Abstract
Approximately 100 million elderly will enter Medicare over the next 25 years. We consider the potential benefits of interventions that would reduce or eliminate the most important risk factors for disease and spending. Effective control of hypertension could reduce health care spending $890 billion for these cohorts while adding 75 million disability-adjusted life years (DALYs). Eliminating diabetes would add 90 million life-year equivalents at a cost of $2,761 per DALY. Reducing obesity back to levels seen in the 1980’s would have little effect on mortality, but yields great improvements in morbidity (especially heart disease and diabetes) with a cost savings of over $1 trillion. Smoking cessation will have the smallest impact, adding 32 million DALYs at a cost of $9.045 per DALY. While smoking cessation reduces lung disease and lung cancer, but these are relatively low prevalence compared to the other diseases. Its impact on heart disease is negligible. The effects on overall social welfare are unknown, since we do not estimate the costs of these interventions, the costs of any behavioral modification, or the welfare loss due to providers from lower medical spending.
Keywords: prevention, aging, medical technology, cost-effectiveness
Chronic illness imposes substantial burden on the elderly. Approximately 80% of the senior population has one or more chronic illnesses, and 25% are limited in their ability to perform activities of daily living because of chronic conditions (Fried, Freedman et al. 1997). Direct medical costs for persons with chronic conditions account for nearly 70% percent of national medical expenditures (Mockenhaupt and Ory 1998). This burden is further exacerbated by trends in younger cohorts. The past few decades have witnessed alarming increases in obesity and diabetes among the young (Mokdad, Ford et al. 2000; Mokdad, Serdula et al. 2000), and disability rates are rising within all demographic and economic groups (Lakdawalla, Bhattacharya et al. 2004). So not only will the number of elderly double in the next 25 years, the constellation of disease and disability facing them will be much greater.
Recent biomedical innovations hold considerable promise to improve the treatment of chronic illness, but their timing, cost, and availability is unclear. On the other hand, existing preventive practices and services could alleviate some of this burden by eliminating or forestalling expensive disease. The type of prevention is important, and this paper considers several risk factors linked to heart disease, one of the leading causes of mortality in the elderly. Improvements in health will allow the elderly to live longer and accrue more expenses and, as it is sometimes argued, ultimately incur more health care costs, as Lubitz et al. (2003) found in their study of disability (Lubitz, Cai et al. 2003). Our model, which is based on approximately 90,000 person-years of data for 40,000 Medicare beneficiaries, accounts for potential mortality improvements, as well as improvements in quality of life.
Using this model, we simulate the effects of interventions to reduce hypertension, smoking, obesity, and diabetes among the elderly. The results demonstrate that prevention among the elderly can be very cost-effective, but even the most effective interventions will not reduce health care spending, except in the case of obesity. The next section of this paper discusses our model in more detail.
METHODS
The future elderly model (FEM) begins with a representative sample of approximately 100,000 aged Medicare beneficiaries from the 1992–2000 Medicare Current Beneficiary Survey (MCBS), and tracks their health and spending over the course of their lifetime (after age 65). More detail about the model is provided in a technical appendix, so we provide only the most salient details here. Starting in 2005, we predict health care spending for everyone in this representative cohort. These predictions come from pooled weighted least squares regressions of total health care spending on risk factors, self-reported conditions, functional status, and interactions of conditions and functional status, with spending inflated to constant dollars using the medical component of the consumer price index.
We age our cohort by simulating health and functional outcomes in the subsequent year. This process first requires knowledge of the underlying risk of changing health. We model annual mortality and the development of several health conditions: stroke, heart disease, arthritis, Alzheimer’s disease, hypertension, diabetes, lung disease, lung cancer, breast cancer, prostate cancer, colon cancer, or other cancer. The definitions of health conditions are derived from questions in the MCBS of the form “Has a doctor ever told you….” Heart conditions include angina pectoris or coronary heart disease, myocardial infarction, and other heart conditions such as congestive heart failure, problems with the valves in the heart, or problems with the rhythm of heartbeat. Lung disease includes emphysema, asthma, or chronic obstructive pulmonary disorder. Arthritis does not include rheumatoid arthritis.
We measure functional status by limitations in activities of daily living (ADLs) and limitations in instrumental activities of daily living (IADLs). The ADL measure is based on a battery of questions asking respondents if they had any difficulty dressing; eating; bathing or showering; getting into and out of a chair; walking; and using the toilet. For IADLs, respondents were asked if they had any difficulty using the phone, doing light housework, doing heavy housework, making meals, shopping or managing money. Based on responses to these questions, we constructed a hierarchical measure of physical functioning: no limitations, limited in at least on instrumental activity of daily living, limited in 1 or 2 activities of daily living, limited in 3 or more activities of daily living, or living in a nursing home. The inclusion of nursing home as a level of physical functioning is necessary because of a dramatic drop in the report of ADL limitations among nursing home residents in 1997 and thereafter attributable to changes in the survey design. In 1996, 73% of nursing home respondents reported 3 or more limitations in activities of daily living.
Both functional status and the likelihood of developing a health condition depend on risk factors (gender, education, race, ethnicity, education, obesity (BMI>=30), overweight (25<=BMI<30), underweight (BMI<20), ever having smoked and currently smoking); other health conditions where clinically warranted, and age. We treated all health conditions as “absorbing” — i.e., once people got an illness, they had it forever and therefore could not get it again — and modeled transitions into these conditions. This assumption was consistent with the way the data were obtained (“Has a doctor ever told you…”) and with the course of most chronic diseases. However, we allowed for transitions into and out of functional states, but the probabilities were allowed to depend on functional status in the previous two years (second-order Markov) to capture inertia in physical functioning scores.
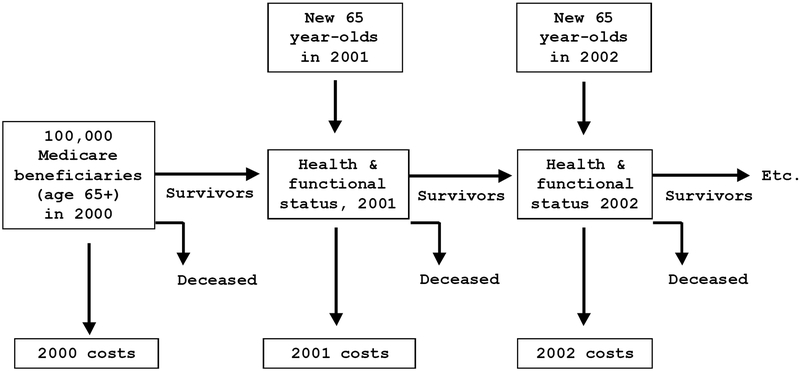
Using these models, we then predicted each person’s probability of dying, getting a new disease, or entering a new functional state using Monte Carlo techniques as summarized in Figure 1. As our initial sample ages, it becomes less representative of the Medicare population. We annually replenish our sample through 2030 with a new cohort of 65-year-olds using data on the health of younger cohorts from the 1982 to 1996 National Health Interview Surveys to predict the health of new Medicare entrants. For example, the health of 65-year olds in 2026 will depend on the health of 35-year olds in 1996, appropriately trended.
