Abstract
AIM:
The main objective is isolation and molecular characterisation of Malassezia spp. from pityriasis versicolor (PV) patients with special emphasis to risk factors in Diyala province, Iraq.
METHODS:
Fifty patients (32 males and 18 females) presented with PV, the age ranged (15-45) years were included. Direct wet mount using KOH 10%, culture of skin scraping and PCR were used for confirmatory diagnosis.
RESULTS:
Malassezia spp. was isolated from (54%) of skin scraping; M. furfur (32%); M. pachydermatis (8%) and M. globosa (14%). The age group (15-22) years were frequently exposed to Malassezia infection. A significant inverse correlation was reported between age and exposure to Malassezia spp. Infection. Males were frequently exposed to Malassezia infection, (40%). A significant correlation was reported between gender and exposure to Malassezia spp. Infection. Females were at risk of getting Malassezia infection (2.619) time than males. Patient resident in the urban area frequently exposed to Malassezia infection, (34%). Patients resident in the rural area appears to be at risk of getting Malassezia infection (1.093) time than those in an urban area. Patient with good economic status was frequently exposed to Malassezia infection, (36%). Patients with middle economic status appear to be at risk of getting Malassezia infection (0.42) time than those with good economic status. Patients with primary education were frequently exposed to Malassezia infection, (22%). A significant correlation was reported between education level and exposure to Malassezia spp. Infection. No significant correlation was reported between economic status; type of job; source of water; contact with dogs and birds and Malassezia spp. Infection.
CONCLUSION:
M. furfur, M. pachydermatis and M. globosa represent the most common Malassezia spp. causing PV. Using of PCR is very critical to confirm the diagnosis of Malassezia spp. Malassezia infection inversely correlated with age and positively correlated with females gender and education. The residency in a rural area and middle economic status increase the possibility of infection. Infection was not affected by the source of water; job and contact with dogs and birds.
Keywords: Pityriasis versicolor, Malassezia spp, Molecular diagnosis, Risk factors, Iraq
Introduction
The family Malassezia involves a gathering of superficial dimorphic fungi occurring as normal skin biota on the human body; however, they can also cause infection or are related to certain skin ailments. Rarely, they can become invasive, to cause opportunistic systemic infection in the presence of certain predisposing factors [1]. However, the complexity of the interaction of a unicellular eukaryotic creature (Malassezia) with a tissue of a multicellular living being (skin) makes understanding the communications and advancement of infection a complex process [2].
Malassezia yeasts inhabit various body sites including scalp, forehead, shoulder, abdomen, lower axilla, groin and forearm, due to an increased thickness of sebaceous organs at these locales [3]. It has been reported that Malassezia yeasts are related to dermatoses such as pityriasis versicolor, seborrheic dermatitis and Malassezia folliculitis, recently, reported is the implication of Malassezia yeasts in atopic dermatitis and psoriasis [4]. Pityriasis versicolor (PV) is a superficial fungal infection, characterized by changes in skin pigment due to colonization of the stratum corneum by a dimorphic lipophilic fungus of the normal flora of the skin, and its characteristic by multiple macules and/or patches of variable appearance (hypopigmented, hyperpigmented, dark brown or erythematous) surrounded by normal skin [5]. Pityriasis versicolor is a worldwide in distribution, particularly in tropical areas. Infection by pityriasis versicolor has varied according to age and gender. It usually infects adults due to increased sebum secretion after puberty.
The main objective of this research is isolation and molecular characterisation of Malassezia spp. from pityriasis versicolor (PV) patients with special emphasis to risk factors in Diyala province, Iraq
Material and Methods
Study Area and Study Population
This study was performed in Baqubah city -Diyala province 33°45’34.71” N; 44°36’23.97” E, Northeast. The study included 50 pityriasis versicolor patients, age ranged (15-45) years; (32 males and 18 females) attended to the dermatology outpatient’s clinic, Baqubah teaching hospital from 14 of October 2017 to 13 of January 2018.
Collection of Samples
Samples used were hair and skin scrapings. Forceps and surgical blades were used for collecting hair and skin samples. The scales were collected in sterile empty Petri dishes. [6].
Wet mount KOH
Scales specimens were subjected to direct examination by placing on a clean slide mounted with a drop of 10% KOH, covered with a coverslip. The slide was warmed gently and examined under a microscope (40X). Characteristic spaghetti and meatballs appearance (shortly curved hyphae and round yeasts) confirmed the diagnosis of PV [7].
Culture
Scales were inoculated onto Sabouraud’s dextrose agar (SDA), containing 0.05 gm/L chloramphenicol, Penicillin at a concentration of 0.4 ml/L and Streptomycin at a concentration of 2 ml/L with olive oil and without olive oil. The vials were incubated at 37°C for 1-2 weeks. All the slants examined on days 3, 7 and then at weekly intervals up to three weeks for any developing colonies [8].
Staining
For examination, 5 ml of sterile distilled water was mixed with a loopful of actively growing yeast, and the concentration was adjusted to about 105 cell/ml Smears from the colonies were stained with Gram’s stain and examined under a microscope for identification of Malassezia [9]. Lactophenol cotton blue stain used for microscopic observation of yeast cells, the suspension of yeast cells were prepared, loopful of culture were stained with lactophenol cotton blue on a sterile glass slide.
