Abstract
Previous research has shown that livestock exposed to ergot alkaloids results in decreased vasoactivity of gastrointestinal and peripheral vasculature. Little is known regarding the effect ergot alkaloid exposure during gestation may have on vasculature supporting the fetus. The objective of this study was to evaluate contractile responses of uterine and umbilical arteries collected from ewes consuming ergot alkaloids during gestation. On day 35 of gestation, 36 Suffolk ewes (78.24 ± 9.5 kg) were assigned to endophyte-infected (E+) or endophyte-free (E−) tall fescue seed treatments that were fed either throughout or switched on day 86 of gestation, creating four seed treatments E+E+, E+E−, E−E+, and E−E−. Ewes were fed E+ tall fescue seed to provide 1.77 mg of total ergovaline ⋅ hd−1 ⋅ d−1 with E− ewes receiving the same quantity of E− seed. Gestation was terminated on day 133, and sections of uterine artery and umbilical cord were surgically collected. Only collections from 28 ewes (n = 7/treatment) were of sufficient viability to proceed with the contractility experiments. Arteries were cleaned, sliced into 2-mm cross sections, and suspended in multi-myograph chambers containing 5 mL of continuously oxygenated Krebs–Henseleit buffer. Vessels were exposed to increasing concentrations (5 × 10−8 to 1 × 10−4 M) of norepinephrine, serotonin, ergotamine, and ergovaline (5 × 10−9 to 1 × 10−5M; extract of tall fescue seed) in 15-min intervals. Increasing concentrations of norepinephrine generated a contractile response by the uterine artery (P < 0.05), but no response in the umbilical artery. Increasing concentrations of serotonin resulted in negligible responses in uterine preparations, whereas umbilical artery preparations were responsive (P < 0.05) to serotonin. Ewes receiving E+E+ and E−E+ treatments had decreased vasoactivity in umbilical arteries to serotonin with a dextral shift in concentrations where the response curve initiated (P < 0.05). Interestingly, uterine arteries were not responsive to exposure to ergotamine or ergovaline, whereas umbilical arteries were responsive (P < 0.05). Umbilical arteries collected from ewes receiving E−E− and E+E− were more vasoactive to ergot alkaloids (P < 0.05) than other treatments. These findings indicate that maternal blood supply to the placenta appears protected from negative effects of ergot alkaloids; however, umbilical vasculature is not, and this could adversely influence fetal growth.
Keywords: ergot alkaloids, ewe, gestation, vasoconstriction
INTRODUCTION
Ergot alkaloids produced by a fungal endophyte (Epichloë coenophiala) found in tall fescue (Lolium arundinaceum) are responsible for reductions in reproductive performance (Porter and Thompson, 1992; Strickland et al., 2011). Duckett et al. (2014) demonstrated that lambs born from ewes fed toxic endophyte-infected tall fescue during gestation had reduced birth, organ, and muscle weights compared with controls. Throughout gestation, uterine and umbilical blood flows increase to support the growing fetus (Ford, 1995), making blood flow critical to nutrient availability necessary to support fetal growth and development (Wu et al., 2006). Reductions in fetal growth in ruminants carrying multiple fetuses have been connected with decreased uterine and placental blood flows (Ferrell and Reynolds, 1992).
Ergot alkaloids have been associated with vasoconstriction resulting in impaired blood flow (Klotz, 2015). Using bovine umbilical and uterine arteries, Dyer (1993) showed the vasoconstrictive effects of ergovaline, a predominant ergot alkaloid produced by E. coenophiala (Lyons et al., 1986; Yates and Powell, 1988), are mediated by the serotonin (5HT) receptor 5HT2. Schöning et al. (2001) further demonstrated that 5HT2A-derived vasoactivity in rodent peripheral vascular models is altered with exposure to ergovaline. Specifically, exposure to ergovaline and other ergopeptine alkaloids has been shown to reduce the vasoactivity of gut vasculature (Egert et al., 2014; Jia et al., 2015) and peripheral vasculature (Klotz et al., 2012; Klotz et al., 2016). Thus, it was hypothesized that maternal exposure to ergot alkaloids during gestation will reduce vasoactivity of uterine and umbilical vasculature and result in reduced blood flow causing intra-uterine growth restriction. The objective of this study was to evaluate contractile responses and gene expression associated with vasoactivity in uterine and umbilical arteries collected from ewes consuming ergot alkaloids during different periods of gestation.
