Abstract
Germline mutations in the tumor suppressor gene, BRCA-1 associated protein (BAP1), underlie a tumor predisposition syndrome characterized by increased risk for numerous cancers including uveal melanoma, melanocytic tumors and mesothelioma, among others. In the present study we report the identification of a novel germline BAP1 mutation, c.1777C>T, which produces a truncated BAP1 protein product and segregates with cancer. Family members with this mutation demonstrated a primary clinical phenotype of autosomal dominant, early-onset melanocytic neoplasms with immunohistochemistry (IHC) of these tumors demonstrating lack of BAP1 protein expression. In addition, family members harboring the BAP1 c.1777C>T germline mutation developed other neoplastic disease including thyroid cancer. IHC analysis of the thyroid cancer, as well, demonstrated loss of BAP1 protein expression. Our investigation identifies a new BAP1 mutation, further highlights the relevance of BAP1 as a clinically important tumor suppressor gene, and broadens the range of cancers associated with BAP1 inactivation. Further study will be required to understand the full scope of BAP1-associated neoplastic disease.
Keywords: Cancer genetics, BAP1, tumor predisposition syndrome, thyroid cancer, melanoma
Introduction
BRCA-1 associated protein (BAP1) functions as a tumor suppressor gene by modulating a number of cellular processes including cell cycle progression, cell growth, DNA repair and stem cell dynamics (1–5). Germline mutations in BAP1 have been implicated in causing an autosomal dominant tumor predisposition syndrome (6) (OMIM #6143237) that is associated with predisposition to uveal melanomas, melanocytic tumors and mesotheliomas (7–10). Since the original description of the BAP1 predisposition syndrome additional cancers such as renal cell carcinoma, cutaneous melanoma and basal cell carcinoma, have been linked with germline mutations of BAP1 (11–14). In the present study we report a novel germline BAP1 mutation, c.1777C>T, identified in a patient with multiple cancers and a family history remarkable for autosomal dominant, early age melanocytic tumors and cutaneous melanomas. The c.1777C>T mutation introduces a premature stop codon into the BAP1 open reading frame with resultant expression of a truncated protein. Immunohistochemical (IHC) assessment of the melanocytic tumors from this family documented loss of BAP1 protein expression within neoplastic cells. Additionally, IHC analysis of a thyroid papillary carcinoma and a thyroid adenoma arising in the proband carrying the c.1777C>T mutation germline mutation demonstrated loss of BAP1 protein expression in these tumors. The results of our investigation suggest that loss of BAP1 tumor suppression contributes to the development of thyroid cancers, expands the clinical phenotype of the BAP1-associated tumor predisposition syndrome and underscores the increasing importance of recognizing this syndrome in the evaluation of suspected hereditary cancers. Finally, the current investigation provides further insight in the molecular pathobiology associated with loss of BAP1 expression.
Materials and methods
Clinical genetic testing
Clinical germline testing of CDKN2A was performed in a CLIA-certified laboratory (Myriad genetics, Salt Lake City, Utah).
BAP1 sequencing and mutation analysis
Genomic DNA was extracted from 5 ml whole blood by protein salting out and nucleic acid precipitation (15). For patients who were deceased at the time of the study (patients III.3 and III.9) genomic DNA was obtained from the normal tissue portions of formalin-fixed, paraffin-embedded (FFPE) tumor blocks using the RecoverAll Total Nucleic Acid Isolation Kit for FFPE (Life Technologies, Grand Island, NY). Exons 1–17 of the BAP1 gene (NCBI Reference Sequence: NM_004656.3) were amplified employing a PCR protocol adapted from Wiesner et al. (9); Sanger sequencing of amplified fragments in forward and reverse directions was performed by Genewiz (La Jolla, California). Electropherogram results were analyzed and mutations identified using Mutation Surveyor 2.61.