Catalase
Catalase test was applied by using a drop of 3% hydrogen peroxide, and the production of gas bubbles was considered a positive reaction [10].
Tween assimilation tests
According to the method reported by [11], yeast cells of (2 x 10 to 3 x 10 CFU/ml) was suspended in 1ml sterile distilled water and poured into plate containing SDA with 0.05 gm/L chloramphenicol, Penicillin at concentration of 0.4 ml/L and Streptomycin at concentration of 2 ml/L cooled at about 50ºC. The inoculum was then spread evenly. After solidification, four holes were made using a 2 mm diameter punch and filled with 5 μl of Tween 20, 40, 60 and 80, respectively. The plates were incubated for 1 week at 32ºC. The utilisation of Tween was assessed by the degree of growth and/or reaction (precipitation) of the lipophilic yeasts around the wells [11].
Assimilation of Castor oil
Assimilation of castor oil was examined using the agar diffusion test. Ten ml sterile Brain Heart Agar (BHI), was melted and allowed to cool to about 50ºC. Yeast cells of (2 x 10 to 3 x 10 CFU/ml) were suspended in 1ml sterilised distilled water and poured into a plate containing the medium was then spread evenly. Once the medium had solidified, one hole was made using a sterile 6mm punch and filled with 50μl of castor oil. The plates were incubated at 32ºC for 10 days and assessed for growth around the individual wells after 2, 4, 6, 8 and 10 days [6].
Splitting of Esculin
Glucosidase activity was assayed by using the esculin agar tube. Using a loop, the yeast inoculum was deeply inoculated into the agar and incubated at 32ºC for 5 days. The splitting of esculin into esculetin and glucose is revealed by the darkening of the medium with the liberation of soluble ferric salt incorporated in the medium [10].
Tryptophan test (Pigment Induction medium)
Yeast cells were cultured on m Dixons medium which was prepared earlier addition of 0.6% of tryptophan instead of peptone to the original medium. After sterilisation and cooling at room temperature, the suspension was smeared on the agar medium using a sterile swab. The plates were incubated at 32ºC for 2 to 4 weeks [12].
Preservation of the Malassezia isolates
Malassezia spp. isolates were grown in the universal bottle containing 5ml of SDA used as slants, and they were enveloped with parafilm and stocked at 4ºC for 3 months [13].
DNA Extraction
DNA was extracted from Malassezia spp. By using the QIAamp DNA Mini Kit (Qiagen, Germany) according to the protocol stated by the kit manufacturer [14].
Concentration and purity of DNA
DNA was extracted from a hundred isolated of Malassezia spp. And they were concentrated in one tube. The concentration and the purity of the DNA samples were determined by Quantus Fluorometer at (9.9 ng/µl and 57 ng/µl) was used to detect the concentration of extracted DNA to detect the goodness of samples for downstream applications. For 1 µl of DNA, 199 µl of diluted QuantyFlour Dye was mixed. After 5min incubation at room temperature, DNA concentration values were detected, according to the protocol stated by the kit manufacturer (Promega, U.S.A).
Primers selection and preparation
Universal primers ITS3 (GCATCGATGAAGAACGCAGC) and ITS4 (TCC TCC GCT TATTGA TAT GC). The 5.8S rDNA and the it’s 2 region, amplified from type, neotype, reference and clinical isolates of Malassezia species by using the ITS 3 and 4 primers (Gaitanis, G et al., 2002) [2]. We’re synthesised by (QIAGEN, Germany).
PCR working solution
Optimisation of PCR was accomplished after several trials. Thus the following mixture was adopted amplification reactions were produced in the 25μl final volume containing 12.5 μl Go Taq® master mix (Promega, USA), 2μl of the primers and 2μl DNA template and complete the volume by 8.2 ul nuclease-free water
Programmable thermal controller
Program for amplifying the 5.8S rDNA and the it’s 2 region, amplified from the type of ITS3 and ITS4 for Malassezia spp. For identification of Malassezia spp.,an initial denaturation step at 95°C for five minutes was followed by thirty cycles of denaturation at 95°C for thirty seconds, annealing at 55°C for thirty seconds, and extension at 72°C for thirty seconds, with a final extension step of 72°C for seven minutes [15].
Agarose Gel Electrophoresis
After PCR amplification, agarose gel electrophoresis was adopted to confirm the presence of amplification. PCR was completely dependable on the extracted DNA criteria, according to the protocol stated by the kit manufacturer (Promega, U.S.A).
Statistical Data Analysis
Patients demography and cross tabulation were calculated by Statistical Package for the Social Sciences for Windows version 17 (SPSS, Armonk, NY: IBM Corp.). Pearson’s chi-square and Pearson’s correlation coefficient were used for the correlation between the variables of the two tests. A p value of ≤ 0.05 and ≤ 0.01 (two-tailed) was set to be statistically significant
Results
Table 1 shows the morphological characteristics and physiological characteristics of Malassezia spp. Isolated from human samples. According to these features, the total number of Malassezia spp. Isolated from a human was 27/50, (54%), M. furfur isolated from 16/50, (32%); M. pachydermatis 4/50, (8%) and M. globosa were isolated from 7/50, (14%). These results were confirmed by PCR as shown in Figure 1.
