Abstract
The remodeling of biological membranes is crucial for a vast number of cellular activities and is an inherently multiscale process in both time and space. Seminal work has provided important insights into nanometer-scale membrane deformations, and highlighted the remarkable variation and complexity in the underlying molecular machineries and mechanisms. However, how membranes are remodeled at the micron-scale, particularly in vivo, remains poorly understood. Here we discuss how using regulated exocytosis of large (1.5 – 2.0 μm) membrane-bound secretory granules in the salivary gland of live mice as a model system, has provided evidence for the importance of the actomyosin cytoskeleton in micron-scale membrane remodeling in physiological conditions. We highlight some of these advances, and present mechanistic hypotheses for how the various biochemical and biophysical properties of distinct actomyosin networks may drive this process.
Keywords: actomyosin, micron-scale, membrane remodeling, intravital microscopy, regulated exocytosis, in vivo, cytoskeleton
Graphical Abstract
During regulated exocytosis in salivary gland acinar cells in vivo, after large (~1.5 μm) membrane-bound secretory granules fuse with the cell apical plasma membrane (APM), the secretory granule membrane integrates into the APM. This integration involves a directional flow of membrane that is energetically unfavorable, requiring forces generated by the actomyosin cytoskeleton.
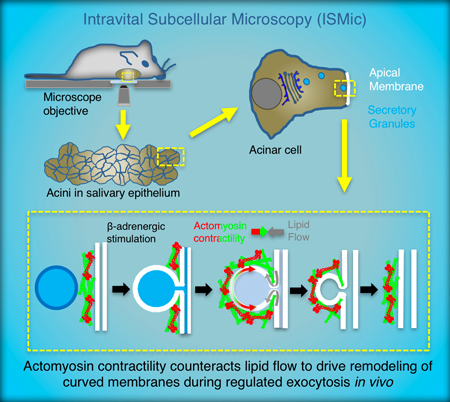
1. Introduction
Biological membranes are self-sealing, semi-permeable molecular sheets, composed primarily of a lipid bilayer and a variety of embedded or bound proteins. Membranes surround each cell and its organelles, allowing the precise and simultaneous coordination of the thousands of biochemical reactions required for eukaryotic life. Although membranes were initially perceived as rigid and static structures, we now know that they are constantly remodeled in response to a variety of cues, undergoing changes in shape and composition. Membrane remodeling is essential for basic cellular processes such as intracellular trafficking, cytokinesis and cell motility, which ultimately control physiological processes such as immune response and development, and pathological processes such as neurodegeneration, and tumor progression.[1, 2] This diversity of functions requires that membranes be remodeled across a range of spatial and temporal scales, a feat accomplished by the concerted action of three processes occurring within, or at the interface of, the lipid bilayer: 1) the modification of the lipid bilayer composition via lipid-modifying enzymes such as lipid kinases and phospholipases;[3, 4] 2) the insertion or removal of proteins capable of altering the biophysical properties of the bilayer, such as BAR-domain containing and ESCRT proteins;[5 - 8] and 3) the assembly/actions of protein complexes which are capable of generating forces on the bilayer, such as clathrin or the actomyosin cytoskeleton.[9, 10] Our focus has been on elucidating the role of the cytoskeleton in membrane remodeling; particularly on how actin, myosin and accessory components assemble and interact to alter the shape and dynamics of membranes. Further, we focus exclusively on unravelling this process in a true physiological context, using the process of regulated exocytosis in the salivary gland of live mice as our in vivo mammalian model system.
2. Micron-scale membrane remodeling during regulated exocytosis in murine exocrine glands
During protein secretion, proteins are released from the trans Golgi network in membrane-bound vesicles and transported constitutively to the cell periphery. Here, the vesicles either fuse immediately with the apical plasma membrane (APM) and release their contents into the extracellular space (constitutive exocytosis), or accumulate until an extracellular stimulus triggers their fusion with the plasma membrane (regulated exocytosis). [11] In both cases, secretion occurs via the docking and fusion of the vesicle membrane with the APM, and the simultaneous release of vesicular contents via a fusion pore. While constitutive exocytosis occurs in all cells, regulated exocytosis occurs in four specialized secretory tissues: neuronal, endocrine, exocrine and hematopoietic. There is remarkable diversity in the dimensions and contents of secretory vesicles across different specialized secretory cells (reviewed in [12]), from 50 – 100 nm neurotransmitter-containing vesicles produced in neuronal cells that are released in miliseconds, to much larger (1.5 – 2 μm) secretory vesicles, called secretory granules (SGs), produced in exocrine cells containing bulky glycosylated cargos that are secreted in minutes.
