upper and lower quartiles. Aedes aegypti strains with elevated enzymatic activity compared with the Rockefeller strain are marked with *(Dunnett test P < 0.000).
Mosquito samples: 1.Rockefeller; 2. DDT F30; 3. Propoxur F29; 4. Lambdacyhalothrin F29; 5. Buga F1; 6. Yumbo F2; 7. Palmira F2; 8. Cali (PdC) F2; 9. Girón F1; 10. Medellín (BF) F2; 11. Medellín (AF) F2
When were compared with the susceptible Rockefeller strain, the activity of α- esterases was elevated in DDT F30, Yumbo F2 and Medellin (BF) F2 mosquito strains, while the activity of β- esterases was elevated only in mosquitoes from the DDT F30 strain (Dunnett test P < 0.05). P450 monooxygenases content was significantly higher in two out of seven field strains, the field-collected mosquitoes from Yumbo F2 and Medellín (BF) F2, (Dunnett's test P < 0.05) (Figure 2).
3.3. Kdr mutations
3.3.1. Detection of kdr mutations and allelic frequencies of Val101lle and Phe1534Cys
Both kdr mutations alleles, Ile1016 and Cys1534, were observed in all the evaluated strains (Table 2). Allelic frequencies of Ile1016 ranged 0.02-0.72, and Cys1534 ranged 0.44-0.99. Frequencies of Cys1534 were higher than Ile1016 in all samples. The Cys1534 allele was close to fixation in three strains (Lambdacyhalothrin F29, Yumbo F2 and Palmira F2) with frequencies of 0.99.
Table 2.
Allelic frequencies of Val1016Ile and Phe1535Cys in laboratory-selected strains and field-collected strains of Aedes (Stegomyia) aegypti from Colombia.
| Val1016Ile |
Phe1535Cys |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | n | Genotype | Allele frequency |
HWE x2 (df)* P-value |
n | Genotype | Allele frequency |
HWE x2 (df)* P- value |
||||||
| GG | GA | AA | Val 1016 |
Ile 1016 |
TT | TG | GG | Phe 1534 |
Cys 1534 |
|||||
| Laboratory-Selected strains | ||||||||||||||
| Rockefeller | 48 | 46 | 0 | 2 | 0.96 | 0.04 | 0.000* | 47 | 26 | 1 | 20 | 0.56 | 0.44 | 0.000* |
| DDT F30 | 51 | 46 | 0 | 5 | 0.9 | 0.1 | 0.000* | 50 | 2 | 16 | 32 | 0.2 | 0.8 | 1.000 |
| Propoxur F29 | 54 | 36 | 1 | 17 | 0.68 | 0.32 | 0.000* | 54 | 0 | 3 | 51 | 0.03 | 0.97 | 0.834 |
| Lambdacyalothrin F29 | 54 | 15 | 0 | 39 | 0.28 | 0.72 | 0.000* | 54 | 0 | 1 | 53 | 0.01 | 0.99 | 0.945 |
| Field-collected strains | ||||||||||||||
| Buga F1 | 52 | 38 | 0 | 14 | 0.73 | 0.27 | 0.000* | 54 | 0 | 13 | 41 | 0.12 | 0.88 | 0.315 |
| Yumbo F2 | 53 | 15 | 0 | 34 | 0.31 | 0.69 | 0.000* | 54 | 0 | 1 | 53 | 0.01 | 0.99 | 0.945 |
| Palmira F2 | 50 | 23 | 0 | 27 | 0.48 | 0.52 | 0.000* | 54 | 0 | 1 | 53 | 0.01 | 0.99 | 0.945 |
| Cali (pdC) F2 | 54 | 41 | 0 | 13 | 0.76 | 0.24 | 0.000* | 54 | 0 | 28 | 26 | 0.26 | 0.74 | 0.010* |
| Girón F1 | 54 | 25 | 0 | 29 | 0.46 | 0.54 | 0.000* | 54 | 0 | 6 | 48 | 0.06 | 0.94 | 0.666 |
| Medellín (BF) F2 | 51 | 15 | 0 | 36 | 0.29 | 0.71 | 0.000* | 54 | 0 | 19 | 35 | 0.18 | 0.82 | 0.117 |
| Medellín (AF)F2 | 53 | 52 | 0 | 1 | 0.98 | 0.02 | 0.000* | 54 | 18 | 24 | 12 | 0.56 | 0.44 | 0.462 |
| Total samples | 574 | 352 | 1 | 216 | 583 | 46 | 113 | 424 | ||||||
GG Homozygote for Val1016 susceptible; GA Heterozygote Val1016/Ile1016; AA Homozygote for Ile1016 resistant. TT Homozygote for Phe1534 susceptible; TG Heterozygote Phe1535/Cys1534; GG Homozygote for Cys1534 resistant
