Abstract
One of the techniques utilised in the management of cancer in all stages is multiple biomedical imaging. Imaging as an important part of cancer clinical protocols can provide a variety of information about morphology, structure, metabolism and functions. Application of imaging technics together with other investigative apparatus including in fluids analysis and vitro tissue would help clinical decision-making. Mixed imaging techniques can provide supplementary information used to improve staging and therapy planning. Imaging aimed to find minimally invasive therapy to make better results and reduce side effects. Probably, the most important factor in reducing mortality of certain cancers is an early diagnosis of cancer via screening based on imaging. The most common cancer in women is breast cancer. It is considered as the second major cause of cancer deaths in females, and therefore it remained as an important medical and socio-economic issue. Medical imaging has always formed part of breast cancer care and has used in all phases of cancer management from detection and staging to therapy monitoring and post-therapeutic follow-up. An essential action to be performed in the preoperative staging of breast cancer based on breast imaging. The general term of breast imaging refers to breast sonography, mammography, and magnetic resonance tomography (MRT) of the breast (magnetic resonance mammography, MRM).
Further development in technology will lead to increase imaging speed to meet physiological processes requirements. One of the issues in the diagnosis of breast cancer is sensitivity limitation. To overcome this limitation, complementary imaging examinations are utilised that traditionally includes screening ultrasound, and combined mammography and ultrasound. Development in targeted imaging and therapeutic agents calls for close cooperation among academic environment and industries such as biotechnological, IT and pharmaceutical industries.
Keywords: Breast Imaging, Cancer detection, Mammography, Ultrasonography MRI, Breast Scintimammography
Introduction
Cancer is the cause of one in eight deaths worldwide, and about 12 million new cases are diagnosed yearly. In spite of using aggressive treatment strategies, the rate of mortality has remained high and thus; there need more studies about developing new approaches to cancer management [1], [2], [3]. One of the major tools used in comprehensive cancer care is medical imaging. It brings many advantages including monitoring capability in real time, no need to tissue destruction, minimal-invasive procedure, and usability over broad periods and size ranges that are usually needed in pathological and biological processes. The main factor in reducing mortality and cancer management costs is an early diagnosis. Leonard Fass [4] study showed that imaging through symptomatic disease management and screening plays an important role in cancer management. This is shown schematically in Figure 1.
Figure 1.
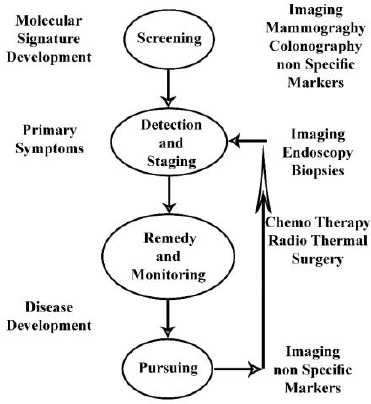
The role of Imaging in cancer management [4]
Another tool used in all stages of cancer management is biomedical imaging. It can be used in prediction, staging, screening, prognosis, biopsy guidance for diagnosis, plan of treatment, therapy guidance, therapy response recurrence and palliation [5], [6], [7], [8], [9].
Translational studies link fundamental science to clinical applications; therefore, resulted in significant health consequences [3], [10] (Figure 2). With regards to the wide range of usage in the management of patients with cancer for diagnosis, staging, therapy planning, monitoring, and surveillance, imaging considered as an essential component of clinical cancer protocols [11]. Current clinical imaging techniques have some limitations such as using of computed tomography (CT) or magnetic resonance imaging (MRI) for studying in vivo biological changes in relation with prognosis, carcinogenesis and therapy response [12].
Figure 2.
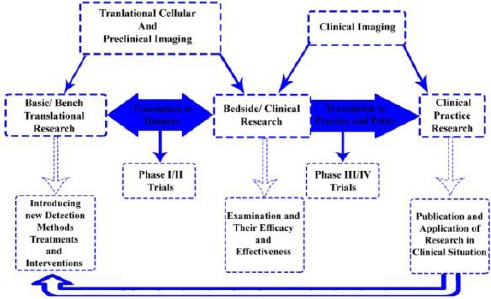
Map of translational research based on practice. (The working model of bench-to-bedside translational research is described in this diagram); Forward Translation: Molecular explorations translated into the clinical application; Reverse Translation: Scientific questions arise from relevant clinical findings resulted in research progress
Full spatial mapping of gene expression and molecular activities within cells and tissues are required to get health benefits of understanding the genome and proteome. To this end, structural and functional imaging of molecules is vital. To recognise the existence of cancer, the tumour stage, its aggressiveness and response to therapy, imaging biomarkers are under development [13]. Several medicinal treatments are under development, classified as anti-hormonal, cytotoxic, immunotherapeutic and molecularly targeted. The molecularly targeted treatments include angiogenesis inhibitors, multi-targeted tyrosine kinase inhibitors, cell cycle inhibitors, signal transduction inhibitors, apoptosis inducers and epigenetic modulators [14]. Several targeted agents have been developed as cancer markers comprising of αvβ3 integrin, carcinoembryonic antigen (CEA), epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), prostate stimulating membrane antigen (PSMA), somatostatin receptors, MC-1 receptor, folate receptors and transferrin receptors. In vitro imaging methods (i.e. imaging mass spectrometry (IMS)) by their ultra-high resolution can be used to specify the spatial distribution of proteins, peptides, and drugs in tumour tissue specimens. The main focus of this review is the clinical imaging methods [8].
Developments in our ability to analyse molecular processes, such as gene and protein expression, cellular and molecular biochemistry, make it possible of a better understanding of breast cancer biology and recognition of new therapies for patients [15]. Measuring biological processes in vivo without disturbing them, helps in better characterisation of tumour biology. It also lets us evaluate the way biological, and cytotoxic therapies change key pathways through which tumour responds and generate resistance. Molecular imaging can carefully characterise the properties of the tumour, and biological processes hence play an important role in clinical care from detection to staging, breast cancer knowledge, evaluation of therapeutic objectives, and assessment of responses to various therapies [16].
The elasticity of cancer tissues is lower than normal tissues. This difference in elasticity makes ultrasound elastography suitable to the differential diagnosis of prostate cancer, liver fibrosis and breast cancer [17], [18]. Lots of clinical imaging systems work based on the different response of body tissues and fluids to electromagnetic radiation. But ultrasound works through frequency variation, dispersion and reflection of acoustic waves. The interaction of ultrasound and tissues makes it able to illustrate tissue elasticity. Another application of ultrasound is in thermal therapy, and its mediating ability of gene expression and gene transfer is well known [19]. Imaging of pancreatic masses, lymph nodes, adrenal and submucosal tumours by endoscopic ultrasound elastography is possible and can avoid fine needle aspiration biopsies [20].
In their study about breast cancer imaging techniques, Poplach et al., [21], [22] stated that imaging techniques based on non-ionizing electromagnetic radiation such as microwave imaging spectroscopy and near-infrared spectroscopy among others had been mainly investigated for breast imaging. However physical properties of imaging systems involving sensitivity, temporal and spatial resolution are different. The most sensitive clinical imaging techniques are PET and nuclear medicine which their sensitivity varied from nanomole/kilogram to picomole/kilogram. The sensitivity of X-Ray systems such as CT is millimole/kilogram whereas the sensitivity of MR is around 10 µmol/kg [23].