Figure 1.
Overview of the Simulation Model
PREVENTION SCENARIOS
In previous work, we conducted systematic literature searches and elicited consensus from several panels of distinguished experts to identify the medical technologies that would most impact the elderly over the next thirty years. This process identified 34 specific medical technologies most likely to affect the health of the future elderly in three clinical domains: cardiovascular disease, neurological disorders, and cancer and the biology of aging. We then used the FEM to forecast the likely consequences of these technologies (Goldman, Shang et al. forthcoming).
Throughout this process, however, our experts noted that lifestyle changes and better prevention could have the most dramatic effects on population health. They predicted that increased compliance with evidence-based effective medicine—through the use of computerized feedback, guidelines embedded in computerized medical record keeping software, better information technologies, expanded use of continuous quality improvement techniques, and the public release of performance data—would prevent disease progression and increase patient compliance with therapies with known efficacy. Furthermore, they predicted that continued investments in public health could affect outcomes more than many of the technologies they considered. We developed four prevention scenarios for the elderly population based on their recommendations: smoking cessation, hypertension control, diabetes control, and obesity control.
Scenario 1: Smoking Cessation
Several smoking-related interventions—including physician advice, psychosocial therapies, and pharmacotherapy—have been found to reduce smoking rates in the general population. (AHCPR 2000; Stead, Lancaster et al. 2003; Silagy, Lancaster et al. 2004; Stead and Lancaster 2004; Lancaster and Stead 2005; Stead and Lancaster 2005). Whether these interventions will work in the elderly is less clear. One randomized trial provided Medicare beneficiaries yearly preventative visits for two years and optional counseling visits to their primary care provider (Burton, Paglia et al. 1995). After controlling for sociodemographic factors and baseline health, a logistic regression model found no significant effect of intervention on smoking cessation. Another randomized trial compared usual care with physician-delivered brief quit-smoking advice and counseling for 659 smokers aged 50 to 74 (Morgan, Noll et al. 1996) and found a 7 percentage point improvement in quit rates (p<0.01). The Agency for Healthcare Policy and Research concluded in 2000 that several different modalities are effective in treating tobacco use in adults aged 50 and older (AHCPR 2000). Telephone counseling was especially promising because of particular concerns of this population, such as mobility (AHCPR 2000). But older patients make more frequent medical visits to their health care provider. Therefore primary physician plays an important role in motivating those who smoke to quit (Boyd 1996). Furthermore, nicotine replacement therapy is especially appropriate for older smokers who are addicted to nicotine (Boyd 1996).
We implement this scenario by assuming a smoking cessation program that is 100% effective for the elderly. Assuming such an extreme rate of effectiveness—which clearly exceeds the best behavioral and pharmaceutical therapies currently available—is useful as a way to determine the greatest benefit that might be derived from elderly smoking prevention.
Scenario 2: Controlling hypertension
Numerous studies have shown that hypertension is a major risk factor for heart disease and stroke. However, with the advances of medical technologies, effective treatments for hypertension, such as diuretics, β-blockers, angiotensin-converting enzyme (ACE) inhibitors and calcium channel antagonists, can significantly reduce these risks in the general population (Collins, Peto et al. 1990; MacMahon, Peto et al. 1990; Psaty, Smith et al. 1997; Neal, MacMahon et al. 2000; Yusuf, Sleight et al. 2000; PROGRESS Collaborative Group 2001). Studies designed to elucidate the effects among elderly patients have also found similar benefits (SHEP Cooperative Research Group 1991). In a randomized double-blinded comparison of placebo and active treatment for older patients with isolated systolic hypertension (Staessen, Fagard et al. 1997), active treatment reduced the rate of fatal and non-fatal cardiac endpoints by 26%.
We implement this scenario by assuming that hypertension is effectively treated in all elderly with the condition. As with smoking, assuming such an extreme rate of effectiveness is useful as a way to determine the greatest benefit that might be derived from elderly smoking prevention. Unlike smoking, however, the intervention is not unknown in this case. We find in our analysis that the odds ratios of hypertension on the risk of stroke and heart disease are 1.28 and 1.59 respectively. These estimates are consistent with effects derived from randomized clinical trials of anti-hypertensive treatments.
Scenario 3: Obesity reduction
Weights have increased markedly in the United States in the past two decades (CDC 2004). Increased weight results from a combination of increased caloric intake and reduced energy expenditure. Therapies are directed to one or both of these margins. Most therapeutic interventions have not been separately evaluated in the elderly, so we consider their impact in the general population. Diet and increased physical activity have been shown to reduce weight (National Heart 1998), the difficulty with these techniques is their durability. The majority of patients regain the initial weight loss within two to five years (Wing, Epstein et al. 1985; Wadden, Sternberg et al. 1989; Skender, Goodrick et al. 1996; Maggio and Pi-Sunyer 1997). Attention has thus focused on more intensive interventions to sustain weight loss.
Three classes of therapy have been examined. Behavioral and cognitive-behavioral therapy have been shown to be effective in reducing obesity, especially when combined with a diet/exercise approach (Shaw, O’Rourke et al. 2005). Pharmacotherapy has been of limited impact in the treatment of overweight and obesity. Once-popular pharmaceuticals such as fenfluramine and dexfenfluramine have been withdrawn from the market after reports linked them with valvular heart disease. Some studies have shown modest effectiveness of orlistat and sibutramine in promoting weight loss, but clinical trials for these agents are complicated by high attrition rates, likely a result of substantial side effects (Padwal, Li et al. 2004). For the morbidly obese, surgical interventions including gastic bypass and gastroplasty have been shown to result in substantial weight loss, as much as 23–28 kg. The risks associated with surgery limits its effectiveness in the moderately obese population, however.
Our simulated prevention scenario assumes that rates of obesity were cut in half, consistent with rates observed in the 1980’s. This extreme assumption is meant to emphasize the possible value of very efficacious BMI prevention.
Scenario 4: Diabetes Control
More than 10 percent of people over age 65 have clinical diabetes, the vast majority of which is type 2 diabetes. Diabetes has been among the top ten causes of death in the United States for several decades, and is the leading cause of end-stage renal disease and visual loss among individuals under age 65. In 1997, diabetes was responsible for approximately 2.3 million hospital admissions, 14 million hospital days, and 70 million nursing home days. Direct medical expenditures on diabetic care have been estimated at $44 billion (American Board of Family Practice 1997; Lebovitz 1997; American Diabetes Association 1998). One matched cohort analysis indicated that the annual excess expenditures for diabetic patients totaled $3,500 per person (Selby, Ray et al. 1997).
Our simulation presumes that diabetes can be perfectly controlled in the population, through both a combination of prevention and better treatment that would reduce the risks to those of a person with non-diabetes. This scenario—like the others—is an extreme case but is useful for gauging the relative value of prevention.
