Abstract
BACKGROUND:
Scleromyxedema, also referred to as the Arndt-Gottron (S-AG) syndrome or the systemic form of Lichen myxedematosus (LM), is a cutaneous mucinosis with a chronic course and high lethality from systemic involvement of other organs and systems. Interesting in several aspects is the association between scleromyxedema and viral hepatitis about: 1) hepatitis virus infection as a possible etiological factor for the development of scleromyxedema, 2) antiretroviral therapy for the treatment of hepatitis as a method of reversing scleromyxedema and 3) antiviral drugs as inducers of scleromyxedema.
CASE REPORT:
We present a 53-year old patient who for nine months had been on tenofovir disoproxil 245 mg (0-0-1) therapy for chronic hepatitis B. Three months after the start of antiviral therapy (i.e. for a period of 6 months), the patient observed swelling, itching and hardening of the skin on the face, ears and hands, which subsequently spread throughout the trunk. Subsequent histological study of a skin biopsy revealed changes of scleromyxedema at an advanced stage, though immunoelectrophoresis of serum and urine excluded the presence of paraproteinaemia or para proteinuria. Systemic antihistamine and topical corticosteroid therapy were instituted. Bone involvement with possible plasmacytoma was excluded, and a myelogram showed evidence of an erythroblastic reaction of bone marrow.
CONCLUSION:
We believe that drug-induced scleromyxedema is a rare but possible phenomenon. We describe the first case of tenofovir-induced scleromyxedema within the framework of chronic hepatitis B treatment.
Keywords: Scleromyxedema, Arndt - Gottron syndrome, Tenofovir, Hepatitis B, Diabetes mellitus, Survival benefit, Pathogenetic relationship, Treatment
Introduction
Scleromyxedema, a systemic form of lichen myxedematosus (LM) [1], is associated with significant mortality [2], [3], [4]. Interesting in this regard is the association of scleromyxedema with hepatitis virus [5]. Scleromyxedema may occur secondarily in patients with viral hepatitis C [5], [6]. According to some authors, antiviral therapy for the treatment of hepatitis leads to the reversal of scleromyxedema and, according to others, treatment with interferon alpha 2 leads to worsening of LM [7]. We describe a patient in whom we believe there is a possible association between the development of scleromyxedema and the use of tenofovir disoproxil for hepatitis B.
Case report
We present a 53-year-old man with type 2 diabetes mellitus, chronic hepatitis B, hepatic cirrhosis, duodenal ulcer, mild splenomegaly, chronic cholecystitis and hepatitis B associated nephropathy. The patient was receiving treatment with insulin degludec 30 IU-0 -0 and insulin aspart 10 IU-14 IU-14 IU, and for the past nine months, he received tenofovir disoproxil 245 mg (0-0-1) for treatment of chronic hepatitis B. The patient was hospitalized for swelling, pruritus and hardening of the skin on the face, ears and hands, which subsequently spread to involve the trunk. Skin complaints began 3 months after the start of therapy with tenofovir. Dermatological examination revealed significant thickening and hardening in the areas of the face, neck, body and extremities, and generalised lichenoid papules were also found (Figure 1a, 1b, 1c, and 1d).
Figure 1.
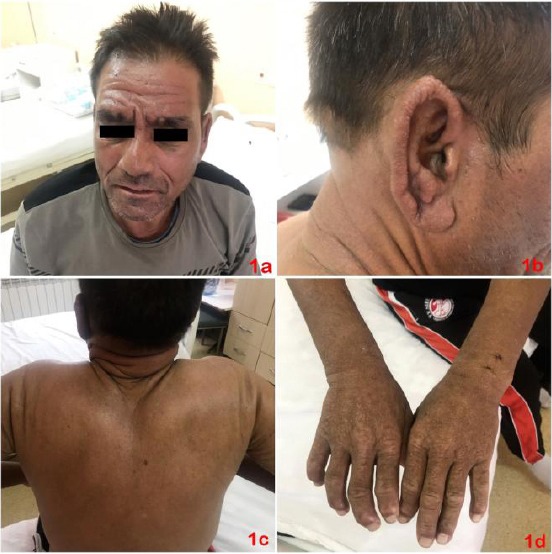
a) Hardening of the face skin; b) Skin-colored small papules on the ear skin; c) Hardening of the skin on the back and neck; d) Multiple disseminated papules on the skin of the hands and arthropathy
Based on clinical data, scleromyxedema, scleredema of Buschke and lichen amyloidosis were considered as possible diagnoses. A skin biopsy showed numerous fibroblasts and irregularly arranged collagen bundles with prominent mucin deposition (Figure 2), consistent with an advanced stage of scleromyxedema.