If P < 0,05 not consistent with Hardy Weinberg Equilibrium (HWE)
Mosquitoes from the lambdacyhalothrin F29 laboratory strain had the highest frequency of both mutations Ile1016 (0.72) and Cys1534 (0.99), while the highest frequencies in the field mosquitoes were detected in Medellin (BF) F2 (0.71 and 0.82, respectively). Both strains were resistant to all PYRs and DDT. In contrast the laboratory strain DDT F30 and field strain Medellin (AF) F2 had the lowest frequency of Ile1016. The latter one had the lowest frequency of Cys1534 as well. Medellin (AF) F2 was susceptible to almost all insecticides evaluated, except to DDT (Table 2).
Overall, the homozygous wild type Val1016/Val1016 genotype predominated in the evaluated strains. This genotype was found in 61% of the samples. While the homozygous resistant Ile1016/Ile1016 was found in 37% of the samples. The heterozygous Val1016/Ile1016 was absent in most of the samples. Only one heterozygous sample from the Propoxur F29 strains was found. On the other hand, the homozygous mutant Cys1534/Cys1534 was more frequent in all samples (73%). While the homozygous for the wild type phenotype Phe1534/Phe1534 was found in three out of the eleven evaluated strains (Table 2).
Correlation analyses were carried out between percentage of mortality to PYRs and DDT insecticides and the frequencies of Ile1016 and Cys1534 alleles are shown in the Supplementary material 2 (Pearson analyses table). Negative correlation between percentage of mortality and frequencies of Ile1016 allele was found with permethrin (Pearson r-0.765; P = 0.006) and lambdacyhalothrin (Pearson r-0.709; P = 0.014) insecticides. For the allele Cys1534 the negative correlation was found only with permethrin insecticide (Pearson r-0.762; P = 0.006).
3.3.2. Dilocus haplotypes frequencies
All mosquitoes evaluated were genotyped for both kdr loci. The nine dilocus genotypes (Three 1016 genotypes } Three 1534 genotypes) (Supplementary material 3 (Dilocus genotypes table)). The 36% of mosquitos evaluated expressed both mutations (double homozygous mutants). In contrast the double homozygous wild type (Val/Val + Phe/Phe) was absent in most of the samples. Frequencies of dilocus haplotypes analysis at loci 1,016 and 1,534 are show in the Table 3. The double homozygous Ile1016/Cys1534 (IC) haplotype was observed in higher frequency (>0.5) in the strains with higher resistance to PYRs (Lambdacyhalothrin F29 Yumbo F2, Girón and Medellin BF). While the Ile1016/Phe1534 (IF), was absent in all laboratory- selected strains and two out of the seven field- strains. These results are possibly due to the lack of homozygotes for both alleles in most populations (Table 3).
4. Discussion
This study has demonstrated that multiple mechanisms of PYRs resistance have developed in different Colombian Ae. aegypti field populations, namely, enhanced GST expression and mutations in the kdr gene. While, the laboratory- selected resistance strains (lambdacyhalothrin F29, Propoxur F29 and DDT F30) were mainly associated with kdr selection rather than detoxification enzyme expression.
