Abstract
Background
Agitation is common after subarachnoid hemorrhage (SAH) and may be independently associated with outcomes. We sought to determine whether the duration of agitation and fluctuating consciousness were also associated with outcomes in patients with SAH.
Methods
We identified all patients with positive Richmond Agitation Sedation Scale (RASS) scores from a prospective observational cohort of patients with SAH from 2011–2015. Total duration of agitation was extrapolated for each patient using available RASS scores, and 24-hour mean and standard deviation (SD) of RASS scores were calculated for each patient. We also calculated each patient’s duration of substantial fluctuation of consciousness, defined as the number of days with 24-hour RASS SD >1. Patients were stratified by 3-month outcome using the modified Rankin Scale (mRS), and associations with outcome were assessed via logistic regression.
Results
There were 98 patients with at least one positive RASS score, with median total duration of agitation 8 hours (interquartile range [IQR] 4–18), and median duration of substantially fluctuating consciousness 2 days (IQR 1–3). Unfavorable 3-month outcome was significantly associated with a longer duration of fluctuating consciousness (odds ratio [OR] per day, 1.51; 95% confidence interval [CI], 1.04–2.20; p=0.031), but a briefer duration of agitation (OR per hour, 0.94; 95% CI, 0.89–0.99; p=0.031).
Conclusion
Though a longer duration of fluctuating consciousness was associated with worse outcomes in our cohort, total duration of agitation was not, and may have had the opposite effect. Our findings should therefore challenge the intensity with which agitation is often treated in SAH patients.
Keywords: Agitation, consciousness, delirium, subarachnoid hemorrhage, stroke, brain injury
Background
Agitation is a common manifestation of hyperactive delirium in both critically ill patients[1] and those with neurological injury, including subarachnoid hemorrhage (SAH).[2] Further, the presence of agitation in patients with SAH and other forms of acute brain injury may have significant consequences, often coinciding with neurological or medical complications and potentially playing a role in long-term outcomes.[2–7] However, it is unclear if the development of agitation and delirium represents an independent effect or an epiphenomenon of disease severity, while the impact of symptom management is similarly unclear.
Among the primary hypotheses for the pathophysiology of delirium is the dysfunction of various neurotransmitter pathways, namely those involving cholinergic, dopaminergic, and serotonergic circuits[8–12]; of note, these neurotransmitter pathways are also implicated in the rehabilitation and recovery from neurological injury.[13,14] Indeed, many pharmacologic treatments for agitation and hyperactive delirium, such as antipsychotic drugs, appear to worsen neurological recovery in experimental models of brain injury.[15,16]
As such, it is important to consider whether the benefits of treating agitation outweigh the potential risks. Additionally, there have been no studies that considered duration or severity of agitation and its effect on outcomes after brain injury in the acute hospital setting. We therefore designed this study to determine whether the duration of agitation and fluctuating consciousness had independent associations with outcomes in patients with SAH, using a large, prospectively collected observational database of patients admitted to our neurological ICU with aneurysmal SAH. Given that previous studies in the post-acute care, rehabilitation setting showed that a longer duration of agitation after brain injury may be associated with worse rehabilitation and functional outcomes,[4,5,17] we hypothesized that a longer duration of agitation and fluctuating consciousness in the acute care setting would also be associated with worse outcomes after SAH.
Methods
Study population
The cohort of patients studied was a subset of the Columbia University Subarachnoid Hemorrhage Outcomes Project (SHOP), using those patients admitted between January 2011 and December 2015 who had any documented Richmond Agitation Sedation Scale (RASS) score > 0. SHOP is a single-center prospective observational cohort study that collects demographic, clinical, radiographic, and outcome data on all adult patients admitted to our hospital with spontaneous SAH, all of which is collectively adjudicated by attending physicians and research staff. The study was approved by the hospital’s Institutional Review Board, and written informed consent was obtained from the patient or a surrogate in all cases.
Clinical management
Clinical management of patients with SAH conformed to American Heart Association guidelines.[18] As part of our institutional nursing protocol, patients who require pharmacologic sedation in the ICU, whether for agitation or otherwise, must have regular documentation of their RASS scores for at least the duration of pharmacologic sedation. In many cases, RASS scores are also documented to indicate a change in clinical status, even in circumstances when no sedation is given.