Immunohistochemistry
Archived, formalin-fixed, paraffin-embedded tumor blocks of the thyroid cancer were employed for IHC studies. H&E staining was carried out using standard methodology. Staining for BAP1 protein was accomplished employing BAP1 antibody (C-4, catalog number: sc-28283) obtained from Santa Cruz Biotechnology (Dallas, Texas). In brief, 4 μm sections were cut and baked at 60 degrees for 1 hour. The slides were then processed through the Leica BOND-III automated IHC and ISH system (Buffalo Grove, Illinois). Post processing, the IHC slides were dehydrated and cover slipped mounted with Permount adhesive (ThermoFisher Scientific; Waltham, Massachusetts). BAP1 protein IHC staining of melanoma samples was previously performed at an outside institution as part of patient’s prior clinical assessment. Melanoma and melanocytic nevi IHC results described in the present study are based upon clinical reports provided by the patient.
Results
Patient cancer and family history
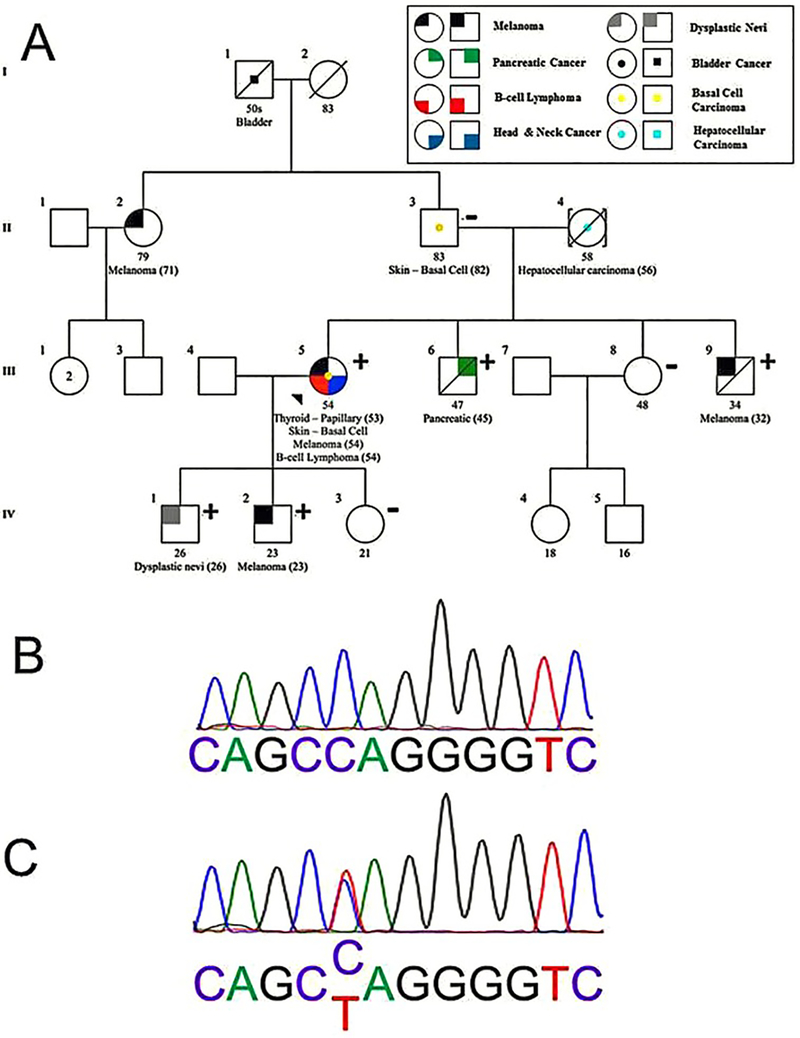
The proband is a 54 year old woman who was referred to the cancer genetics program at the University of Southern California Norris Comprehensive Cancer Center for evaluation of a personal history of multiple cancers including papillary thyroid cancer, basal cell carcinoma, cutaneous melanoma and B-cell lymphoma. A four generation cancer-focused pedigree was generated which demonstrated a multigenerational history of melanoma as well as additional cancers including bladder carcinoma, basal cell carcinoma, hepatocellular carcinoma and pancreatic cancer (Figure 1A). Physical examination of the patient documented greater than 100 moles on the chest and abdomen, but was otherwise unremarkable. Given the personal and family histories, which included melanoma and pancreatic cancer, a concern for familial atypical multiple mole melanoma (FAMM) syndrome arose. Clinical genetic testing for CDKN2A found no pathogenic mutations (Myriad Genetics, Salt Lake City, UT) (16).