Almost all the populations (except Medellin AF) showed resistance to the PYRs type I insecticide permethrin, although this insecticide has not been reported in use for dengue vector control in Colombia (Maestre-Serrano, 2012; Maestre-Serrano et al., 2014). These results have also observed in other studies in Colombia in the Caribbean region (Maestre-Serrano et al., 2014), Casanare (Roldan S Ardila, L Santacoloma, 2013), Choco and Putumayo (Fonseca-González et al., 2011; Santacoloma Varón et al., 2010). The high frequency of permethrin resistance observed is comparable with the DDT resistance observed in the same populations. These results suggest a possible crossresistance between these two insecticides, potentially associated with the previous use of DDT (until the 90’s) in malaria and dengue eradication campaigns (Santacoloma Varón et al., 2010). In Colombia, the phenomenon of widespread resistance to DDT has been widely documented; previous studies in insecticide resistance in the country demonstrated the presence of DDT resistance in Ae. aegypti in all the populations studied (Fonseca-González et al., 2011; Ocampo et al., 2011; Santacoloma Varón et al., 2010). We observed the same pattern, as all the field strains evaluated were highly resistant to DDT, suggesting that the DDT-resistance persists even though this insecticide has not been used for more than 26 years (Maestre-Serrano, 2012).
In Colombia, GST-based resistance had been associated with DDT resistance (Fonseca-González et al., 2011) and may also play a role in resistance to PYRs (Lumjuan et al., 2011; Maestre-Serrano et al., 2014). The biochemical assays showed that the most common enzymatic mechanism observed in the field populations was enhanced GST expression/activity. All the field populations with increased activity of GST were resistant to DDT and most of them were resistant to permethrin, with the exception of the Medellin AF population. Interesting, Medellin AF (High ULV spraying frequency Spanish “alta fumigacion”) has been continuously sprayed with the insecticide malathion; could this protect or reverse PYRs resistance? The way in which GST confer resistance to PYRs has not yet been elucidated, but is believed that GSTs can help to protect against oxidative stress induced by PYRs, thanks to predominantly peroxidase activity in the presence of PYRs (Enayati et al., 2005; Lumjuan et al., 2007).
Although in our results the main enzymatic mechanism observed was GST, which was increased in almost all the field populations, previous studies in Colombia (that did not included GST analyses) observed both high esterases and P450 monooxygenases, that was associated mainly to a broad observed temephos resistance in several places in the country (Ocampo et al., 2011; Santacoloma Varón et al., 2010). Due to the previous findings, vector control programs in the country reduced the use of temephos and started to use other larvicides such as Bacillus thuringiensis or insect growth regulators. Additionally, due to malathion concern in health, the use of PYRs insecticides increased in the country. We hypothesize that this reversal in esterases and P450 monoxigenases activity could be associated with changes in the vector control program in the country.
We observed greater heterogeneity in terms of type II PYRs resistance. The resistance to deltamethrin resistance was observed only in one location and lambdacyhalothrin resistance in three localities. Although resistance to deltamethrin has been documented in the country, mosquitoes are most commonly found to be susceptible (Roldan S Ardila, L Santacoloma, 2013; Santacoloma Varón et al., 2010). This suggests that deltamethrin could still be effective to control mosquitoes from places where there is no resistance. In contrast the lambdacyhalothrin resistance has been commonly detected in Colombia (Granada et al., 2018; Instituto Nacional de Salud, 2014). The presence of resistance to lambdacyhalothrin could be associated to its use since the 1990’s in Colombia in dengue and malaria campaigns (Fonseca-González et al., 2011; Roldan S Ardila, L Santacoloma, 2013; Santacoloma Varón et al., 2010).
The laboratory-selected strains were more homogeneous about PYRs resistance. The laboratory strains Propoxur F29 and Lambdacyhalothrin F29 were resistant to all the evaluated insecticides, PYR, DDT and propoxur, demonstrating that each strain developed cross resistance to other groups of insecticides different from those with which they were selected. However, the biochemical results showed that only the Propoxur F29 strain had a resistance mechanism based on high GST activities, while the kdr mutation study showed high frequencies in both mosquito strains, demonstrating both strains having a resistance mechanism mediated by kdr that confers cross resistance to PYRs and DDT.