Clinical data collection
We prospectively recorded baseline demographic data, prior medical history, and clinical and radiographic findings. The latter was adjudicated by study neurointensivists and included neurological status on admission via the Hunt–Hess scale[19]; the Acute Physiology and Chronic Health Evaluation-2 (APACHE-2) scale; CT scans to evaluate for the modified Fisher score, the presence of hydrocephalus, cerebral edema, and cerebral infarction; and delayed cerebral ischemia (DCI), which has been previously defined.[20] Other treatments and medical and neurological complications are recorded as described previously.[21] Further, data was retrospectively abstracted from the medication administration record as to the total number of days that various sedative medications were given, including propofol, fentanyl, midazolam, dexmedetomidine, haloperidol, and quetiapine.
Measurements
The RASS is a straightforward, easy-to-use 10-point scale measuring level of consciousness, with measurements ranging from -5 (unarousable) to +4 (combative). It has high interrater reliability,[22] though it has been primarily validated as a means of titrating sedative medications.[23] All patients’ RASS scores were retrospectively abstracted from the electronic medical record, including the date and time of the recording. Total duration of agitation (measured in hours) was extrapolated based on available RASS measurements. Further, each patient’s available data was divided into 24-hour epochs, allowing for the calculation of 24-hour mean and standard deviation (SD) of RASS scores. Additional patient-level quantitative markers included the total number of RASS measurements, the number of ICU days during which RASS scores were available, and the number of discrete agitation events (defined as a return to RASS ≤ 0 after any measurement > 0). Post-hoc visual inspection of RASS score trends confirmed that a 24-hour RASS SD > 1 corresponded to substantial fluctuation in a patient’s level of consciousness (Supplemental Figure 1). As a result, the number of days with 24-hour RASS SD > 1 was also recorded.
Outcome variables
Global outcome at 3 months was assessed with a 7-point version of the modified Rankin Scale (mRS) rated from death (6) to symptom-free full recovery (0).[24] Unfavorable outcome was defined as death or moderate-to-severe disability (unable to walk or tend to bodily needs, mRS score 4–6).
Statistical analysis
We used standard descriptive statistics to report patient characteristics, with data that had a normal distribution described with means and standard deviations (SD), and non-normal data described with medians and interquartile ranges (IQR). Differences between continuous variables were analyzed using t-tests or the Mann-Whitney U-test, as appropriate. Differences between categorical variables were analyzed using the Fisher exact test or χ2, as appropriate. Linear regression modeling was used to determine risk factors for a longer duration of agitation. Logistic regression was then performed to determine if duration of agitation and fluctuating consciousness were independently associated with unfavorable 3-month outcome in our study cohort, using age, Hunt-Hess grade, and the development of DCI as covariates in our adjusted model. An additional sensitivity analysis was performed that also included the use of propofol, fentanyl, and dexmedetomidine as covariates.
Missing mRS scores at 3 months due to loss to follow-up (17.3%, n = 17) were replaced via multiple imputation by chained equations.[25] This was done via the ‘mice’ imputation package using predictive mean matching, with 50 datasets created and 20 separate imputations. Statistical significance was set at alpha = 0.05, and odds ratios (OR) and 95% confidence intervals (CI) were included with all analyses. Statistical analyses were performed using R version 3.3.1.
Results
During the time period studied, there were 297 patients in the SHOP database, of whom 98 patients had at least one positive RASS score documented. Patients with positive RASS scores were significantly more likely to have a history of psychiatric disease, as described previously,[2] but were also significantly more likely to have intraventricular hemorrhage (Supplementary Table 1). In the cohort of patients with at least one positive RASS score, mean duration for which RASS data was available was 10.3 days, with a median of 41 total RASS values per patient (IQR 18–72). Mean age was 55.2 (± 13.7) years, 67.3% were female, 78.6% were nonwhite, and mean Hunt-Hess score was 3.0 (± 1.0) (Table 1); only 2 patients from this cohort died during hospitalization.
Table 1.