Figure 1.
(A) Four generation pedigree of family with novel BAP1 mutation, g.1777C>T, documents co-segregation of BAP1 mutation and multiple cancers. Individuals carrying the familial mutation BAP1 g.1777C>T are designated with a “+” and tested individuals proven not to carry the BAP1 g.1777C>T mutation are shown by “−”. The proband, patient III-5, is indicated with an arrow. Her mother, patient II-4, is assumed to be an obligate carrier given the negative test result in her father and absence of available DNA for testing in her deceased mother. Further information for the maternal family to the proband is unavailable because patient II-4 was adopted into the family. (B) Electropherogram depicting portion of germline DNA exon 14 of BAP1 gene from unaffected family member II-3 documenting wildtype BAP1 sequence. (C) Electropherogram of germline DNA obtained from the proband (III-5) illustrating novel BAP1 mutation g.1777C>T, resulting in truncated BAP1 protein. Genotyping of other unaffected family members (III-8 and IV-3) revealed wildtype germline BAP1; genotyping of affected family members (III-6, III-9, IV-1 and IV-2) revealed the novel BAP1 mutation, g.1777C>T.
Dermatologic assessment unveils a BAP1-associated familial cancer syndrome
The patient’s son (IV.1, Figure 1A) with a previous history of an early age dysplastic nevus detected at age 17 was subsequently diagnosed with another dysplastic nevus at age 26. Dermatologic evaluation with biopsy demonstrated compound Spitz proliferation of melanocytes; these histologic features raised concern for loss of expression of the BAP1gene (9). IHC staining of the dysplastic nevi confirmed an absence of BAP1 protein. Within this same time period, the proband’s other son (IV.2, Figure 1A) developed early age melanoma on his knee for which IHC assessment also revealed absent BAP1 protein expression. Dysplastic nevi and melanoma are less common among individuals during the first three decades of life compared with older age cohorts (17,18). The early age of these dermatologic diagnoses together with lack of BAP1 protein expression in the tumor specimens created suspicion for an inherited germline mutation of BAP1; moreover, the constellation of cancer diagnoses within the family raised the possibility of a novel manifestation of the BAP1 tumor predisposition syndrome.
Germline genomic sequencing of the BAP1 exome identifies a novel truncating mutation
Sequencing of the complete BAP1 exome (17 exons) was carried out from germline DNA derived from the proband. A novel point mutation was identified in exon 14, c.1777C>T (p.Q593X), of the BAP1 gene (Figure 1B, C) resulting in the introduction of a truncating, premature stop codon. Additional family members were tested for the presence of this germline mutation and the mutation was identified in family members III.6 (pancreatic cancer positive), III.9 (melanoma positive), IV.1 (dysplastic nevi positive) and IV.2 (melanoma positive). The mutation was absent from patient II.3, the father of the proband. DNA from the proband’s mother was not available; however, the familial patterning of the BAP1 mutation is consistent with obligate transmission via the maternal lineage. Patients IV.3 and III.8, the daughter and sister of the proband, respectively, demonstrated wildtype BAP1 genotypes; notably, both patients IV.3 and III.8 were without a cancer phenotype.