RESULTS
Risk Factors for Disease
Table 1 shows the effects of personal characteristics on incident disease. Each column corresponds to a separate logit estimation of the likelihood of developing the disease in the next year, and each entry is an odds ratio. Since the probabilities of these events are small (these are incidence rates), the odds ratios can be interpreted as relative risks1. Many of these relationships replicate findings from the epidemiological literature, as one would expect. For example, we find that the elderly 85 years of age or older are 1.86 times more likely to develop a stroke than those aged 65 to 74 (p<.01). Hypertension, diabetes, and heart disease all increase the risk of stroke. There are also racial and ethnic differences in the likelihood of developing certain conditions. Hispanics are 50% more likely to develop diabetes and hypertension, even after controlling for other factors. Blacks are more likely to develop arthritis and hypertension, but less likely to develop lung and heart disease (after controlling for hypertension).
Table 1.
Probability of Developing Various Health Conditions as a Function of Individual Characteristics (Ages 65 and older)
| Health condition: | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer: | ||||||||||||
| Risk Factor | Stroke | Heart | Arthritis | Alzheimer’s | HBP | Diabetes | Lung | Lung | Breast | Prostate | Colon | Other |
| Age: | ||||||||||||
| Age 65 to 74 | (reference category) | |||||||||||
| Age 75 to 84 | 1.801*** | 1.511*** | 1.447*** | 3.961*** | 1.185** | 1.090 | 1.244* | 0.818 | 1.220 | 1.366* | 1.845** | 1.413*** |
| Age 85 or above | 1.861*** | 1.924*** | 1.399*** | 7.166*** | 1.193* | 0.765 | 1.011 | 0.419 | 0.879 | 1.452 | 1.810* | 1.067 |
| Male | 1.129 | 1.090 | 0.738*** | 1.102 | 0.808*** | 1.132 | 0.836* | 1.534 | 1.077 | 1.178 | ||
| Black | 1.082 | 0.806* | 1.282** | 1.195 | 1.590*** | 1.180 | 0.580** | 0.948 | 1.318 | 1.346 | 0.498 | 0.584** |
| Hispanic | 0.829 | 0.943 | 0.954 | 0.767 | 1.490*** | 1.502* | 1.386 | 1.480 | 0.995 | 0.913 | 0.870 | 0.686 |
| Urban | 1.051 | 0.931 | 1.012 | 1.012 | 0.908 | 0.801* | 0.900 | 1.187 | 2.314** | 0.955 | 1.018 | 1.158 |
| Education: | ||||||||||||
| Less than high school | 1.083 | 1.076 | 0.955 | 1.579*** | 0.931 | 1.142 | 1.132 | 1.544 | 0.608* | 1.124 | 0.576** | 0.892 |
| High degree | (reference category) | |||||||||||
| College graduate | 0.884 | 1.122 | 1.013 | 1.182 | 0.904 | 1.042 | 0.748 | 1.155 | 1.336 | 1.376 | 0.982 | 1.066 |
| Smoking: | ||||||||||||
| Ever smoked | 1.075 | 1.065 | 2.043*** | 2.536** | ||||||||
| Current smoking | 1.445** | 1.057 | 1.880*** | 2.762*** | ||||||||
| Body mass index: | ||||||||||||
| Obese (bmi>=30) | 0.806 | 1.353*** | 1.484*** | 1.584*** | 3.216*** | 1.461** | 1.185 | 1.272 | 1.117 | 1.273 | 1.126 | |
| Overweight (25<=bmi<30) | 0.927 | 1.119 | 1.175** | 1.233** | 1.611*** | 1.290* | 1.525 | 1.229 | 1.041 | 1.265 | 1.034 | |
| Normal (20<=bmi<25) | (reference category) | |||||||||||
| Underweight (bmi<20) | 1.021 | 0.868 | 0.716*** | 0.760* | 0.534* | 1.817*** | 1.145 | 0.782 | 0.728 | 0.888 | 0.907 | |
| Health conditions: | ||||||||||||
| Hypertension | 1.275** | 1.590*** | ||||||||||
| Diabetes | 1.405** | 1.299*** | 1.382*** | |||||||||
| Heart disease | 1.289** | |||||||||||
| No. of observations | 46,677 | 28,498 | 22,002 | 51,174 | 23,449 | 43,907 | 45,100 | 51,735 | 28,106 | 20,274 | 50,757 | 47,226 |
Note: Each column shows the odds ratios from a separate logit regression of developing the disease shown in the column heading as a function of the variables shown in the rows. Since these logits measure incident disease--the probability of developing the disease in the next year--the sample will differ depending on the number of people in the 1992–2000 Medicare Current Beneficiary Survey who do not have the health condition in the prior year. Because the incidence rates are low, these can be interpreted as relative risks; so, for example, obese people have a 59% higher risk of developing hypertension than those of normal weight. HBP stands for high blood pressure; heart conditions include angina pectoris or coronary heart disease, myocardial infarction, and other heart conditions such as congestive heart failure, problems with the valves in the heart, or problems with the rhythm of heartbeat; lung disease includes emphysema, asthma, or chronic obstructive pulmonary disorder; arthritis does not include rheumatoid arthritis.
Of particular interest for this paper are the effects of body mass index, smoking, hypertension, and diabetes. Obesity—defined as a body mass index of 30 kg/m2 or more—is strongly associated with increased risk of diabetes (222% increase), hypertension (58%), lung disease (46%), arthritis (48%), and heart disease (35%) relative to those of normal weight. It is also important to note that the higher risk of heart disease is significant even after one adjusts for the presence of hypertension and diabetes. The adjustment may also explain why obesity is not associated with an increased risk of stroke after controlling for these two conditions. We also see no relationship between elderly obesity and cancer, despite some preliminary epidemiologic evidence suggesting otherwise. Individuals with a BMI of at least 30 kg/m2 had an elevated risk of pancreatic cancer compared with those with a BMI of less than 23 kg/m2 in two prospective cohort studies (Michaud, Giovannucci et al. 2001), but such cancer is relatively rare and so will not drive this relationship in our analysis.
Current smoking status and ever smoked are included as risk factors in our analysis. There are several reasons for this decision. The first is that any exposure to smoking may have lifetime consequences, especially if the smoking habit was heavy and persisted over a long period of time. Smoking cessation programs directed at the elderly will not eliminate this past behavior—and its concomitant health risk—they will merely change current status. Second, there is some selection in current smoking status. People who are ill might cease smoking, and so current smoking by itself in these analyses would be subject to bias (since sicker people are less likely to be smoking). The inclusion of past smoking provides an (admittedly imperfect) control for this behavior. One could also argue that our sample is still subject to mortality selection, in the sense that many of the smokers have died off or become very ill before entering our (elderly) sample. In fact, this is not a bias for which we need to adjust, since our purpose is to model smoking cessation efforts for the elderly. If all the smokers are already very sick, then smoking cessation will be ineffective and we want to capture that fact.
We do find that current smoking significantly increases risk of stroke (45%), lung disease (88%), and lung cancer (177%). Past smoking has similar effects on lung disease and lung cancer, but does not seem to affect stroke risk. We do not find that smoking among the elderly affects the risk of heart disease, a results that is consistent with epidemiological evidence. There are two points here. First, while smoking clearly increases the risk of heart disease in younger populations, this effect is attenuated at older ages. Second, this does not mean that the smoking does not improve survival among those who already have heart disease (as the CASS study showed).