Because cancer is a disease with multiple factors, imaging has to display the variant mechanisms and stages of pathogenesis. Tumour volume characteristics can only show response delay to therapy and can’t indicate the metabolism and other parameters. Thus its application alone as a measure of disease development is not inadequate. That’s the reason for using multiple imaging methods for managing cancer. Multiple imaging techniques such as PET/CT incorporates the benefits of PET (i.e. metabolic sensitivity) and CT (i.e. temporal and spatial resolution). Integration of imaging techniques such as SPECT/CT, PET/CT, PET and MR and ultrasound and MR in the future will require developing standard approaches to perform longitudinal studies about the response to therapy [25].
At last, integrating various imaging technologies will lead to diagnostic orthogonality. It is supposed that the combination of such information not only provides a mean to validate individual technologies in the future but also forms a part of the diagnostic process, particularly for prediction, early detection and early therapy response of the disease [17]. In this review, the most recent technologies in the world’s newest research studies are introduced. Also, new scientific methods for the examination, diagnosis and evaluation of benign and malignant tumours are presented.
Digital imaging systems
Full field digital mammography is one of the helpful digital imaging systems for breast cancer diagnoses. It has several privileges over film-based techniques for screening of breast [26] including increased dynamic range, improved sensitivity for dense breasts, lower dose, digital archiving, computer-aided detection/diagnosis, telemedicine, tomosynthesis, softcopy review, 3-D visualisation techniques and reducing breast compression pressure. Overlapping and obscuring malignancies by normal structures like glandular tissue in the breast, particularly those placed deep in the breast is a potential constraint of 2D mammograms. This can cause cancers to be skipped in the scan.
On the contrary, overlapping normal tissues can be misunderstood with tumours in an X-ray image and caused extra imaging, performing unessential biopsies, higher costs of healthcare and patient anxiety. The combination of tomosynthesis and digital mammography can result in lower false negatives and increasing true positives [27]. Also, using 3D X-ray techniques along with tomosynthesis can reduce breast compression. Other 3D methods to produce stereoscopic images are available. Digital X-ray images of the breast taken at two different angles by about eight degrees’ difference can produce stereoscopic mammograms. The radiologist through these images can see the breast interior structure in three dimensions and make better distinguish between benign and malignant lesions. Accordingly, one can say that this technique has a greater detection rate and lower false positives compared to regularly 2D mammography [28]. In the other side, a higher number of images in this technique increases the risk of exposure high radiation dose.
Jong [29] stated that Contrast-enhanced mammography is an investigational technique that uses iodinated contrast agents. This technique is built on the principle that the quick growth of tumours necessitates enhanced blood reserve across angiogenesis. If the compression device is not activated, then contrast should be administered. Angiogenesis regions resulted in the accumulation of the contrast agent. Diekmann and Bick [30] uttered that contrast-enhanced mammography with tom synthesis is an imaging contrast distribution technique in breast tissue. There are two methods to evaluate images. The first one is to look for maximum iodine concentration in the image, generally 1 minute after injection. The regions with high absorption demonstrate the growing activity of tissues, and likely the malignant ones. The kinetic analysis technique can track iodine contrast agent flow inside of a tissue region and outside of it. The speed of wash-in and wash-out of iodine in malignant cancers is high, while iodine uptake of benign tissues is slow, took about 5 min in this study. This is identical with perfusion imaging by MRI using gadolinium-based contrast agents.
Dual-energy contrast mammography has the ability of better breast lesions diagnosis at lower radiation doses than non-contrast enhanced mammography, but this is not identified for contrast-enhanced MRI and needs to be evaluated. Combination of tomosynthesis with contrast-enhanced mammography may lead to better detection of preliminary and secondary lesions and also the potency to monitor therapy [31], [32].
To enhance lesion detection efficiency, computer-aided detection (CAD) is used. Dual-energy techniques have some advantages include removing the structural noise, contrasting media that magnify the area around the tumour, and improving lesions detectability. CAD aims to facilitate recognise lesions, particularly in locations where obtaining a second reading is difficult. CAD is more suitable for recognising micro-calcifications but less effective for breast masses. Competent breast cancer experts who are capable of differentiating benign lesions from malignant ones can better utilise CAD [33]. CAD has great sensitivity for breast cancer detection on initially and short-term follow-up digital mammograms. Reproducibility for true positive CAD marks is significantly higher than for false positive ones [34]. Recently, a mega-study composed of 231,221 mammograms showed that CAD improves the efficiency of the single reader and increases sensitivity just with a little increment in recall rate [35].
Increased sensitivity and specificity is possible using dual modality systems that combine ultrasound and X-ray systems [36]. Mammography has no sensitivity in imaging young compact breasts because the encompassing fibroglandular tissue decreases the manifestation of lesions. Moreover, screening by ultrasound can considerably increase the detection rate of small cancers. Also it can represent considerably more cancers at a smaller size and lower phases compared to physical examination alone, which identifies hardly any cancers. Increasing breast compression that is naturally greater in older women with dense breasts significantly declines the mammographic sensitivity for breast cancer. The detection sensitivity of full field digital mammography systems for dense breasts is better than film-based systems. The American Cancer Society recommends that even women with a good health condition should continue screening mammography [37].
Breast Cancer Magnetic resonance imaging (MRI)
The application of magnetic resonance systems in cancer detection, monitoring therapy response, staging, least-invasive therapy guidance and biopsy guidance has been documented [38]. Imaging techniques using in cancers are based on diffusion-weighted imaging, perfusion imaging using contrast agents, exogenous spectroscopic imaging with hyperpolarised contrast agents, endogenous spectroscopic imaging, blood oxygen level determination (BOLD) imaging, magnetic resonance elastography, relaxivity-based imaging with and without contrast agents [39].
The first investigators who used MRI for breast cancer examination were Ross et al., [40]. Today, breast MRI is considered as a supplementary technique along with ultrasound and mammography. So, MRI technology of the breast has greatly improved by the extension of advanced surface and gradient coils, contrast agents, parallel imaging and novel fast imaging sequences. Simultaneous imaging of both breasts are possible using new surface coils, allows indicating involvement of the contralateral breast. Breast MRI sensitivity for breast cancer detection is higher than ultrasound or mammography. Because of high expenses, hard accessibility, and high false positives of MRI, it is not widely used as a screening test for breast cancer, but in special cases [41]. Breast MRI is not based on ionising radiation. As a result, it is suggested in the repeated screening of patients with high risk of radiation-induced DNA mutations. Breast MRI is applied to screen women with very dense breast tissue, women with a family background of breast cancer, or women with silicone implants that may conceal pathology in mammography. It can also be used for seeking recurrence in patients with scar tissue. The American Cancer Society has strongly confirmed using MRI to identify contralateral disease extension and lymph node involvement in breast cancer [37].
Because of its ability to show multi-focal tumours, chest wall involvement, retraction of the skin and lymph node metastases, MRI is the most accurate instrument for the local staging of breast cancer. Compared to other methods, it performs better in imaging invasive lobular carcinoma. Magnetic resonance imaging due to its ability in easier analysing of pathological response as well as a low rate of reoperation needed for positive margins is preferable than mammography and ultrasound [42]. This suggests one important role of MRI, i.e. facilitate decision-making about performing conserving surgery or mastectomy.
Strong paramagnetic nature of gadolinium makes it possible to alter the magnetic situation of hydrogen atoms inside water molecules. High concentrations of gadolinium components due to susceptibility effects resulted in local changes in the magnetic field. Dynamic studies of wash-in and wash-out are possible using contrast-enhanced MR. The maximum effect is accrued after rapid intravenous injection during the first passage of a bolus of contrast agent. This creates darkness on gradient echo T2*-weighted images in highly perfused areas of tissues. But tissues with a high contrast agent uptake appear bright on T1-weighted images.
