SYNOPSIS
Sepsis is characterized by characterized by early massive catabolism, lean body mass (LBM) loss and escalating hypermetabolism persisting for months to years. Early enteral nutrition should attempt to correct micronutrient/vitamin deficiencies, deliver adequate protein and moderated non-protein calories as well-nourished patients generate endogenous energy. Post-resuscitation, increasing protein/calories are needed to attenuate LBM loss and promote recovery. Malnutrition screening is essential and parenteral nutrition can be safely added when enteral nutrition is failing based on pre-illness malnutrition. Following ICU discharge, significant protein/calorie delivery Is required for months to years to facilitate functional and LBM recovery, with high protein oral supplements being essential to achieve adequate nutrition.
Keywords: PROTIEN, PARENTERAL NUTRITION, ENTERAL NUTRITION, CALORIES, LEAN BODY MASS, LIPIDS
I. Introduction
Sepsis, requiring care in the intensive care unit (ICU), is characterized by an acute catabolic response leading to rapid mobilization of energy stores as muscle, glycogen, and lipid stores are broken down to drive glucose production [1, 2]. This contributes to rapid loss of lean body mass (LBM) contributing to muscle wasting, weakness, and loss of physical function commonly known as ICU-acquired weakness (ICU-AW) or Post-ICU syndrome (PICS) [3]. This LBM loss is exacerbated by sepsis-induced anorexia and the inability to take nutrients by mouth volitionally for days to months [4]. Unless nutrition therapy is provided via enteral or parenteral routes, patients also accumulate a rapidly evolving energy deficit which further contributes to muscle wasting and worsened outcomes [5–7]. This illness, and unfortunately iatrogenic, starvation is superimposed upon the marked inflammatory and endocrine-mediated acute phase stress response. A critically ill (burns) patient can lose as much as a kilogram of LBM per day [8]. Other ICU patients also suffer significant LBM loss, much of it in the first 7–10 days of their ICU stay [9]. Patients often regain weight post-ICU stay, but much of this is only fat mass rather than functional LBM [10]. This is not surprising as data from burn ICU patients demonstrate that catabolism and subsequent increasing hypermetabolism following injury can persist for up to two years following discharge from hospital; this can markedly hinder recovery of LBM and function [8].
This evolutionarily conserved stress response allows the injured or septic human to generate energy to escape an attacker and recover from initial illness in a period where food gathering and consumption would initially be limited. Prior to the relatively recent (evolutionarily) development of ICU and hospital care, this period of cachexia and catabolism was self-limited, likely to a few days. The injured or infected (septic) human either escaped its attacker, and then either improved and re-initiated volitional nutrition intake or death occurred. However, modern ICU care now allows prolonged survival from sepsis via the ability to provide vital organ support for extended periods of time, making previously unsurvivable septic insults now survivable. In fact, innovations in ICU care have recently led to an almost yearly reduction of hospital mortality from sepsis [11]. However, these same data reveal many patients with sepsis are not returning home to functional lives post-ICU discharge, but instead to rehabilitation settings where it is unclear if they ever returned to a meaningful quality of life (QoL). In fact, in the same period that in-hospital ICU mortality appears to be declining, there has been a tripling in the number of patients going to rehabilitation settings [11]. Up to 40% of mortality within the first year of ICU stay occurs following ICU discharge [12]. Unfortunately, for those who do survive, nearly half will not return to work in the first year post-discharge [13], often due to PICS and ICU-AW [3].
A growing body of data indicates that persistent underfeeding throughout the ICU stay, particularly protein underfeeding, may significantly contribute to long-term mortality and QoL impairment months later [5, 14–16]. If we are to optimize recovery from sepsis and critical care we need to consider basic metabolism and a historic understanding of starvation and recovery to employ targeted nutritional care to our critically ill sepsis patients. The focus of modern ICU nutrition therapy and research efforts should emphasize the realization that nutritional needs change over the course of a septic illness as catabolism persists and increasing hypermetabolism evolves and persists, often for months to years [9]. Finally, screening for pre-illness malnutrition and the presence of nutritional risk (as defined by scores such as the NUTRIC score [17, 18] or (computed tomography) CT LBM analysis [19]) is essential at diagnosis of sepsis. In patients found to have pre-existing sarcopenia or malnutrition, parenteral nutrition (PN), with adequate protein delivery and modern balanced lipids, can be safely added when EN is failing.
II. Management Goals for Nutrition in Sepsis (See Table 1A, 1B, 1C, and Figure 1)
Table 1.
| A. Nutritional interventions in sepsis: Acute Phase- (First 24–96 H in ICU Until Resuscitated) | |||
|---|---|---|---|
|
| |||
| Nutritional intervention | Recommended Delivery/Dose | Rationale/Recent Evidence | Refs |
|
| |||