MATERIALS AND METHODS
All experimental procedures involving live ewes and perinatal lambs were approved by the Clemson University Institutional Animal Care and Use Committee and conducted at the Clemson University Small Ruminant Facility.
Ewes and Tall Fescue Seed Treatments
This work was part of a large study originally described by Britt et al. (2019). Briefly, 36 Suffolk ewes naïve to endophyte-infected tall fescue that were estrus-synchronized, exposed to a single Suffolk ram, and confirmed pregnant on day 35 (n = 36; BW = 78.2 ± 9.5 kg) were used in this study. Ewes were divided into 9 groups of 4 each based on synchronization date (synchronized using intravaginal controlled internal drug release (CIDR) insert (Eazi-Breed CIDR, Zoetis Animal Health) followed by a prostaglandin F2α injection (12.5 mg; Lutalyse; Pfizer, New York, NY)) and were assigned to a fescue seed treatment. Treatments were endophyte-infected (E+) or endophyte-free (E–) tall fescue seed treatments fed at two stages of gestation (day 35–85 and day 86–133). Ground E+ and E– seed were analyzed for ergovaline content using HPLC as described by Klotz et al. (2018) and contained 4.14 and 0 μg/g DM of ergovaline + ergovalinine, respectively. Ewes were individually fed a TMR (corn, cottonseed hulls, and soybean meal) daily with 0.428 kg seed mixed into the ration so that the E+ ewes received 1.77 mg ergovaline ⋅ hd−1 ⋅ d−1 and E– ewes received the same amount of endophyte-free tall fescue seed with 0 mg ⋅ hd−1 ⋅ d−1 of ergovaline + ergovalinine. The dose of ergovaline in the TMR was based on previous work of Duckett et al. (2014). Complications associated with abortion (n = 1), still births (n = 2), ketosis (n = 1), and incorrect laboratory gas (n = 4) resulted in collections of viable vessels from n = 7 ewes per treatment being included in the myograph portion of the study.
Blood Vessel Collection and Myography
On day 133, gestation was surgically terminated as described by Britt et al. (2019). Briefly, a lower midline incision between the umbilicus and pubic symphysis was made and the gravid uterus was exteriorized. Using the first fetus identified and removed from the uterus, the median portion of the umbilical cord was identified and a 4- to 5-cm section was ligated with 0 silk suture (Ethicon Inc., Somerville, NJ). This isolated section was cut from the cord and placed into a modified Krebs–Henseleit oxygenated buffer solution (95% O2/5% CO2; pH = 7.4; mM composition = D-glucose, 11.1; MgSO4, 1.2; KH2PO4, 1.2; KCl, 4.7; NaCl, 118.1; CaCl2, 3.4; and NaHCO3, 24.9; Sigma Chemical Co., St. Louis, MO) maintained at 4°C. The cranial and caudal branch of uterine artery extending off of the broad ligament was identified using the same horn that the fetus was removed from, and a 4- to 5-cm section was ligated with silk suture, excised, and also placed in 4°C Krebs–Henseleit oxygenated buffer solution.