Risk Factors for Mortality and Functional Status
Table 2 shows the odds ratios associated with dying or functional limitations for various risk factors. These can be interpreted as relative risks since the raw probability of dying is 5.0% for all elderly in the MCBS2 (Zhang and Yu 1998). The risk of dying increases substantially with age, and men have higher risk than women (although the age profile is similar as demonstrated by the insignificant age-gender interactions). There is a gradient by education (those with more education are less likely to die), even after controlling disease, functional status, and other risk factors. This finding is consistent with evidence of healthy behavior—such as better adherence to medication—found in other studies and not controlled for here (Goldman and Smith 2002). Current and past smoking both increase the risk of death—even after controlling for lung diseases, lung cancer, and other conditions—by 21 and 25 percent, respectively.
Table 2.
Probability of Dying or Developing Functional Limitations, Ages 65 and Older
| Risk Factor | Mortality | 1 or more IADLs | 1 or more ADLs | 3 or more ADLs | Nurs. Home Entry |
|---|---|---|---|---|---|
| Age | |||||
| Age 65 to 74 | (reference category) | ||||
| Age 75 to 84 | 2.069*** | 1.831*** | 1.954*** | 1.828*** | 3.827*** |
| Age 85 or older | 3.592*** | 2.968*** | 3.472*** | 2.977*** | 9.552*** |
| Gender & age interactions: | |||||
| Male | 1.958*** | 0.461*** | 0.845** | 0.938 | 1.069 |
| Male * Age 65 to 74 | (reference category) | ||||
| Male * age 75 to 84 | 0.845 | 1.050 | 0.961 | 0.972 | 0.928 |
| Male * Age 85 or older | 0.818 | 1.550*** | 1.087 | 1.025 | 0.870 |
| Black | 1.049 | 1.075 | 1.087 | 1.047 | 0.713** |
| Hispanic | 0.904 | 0.883 | 0.992 | 1.075 | 0.412*** |
| Urban | 0.961 | 0.882*** | 0.995 | 1.045 | 0.817** |
| Education: | |||||
| Less than high school | 1.103* | 1.295*** | 1.119** | 1.208*** | 1.153* |
| High degree | (reference category) | ||||
| College graduate | 0.856* | 0.831*** | 0.827** | 1.023 | 0.656** |
| Smoking: | |||||
| Ever smoked | 1.245*** | 1.054 | 1.078 | 0.955 | 0.953 |
| Current smoking | 1.207** | 1.226*** | 1.255*** | 1.000 | 1.169 |
| Body mass index: | |||||
| Obese (bmi>=30) | 0.676*** | 1.550*** | 1.668*** | 1.248** | 0.710** |
| Overweight (25<=bmi<30) | 0.721*** | 1.065 | 1.217*** | 0.936 | 0.746*** |
| Normal (20<=bmi<25) | (reference category) | ||||
| Underweight (bmi<20) | 1.664*** | 1.301*** | 1.140 | 1.202* | 1.290** |
| Cancers: | |||||
| Lung cancer | 4.316*** | 1.154 | 1.584* | 1.285 | 1.605 |
| Prostate cancer | 1.368** | 1.183 | 1.204 | 1.050 | 1.258 |
| Breast cancer | 1.055 | 1.261** | 0.978 | 1.134 | 0.996 |
| Colon cancer | 1.306* | 1.083 | 1.025 | 1.113 | 1.196 |
| Other cancer | 1.522*** | 1.264*** | 1.238*** | 1.160 | 0.868 |
| Health conditions: | |||||
| Heart disease | 1.411*** | 1.513*** | 1.207*** | 1.175** | 0.893 |
| Diabetes | 1.628*** | 1.528*** | 1.408*** | 1.266*** | 1.421*** |
| Hypertension | 1.073 | 1.268*** | 1.229*** | 1.143* | 0.865* |
| Stroke | 1.278*** | 1.851*** | 1.552*** | 1.732*** | 1.536*** |
| Lung disease | 1.312*** | 1.895*** | 1.546*** | 1.198** | 1.019 |
| Alzheimer’s disease | 1.253*** | 1.418** | 1.986*** | 2.361*** | 3.484*** |
| Osteoarthritis | 0.775*** | 1.778*** | 1.693*** | 1.257*** | 0.757*** |
| Disability: | |||||
| No limitations | (reference category) | ||||
| IADLs only | 1.594*** | 2.376*** | 3.149*** | 3.191*** | |
| 1 or 2 ADLs | 2.410*** | 9.007*** | 3.615*** | ||
| 3 or more ADLs | 4.532*** | 6.464*** | |||
| Nursing home | 6.694*** | ||||
| No. of observations | 57,626 | 46,232 | 37,351 | 47,275 | 53,342 |
Note: Each column shows the odds ratios from a separate logit regression of dying or developing a functional limitation. Since these logits measure incident death or disability, the sample differs across columns. Because the incidence rates are low, each entry can be interpreted as a relative risk; so, for example, patients with Alzheimer’s disease have a 248% greater chance of entering a nursing home ceteris paribus. In the FEM, functional status will be actually be modelled using an ordered logit (no limitations, IADL limitations only, 1 or 2 ADL limitations, 3 or more ADL limitations, or nursing home). Independent logit estimation is conducted here because of the ease of interpretation. (ADL and IADL limitations are not available for those in the nursing home after 1997). Heart disease includes angina pectoris or coronary heart disease, myocardial infarction, and other heart conditions such as congestive heart failure, problems with the valves in the heart, or problems with the rhythm of heartbeat; lung disease includes emphysema, asthma, or chronic obstructive pulmonary disorder; arthritis does not include rheumatoid arthritis.
The presence of many chronic diseases and cancers is associated with higher risk of death. Interestingly, hypertension and breast cancer among the elderly do not increase the risk of death, although the hypertension effect should be interpreted as an effect after controlling for heart disease. (We saw earlier that hypertension has a large effect on developing heart conditions.) Diabetes increases the risk of death by 63%. In part this may be explained by our lack of controls for kidney-related diseases. Functional status also has a strong association with mortality, especially residence in a nursing home. Much of this effect arises because functional status proxies for unobserved illness, although there may be a causal component.