Takayoshi et al., [43] argued that myocardial perfusion imaging could be used in the assessment of two-sided micro-calcifications following by stereotactic vacuum-assisted biopsy. They further stated that it could illustrate the existence of malignant micro-calcifications seen on mammography. Naoyo Nishida et al., [44] declare that evaluating neo-angiogenesis requires using dynamic contrast MRI with gadolinium-based contrast agents that is related to microvessel density, histopathology and response to chemotherapy [45], [46], [47]. The time/signal intensity graphs at the maximal enhancement site for each progression lesion are achieved closely. Three types of time/signal intensity curves identified by Riham H. et al., [48] as below:
(I) continual enhancement curves are normally related to benign lesions.
(II) Curves with a quick contrast uptake following with a plateau state can be representative of both benign and malignant lesions.
(III) Curves with a quick contrast uptake and wash-out are usually pertained to malignant lesions.
Using agents that block angiogenesis, MR perfusion imaging can be used directly and indirectly to monitor therapy. Because of the potential ability of anti-angiogenic therapy in temporarily normalising the abnormalities in structure and function of tumour vascular system, it can terminate angiogenic blood vessels. Due to the less permeability of normalised blood vessels, their efficacy in drug and oxygen delivery is better. Pericytes that are vascular smooth muscle cells has an essential role in blood vessel formation. Further, they can help the normalised vessels to remain strong [49]. The strong vessels can diminish the intravasation of cancer cells, hence decrease the risk of haematogenous metastasis. Severity time curves for various breast tissues are illustrated in Figure 3.
Figure 3.
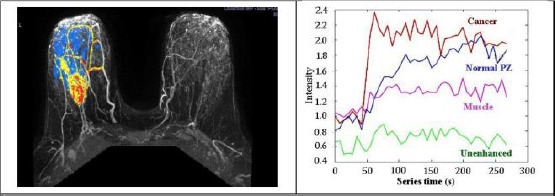
Time/severity curves of breast MR contrast uptake (derived from; Riham H. et al., [48])
American Breast Imaging Reporting and Database system [50] has declared that enhancement of heterogeneous or rim is an indication for malignancy and irregularly shaped speculated masses. Nunes [51], on the other hand, stated that a nonmass asymmetrical enhancement with a segmental or regional pattern could be considered as a powerful symptom of in situ ductal carcinoma. Liberman et al. [52] reported in their study that non-enhancing septa or smooth borders which are observed in many fibroadenomas suggest benign lesions. They further stated that small lesions with the size of smaller than 5 mm often have no clinical significance.
Diffusion-weighted imaging Method
Diffusion-weighted imaging (DWI) MRI formerly used in cytotoxic oedema detecting in stroke. Today, it is also used to measure Brownian movement (the diffusion of water molecules). It is expected that this technique can be used for the identification of tumours and metastases as well as classifying breast lesions as benign or malignant [53]. Both quantitative and qualitative information on the changes developed at a cellular level can be obtained using DWI MRI. This information shows the effect of cell membrane integrity and tumour cellularity. Thanks to recent advancements, this technique is extensively applied for tumour assessment inside the body and have developed whole body DWI. Here is an example of osseous and soft tissue metastatic lesions; Figure 4-1 and 4-2 illustrate a 42 years old female with breast cancer and massive metastatic nodal spread along some parts of her body that was presented with lower back pain, right sciatica [54].
Figure 4-1.
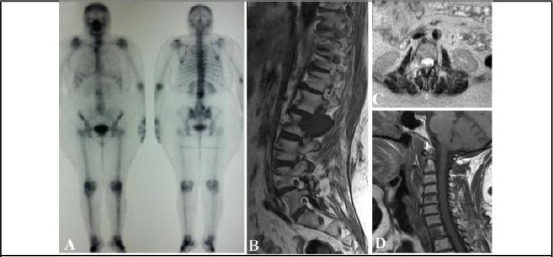
An instance case of metastatic breast cancer; A) The anterior and posterior projections of the patient, focal zones of hot uptake at D1, L2 and right aspect of the manubrium sterni are shown in a bone scan; B) Sagittal T1W image of the dorsolumbar spine. A sizable hypointense marrow lesion can be seen in the right posterolateral aspect of the L2 vertebra.
Further, a pedicle with expansion and extra-osseous soft tissue component are demonstrated; C) Axial T2W image at L2 level. Right sided infiltrative process with intra and retrospinal soft tissue extensions are shown; D) Sagittal T1W image of the cervical spine. Hypointense metastatic lesions at C5 and D1 are demonstrated
Figure 4-2.
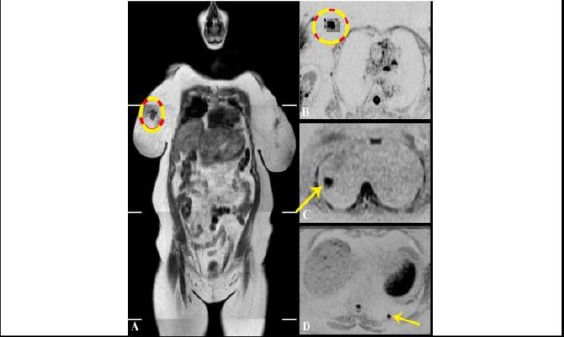
The previous case of metastatic breast cancer; A) WB-MRI coronal T1W image. The primary breast lesion can be seen at upper inner quadrant of the right breast. It is showed by dark signal and irregular outlines; B) Corresponding axial inverted DWI. Breast lesion are shown as a flared out zone of evident diffusion restriction. The mean ADC value of the zone is 0.91 x 10-3 mm2/sec; C) & D) Axial inverted DWI at different levels. The pulmonary nodules are shown as rounded focal zones of diffusion restriction at lateral segment of right middle lung lobe C) and posterior segment of left lower lung lobe D) giving a low mean ADC value ranging between 0.97 and 1.03 x 10-3 mm2/sec
According to Galbán et al., [55], tumour tissues cause a disruption in water molecule diffusion, as a result, the rate of apparent diffusion constant (ADC) reduces, resulted in high signal in DWI images. Positive response to therapy is represented by a rise in ADC. The number of killed cells is directly related with increase of water ADC after therapy. Water liberation into the extracellular space in result of cell necrosis is a possible cause of this relationship.
Buijs et al., [56] stated that response evaluation of metastatic breast cancer to chemoembolization is possible by ADC Measurement. ADC values of normal tissues would not change, whereas this value for tumor tissue showed an increase after transarterial chemoembolization. In the other hand, a full response cannot be obtained in solid tumors using MRI, even if volume changes viewed with contrast-enhanced. Response to radiation therapy in the cases with High DWI MRI may be possible [37]. The response of tumours having a high diffusion constant due to their wide necrotic region is worse. Palpation is applied as a part of the clinical diagnosis of prostate, thyroid, breast and abdominal pathologies. It determines the rigidity of a region compared to the nearby tissues. The regions with increased ADC are obtained by partial responders. Whole-body MRI is comparable with PET/CT and scintigraphy in the identification of sclerotic metastases, which are usual processes in breast and prostate cancers as well as multiple myeloma. Currently, PET/CT is applied for soft-tissue metastatic disease, while diffusion-weighted MRI methods have the potential to be used in the future.