Preparation of arterial samples for myograph experiments consisted of removal of external fat and connective tissue. Cleaned segments were cut into 2- to 3-mm cross sections using an adjustable acrylic channel tissue matrix (Braintree Scientific, Braintree, MA). Prior to mounting in a myograph, cross-sections were examined using a dissecting microscope (Stemi 2000-C, Carl Zeiss Inc., Oberkochen, Germany) at 12.5× magnification to measure dimensions for assurance of consistent segment size and to verify physical integrity of tissue. Cross-sections were suspended horizontally in a 5-mL tissue bath (DMT610M multichamber myographs, Danish Myo Technologies, Atlanta, GA) containing continuously oxygenated modified Krebs–Henseleit buffer (95% O2/5% CO2; pH = 7.4; 37°C), with 3 × 10–5 M desipramine and 1 × 10–6 M propranolol (Sigma Chemical Co.) to inactivate catecholamine–neuronal uptake and β-adrenergic receptors, respectively. After equilibration to 1 g of tension (~1.5 h), umbilical and uterine arteries were exposed to an addition of 120 mM KCl to verify tissue viability and for subsequent use as a reference for normalization of resultant contractile response data.
Uterine and umbilical artery cross sections were run in duplicate from each ewe for each treatment. Following recovery from the 120 mM KCl addition and reestablishment of the 1-g baseline tension, agonist or alkaloid additions occurred in 15-min intervals. Each 15-min interval consisted of a 9-min treatment incubation period, followed by a washout period during which 5-mL aliquots of buffer without treatment were incubated with an arterial segment for two consecutive 2.5-min periods, followed by a final buffer replacement and 1-min incubation prior to the next addition.
Treatment additions consisted of the ergot alkaloids ergovaline and ergotamine and the biogenic amines norepinephrine and serotonin. Ergovaline was provided in the form of a toxic endophyte-infected tall fescue seed extract produced as described by Ji et al. (2014) and validated against pure ergovaline in bovine lateral saphenous veins by Foote et al. (2012). This extract was diluted based on the measured concentration of ergovaline. Ergotamine tartrate (Sigma-Aldrich, St. Louis, MO; 45510; >97.0% purity) was diluted with DMSO (Sigma-Aldrich; D8418). Serotonin (5HT) hydrochloride (Sigma-Aldrich; H9523) and (±)-norepinephrine (+)-bitartrate (Sigma-Aldrich; A0937) were solubilized and diluted with purified water. The contractile response curves for ergotamine, 5HT, and norepinephrine were constructed with 8 consecutive additions every 15 min at fixed concentrations ranging from 5 × 10–8 to 1 × 10–4 M in the myograph tissue bath. The response curve for ergovaline consisted of eight additions that ranged from 5 × 10–9 to 1 × 10–5 M because of limiting ergovaline concentrations in the tall fescue seed extract stock.
Isometric contractions of uterine and umbilical artery preparations were recorded as grams of tension in response to exposure to the KCl reference and the ergovaline, ergotamine, 5HT, and norepinephrine treatments. Data were digitally recorded using a Powerlab 16/35 (ADInstruments, Colorado Springs, CO) and Chart software (Version 7.2, ADInstruments). Contractile response was recorded as the greatest response, in grams, during the 9-min incubation following treatment addition and adjusted by baseline tension recorded just prior to the addition of 120 mM KCl. Treatment response data were normalized as a percentage of the maximal contraction produced by KCl addition. Concentration–response curves were constructed by plotting the normalized data using GraphPad Prism (version 5.0f; San Diego, CA). This graphical presentation employed a nonlinear regression (sigmoidal concentration–response curve) to fit a line to contractile response data points for a treatment using the 3-parameter equation:
where the “top” and “bottom” are plateaus in the units of the y-axis. This calculation permitted the calculation of a compound’s potency for umbilical and uterine arteries expressed as the concentration the compound required to produce 50% of the observed contractile response (EC50).