Baseline characteristics, including demographics, clinical predictors, agitation-related characteristics, and sedative medication use for patients with any positive RASS score during their ICU admission.
| Characteristics | All patients | Stratified by outcome at 3 monthsb | ||
|---|---|---|---|---|
| Favorable (mRS 0-3) (n = 60) |
Unfavorable (mRS 4-6) (n = 21) |
P-value | ||
| Demographics/clinical predictors | ||||
| Age, mean (SD) | 55.2 (13.7) | 54.1 (12.2) | 62.1 (14.1) | 0.027 |
| Male, % | 32.7% | 36.7% | 33.3% | 0.79 |
| Non-white ethnicity, % | 78.6% | 80.0% | 81.0% | 0.93 |
| History of psychiatric diagnosis, % | 30.9% | 30.0% | 33.3% | 0.79 |
| Hunt and Hess score, mean (SD) | 3.0 (1.0) | 2.9 (1.1) | 3.8 (0.8) | < 0.001 |
| Modified Fisher score, mean (SD) | 1.7 (1.1) | 1.8 (1.0) | 2.1 (1.1) | 0.28 |
| Intraventricular hemorrhage, % | 71.8% | 68.6% | 76.2% | 0.58 |
| APACHE-2 score, mean (SD) | 15.0 (6.0) | 14.0 (5.3) | 19.6 (5.2) | < 0.001 |
| Agitation-related characteristics | ||||
| Duration of RASS recording, days, mean (SD) | 10.3 (6.0) | 9.3 (4.5) | 15.2 (7.9) | 0.003 |
| Total number of RASS values, median (IQR) | 41 (18-72) | 36 (18-64) | 37 (23-72) | 0.008 |
| Early onset agitation (day 0-3), % | 52.0% | 55.0% | 47.6% | 0.57 |
| Isolated episode of agitation, % | 50.0% | 45.0% | 52.4% | 0.57 |
| Discrete agitation episodes, median (IQR) | 2 (1-4) | 2 (1-4) | 2 (1-3) | 0.12 |
| Duration of agitation, hours, median (IQR) | 8 (4-18) | 10 (4-23) | 6 (1-11) | 0.044 |
| Duration of substantial fluctuating consciousnessa, days, median (IQR) | 2 (1-3) | 1.5 (0-3) | 3 (2-4) | 0.053 |
| Duration of medication use, days, mean (SD) | ||||
| Quetiapine | 2.4 (4.8) | 3.0 (5.4) | 2.1 (4.5) | 0.44 |
| Haloperidol | 0.5 (2.4) | 0.7 (3.1) | 0 (0) | 0.07 |
| Olanzapine | 0.4 (2.3) | 0.3 (1.5) | 0.1 (0.7) | 0.44 |
| Midazolam | 1.2 (1.9) | 1.0 (1.1) | 2.0 (3.6) | 0.24 |
| Lorazepam | 0.7 (1.5) | 0.7 (1.7) | 0.5 (1.2) | 0.54 |
| Fentanyl | 6.8 (9.2) | 5.9 (6.6) | 12.7 (15.0) | 0.055 |
| Propofol | 3.1 (5.0) | 2.4 (4.1) | 6.4 (7.4) | 0.026 |
| Dexmedetomidine | 4.2 (5.2) | 3.8 (4.6) | 7.8 (6.9) | 0.020 |
Abbreviations: RASS, Richmond Agitation-Sedation Scale; ICU, intensive care unit; SD, standard deviation; APACHE-2, Acute Physiology and Chronic Health Evaluation-2 score; IQR, interquartile range; mRS, modified Rankin Scale.
Defined as 24-hour RASS SD > 1.
Outcome at 3 months not available for 17 patients, who were omitted from univariate comparison.
The median number of discrete agitation episodes was 2 (IQR 1–4), and 52.0% had their first onset of agitation in the first three days of hospitalization. The median total duration of agitation among these patients was 8 hours (IQR 4–18), while the median duration of high RASS variability was 2 days (IQR 1–3). Among these patients, 24 had a RASS score of -5 (indicating a comatose state) recorded at any time, though only 9 patients were comatose for greater than 6 hours, and 6 patients for greater than 24 hours. Fentanyl was the most frequently used sedative medication, followed by dexmedetomidine, propofol, and quetiapine. Linear regression modeling showed that intraventricular hemorrhage, fever, pulmonary edema, and a history of psychiatric disease were significant risk factors for a longer duration of agitation (Supplementary Table 2).
