Platelet counts of all women with an uncomplicated pregnancy gradually decrease at approximately the same rate throughout their pregnancy.1 Dilution of platelets by plasma volume expansion is an apparent cause for lower platelet counts, but multiple additional physiologic changes during pregnancy may also contribute to the lower platelet counts.
Blood circulation through the placenta is similar to blood circulation through the spleen. In the spleen, some blood flows directly from arterioles to venules, without intervening capillaries, while other blood is shunted into the low pressure pools in the sinusoids. Plasma preferentially flows directly to the venules, described as “plasma skimming”, while the blood cells are sequestered in the sinusoids.2 Platelet counts in splenic blood are 7-fold greater than platelet counts in peripheral blood.3 In normal adults, one-third of all circulating platelets are transiently sequestered within the splenic sinusoids. Increased spleen size results in greater splenic sequestration of platelets and lower peripheral blood platelet counts.3
In the placenta some blood also flows directly from arterioles to venules, without intervening capillaries, while other blood is shunted into the low pressure pools in the intervillous spaces. These similar patterns of circulation suggest that there may be increased sequestration of blood cells in the placental intervillous spaces, similar to the splenic sinusoids. Although platelets sequestered within splenic sinusoids return to the circulation,3 platelets sequestered within the intervillous spaces may be consumed by incorporation into the perivillous fibrinoid. Perivillous fibrinoid is present in all term placentas. It is a product of coagulation that fills gaps in the syncytiotrophoblast cells lining the surface of mature villi and maintains the integrity of the villous surface.4,5
Platelet counts of women with twin pregnancies are lower than platelet counts of women with singleton pregnancies at each trimester and at delivery.1 Women with twin pregnancies have larger placentas, or may have two placentas. This suggests that larger placentas in women with twin pregnancies may contribute to their greater platelet count decrease.
Based on these observations, we postulated that blood cells are sequestered within the intervillous spaces of the placenta and that platelets are then consumed by incorporation into perivillous fibrinoid. To test this hypothesis, we compared blood cell counts in maternal peripheral blood to blood obtained from the placental intervillous space.
We collected placentas from 40 women with uncomplicated singleton pregnancies who had a scheduled cesarean delivery for routine indications at the Oklahoma University Medical Center. The mother’s complete blood count (CBC) from the day of delivery was recorded. At delivery, placentas were immediately immersed in a solution of 4.5 mmol ethylenediaminetetraacetic acid (EDTA) in 0.85% sodium chloride to prevent blood coagulation within the intervillous space. Maternal placental blood was collected from the intervillous space6 and placental tissue samples for microscopy were also collected at the same time. Contamination of maternal blood with fetal blood was determined by the percent of fetal red blood cells in each sample. Then a CBC was performed on the placental blood sample with the lowest percentage of fetal blood. A stained smear of the placental blood was examined for platelet clumping; if clumping occurred, the sample was not used. To correct for dilution of placental blood by the EDTA solution, we used the ratio of hemoglobin concentration in the peripheral blood to placental blood to adjust the placental white blood cell and platelet counts. To determine if contamination of maternal blood from the intervillous space by fetal blood influenced the blood counts, we separately analyzed blood counts on the 20 women with placental blood samples that had fetal blood contamination less than the median value. This study was approved by the University of Oklahoma Institutional Review Board (approval #4156).
The median time between delivery and placental blood sample collection was 18 minutes (range, 13–82 minutes). The placental weight (median 501 gm, range 364–938) was more than two-fold the weight of a normal adult spleen (150–200 gm). The median total white blood cell count, neutrophil count, and lymphocyte count, corrected for dilution by the EDTA solution, were all higher in placental blood than in the peripheral venous blood. These differences were significant when the placental blood of the 20 women with less fetal blood contamination was analyzed (Table 1). The median corrected platelet count in placental blood of all 40 women was lower than the platelet count in peripheral venous blood. The median corrected platelet count in placental blood of the 20 women with less fetal blood contamination was also lower than the platelet count in peripheral venous blood, however the difference was not significant, possibly due to the smaller sample size and the variation among the platelet counts.