Example of cross-resistance between carbamates and PYRs mediated by kdr mutations was observed in mosquitos Ae. aegypti from Santiago de Cuba that were selected with propoxur during 14 generations, frequencies of the Ile1016 allele increased from 0.033 in the original strain to 0.40, showing resistance to PYRs. The most parsimonious explanation offered by the researchers is that the Ile1016 allele is linked to a loci conditioned by resistance to propoxur and that selection with propoxur leads to a rapid increase of this allele linked to kdr mutations (Saavedra-Rodriguez et al., 2007).
In all mosquitoes evaluated, the Val1016Ile and Phe1534Cys kdr mutations were present. In our observations the allelic frequencies of Cys1534 were higher than those for the Ile1016 allele. This same pattern has been documented in other populations of Ae. aegypti from Venezuela where, the Cys1534 allele has been found almost fixed in several populations, with frequencies ranging between 0.35-1.00 (Alvarez et al., 2015). In the Guadalupe and San Martin Islands similar observation have been made, where the ranges of the Cys1534 were between 0.92-0.98 (Goindin et al., 2017); recently in Jamaica, this allele was determined to be fixed in all Ae. aegypti populations examined (Francis et al., 2017). In Colombia, previous reports showed that the Cys1534 frequencies ranged between 0.56 to 0.63 in Ae. aegypti from Antioquia, La Guajira and Meta (Granada et al., 2018).
The allele Ile1016 has registered a rapid increase and dispersion in Ae. aegypti populations in some Latin- America countries (Brito et al., 2018; García et al., 2009; Saavedra-Rodriguez et al., 2007; Vera-Maloof et al., 2015). These studies suggest that Val1016Ile mutation is easily selected (García et al., 2009; Vera-Maloof et al., 2015). This mutation was described for the first time in Colombia in 2014 in Ae. aegypti from the Caribbean region, with frequencies ranging between (0.09-0.35) (Maestre-Serrano et al., 2014). Later the allele Ile1016 was describing in three populations of Colombia, and was observed in similar frequencies as a new mutation kdr found, the Val419Ile. The presence of both mutations was associated with lambdacyhalothrin resistance (Granada et al., 2018).
Even though in all mosquitos samples both kdr mutants were observed, albeit with different frequencies, not all the mosquitoes were found to be resistant to PYRs. The correlation analyses suggests that in our samples, the high frequencies of both Ile1016 (>0.5) and Cys1534 could be involved with the permethrin and lambdacyhalothrin resistance, while the high frequency of Cys1534 alone were correlated with permethrin resistance. In a previous study in Colombia, a correlation was observed between high frequencies of Ile1016 and the resistance ratios (RR) to deltamethrin, cyflutrin and permethrin, but not to lambdacyhalothrin and DDT (Maestre-Serrano et al., 2014). In our study, the susceptible strains (Rockefeller and Medellin AF), had the lowest allelic frequencies for Cys1534 (0.44) and Ile1016 (0.02-0.04). Despite have both kdr mutations did not show any correlation with resistance to PYRs.
Our results may suggest that the presence of the Cys1534 mutation alone was not sufficient to generate resistance to permethrin, and it is necessary that both mutations be in high frequencies. Vera-Maloof et al., 2015 hypothesized that the Cys1534 allele appears rapidly in populations and its frequency increases much faster than Ile1016 and that resistance to PYRs requires the sequential evolution of these two mutations. Mutations in domains II and III are synergistic so that the double mutants Ile1016/Cys1534 (IC) have greater resistance to PYRs than the single kdr mutation (Brito et al., 2018; Saavedra-rodriguez et al., 2018; Vera-Maloof et al., 2015).
In Colombia, natural populations of Ae. aegypti have the ability to develop resistance to PYRs insecticides used in public health. The presence and combination of increased activity of GST and both kdr mutations associated with PYRs resistance, makes it necessary to develop new strategies for vector management. It is important to maintain continuous monitoring of resistance when PYRs are used in vector control programs.