We focus here specifically on the latter, and in particular on regulated exocytosis in secretory cells of the mouse salivary gland (known as acinar cells). During this process (Figure 1A and1B), large SGs fuse to specialized domains of the acinar cell APM, that sheathe a network of intercellular canaliculi. This fusion occurs in response to extracellular signals from β-adrenergic receptors, and can be robustly stimulated by administration the β-adrenergic receptor agonist isoproterenol. After fusion, the SG membrane undergoes a gradual integration into the APM (Figure 1A), taking in the range of ~ 1 minute, and exhibiting linear kinetics.[12 - 15] It is important to note that the physiology and kinetics of this process cannot be recapitulated in in vitro or ex vivo systems. For example, the incidence of compound exocytosis, which is observed in vitro and ex vivo,[16, 17] does not occur in vivo.[13] The development of intravital subcellular microscopy (ISMic), which allows the imaging of subcellular events in live anesthetized rodents (Figure 1B), has thus been pivotal in enabling secretion in the salivary gland to be used as a robust, inducible model system to elucidate the dynamics, molecule composition and mechanisms of membrane remodeling by the actomyosin cytoskeleton in physiological conditions.[13, 14, 18 – 20]
Figure 1-. Kinetics of regulated exocytosis in the live murine salivary gland.
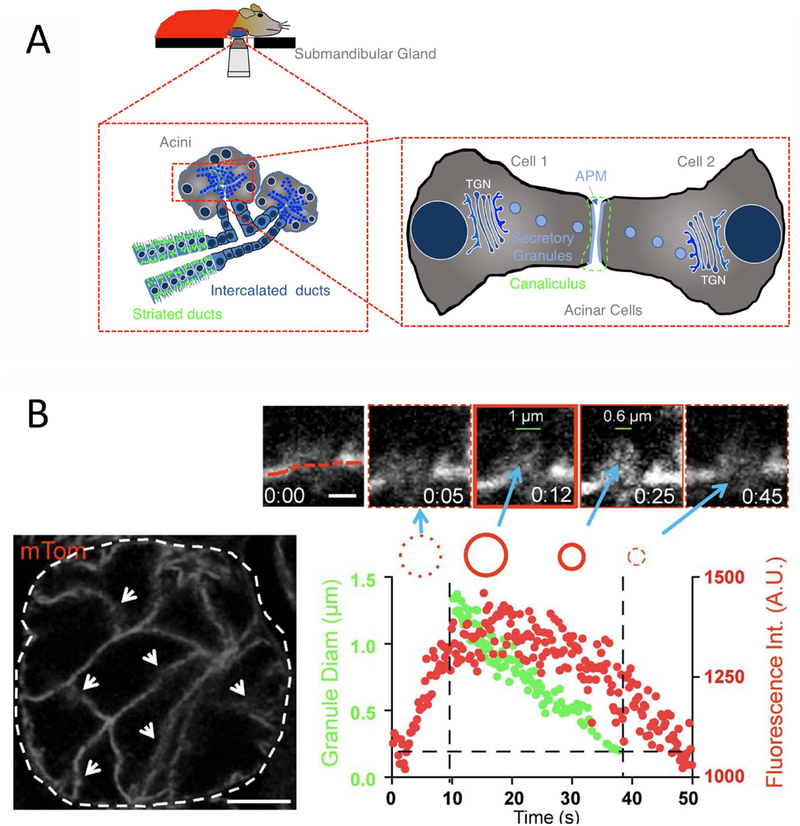
(A) Illustration of a mouse salivary gland exposed, post-surgery, and positioned on an objective for ISMic; Below, left: Schematic to illustrate a zoom in on two acini, the smallest secretory unit of the salivary gland, comprised of specialized secretory acinar cells. Blue, filled circles represent secretory granules residing near the apical plasma membrane (APM, blue lines) of acinar cells until regulated exocytosis is triggered; Right: A further zoom in on two acinar cells to highlight the APM (blue) around narrow, tube-like intercellular canaliculi (encircled by green dotted line). (B) Left- A confocal fluorescence image of a single acinus (dashed line), with the acinar cell APMs demarcated by arrows (scale bar, 20 μm). Top- Zoom in on a single fused SG to show kinetics of integration (blue arrows). APM delineated by red dashed line, scale bar, 1 μm). Graph- SG fluorescence intensity (Int.; red dots) and diameter (Diam; green dots) as a function of time (reproduced from [14]). (B) Left:
3. Role of the actomyosin cytoskeleton on post-fusion SGs: Current status