Table 1.
Comparison of peripheral and placental intervillous space blood counts in women with scheduled cesarean sections
| Blood cells | Peripheral blood | Placental blood (corrected*) | Absolute difference between medians | P-value† |
|---|---|---|---|---|
| Median (range) | ||||
| All 40 women with uncomplicated deliveries and acceptable placental blood samples | ||||
| Hemoglobin (g/dL) | 11.9 (8.0–14.4) | |||
| White blood cells (103/µL) | 9.50 (5.60–12.99) | 9.94 (1.64–23.16) | +0.44 | 0.0285 |
| Neutrophils (103/µL) | 6.50 (3.46–9.51) | 6.40 (0.11–17.61) | −0.10 | 0.2231 |
| Lymphocytes (103/µL) | 2.05 (1.21–4.81) | 2.44 (0.04–6.69) | +0.39 | 0.0012 |
| Platelets (103/µL) | 233 (98–340) | 164 (18–728) | −69 | 0.0285 |
| The 20 women with placental blood samples that had fetal blood contamination ≤13.5%** | ||||
| Hemoglobin (g/dL) | 11.6 (9.1–14.4) | |||
| White blood cells (103/µL) | 9.56 (5.6–12.99) | 11.52 (5.92–23.16) | +1.96 | 0.0002 |
| Neutrophils (103/µL) | 6.65 (3.75–9.51) | 7.69 (3.55–17.61) | +1.04 | 0.0032 |
| Lymphocytes (103/µL) | 1.99 (1.29–4.81) | 2.54 (1.42–5.07) | +0.55 | 0.0020 |
| Platelets (103/µL) | 233 (98–324) | 179 (73–728) | −54 | 0.1992 |
White blood cell and platelet counts in blood from the placental intervillous space were corrected for the amount of dilution by the EDTA anticoagulant solution, based on the relative hemoglobin concentrations in the peripheral blood and placental intravillous space blood. The median correction ratio for all 40 placental blood samples (peripheral hemoglobin/placental hemoglobin) was 1.9 (range, 0.69 – 4.31).
P-values were obtained from the Wilcoxon signed-rank test.
The median fetal blood contamination of the placental intervillous blood samples was 13.5%. To determine the effect of fetal blood contamination on white blood cell and platelet counts, data for the 20 placental blood samples with ≤13.5% contamination by fetal blood are presented.
Microscopic examination of all 40 placentas documented that they were normal. None of the placentas had excessive subchorionic or perivillous fibrinoid, intervillous or subchorionic thrombi, or inflammatory infiltrates. Immunohistochemical stains using a rabbit monoclonal antibody to the platelet membrane glycoprotein IIIa (CD61) identified platelets within the perivillous fibrinoid (Figure 1). These images suggest that platelets are consumed in the process of blood coagulation and fibrinoid generation to seal the gaps in the syncytiotrophoblastic surface of the terminal villi.
Figure 1.