Patients in our study cohort were then stratified into those with favorable and unfavorable outcomes at 3 months. On univariate analysis, patients with unfavorable outcomes were more likely to be older, have higher admission Hunt-Hess and APACHE-2 scores, shorter duration of agitation, and longer duration of propofol and dexmedetomidine use; duration of substantial fluctuation of consciousness did not reach statistical significance when imputed outcomes were not included (p=0.053; Table 1). However, in an adjusted logistic regression model using imputed outcomes, longer duration of fluctuating consciousness and shorter total duration of agitation were both associated with unfavorable outcome at 3 months (Table 2). In an additional sensitivity analysis that included the use of propofol, fentanyl, and dexmedetomidine, a shorter duration of agitation remained significantly associated with unfavorable outcome, though duration of fluctuating consciousness was no longer statistically significant (Table 3).
Table 2.
Results of a logistic regression model testing associations between duration of agitation and substantial fluctuating consciousness with unfavorable outcome at 3 months (modified Rankin Scale 4-6).
| Odds Ratio (95% CI) | p-value | |
|---|---|---|
| Duration of agitation, per hour | 0.94 (0.89-0.99) | 0.031 |
| Duration of substantial fluctuating consciousnessa, days | 1.51 (1.04-2.20) | 0.031 |
| Age, per year | 1.07 (1.02-1.13) | 0.010 |
| Hunt-Hess grade | 3.58 (1.06-12.14) | 0.041 |
| Delayed cerebral ischemia | 3.87 (1.09-13.75) | 0.037 |
Substantial fluctuating consciousness defined as 24-hour RASS SD > 1.
Abbreviations: RASS, Richmond Agitation Sedation Scale; SD, standard deviation; CI, confidence interval.
Table 3.
Results of a sensitivity analysis testing associations between duration of agitation and substantial fluctuating consciousness with unfavorable outcome at 3 months (modified Rankin Scale 4-6), with a logistic regression model that included sedative use.
| Odds Ratio (95% CI) | p-value | |
|---|---|---|
| Duration of agitation, per hour | 0.94 (0.89-1.00) | 0.046 |
| Duration of substantial fluctuating consciousnessa, days | 1.38 (0.94-2.03) | 0.097 |
| Age | 1.06 (1.01-1.12) | 0.018 |
| Hunt-Hess grade | 2.78 (0.67-11.45) | 0.16 |
| Delayed cerebral ischemia | 3.07 (0.80-11.72) | 0.10 |
| Propofol use | 2.54 (0.47-13.60) | 0.28 |
| Fentanyl use | 0.36 (0.02-5.81) | 0.47 |
| Dexmedetomidine use | 2.17 (0.37-12.89) | 0.39 |
Substantial fluctuating consciousness defined as 24-hour RASS SD > 1.
Abbreviations: RASS, Richmond Agitation Sedation Scale; SD, standard deviation; CI, confidence interval.
Discussion
We found that there may be a possible association between agitation-related measurements and outcomes in patients with subarachnoid hemorrhage, suggesting value in quantitative measurements beyond merely identifying the presence of agitation and delirium. Specifically, we found that the overall duration of agitation and the duration of fluctuating consciousness may have opposite associations with outcome (Figure 1). We also found that certain clinical factors were associated with an increasing duration of agitation, including some that were predictable, like fever, intraventricular hemorrhage, and a history of psychiatric disease, all of which likely represent risk factors for the development of agitation. However, a longer duration of agitation was also unexpectedly associated with the development of pulmonary edema, which may be more likely to represent a sequela of prolonged agitation and its accompanying surge of catecholamines.
Figure 1.