Immunohistochemical analysis implicates deficient BAP1 tumor suppression in the development of thyroid cancer
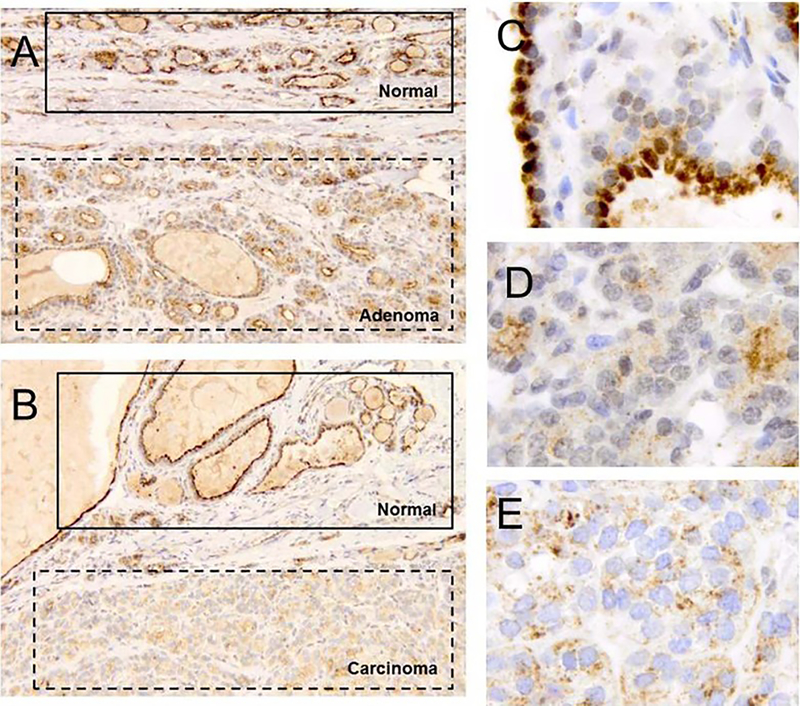
The tumor spectrum defining BAP1 predisposition syndrome includes, prominently, the constellation of uveal melanoma, cutaneous melanoma, melanocytic tumors, mesothelioma renal cell carcinoma and basal cell carcinomas (8,10,13,19,20). Although thyroid cancers have been identified in families of known BAP1 mutation carrier (21), to date, loss of BAP1 protein expression or bi-allelic BAP1 gene deletion has yet to be documented for these specific tumors. In the current family, the proband developed both a thyroid adenoma and thyroid papillary cancer. Demonstration of loss of BAP1 protein expression in these tumors would provide evidence for expanding the spectrum of cancers arising from absence of BAP1 tumor suppression. We observed intact BAP1 expression in normal tissue derived from the thyroid specimen (Figure 2A–C) and absence of nuclear expression in both the follicular adenoma (Figure 2A, D) and the papillary thyroid carcinoma (Figure 2B, E). These results are consistent with loss of BAP1 tumor suppression driving the formation of the thyroid adenoma and papillary carcinoma.
Figure 2.
(A) BAP1 IHC staining (100x) of normal thyroid tissue together with thyroid adenoma originating from proband patient (III-5) reveals strong nuclear staining of BAP1 in normal tissue compared with absence of staining in adenoma. (B) BAP1 IHC staining (100x) of normal thyroid tissue together with papillary thyroid carcinoma originating from proband patient (III-5) demonstrates strong nuclear staining of BAP1 in normal tissue compared with absence of staining in carcinoma. (C) Higher power image (630x) of normal thyroid tissue documents strong nuclear staining of BAP1. (D) Higher power image (630x) of thyroid adenoma demonstrates absence of BAP1 nuclear staining. (E) Higher power image (630x) of papillary thyroid carcinoma reveals absence of BAP1 nuclear staining.
Discussion
In the current study we identify a novel germline BAP1 mutation, c.1777C>T, resulting in a truncated protein, in a family with multiple cancers. These findings further define the evolving tumor spectrum characterizing the BAP1-related tumor predisposition syndrome and provide insight into the pathobiology of disrupted BAP1 tumor suppression.