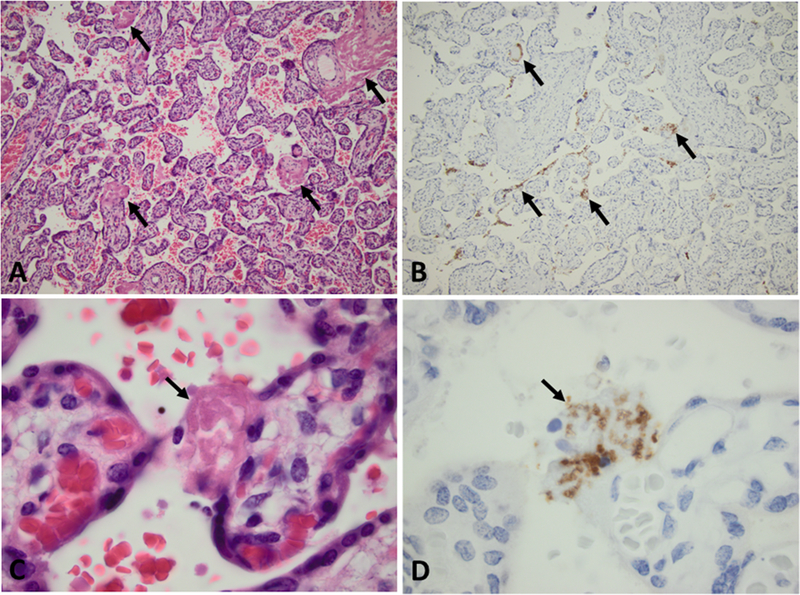
Placental morphology and immunohistochemical staining of sections of a placenta obtained at delivery and included in this study. Placental sections were stained with hematoxylin and eosin (H&E) to demonstrate the structure of the villi and perivillous fibrinoid, and also with a rabbit anti-CD61 (platelet membrane glycoprotein IIIa) monoclonal antibody by ProPath referral pathology service, Dallas, TX. A. Lowmagnification view of the placental parenchyma illustrating mature terminal villi. Perivillous fibrinoid filling gaps in the syncytiotrophoblast surface of the villi are identified by the homogeneous pink stain. Representative examples of the perivillous fibrinoid are indicated by the arrows. (H&E, 10X objective) B. An adjacent location in the same placental section stained with anti-CD61 (brown color) demonstrating platelets within the perivillous fibrinoid, in a linear pattern along the villous surfaces throughout this section. Representative examples of platelet staining are indicated by the arrows. (CD61 immunostaining, 10X objective) C. High-magnification of the placental parenchyma illustrates a mature terminal villus. The arrow indicates perivillous fibrinoid sealing a gap in the syncytiotrophoblast surface. (H&E, 100X objective) D. An adjacent location stained with anti-CD61 (brown color) demonstrating platelets within the perivillous fibrinoid, indicated by the arrow. (CD61 immunostaining, 100X objective)
The higher white blood cell counts in placental blood compared to peripheral venous blood are consistent with the sequestration of blood cells within the intervillous space, similar to the sequestration of blood cells in splenic sinusoids.3 The lower or equivalent platelet counts in placental blood compared to peripheral venous blood and the immunohistochemistry identification of platelets within the perivillous fibrinoid are consistent with the hypothesis that platelets are not only sequestered within the placental intervillous space but also consumed by incorporation into the perivillous fibrinoid, which maintains integrity of the syncytiotrophoblast surface. These data support the hypothesis that the sequestration and consumption of maternal platelets within the placental intervillous space contribute to lower platelet counts during pregnancy.
Acknowledgments
Support: This project was supported in part by 1K01HL135466–01 from the National Heart, Lung and Blood Institute (Terrell)
Footnotes
Ethics statement: This study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board (approval #4156).
Conflict of Interest and Financial Disclosure: All authors have no conflicts of interest or financial conflicts with this study.
REFERENCES
- 1.Reese JA, Peck JD, Deschamps DR, et al. Platelet counts during pregnancy. New Eng J Med 2018;379:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonsson V, Bock JE, Nielsen JB. Significance of plasma skimming and plasma volume expansion. J Appl Physiol 1992;72:2047–51. [DOI] [PubMed] [Google Scholar]
- 3.Aster RH. Pooling of platelets in the spleen: Role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest 1966;45:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann P, Huppertz B, Frank H-G. The fibrinoids of the human placenta: origin, composition and functional relevance. Ann Anatomy 1996;178:485–501. [DOI] [PubMed] [Google Scholar]
- 5.Benirschke K, Burton GJ, Baergen RA. Pathology of the Human Placenta Heidelberg, Germany: Springer-Verlag; 2012. [Google Scholar]
- 6.Othoro C, Moore JM, Wassenuehler K, et al. Evaluation of various methods of maternal placental blood collection for immunology studies. Clin Vaccine Immunology 2006;13:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