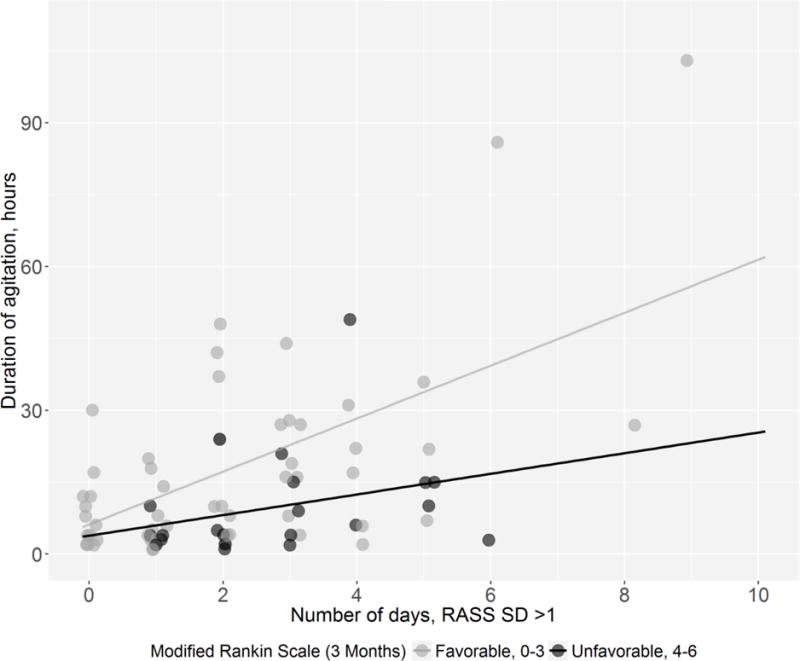
Comparison of agitation duration (in hours) and duration of substantial fluctuation of consciousness (in days) in patients with favorable vs. unfavorable outcomes.
Considering previous literature from the post-acute care setting that showed an association between higher levels of agitation and worse outcomes,[4,5,17] our results were surprising, as they suggested that a longer duration of agitation in the acute setting was not necessarily associated with unfavorable outcomes, and may even be associated with more favorable outcomes. This may represent a novel, hypothesis-generating finding, as there are no studies to our knowledge that investigate the duration and intensity of agitation after acute brain injury while in the hospital setting.
One potential interpretation of our result is that persistent or recurrent agitation in patients with higher grade subarachnoid hemorrhage may be an indication of recovery potential, in comparison to those with persistently depressed consciousness who are unable to mount such a response. However, there are many other contributing factors that may be difficult to disentangle among these high-grade patients, including mixed forms of delirium, a multitude of medication effects, and heterogeneity of brain injury locations. Additionally, it may still be the case that agitation in the acute setting is merely associated with survival in higher grade SAH patients, as the association between functional outcomes and persistent agitation in the post-acute setting remains unclear for this subset of patients.
It is perhaps less surprising that a longer duration of fluctuating consciousness was associated with worse outcomes. Prolonged fluctuations of consciousness may represent protracted states of delirium, or else repeated attempts made to treat agitation with pharmacologic sedation, the long-term effects of which are still unclear. Additionally, for patients who receive continuous anesthetic infusions, the phenomenon of frequent awakening trials for clinical assessments followed by compensatory increases of sedation due to rebound agitation may also play a role. Though the sedative infusions themselves were not significantly associated with outcome in our sensitivity analysis, further prospective studies that address their long-term effects in this patient population are warranted.
Having quantitative markers tracked continuously may allow further insights into the management of agitation and delirium, and our study suggests that there may be some utility in using RASS scores in this way. Though the RASS is already a clinically established tool used to monitor the effects of sedating medications,[23] it also appears to be a feasible method of monitoring changes in level of consciousness in patients with acute brain injury, regardless of whether sedating medications are actively being administered. Such an approach may be worthy of further investigation in studies related to delirium in patients with neurological injury, particularly because some components of widely-used delirium screening instruments like the Confusion Assessment Method for the ICU (CAM-ICU) may not be feasible in patients with severe neurological deficits.[26] Since fluctuations in consciousness make up a significant component of such screening tests, and since the RASS score is easily measurable even in patients with neurological injury, further studies are warranted in the use of RASS score trends as a marker of delirium in such patients. This may even apply to patients who are classified as comatose at a given time, as a subset of our patients fluctuated between comatose and agitated states.