Figure 2.
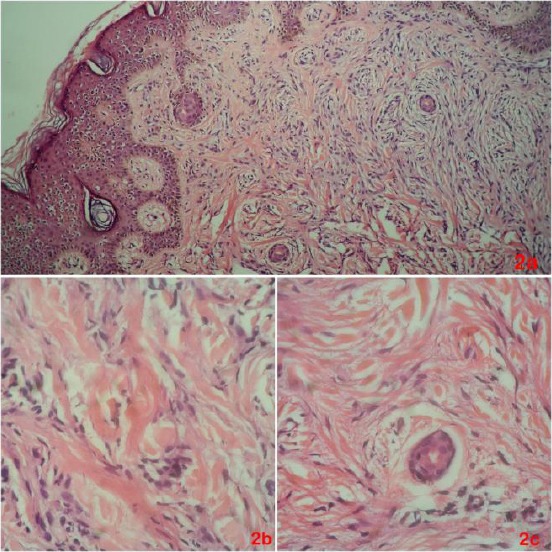
a) This skin biopsy shows a combination of numerous fibroblasts, mucin, and irregularly arranged collagen bundles; b) At higher magnification, there are irregularly arranged collagen and scattered spindled cells, representing fibroblasts, within a mucinous background; c) This image shows details of fibroblasts around the cross-sectional profile of an eccrine sweat duct
Double antihistamine therapy was initiated due to the presence of severe itching, and flumetasone pivalate/clioquinol was administered topically. The consultation was obtained from a gastroenterologist, who concluded that, given the patient’s ongoing chronic hepatitis B and posthepatic cirrhosis, it would not be appropriate to start systemic corticosteroid therapy because of its immunosuppressive effect. Immunoelectrophoresis of serum and urine excluded paraproteinaemia or para proteinuria. During the hospitalisation, additional tests were performed. Skull and pelvic radiography excluded possible bone involvement with plasmacytoma, and ultrasound of the abdominal organs showed no paraneoplastic process.
Laboratory data included CEA - 2.87 μg/ml (0-5), PSA-0.178 μg/ml (0-3,100), and AST-31 IU (0-200). A myelogram showed evidence of an erythroblastic reaction of bone marrow, a mild leukemoid reaction of granulocyte-neutrophil type, and a mild eosinophilic bone marrow response-consistent with reactive changes. The patient was referred to the haematology and oncology clinic for bone marrow puncture, bone scintigraphy, and further therapeutic recommendations. Ambulatory systemic therapy with Azathioprine 2 x 50 mg/day and topical-flumethasone pivalate/clioquinol was initiated. After a few months of treatment with Azathioprine 2 x 50 mg/day, a slight improvement in major symptoms was observed. It is planned to discontinue tenofovir therapy after consultation with a gastroenterologist. The viral load following azathioprine therapy was 0, but the therapy was stopped and after a 1-week treatment with intravenous immunoglobulin will be initiated. Treatment with intravenous IgG will be performed as follows: 2 g per kg/every month, divided into five days with 430 mg/day for 6 months.
Discussion
Scleromyxedema or Arndt-Gottron (S-AG) syndrome is a cutaneous mucinosis that mainly affects adults between 30 and 70 years of age and whose aetiology is not fully understood [2]. The disease is defined as a systemic form of Lichen myxedematosus [5]. It is believed that in 80% of patients there is monoclonal gammopathy and there is probably an immune response of B-cells to antigenic mucin deposits in the dermis [3]. However, it is possible to observe scleromyxedema without underlying paraproteinemia, as in our patient [3]. A major sign of this disease is the histopathological triad of dermal mucin deposition, fibroblast proliferation, and fibrosis [2]. The clinical condition is characterised by widespread lichenoid papules which coalesce to form generalised plaques that cause extensive thickening and hardening of the skin [2], [3]. There may also be an atypical clinical presentation with cutaneous and subcutaneous nodules [8]. According to prevailing literature, the absence of thyroid disease should also be considered as one of the main criteria for the diagnosis of scleromyxedema [1].
Scleromyxedema is a chronic disease that may be accompanied by severe systemic manifestations such as gastrointestinal, neurological, cardiac, muscular, renal, pulmonary, or ophthalmological disorders, and these associations may cause significant disability and may be fatal [3], [4].