| Early Enteral Nutrition | Protein - ~ 1.0 g/kg/d | -Prevent LBM wasting, weakness and infections to improve recovery | 9, 14, 16, 24–28 |
| Non-Protein Kcals- ~15 kcal/kg/day (in well-nourished patients) | -Concern for ↑↑ protein dose (> 1.2–1.5 g/kg/d?) (Day 1–4?) creating risk due to impaired autophagy | ||
| -Benefit for key role of elevating lipid dose in non-protein kcal delivery in day 1–4? | |||
|
| |||
| Parenteral Nutrition | -Well-Nourished: Consider Delay Until Day 3–7 if < 60% EN Protein/Kcal Goal | -Prevent caloric deficit early to reduce LBM loss, enhance recovery, physical function, and QoL. | 2, 9, 15–18, 22, 24, 25 29–31, 34, |
| -Malnourished Pts: Start at ICU Admit Goal: ~ 1.2 g/kg/d Protein Total Kcals ~15–20 kcal/kg/d | -Signal of benefit in pts failing EN, EN contraindications, or pre-ICU malnutrition | ||
| -TPN does not increase risk of infection over EN or other IV fluid delivery | |||
|
| |||
| Prokinetics and/or post pyloric feeding | Consider Metocloperamide or Erythromycin for GRVs>500 or feeding intolerance symptoms | -Inconclusive- Post-pyloric feeding may reduce aspiration in Meta-Analysis. However, Post-Pyloric Feeding Equivalent to Gastric Feeding in recent RCT on aspiration risk and EN delivery | 24, 37–39 |
| Consider Post-Pyloric Feeding for GRVs > 500, feeding intolerance symptoms, may reduce silent aspiration if tube past 3rd portion of duodenum? | -Future new efficacious and low side-effect prokinetics needed | ||
|
| |||
| Supplemental parenteral feeding during first week in ICU | -Well-Nourished: Consider Delay Until Day 3–7 if < 60% EN Protein/Kcal Goal | -Prevent caloric deficit early to enhance recovery | 2, 6, 9, 15–18 24,25, 29–31, 34 |
| -Malnourished Pts: Start at ICU Admit Goal: ~ 1.2 g/kg/d Protein Total Kcals ~15–20 kcal/kg/d | -No clear benefit of higher Kcal doses (> 25 kcal/kg/d) in well-nourished ICU pts receiving dextrose-predominant, low protein PN in first 3 d. | ||
| Start at ICU admit in malnourished pts with NUTRIC > 5 (w/o IL-6) and/or NRS ≥ 5 | -Potential benefit in pts with contraindication to EN or failing EN, especially malnourished pts at ICU admit | ||
|
| |||
| More protein (>1.2 g g/kg/day) early (Day 1–4 in ICU) | Protein - ~ 1.0 g/kg/d | Key Area of Controversy | 2, 5, 6, 14,15, 23, 24,25,28, 34,41 |
| Until further research is completed on effects of very early protein delivery | -Spare endogenous protein to reduce LBM loss, facilitate early mobility and enhance recovery | ||
| -Concern for ↑↑ protein dose (> 1.2–1.5 g/kg/d?) (Day 1–4?) creating risk due to impaired autophagy | |||
|
| |||
| Thiamine | Strongly consider repletion all pts in septic shock requiring vasopressors: | -~35% of septic shock pts may be thiamine deficient | 52–54 |
| Dose: 200 mg IV Thiamine × 7 d | -In thiamine deificent pts, thiamine replacement reduced mortality from septic shock | ||
| -Thiamine, Vit. C and low does sterroids may reduce mortialty | |||
|
| |||
| Vitamin D | -Vitamin D Level measured at ICU admit in ALL pts | -Many pts worldwide are Vitamin D deficient and Vitamin D is essential to immune function and muscle restoration and function | 59–64 |
| -Vitamin D < 20 should receive 100,000 Units of Vitamin D2 or D3 for 5 d in first week and then 1–2× weekly (monitoring levels) for ICU stay | -Data in ICU shows Vitamin D deficiency has significant relationship to adverse ICU outcomes | ||
| -Recent large RCT in ICU shows mortality benefit to repletion | |||
|
| |||
| Balanced TPN Lipids (Fish/Olive Oil) | -Recommend use of balanced lipid solutions containing fish oil and/or olive oil to minimize soy lipid content | Soy lipids are:
|
32,33, 72,73 |
| - Pure soy lipid should not be used in sepsis or critical care setting for PN nutrition delivery | -Meta-Analysis data and recent RCTs support use of balanced lipids with reduced infections and LOS | ||
|
| |||
| Anti-oxidants | Possible role for Vitamin C in Septic shock with thiamine and low dose steroids- | -Prevent organ failure/fluid leak | 54–58 |
| (Vitamin C: 1.5 g IV q 6 h for 4 d or until discharge from the ICU) | -No clear benefit; for selenium or cocktail use possibly dependent on dose and pre-illness deficiency status -More confirmatory literature needed for Vit. C | ||
|
| |||
| Trace Element/Micronutrients | Routine administration of IV micronutrients/vitamins plus electrolyte replacement justified during acute phase of ICU until full enteral intake reached | - Meaningful number of pts are or may become deficient in trace elements at ICU admit | 22,49 |
| -Depletion can lead to “refeeding syndrome” – with thiamine, Mg, K, and PO4 deficiencies and potentially fatal complications, (i.e. cardiac failure, lactic acidosis, and respiratory failure) | |||
|
| |||
| Glutamine | Do not use early in shock, on vaspopressors, or in renal failure (especially pre-dialysis?) | -Resupply conditional deficiency to improve outcome | 57, 67–71 |
| - Inconclusive and potentially harmful in higher doses (> 0.5 g/kg/d EN/oral and > 0.35 g/kg/d IV), early in shock or renal failure | |||
| - Multiple meta-analysis support continued safety and use in TPN pts not in shock or renal failure at appropriate doses (< 0.35 g/kg/d) | -Ongoing trials in burn injury indicate safety of EN/oral GLN and potential benefit | ||
| B. Nutritional interventions in Sepsis: Chronic and Recovery Phase- (Post-Resuscitation – Hospital Discharge) | |||
|---|---|---|---|
|
| |||
| Nutritional intervention | Recommended Delivery/Dose | Rationale/Recent Evidence | refs |