Magnetic resonance elastography
Magnetic resonance elastography (MRE) by transmission of shear waves and imaging their propagation using MRI, measures the mechanical characteristic (stiffness) of soft tissues [57]. It is a non-invasive medical imaging technique. The stiffness of diseased tissues is often more than the normal tissues around it. This is done while the application of acoustic waves by adjusting motion-sensitive phase contrast MRI sequences. The range of acoustic waves frequencies is among 100 Hz to 1 kHz. Propagation of shear waves is a function of the shear modulus of the tissue [58]. MRE produces images of shear wave propagations with variable wavelengths. Some researchers based on these criteria have investigated the application of MRE in breast cancer detection as a constant method of detection. They reported that; breast cancer tissues are much harder than healthy fibroglandular tissue. Physicians used this characteristic for screening and diagnosis of many diseases, through palpation. MRE, through a non-invasive and objective way, calculates the mechanical parameter while elicited by palpation, [59], [60], [61].
Apoptosis/Receptor imaging
Binding agents to cell surface proteases that attract phagocytes to dying cells are also used to direct imaging of apoptosis. To perform optical and nuclear medicine imaging, Annexin V has been utilised. Zhao et al., [62] reported the using of targeted super-paramagnetic iron oxide (SPIO) to perform receptor imaging. This is a practical method for breast cancer imaging. Artemov et al., [38], for example, used targeted iron oxide to image tyrosine kinase Her-2/neu receptor in breast cancer cells. The targeted agent used to MR contrast was Streptavidin-conjugated super-paramagnetic nanoparticles. Boosted T2 MR contrast were generated for Her-2/neu-expressing cells via directing these nanoparticles to receptors which previously classified as a biotinylated monoclonal antibody. The expression level of Her-2/neu receptors governs the contrast of MR images. The level of expression attained independently using fluorescence-activated cell sorting (FACS) analysis. One of the limitations of MRI for direct imaging is its sensitivity but could utilize in pre-clinical imaging. Such target-mediated imaging is successfully applied in nuclear medicine imaging, include imaging of breast cancer tumours [63], [64]. In this method, the molecular expression pattern of tumours is targeted. Synthesised target ligands composed of antibodies, peptide analogues, nanobodies and affibodies can target those molecules (for example, enzymes, transporters and receptors) overexpressed on cancer cells. They stick to the target with high affinity and specificity (Figure 5).
Figure 5.
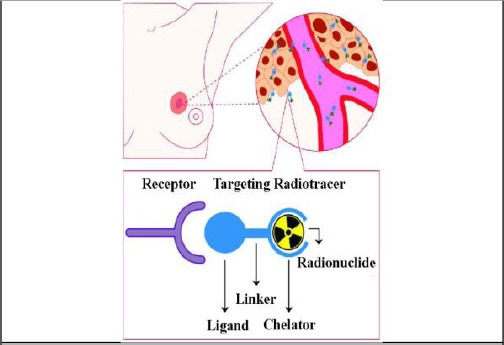
Schematic of receptor targeted nuclear imaging. Ligands can bind those targets that are overexpressed on breast cancer (BC) cells. These ligands can be joint to a chelator, mostly by a linker
Magnetic Resonance Spectroscopy
An additional technique to improve the accuracy of a breast MRI study is Magnetic resonance spectroscopy (MRS). Following conventional MRI imaging of a breast, a chemical “spectrum” is evaluated in a suspicious region to provide additional information about the chemical content of that region. This information can be used to distinguish benign from malignant masses, and to determine the faster respond of a mass to chemotherapy treatments and MRI alone [65]. The molecules such as hydrogen ions or protons are analysed in MRS, but the more common method is Proton spectroscopy. Localising tumours, detecting a response to therapy and directing biopsies in the breast are the potential role of Spectroscopic imaging and 3D MR proton spectroscopy [66]. Integrating MR spectroscopic imaging with MR anatomic imaging lets to localise the spatial position of metabolites. The main phospholipid in cell membranes is Choline. It is known as a potential marker of cell division and helps in phosphatidylcholine formation. According to Chung-Ho et al., [67], the present of carcinogenesis in human breast epithelial cells causes membrane choline phospholipid metabolism to be changed progressively. Yeung et al., [68] in their study about the application of proton MR spectroscopy in preliminary observations and specifying of histopathologic subtypes in axillary node metastases of breast cancer, noticed that high choline levels are related to increased lymph node metastases and invasive ductal carcinomas of the breast. An example of proton spectroscopy for a breast lesion on MRI and the corresponding MRS spectrum is illustrated in Figure 6 [69].
Figure 6.
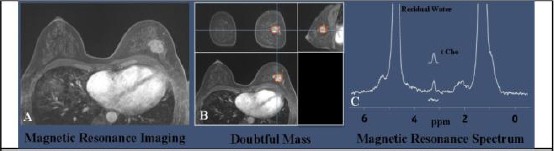
Imaging of a breast lesion using MRI anatomic image and proton spectroscopy. (Derived from; Haddadin et al. [69]); A) Magnetic resonance spectroscopy derived from a post-contrast gradient echo MRI of a 51-year-old woman with invasive ductal carcinoma; B) Detected Areas with doubtful masses; C) The resonances of corresponding water and lipid suppressed spectrum acquired
Bartella and Huang [70] further showed that monitoring and predicting response to chemotherapy as well as reducing the number of breast biopsies is accessible, provided that choline levels are used to differentiate benign from malignant tumours. Change monitoring in these metabolites can help to observe therapy response or malignant transformation. Owing to the low concentration of these isotopes inside tissues, the obtained signal is weak, and also, acquisition of signals takes very long times. As a result, clinical application of spectroscopy of endogenous has been limited [71], [72].
Breast Ultrasonography
Ultrasound is an imaging examination. Application of ultrasound in the detection of tumours in the breast, thyroid, prostate, pancreatic, liver, uterine, ovarian, and kidney is very common and is considered as a common diagnostic imaging method. During performing the ultrasound test, sound waves with high frequency passed across the breast and turned into images that are shown on a display screen. Ultrasound is used to complete other screening tests and is not a screening test for breast cancer by itself [73]. When observing an abnormality on mammography, or when felting it by physical examination, the best method to identify whether the irregularity is rigid (for example, a benign fibroadenoma or cancer) or fluid-filled (such as a benign cyst) is performing an ultrasound test. Ultrasound cannot be used to determine if a solid lump is cancerous. Visualisation of lesions is enhanced in volume ultrasound. Another main application of ultrasound is to guide biopsies. With no need to ionising radiation, ultrasound is suitable for follow up studies to check for recurrence [74]. Some of the recent developments in ultrasound include ultrasound elastography, targeted microbubble contrast agents, photoacoustic imaging and locally activated ultrasound [73], [75].
Breast Scintimammography
Specialising imaging systems would be an area of developments in the context of nuclear medicine in future. Development of specialised gamma cameras, for example, could help in imaging of breast and axillary nodes by the use of scintimammography. This is because of the need to prevent dispersing from extra-mammary sources and has a significant role in breast imaging using radiotracers. It is also the prevailing effect when imaging near the chest wall by the use of mammoscintigraphy [76]. Another technique for breast imaging is Scintimammography. It is used to identify cancer cells in women breasts who have abnormal mammograms, or those with post-operative scar tissue, dense breast tissue, or breast implants. Radiopharmaceuticals images for scintimammography are taken using traditional gamma cameras that are also called a large field of view cameras [77]. Due to the large passive area at the edge of these camera detectors that prevents it from imaging breast tissue closing to the chest wall, scintimammography is generally performed either in patient’s supine position, and the camera takes a lateral image of the breast, or in the prone position so that the breast hangs freely. Therefore, with no breast compression, the sensitivity for detecting smaller lesions is decreased [78], [79], [80]. Breast Mammography and scintimammography of carcinoma used in the scintimammography in a woman are shown in Figure 7. A woman in the figure received a small amount injection of a radioactive substance which is absorbed by cancer cells. A gamma camera is used to image the breasts [81]. To overcome the restrictions of traditional scintimammography, specific breast-specific gamma camera imaging (BSGI) systems have been developed. The cameras using in these systems have a narrow field of view, causing improved flexibility of movement and increased resolution in comparison to conventional gamma cameras [76].