The BAP1 gene lies on the reverse strand of chromosome 3 spanning 3p21.31-p21.2 (22). BAP1 encodes a ubiquitin carboxy-terminal hydrolase which modulates cell proliferation and cell cycle progression acting as a tumor suppressor gene via association with and/or deubiquitination of a host of transcription-regulating proteins including among others ASXL1, ASXL2, FOXK1, OGT and HCF-1 (2,23,24). The open reading frame of BAP1 spans 2166 nucleotides with 17 exons comprising multiple functional domains, including ubiquitin carboxyl-terminal hydrolase (UCH)(exons 1–7), the host cell factor-1 binding domain (HCF1-BD)(exon 10) and the C-terminal domain (exons 13–17); the C-terminal domain contains the nuclear localization signals 1 and 2 and the UCH37-like domain (ULD) (6,22,25). Previously identified germline BAP1 mutations are mostly nonsense or frameshift mutations; the c.1777C>T mutation described in the current study is a nonsense mutation. These mutations result in premature stop codons which give rise to truncated proteins and likely nonsense-mediated mRNA decay (26). The germline BAP1 mutations described to date are evenly distributed across the open reading frame of the BAP1 gene, although a disproportionate number of mutations are situated on exon 13 as it represents the largest exon. Approximately one half of reported germline BAP1 mutations occur within a functional domain; and these mutations exhibit proportional prevalence across these domains (6).
Germline loss of BAP1 expression has been documented to underlie a number of familial cancer occurrences (27). The tumors originally associated with mutated germline BAP1 include melanocytic tumors (9), uveal melanomas (7) and mesotheliomas (8). At that same time, it was postulated that germline BAP1 mutations likely additionally promote cutaneous melanoma (9), and might predispose to a constellation of additional cancers (8). And since the initial reports of these cancers linked with germline mutated BAP1, the scope of associated cancers has, indeed, significantly expanded (28).
The strongest causative association between loss of BAP1 expression and a specific cancer are those cancers for which bi-allelic mutations/deletions and/or disruption of BAP1 protein expression have been demonstrated in the tumor. These neoplasms include, in addition to the originally documented cancers, renal cell carcinoma, basal cell carcinoma, lung cancer, tumors of the CNS and sarcomas (8,10–14,20,21). The present study enlarges this collection also to encompass papillary thyroid cancer and thyroid adenoma.
The current observation that loss of BAP1 tumor suppression drives progression of thyroid cancer additionally clarifies the spectrum of the BAP1 tumor predisposition syndrome and also elucidates the pathobiology resulting from mutation of the gene. In the proband we observed that loss of BAP1 expression occurred in both a follicular adenoma as well as an adjacent papillary thyroid cancer. Follicular thyroid cancer may develop via an adenoma to carcinoma progression sequence (29). Both RAS gene mutations and PAX8/PPARγ gene fusions have previously been implicated as initiating events of this molecular sequence (30); the current study raises speculation that loss of BAP1 expression may represent an alternate early event in this progression pathway. The question arises whether loss of BAP1 expression may also drive similar early adenoma-initiating events in other tissues. The occurrence of the thyroid neoplasms in the proband is further remarkable; it is also unique for tumors of two different histologies to develop synchronously within the thyroid gland (31–33). This circumstance may belie singular molecular pathways which are fostered by the underlying germline BAP1 mutation; further elucidation of other somatic mutations in these thyroid tumors may provide further insight into such pathways.
The proband, in addition to diagnoses of melanoma and thyroid cancer, also developed B-cell lymphoma. Relative to solid tumors, there exist few reports of hematopoietic cancers associated with germline mutations of BAP1. Previous associations are limited to histories of family members of patients carrying germline BAP1 mutations having leukemia (34,35) and one unspecified hematologic cancer (21). Laboratory investigations, however, unambiguously document that loss of BAP1 expression can disrupt normal bone marrow function, cause myeloid transformation and give rise to myeloid dysplasia (24). The present study broadens the list of hematopoietic cancers associated with individuals carrying germline BAP1 mutations; our investigation suggests that loss of BAP1 expression may disrupt not merely ontogeny of the myeloid cell lineage but may extend to lymphoid cell lineage causing B cell lymphoma, as well.