Table 1.
Diagnosis of Malassezia spp. from human samples by morphological and physiological characteristics compared with conventional PCR using ITS3&ITS4 primers
| Characteristics | Malassezia Isolates | |||
|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | ||
| Morphological characteristics | Colony morphology | Mat, dull, smooth, umbonate or slightly folded with convex elevation | Mat, convex, umbonate (sometimes) | Raised, folded, rough |
| Pigmentation | + | + | + | |
| Cell shape | Oval, cylindrical spherical | Oval | Spherical | |
| Physiological characteristics | Growth on SDA | |||
| Growth on SDA-castor oil | + | + | + | |
| Growth on Esculin agar | + | + | + | |
| Growth on mDA 32°C | + | + | + | |
| Growth on mDA 37°C | + | + | + | |
| Growth on mDA 41°C | + | + | + | |
| Catalase reaction | + | + | + | |
| Utilization of Tween 20 | + | + | + | |
| Utilization of Tween 40 | + | + | + | |
| Utilization of Tween 60 | + | + | + | |
| Utilization of Tween 80 | + | + | + | |
| Total number of Malassezia isolates | 16 (32%) | 4 (8%) | 7 (14%) | |
| Diagnosis by Conventional PCR using ITS3&ITS4 primers | 16 (32%) | 4 (8%) | 7 (14%) | |
Figure 1.
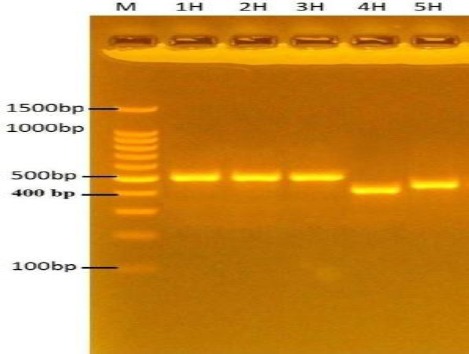
Agarose gel electrophoresis (2%) for 100 v/mAmp for 80 min of Malassezia spp. DNA products generated through ITS 3 (GCATCGATGAAGAACGCAGC), and ITS4 (TCCTCCGCTTATTGATATGC) primers, stained with Ethidium bromide; M: Molecular marker (100bp); lanes 1H, 2H, 3H: M. furfur (509bp); lanes H4: M. globosa (430bp); lanes H5: M. pachydermatis (483bp)
As shown in Table 2, the age group (15-22) years was more frequently exposed to Malassezia infection, followed by (23-30) year’s age group. The age group (39-42) years was the minimally exposed to infection compared with other groups. M. furfur was the predominant species recovered from PV cases while M. pachydermatis was the least once. The isolation rate was drastically decreased while the age of patients approximates to forties. No significant difference was reported between age groups exposed to Malassezia spp. Infection. An inverse significant correlation was reported between age and exposure to Malassezia spp. Infection.
Table 2.
Age as a possible risk factor associated with Malassezia infection in human
| Descriptive statistic of patient’s age | |||||
|---|---|---|---|---|---|
| Minimum (years) | 15 | ||||
| Maximum (years) | 45 | ||||
| Mean ± Std. Deviation | 27.82 ± 8.39 | ||||
| Age group (years) | Malassezia spp. infection | ||||
| Positive No. (%) | Total No. (%) of positive cases | Negative No. (%) | |||
| M. furfur | M. pachydermatis | M. globosa | |||
| 15-22 | 7 (10%) | 1 (2%) | 3 (6%) | 11 (22%) | 5 (10%) |
| 23-30 | 4 (8%) | 2 (4%) | 3 (6%) | 9 (18%) | 7 (14%) |
| 31-38 | 3 (6%) | 1 (2%) | 0 (0%) | 4 (8%) | 8 (16%) |
| 39-42 | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 2 (4%) |
| 43-46 | 1 (2%) | 0 (0%) | 1 (2%) | 2 (4%) | 1 (2%) |
| Total No. (%) | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) |
| χ2 | 27.523 | ||||
| P value | 0.154 | ||||
| R | -0.284 | ||||
| P value | 0.046 | ||||
As shown in Table 3, males were more frequently exposed to Malassezia infection, 20/50, (40%). M. furfur was the predominant species recovered from males with PV cases, 11/50, (22%) while M. globosa recovered from 5/50, (10%) and M. pachydermatis was the least once, isolated from 4/50, (8%).
Table 3.
Gender as a possible risk factor associated with Malassezia infection human
| Gender | Positive No.(%) | Total No. of positive cases | Negative No.(%) | ||||
|---|---|---|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | |||||
| Male | 11 (22%) | 4 (8%) | 5 (10%) | 20 (40%) | 12 (24%) | ||
| Female | 5 (10%) | 0 (0%) | 2 (4%) | 7 (14%) | 11 (22%) | ||
| Total No. (%) | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) | ||
| χ2 | 3.970 | ||||||
| P value | 0.265 | ||||||
| R | 0.270 | ||||||
| P value | 0.05 | ||||||
| Risk Estimate | 95% Confidence Interval | ||||||
| Odds Ratio for gender (female / male) | Value | Lower | Upper | ||||
| 2.619 | 0.799 | 8.588 | |||||
No significant difference was reported between genders exposed to Malassezia spp. Infection. A significant correlation was reported between gender and exposure to Malassezia spp. Infection. Females appear to be at risk of getting Malassezia infection at (2.619) time than males.