Figure 7.
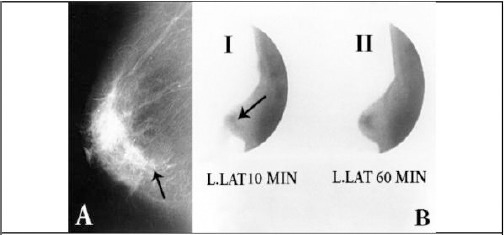
Ductal carcinoma in situ of the left breast, comedo, solid and cribriform type [81]. A) Mammography, medio-lateral projection. Microcalcifications (transparent arrow) behind the nipple; B) Scinti-mammography, left lateral projection: 99m Tc-(V) DMSA at 10 min and 60 min (i-ii)
Brem et al., [79] argued that the sensitivity of a specific gamma camera to detect breast cancer in patients with two-sided mammograms is comparable to MRI. Sweeney and Sacchini [82], on the other hand, believed that this superiority is because of using breast-specific gamma camera imaging system. Some recommendations for scintimammograph application in recent studies are as follows:
- In patients having lesions with a low probability of malignancy who referred for biopsy.
- In patients with breast cancer who their disease is confirmed to detect axillary lymph node metastases.
- As an additional test to mammography in patients who have palpable masses or suspicious mammograms to distinguish benign and malignant breast lesions.
- In patients whom mammography showed the benign probability, and are recommended for repeating mammography (in 3-6 months).
- In patients whose, dense breast tissue is found on mammography but is hard to evaluate it on mammography.
Conclusion
One of the techniques utilised in all stages of cancer management is multiple biomedical imaging. Imaging as an important part of cancer clinical protocols can provide a variety of information about morphology, structure, metabolism and functions. Application of imaging technics along with other detective apparatus including in vitro tissue and fluids analysis would help clinical decision-making. Mixed imaging techniques can provide supplementary information used to improve staging and therapy planning. Imaging aimed to find minimally invasive therapy to make better results and reduce side effects. Some of the research areas with the potential of clinical use in the next decade include gene therapy expression, targeted imaging of receptors, and cancer stem cells. Further development in technology will lead to increase imaging speed to meet physiological processes requirements. Development in targeted imaging and therapeutic agents calls for close cooperation among academia and industries (biotechnological, IT and pharmaceutical industries).
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9e29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252e71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Forootan M, Tabatabaeefar M, Mosaffa N, Rahimzadeh Ashkalak H, Darvishi M. Investigating Esophageal Stent-Placement Outcomes in Patients with Inoperable Non-Cervical Esophageal Cancer. J Cancer. 2018;9(1):213–218. doi: 10.7150/jca.21854. https://doi.org/10.7150/jca.21854 PMid:29290788 PMCid:PMC5743730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fass L. Imaging and cancer:a review. Molecular oncology. 2008;2(2):115–52. doi: 10.1016/j.molonc.2008.04.001. https://doi.org/10.1016/j.molonc.2008.04.001 PMid:19383333 PMCid:PMC5527766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ehman RL, Hendee WR, Welch MJ, Dunnick NR, Bresolin LB, Arenson RL, Baum S, Hricak H, Thrall JH. Blueprint for imaging in biomedical research. Radiology. 2007;244(1):12–27. doi: 10.1148/radiol.2441070058. https://doi.org/10.1148/radiol.2441070058 PMid:17507725. [DOI] [PubMed] [Google Scholar]
- 6.Mohammadifard M, Saburi A. The role of other imaging modalities in evaluating the tubal patency. J Hum Reprod Sci. 2014;7(2):154–155. doi: 10.4103/0974-1208.138877. https://doi.org/10.4103/0974-1208.138877 PMid:25191032 PMCid:PMC4150145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javedani Masrour M, Shafaie A, Yoonesi L, Aerabsheibani H, Javedani Masrour S. Evaluating Endometrial Thickness and Vascular Ultrasound Pattern and Pregnancy Outcomes in Intrauterine Insemination Cycle. Asian Journal of Pharmaceutical Research and Health Care. 2016;8(S1):24–29. https://doi.org/10.18311/ajprhc/2016/7718. [Google Scholar]
- 8.Younesi L, Shahnazari R. Chorionic Bump in First-trimester Sonography. J Med Ultrasound. 2017;25(4):221–226. doi: 10.1016/j.jmu.2017.04.004. https://doi.org/10.1016/j.jmu.2017.04.004 PMid:30065496 PMCid:PMC6029338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brindle K. New approaches for imaging tumour responses to treatment. Nature Reviews Cancer. 2008;8(2):94–107. doi: 10.1038/nrc2289. https://doi.org/10.1038/nrc2289 PMid:18202697. [DOI] [PubMed] [Google Scholar]
- 10.Heryanto YD, Achmad A, Taketomi-Takahashi A, et al. 2015. In vivo molecular imaging of cancer stem cells. Am J Nucl Med Mol Imaging. 2014;5:14e26. [PMC free article] [PubMed] [Google Scholar]
- 11.Desar IM, Van Herpen CM, Van Laarhoven HW, Barentsz JO, Oyen WJ, Van Der Graaf WT. Beyond RECIST:molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer treatment reviews. 2009;35(4):309–21. doi: 10.1016/j.ctrv.2008.12.001. https://doi.org/10.1016/j.ctrv.2008.12.001 PMid:19136215. [DOI] [PubMed] [Google Scholar]
- 12.Yang DJ, Pham L, Liao MH, et al. 2014. Advances in molecular pathway-directed cancer systems imaging and therapy. Biomed Res Int. 2014;2014:639475. doi: 10.1155/2014/639475. https://doi.org/10.1155/2014/639475 PMid:25587538 PMCid:PMC4283426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JJ, Sorensen AG, Thrall JH. Biomarkers in imaging:realising radiology's future. Radiology. 2003;227(3):633–8. doi: 10.1148/radiol.2273020518. https://doi.org/10.1148/radiol.2273020518 PMid:12663828. [DOI] [PubMed] [Google Scholar]
- 14.Hehenberger M, Chatterjee A, Reddy U, Hernandez J, Sprengel J. IT solutions for imaging biomarkers in biopharmaceutical research and development. IBM systems journal. 2007;46(1):183–98. https://doi.org/10.1147/sj.461.0183. [Google Scholar]
- 15.O'Connor M, Rhodes D, Hruska C. Molecular breast imaging. Expert Rev Anticancer Ther. 2009;9:1073–1080. doi: 10.1586/era.09.75. https://doi.org/10.1586/era.09.75 PMid:19671027 PMCid:PMC2748346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mankoff DA. A defi nition of molecular imaging. J Nucl Med. 2007;48:18N–21N. [PubMed] [Google Scholar]