RNA Isolation and Real-Time PCR
Cleaned blood vessels not used for myograph experiments were placed in foil packets, heat sealed, snap frozen in liquid N2, and stored at –80°C until processed for RNA. Samples were ground in liquid N2 using pre-sterilized, chilled mortar and pestles. For isolation of RNA, TRI reagent (Molecular Research Center, Inc., Cincinnati, OH) was added to ground blood vessel samples and either stored at –80°C or followed by chloroform extraction, RNA precipitation, and an ethanol rinse (Chomczynski and Sacchi, 1987). Isolated RNA was cleaned of impurities using RNeasy clean up kit (Qiagen, Valencia, CA). Cleaned RNA was quantified (NanoDrop; ND-1000; Thermo Fisher Scientific, Inc., Waltham, MA) and RNA integrity was confirmed using an Experion Automated Electrophoresis System (Bio-Rad Laboratories, Inc., Hercules, CA) and an Experion RNA StdSens kit (Bio-Rad laboratories). The RNA sample quality was considered adequate for use if the RNA quality indicator (RQI) was >8. For the current study, the mean RQI was for uterine and umbilical arteries was 9.7 ± 0.02 and 9.3 ± 0.03, respectively.
Reverse transcription was used to transcribe RNA into first-strand cDNA (Accuscript First Strand cDNA synthesis kit; Agilent Technologies, La Jolla, CA). Resultant cDNA was quantified using a fluorometer (Qubit 2.0; Life Technologies, Carlsbad, CA) and all samples of cDNA were diluted to a uniform 50 ng/μL concentration and further diluted to 1 ng/μL concentration for quantitative real-time PCR analysis (qPCR). One microliter of each 50 ng/μL sample was combined into a single composite sample that was again quantified and serially diluted for use as standard curve samples to 50, 10, 5, 1, 0.5, and 0.1 ng of composite cDNA.
Ovine-specific nucleotide sequences were obtained from previously published sequences in the National Center for Biotechnology Information (NCBI) database. Exon junction sites for each gene were determined using the NCBI Browser (http://www.ncbi.nlm.nih.gov/sutils/splign/splign.cgi) when available. Purchased primers (Table 1) were designed to incorporate sequences containing these junctions using PrimerQuest software (Integrated DNA Technologies, Inc., Coralville, IA).
Table 1.
Gene names, Ovis aires accession numbers (National Center for Biotechnology Information), and forward and reverse primer sequences for genes assessed with real-time PCR
| Gene1 | Symbol | Accession number | Primer sequence 5′ → 3′ (forward; reverse) | Amplicon (bp) |
|---|---|---|---|---|
| β-Actin | ACTB | NM_001009784.1 | TCC ACC GCA AAT GCT TCT CAC GAG GCC AAT CTC ATC TC | 99 |
| Glyceraldehyde 3-phosphate dehydrogenase | GAPDH | NM_001190390.1 | AGT TCC ACG GCA CAG TCA AG AGG ATC TCG CTC CTG GAA GAT | 83 |
| Ribosomal protein, larger, P0 | RPLP0 | XM_004017413.2 | CTC TGG AGA AAC TGT TGC CT TAT TGG CCA GCA GCA TGT | 96 |
| α-Adrenergic receptor 1A | ADRA1A | XM_015093082.1 | GCC CAT TGG GTC TTT CTT TC GGG TTG ATG CAG CTG TTT AG | 93 |
| 5-Hydroxytryptamine receptor 1B | HTR1B | XM_015097299.1 | CCA CGG AAT TGA CTG AGA TAG G GGG AAG GAG AGT GCA AAT GA | 138 |
| 5-Hydroxytryptamine receptor 1D | HTR1D | XM_015093689.1 | GGG AGA GGA AAG CCA CAA A GGA CCA GAG ATG CTA CAA AGA A | 87 |
| 5-Hydroxytryptamine receptor 2A | HTR2A | XM_004012066.3 | GCA GAA TGC CAC CAA CTA TTT C ACC GGT ACC CAT AGA GGA TG | 107 |
| Guanine nucleotide-binding protein G(s) subunit alpha | GNAS | XM_012188911.1 | CGC ACC ATC TCT GTG ATT CT GCG AGC AAA TTC TGG AAA GTA G | 105 |
| G protein subunit alpha q | GNAQ | XM_015093177.1 | GTA CGA GCA CAA TAA GGC TCA CTC GTC GTC TGT CAT AGC ATT C | 143 |
| G protein subunit alpha 11 | GNA11 | XM_015095900.1 | GTG GAG TCA GAC AAC GAG AA GAG GAA GAG GAT GAC AGA TGA G | 102 |
| Nitric oxide synthase 3 | NOS3 | NM_001129901.1 | TCC CTG TAC TAT CTC ATC CTC TC CTG ACT GTA GGC TCT GGA ATA C | 101 |
1 β-Actin, GAPDH, and RPLP0 are reference genes used to normalize gene expression of target genes.