As shown in Table 4, patient resident in urban area were more frequently exposed to malassezia infection, 17/50, (34%). M. furfur was the predominant species recovered from patients with PV cases, 11/50, (22%) while M. globosa recovered from 4/50, (8%) and M. pachydermatis was the least once, isolated from 2/50, (4%).
Table 4.
Residence as a possible risk factor associated with Malassezia infection in human
| Residence | Positive NO.(%) | Negative No.(%) | ||||||
|---|---|---|---|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | Total No. of positive cases | |||||
| Rural | 5 (10%) | 2 (4%) | 3 (6%) | 10 (20%) | 9 (18%) | |||
| Urban | 11 (22%) | 2 (4%) | 4 (8%) | 17 (34%) | 14 (28%) | |||
| Total No.(%) | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) | |||
| χ2 | 0.023 | |||||||
| P value | 0.879 | |||||||
| R | -0.021 | |||||||
| P value | 0.882 | |||||||
| Risk Estimate | 95% Confidence Interval | |||||||
| Odds Ratio for Residence (rural / urban) | Value | Lower | Upper | |||||
| 1.093 | 0.348 | 3.435 | ||||||
Neither significant difference nor correlation was reported between residency and exposure to malassezia spp. infection. Patients residency in rural area appear to be at risk of getting malassezia infection at (1.093) time than those in urban area.
As shown in Table 5, patient with good economic status were more frequently exposed to malassezia infection, 18/50, (36%). M. furfur was the predominant species recovered from patients with PV cases, 6/50, (12%) while M. globosa recovered from 4/50, (8%). Neither significant difference nor correlation was reported between economic status and exposure to Malassezia spp. Infection. Patients with middle economic status appear to be at risk of getting Malassezia infection at (0.42) time than those with good economic status.
Table 5.
Family economic status as a possible risk factor associated with Malassezia infection in human
| Economic Status | Positive No.(%) | Negative NO.(%) | |||||
|---|---|---|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | Total No. of positive cases | ||||
| Middle | 6 (12%) | 0 (0%) | 3 (6%) | 9 (18%) | 4 (8%) | ||
| Good | 10 (20%) | 4 (8%) | 4 (8%) | 18 (36%) | 18 (36%) | ||
| Very good | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | ||
| Total No.(%) | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) | ||
| χ2 | 5.424 | ||||||
| P value | 0.491 | ||||||
| R | -0.086 | ||||||
| Risk Estimate | 95% Confidence Interval | ||||||
| Odds Ratio for economic status (middle/good) | Value | Lower | Upper | ||||
| 0.421 | 0.110 | 1.612 | |||||
As shown in Table 6, patients with Primary education were more frequently exposed to Malassezia infection, 11/50, (22%). M. furfur was the predominant species recovered from patients with PV cases, 8/50, (16%) while M. globosa recovered from 3/50, (6%). Illiterate patients were less frequently exposed to Malassezia infection, 2/50, (4%). M. pachydermatis was the predominant species recovered from patients with PV cases, 2/50, (4%).
Table 6.
Patients education status as possible risk factors associated with Malassezia infection in human
| Education level | Malassezia spp | Total No. (%) of negative cases | |||
|---|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | Total No. (%) of positive cases | ||
| Illiterate | 0 (0%) | 2 (4%) | 0 (0%) | 2 (4%) | 4 (8%) |
| Primary | 8 (16%) | 0 (0%) | 3 (6%) | 11 (22%) | 12 (24%) |
| Secondary | 6 (12%) | 1 (2%) | 2 (4%) | 9 (18%) | 6 (12%) |
| Higher Education | 2 (4%) | 1 (2%) | 2 (4%) | 5 (10%) | 1 (2%) |
| Total | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) |
| χ2 | 3.785 | ||||
| P value | 0.286 | ||||
| R | 0.280 | ||||
| P value | 0.05 | ||||
No significant differences were reported between education status and Malassezia spp. Significant correlation was reported between education level and exposure to Malassezia spp. Infection.
As shown in Table 7, Students were more frequently exposed to Malassezia infection, 10/50, (20%). M. furfur was the predominant species recovered from patients with PV cases, 8/50, (16%) while M. globosa and M. pachydermatis recovered from 1/50, (2%). Patients with the academic job were less frequently exposed to Malassezia infection, 2/50, (4%). M. pachydermatis and M. globosa were the predominant species recovered from patients with PV cases, 2/50, (4%). Neither significant difference nor correlations were reported between the type of job and Malassezia spp. Infection.
Table 7.