F-actin and non-muscle myosin II (NMII) have been reported to be necessary to extrude the cargo into the extracellular space upon fusion with the plasma membrane in numerous in vitro model systems including surfactant secretion in the lung, the secretion of the Von-Willebrand factor from the Weibel-Palade bodies[21] and in lacrimal glands.[22] In cells from the exocrine pancreas, the same components have been reported to regulate the dynamics of the fusion pore.[23] The actomyosin machinery has also been reported to play a role during both exocytosis and compensatory endocytosis in Xenopus oocytes.[24, 25]
Using ISMic, we previously showed that F-actin and non-muscle myosin II (NMII) localize to the surface of SGs seconds after fusion with the APM.[12, 13] We found that the pharmacological inhibition of F-actin assembly and NMII motor activity did not prevent SGs from fusing to the APM, but resulted in increased SG diameter post-fusion, and halted SG integration.[13, 26, 27] More specifically, without F-actin the SGs undergo a biphasic expansion: an initial linear expansion, followed by a stepwise more significant enlargement (Figure 2A). We proposed that the hydrostatic pressure generated by fluid secretion was responsible for the first enlargement, and the second phase was due to compound exocytosis. Blocking myosin activity, on the other hand, resulted in an initial expansion and delay, but no compound exocytosis.
Figure 2-. Effects of F-actin, NMIIA and NMIIB inhibition on SG integration during regulated exocytosis.
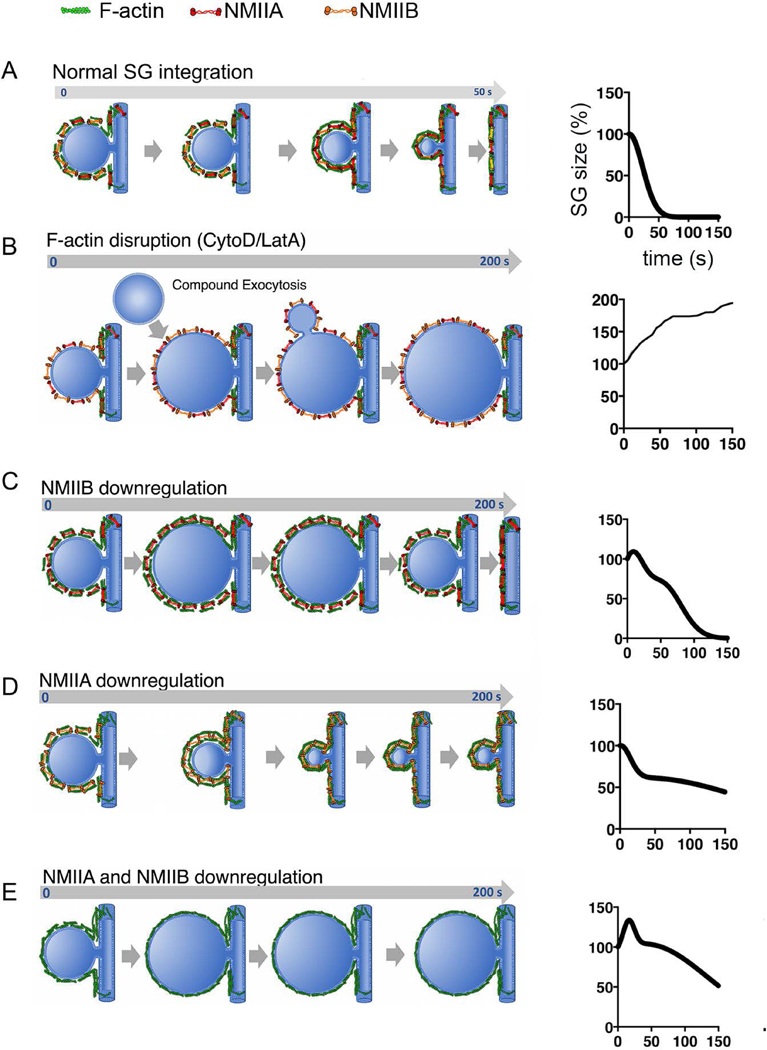
(A) Left- Under normal conditions, the SG integrates fully into the APM within 1 minute of fusion. Right- Fit of kinetic data of SG integration from [14]. (B) Left- F-actin inhibition leads to a biphasic SG expansion of the SG, likely due to the absence of a scaffold upon which NMII can exert contractile forces, and occurrence of compound exocytosis (which does not occur when F-actin is present). The SG eventually integrates, suggesting the pore remains open. Right- Fit of kinetic data of SG integration from [14]. (C) Left- When NMIIB is downregulated, the SG initially expands, but ultimately collapses after ~200 s, suggesting NMIIB is needed for initial integration, but not for late integration or opening/maintenance of the fusion pore. Right- Fit of kinetic data of SG integration from [14]. (D) Left- When NMIIA is down regulated, initial integration kinetics are normal, but the SGs do not collapse, suggesting that NMIIA is needed for late integration and/or opening/maintenance of the fusion pore. Right- Fit of kinetic data of SG integration from [14]. (E) Left- In the absence of both NMIIA and NMIIB, SGs expand upon fusion with the APM, and do not integrate. Right- Fit of kinetic data of SG integration from [14].