Genes encoding for β-actin (ACTB), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), ribosomal protein, larger, P0 (RPLP0), α-adrenergic receptor 1A (ADRA1A), 5-hydroxytryptamine receptors 1B, 1D, and 2A (HTR1B, HTR1D, HTR2A, respectively), and the associated G-proteins (GNAS), (GNAQ), (GNA11), and nitric oxide synthase 3(NOS3) were evaluated for expression levels using qPCR with FAST SYBR Green Master Mix (Applied Biosystems, Foster City, CA; 4385612) using a StepOnePlus Real Time PCR system (Applied Biosystems).
Each sample reaction was run as one gene per plate in triplicate and contained 10 µL of FAST SYBR Green Master Mix, 1.2 µL each of the forward and reverse primer (600 nm; as determined by primer optimization), 6.6 µL nuclease free water, and 1.0 µL of diluted cDNA (1 ng) in a final reaction volume of 20 µL. Thermal profile for quantitative real-time PCR was 20 s at 95°C, followed by 40 cycles of 3 s at 95°C and 30 s at 60°C. Dissociation or melt curves were generated to ensure only a single product was amplified. The melt curve stage was run using initial denaturing step at 95°C for 15 s, cooling to 60°C for 60 s, and then increasing 1°C every 0.3 s to 95°C for 15 s. All genes investigated produced a single melt peak using the primer pairs included in Table 1. In addition to the melt curve, no-template control triplicates were included on each plate to ensure the absence of cross-contamination of reagents containing DNA template and primer dimer formation. Mean PCR efficiency for genes assessed was 100.48 ± 1.58%. Relative quantitation of mRNA abundance of target genes was conducted using relative standard curve method (Applied Biosystems, 2010), to determine cycle threshold values (Ct) that were normalized to a mean of three reference genes ACTB, GAPDH, and RPLP0 as described previously by Vandesompele et al. (2002) and Hellemans et al. (2007) using qbase+ 3.0 (Biogazelle; Ghent, Belgium).
Statistical Analysis
All statistical analyses were conducted using the Mixed models of SAS (SAS 9.3; SAS Inst. Inc., Cary, NC) and tests of fixed effects used the Satterthwaite approximation of denominator degrees of freedom. Contractile response data to biogenic amines norepinephrine and serotonin and tall fescue extract (ergovaline) and ergotamine treatments were analyzed as a completely randomized design with a split-plot treatment design. Whole plot experimental unit was ewe with seed treatment as the treatment factor. The blood vessels collected were the subplot experimental units and the concentration of biogenic amine or ergot alkaloid treatments were used as the subplot treatment factor. The model included fixed effects of seed treatment, myograph treatment concentration, and the interaction. For the variables EC50 and vessel dimensions (inside and outside diameter), analysis of variance was conducted as a completely randomized design for the main effect of seed treatment. The model was assessed to determine whether inclusion of covariates for fetal number and genotype of D2 dopamine receptor (Wilbanks et al., 2018) improved control for variation. Neither covariate was included in the model, as neither was significant and did not influence conclusions regarding contractile response.
Pairwise comparisons of least squares means (±SEM) were conducted only if the probability of a greater F-statistic in the ANOVA was significant for the tested effect. If significant, means separation was conducted using least significant difference (LSD) feature in SAS and comparisons were considered significant at P ≤ 0.05 and a tendency for significance at P ≤ 0.1, unless reported otherwise.