Patient’s job as possible risk factors associated with Malassezia infection in human
| Job | Malassezia spp | Total No. (%) of positive cases | Total No. (%) of negative cases | ||
|---|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | |||
| Free Work (Self-employer) | 2 (4%) | 0 (0%) | 2 (4%) | 4 (8%) | 3 (6%) |
| Farmer | 2 (4%) | 2 (4%) | 1 (2%) | 5 (10%) | 3 (6%) |
| Housewife | 2 (4%) | 0 (0%) | 1 (2%) | 3 (6%) | 7 (14%) |
| Military | 2 (4%) | 0 (0%) | 1 (2%) | 3 (6%) | 6 (12%) |
| Student | 8 (16%) | 1 (2%) | 1 (2%) | 10 (20%) | 3 (6%) |
| Education | 0 (0%) | 1 (2%) | 1 (2%) | 2 (4%) | 1 (2%) |
| Total | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) |
| χ2 | 18.981 | ||||
| P value | 0.215 | ||||
| R | 0.063 | ||||
| P value | 0.663 | ||||
As shown in Table 8, patient utilizing filtrated water were more frequently exposed to Malassezia infection, 21/50, (42%). M. furfur was the predominant species recovered from patients with PV cases, 14/50, (28%) while M. globosa 4/50, (8%) and M. pachydermatis recovered from 3/50, (6%). patients utilizing river water were less frequently exposed to malassezia infection, mainly M. furfur, 1/50, (2%). Neither significant difference nor correlations were reported between source of water and Malassezia spp. infection.
Table 8.
Source of water as possible risk factors associated with Malassezia infection in human
| Source of water | Malassezia spp | Total No. (%) of positive cases | Total No. (%) of negative cases | ||
|---|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | |||
| Tap Water | 1 (2%) | 1 (2%) | 3 (6%) | 5 (10%) | 2 (4%) |
| Filtrated | 14 (28%) | 3 (6%) | 4 (8%) | 21 (42%) | 21 (42%) |
| River Water | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) |
| Total | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) |
| χ2 | 8.592 | ||||
| P value | 0.198 | ||||
| R | -0.219 | ||||
| P value | 0.126 | ||||
As shown in Table 9, patients who do not have any type of contact with animals were more frequently exposed to malassezia infection, 15/50, (30%). M. furfur was the predominant species recovered from patients with PV cases, 10/50, (20%) while M. globosa 4/50, (8%) and M. pachydermatis recovered from 1/50, (2%). patients who have contact with dogs only were exposed equally to M. furfur 1 /50, (2 %) and M. pachydermatis recovered from 1/50, (2%) infection. Neither significant difference nor correlations were reported between contact with animals and Malassezia spp. infection.
Table 9.
contact with animals as a possible risk factors associated with Malassezia infection in human
| contact with Animals | Malassezia spp | Total No. (%) of positive cases | Total No. (%) of negative cases | ||
|---|---|---|---|---|---|
| M. furfur | M. pachydermatis | M. globosa | |||
| No contact | 10 (20%) | 1 (2%) | 4 (8%) | 15 (30%) | 13 (26%) |
| Bird | 3 (6%) | 1 (2%) | 1 (2%) | 5 (10%) | 6 (12%) |
| Dogs | 1 (2%) | 1 (2%) | 0 (0%) | 2 (4%) | 2 (4%) |
| Birds and dogs | 2 (4%) | 1 (2%) | 2 (4%) | 5 (10%) | 2 (4%) |
| Total | 16 (32%) | 4 (8%) | 7 (14%) | 27 (54%) | 23 (46%) |
| χ2 | 5.243 | ||||
| P value | 0.813 | ||||
| R | 0.185 | ||||
| P value | 0.199 | ||||
Discussion
Malassezia species are part of the resident skin flora of humans and other warm-blooded vertebrates that are discovered in 75-80 % of healthy subjects [16]. These yeasts are associated with a wide spectrum of clinical manifestations from benign skin conditions, such as pityriasis versicolor to fungemia in the immunocompromised host [17]. A molecular analysis-based nonculture method appears to be the most reliable and appropriate method by which to analyse Malassezia spp since the isolation media and procedures used does not influence such a method [18]. Therefore molecular methods have revolutionised the study of diseases caused by Malassezia species because the organisms are fastidious and difficult to identify. Therefore molecular methods overcome the limits of the conventional methods [19].
The current study showed that M.furfur was isolated from (32%) which was more common than M. globosa which isolated from (14%) and M. pachydermatis which was isolated from (8%).
This study comes in line with that reported by Al-ammari et al., [20], in Baghdad, with different clinical presentations including steroids acne, folliculitis, seborrheic dermatitis and atopic dermatitis. In another study, in Diyala province-Iraq; Al-Ezzy et al., [21], found that Malassezia was isolated from (61.7%) of patients with PV specimens with similar age group distribution during 2017 in Baqubah teaching hospital. Current result comes by Shah in India [17], who recovered Malassezia spp from (50.35%) of PV patients. Current result disagrees with Shah and Chaudhary et al. from India [17], [22], reported the recovery of M. globosa from the majority of PV cases and stated that M. globosa is more pathogenic than other Malassezia species as it has greater enzymatic activity, involving lipase and esterase production.