A rare variant that can be associated with a high risk of mortality is the so-called ’dermato-neuro syndrome’ [9]. It is characterised by a prodromal flu-like period, followed by fever, convulsions and coma [9].
Of interest are the rare cases, such as this one, in which scleromyxedema is present within the framework of a hepatitis virus infection [5]. Much of the literature on this association describes scleromyxedema secondary to hepatitis C virus and successful treatment with antiviral therapy [5], [6]. According to these sources, hepatitis C infection should be considered as a possible etiological factor for the development of scleromyxedema [5], [6]. However, it is also possible that lichen myxedematosus may worsen in the setting of chronic hepatitis C therapy treated with interferon alfa-2a [7]. Also, there is a reported case of scleromyxedema development as a result of interferon beta-1a (IFN beta-1a) therapy following a diagnosis of multiple sclerosis (MS) [10]. At present, the mechanism by which IFN beta-1a may lead to the development of scleromyxedema has not been established [10]. In any event, we believe that the possibility of drug-induced scleromyxedema is worthy of serious consideration.
Also described in the literature is a case of papular mucinosis (PM) in association with HIV infection in a patient with highly active antiretroviral therapy (HAART) with tenofovir disoproxil/emtricitabine and lopinavir/ritonavir [11]. According to studies: 1) antiretroviral therapy (including tenofovir) in patients with HIV infection and lichen myxedematosus leads to a complete resolution of papular mucinosis and 2) tenofovir prevents hepatic fibrosis development and protects against bleomycin-induced dermal fibrosis by downregulating adenosine levels in the liver and skin [12], [13]. These data indicate that tenofovir, through a variety of mechanisms, may affect the pathogenetic chain of events leading to cutaneous mucinosis and fibrosis. Based on the previous data, we believe that we describe the first case of scleromyxedema induced by treatment with tenofovir for hepatitis B.
At the moment there is no specific definitive treatment of scleromyxedema [4]. There is a wide range of treatment options, such as high-dose corticosteroids, a variety of immunosuppressive and chemotherapeutic drugs, PUVA and UVA1 phototherapy, interferon-α, retinoids, thalidomide, bortezomib, and autologous stem cell transplantation, but intravenous immunoglobulin (IVIg) treatment is considered to be relatively effective and safe treatment, and appears to be the treatment of choice for scleromyxedema [3], [4], [8]. Also, some authors suggest that treatment with intravenous immunoglobulins is considered and recommended as First Line therapy [14]. They recommend a dose of 2 g per kg, divided into four/five partial doses on four/five days, with the interval between cycles from 4 weeks to maximally 6 weeks (as well as the one assigned to our patient) [14].
In conclusion, the exact mechanisms under which antiviral drugs affect skin deposits of mucin and cutaneous fibrosis have not yet been established. There are contradictory data according to which, on the one hand, drug therapy for hepatitis C worsens LM, and on the other, treatment with tenofovir in HIV-infected patients leads to a reversal of cutaneous mucinosis. However, they indicate that the pathogenesis of scleromyxedema may be associated with different drug groups. We believe that we are presenting the first case of tenofovir-induced scleromyxedema.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Rongioletti F, Rebora A. Updated classification of papular mucinosis, lichen myxedematosus, and scleromyxedema. J Am Acad Dermatol. 2001;44(2):273–81. doi: 10.1067/mjd.2001.111630. https://doi.org/10.1067/mjd.2001.111630 PMid:11174386. [DOI] [PubMed] [Google Scholar]