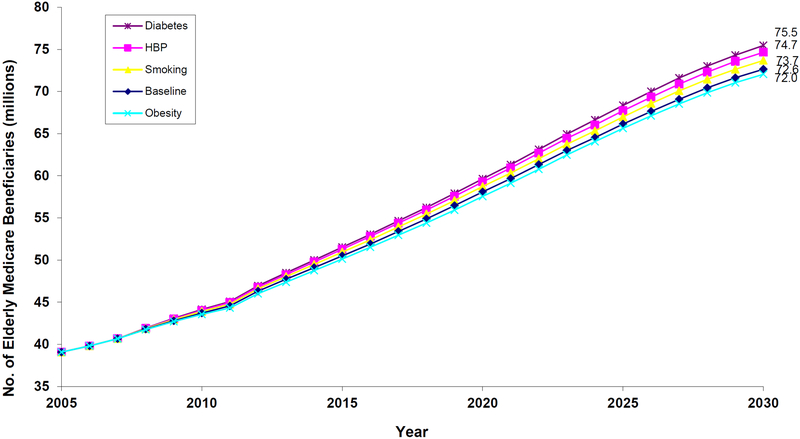
Population
Figure 2 shows the number of elderly Americans aged 65 or older with Parts A and B Medicare coverage. Under the status quo, this population will reach 72.6 million by 2030; an estimate that corresponds closely to the 71.4 million estimate of Part A and B enrollees from the 2005 Trustees’ report. The inflection point at 2012 in population growth is due to the baby boomers entering Medicare. More importantly for this paper, however, is the effect prevention would have on our baseline estimate. We have 4 scenarios:
Hypertension control (100% effective)
Diabetes control (100% effective)
Smoking cessation (100% effective)
Obesity reduction to 1980 levels (50% reduction)
Figure 2.
Projections of the Elderly Medicare Population Under Various Prevention Scenarios, 2005–2030
Notes: figure shows the number of elderly Americans aged 65 or older with Parts A and B Medicare coverage. Projections are made using the future elderly model. Under the status quo, this population will reach 72.6 million by 2030. 100% effective diabetes control, the population would be 2.9 million greater; with 100% effective hypertension control, 2.1 million greater; with 100% effective smoking cessation, 1.1 million greater. A 50% reduction in obesity prevalence would reduce the population by 0.6 million. The (very small) reduction in the obesity scenario is due to the slightly protective effects of higher body mass in the elderly population, which counteracts the beneficial health effects of weight reduction.
If diabetes could be controlled completely effectively, this population would be 2.9 million higher—a 4% increase. Other interventions would increase the population, but not by as much. Hypertension control would increase the 2030 beneficiary population by 2.1 million (3%); universal smoking cessation, by 1.1 million (2%). Obesity reduction would have a small effect on population, reducing it by 0.6 million (1%) in 2030. This reduction can be attributed to the slightly protective effects of higher body mass in the elderly population, which counteracts the beneficial health effects of weight reduction.
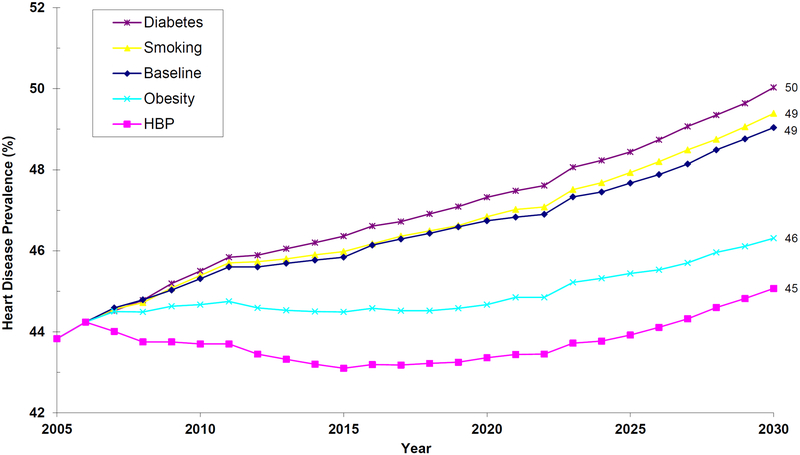
Health
Figure 3 summarizes the results for heart disease. Under the status quo, heart disease prevalence will rise to 49% by 2030. Hypertension control would have the greatest impact on heart disease. By 2030, the rate would be reduced by 4 percentage points. Obesity control also would result in a 3 percentage-point improvement. Smoking cessation would not have an appreciable impact on heart disease in the elderly, for two reasons. First, the clinical evidence (and Table 1) demonstrate that smoking cessation is less beneficial in preventing heart disease at older ages. Second, smoking will keep people alive somewhat longer, and age is an independent risk factor for heart disease. A similar story obtains for diabetes—it is not as strong a risk factor as hypertension (Table 1), and because diabetes is having the greatest effect on the population, the age effect is working against the beneficial effects of disease reduction.
Figure 3.
Projections of Heart Disease Prevalence in the Elderly Medicare Population, 2005–2030
Notes: The figure shows prevalence of heart disease among Medicare beneficiaries aged 65 or older with Parts A and B Medicare coverage. Projections are done using the future elderly model. Respondents are assumed to have heart disease if they self-reported that a doctor ever told them they had angina pectoris or coronary heart disease; myocardial infarction; or another heart condition such as congestive heart failure, problems with the valves in the heart, or problems with the rhythm of heartbeat. Under the status quo, heart disease prevalence will rise to 49% by 2030. With effective hypertension control, there will be a 4 percentage-point improvement; and with effective obesity control, a 3 percentage-point improvement. Smoking cessation will not reduce the prevalence of heart disease in the elderly—a finding consistent with the evidence that smoking cessation is less beneficial in preventing heart disease at older ages. Diabetes will raise the prevalence of heart disease because people are living longer and age is an independent risk factor for disease.
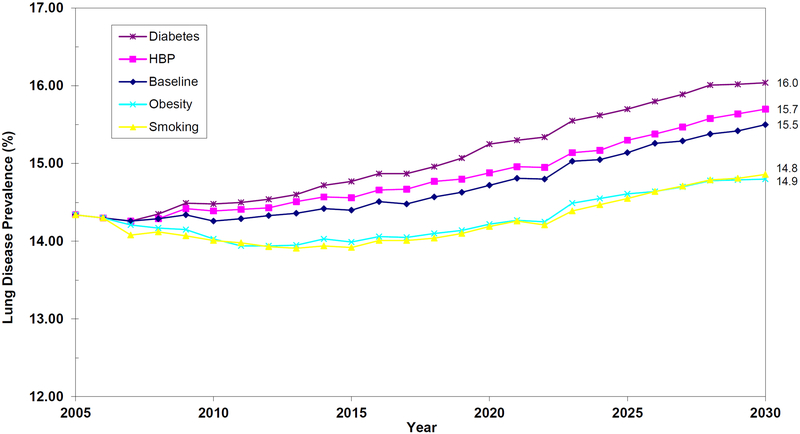
Figure 4 shows the effects of prevention on the prevalence of lung disease. Smoking and obesity control will have modest beneficial effects on the prevalence of lung disease. The modest effects of smoking are somewhat surprising; however, it should be noted that less than 20% of the entering Medicare cohorts smoke. Thus, while smoking is a strong risk factor for emphysema, asthma, and COPD, it only contributes to a minority of incident cases. (A natural analogy is the observation that only about 50% of lung cancer patients were smokers, despite the fact that smoking is such an important risk factor.)
Figure 4.
Projections of Lung Disease Prevalence in the Elderly Medicare Population, 2005–2030
Notes: The figure shows prevalence of lung disease among Medicare beneficiaries aged 65 or older with Parts A and B Medicare coverage. Projections are done using the future elderly model. Respondents are assumed to have lung disease if they self-reported that a doctor ever told them they had asthma, emphysema, or chronic obstructive pulmonary disease. Smoking and obesity control will have modest beneficial effects on the prevalence of lung disease.