- 17.Zhi H, Ou B, Luo BM, Feng X, Wen YL, Yang HY. Comparison of ultrasound elastography, mammography, and sonography in the diagnosis of solid breast lesions. Journal of ultrasound in medicine. 2007;26(6):807–15. doi: 10.7863/jum.2007.26.6.807. https://doi.org/10.7863/jum.2007.26.6.807 PMid:17526612. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi M, Miyagawa T, Matsumura T, Kawazoe N, Ishikawa S, Shimokama T, Shiina T, Miyanaga N, Akaza H. The impact of real-time tissue elasticity imaging (elastography) on the detection of prostate cancer:clinicopathological analysis. International journal of clinical oncology. 2007;12(4):250–5. doi: 10.1007/s10147-007-0669-7. https://doi.org/10.1007/s10147-007-0669-7 PMid:17701002. [DOI] [PubMed] [Google Scholar]
- 19.Pallwein L, Mitterberger M, Struve P, Pinggera G, Horninger W, Bartsch G, Aigner F, Lorenz A, Pedross F, Frauscher F. Real?time elastography for detecting prostate cancer:preliminary experience. BJU international. 2007;100(1):42–6. doi: 10.1111/j.1464-410X.2007.06851.x. https://doi.org/10.1111/j.1464-410X.2007.06851.x PMid:17552952. [DOI] [PubMed] [Google Scholar]
- 20.Saftoiu A, Vilman P. Endoscopic ultrasound elastography-a new imaging technique for the visualization of tissue elasticity distribution. Journal of Gastrointestinal and Liver Diseases. 2006;15(2):161–165. PMid:16802011. [PubMed] [Google Scholar]
- 21.Poplack SP, Paulsen KD, Hartov A, Meaney PM, Pogue BW, Tosteson TD, Grove MR, Soho SK, Wells WA. Electromagnetic breast imaging:average tissue property values in women with negative clinical findings. Radiology. 2004;231(2):571–80. doi: 10.1148/radiol.2312030606. https://doi.org/10.1148/radiol.2312030606 PMid:15128998. [DOI] [PubMed] [Google Scholar]
- 22.Poplack SP, Tosteson TD, Wells WA, Pogue BW, Meaney PM, Hartov A, Kogel CA, Soho SK, Gibson JJ, Paulsen KD. Electromagnetic breast imaging:results of a pilot study in women with abnormal mammograms. Radiology. 2007;243(2):350–9. doi: 10.1148/radiol.2432060286. https://doi.org/10.1148/radiol.2432060286 PMid:17400760. [DOI] [PubMed] [Google Scholar]
- 23.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proceedings of the National Academy of Sciences. 2007;104(28):11760–5. doi: 10.1073/pnas.0703875104. https://doi.org/10.1073/pnas.0703875104 PMid:17601776 PMCid:PMC1913863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyer T, Townsend DW, Blodgett TM. Dual-modality PET/CT tomography for clinical oncology. The Quarterly Journal of Nuclear Medicine and Molecular Imaging. 2002;46(1):24–34. [PubMed] [Google Scholar]
- 25.D'Hallewin MA, El Khatib S, Leroux A, Bezdetnaya L, Guillemin F. Endoscopic confocal fluorescence microscopy of normal and tumor bearing rat bladder. The Journal of urology. 2005;174(2):736–40. doi: 10.1097/01.ju.0000164729.36663.8d. https://doi.org/10.1097/01.ju.0000164729.36663.8d PMid:16006967. [DOI] [PubMed] [Google Scholar]
- 26.Pisano ED Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83. doi: 10.1056/NEJMoa052911. https://doi.org/10.1056/NEJMoa052911 PMid:16169887. [DOI] [PubMed] [Google Scholar]
- 27.Bohndiek SE, Cook EJ, Arvanitis CD, Olivo A, Royle GJ, Clark AT, Prydderch ML, Turchetta R, Speller RD. A CMOS active pixel sensor system for laboratory-based x-ray diffraction studies of biological tissue. Physics in Medicine &Biology. 2008;53(3):655–672. doi: 10.1088/0031-9155/53/3/010. https://doi.org/10.1088/0031-9155/53/3/010 PMid:18199908. [DOI] [PubMed] [Google Scholar]
- 28.Getty D, D'Orsi CJ, Pickett R, Newell M, Gundry K, Roberson S. Improved accuracy of lesion detection in breast cancer screening with stereoscopic digital mammography. InRSNA Meeting. 2007 (abstract SSG01-04) 2007. [Google Scholar]
- 29.Jong RA, Yaffe MJ, Skarpathiotakis M, Shumak RS, Danjoux NM, Gunesekara A, Plewes DB. Contrast-enhanced digital mammography:initial clinical experience. Radiology. 2003;228(3):842–50. doi: 10.1148/radiol.2283020961. https://doi.org/10.1148/radiol.2283020961 PMid:12881585. [DOI] [PubMed] [Google Scholar]
- 30.Diekmann F, Bick U. Tomosynthesis and contrast-enhanced digital mammography:recent advances in digital mammography. European radiology. 2007;17(12):3086–92. doi: 10.1007/s00330-007-0715-x. https://doi.org/10.1007/s00330-007-0715-x PMid:17661053. [DOI] [PubMed] [Google Scholar]
- 31.Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography:feasibility. Radiology. 2003;229(1):261. doi: 10.1148/radiol.2291021276. https://doi.org/10.1148/radiol.2291021276 PMid:12888621. [DOI] [PubMed] [Google Scholar]
- 32.Kwan ALC, et al. Flynn M.J, editor. Medical Imaging 2005:Physics of Medical Imaging. Proceedings of the SPIE. 2005;5745:1317–1321. https://doi.org/10.1117/12.595887. [Google Scholar]
- 33.Campadelli P, Casiraghi E, Artioli D. A fully automated method for lung nodule detection from postero-anterior chest radiographs. IEEE transactions on medical imaging. 2006;25(12):1588–603. doi: 10.1109/tmi.2006.884198. https://doi.org/10.1109/TMI.2006.884198 PMid:17167994. [DOI] [PubMed] [Google Scholar]
- 34.Kim SJ, Moon WK, Cho N, Cha JH, Kim SM, Im JG. Computer-aided detection in full-field digital mammography:sensitivity and reproducibility in serial examinations. Radiology. 2008;246(1):71–80. doi: 10.1148/radiol.2461062072. https://doi.org/10.1148/radiol.2461062072 PMid:18096530. [DOI] [PubMed] [Google Scholar]
- 35.Gromet M. Comparison of computer-aided detection to double reading of screening mammograms:review of 231,221 mammograms. American Journal of Roentgenology. 2008;190(4):854–9. doi: 10.2214/AJR.07.2812. https://doi.org/10.2214/AJR.07.2812 PMid:18356428. [DOI] [PubMed] [Google Scholar]
- 36.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them:an analysis of 27,825 patient evaluations. Radiology. 2002;225(1):165–75. doi: 10.1148/radiol.2251011667. https://doi.org/10.1148/radiol.2251011667 PMid:12355001. [DOI] [PubMed] [Google Scholar]
- 37.Mardor Y. Proceedings of the American Association for Cancer Research 41 (abstract 2547) 2003 PMid:12637476. [Google Scholar]
- 38.Artemov D, Mori N, Okollie B, Bhujwalla ZM. MR molecular imaging of the Her?2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles. Magnetic Resonance in Medicine:An Official Journal of the International Society for Magnetic Resonance in Medicine. 2003;49(3):403–8. doi: 10.1002/mrm.10406. https://doi.org/10.1002/mrm.10406 PMid:12594741. [DOI] [PubMed] [Google Scholar]
- 39.Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized 13 C magnetic resonance imaging and spectroscopy. Nature medicine. 2007;13(11):1382–1387. doi: 10.1038/nm1650. https://doi.org/10.1038/nm1650 PMid:17965722. [DOI] [PubMed] [Google Scholar]