5. Conclusion
Pyrethroid resistance in Ae. aegypti from Colombia are mediated by multiple mechanisms. During the period of the study in which the samples of the field populations were collected (2014-2015), the principal mechanisms associated with PYRs resistance were high activity of the GSTs enzymes and high frequencies of both kdr mutations (Val1016Ile and Phe1534Cys). Resistance mechanisms can vary over time in response to insecticides applied by control programs. These mechanisms appear in response to the selective pressure exerted by the insecticides used, which is why we must constantly monitor these mechanisms over time.
Supplementary Material
Highlights.
Resistance to pyrethroids insecticide was detected in Ae. aegypti from Colombia
Pyrethroids resistance is mediated mainly by GST enzymes and kdr mutations
DDT insecticide resistance may induce cross resistance to pyrethroids in Colombia
Cross-resistance between propoxur and lambdacyhalothrin selected strains was observed
Changes in insecticide use in Colombia has modified reported resistance mechanisms
Acknowledgements
We are grateful to the Coordinators and the staff of Vector-Borne Diseases Sections from Departmental Health Secretaries of Valle del Cauca, Antioquia, and Santander. Special thanks to the Entomologists María Elena Cuellar (Departmental Health Secretaries of Valle del Cauca), Guillermo Rua (Universidad de Antioquia) and Jonny E. Duque (CINTROP-UIS) for the help in the samples field collections. To Alma Solis and Francisco Santoyo for assistance in the biochemical assays (CRISP-INSP Mexico) and Luis E. Ramirez (CIDEIM) for maintenance of the Ae. aegypti colonies and assistance with CDC assays.
Funding: The first author obtained a PhD scholarship [N° 567-2012] from COLCIENCIAS during 2013-2017. This project was supported partially supported by Banco de la República de Colombia. [Project N° 3787- 2016] and financed through the program “Desarrollo investigación aplicada para contribuir a un modelo efectivo y sostenible de intervención del dengue en Santander, Casanare y Valle del Cauca” (Development of applied research to contribute to an effective and sustainable model of dengue intervention in Santander, Casanare and Valle del Cauca), financed by “Sistema General de Regalías (SGR), Fondo de Ciencia, Tecnología e Innovación (CTel) aprobado por el Órgano Colegiado de Administración y Decisión (OCAD), mediante el Acuerdo 009 de 2013”. Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under Award Number [D43TW006589]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have declared no conflict of interest.
References
- Aguirre-Obando OA, Martins AJ, Navarro-Silva MA, 2017. First report of the Phe1534Cys kdr mutation in natural populations of Aedes albopictus from Brazil. Parasit. Vectors 10, 160 10.1186/s13071-017-2089-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alvarez LC, Ponce G, Saavedra-Rodriguez K, Lopez B, Flores AE, 2015. Frequency of V1016I and F1534C mutations in the voltage-gated sodium channel gene in Aedes aegypti in Venezuela. Pest Manag. Sci 71, 863–869. 10.1002/ps.3846 [DOI] [PubMed] [Google Scholar]