There are several limitations and possible sources of bias in our study. First, the frequency and duration of RASS monitoring was not standardized for all patients. As a result, some patients who experienced agitation during their ICU admission may have been missed, and we focused our primary analysis only on those patients with documented positive RASS scores to reduce the risk of misclassification bias. Additionally, though the RASS does have very high interrater reliability among bedside nurses,[22] nurses at our institution do not receive formal, standardized training on its use. Second, patients with unfavorable outcomes had a significantly longer duration of RASS monitoring (and therefore a higher number of total RASS values that were recorded), likely due to their higher illness severity and likelihood of requiring prolonged anesthetic infusions. This may have potentially led to some degree of confounding by indication. However, the total duration of agitation was higher in the group of patients with favorable outcomes and fewer RASS values, so that it may be more likely that their duration of agitation was underestimated. Third, duration of agitation was not prospectively measured, so our method of retrospective extrapolation based on available RASS measurements offers only a rough estimate. Fourth, the generalizability of our findings may be limited, as the majority of our patients are non-white, and we may also have clinical treatment protocols unique to our institution. Fifth, we lacked data on cumulative dosages of anesthetics and other sedating medications, information which may have strengthened our findings. Finally, there are multiple factors that may have contributed to fluctuations in consciousness other than sedative medications, including medical complications like infections, hydrocephalus, or delayed cerebral ischemia. Unfortunately, our data did not include details regarding the timing of such events.
Conclusions
Our findings suggest that a longer duration of fluctuating consciousness, but not total duration of agitation itself, may be associated with worse outcomes after SAH. Our findings should therefore challenge the intensity with which agitation is often treated in SAH patients. Additionally, monitoring for and analyzing fluctuations in consciousness can be a relatively straightforward tool to add to a clinician’s armamentarium in the management of patients with acute brain injury. Further prospective studies looking for associations between these measures of consciousness and long-term outcomes are warranted in patients with acute brain injury, with additional consideration of using RASS measurements as part of a multimodal approach using other delirium screening tools to identify and monitor delirium symptoms over time.
Supplementary Material
Acknowledgments
Funding/Support: This publication was supported by the DANA foundation (JC, JMS), the NLM of the NIH under Award Number R01 LM011826 (JC), and NIH grant K01 ES026833 (SP).
Footnotes
Conflicts of interest: Dr. Claassen reported receiving honoraria from serving on the Advisory Board of SAGE for study development.
References
- 1.Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, Davidson JE, Devlin JW, Kress JP, Joffe AM, Coursin DB, Herr DL, Tung A, Robinson BRH, Fontaine DK, Ramsay MA, Riker RR, Sessler CN, Pun B, Skrobik Y, Jaeschke R. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Critical care medicine. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Reznik ME, Schmidt JM, Mahta A, Agarwal S, Roh DJ, Park S, Frey HP, Claassen J. Agitation After Subarachnoid Hemorrhage: A Frequent Omen of Hospital Complications Associated with Worse Outcomes. Neurocritical care. 2016:1–8. doi: 10.1007/s12028-016-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogner J, Barrett RS, Hammond FM, Horn SD, Corrigan JD, Rosenthal J, Beaulieu CL, Waszkiewicz M, Shea T, Reddin CJ, Cullen N, Giuffrida CG, Young J, Garmoe W. Predictors of Agitated Behavior During Inpatient Rehabilitation for Traumatic Brain Injury. Archives of physical medicine and rehabilitation. 2015;96(8, Supplement):S274–S281.e274. doi: 10.1016/j.apmr.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Venkateshwara G, Nair KPS, Khan M, Saad R. Agitation after traumatic brain injury and predictors of outcome. Brain Injury. 2014;28(3):336–340. doi: 10.3109/02699052.2013.873142. [DOI] [PubMed] [Google Scholar]
- 5.Nott MT, Chapparo C, Baguley IJ. Agitation following traumatic brain injury: An Australian sample. Brain Injury. 2006;20(11):1175–1182. doi: 10.1080/02699050601049114. [DOI] [PubMed] [Google Scholar]