|
| |||
| Enteral Nutrition | Protein – 1.2–2.0 g/kg/d | -Prevent ongoing LBM wasting, weakness and infections to improve recovery | 7, 9, 14–16, 24, 28 |
| Non-Protein Kcals- 25–30 kcal/kg/day (ideally guided by indirect calorimetry) | -Facilitate Early Mobility and Physical Therapy | ||
| In Recovery Phase- Pts likely require greater Kcal and protein delivery | -Minnesota Starvation Study shows > 4000 kcal/d required for recovery | ||
|
| |||
| Parenteral Nutrition | -Well-Nourished: Consider Delay Until Day 3–7 if < 60% EN Protein/Kcal Goal | -Prevent caloric deficit early to reduce LBM loss, enhance recovery, physical function, and QoL. | 2, 9, 15, 16–18, 22, 24, 25, 29–31, 34 |
| -Malnourished Pts: Start at ICU Admit Goal: ~ 1.2 g/kg/d Protein Total Kcals ~15–20 kcal/kg/d | -Signal of benefit in pts failing EN, EN contraindications, or pre-ICU malnutrition | ||
| -TPN does not increase risk of infection over EN or other IV fluid delivery | |||
|
| |||
| Oral Nutrition | High Protein Oral Nutrition Supplements should be provided to all pts 2–3 × day when oral nutrition initiated | -Oral intake is exceedingly poor in ICU patients | 4, 7, 9, 43–48 |
| -Recent large RCT, large database observational data and meta-analysis shows reduced mortality, complications, LOS, hospital costs | |||
| -Minnesota Starvation Study shows > 4000 kcal/d required for recovery | |||
|
| |||
| Supplemental Parenteral Feeding | -Well-Nourished: Consider Delay Until Day 3–7 if < 60% EN Protein/Kcal Goal | -Prevent caloric deficit early to enhance recovery | 2, 6, 9, 15–18 24,25, 29–31, 34 |
| -Malnourished Pts: Start at ICU Admit Goal: ~ 1.2 g/kg/d Protein Total Kcals ~15–20 kcal/kg/d | -No clear benefit of higher Kcal doses (> 25 kcal/kg/d) in well-nourished ICU pts receiving dextrose-predominant, low protein PN in first 3 d. | ||
| Start at ICU admit in malnourished pts with NUTRIC > 5 (w/o IL-6) and/or NRS ≥ 5 | -Potential benefit in pts with contraindication to EN or failing EN, especially malnourished pts at ICU admit | ||
|
| |||
| Vitamin D | -Vitamin D Level measured at ICU admit in ALL pts | -Many pts worldwide are Vitamin D deficient and Vitamin D is essential to immune function and muscle restoration and function | 59–64 |
| -Vitamin D < 20 should receive 100,000 Units of Vitamin D2 or D3 for 5 d in first week and then 1–2× weekly (monitoring levels) for ICU stay | -Data in ICU shows Vitamin D deficiency has significant relationship to adverse ICU outcomes | ||
| -Recent large RCT in ICU shows mortality benefit to repletion | |||
|
| |||
| Balanced TPN Lipids (Fish/Olive Oil) | -Recommend use of balanced lipid solutions containing fish oil and/or olive oil to minimize soy lipid content | Soy lipids are:
|
32,33, 72,73 |
| - Pure soy lipid should not be used in sepsis or critical care setting for PN nutrition delivery | Meta-Analysis data and recent RCTs support use of balanced lipids with reduced infections and LOS | ||
|
| |||
| Glutamine | Do not use early in shock, on vaspopressors, or in renal failure (especially pre-dialysis?) | -Resupply conditional deficiency to improve outcome | 57, 67–71 |
| - Inconclusive and potentially harmful in higher doses (> 0.5 g/kg/d EN/oral and > 0.35 g/kg/d IV), early in shock or renal failure | |||
| - Multiple meta-analysis support continued safety and use in TPN pts not in shock or renal failure at appropriate doses (< 0.35 g/kg/d) | -Ongoing trials in burn injury indicate safety of EN/oral GLN and potential benefit | ||
| C. Nutritional Interventions In Sepsis: Post-Hospital Discharge | |||
|---|---|---|---|
|
| |||
| Nutritional intervention | Recommended Delivery/Dose | Rationale/Recent Evidence | refs |
|
| |||
| Oral Nutrition | High Protein Oral Nutrition Supplements should be provided to all pts 2–3 × day for 3 months- 1 year post discharge | -Oral intake is exceedingly poor in ICU patients | 4, 7, 9, 43–48 |
| Protein Goal- 1.2–2.0 g/kg/d | -Recent large RCT, large database observational data and meta-analysis shows reduced mortality, complications, LOS, hospital costs | ||
| Kcal Goal- May be 4000–5000 kcal/day based on Minnesota Starvation Study | -Minnesota Starvation Study shows > 4000 kcal/d required for recovery | ||
| Post-ICU hypermetabolism and catabolism can persist for months to years post-ICU discharge | |||
|
| |||
| Vitamin D | -Vitamin D Level measured at ICU admit in ALL pts | -Many pts worldwide are Vitamin D deficient and Vitamin D is essential to immune function and muscle restoration and function | 59–64 |
| -Vitamin D < 20 should receive 100,000 Units of Vitamin D2 or D3 for 5 d in first week and then 1–2× weekly (monitoring levels) for ICU stay and likely in post-hospital period | -Data in ICU shows Vitamin D deficiency has significant relationship to adverse ICU outcomes | ||
| -Recent large RCT in ICU shows mortality benefit to repletion | |||
Abbreviations: LBM- Lean Body Mass, EN- Enteral Nutrition, PN- Parenteral Nutrition, TPN- Total Parenteral Nutrition, d- day, pt- patient, QoL- Quality of Life, RCT- Randomized Controlled Trial, GLN- Glutamine, LOS- Length of Stay
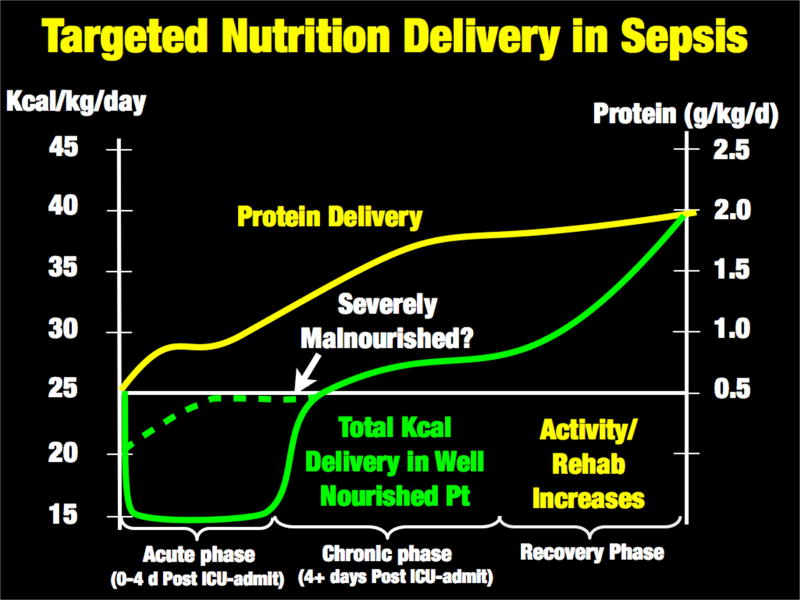
Figure 1.
Proposal for Targeted Nutrition Delivery in Sepsis
A. Acute catabolic phase of sepsis (Table 1A and Figure 2)
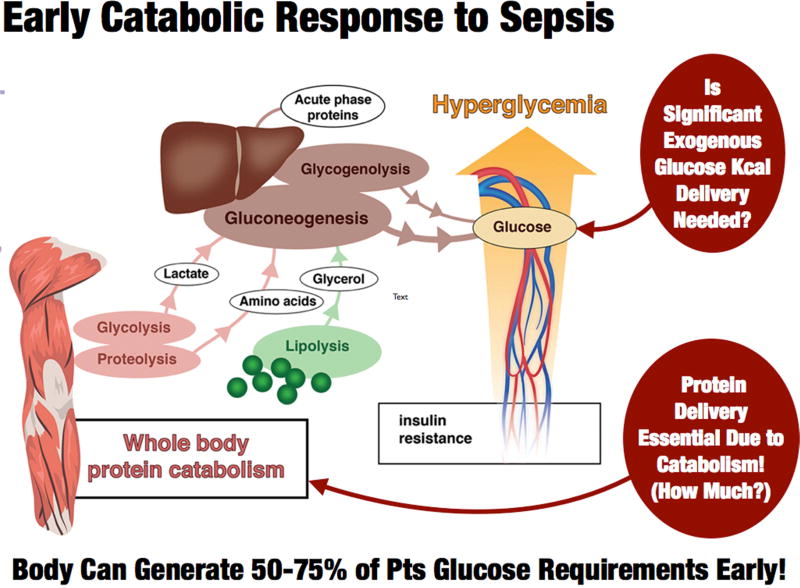
Figure 2.
Early Acute Phase Catabolic Response to Sepsis:
Adapted from Adapted from: Anesthesiology 2015; 123:1455–72.