- Atencia MC, Pérez M. de J., Jaramillo MC, Caldera SM, Cochero S, Bejarano EE, 2016. Primer reporte de la mutación F1534C, asociada con resistencia cruzada a DDT y piretroides, en Aedes aegypti de Colombia. Biomédica 36 10.7705/biomedica.v36i3.2834 [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething P, Brady O, Messina J, Farlow A, Moyes C, 2012. The global distribution and burden of dengue. NIH-PA Author Manuscr. Nat 496, 504–507. 10.1038/nature12060.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MR, Williams BA, Rusiecki JA, Blair A, Beane Freeman LE, Hoppin JA, Dosemeci M, Lubin J, Sandler DP, Alavanja MC, 2010. Occupational exposure to terbufos and the incidence of cancer in the Agricultural Health Study. Cancer Causes Control 21, 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M, 1976. A rapid and sensitive method for the quantiWcation of microgram quantities of protein utilizing the principle of protein– dye binding. Anal. Biochem 72, 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Brengues C, Hawkes NJ, Chandre F, McCarroll L, Duchon S, Guillet P, Manguin S, Morgan JC, Hemingway J, 2003. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutatuions in the voltage-gated sodium channel gene. Med. Vet. Entomol 17, 87–94. 10.1046/j.1365-2915.2003.00412.x [DOI] [PubMed] [Google Scholar]
- Brito LP, Carrara L, De Freitas RM, Bento J, Lima P, Martins AJ, 2018. Levels of Resistance to Pyrethroid among Distinct kdr Alleles in Aedes aegypti Laboratory Lines and Frequency of kdr Alleles in 27 Natural Populations from Rio de Janeiro, Brazil: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogdon William G., Chan A, 2010. Instrucciones para la Evaluacion de la Resistencia a Insecticida en V mediante del Ensayo Biologico de la - 1–28. [Google Scholar]
- Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, Dupont-Rouzeyrol M, Lourenço-de-Oliveira R, Failloux AB, 2016. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis 10, 1–11. 10.1371/journal.pntd.0004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MJ, Colpitts TM, Fikrig E, 2014. Role of the Vector in Arbovirus Transmission. Annu. Rev. Virol 1, 71–88. 10.1146/annurev-virology-031413-085513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusfour I, Thalmensy V, Gaborit P, Issaly J, Carinci R, Girod R, 2011. Multiple insecticide resistance in aedes aegypti (Diptera: Culicidae) populations compromises the effectiveness of dengue vector control in French Guiana. Mem. Inst. Oswaldo Cruz 106, 346–352. 10.1590/S0074-02762011000300015 [DOI] [PubMed] [Google Scholar]
- Enayati AA, Ranson H., Hemingway J, 2005. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol 14, 3–8. 10.1111/j.1365-2583.2004.00529.x [DOI] [PubMed] [Google Scholar]
- Fonseca-González I, Quiñones ML, Lenhart A, Brogdon WG, 2011. Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag. Sci 67, 430–437. 10.1002/ps.2081 [DOI] [PubMed] [Google Scholar]
- Francis S, Karla SR, Perera R, Paine M, Black WC, Delgoda R, 2017. Insecticide resistance to permethrin and malathion and associated mechanisms in Aedes aegypti mosquitoes from St. Andrew Jamaica. PLoS One. 10.1371/journal.pone.0179673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García GP, Flores AE, Fernández-Salas I, Saavedra-Rodríguez K, Reyes-Solis G, Lozano-Fuentes S, Bond JG, Casas-Martínez M, Ramsey JM, García-Reóon J, Domínguez-Galera M, Ranson H., Hemingway J, Eisen L, Black WC IV, 2009. Recent rapid rise of a permethrin knock down resistance allele in Aedes aegypti in M??xico. PLoS Negl. Trop. Dis 3 10.1371/journal.pntd.0000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt TR, Rodríguez S, Day IN, 2007. Cubic exact solutions for the estimation of pairwise haplotype frequencies: implications for linkage disequilibrium analyses and a web tool “CubeX.” BMC Bioinformatics 8, 428 10.1186/1471-2105-8-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goindin D, Delannay C, Gelasse A, Ramdini C, Gaude T, Faucon F, David J-P, Gustave J, Vega-Rua A, Fouque F, 2017. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect. Dis. Poverty 6, 38 10.1186/S40249-017-0254-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granada Y, Mejía-Jaramillo A, Strode C, Triana-Chavez O, 2018. A Point Mutation V419L in the Sodium Channel Gene from Natural Populations of Aedes aegypti Is Involved in Resistance to λ-Cyhalothrin in Colombia. Insects 9, 23 10.3390/insects9010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisales N, Poupardin R, Gomez S, Fonseca-Gonzalez I, Ranson H, Lenhart A, 2013. Temephos Resistance in Aedes aegypti in Colombia Compromises Dengue Vector Control. PLoS Negl. Trop. Dis 7 10.1371/journal.pntd.0002438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ, 2002. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res 33, 330–342. 10.1016/S0188-4409(02)00378-8 [DOI] [PubMed] [Google Scholar]
- Instituto Nacional de Salud, 2014. Instituto Nacional de Salud. Informe epidemiólogico “Red de vigilancia de la resistencia a insecticidas de uso en salud pública en Colombia 2004-2014.” Inst. Nac. Salud 27. [Google Scholar]
- Karunamoorthi K, Sabesan S, 2013. Insecticide Resistance in Insect Vectors of Disease with Special Reference to Mosquitoes: A Potential Threat to Global Public Health. Heal. Scope Int. Quarerly J. 2, 4–18. 10.17795/jhealthscope-9840 [DOI] [Google Scholar]
- Liu N, 2015. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol 60, 537–559. 10.1146/annurev-ento-010814-020828 [DOI] [PubMed] [Google Scholar]
- Lumjuan N, Rajatileka S, Changsom D, Wicheer J, Leelapat P, Prapanthadara L. aied, Somboon P, Lycett G, Ranson H, 2011. The role of the Aedes aegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect Biochem. Mol. Biol 41, 203–209. 10.1016/j.ibmb.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Lumjuan N, Stevenson BJ, Prapanthadara L. aied, Somboon P, Brophy PM, Loftus BJ, Severson DW, Ranson H, 2007. The Aedes aegypti glutathione transferase family. Insect Biochem. Mol. Biol 37, 1026–1035. 10.1016/j.ibmb.2007.05.018 [DOI] [PubMed] [Google Scholar]
- Maestre-Serrano R, 2012. World ‘ s largest Science, Technology & Medicine Open Access book publisher Susceptibility Status of Aedes aegypti to Insecticides in Colombia Pest Engineering, Farzana Perveen (Ed.). 10.5772/31243 [DOI] [Google Scholar]
- Maestre-Serrano R, Gomez-Camargo D, Ponce-Garcia G, Flores AE, 2014. Susceptibility to insecticides and resistance mechanisms in Aedes aegypti from the Colombian Caribbean Region. Pestic. Biochem. Physiol 116, 63–73. 10.1016/j.pestbp.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Mattar S, Miranda J, Pinzon H, Tique V, Bola??os A, Aponte J, Arrieta G, Gonzalez M, Barrios K, Contreras H, Alvarez J, Aleman A, 2015. Outbreak of chikungunya virus in the north Caribbean area of Colombia: Clinical presentation and phylogenetic analysis. J. Infect. Dev. Ctries 9, 1126–1132. 10.3855/jidc.6670 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement 2535, 1–8. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes C, Vontas J, Martins A, Ng L, Koou S, Dusfour I, 2017. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis 1–20. 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo CB, Salazar-Terreros MJ, Mina NJ, McAllister J, Brogdon W, 2011. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Trop 118, 37–44. 10.1016/j.actatropica.2011.01.007 [DOI] [PubMed] [Google Scholar]
- Padilla JC; Rojas DP; Saenz R, 2012. Dengue en Colombia, First, ed. Guías de Impresión Limitada, Bogota. [Google Scholar]