- 6.Caeiro L, Ferro JM, Albuquerque R, Figueira ML. Delirium in the first days of acute stroke. Journal of neurology. 251(2):171–178. doi: 10.1007/s00415-004-0294-6. [DOI] [PubMed] [Google Scholar]
- 7.Oldenbeuving AW, de Kort PLM, Jansen BPW, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: Incidence, risk factors, and outcome. Neurology. 2011;76(11):993–999. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 8.van der Mast RC, Fekkes D. Serotonin and amino acids: partners in delirium pathophysiology? Seminars in clinical neuropsychiatry. 2000;5(2):125–131. doi: 10.153/scnp00500125. [DOI] [PubMed] [Google Scholar]
- 9.Hshieh TT, Fong TG, Marcantonio ER, Inouye SK. Cholinergic Deficiency Hypothesis in Delirium: A Synthesis of Current Evidence. The journals of gerontology Series A, Biological sciences and medical sciences. 2008;63(7):764–772. doi: 10.1093/gerona/63.7.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldonado JR. Pathoetiological Model of Delirium: a Comprehensive Understanding of the Neurobiology of Delirium and an Evidence-Based Approach to Prevention and Treatment. Critical Care Clinics. 24(4):789–856. doi: 10.1016/j.ccc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Ali S, Patel M, Jabeen S, Bailey RK, Patel T, Shahid M, Riley WJ, Arain A. Insight into Delirium. Innovations in Clinical Neuroscience. 2011;8(10):25–34. [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson TN, Raeburn CD, Angles EM, Moss M. Low Tryptophan Levels Are Associated with Post-Operative Delirium in the Elderly. American journal of surgery. 2008;196(5):670–674. doi: 10.1016/j.amjsurg.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein LB. Neurotransmitters and motor activity: Effects on functional recovery after brain injury. NeuroRx. 2006;3(4):451–457. doi: 10.1016/j.nurx.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chollet F, Tardy J, Albucher J-F, Thalamas C, Berard E, Lamy C, Bejot Y, Deltour S, Jaillard A, Niclot P, Guillon B, Moulin T, Marque P, Pariente J, Arnaud C, Loubinoux I. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. The Lancet Neurology. 10(2):123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 15.Feeney D, Gonzalez A, Law W. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217(4562):855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LB, Bullman S. Differential Effects of Haloperidol and Clozapine on Motor Recovery after Sensorimotor Cortex Injury in Rats. Neurorehabilitation and Neural Repair. 2002;16(4):321–325. doi: 10.1177/154596830201600402. [DOI] [PubMed] [Google Scholar]
- 17.Bogner JA, Corrigan JD, Fugate L, Mysiw WJ, Clinchot D. Role of Agitation in Prediction of Outcomes After Traumatic Brain Injury. American Journal of Physical Medicine & Rehabilitation. 2001;80(9):636–644. doi: 10.1097/00002060-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2012 doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 19.Hunt William E, Hess Robert M. Surgical Risk as Related to Time of Intervention in the Repair of Intracranial Aneurysms. Journal of Neurosurgery. 1968;28(1):14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 20.Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, Connolly ES, Mayer SA. Effect of Cisternal and Ventricular Blood on Risk of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage:: The Fisher Scale Revisited. Stroke. 2001;32(9):2012–2020. doi: 10.1161/hs0901.095677. [DOI] [PubMed] [Google Scholar]
- 21.Wartenberg KE, Schmidt JM, Temes RE, Frontera JA, Kowalski RG, Ostapkovich N, Sheth SJ, Parra A, Connolly ES, Mayer SA. Medical complications after subarachnoid hemorrhage: Frequency and impact on outcome. Stroke. 2005;36(2):521–521. [Google Scholar]
- 22.Ely E, Truman B, Shintani A, et al. Monitoring sedation status over time in icu patients: Reliability and validity of the richmond agitation-sedation scale (rass) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 23.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation–Sedation Scale. American Journal of Respiratory and Critical Care Medicine. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 24.de Haan R, Limburg M, Bossuyt P, van der Meulen J, Aaronson N. The Clinical Meaning of Rankin ‘Handicap’ Grades After Stroke. Stroke. 1995;26(11):2027–2030. doi: 10.1161/01.str.26.11.2027. [DOI] [PubMed] [Google Scholar]
- 25.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? International Journal of Methods in Psychiatric Research. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riker RR, Fugate JE. Clinical Monitoring Scales in Acute Brain Injury: Assessment of Coma, Pain, Agitation, and Delirium. Neurocritical care. 2014;21(2):27–37. doi: 10.1007/s12028-014-0025-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