Current isolation rate come in contrary with that reported by Chaudhary et al. and Archana et al. from India [22], [23], who recorded that the isolation rate of Malassezia species in Indian patients with PV was (70% and 96.66%) respectively. Also, current result comes in contrary with that reported by Shokohi from Iran [8], who recorded (88.4%) as isolation rate from PV cases.
The current study revealed that the age group (15-22) years was frequently exposed to Malassezia infection, followed by (23-30) years age group.The age group (39-42) years was the minimally exposed to infection compared with other groups. This result come in accordance with that reported in India and Iraq by [17], [23], [24], [25], [26], [27], [28]. M. furfur was the predominant species recovered from PV cases while M. pachydermatis was the least once. The isolation rate was drastically decreased while the age of patients approximates to forties which come in line with Al-ammari from Iraq and Akaza from Japan [20], [28] and disagree with Shah from India, [17] who stated that M. globosa was the predominant yeast recovered from PV cases. Also disagree with Archana from India, [23] stated that M. furfur represent the second cause of PV after M. sympodialis. An inverse significant correlation was reported between age and exposure to Malassezia spp. Infection, which agrees with Al-ammari from Iraq and Thayikkannu et al., from India [20], [25].
The current study revealed that males were more frequently exposed to malassezia infection, (40%). M. furfur was the predominant species recovered from males with PV cases, (22%) which come in accordance with studies from Iraq and India [20], [21], [23], [24], [26], [27], [29]. While M. globosa recovered from, (10%) and M. pachydermatis was the least once, isolated from, (8%). Significant correlation was reported between gender and exposure to malassezia spp infection which come in accordance with Al-Ezzy from Iraq [21]. On the other hand, Hasan et al., [24], stated that military personnel, athletes, and those doing hard works that usually associated with hyper sweating were more vulnerable to pityriasis versicolor infection. Furthermore, sebaceous glands secretion is increased in males 15 years and older compared to females, and that may also promote pityriasis versicolor infection in males.
Females appear to be at risk of getting malassezia infection at (2.619) time than males which come in accordance with Al-Ammari from Iraq [20], stated that many factors play role in Malassezia pathogenicity such as increased sebum production, hormonal fluctuations, stress, illness, infrequent shampooing, food allergies, vitamin B deficiency, hair curlers and blow dryers, cold weather (winter), use of hair sprays, gels and hair coloring chemicals which more probably resented in females and hence they become more susceptible to Malassezia infections than males.
Current study revealed that patient resident in urban area were more frequently exposed to malassezia infection, (34%) which come in line with Devendrappa and Javed from India [26]. M. furfur was the predominant species, (22%) while M. globosa recovered from, (8%) and M. pachydermatis was the least once, isolated from, (4%). This result disagree with Hasan et al., from Iraq, [24], who reported no significant correlation. On the other hand current study revealed that patients residency in rural area appear to be at risk of getting malassezia infection at (1.093) time than those in urban area. This result come in accordance with Sharma from India [30], stated that geographical variations have been observed in the densities of different Malassezia species on the skin. Malassezia infections more commonly reported in the warm and humid tropical and subtropical climates which is more suited for its growth. The higher incidence in urban people was mainly due to the location of the hospital in the city. As the health facilities are also available in rural areas, the attendance of the patients from these areas to this hospital was reduced; probably this could be the reason for the low incidence from rural areas. Another factor could be that the rural population is not bothered about the cosmetic aspect of this disease.
The current study revealed that no correlation was reported between economic status and exposure to Malassezia spp. Infection. This may be attributed to factors other than economy play a vital role in infection such as psychological and immunological stress.
The current study revealed that patients with primary education as well as students were more frequently exposed to Malassezia infection. Illiterate patients and patients with an academic job were less frequently exposed to Malassezia infection, (4%). A significant correlation was reported between education level and exposure to Malassezia spp while Malassezia spp. Infection does not correlate with the type of job. This result may be attributed to the limited number of illiterate patients seeking medical consultations as well as investigations related to skin infections possibly due to they consider this is a type of luxury checkup. On the other hand, the limited number of patients with higher education reflects their knowledge about the complications of cutaneous infections as well as their attention to check any skin abnormalities such as hyperpigmentation or hypopigmentation or even scales. Type of patient’s job may facilitate the flaring of infection indirectly by acting as a stress factor which acts with other genetic, hormonal, and environmental factors such as humidity and temperature to establishing a clinical presentation of PV.
The current study revealed those patients utilising filtrated water were more frequently exposed to Malassezia infection. M. furfur was the predominant species recovered from patients with PV. Patients utilizing river water were less frequently exposed to Malassezia infection, mainly M. furfur, (2%) although, no significant correlation was reported between the source of water and Malassezia spp. Infection. Current result comes in agreement with Babic et al., from Slovenia [31], who stated that fungi including genus Malassezia can form fungal biofilms within 48 h in tap systems in private homes, hospitals or industrial network was confirmed for opportunistic and pathogenic species from the genera Malassezia. Once established, biofilms are difficult to be fully removed from the pipe system, which on the long-term leads to altered taste and odour of water, production of allergenic or irritating compounds, and mycotoxins with an effect on human health [31].