1. Acute Phase - Adequate Protein and Moderated Non-Protein Calories
As stated above, the early or “acute phase” of sepsis is characterized by massive mobilization of the body’s calorie reserves as muscle, glycogen, and lipid stores are broken down to generate glucose to support ATP production [1, 2]. (See Figure 2) This metabolic response to stress can generate 50–75% of glucose needs during illness [2], and is not suppressed by feeding or intravenous glucose infusion [16]. Further, the early acute phase of sepsis and trauma are not hypermetabolic states; rather, patients have a total energy expenditure (TEE) to resting energy expenditure (REE) ratio of 1.0 and 1.1 for sepsis and trauma, respectively [20]. Thus, caloric need does not consistently increase in the early phases of sepsis. In fact, the more severe the septic shock, the lower the REE, as the body “hibernates” and reduces metabolism in response to severe sepsis [21]. This is shown in Table 1 in the context of caloric needs from the World Health Organization in health, and the landmark Minnesota Starvation Study [7], Data from Uehara et al demonstrate that REE in the first 2–5 days (acute phase) in elderly sepsis patients (mean age: 67) is ~1850 kcal/day with a TEE of ~1920 kcal/day (giving a TEE of 25 kcal/kg). These data and other recent trials [22] suggest we should consider feeding less non-protein calories early in the acute phase (first 24–96 h) of critical illness, and markedly increase calorie delivery during recovery as illustrated in Figure 1. At the same time, it is well known that protein losses increase 4-fold in the first 24 hours of critical illness [23] and health carers are exceedingly poor at meeting these needs [23]. Unfortunately, large international surveys indicate ICU practitioners deliver an average of 0.6 g/kg/day of protein for the first two weeks following ICU admission [6]. This is 33–50% of the latest ICU guideline-recommended protein delivery of 1.2–2.0 g/kg/day [24]. In contrast to conventional teaching, the delivery of additional non-protein calories does not significantly improve nitrogen balance in illness beyond delivery of 50% of predicted REE [16]. A secondary analysis of the pediatric PEPANIC trial by the Van Den Berghe group demonstrates that very early higher protein delivery may be associated with adverse outcomes, related possibly to inhibition of autophagy [25]. Of note, increased lipid delivery early in critical illness was associated with earlier ICU discharge. This leaves the clinician in a challenging position with an essential need to provide protein during ICU recovery, yet it remains unclear currently how much to give and when to escalate protein delivery to guideline goals. Thus, an ideal “targeted” feeding strategy may perhaps be ~15 kcal/kg/day of total energy needs during early ICU stay (acute phase – day 1–4), while ensuring patients receive an optimal lower protein delivery (~1.0 g/kg/day) as early as possible post-ICU admission. (Figure 1). Reduced calorie/protein delivery during the acute phase may not however be applicable in severely malnourished patents (i.e. patients with significant pre-ICU weight loss or NUTRIC Score (without IL-6 levels) ≥5) who are unlikely to have the metabolic reserve to generate endogenous energy needs [18, 24]. Ironically, the most recent SCCM/ASPEN guidelines emphasize these points suggesting hypocaloric PN (≤20 kcal/kg/day or 80% of estimated energy needs) with adequate protein (≥ 1.2 g protein/kg/day) be considered in patients requiring PN over the first week in critical care [24]. In early sepsis they suggest provision of trophic feeds (defined as 10–20 kcal/hour, up to 500 kcal/day) for the initial phase of sepsis, advancing as tolerated after 24–48 hours to >80% of target energy needs with early delivery of 1.2 to 2 g protein/kg/day [24]. These data for moderated non-protein calorie delivery are driven by recent large RCTs showing equivalent outcomes from trophic versus higher energy feeding (non-protein kcal delivery) [26, 27]. The need for additional protein intake has been well-described by Hofer et al in a number of recent publications questioning whether it is actually “protein-deficit” and not calorie deficit that is important in improving outcome in critical illness [14, 15, 28]. Given limited higher protein, lower kcal EN options, TPN or EN protein supplements may be required. TPN is now a significantly more viable option to achieve this goal as three recent large trials of both supplemental and full TPN support versus EN in the ICU setting demonstrated no increase in infection risk with TPN [29–31]. This is likely due to improvements in glucose control, central line infection control measures and, potentially, improved (non-pure soy based) balanced lipid formulations that reduce infection compared to pure soy lipid [32, 33]. In support of early TPN use, the new SCCM/ASPEN guidelines indicate any patient at high nutrition risk (NRS 2002 ≥5 or Nutric score (w/o IL-6 score) ≥5) or found to be severely malnourished when EN is not feasible, exclusive PN should be initiated as soon as possible following ICU admission [24].
B. Chronic and recovery phase of sepsis: significantly increased protein and calorie needs (See Table 1B and Figure 1)
1. Chronic phase - post-resuscitation increase in nutrition delivery
As successful resuscitation of the acute phase of sepsis occurs and thev patient stabilizes, an increasing amount of protein (1.2 – 2.0 g/kg/d) and calories (25–30 kcal/kg/d) needs to be delivered to reduce further loss of LBM, allow for early mobilization, and encourage functional recovery (Figure 1). The concept of adequate protein and calorie delivery improving QoL is well-described in a recent study of ICU patients mechanically ventilated >8 days [34]. After adjustment for covariates, patients receiving inadequate nutrition over the first ICU week (<50% of predicted calorie/protein need) had an increased mortality compared to those patients receiving adequate nutrition delivery (>80% of calorie/protein needs). These data also demonstrate that for every 25% increase in calorie/protein delivery in the first ICU week there was an improvement in 3-month post-ICU physical QoL scores (as measured by the SF-36 acore) with medical ICU patients showing significant improvements in both 3 and 6 month SF-36 scores [34].
2. Recovery phase - continued increase in nutrition delivery needs? - role of the Minnesota Starvation study in ICU recovery
As patients improve and enter the “recovery phase”, caloric intake likely needs to increase further, with implementation of aggressive rehabilitation and exercise interventions. The landmark “Minnesota Starvation Study” performed at the end of World War II [7, 35] (a study all medical students and hospital practitioners should be taught or read for themselves) provides essential data on the nutritional needs required to recover from the fundamental severe LBM loss observed post-sepsis. This seminal study demonstrates that a healthy 70 kg human, following significant weight loss, requires an average of 5000 kcal/day for 6 months to 2 years to fully regain lost muscle mass and weight [7]. As many ICU patients suffer similar marked weight/LBM loss, in addition to prolonged hypermetabolism and catabolism (which Minnesota subjects did not have as they were healthy volunteers), we must recognize that significant calorie/protein delivery will be required to restore this lost LBM and improve QoL. During the recovery phase of critical illness, the body experiences a massive increase in metabolic needs, with TEE increasing as much as ~1.7-fold above REE [20]. In the 2nd week following sepsis this increases to a TEE of ~3250 kcal/d or 47 kcal/kg/day – virtually identical to WHO requirements for normal, healthy humans. In younger trauma patients (mean age: 34), Uehara et al described an even greater increase in caloric need in the second week post-injury to an average of ~4120 kcal/day or 59 kcal/kg/day. This is nearly identical to the 4000 kcal/day that Ansel Keys demonstrated was needed to recover from starvation in the young Minnesota subjects (Table 1).
C. Current practice of nutrition in sepsis and ICUs worldwide: Do we already “hypocalorically” feed our patients beyond the acute phase?
Extensive data for current international nutrition delivery in critical care are available from the International Nutrition Survey conducted regularly by the Canadian Critical Care Nutrition Group (www.criticalcarenutrition.com). These data reveal that ‘average calories delivered in ICU over the first 12 days is 1034 kcals and 47 g of protein’ (Table 1) [6]. This period is far longer than the first 1–4 days of the acute phase where hypocaloric feeding (with moderated “adequate” protein) may make physiologic sense. More troubling is the fact that this total is far lower than the 1800 kcal/day calories and ~0.8 g/kg/day protein that led to severe starvation in the Minnesota Starvation Study! Thus, drawing comparison in nutrition delivery between ICUs worldwide and the landmark Starvation Study:
| Minnesota Starvation Study (Starvation Period) |
| 1800 kcal/d |
| 0.75–0.8 g/kg/protein |
| ICU Patients worldwide for first 12 days in ICU |
| 1034 kcal/d |
| 0.6 g/kg/protein |
These data confirm that ICU patients worldwide average far less energy and protein then healthy subjects in the legendary Minnesota Starvation Study. This study would likely never be repeated today due to the ethics of inducing potentially life-threatening starvation in healthy volunteers. We know that starvation in humans leads to slowing of metabolic rate and reduced protein catabolism over time. Unfortunately, after the first ICU week critical illness leads to significant hypermetabolism and severe ongoing protein losses. Moreover, 30–50% of ICU patients are malnourished at hospital admission [36] (unlike the well-nourished men in Key’s Minnesota Starvation study), thus greatly increasing the risk of ongoing in-hospital starvation. We must critically examine and measure actual practice in our individual ICUs as most already “underfeed” calories and protein well beyond the acute phase. Methods to improve EN including pro-kinetic agents [37] and post-pyloric feeding have not been successful in addressing this global ICU iatrogenic malnutrition. New guidelines calling for the abandonment of checking gastric residual volumes (GRVs) [38], or changing GRV cut-offs to >500 ml before feeding is stopped, may show promise to help improve EN delivery [24]. In a recent RCT, post-pyloric feeding did not reliably prevent aspiration or increase EN delivery [39] so gastric feeding should be the primary route to deliver EN. Finally, could iatrogenic malnutrition in the ICU likely explain in part the increasing number of ICU survivors who ultimately become “victims” of Post-ICU syndrome (PICS), never to walk again or return to a meaningful quality of life post-ICU discharge [3, 13, 40, 41]?