Pancreatic cancer has been reported previously in family members of patient’s having deleterious BAP1 mutations (8,21). This association suggests the possibility that loss of BAP1 tumor suppression may also drive the progression of pancreatic cancer. In the current study we document the occurrence of a BAP1 mutation in a patient (patient III.6, Figure 1) with pancreatic cancer which further strengthens this association. Unfortunately, insufficient pancreatic cancer tissue from this patient was available to perform IHC analysis to assess loss of BAP1 protein expression. We speculate that future studies may unambiguously implicate loss of BAP1 tumor suppression in the development of pancreatic cancer.
The father of the proband (Figure 1, patient II.3) did not carry a BAP1 mutation, suggesting a high probability that this mutation originated in the mother unless the BAP1 mutation was de novo (Figure 1, patient II.4). We note that the mother developed hepatocellular carcinoma. This clinical presentation raises speculation that loss of BAP1 tumor suppression may also play a role in the oncogenesis of hepatocellular carcinoma; unfortunately, no tissue specimens were available from the mother to test this hypothesis.
In summary, the burgeoning spectrum of tumors associated with germline mutations of BAP1 together with the observation that BAP1 is ubiquitously expressed among tissues (36), suggest that the gene may function as a generalized tumor suppressor gene. That there manifests some diversity with regard to what tumors develop within and among families with germline mutations of BAP1 may also imply that the gene exhibits variable penetrance and/or exerts a modifier effect within the context of a landscape of additional familial genetic variation (37,38). Therefore, it is reasonable to consider germline BAP1 mutations as a possible causative mechanism in families exhibiting a familial cancer syndrome but whose genetic workup identifies no typical genetic abnormality. It may be anticipated that as whole exome sequencing becomes more widely adopted as a part of medical genetic and, more specifically, cancer genetic workups, the identification of additional germline BAP1 mutations and clarification of the molecular mechanisms contributing to the development of familial cancer syndrome will be forthcoming (39–41).
Acknowledgments
This study was funded by the ASCO Young Investigator Awards (to K.M.), USC Norris Comprehensive Cancer Center Support Grant CA014089 (to S.B.G.) and the Anton B. Burg Foundation (to S.B.G.).
References
- 1.Eletr ZM, Wilkinson KD. An emerging model for BAP1’s role in regulating cell cycle progression. Cell Biochem Biophys 2011;60:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machida YJ, Machida Y, Vashisht AA, et al. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem 2009;284:34179–34188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan H, Jia R, Zhang L, et al. BAP1 regulates cell cycle progression through E2F1 target genes and mediates transcriptional silencing via H2A monoubiquitination in uveal melanoma cells. Int J Biochem Cell Biol 2015;60C:176–184. [DOI] [PubMed] [Google Scholar]
- 4.Yu H, Pak H, Hammond-Martel I, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci U S A 2014;111:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strikoudis A, Guillamot M, Aifantis I. Regulation of stem cell function by protein ubiquitylation. EMBO Rep 2014;15:365–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbone M, Ferris LK, Baumann F, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med 2012;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011;43:1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiesner T, Obenauf AC, Murali R, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 2011;43:1018–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Rahman MH, Pilarski R, Cebulla CM, et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet 2011;48:856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popova T, Hebert L, Jacquemin V, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet 2013;92:974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Fouchardiere A, Cabaret O, Savin L, et al. Germline BAP1 mutations predispose also to multiple basal cell carcinomas. Clin Genet 2015;88:273–277. [DOI] [PubMed] [Google Scholar]
- 13.Wadt KA, Aoude LG, Johansson P, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet 2015;88:267–272. [DOI] [PubMed] [Google Scholar]