The current study revealed that patients who do not have any type of contact with animals were more frequently exposed to Malassezia infection and M. furfur was the predominant species which explain that the source of infection not restricted to birds or dogs. Patients who have contact with dogs only were equally exposed to M. furfur, and M. pachydermatis which reflect that dogs not only the source of infection but also other factors such as this may be attributed to several factors includes but not limited to, humidity and high temperature, hyperhidrosis, familial susceptibility, and immunosuppression [32]. A range of skin microenvironmental factors, such as the bacterial microbiota present, pH, salts, immune responses, cutaneous biochemistry, and physiology, may play a role in adherence and growth of Malassezia species, favouring distinct genotypes depending on the geographical area and/or the skin sites [33]. No significant correlation was reported between contact with animals and Malassezia spp — infection which comes in line with Al-Ezzy from Iraq [21].
In conclusion, M. furfur, M. pachydermatis and M. globosa represent the most common Malassezia spp. causing PV. Using of PCR is very critical to confirm the diagnosis of Malassezia spp. Malassezia infection inversely correlated with age and positively correlated with females gender and education. The residency in a rural area and middle economic status increase the possibility of infection. Infection was not affected by the source of water; job and contact with dogs and birds.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Inamadar AC, Palit A. The genus Malassezia and human disease. Indian Journal of Dermatology, Venereology, and Leprology. 2003;69(4):265. [PubMed] [Google Scholar]
- 2.Gaitanis G, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in the skin and systemic diseases. Clinical microbiology reviews. 2012;25(1):106–41. doi: 10.1128/CMR.00021-11. https://doi.org/10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varada S, Dabade T, Loo DS. Uncommon presentations of tinea versicolor. Dermatology practical &conceptual. 2014;4(3):93. doi: 10.5826/dpc.0403a21. https://doi.org/10.5826/dpc.0403a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson T. The human skin microbiome:cause or effect?The role of Malassezia in human skin health and disease. Medical Mycology Oxford Univ Press Great Clarendon St, Oxford Ox2 6dp, England. 2018 [Google Scholar]
- 5.Rai M, Wankhade S. Tinea versicolor–an epidemiology. J Microbial Biochem Technol. 2009;1(1):51–6. https://doi.org/10.4172/1948-5948.1000010. [Google Scholar]
- 6.Khosravi A, Eidi S, Katiraee F, Ziglari T, Bayat M, Nissiani M. Identification of different Malassezia species isolated from patients with Malassezia infections. World Journal of Zoology. 2009;4(2):85–9. [Google Scholar]
- 7.ElShabrawy WO, Saudy N, Sallam M. Molecular and Phenotypic Identification and Speciation of Malassezia Yeasts Isolated from Egyptian Patients with Pityriasis Versicolor. Journal of clinical and diagnostic research:JCDR. 2017;11(8):DC12. doi: 10.7860/JCDR/2017/27747.10416. https://doi.org/10.7860/JCDR/2017/27747.10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shokohi T, Afshar P, Barzgar A. Distribution of Malassezia species in patients with pityriasis versicolor in Northern Iran. Indian journal of medical microbiology. 2009;27(4):321. doi: 10.4103/0255-0857.55445. https://doi.org/10.4103/0255-0857.55445. [DOI] [PubMed] [Google Scholar]
- 9.Tu WT, Chin SY, Chou CL, Hsu CY, Chen YT, Liu D, et al. Utility of Gram staining for diagnosis of Malassezia folliculitis. The Journal of dermatology. 2018;45(2):228–31. doi: 10.1111/1346-8138.14120. https://doi.org/10.1111/1346-8138.14120. [DOI] [PubMed] [Google Scholar]
- 10.Karhoot J, Noaimi A, Ahmad W. Isolation and identification of Malassezia species in patients with pityriasis versicolor. The Iraqi Postgraduate Medical Journal. 2012;11(6):724–30. [Google Scholar]
- 11.Darling MJ, Lambiase MC, Young RJ. Tinea versicolor mimicking pityriasis rubra pilaris. CUTIS-NEW YORK. 2005;75(5):265. [PubMed] [Google Scholar]
- 12.Gouda A. M. Sc. thesis. College of Medicine, University of Ain Shams; 2008. Malassezia species isolated from lesional and non lesional skin in patients with pityriasis versicolor. [Google Scholar]
- 13.Petti CA, Carroll KC. Procedures for the Storage of Microorganisms. InManual of Clinical Microbiology. (10th Edition) 2011:124–131. https://doi.org/10.1128/9781555816728.ch9. [Google Scholar]
- 14.Qiagen. QIAamp®DNA Mini and Blood Mini Handbook. Germany Qiagen; 2016. [Google Scholar]
- 15.Mirhendi H, Makimura K, Khoramizadeh M, Yamaguchi H. A one-enzyme PCR-RFLP assay for identification of six medically important Candida species. Nippon Ishinkin Gakkai Zasshi. 2006;47(3):225–9. doi: 10.3314/jjmm.47.225. https://doi.org/10.3314/jjmm.47.225. [DOI] [PubMed] [Google Scholar]