These data demand that we ask whether our septic patients have been unable to recover their QoL post-ICU for months to years due to our lack of understanding of their fundamental metabolic needs in different phases of their illness, especially following ICU and hospital discharge?
D. ICU/Hospital discharge nutrition delivery to optimize recovery (Table 1C and Figure 2)
Can patients discharged from critical care following sepsis consume adequate calories and protein to enable optimal recovery? In the week following endotracheal extubation, an observational study demonstrated an average spontaneous calorie intake of 700 kcal/day; the entire population studied consumed <50% of calorie/protein needs for 7 days [4]. This study also emphasizes the importance of closely observing food intake in post-operative patients. In patients who have lost significant weight following surgery or illness, a considerable period of significantly increased calorie and protein delivery is required for recovery [42]. To address this, a large body of data demonstrates that oral nutrition supplements (ONS) must become a fundamental part of our post-ICU and hospital discharge care. A meta-analysis in a range of hospitalized patients demonstrates that ONS reduces mortality, reduces hospital complications, reduce hospital readmissions, shortens length of stay, and reduces hospital costs [43–46]. A large hospital database analysis of ONS use in 724,000 patients matched with controls not receiving ONS showed a 21% reduction in hospital LOS; for every $1 spent on ONS, $52.63 was saved in hospital costs [47]. Finally, a recent large RCT of 652 patients in 78 centers studied the effect of high protein ONS (HP-ONS) with β-hydroxy-β-methylbutyrate (HP-HMB) versus placebo in elderly, malnourished (Subjective Global Assessment [SGA] class B or C) hospitalized adults. HP-HMB reduced 90-day mortality by ~50% relative to placebo (4.8% vs. 9.7%; relative risk 0.49, 95% confidence interval [CI], 0.27 to 0.90; p = 0.018). The number-needed-to-treat to prevent 1 death was 20.3 (95% CI: 10.9, 121.4) [48]. As it is well known that ICU patients recovering from sepsis will not consume sufficient calories and protein to recover optimally, the use of HP-ONS will be essential. It is strongly recommended for all patients once oral intake is resumed for at least 3 months (and up to one year) following ICU discharge.
E. Correction of vitamin/micronutrient deficiencies and specific nutrient delivery (Tables 1A, 1B, 1C)
In addition to protein and calorie needs a new and growing body of literature is identifying nutrients that should and should not be administered in the early acute phase of sepsis. These will be discussed specifically below.
1. Micronutrients and electrolytes
Recent literature demonstrates a meaningful number of patients may be deficient in trace elements at ICU admissions, or become deficient during their stay [49]. Refeeding syndrome is a real and present danger to the malnourished ICU patient. This must be monitored via evaluation of electrolytes (phospate, potassium, magnesium) and repletion when needed [50, 51]. The van den Berghe group advocates for continuous infusion of trace elements - “routine administration of intravenous micronutrients and vitamins plus electrolyte replacement is justified during the acute phase of critical illness until full enteral intake is reached” [49].
2. Thiamine
Thiamine is an essential vitamin for aerobic nutrient metabolism, playing a vital role in the Krebs’ cycle and the pentose-phosphate shuttle [52]. New data indicate that thiamine deficiency occurs in up to 35% of septic shock patients [53]. A recent randomized, double-blind, controlled trial showed that administration of 200 mg thiamine to patients with septic shock did not improve lactate levels or other outcomes overall [53]. However, in thiamine-deficient patients, there was a statistically significant decrease in mortality over time, and a reduction in lactate at 24 hours, in those receiving thiamine (p = 0.047). These data has been supplemented by a recent retrospective before-after clinical study, showing significantly reduced mortality in septic shock patients receiving thiamine, vitamin C and low dose steroids [54]. Hospital mortality was 8.5% (4/47) in the treatment group compared to 40.4% (19/47) in the earlier control group (p <0.001). These trial data do however require confirmatory larger RCTs. As thiamine measurement is costly and not routinely performed, and thiamine itself is quite inexpensive and carries almost no risk, a recommendation for all patients with septic shock to receive 200 mg thiamine for 7 days post-ICU admission seems reasonable to improve outcomes, though with the caveat that additional data are needed.
3. Vitamin C and antioxidants
As mentioned above, a potential benefit of Vitamin C with thiamine and low-dose steroids has recently been described [54]. The doses of Vitamin C used in this Marik trial are high, yet appeared to be safe and can be considered for use. Some concern for oxalate nephropathy should be considered, especially in patients with significant renal dyfunction, although the Marik group has denied any incidence of this is in their short term Vitamin C use. This practice has been seemingly safe in short-term use in the burn setting [55]. Consistent use of Vitamin C at this level, as is often done in burn patients to reduce fluid leak and fluid requirements [55], may challenge some ICU pharmacies to keep up with demand as this practice will be new to many centers. Routine use of selenium and other antioxidants has shown promise in meta-analysis [56], however recent large RCTs have not shown benefit [57, 58].
4. Vitamin D
A rapidly growing body of data demonstrates a significant proportion of the population of the US and other industrialized nations is Vitamin D deficient [59]. Data in ICU and surgical patients show that Vitamin D deficiency has a significant association with postoperative complications and adverse ICU outcomes [60–62]. A key recent RCT found that ICU patients with Vitamin D levels <12 ng/ml experienced a significant improvement in hospital survival with aggressive supplementation of Vitamin D3 given orally or via the nasogastric tube at a single dose of 540 000 IU followed by monthly maintenance doses of 90 000 IU for 5 months [63]. This will be difficult for many centers to administer if concentrated Vitamin D solutions are not available. A recent double blinded pilot RCT of 50,000 IU vitamin D3 or 100,000 IU vitamin D3 daily for 5 consecutive days enterally (total vitamin D3 dose = 250,000 IU or 500,000 IU, respectively) reported a significant decrease in hospital length of stay in the 50,000 IU D3/day (25 ± 14 days) and 100,000 IU D3/day (18 ± 11 days) groups compared to the placebo group (36 ± 19 days); p=0.03) [64]. Vitamin D levels are thus recommended to be checked at ICU admission and once weekly thereafter in all septic shock patients. For patients found to be deficient (<30 ng/ml) a repletion dose of 100,000 units of Vitamin D2 or D3 for five days in the first week and one-two times per week thereafter (monitoring levels) for the duration of the ICU stay is reasonable. Larger trials on the role of vitamin D supplementation in sepsis and critical illness are currently underway.
5. Glutamine
Glutamine (GLN) is the most abundant non-essential free amino acid [65]. Low GLN levels have been associated with poor outcomes [66]. Thus, GLN has been labeled a “conditionally essential” amino acid during prolonged critical illness, leading to the hypothesis that GLN supplementation would improve outcomes [65]. However, signals showing a risk of harm have come from two large-scale multicenter trials evaluating mortality utilizing a combination of high-dose intravenous/enteral GLN (the REDOXS study) [57] or a high-dose enteral mixture of different nutrients including GLN (the METAPLUS trial) [67]. These new trials were both targeted to investigate GLN (and other nutrients) as primary pharmaconutrients, and not as supplementation to PN. These data suggest that patients in the early phase of sepsis, on vasopressors, or in renal failure (especially without dialysis) should not get supplemental GLN. Two recent meta-analyses [68], [69] have confirmed that traditional PN supplementation with intravenous GLN is safe, reduces mortality and LOS, and improves outcome. Based on nine level 1 and 19 level 2 studies, the authors concluded, “When PN is prescribed to ICU patients, parenteral GLN supplementation should be considered”. Patients in need of PN, and those with burns, trauma or malignancies, may continue to benefit from supplemental GLN, administered either intravenously <0.35 g/kg/day or enterally <0.5 g/kg/day [70, 71]. TPN routinely contains only 19 amino acids so GLN must be supplemented, and not given pharmacologically, in a stable form to provide complete nutrition.