- 40.Ross RJ, Thompson JS, Kim K, Bailey RA. Nuclear magnetic resonance imaging and evaluation of human breast tissue:preliminary clinical trials. Radiology. 1982;143(1):195–205. doi: 10.1148/radiology.143.1.7063727. https://doi.org/10.1148/radiology.143.1.7063727 PMid:7063727. [DOI] [PubMed] [Google Scholar]
- 41.Brindle KM. Molecular imaging using magnetic resonance:new tools for the development of tumour therapy. The British journal of radiology. 2003;76(suppl_2):S111–7. doi: 10.1259/bjr/50577981. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharyya M, Ryan D, Carpenter R, Vinnicombe S, Gallagher CJ. Using MRI to plan breast-conserving surgery following neoadjuvant chemotherapy for early breast cancer. British journal of cancer. 2008;98(2):289–293. doi: 10.1038/sj.bjc.6604171. https://doi.org/10.1038/sj.bjc.6604171 PMid:18219287 PMCid:PMC2361466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uematsu T, Yuen S, Kasami M, Uchida Y. Dynamic contrast-enhanced MR imaging in screening detected microcalcification lesions of the breast:is there any value? Breast cancer research and treatment. 2007;103(3):269–81. doi: 10.1007/s10549-006-9373-y. https://doi.org/10.1007/s10549-006-9373-y PMid:17063274. [DOI] [PubMed] [Google Scholar]
- 44.Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vascular health and risk management. 2006;2(3):213–219. doi: 10.2147/vhrm.2006.2.3.213. https://doi.org/10.2147/vhrm.2006.2.3.213 PMid:17326328 PMCid:PMC1993983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leach MO. Application of magnetic resonance imaging to angiogenesis in breast cancer. Breast Cancer Research. 2001;3:22–27. doi: 10.1186/bcr266. https://doi.org/10.1186/bcr266 PMid:11300102 PMCid:PMC138673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Padhani AR, O'Donell A, Hayes C, Judson I, Workman P, Hannah A, Leach MO, Husband JE. Changes in tumour vascular permeability with antiangiogenesis therapy:observations on histogram analysis. Proceedings of the Eighth International Society of Magnetic Resonance in Medicine. 2000:108. [Google Scholar]
- 47.Padhani AR, Hayes C, Assersohn L, Powles T, Leach MO, Husband JE. Proceedings of the 8th International Society for Magnetic Resonance in Medicine. Denver: 2000. Response of breast carcinoma to chemotherapy:MR permeability changes using histogram analysis; p. 2160. [Google Scholar]
- 48.El Khouli RH, Macura KJ, Jacobs MA, Khalil TH, Kamel IR, Dwyer A, Bluemke DA. Dynamic contrast-enhanced MRI of the breast:quantitative method for kinetic curve type assessment. American Journal of Roentgenology. 2009;193(4):W295–300. doi: 10.2214/AJR.09.2483. https://doi.org/10.2214/AJR.09.2483 PMid:19770298 PMCid:PMC3034220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7(4):452–64. doi: 10.1215/S1152851705000232. https://doi.org/10.1215/S1152851705000232 PMid:16212810 PMCid:PMC1871727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American College of Radiology. American College of Radiology. fourth ed. Reston, VA: 2004. Breast Imaging Reporting and Data System (BI-RADS) [Google Scholar]
- 51.Nunes LW. Architectural-based interpretations of breast MR imaging. Magnetic resonance imaging clinics of North America. 2001;9(2):303–20. PMid:11493421. [PubMed] [Google Scholar]
- 52.Liberman L, Mason G, Morris EA, Dershaw DD. Does size matter?Positive predictive value of MRI-detected breast lesions as a function of lesion size. American Journal of Roentgenology. 2006;186(2):426–30. doi: 10.2214/AJR.04.1707. https://doi.org/10.2214/AJR.04.1707 PMid:16423948. [DOI] [PubMed] [Google Scholar]
- 53.Pavilla A. Process the signal and the image. University Rennes; 2017. Simultaneous quantification of diffusion and cerebral perfusion in magnetic resonance imaging:application to the diagnosis of ischemic stroke. PMid:28608327. [Google Scholar]
- 54.Hamid A, Ali WR. A comparative study between whole body magnetic resonance imaging and bone scintgraphy in detection of bone metastases in patients with known breast or lung cancer. The Egyptian Journal of Hospital Medicine. 2013;31(762):200–215. https://doi.org/10.12816/0000837. [Google Scholar]
- 55.Galban CJ, Hoff BA, Chenevert TL, Ross BD. Diffusion MRI in early cancer therapeutic response assessment. NMR in biomedicine. 2017;30(3):e3458. doi: 10.1002/nbm.3458. https://doi.org/10.1002/nbm.3458 PMid:26773848 PMCid:PMC4947029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buijs M, Kamel IR, Vossen JA, Georgiades CS, Hong K, Geschwind JF. Assessment of Metastatic Breast Cancer Response to Chemoembolization with Contrast Agent-enhanced and Diffusion-weighted MR Imaging. Journal of Vascular and Interventional Radiology. 2007;18(8):957–63. doi: 10.1016/j.jvir.2007.04.025. https://doi.org/10.1016/j.jvir.2007.04.025 PMid:17675611. [DOI] [PubMed] [Google Scholar]
- 57.Kruse SA, Smith JA, Lawrence AJ, Dresner MA, Manduca AJ, Greenleaf JF, Ehman RL. Tissue characterization using magnetic resonance elastography:preliminary results. Physics in Medicine &Biology. 2000;45(6):1579. doi: 10.1088/0031-9155/45/6/313. https://doi.org/10.1088/0031-9155/45/6/313. [DOI] [PubMed] [Google Scholar]
- 58.Plewes DB, Bishop J, Samani A, Sciarretta J. Visualization and quantification of breast cancer biomechanical properties with magnetic resonance elastography. Physics in Medicine &Biology. 2000;45(6):1591–1610. doi: 10.1088/0031-9155/45/6/314. https://doi.org/10.1088/0031-9155/45/6/314. [DOI] [PubMed] [Google Scholar]
- 59.Sinkus R, Lorenzen J, Schrader D, Lorenzen M, Dargatz M, Holz D. High-resolution tensor MR elastography for breast tumour detection. Physics in Medicine &Biology. 2000;45(6):1649–1664. doi: 10.1088/0031-9155/45/6/317. https://doi.org/10.1088/0031-9155/45/6/317. [DOI] [PubMed] [Google Scholar]
- 60.McKnight AL, Kugel JL, Rossman PJ, Manduca A, Hartmann LC, Ehman RL. MR elastography of breast cancer:preliminary results. American journal of roentgenology. 2002;178(6):1411–7. doi: 10.2214/ajr.178.6.1781411. https://doi.org/10.2214/ajr.178.6.1781411 PMid:12034608. [DOI] [PubMed] [Google Scholar]
- 61.Xydeas T, Siegmann K, Sinkus R, Krainick-Strobel U, Miller S, Claussen CD. Magnetic resonance elastography of the breast:correlation of signal intensity data with viscoelastic properties. Investigative radiology. 2005;40(7):412–20. doi: 10.1097/01.rli.0000166940.72971.4a. https://doi.org/10.1097/01.rli.0000166940.72971.4a PMid:15973132. [DOI] [PubMed] [Google Scholar]