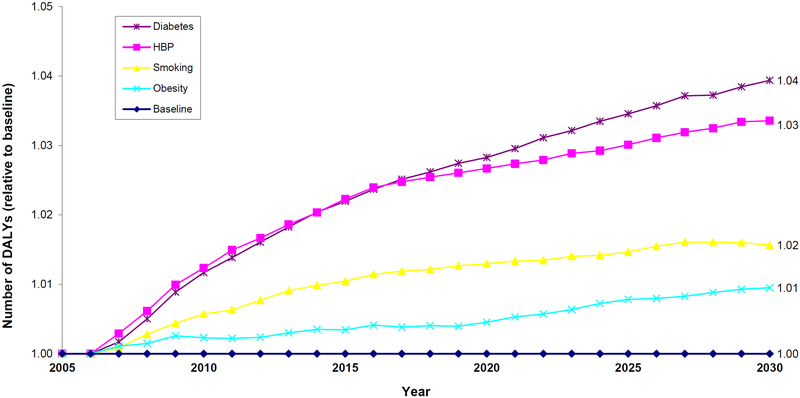
Figure 5 incorporates these health and population changes into an overall effect on disability-adjusted life years (DALYs). The results are shown relative to the status quo (baseline=1.0) Overall improvements are greatest in the diabetes and hypertension control scenarios. Modest improvements also occur with smoking cessation. Obesity control does not improve DALYs because-while obesity is associated with reduced physical functioning—it also worsens survival. These effects offset in the obesity reduction scenario.
Figure 5.
Projections of Relative Disability-Adjusted Life Years of the Elderly Medicare Population, 2005–2030
Notes: The figure shows the total level of DALYs, relative to the status quo (baseline=1.0), of Medicare beneficiaries aged 65 or older with Parts A and B Medicare coverage, which is a combination of both changes in survival and functioning. Projections are done using the future elderly model. Respondents in a nursing home are assumed to have a DALY score of 0.4; otherwise, the score is measures as follows: 3 or more ADLs (0.6); 1 or 2 ADLs (0.8); and 1 or more IADLs (0.9); and 1.0 if they are not limited at all in activities of daily living or instrumental activities of daily living. Average functioning is most improved in the diabetes and hypertension control scenarios. Total DALYs also increase with smoking cessation. Obesity control does not improve DALYs because-while obesity is associated with reduced physical functioning -it also worsens survival. These effects offset in the obesity reduction scenario.
Spending
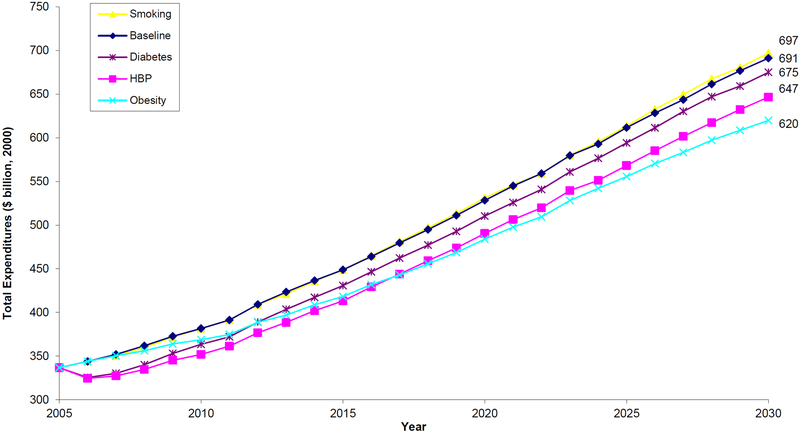
Figure 6 summarizes the effects of prevention on elderly medical spending. The figure shows total spending by elderly (65 years of age or older) Medicare beneficiaries enrolled in Parts A and B. Spending is measured in 2000 dollars. Under the status quo, total spending would rise to $691.1 billion in 2030. The greatest annual savings come from obesity reduction and effective hypertension control, which would reduce spending in 2030 by $71 billion and $44 billion respectively.
Figure 6.
Projections of Total Medical Expenditures by the Elderly Medicare Population, 2005–2030
Notes: The figure shows total spending by elderly (65 years of age or older) Medicare beneficiaries enrolled in Parts A and B. Spending is measured in 2000 dollars. Under the status quo, total spending would rise to $691 billion in 2030. The greatest annual savings come from obesity reduction and effective hypertension control, which would reduce spending in 2030 by $71 billion and $44 billion respectively.
The potential benefits of prevention are summarized in Table 3. These estimates exclude the costs of prevention, which include not only the costs of a possible treatment, but also the costs of behavioral modification, both of which may be substantial. For example, obesity reduction and smoking cessation might entail behavioral changes that are costly to the people who may have to modify their diet or smoking habits. Thus, our potential benefits in Table 3 can be thought of as the consequences if a pill were developed that perfectly mitigated the consequences of diabetes, hypertension, smoking, or reduced obesity back to 1980 levels without any side effects or behavioral change. How much would such a pill be worth?
Table 3.
Effects of Prevention on the Health and Spending of Medicare Entrants, 2005–2030
| All Medicare Entrants: | Per Entrant: | Per Treated: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | Change in total DALYs (millions) | Change in total spending (billions) | Change in Lifetime DALYs | Change in lifetime spending | Change in Lifetime DALYs | Change in lifetime spending | Cost/DALY | |||
| Eliminating Hypertension | 75.3 | −890 | 0.69 | −8,197 | 0.81 | −9,543 | Cost saving | |||
| Smoking cessation | 32.4 | 293 | 0.30 | 2,699 | 1.99 | 18,006 | 9,045 | |||
| Reducing obesity | 16.4 | −1,201 | 0.15 | −11,055 | 0.44 | −32,474 | Cost saving | |||
| Eliminating diabetes | 90.0 | 246 | 0.82 | 2,263 | 1.75 | 4,831 | 2,761 | |||
Notes: Effects are computed based on lifetime spending and health patterns for the 108 million elderly Medicare beneficiaries who will enter the program between 2005 and 2030. Spending estimates do not include the costs of prevention or monetized welfare loss of any behavioral changes. Hypertension prevention and obesity reduction save money and improve health. The per entrant estimates are averaged over the entire population, not just those who are treated. Eliminating hypertension and diabetes, for example, would, on average, add about three-quarters of a life year for all Medicare beneficiaries.
The estimates are computed for the 108 million Medicare beneficiaries who will enter the program between 2005 and 2030. In the case of hypertension, such prevention would result in 75 million additional disability-adjusted life years and would result in lower medical spending of $890 billion (2000 dollars). The health benefits of diabetes are even greater; 90 million disability-adjusted life years would be saved. In the case of diabetes prevention, however, spending would exceed the status quo by $246 billion, yielding a cost per DALY of $2,761. Smoking cessation would save 32 million disability-adjusted life-years but costs would rise by $293 billion, yielding a cost per DALY of $9,045. Obesity prevention would increase DALYs by the least, but the cost savings are relatively large: $1.2 trillion.