6. Lipids
Current use of pure soy lipid as part of parenteral nutrition should likely be abandoned as it is immunosuppressive and pro-inflammatory [72, 73]. This is particularly true given the now worldwide availability of balanced lipid solutions containing various combinations of fish and/or olive oil. There are also data supporting a benefit of utilizing fish oil containing balanced lipid formulations versus soy lipid alone in patients requiring TPN in the ICU or post-operative setting. These data include a recent meta-analysis of 23 RCTs, including 1502 surgical and ICU patients, which demonstrated that fish oil-containing lipids reduced length of stay and infectious complications versus traditional soy-only lipids [32]. A more recent meta-analysis of 10 RCTs demonstrated that fish oil-based intravenous lipids significantly reduced infections in critical illness [33]. It is thus recommended that when TPN is utilized, a modern, balanced lipid that reduces soy lipid content should be given.
III. Conclusions
In conclusion, to optimize nutrition delivery we need to consider basic metabolism and our historic understanding of data for recovery from severe LBM loss (starvation) to employ targeted nutritional care in sepsis. If we are to optimize patient outcomes and start creating “survivors and not victims” following sepsis and intensive care, we must continue to evolve our delivery of “personalized” nutritional needs, which almost assuredly change over the course of illness. The presence of nutritional risk and metabolic reserve as defined by the NUTRIC score and CT scan- or ultrasound-guided LBM assessment should guide how we feed our patients, with high risk (NUTRIC ≥5 or sarcopenic patients) getting aggressive early calorie and protein delivery via early EN and/or PN. Furthermore, we must all read and revel in the defining achievement that is the Minnesota Starvation Study [7] and learn from its landmark lessons. Most important among these is that even healthy subjects require significant calories (typically >4000 kcal/day) to recover from massive weight and LBM loss, such as occurs following sepsis. How many of our care protocols, or our patients, acknowledge or achieve this well-described goal? Is it possible this lack of understanding of caloric and protein needs during recovery, and thus suboptimal provision, has led to the extremely poor long-term outcomes and QoL that follows ICU care? Only time and further research will tell for sure. This increase in calorie delivery should be targeted to when patients are recovering. Use of emerging metabolic cart technology [74] and, perhaps, even bedside C13 breath testing, to target over-/under-feeding and substrate delivery [75, 76] will help guide this in the future. Finally, we must learn to target and incorporate nutritional therapies such as vitamin D, probiotics, and anabolic/anti-catabolic agents to optimize our patients’ chances of survival and to thrive against all evolutionary odds. We have long known Mother Nature does not want our ICU patients to win this war and become “survivors…and not victims”. But, to begin winning this war on long-term ICU outcomes and give our patients back the lives they came to us to restore, we must ensure we are giving the right nutrition, to the right patient, at the right time!
Table 2.
Summary of Caloric Needs of Critically Ill and Healthy Individuals in the Context of the Minnesota Starvation Study and Actual Current ICU Calories
| Mean REE (kcal/d) | TEE (kcal/d) |
TEE/wght (kcal/kg/d) | |
|---|---|---|---|
|
| |||
| Uehara et al ICU Study12 | |||
| Sepsis Patients | |||
| (Mean age: 67) | |||
| Week 1 | ~1854 | 1927 +/− 370 | 25 +/− 5 |
| Week 2 | 3257 +/− 370 | 47 +/− 6 | |
| Trauma Patients | |||
| (Mean Age: 34) | |||
| Week 1 | ~2122 | 2380 +/− 422 | 31 +/−6 |
| Week 2 | 4123 +/− 518 | 59 +/−7 | |
|
| |||
| WHO Calorie Requirements Healthy Subjects* | |||
| Men | ~3000 | 44 (Range: 35–53) | |
| Women | ~2500 | 36 (Range: 29–4) | |
|
| |||
| Minnesota Starvation Study Calorie Delivery | Delivered Energy (Kcal/d) | Delivered Energy/Wght (Kcal/kg/d) | |
| Baseline Period | 3200 | ~50 | |
| Starvation Period | ~1800 | 23–30 | |
| Recovery Period Delivery (For recovery to occur) | ~4000 | ~60 | |
|
| |||
| Actual Average Kcal/d | 1034 kcal/d | ||
| Delivered In Critically Ill Patients | |||
| Over First 12 Days of ICU Stay15 | |||
- Data for healthy 70 kg person with intermediate physical activity (1.75 physical activity level (PAL) factor)-Reference:http://www.fao.org/docrep/007/y5686e/y5686e00.htm#Contents)
KEY POINTS.
Sepsis is characterized by early massive catabolism, lean body mass (LBM) loss and escalating hypermetabolism persisting for months-years.
Early enteral nutrition should attempt to correct micronutrient/vitamin deficiencies, deliver adequate protein (~1.0 g/kg/d) and moderated non-protien calories (~15 kcal/kg/d) as well-nourished patients generate significant endogenous energy.
Post-resuscitation, increasing protein (1.5 – 2.0 g/kg/d) and calories are needed to attenuate LBM loss, promote early mobility and recovery.
Following ICU, significant protein/calorie delivery for months-years Is required to facilitate functional and LBM recovery, with high protein oral supplements being essential to achieve adequate nutrition (> 3000 kcal/d and higher-protein (> 1.5 g/kg/d) likely needed)
Screening for pre-illness malnutrition is essential with supplemental parenteral nutrition added if protein/calorie goals not met with timeliness dependent on pre-illness nutrition/LBM status.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
PEW- Is an associate editor of Clinical Nutrition (Elsevier). Has received grant funding related to this work from the NIH NHLBI R34 HL109369, Canadian Institutes of Health Research, Baxter, Fresenius, Lyric Pharmaceuticals, Isomark Inc and Medtronics. Dr. Wischmeyer has served as a consultant to Nestle, Abbott, Fresenius, Baxter, Medtronics, Nutricia, and Lyric Pharmaceuticals, and Takeda for research related to this work. Dr. Wischmeyer has limited ownership shares in Isomark for his consulting work with Isomark, which has otherwise been unpaid in nature. Dr. Wischmeyer has received honoraria or travel expenses for lectures on improving nutrition care in illness from Abbott, Fresenius and Medtronics.