More recently[14] we determined that two isoforms of NMII, namely NMIIA and NMIIB, localize to fused SGs and, notably, that knockdown of either isoform had differential effects on the kinetics of SG integration. Specifically, knockdown of NMIIB (Figure 2B) resulted in an initial increase in the diameter of fused SGs, and a two-fold increase in the time required for complete SG integration.[14, 15] Conversely, in the absence of NMIIA (Figure 2C), the kinetics of initial integration remained unaffected until SGs reached 50–60 % of their initial diameter, at which point integration was stalled.[14, 15] Ablation of both NMIIA and NMIIB (Figure 2D) blocked integration in all but a small percentage of SGs.[14, 15] Finally, we made the intriguing discovery that the recruitment of NMII to fused SGs does not require the presence of F-actin.[14, 15]
Together, these data suggest that the actomyosin cytoskeleton provides mechanical forces on the SG membrane that are required for normal exocytosis to occur. Since, unlike exocytosis of Weibel– Palade bodies or surfactant in lung alveolar cells, which require the compression of an actomyosin coat to drive cargo expulsion,[21] the cargo in salivary gland SGs is not densely packed or cross-linked,[28] the actomyosin forces are likely required for membrane integration rather than squeezing out of SG contents.
These data also raise new questions concerning the functional mechanisms and spatio-temporal organization of the actomyosin cytoskeleton, as well as interactions with other molecular components during SG integration. While F-actin-independent recruitment of NMII is not unprecedented,[14] the identity of the NMII receptor on the surface of SGs is yet to be determined, highlighting the importance of an in-depth characterization of the molecular machinery on the SG surface to fully understand the integration process. Below we discuss some biophysical considerations, hypothesize likely molecular scenarios, and propose experiments to test their validity.
4. SG integration- mechanical and geometric considerations
The rate-limiting steps controlling the amount of secreted cargo during regulated exocytosis are: i) the number of fusion events that occur, and ii) the rate of content release and membrane integration, post fusion. Given the large size (1.5 – 2 μm) of the SGs relative to “tubular” APM (0.3 – 0.4 μm), the latter step is of particular importance, raising a number of intriguing biophysical questions. For example, in mast cells, it was suggested that granules are under significant tension, causing a lipid flux from plasma to SG membranes when they are connected by a fusion pore.[30, 31, 32] Is there a similar difference in membrane tension between the SG membranes and the APM in the salivary gland? And, given the diameter of the SG relative to the canaliculi, is the integration of the SG membrane into the APM energetically favorable?