RESULTS
Uterine artery blood vessels collected from ewes that were receiving E+ seed in the diet at the time of collection had uterine arteries with smaller inside and outside diameters compared to those receiving E– seed (P < 0.01; Table 2). For the E+E+ ewes this translated to an overall smaller vessel when considering the thickness of the vessel wall, whereas the E–E+ ewes did not differ from E–E–, even with the smallest diameter inside the uterine artery (P < 0.05). Due to a very small lumen across all seed treatments, only the outside diameter of umbilical arteries was obtainable (Table 2). Umbilical arteries removed from both groups of ewes receiving E+ seed at the time of collection had smaller outside diameters than those groups of ewes receiving E– seed (P < 0.01).
Table 2.
Vascular dimensions of blood vessels collected from ewes fed endophyte-infected (E+) or endophyte-free (E–) seed from day 35 to d 133 or switched on day 86 of gestation1
| Variable2 | Seed treatment | SEM | P-value | |||
|---|---|---|---|---|---|---|
| E–E– | E+E– | E–E+ | E+E+ | |||
| Uterine artery | ||||||
| Inside diameter, mm | 1.25a | 1.31a | 1.07b | 1.14b | 0.03 | < 0.01 |
| Outside diameter, mm | 2.91a | 2.83a | 2.71b | 2.55c | 0.04 | < 0.01 |
| Wall thickness, mm | 0.83a | 0.75b | 0.82a | 0.70c | 0.01 | < 0.01 |
| Umbilical artery | ||||||
| Outside diameter, mm | 3.06a | 3.1a | 2.73b | 2.76b | 0.05 | < 0.01 |
1Unable to collect umbilical artery inside diameter.
2Wall thickness = (outside-inside diameter)/2.
abValues not sharing like superscripts within a row are different (P < 0.05).
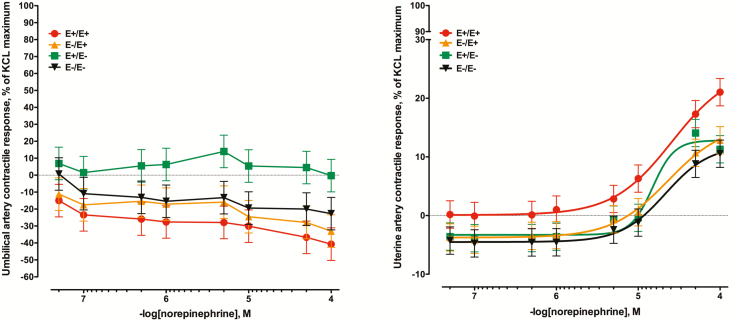
When umbilical arteries were exposed to increasing concentrations of norepinephrine, there was no measurable positive contractile response (Figure 1A). Although there was a seed treatment × norepinephrine concentration interaction (P = 0.02), this was primarily due to variable relaxation across seed treatments due to an absence of contractile response during the 2.5 h of norepinephrine additions. Conversely, uterine artery was responsive to norepinephrine, but significant increases in contractile response did not occur until the final three additions (P < 0.05; Figure 1B). Further, there was no seed treatment × norepinephrine concentration interaction for the uterine artery (P = 0.94). There was a tendency for effect of seed treatment (P = 0.07) with E+E+ arteries exhibiting the greatest contractile response, while the remaining three seed treatments did not differ.
Figure 1.
Mean contractile responses of (A) umbilical artery and (B) uterine artery to increasing concentrations of norepinephrine using vessels collected from ewes (n = 7/ treatment) that had been fed endophyte-infected (E+) or endophyte-free (E–) tall fescue seed from day 35 to day 133 of pregnancy (E+/E+; E–/E–) or had the treatment switched on day 86 (E+/E–; E–/E+). Seed treatment × norepinephrine concentration was significant for umbilical artery (P = 0.02), but not for uterine artery (P = 0.94). There was a tendency for an effect of seed treatment for uterine artery (P = 0.07).