- 14.Njauw CN, Kim I, Piris A, et al. Germline BAP1 inactivation is preferentially associated with metastatic ocular melanoma and cutaneous-ocular melanoma families. PLoS ONE 2012;7:e35295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton Bishop JA, Gruis NA. Genetics: what advice for patients who present with a family history of melanoma? Semin Oncol 2007;34:452–459. [DOI] [PubMed] [Google Scholar]
- 17.Sagebiel RW, Banda PW, Schneider JS, et al. Age distribution and histologic patterns of dysplastic nevi. J Am Acad Dermatol 1985;13:975–982. [DOI] [PubMed] [Google Scholar]
- 18.Lachiewicz AM, Berwick M, Wiggins CL, et al. Epidemiologic support for melanoma heterogeneity using the surveillance, epidemiology, and end results program. J Invest Dermatol 2008;128:1340–1342. [DOI] [PubMed] [Google Scholar]
- 19.Wiesner T, Fried I, Ulz P, et al. Toward an improved definition of the tumor spectrum associated with BAP1 germline mutations. J Clin Oncol 2012;30:e337–e340. [DOI] [PubMed] [Google Scholar]
- 20.Farley MN, Schmidt LS, Mester JL, et al. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res 2013;11:1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilarski R, Cebulla CM, Massengill JB, et al. Expanding the clinical phenotype of hereditary BAP1 cancer predisposition syndrome, reporting three new cases. Genes Chromosomes Cancer 2014;53:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen DE, Proctor M, Marquis ST, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998;16: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 23.White AE, Harper JW. Cancer. Emerging anatomy of the BAP1 tumor suppressor system. Science 2012;337:1463–1464. [DOI] [PubMed] [Google Scholar]
- 24.Dey A, Seshasayee D, Noubade R, et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 2012;337:1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misaghi S, Ottosen S, Izrael-Tomasevic A, et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol 2009;29:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker KE, Parker R. Nonsense-mediated mRNA decay: terminating erroneous gene expression. Curr Opin Cell Biol 2004;16:293–299. [DOI] [PubMed] [Google Scholar]
- 27.Cheung M, Talarchek J, Schindeler K, et al. Further evidence for germline BAP1 mutations predisposing to melanoma and malignant mesothelioma. Cancer Genet 2013;206:206–210. [DOI] [PubMed] [Google Scholar]
- 28.Rai K, Pilarski R, Cebulla CM, et al. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet 2015;doi: 10.1111/cge.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid KW, Farid NR. How to define follicular thyroid carcinoma? Virchows Arch 2006;448:385–393. [DOI] [PubMed] [Google Scholar]
- 30.Eberhardt NL, Grebe SK, McIver B, et al. The role of the PAX8/ PPARgamma fusion oncogene in the pathogenesis of follicular thyroid cancer. Mol Cell Endocrinol 2010;321:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan N, Walden G, Lazic D, et al. Collision tumours of the thyroid: an interesting case and review of the literature. Head Neck 2014;37:E125–E129. [DOI] [PubMed] [Google Scholar]
- 32.Walvekar RR, Kane SV, D’Cruz AK. Collision tumor of the thyroid: follicular variant of papillary carcinoma and squamous carcinoma. World J Surg Oncol 2006;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadat Alavi M, Azarpira N. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumor with lymph node metastasis: a case report. J Med Case Rep 2011;5:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maerker DA, Zeschnigk M, Nelles J, et al. BAP1 germline mutation in two first grade family members with uveal melanoma. Br J Ophthalmol 2014;98:224–227. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro C, Campelos S, Moura CS, et al. Well-differentiated papillary mesothelioma: clustering in a Portuguese family with a germline BAP1 mutation. Ann Oncol 2013;24:2147–2150. [DOI] [PubMed] [Google Scholar]
- 36.Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissuebased map of the human proteome. Science 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 37.Weatherall DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet 2001;2:245–255. [DOI] [PubMed] [Google Scholar]
- 38.Nadeau JH. Modifier genes in mice and humans. Nat Rev Genet 2001;2:165–174. [DOI] [PubMed] [Google Scholar]
- 39.Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Hum Genet 2014;59:5–15. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Muzny DM, Xia F, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 2014;312:1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Need AC, Shashi V, Hitomi Y, et al. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet 2012;49:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]