We previously established a mechanical model that systematically delineates the mechanical conditions controlling the fate of post-fusion vesicles.[33] The model, based on considerations of elastic energy intrinsic to the membrane, predicts that the geometry of post-fusion vesicles significantly influences the integration process of the vesicular membrane into the APM: a larger vesicle with a relatively narrower fusion pore is predicted to energetically favor vesicle fission, whereas a relatively smaller vesicle with a wider fusion pore promotes full-integration of the post-fusion vesicle into the APM.[33] Interestingly, the fate of post-fusion vesicles can be modulated by mechanical forces external to the membrane, whereby increasing relative tension on the SG membrane tips the balance toward full integration into APM. While we do not know the precise dimensions of the fusion pore in fused SGs, the diameter of the canaliculus before fusion is in the range of 0.3 – 0.4 μm. The APM ensheathing the canaliculi thus has a higher curvature energy than the SG membrane and, as a result, is under a higher pressure. In this situation, the flow of lipids will tend to be towards the SG membrane, suggesting that complete integration of large SG membranes into APM is not energetically favorable (Figure 3A). Further understanding can be gained by acquiring more accurate dimensions of the fusion pore, for example by electron microscopy. Additionally, labeling the SG membrane distinctly from the APM would allow a quantitative analysis of the velocity of lipid flow after SG fusion. These data could then be used to determine definitively whether the SG membrane is under tension. Because the exact lipid composition of the membrane around SGs pre-fusion is still not known, such an experiment would first require a lipidomics screen of purified, unfused SGs, which is challenging but feasible, as the procedure for achieving high purity isolated secretory granules from rodent salivary glands have been already established.[34] Finally, it has been shown that as SG fusion progresses, the diameter of the APM increases,[26, 27] likely as excess membrane is integrated faster than compensatory endocytosis can recycle it; an experiment measuring the kinetics of SG fusion in the expanded APM would also inform on the role of membrane tension in the SG integration process.
Figure 3-.
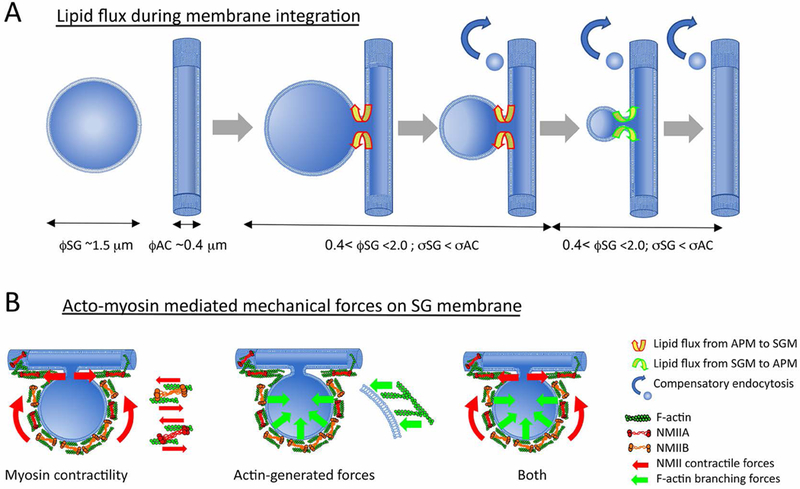
(A) Model illustrating how the dimensions and geometries of the SG, fusion pore and AC, influence SG membrane integration into the APM. The membrane tension of the early post-fusion SG, with a diameter of ~1.5 μm (øSG) relative to the membrane tension of the APM surrounding the canaliculi with diameter ~0.4 μm (øAC) is predicted to energetically favor lipid flow from the APM towards the SG (yellow and red arrows). When the SG diameter is in the range of the canalicular diameter, øSG and øAC energetically favors lipid flow from the SG membrane towards the APM (yellow and green arrows), favoring SG integration. This suggests that external mechanical forces are necessary for complete integration of large SG membranes into canalicular APM. (B) Actomyosin mediated mechanical forces on the SG membrane to facilitate integration may be generated by either NMIIA/NMIIB contractile forces; forces generated by actin polymerization and branching; or both.
5. The actomyosin network on the SG surface: contractile or scaffolding?
Given the canonical synergistic role of actomyosin in producing forces, it is tempting to postulate that the two NMII isoforms use the F-actin scaffold to generate contractile forces (on the actin filaments), in effect forcing the SG membrane to integrate into the APM, at least until the SG reaches the dimensions at which it can spontaneously collapse (Figure 3B).