- Parra B, Lizarazo J, Jiménez-Arango JA, Zea-Vera AF, González-Manrique G, Vargas J, Angarita JA, Zuñiga G, Lopez-Gonzalez R, Beltran CL, Rizcala KH, Morales MT, Pacheco O, Ospina ML, Kumar A, Cornblath DR, Muñoz LS, Osorio L, Barreras P, Pardo CA, 2016. Guillain-Barrá Syndrome Associated with Zika Virus Infection in Colombia. N. Engl. J. Med 375, 1513–1523. 10.1056/NEJMoa1605564 [DOI] [PubMed] [Google Scholar]
- Penilla RP, Rodriguez AD, Hemingway J, Torres JL, Arredondo-Jimenez JI, Rodriguez MH, 1998. Resistance management strategies in malaria vector mosquito control. Baseline data for a large-scale field trial against Anopheles albimanus in Mexico. Med Vet Entomol 12, 217–233. [DOI] [PubMed] [Google Scholar]
- Ranson H, Burhani J, Lumjuan N, Black WC IV, 2008. Review Insecticide resistance in dengue vectors. TropIKA 1–12. [Google Scholar]
- Ardila Roldan S, Santacoloma L, Η.B., 2013. Estado de la susceptibilidad a insecticidas de uso en salud Pública en poblaciones naturales de Aedes aegypti (Diptera: Culicidae) del departamento del Casanare, Colombia. Biomedica 33. [DOI] [PubMed] [Google Scholar]
- Saavedra-rodriguez K, Maloof FV, Corey LC, Garcia-rejon J, Lenhart A, Penilla P, Rodriguez A, Sandoval AA, Flores AE, Ponce G, Lozano S, W.C.B. Iv, 2018. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico 1–9. 10.1038/S41598-018-25222-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Strode C, Flores AE, Garcia-Luna S, Reyes-Solis G, Ranson H, Hemingway J, Black IV WC, 2014. Differential transcription profiles in Aedes aegypti detoxification genes after temephos selection. Insect Mol. Biol 23, 199–215. 10.1111/imb.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Fernandez-Salas I, Bisset J, Rodriguez M, Mccall PJ, Donnelly MJ, Ranson H, Hemingway J, Black IV WC, 2007. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol. Biol 16, 785–798. 10.1111/j.1365-2583.2007.00774.X [DOI] [PubMed] [Google Scholar]
- Santacoloma L, Chaves B, Brochero HL, 2012. Estado de la susceptibilidad de poblaciones naturales del vector del dengue a insecticidas en 13 localidades de Colombia. Biomédica 32, 333–343. 10.7705/biomedica.v32i3.680 [DOI] [PubMed] [Google Scholar]
- Santacoloma Varón L, Chaves Córdoba Bernardo, Brochero H, 2010. Susceptibilidad de Aedes aegypti a DDT, deltametrina y lambdacialotrina en Colombia. Rev. Panamericaca Slaud Publica 27, 66–73. 10.1590/S1020-49892010000100010 [DOI] [PubMed] [Google Scholar]
- Sritharan J, Demers PA, Harris SA, Cole DC, Peters CE, Villeneuve PJ, Sritharan J, 2017. Occupation and risk of prostate cancer in Canadian men: A case-control study across eight Canadian provinces. Cancer Epidemiol 48, 96–103. 10.1016/j.canep.2017.04.006 [DOI] [PubMed] [Google Scholar]
- Vera-Maloof FZ, Saavedra-Rodriguez K, Elizondo-Quiroga AE, Lozano-Fuentes S, Black IV WC, 2015. Coevolution of the Ile1,016 and Cys1,534 Mutations in the Voltage Gated Sodium Channel Gene of Aedes aegypti in Mexico. PLoS Negl. Trop. Dis 9, 1–22. 10.1371/journal.pntd.0004263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2006. PESTICIDES APPLICATION AND THEIR APPLICA For the control of vectors. World Heal. Organ. 1, 125 10.1111/j.1439-0310.2006.01165.x [DOI] [Google Scholar]
- WHO, 2005. Guidelines for laboratory and field testing of mosquito larvicides. World Heal. Organ. 1–41. https://doi.org/Ref:WHO/CDS/WHOPES/GCDPP/2005.11 [Google Scholar]
- WHO/CDS/WHOPES/GCDPP, 2005. Safety of Pyrethroids for Public Health Use. World Heal. Organ. https://doi.org/who/cds/whopes/gcdpp/2005.10 [Google Scholar]
- Yanola J, Somboon P, Walton C, Nachaiwieng W, Somwang P, Prapanthadara L, 2011. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop. Med. Int. Health 16, 501–509. 10.1111/j.1365-3156.2011.02725.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.