- 2.Sala A, Cunha P, Pinto C, Alves C, Paiva I, Araujo A. Scleromyxedema:clinical diagnosis and autopsy findings. A Bras Dermatol. 2016;91(5 Suppl 1):48–50. doi: 10.1590/abd1806-4841.20164527. https://doi.org/10.1590/abd1806-4841.20164527 PMid:28300892 PMCid:PMC5324991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saniee S, Davarnia G. Scleromyxedema without Paraproteinemia:Treatment with Thalidomide and Prednisolone. Case Rep Dermatol. 2016;8(3):327–332. doi: 10.1159/000452319. https://doi.org/10.1159/000452319 PMid:27990110 PMCid:PMC5156886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rongioletti F, Merlo G, Cinotti E, Fausti V, Cozzani E, Cribier B, Metze D, Calonje E, Kanitakis J, Kempf W, Stefanato C, Marinho E, Parodi A. Scleromyxedema:a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69(1):66–72. doi: 10.1016/j.jaad.2013.01.007. https://doi.org/10.1016/j.jaad.2013.01.007 PMid:23453242. [DOI] [PubMed] [Google Scholar]
- 5.Smith J, Kalimullah F, Erickson C, Peng L. Scleromyxedema secondary to hepatitis C virus and successfully treated with antiviral therapy. Dermatol Online J. 2015;21(9) [PubMed] [Google Scholar]
- 6.Azfar N, Khan A, Zaman T, Jahangir M. Scleromyxedema in an HCV-positive male. J Pak Assoc Dermatol. 2008;18:116–118. [Google Scholar]
- 7.Rongioletti F, Rebora A. Worsening of lichen myxedematosus during interferon alfa-2a therapy for chronic active hepatitis C. J Am Acad Dermatol. 1998;38(5 Pt 1):760–1. doi: 10.1016/s0190-9622(98)70205-1. https://doi.org/10.1016/S0190-9622(98)70205-1. [DOI] [PubMed] [Google Scholar]
- 8.Dolenc-Voljč M, Jurčić V, Hočevar A, Tomšič M. Scleromyxedema with subcutaneous nodules:successful treatment with thalidomide and intravenous immunoglobulin. Case Rep Dermatol. 2013;5(3):309–15. doi: 10.1159/000356469. https://doi.org/10.1159/000356469 PMid:24348379 PMCid:PMC3843934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming K, Virmani D, Sutton E, Langley R, Corbin J, Pasternak S, Walsh N. Scleromyxedema and the dermato-neuro syndrome:case report and review of the literature. J Cutan Pathol. 2012;39(5):508–17. doi: 10.1111/j.1600-0560.2012.01882.x. https://doi.org/10.1111/j.1600-0560.2012.01882.x PMid:22515222. [DOI] [PubMed] [Google Scholar]
- 10.Kumar N, Rodriguez M. Scleromyxedema in a patient with multiple sclerosis and monoclonal gammopathy on interferon beta-1a. Mult Scler. 2004;10(1):85–6. doi: 10.1191/1352458504ms987cr. https://doi.org/10.1191/1352458504ms987cr PMid:14760958. [DOI] [PubMed] [Google Scholar]
- 11.Abbott R, Calonje E, Almaani N, Kulasegram R, McGibbon D. Widespread papules in a patient with human immunodeficiency virus. Papular mucinosis (PM) in association with HIV infection. Clin Exp Dermatol. 2010;35(7):801–2. doi: 10.1111/j.1365-2230.2010.03830.x. PMid:20831608. [DOI] [PubMed] [Google Scholar]
- 12.Feig J, Mediero A, Corciulo C, Liu H, Zhang J, Perez-Aso M, Picard L, Wilder T, Cronstein B. The antiviral drug tenofovir, an inhibitor of Pannexin-1-mediated ATP release, prevents liver and skin fibrosis by downregulating adenosine levels in the liver and skin. PLoS One. 2017;12(11):e0188135. doi: 10.1371/journal.pone.0188135. https://doi.org/10.1371/journal.pone.0188135 PMid:29145453 PMCid:PMC5690602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girón J, Dean M. Resolution of papular mucinosis in a person with HIV infection. AIDS Read. 2007;17(8):418–20. PMid:17717886. [PubMed] [Google Scholar]
- 14.Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, Cutolo M, Rongioletti F, Denton C, Rudnicka L, Frasin L, Smith V, Gabrielli A, Aberer E, Bagot M, Bali G, Bouaziz J, Braae Olesen A, Foeldvari I, Frances C, Jalili A, Just U, Kähäri V, Kárpáti S, Kofoed K, Krasowska D, Olszewska M, Orteu C, Panelius J, Parodi A, Petit A, Quaglino P, Ranki A, Sanchez Schmidt J, Seneschal J, Skrok A, Sticherling M, Sunderkötter C, Taieb A, Tanew A, Wolf P, Worm M, Wutte N, Krieg T. European dermatology forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 2:Scleromyxedema, scleredema and nephrogenic systemic fibrosis. J Eur Acad Dermatol Venereol. 2017;31(10):1581–1594. doi: 10.1111/jdv.14466. https://doi.org/10.1111/jdv.14466 PMid:28786499. [DOI] [PubMed] [Google Scholar]