In an alternative scenario, it is possible that F-actin and NMIIA/NMIIB form a protective scaffold around the SG immediately after fusion. In this case, rather than providing contractile forces to push the SG membrane, NMII crosslinking and/or contractility maintain the integrity of the scaffold, preventing the SG from expanding due to either compound exocytosis, swelling due to hydrostatic pressure, or lipid convection from the APM into the SG membrane due to a tension gradient. The forces to push the SG membrane into the APM may instead be propagated by: 1) the continuous depolymerization of actin, leading to the constant rearrangement and decrease in size of the actomyosin scaffold,[35] with the resulting tension forcing the membrane constrained within to integrate with the APM; or 2) the polymerization of a distinct population of actin, with the barbed end of F-actin pushing against the SG surface (Figure 3B). Depolymerization-based force generation has been reported in cytokinetic actin ring compression during cell division,[36] cell migration,[37] and in more recent work on fused lamellar bodies in alveolar secretory cells.[38] Support for this model can be provided by the finding that the F-actin depolymerizing factor ADF/cofilin was found on the surface of SGs,[38] although its role in SG integration was not assessed. In the case of actin polymerization, the forces generated depend critically on the network architecture.[39] It has been shown that linear polymerizing filaments of F-actin, such as those contained in filopodia, can produce high speeds, but represent a weak configuration for force generation, in the piconewton range,[40] while 2D branched networks, created by Arp2/3 actin-nucleating complexes produce much higher forces at lower displacement (in the nanonewton range), and polymerization parallel to a surface is suggested to lead to strong orthogonal forces.[39] The role for actin polymerization and/or branching in SG membrane integration can be interrogated by assessing effects of pharmacological and/or genetic inhibition of actin nucleating, polymerizing and branching proteins. Tracking the recruitment and localization of these molecules, initially by immunofluorescence, and ultimately through ISMic in transgenic or knockin mice expressing tagged-proteins, will provide insights into their specific roles in the membrane integration process. Since actomyosin network architecture ultimately determines the productive force, 4D super-resolution ISMic of tagged NMII and markers for F-actin will be an important next step to elucidate the organization of these proteins, and monitor changes over time.
6. Actin-independent recruitment and isoform-specific roles of NMII on fused SGs
The presence of NMII on SGs lacking F-actin[14] implies the existence of an alternate NMII receptor on the SG surface, whose identity is not yet clear. Recent works reporting F-actin independent NMII recruitment include a study on yeast cytokinesis where NMII is recruited to the contractile ring by IQGAP-Rng and aniliin,[41] and a study on Drosophila development showing increased membrane tension is sufficient to promote the recruitment and activation of NMII at the cell cortex.[42] Strategies to uncover the NMII receptor on the SG surface include a proteomics and lipidomics screen of purified, fused granules for likely candidates. Additionally, our data showed that knockdown of either NMII isoform had distinct effects on SG membrane integration. One approach to assess whether the two isoforms play distinct roles is to track each isoform separately on the SG surface during the integration process. By generating transgenic mice that express each isoform tagged with a different fluorophore, the relative timing of recruitment and spatial localization can inform on whether the isoforms work independently or form co-filaments with varying stoichiometries.
The opening of the fusion pore after SG fusion requires factors such as SNAREs and has been shown to be F-actin independent.[43] However, it is possible that NMIIA plays a role in maintaining the pore in an open state during the entirety of the SG integration process, as fusion pore geometry, dimensions and even composition keep evolving probably due to the lipid flow. Indeed, such role for NMIIA in maintaining an open exocytic fusion pore has been proposed in pancreatic acinar cells[23] and in neuroendocrine cells,[44] and is consistent with the conclusion from our mechanical model.[33] This would fit with our data showing that while SGs fuse and begin to integrate in absence of NMIIA, their inability to fully integrate may be because pore maintenance later in the integration process is defective. Electron microscopy analysis of fused SGs from acinar cells will be important to investigate how the dimensions of the fusion pore are altered in the absence of NMIIA.
7. Conclusion and Outlook
The role of actin assembly has been well documented in membrane remodeling events such as invagination during clathrin- and caveolin-dependent endocytosis, and vesicle fission.[45 - 47] Indeed, important studies on endocytosis have provided numerous insights into the coordination of F-actin polymerization, organization and dynamics with nanometer-scale membrane changes. However, it is still not clear how these processes play out in membrane remodeling at the micron-scale, including at the cleavage furrow of most dividing animal cells , during autophagy,[48] lipid droplet formation,[49] micropinocytosis,[50] or during certain forms of regulated exocytosis.[12] This information is especially sparse in the realm of live multicellular organisms, due to the lack of appropriate model systems and technologies. This is now changing due to important advances in both avenues in recent years, allowing an unprecedented view into micron-scale membrane remodeling in a physiological context. We would argue that the process of regulated exocytosis in the mouse salivary gland provides a unique and robust model system to further investigate specifics of the recruitment of cytoskeletal players, and their concomitant interactions to drive the remodeling of these micron-scale curved membranes, in vivo.