References
- 1.Gillis C, Carli F. Promoting Perioperative Metabolic and Nutritional Care. Anesthesiology. 2015;123(6):1455–1472. doi: 10.1097/ALN.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 2.Preiser JC, van Zanten AR, Berger MM, Biolo G, Casaer MP, Doig GS, Griffiths RD, Heyland DK, Hiesmayr M, Iapichino G, et al. Metabolic and nutritional support of critically ill patients: consensus and controversies. Crit Care. 2015;19:35. doi: 10.1186/s13054-015-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinglas VD, Aronson Friedman L, Colantuoni E, Mendez-Tellez PA, Shanholtz CB, Ciesla ND, Pronovost PJ, Needham DM. Muscle Weakness and 5-Year Survival in Acute Respiratory Distress Syndrome Survivors. Crit Care Med. 2017;45(3):446–453. doi: 10.1097/CCM.0000000000002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson SJ, Tsai AA, Scala CM, Sowa DC, Sheean PM, Braunschweig CL. Adequacy of oral intake in critically ill patients 1 week after extubation. J Am Diet Assoc. 2010;110(3):427–433. doi: 10.1016/j.jada.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Wei X, Day AG, Ouellette-Kuntz H, Heyland DK. The Association Between Nutritional Adequacy and Long-Term Outcomes in Critically Ill Patients Requiring Prolonged Mechanical Ventilation: A Multicenter Cohort Study. Crit Care Med. 2015;43(8):1569–1579. doi: 10.1097/CCM.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 6.Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, Heyland DK. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 7.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation. I–II. Minneapolis, MN: University of Minnesota Press; 1950. [Google Scholar]
- 8.Stanojcic M, Finnerty CC, Jeschke MG. Anabolic and anticatabolic agents in critical care. Curr Opin Crit Care. 2016;22(4):325–331. doi: 10.1097/MCC.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wischmeyer PE. Are we creating survivors…or victims in critical care? Delivering targeted nutrition to improve outcomes. Curr Opin Crit Care. 2016;22(4):279–284. doi: 10.1097/MCC.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 10.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 11.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. Jama. 2014;311(13):1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 12.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31(9):2316–2323. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 13.Kamdar BB, Huang M, Dinglas VD, Colantuoni E, von Wachter TM, Hopkins RO, Needham DM, National Heart L Blood Institute Acute Respiratory Distress Syndrome N. Joblessness and Lost Earnings After ARDS in a 1-Year National Multicenter Study. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201611-2327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. The American journal of clinical nutrition. 2012;96(3):591–600. doi: 10.3945/ajcn.111.032078. [DOI] [PubMed] [Google Scholar]
- 15.Hoffer LJ, Bistrian BR. What is the best nutritional support for critically ill patients? Hepatobiliary Surg Nutr. 2014;3(4):172–174. doi: 10.3978/j.issn.2304-3881.2014.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshima T, Deutz NE, Doig G, Wischmeyer PE, Pichard C. Protein-energy nutrition in the ICU is the power couple: A hypothesis forming analysis. Clin Nutr. 2016;35(4):968–974. doi: 10.1016/j.clnu.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Rahman A, Hasan RM, Agarwala R, Martin C, Day AG, Heyland DK. Identifying critically-ill patients who will benefit most from nutritional therapy: Further validation of the "modified NUTRIC" nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–162. doi: 10.1016/j.clnu.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268. doi: 10.1186/cc10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Twisk JW, Oudemans-van Straaten HM, Weijs PJ. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20(1):386. doi: 10.1186/s13054-016-1563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uehara M, Plank LD, Hill GL. Components of energy expenditure in patients with severe sepsis and major trauma: a basis for clinical care. Crit Care Med. 1999;27(7):1295–1302. doi: 10.1097/00003246-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Critical care medicine. 1993;21(7):1012–1019. doi: 10.1097/00003246-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, et al. Early versus late parenteral nutrition in critically ill adults. The New England journal of medicine. 2011;365(6):506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 23.Fürst P. Protein and amino acid metabolism: Composition of stressed and nonstressed states. In: Cresci G, editor. Nutrition support for the critically ill patient. Boca Raton: Taylor & Francis (CRC); 2005. p. 29. [Google Scholar]
- 24.McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 25.Vanhorebeek I, Verbruggen S, Casaer MP, Gunst J, Wouters PJ, Hanot J, Guerra GG, Vlasselaers D, Joosten K, Van den Berghe G. Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med. 2017 doi: 10.1016/S2213-2600(17)30186-8. [DOI] [PubMed] [Google Scholar]
- 26.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical. Trials N, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. Jama. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, Mehta S, McIntyre L, Solaiman O, Sakkijha MH, et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med. 2015;372(25):2398–2408. doi: 10.1056/NEJMoa1502826. [DOI] [PubMed] [Google Scholar]
- 28.Hoffer LJ, Bistrian BR. Energy deficit is clinically relevant for critically ill patients: no. Intensive Care Med. 2015;41(2):339–341. doi: 10.1007/s00134-014-3518-y. [DOI] [PubMed] [Google Scholar]
- 29.Doig GS, Simpson F, Sweetman EA, Finfer SR, Cooper DJ, Heighes PT, Davies AR, O'Leary M, Solano T, Peake S, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013;309(20):2130–2138. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 30.Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, Thibault R, Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381(9864):385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 31.Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, Bellingan G, Leonard R, Mythen MG, Rowan KM, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med. 2014;371(18):1673–1684. doi: 10.1056/NEJMoa1409860. [DOI] [PubMed] [Google Scholar]
- 32.Pradelli L, Mayer K, Muscaritoli M, et al. n-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care. 2012;16:R184. doi: 10.1186/cc11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manzanares W, Langlois PL, Dhaliwal R, Lemieux M, Heyland DK. Intravenous fish oil lipid emulsions in critically ill patients: an updated systematic review and meta-analysis. Crit Care. 2015;19:167. doi: 10.1186/s13054-015-0888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei X, Day AG, Ouellette-Kuntz H, Heyland DK. The Association Between Nutritional Adequacy and Long-Term Outcomes in Critically Ill Patients Requiring Prolonged Mechanical Ventilation: A Multicenter Cohort Study. Critical care medicine. 2015 doi: 10.1097/CCM.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 35.Kalm LM, Semba RD. They starved so that others be better fed: remembering Ancel Keys and the Minnesota experiment. J Nutr. 2005;135(6):1347–1352. doi: 10.1093/jn/135.6.1347. [DOI] [PubMed] [Google Scholar]
- 36.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 37.MacLaren R, Kiser TH, Fish DN, Wischmeyer PE. Erythromycin vs metoclopramide for facilitating gastric emptying and tolerance to intragastric nutrition in critically ill patients. JPEN J Parenter Enteral Nutr. 2008;32(4):412–419. doi: 10.1177/0148607108319803. [DOI] [PubMed] [Google Scholar]
- 38.Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, Clavel M, Frat JP, Plantefeve G, Quenot JP, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. Jama. 2013;309(3):249–256. doi: 10.1001/jama.2012.196377. [DOI] [PubMed] [Google Scholar]
- 39.Davies AR, Morrison SS, Bailey MJ, Bellomo R, Cooper DJ, Doig GS, Finfer SR, Heyland DK Investigators ES, Group ACT. A multicenter, randomized controlled trial comparing early nasojejunal with nasogastric nutrition in critical illness. Crit Care Med. 2012;40(8):2342–2348. doi: 10.1097/CCM.0b013e318255d87e. [DOI] [PubMed] [Google Scholar]