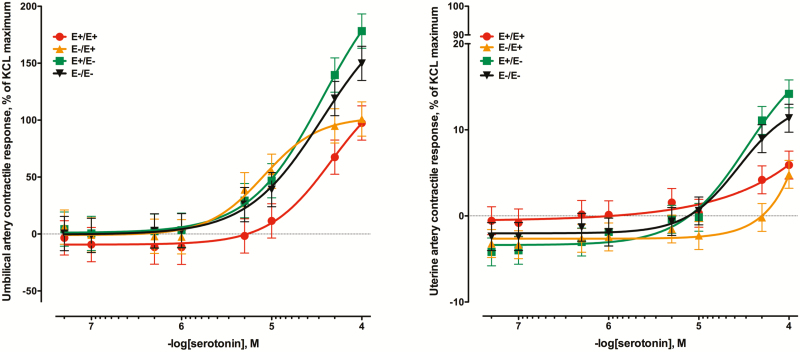
Serotonin was very vasoactive in umbilical artery preparations (Figure 2A) with contractile responses reaching or exceeding 100% of the KCl reference dose. There was a tendency for seed treatment × serotonin concentration interaction (P = 0.07). Ewes that received the E+ seed throughout gestation had significantly less contractile response compared to either E– treatment at the time of collection. Ewes that were switched to E+ on day 86 (E–E+) did not differ from the E– treatments up to the 1 × 10–5 M addition of serotonin but did not continue to increase with the final two additions of serotonin and were less than the E–E– and E+E– (P < 0.05). The contractile response of the uterine artery to increasing concentrations of serotonin were generally less (Figure 2B) than those responses observed in umbilical arteries (although these data were not directly compared). There was a seed treatment × serotonin concentration interaction for the uterine artery (P = 0.02) and, like the umbilical artery, the two treatments receiving E+ seed at the time of vessel collection had less contractile intensity to the high concentrations of serotonin (P < 0.05).
Figure 2.
Mean contractile responses of (A) umbilical artery and (B) uterine artery to increasing concentrations of serotonin using vessels collected from ewes (n = 7/treatment) that had been fed endophyte-infected (E+) or endophyte-free (E–) tall fescue seed from day 35 to day 133 of pregnancy (E+/E+; E–/E–) or had the treatment switched on d 86 (E+/E–; E–/E+). There was a tendency for a seed treatment × serotonin concentration interaction for umbilical artery (P = 0.07) and the interaction was significant for uterine artery (P < 0.01).
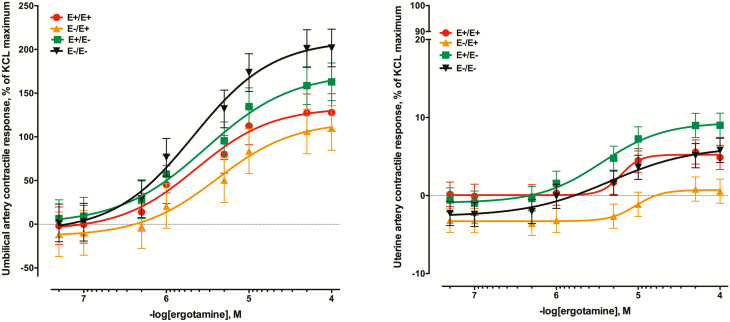
The contractile response of umbilical arteries to increasing concentrations of the ergot alkaloid ergotamine were substantial (P < 0.01; Figure 3A). There was not a seed treatment × ergotamine concentration interaction (P = 0.14) although the curves for ewes receiving E+ were below those for E–. While there was a contractile response to increasing concentrations of ergotamine in uterine arteries (Figure 3B), the greatest contractile response was 9.01 ± 1.5% (E+E–) of the KCl maximum (compared to 201.6 ± 21.5% for the E–E– umbilical artery). There was a seed treatment × ergotamine interaction (P < 0.01) and the uterine arteries collected from E–E+ ewes had the least contractile response to ergotamine (P < 0.05) barely exceeding 0% response (0.56 ±1.5%).