Abbreviations:
- APM
apical plasma membrane
- SG
secretory granule
- ISMic
intravital subcellular microscopy
- NMII
nonmuscle myosin II
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003. March 6;422(6927):37–44. [DOI] [PubMed] [Google Scholar]
- 2.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005. December 1;438(7068):590–6. [DOI] [PubMed] [Google Scholar]
- 3.Shnyrova AV, Frolov VA, Zimmerberg J. Domain-driven morphogenesis of cellular membranes. Curr Biol CB. 2009. September 15;19(17):R772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozlov MM, Campelo F, Liska N, Chernomordik LV, Marrink SJ, McMahon HT. Mechanisms shaping cell membranes. Curr Opin Cell Biol. 2014. August;29:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley WP, Yamada KM. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr Opin Genet Dev. 2013. August;23(4):408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suetsugu S, Kurisu S, Takenawa T. Dynamic Shaping of Cellular Membranes by Phospholipids and Membrane-Deforming Proteins. Physiol Rev. 2014. October 1;94(4):1219–48. [DOI] [PubMed] [Google Scholar]
- 7.Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013. September 1;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiaruttini N, Redondo-Morata L, Colom A, Humbert F, Lenz M, Scheuring S, Roux A. Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation. Cell. 2015. November 5;163(4):866–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatch AL, Gurel PS, Higgs HN. Novel roles for actin in mitochondrial fission. J Cell Sci. 2014. November 1;127(21):4549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korobova F, Gauvin TJ, Higgs HN. A role for myosin II in mammalian mitochondrial fission. Curr Biol CB. 2014. February 17;24(4):409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003. April;83(2):581–632. [DOI] [PubMed] [Google Scholar]
- 12.Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci CMLS. 2013. June;70(12):2099–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masedunskas A, Sramkova M, Parente L, Sales KU, Amornphimoltham P, Bugge TH, Weigert R. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc Natl Acad Sci U S A. 2011. August 16;108(33):13552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milberg O, Shitara A, Ebrahim S, Masedunskas A, Tora M, Tran DT, Chen Y, Conti MA, Adelstein RS, Ten Hagen KG, Weigert R. Concerted actions of distinct nonmuscle myosin II isoforms drive intracellular membrane remodeling in live animals. J Cell Biol. 2017. July 3;216(7):1925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebrahim S, Milberg O, Weigert R. Isoform-specific roles of NMII drive membrane remodeling in vivo. Cell Cycle Georget Tex. 2017. October 18;16(20):1851–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasai H, Kishimoto T, Nemoto T, Hatakeyama H, Liu T-T, Takahashi N. Two-photon excitation imaging of exocytosis and endocytosis and determination of their spatial organization. Adv Drug Deliv Rev. 2006. September 15;58(7):850–77. [DOI] [PubMed] [Google Scholar]
- 17.Pickett JA, Edwardson JM. Compound exocytosis: mechanisms and functional significance. Traffic Cph Den. 2006. February;7(2):109–16. [DOI] [PubMed] [Google Scholar]
- 18.Shitara A, Weigert R. Imaging membrane remodeling during regulated exocytosis in live mice. Exp Cell Res. 2015. October 1;337(2):219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masedunskas A, Porat‐Shliom N, Weigert R. Regulated Exocytosis: Novel Insights from Intravital Microscopy. Traffic. 2012. May 1;13(5):627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masedunskas A, Appaduray MA, Lucas CA, Lastra Cagigas M, Heydecker M, Holliday M, Meiring JCM, Hook J, Kee A, White M, Thomas P, Zhang Y, Adelstein RS, Meckel T, Böcking T, Weigert R, Bryce NS, Gunning PW, Hardeman EC. Parallel assembly of actin and tropomyosin, but not myosin II, during de novo actin filament formation in live mice. J Cell Sci. 2018. March 19;131(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nightingale TD, White IJ, Doyle EL, Turmaine M, Harrison-Lavoie KJ, Webb KF, Cramer LP, Cutler DF. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J Cell Biol. 2011. August 22;194(4):613–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerdeva GV, Wu K, Yarber FA, Rhodes CJ, Kalman D, Schechter JE, Hamm-Alvarez SF. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 2005. October 15;118(Pt 20):4797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhat P, Thorn P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol Biol Cell. 2009. March;20(6):1795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu H-YE, Bement WM. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol Biol Cell. 2007. October;18(10):4096–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003. August;5(8):727–32. [DOI] [PubMed] [Google Scholar]