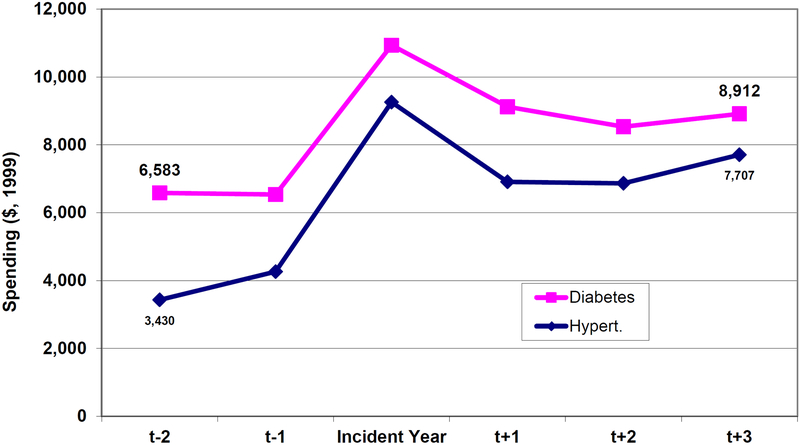
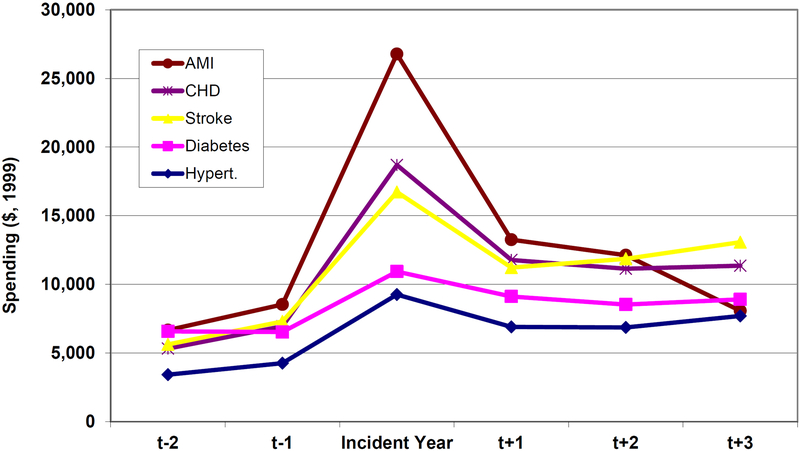
The greater savings from hypertension control compared with diabetes control are worth looking at in more detail. Figure 7 shows the unadjusted mean costs (in 1999 dollars) of incident cases of diabetes and hypertension. People who develop diabetes are sicker than those who develop hypertension prior to disease onset; and hypertension is associated with a larger increase in spending after the incident year. So when we eliminate disease in our prevention scenarios, ceteris paribus, we are keeping more expensive people alive in the diabetes case. And because diabetes has a larger, independent effect on mortality, these people are being kept alive longer (as summarized in the population estimates of Figure 2). Furthermore, hypertension is more strongly associated with heart disease in the elderly, and heart disease is more expensive to treat (Figure 8). So, in the case of hypertension, we avoid more costly disease for each person treated.
Figure 7.
Annual Medical Spending Prior to, and After, Onset of Diabetes and Hypertension
Notes: The figure shows average annual medical spending in 1999 dollars for Medicare beneficiaries 65 years of age or older in the MCBS. Data come from the 1992–1999 MCBS and have been inflated to 1999 dollars using the medical CPI. Note that beneficiaries could be in both groups if they have both illnesses. Incident year defined based on respondents answers to questions of the form “…did a doctor ever tell you you had…”. The relative findings are not sensitive to whether one includes terminal year costs in the calculations. Hypertension patients have higher costs prior to physician diagnosis; and their spike more in that year and subsequently.
Figure 8.
Annual Medical Spending Prior to, and After, Disease Onset for Selected Conditions
Notes: See notes for previous figure. Onset of coronary heart disease and acute myocardial infarction increase costs by the greatest amount.
CONCLUSION
This paper considers the impact of magic bullets. Without data on the efficacy and cost of specific intervention, it cannot be used for cost-utility analysis. Neither can we conduct welfare analysis without considering the costs of behavioral modification, or the implications of lower spending not only for patients but also providers. Still, the results do provide some useful insight for how for prevention-based resources might best be allocated.
First, we find that obesity reduction in the elderly has no effect on mortality, but yields great improvements in morbidity (especially heart disease and diabetes). This finding is consistent with the recent Flegan et al evidence that obesity is not protective at older ages. In fact, the lack of a mortality effect is precisely what yields such large reductions in medical savings; life is not extended, and but the years of life are relatively disease-free. The bottom line for obesity prevention is that if people want to save lives, they must intervene earlier; but if they want to reduce costs; they can wait until older age.
Diabetes reduction, on the other hand, has opposite implications. Diabetes has a big impact on mortality, but not as much on morbidity (at older ages). The result is that controlling diabetes will increase costs. The question then becomes whether it can do so in a cost-effective manner, and this depends in large part on the particular intervention. Hypertension reduction is an intermediate scenario between diabetes and obesity: it improves both morbidity and mortality, and it does so by a great amount. Smoking cessation will have the smallest impact. It improves lung disease and lung cancer, but these are relatively low prevalence compared to the other populations. And only about 15% of the elderly smoke. This is especially true once we take into account efficacy.
Our results suggest that prevention strategies addressing these risk factors in the elderly—especially hypertension and diabetes—could be very cost-effective, especially when viewed in the penumbra of recent policy decisions. For example, Medicare recently approved prophylactic use of implantable cardioverter defibrillators (ICDs) for patients with heart failure or poor function in their left ventricle. The cost of this prevention is at least $50,000 per additional life-year (Al-Khatib, Anstrom et al. 2005). Of course, this would depend on both the efficacy of the intervention, its cost, and the (often substantial) cost of having to change behavior.
Ultimately, a forecasting exercise is less important as a literal “reading of the future” than as an attempt to unpack the competing forces of the present day. Two issues are paramount. First, the rising prevalence of chronic disease among the elderly will continue to drive increases in health care spending. At the same time, advances in bioengineering, genetics, the life sciences, and clinical medicine may lead to rapid improvements in medical care. Other research (Goldman, Shang et al. forthcoming) suggests that many of these will result in modest improvements in health that are often, but not always, worth the cost. Prevention strategies may present one of the few opportunities to improve health and save money.
Acknowledgements
This work was funded by the Centers for Medicare and Medicaid Services (Contract No. 500-95-0056) and the National Institute on Aging through its support of the RAND Roybal Center for Health Policy Simulation (P30AG024968). We would like to thank participants from the NIH Economic Roundtable held on June 1, 2005 at the RAND Corporation in Virginia.
Footnotes
The incidence rates per 1000 population are: heart (52); lung cancer (2); prostate cancer (4); breast cancer (3); colon cancer (3); other cancer (10); diabetes (15); hypertension (63); stroke (13); Alzheimer’s (14); and arthritis (94).