- 16.Jang S-J, Lim S-H, Ko J-H, Oh B-H, Kim S-M, Song Y-C, et al. The investigation on the distribution of Malassezia yeasts on the normal Korean skin by 26S rDNA PCR-RFLP. Annals of dermatology. 2009;21(1):18–26. doi: 10.5021/ad.2009.21.1.18. https://doi.org/10.5021/ad.2009.21.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah A, Koticha A, Ubale M, Wanjare S, Mehta P, Khopkar U. Identification and speciation of Malassezia in patients clinically suspected of having pityriasis versicolor. Indian journal of dermatology. 2013;58(3):239. doi: 10.4103/0019-5154.110841. https://doi.org/10.4103/0019-5154.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Hadidy G, Gomaa N, AboBakar R, Metwally L. Direct molecular identification of Malassezia species from skin scales of patients with seborrheic dermatitis by nested terminal fragment length polymorphism analysis Egypt. J Med Microbiol. 2007;16:437–44. [Google Scholar]
- 19.Talaee R, Katiraee F, Ghaderi M, Erami M, Alavi AK, Nazeri M. Molecular identification and prevalence of Malassezia species in pityriasis versicolor patients from Kashan, Iran. Jundishapur journal of microbiology. 2014;7(8) doi: 10.5812/jjm.11561. https://doi.org/10.5812/jjm.11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Ammari AMM. Relation of Malassezia species with some skin diseases. Baghdad University:Baghdad University; 2012. [Google Scholar]
- 21.Al-Ezzy AIA, Jameel GH, Minnat TR. Isolation of Malassezia Furfur and Evaluation of Ivermectin and Cal-vatia Craniiformis as A Novel Antifungal Agents for Pityriasis Versi-color with Special Refer to Risk Factors in Iraqi Patients. International Journal of Current Pharmaceutical Review and Research. 2017;8(4):311–9. [Google Scholar]
- 22.Chaudhary R, Singh S, Banerjee T, Tilak R. Prevalence of different Malassezia species in pityriasis versicolor in central India. Indian Journal of Dermatology, Venereology, and Leprology. 2010;76(2):159. doi: 10.4103/0378-6323.60566. https://doi.org/10.4103/0378-6323.60566. [DOI] [PubMed] [Google Scholar]
- 23.Archana BR, Beena PM, Kumar S. Study of the distribution of malassezia species in patients with pityriasis versicolor in Kolar Region, Karnataka. Indian journal of dermatology. 2015;60(3):321. doi: 10.4103/0019-5154.156436. https://doi.org/10.4103/0019-5154.156436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan A-RS, Abass AA, Khudier MA-K. Clinical and Fungal Study of Pityriasis Versicolor Infection among Patients with Skin Mycoses in Baquba. Iraqi Journal of Community Medicine. 2009;22(1):30–3. [Google Scholar]
- 25.Thayikkannu AB, Kindo AJ, Veeraraghavan M. Malassezia—Can it be Ignored? Indian journal of dermatology. 2015;60(4):332. doi: 10.4103/0019-5154.160475. https://doi.org/10.4103/0019-5154.160475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devendrappa K, Javed MW. Clinical profile of patients with tinea versicolor. International Journal of Research in Dermatology. 2018;4(1):33–7. https://doi.org/10.18203/issn.2455-4529.IntJResDermatol20180017. [Google Scholar]
- 27.Abdul-Hussein AA. Clinical and Pigmentary Variation of Pityriasis Versicolor in Al-Muthana Government's Patients. Medical Journal of Babylon. 2010;7(3-4):383–8. [Google Scholar]
- 28.Akaza N, Akamatsu H, Takeoka S, Sasaki Y, Mizutani H, Nakata S, et al. Malassezia globosa tends to grow actively in summer conditions more than other cutaneous Malassezia species. The Journal of dermatology. 2012;39(7):613–6. doi: 10.1111/j.1346-8138.2011.01477.x. https://doi.org/10.1111/j.1346-8138.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 29.Laschinsky T. Extreme Dandruff and Seborrheic Dermatitis Hair Conditions. 2010 [Google Scholar]
- 30.Sharma M, Sharma R. Profile of dermatophytic and other fungal infections in Jaipur. Indian journal of microbiology. 2012;52(2):270–4. doi: 10.1007/s12088-011-0217-z. https://doi.org/10.1007/s12088-011-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babič MN, Gunde-Cimerman N, Vargha M, Tischner Z, Magyar D, Veríssimo C, et al. Fungal contaminants in drinking water regulation?a tale of ecology, exposure, purification and clinical relevance. International journal of environmental research and public health. 2017;14(6):636. https://doi.org/10.3390/ijerph14060636. [Google Scholar]
- 32.Gupta A, Foley K. Antifungal Treatment for Pityriasis Versicolor. Journal of Fungi. 2015;1:13–29. doi: 10.3390/jof1010013. https://doi.org/10.3390/jof1010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cafarchia C, Gasser R, Figueredo L, Latrofa M, Otranto D. Advances in the identification ofMalassezia. Molecular and cellular probes Molecular and cellular probes. 2011;25:1–7. doi: 10.1016/j.mcp.2010.12.003. https://doi.org/10.1016/j.mcp.2010.12.003. [DOI] [PubMed] [Google Scholar]