- 26.Masedunskas A, Sramkova M, Weigert R. Homeostasis of the apical plasma membrane during regulated exocytosis in the salivary glands of live rodents. Bioarchitecture. 2011. September 1;1(5):225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masedunskas A, Porat-Shliom N, Weigert R. Linking differences in membrane tension with the requirement for a contractile actomyosin scaffold during exocytosis in salivary glands. Commun Integr Biol. 2012. January 1;5(1):84–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorr S-U, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. 2005. June;84(6):500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell. 2007. November;13(5):677–90. [DOI] [PubMed] [Google Scholar]
- 30.Monck JR, Alvarez de Toledo G, Fernandez JM. Tension in secretory granule membranes causes extensive membrane transfer through the exocytotic fusion pore. Proc Natl Acad Sci U S A. 1990. October;87(20):7804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solsona C, Innocenti B, Fernández JM. Regulation of exocytotic fusion by cell inflation. Biophys J. 1998. February;74(2 Pt 1):1061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chizmadzhev YA, Kumenko DA, Kuzmin PI, Chernomordik LV, Zimmerberg J, Cohen FS. Lipid flow through fusion pores connecting membranes of different tensions. Biophys J. 1999. June;76(6):2951–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens T, Wu Z, Liu J. Mechanics of post-fusion exocytotic vesicle. Phys Biol. 2017;14(3):035004. [DOI] [PubMed] [Google Scholar]
- 34.Arvan P, Castle JD. Isolated secretion granules from parotid glands of chronically stimulated rats possess an alkaline internal pH and inward-directed H+ pump activity. J Cell Biol. 1986. October;103(4):1257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma X, Kovács M, Conti MA, Wang A, Zhang Y, Sellers JR, Adelstein RS. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proc Natl Acad Sci U S A. 2012. March 20;109(12):4509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendes Pinto I, Rubinstein B, Kucharavy A, Unruh JR, Li R. Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev Cell. 2012. June 12;22(6):1247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mseka T, Cramer LP. Actin depolymerization-based force retracts the cell rear in polarizing and migrating cells. Curr Biol CB. 2011. December 20;21(24):2085–91. [DOI] [PubMed] [Google Scholar]
- 38.Miklavc P, Ehinger K, Sultan A, Felder T, Paul P, Gottschalk K-E, Frick M. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J Cell Sci. 2015. March 15;128(6):1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dmitrieff S, Nédélec F. Amplification of actin polymerization forces. J Cell Biol. 2016. March 28;212(7):763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophys J. 2005. August;89(2):782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takaine M, Numata O, Nakano K. Fission yeast IQGAP maintains F-actin-independent localization of myosin-II in the contractile ring. Genes Cells Devoted Mol Cell Mech. 2014. February;19(2):161–76. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Gonzalez R, Simoes S de M, Röper J-C, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009. November;17(5):736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi L, Shen Q-T, Kiel A, Wang J, Wang H-W, Melia TJ, Pincet F. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012. March 16;335(6074):1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neco P, Fernández-Peruchena C, Navas S, Gutiérrez LM, de Toledo GA, Alés E. Myosin II contributes to fusion pore expansion during exocytosis. J Biol Chem. 2008. April 18;283(16):10949–57. [DOI] [PubMed] [Google Scholar]
- 45.Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005. February;16(2):964–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005. May 20;121(4):593–606. [DOI] [PubMed] [Google Scholar]
- 47.Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002. September;4(9):691–8. [DOI] [PubMed] [Google Scholar]
- 48.Fengsrud M, Erichsen ES, Berg TO, Raiborg C, Seglen PO. Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy. Eur J Cell Biol. 2000. December;79(12):871–82. [DOI] [PubMed] [Google Scholar]
- 49.Fujimoto T, Parton RG. Not Just Fat: The Structure and Function of the Lipid Droplet. Cold Spring Harb Perspect Biol. 2011. March 1;3(3):a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic Cph Den. 2009. April;10(4):364–71. [DOI] [PubMed] [Google Scholar]