The incidence rates per 1000 population are: IADL limitation (131); one or more ADL limitation (110); 3 or more ADLs (39); nursing home entry (17); and mortality (49). Thus, the odds ratios for some of these measures will overstate relative risks for measures like IADL limitations and one or more ADL limitations.
REFERENCES
- AHCPR (2000). “A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives.” JAMA 283(24): 3244–54. [PubMed] [Google Scholar]
- AHCPR (2000). Clinical Practice Guideline: Treating Tobacco use and Dependence.
- Al-Khatib SM, Anstrom KJ, et al. (2005). “Clinical and economic implications of the Multicenter Automatic Defibrillator Implantation Trial-II.” Ann Intern Med 142(8): 593–600. [DOI] [PubMed] [Google Scholar]
- American Board of Family Practice (1997). Diabetes Mellitus Reference Guide. Lexington, KY, American Board of Family Practice. [Google Scholar]
- American Diabetes Association (1998). “Economic consequences of diabetes mellitus in the US in 1997.” Diabetes Care(21): 296–309. [DOI] [PubMed] [Google Scholar]
- Boyd N (1996). “Smoking cessation: a four-step plan to help older patients quit.” Geriatrics(51): 52–7. [PubMed] [Google Scholar]
- Burton L, Paglia M, et al. (1995). “The effect among older persons of a general preventive visit on three health behaviors: smoking, excessive alcohol drinking, and sedentary lifestyle.” Prev Med 24(5): 492–7. [DOI] [PubMed] [Google Scholar]
- CDC (2004). Health, United States, 2004. Hyattsville, MD, US Department of Health and Human Services, CDC, National Center for Health Statistics. [Google Scholar]
- Collins R, Peto R, et al. (1990). “Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context.” Lancet 335(8693): 827–38. [DOI] [PubMed] [Google Scholar]
- Fried LP, Freedman M, et al. (1997). “Building communities that promote successful aging.” West J Med 167(4): 216–9. [PMC free article] [PubMed] [Google Scholar]
- Goldman DP, Shang B, et al. (forthcoming). “Consequences of Health Trends and Medical Innovation for the Future Elderly.” Health Affairs (Millwood). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DP and Smith JP (2002). “Can patient self-management help explain the SES health gradient?” Proc Natl Acad Sci U S A 99(16): 10929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakdawalla DN, Bhattacharya J, et al. (2004). “Are the young becoming more disabled?” Health Affairs (Millwood) 23(1): 168–76. [DOI] [PubMed] [Google Scholar]
- Lancaster T and Stead L (2005). “Individual behavioural counselling for smoking cessation.” The Cochrane Database of Systematic Reviews 2005, issue 2. [DOI] [PubMed] [Google Scholar]
- Lebovitz HE (1997). Introduction: Goals of Treatment Therapy for Diabetes Mellitus and Related Disorders. Kelly DB. Alexandria, VA, American Diabetes Association. [Google Scholar]
- Lubitz J, Cai L, et al. (2003). “Health, life expectancy, and health care spending among the elderly.” New England Journal of Medicine 349(11): 1048–55. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Peto R, et al. (1990). “Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias.” Lancet 335(8692): 765–74. [DOI] [PubMed] [Google Scholar]
- Maggio CA and Pi-Sunyer FX (1997). “The prevention and treatment of obesity. Application to type 2 diabetes.” Diabetes Care 20(11): 1744–66. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Giovannucci E, et al. (2001). “Physical activity, obesity, height, and the risk of pancreatic cancer.” Jama 286(8): 921–9. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt RE and Ory MG (1998). Medical self-care and managed care.
- Mokdad AH, Ford ES, et al. (2000). “Diabetes trends in the U.S.: 1990–1998.” Diabetes Care 23(9): 1278–83. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Serdula MK, et al. (2000). “The continuing epidemic of obesity in the United States.” Journal of the American Medical Association 284(13): 1650–1. [DOI] [PubMed] [Google Scholar]
- Morgan GD, Noll EL, et al. (1996). “Reaching midlife and older smokers: tailored interventions for routine medical care.” Prev Med 25(3): 346–54. [DOI] [PubMed] [Google Scholar]
- National Heart, L., and Blood Institute Obesity Education Initiative. (1998). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health; Bethesda, MD, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute. [Google Scholar]
- Neal B, MacMahon S, et al. (2000). “Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Blood Pressure Lowering Treatment Trialists’ Collaboration.” Lancet 356(9246): 1955–64. [DOI] [PubMed] [Google Scholar]
- Padwal R, Li SK, et al. (2004). “Long-term pharmacotherapy for obesity and overweight.” Cochrane Database Syst Rev(3): CD004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROGRESS Collaborative Group (2001). “Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.” Lancet 358(9287): 1033–41. [DOI] [PubMed] [Google Scholar]
- Psaty BM, Smith NL, et al. (1997). “Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis.” Jama 277(9): 739–45. [PubMed] [Google Scholar]
- Selby JV, Ray GT, et al. (1997). “Excess costs of medical care for patients with diabetes in a managed care population.” Diabetes Care 20(9): 1396–402. [DOI] [PubMed] [Google Scholar]
- Shaw K, O’Rourke P, et al. (2005). “Psychological interventions for overweight or obesity.” Cochrane Database Syst Rev(2): CD003818. [DOI] [PubMed] [Google Scholar]
- SHEP Cooperative Research Group (1991). “Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP).” Jama 265(24): 3255–64. [PubMed] [Google Scholar]
- Silagy C, Lancaster T, et al. (2004). “Nicotine replacement therapy for smoking cessation.” The Cochrane Database of Systematic Reviews 2004, issue 3. [DOI] [PubMed] [Google Scholar]
- Skender ML, Goodrick GK, et al. (1996). “Comparison of 2-year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions.” J Am Diet Assoc 96(4): 342–6. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Fagard R, et al. (1997). “Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators.” Lancet 350(9080): 757–64. [DOI] [PubMed] [Google Scholar]
- Stead L and Lancaster T (2004). “Antidepressants for smoking cessation.” The Cochrane Database of Systematic Reviews 2004, issue 4. [DOI] [PubMed] [Google Scholar]
- Stead L and Lancaster T (2005). “Group behaviour therapy programmes for smoking cessation.” The Cochrane Database of Systematic Reviews 2005, issue 2. [DOI] [PubMed] [Google Scholar]
- Stead L, Lancaster T, et al. (2003). “Telephone counseling for smoking cessation.” The Cochrane Database of Systematic Reviews 2003, issue 1. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Sternberg JA, et al. (1989). “Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five-year perspective.” Int J Obes 13: 39–46. [PubMed] [Google Scholar]
- Wing RR, Epstein LH, et al. (1985). “Behavior change, weight loss, and physiological improvements in type II diabetic patients.” J Consult Clin Psychol 53(1): 111–22. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, et al. (2000). “Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators.” N Engl J Med 342(3): 145–53. [DOI] [PubMed] [Google Scholar]
- Zhang J and Yu KF (1998). “What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes.” Jama 280(19): 1690–1. [DOI] [PubMed] [Google Scholar]