- 40.Needham DM, Feldman DR, Kho ME. The functional costs of ICU survivorship. Collaborating to improve post-ICU disability. Am J Respir Crit Care Med. 2011;183(8):962–964. doi: 10.1164/rccm.201012-2042ED. [DOI] [PubMed] [Google Scholar]
- 41.Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: new innovations in nutrition and exercise physiology. Crit Care. 2015;19(Suppl 3):S6. doi: 10.1186/cc14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puthucheary ZA, Wischmeyer P. Predicting critical illness mortality and personalizing therapy: moving to multi-dimensional data. Crit Care. 2017;21(1):20. doi: 10.1186/s13054-016-1597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11(2):278–296. doi: 10.1016/j.arr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Elia M, Normand C, Norman K, Laviano A. A systematic review of the cost and cost effectiveness of using standard oral nutritional supplements in the hospital setting. Clin Nutr. 2016;35(2):370–380. doi: 10.1016/j.clnu.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Stratton RJ, Hebuterne X, Elia M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev. 2013;12(4):884–897. doi: 10.1016/j.arr.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Stratton R, Green C, Elia M. Disease-Related Malnutrition: An Evidence-Based Approach to Treatment. Wallingford, UK: CABI Publishing; 2003. [Google Scholar]
- 47.Philipson TJ, Snider JT, Lakdawalla DN, Stryckman B, Goldman DP. Impact of oral nutritional supplementation on hospital outcomes. Am J Manag Care. 2013;19(2):121–128. [PubMed] [Google Scholar]
- 48.Deutz NE, Matheson EM, Matarese LE, Luo M, Baggs GE, Nelson JL, Hegazi RA, Tappenden KA, Ziegler TR, Group NS. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin Nutr. 2016;35(1):18–26. doi: 10.1016/j.clnu.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Casaer MP, Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370(13):1227–1236. doi: 10.1056/NEJMra1304623. [DOI] [PubMed] [Google Scholar]
- 50.Stanga Z, Brunner A, Leuenberger M, Grimble RF, Shenkin A, Allison SP, Lobo DN. Nutrition in clinical practice-the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr. 2008;62(6):687–694. doi: 10.1038/sj.ejcn.1602854. [DOI] [PubMed] [Google Scholar]
- 51.Doig GS, Simpson F, Heighes PT, Bellomo R, Chesher D, Caterson ID, Reade MC, Harrigan PW Refeeding Syndrome Trial Investigators G. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med. 2015;3(12):943–952. doi: 10.1016/S2213-2600(15)00418-X. [DOI] [PubMed] [Google Scholar]
- 52.Frank RA, Leeper FJ, Luisi BF. Structure, mechanism and catalytic duality of thiamine-dependent enzymes. Cell Mol Life Sci. 2007;64(7–8):892–905. doi: 10.1007/s00018-007-6423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donnino MW, Andersen LW, Chase M, Berg KM, Tidswell M, Giberson T, Wolfe R, Moskowitz A, Smithline H, Ngo L, et al. Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit Care Med. 2016;44(2):360–367. doi: 10.1097/CCM.0000000000001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, Vitamin C and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2016 doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135(3):326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 56.Alhazzani W, Jacobi J, Sindi A, Hartog C, Reinhart K, Kokkoris S, Gerlach H, Andrews P, Drabek T, Manzanares W, et al. The effect of selenium therapy on mortality in patients with sepsis syndrome: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2013;41(6):1555–1564. doi: 10.1097/CCM.0b013e31828a24c6. [DOI] [PubMed] [Google Scholar]
- 57.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG Canadian Critical Care Trials G. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368(16):1489–1497. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 58.Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, Moerer O, Weyland A, Marx G, Grundling M, et al. Effect of Sodium Selenite Administration and Procalcitonin-Guided Therapy on Mortality in Patients With Severe Sepsis or Septic Shock: A Randomized Clinical Trial. JAMA Intern Med. 2016;176(9):1266–1276. doi: 10.1001/jamainternmed.2016.2514. [DOI] [PubMed] [Google Scholar]
- 59.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 60.Iglar PJ, Hogan KJ. Vitamin D status and surgical outcomes: a systematic review. Patient Saf Surg. 2015;9:14. doi: 10.1186/s13037-015-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins DM, Wischmeyer PE, Queensland KM, Sillau SH, Sufit AJ, Heyland DK. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN J Parenter Enteral Nutr. 2012;36(6):713–720. doi: 10.1177/0148607112444449. [DOI] [PubMed] [Google Scholar]
- 62.Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill. Crit Care Med. 2014;42(1):97–107. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 63.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Munch A, Warnkross H, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. Jama. 2014;312(15):1520–1530. doi: 10.1001/jama.2014.13204. [DOI] [PubMed] [Google Scholar]
- 64.Han JE, Jones JL, Tangpricha V, Brown MA, Brown LA, Hao L, Hebbar G, Lee MJ, Liu S, Ziegler TR, et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J Clin Transl Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bongers T, Griffiths RD, McArdle A. Exogenous glutamine: the clinical evidence. Crit Care Med. 2007;35(9 Suppl):S545–552. doi: 10.1097/01.CCM.0000279193.23737.06. [DOI] [PubMed] [Google Scholar]
- 66.Rodas PC, Rooyackers O, Hebert C, Norberg A, Wernerman J. Glutamine and glutathione at ICU admission in relation to outcome. Clin Sci (Lond) 2012;122(12):591–597. doi: 10.1042/CS20110520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Zanten AR, Sztark F, Kaisers UX, Zielmann S, Felbinger TW, Sablotzki AR, De Waele JJ, Timsit JF, Honing ML, Keh D, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: a randomized clinical trial. Jama. 2014;312(5):514–524. doi: 10.1001/jama.2014.7698. [DOI] [PubMed] [Google Scholar]
- 68.Wischmeyer PE, Dhaliwal R, McCall M, Ziegler TR, Heyland DK. Parenteral glutamine supplementation in critical illness: a systematic review. Crit Care. 2014;18(2):R76. doi: 10.1186/cc13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr. 2013;32(2):213–223. doi: 10.1016/j.clnu.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Vanek VW, Matarese LE, Robinson M, Sacks GS, Young LS, Kochevar M. A.S.P.E.N. position paper: parenteral nutrition glutamine supplementation. Nutr Clin Pract. 2011;26(4):479–494. doi: 10.1177/0884533611410975. [DOI] [PubMed] [Google Scholar]
- 71.Wischmeyer P. Glutamine Supplementation in Parenteral Nutrition and Intensive Care Unit Patients: Are We Throwing the Baby Out With the Bathwater? JPEN J Parenter Enteral Nutr. 2015;39(8):893–897. doi: 10.1177/0148607115593792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buenestado A, Cortijo J, Sanz MJ, Naim-Abu-Nabah Y, Martinez-Losa M, Mata M, Issekutz AC, Marti-Bonmati E, Morcillo EJ. Olive oil-based lipid emulsion's neutral effects on neutrophil functions and leukocyte-endothelial cell interactions. JPEN J Parenter Enteral Nutr. 2006;30(4):286–296. doi: 10.1177/0148607106030004286. [DOI] [PubMed] [Google Scholar]
- 73.Wischmeyer PE. Alternative lipid emulsions as a new standard of care for total parenteral nutrition: finally available in the United States? Crit Care Med. 2015;43(1):230–231. doi: 10.1097/CCM.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Waele E, Honore PM, Spapen HD. New generation indirect calorimeters for measuring energy expenditure in the critically ill: a rampant or reticent revolution? Crit Care. 2016;20(1):138. doi: 10.1186/s13054-016-1315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butz DE, Weidmann D, Brownsword R, Cook ME, Schoeller DA, Whigham LD. Immediate biofeedback for energy balance via expired breath delta(13)CO2. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2015;2015:8205–8208. doi: 10.1109/EMBC.2015.7320299. [DOI] [PubMed] [Google Scholar]
- 76.Whigham LD, Butz DE, Johnson LK, Schoeller DA, Abbott DH, Porter WP, Cook ME. Breath carbon stable isotope ratios identify changes in energy balance and substrate utilization in humans. International journal of obesity. 2014;38(9):1248–1250. doi: 10.1038/ijo.2014.7. [DOI] [PubMed] [Google Scholar]