- 62.Zhao M, Beauregard DA, Loizou L, Davletov B, Brindle KM. Non-invasive detection of apoptosis using magnetic resonance imaging and a targeted contrast agent. Nature medicine. 2001;7(11):1241–1244. doi: 10.1038/nm1101-1241. https://doi.org/10.1038/nm1101-1241 PMid:11689890. [DOI] [PubMed] [Google Scholar]
- 63.Ambrosini V, Campana D, Tomassetti P, Fanti S. 68 Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. European journal of nuclear medicine and molecular imaging. 2012;39(1):52–60. doi: 10.1007/s00259-011-1989-4. https://doi.org/10.1007/s00259-011-1989-4 PMid:22388622. [DOI] [PubMed] [Google Scholar]
- 64.Bison SM, Konijnenberg MW, Melis M, Pool SE, Bernsen MR, Teunissen JJ, Kwekkeboom DJ, de Jong M. Peptide receptor radionuclide therapy using radiolabeled somatostatin analogs:focus on future developments. Clinical and translational imaging. 2014;2(1):55–66. doi: 10.1007/s40336-014-0054-2. https://doi.org/10.1007/s40336-014-0054-2 PMid:24765618 PMCid:PMC3991004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meisamy S, Bolan PJ, Baker EH, Pollema MG, Le CT, Kelcz F, Lechner MC, Luikens BA, Carlson RA, Brandt KR, Amrami KK. Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging:preliminary results of observer performance study at 4.0 T. Radiology. 2005;236(2):465–75. doi: 10.1148/radiol.2362040836. https://doi.org/10.1148/radiol.2362040836 PMid:16040903. [DOI] [PubMed] [Google Scholar]
- 66.Kurhanewicz J, Vigneron DB, Nelson SJ. Three-dimensional magnetic resonance spectroscopic imaging of brain and prostate cancer. Neoplasia. 2000;2(1-2):166–89. doi: 10.1038/sj.neo.7900081. https://doi.org/10.1038/sj.neo.7900081 PMid:10933075 PMCid:PMC1531872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lau CH, Tredwell GD, Ellis JK, Lam EW, Keun HC. Metabolomic characterisation of the effects of oncogenic PIK3CA transformation in a breast epithelial cell line. Scientific reports. 2017;7:46079. doi: 10.1038/srep46079. https://doi.org/10.1038/srep46079 PMid:28393905 PMCid:PMC5385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeung DK, Yang WT, Tse GM. Breast cancer:in vivo proton MR spectroscopy in the characterization of histopathologic subtypes and preliminary observations in axillary node metastases. Radiology. 2002;225(1):190–7. doi: 10.1148/radiol.2243011519. https://doi.org/10.1148/radiol.2243011519 PMid:12355004. [DOI] [PubMed] [Google Scholar]
- 69.Haddadin IS, McIntosh A, Meisamy S, Corum C, Snyder AL, Powell NJ, Nelson MT, Yee D, Garwood M, Bolan PJ. Metabolite quantification and high?field MRS in breast cancer. NMR in Biomedicine:An International Journal Devoted to the Development and Application of Magnetic Resonance In vivo. 2009;22(1):65–76. doi: 10.1002/nbm.1217. https://doi.org/10.1002/nbm.1217 PMid:17957820 PMCid:PMC2628417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bartella L, Huang W. Proton (1H) MR spectroscopy of the breast. Radiographics. 2007;27(suppl_1):S241–52. doi: 10.1148/rg.27si075504. [DOI] [PubMed] [Google Scholar]
- 71.Hricak H. MRI and choline-PET of the prostate, Refresher Course 88 Deutsche RoentgenKongress. Fortschritte der Rontgenstrahlen. 2007;179(Suppl) [Google Scholar]
- 72.Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annu Rev Biomed Eng. 2005;7:287–326. doi: 10.1146/annurev.bioeng.7.060804.100411. https://doi.org/10.1146/annurev.bioeng.7.060804.100411 PMid:16004573. [DOI] [PubMed] [Google Scholar]
- 73.Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, Wagner WR, Villanueva FS. Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer research. 2005;65(2):533–9. PMid:15695396. [PubMed] [Google Scholar]
- 74.Younesi L, Karimi Dehkordi Z, Safarpour Lima Z, Amjad G. Ultrasound screening at 11-14 weeks of pregnancy for diagnosis of placenta accreta in mothers with a history of cesarean section. E ur J Transl Myol. 2018;28(4):354–361. doi: 10.4081/ejtm.2018.7772. https://doi.org/10.4081/ejtm.2018.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu M, Wang LV. Photoacoustic imaging in biomedicine. Review of scientific instruments. 2006;77(4):041101. https://doi.org/10.1063/1.2195024. [Google Scholar]
- 76.Coover LR, Caravaglia G, Kuhn P. Scintimammography with dedicated breast camera detects and localizes occult carcinoma. Journal of Nuclear Medicine. 2004;45(4):553–8. PMid:15073249. [PubMed] [Google Scholar]
- 77.Rhodes DJ, O'Connor MK, Phillips SW, Smith RL, Collins DA. Molecular breast imaging:a new technique using technetium Tc 99m scintimammography to detect small tumors of the breast. Mayo Clinic Proceedings. 2005;80(1):24–30. doi: 10.1016/S0025-6196(11)62953-4. https://doi.org/10.1016/S0025-6196(11)62953-4. [DOI] [PubMed] [Google Scholar]
- 78.Brem RF, Rapelyea JA, Zisman G, Mohtashemi K, Raub J, Teal CB, Majewski S, Welch BL. Occult breast cancer:scintimammography with high-resolution breast-specific gamma camera in women at high risk for breast cancer. Radiology. 2005;237(1):274–80. doi: 10.1148/radiol.2371040758. https://doi.org/10.1148/radiol.2371040758 PMid:16126919. [DOI] [PubMed] [Google Scholar]
- 79.Brem RF, Petrovitch I, Rapelyea JA, Young H, Teal C, Kelly T. Breast?specific gamma imaging with 99mTc?Sestamibi and magnetic resonance imaging in the diagnosis of breast cancer—a comparative study. The breast journal. 2007;13(5):465–9. doi: 10.1111/j.1524-4741.2007.00466.x. https://doi.org/10.1111/j.1524-4741.2007.00466.x PMid:17760667. [DOI] [PubMed] [Google Scholar]
- 80.O'connor MK, Phillips SW, Hruska CB, Rhodes DJ, Collins DA. Molecular breast imaging:advantages and limitations of a scintimammographic technique in patients with small breast tumors. The breast journal. 2007;13(1):3–11. doi: 10.1111/j.1524-4741.2006.00356.x. https://doi.org/10.1111/j.1524-4741.2006.00356.x PMid:17214787. [DOI] [PubMed] [Google Scholar]
- 81.Papantoniou V, Tsiouris S, Mainta E, Valotassiou V, Souvatzoglou M, Sotiropoulou M, Nakopoulou L, Lazaris D, Louvrou A, Melissinou M, Tzannetaki A. Imaging in situ breast carcinoma (with or without an invasive component) with technetium-99m pentavalent dimercaptosuccinic acid and technetium-99m 2-methoxy isobutyl isonitrile scintimammography. Breast Cancer Research. 2004;7(1):R33. doi: 10.1186/bcr948. https://doi.org/10.1186/bcr948 PMid:15642168 PMCid:PMC1064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sweeney KJ, Sacchini V. Is there a Role for Magnetic Resonance Imaging in a Population?Based Breast Cancer Screening Program? The breast journal. 2007;13(6):543–4. doi: 10.1111/j.1524-4741.2007.00501.x. https://doi.org/10.1111/j.1524-4741.2007.00501.x PMid:17983392. [DOI] [PubMed] [Google Scholar]