Figure 3.
Mean contractile responses of (A) umbilical artery and (B) uterine artery to increasing concentrations of ergotamine using vessels collected from ewes (n = 7/ treatment) that had been fed endophyte-infected (E+) or endophyte-free (E–) tall fescue seed from day 35 to day 133 of pregnancy (E+/E+; E–/E–) or had the treatment switched on day 86 (E+/E–; E–/E+). There was not a seed treatment × ergotamine concentration interaction for umbilical artery (P = 0.14), nor was there a seed treatment effect (P = 0.19), but the effect of ergotamine concentration was significant (P < 0.01). For the uterine artery, the interaction was significant (P < 0.01).
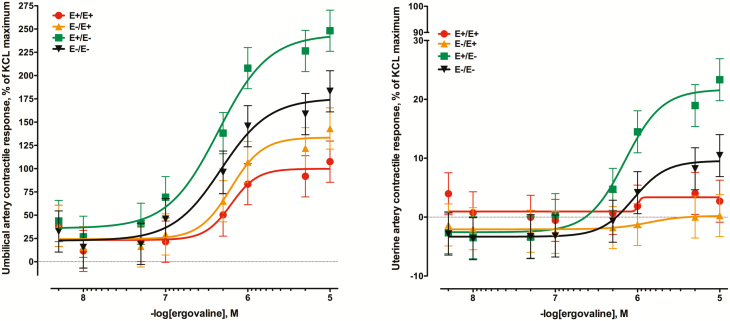
Like ergotamine, the tall fescue seed extract (ergovaline) was vasoactive in umbilical artery preparations (Figure 4A). There was a seed treatment × ergovaline interaction (P < 0.01) with the lowest response curves produced by arteries collected from ewes receiving E+ seed at the end of gestation (P < 0.05). The most responsive group were the umbilical arteries collected from E+E– ewes with maximal responses reaching 248.2 ± 22.1% of the KCl maximum and were greater than even the E–E– group (183.1 ± 22.1%; P < 0.05) at the 1 × 10–4 M addition. Also similar to ergotamine, the uterine artery contractile responses to ergovaline (Figure 4B) were much less than umbilical arteries. There was a seed treatment × ergovaline interaction (P < 0.01) where the uterine arteries collected from E–E+ ewes had the least response to increasing concentrations of ergovaline. Like the umbilical artery responses to ergovaline, the ewes receiving the E+E– seed treatment produced the greatest contractile response to ergovaline (P < 0.05).
Figure 4.
Mean contractile responses of (A) umbilical artery and (B) uterine artery to increasing concentrations of tall fescue seed extract (diluted based on ergovaline concentration) using vessels collected from ewes (n = 7/treatment) that had been fed endophyte-infected (E+) or endophyte-free (E–) tall fescue seed from day 35 to day 133 of pregnancy (E+/E+; E–/E–) or had the treatment switched on day 86 (E+/E–; E–/E+). There was a seed treatment × ergovaline concentration interaction for umbilical artery (P < 0.01) and for uterine artery (P < 0.01).
The EC50 values (Table 3) for the umbilical artery do not include norepinephrine. Because of the lack of response generated by norepinephrine, there was an absence of convergence for these umbilical artery data. For serotonin, ergotamine, and ergovaline there was no effect of seed treatment on EC50 (P ≥ 0.19). The outcome for the uterine artery EC50 (Table 3) was similar to the umbilical artery. There was no effect of seed treatment on the EC50 for norepinephrine, serotonin, or ergovaline (P ≥ 0.45). There was an effect of seed treatment for ergotamine, with the ewes receiving the E–E+ seed treatment having the lowest EC50, or least potent response to increasing concentrations of ergotamine (P < 0.05).
Table 3.
The –log EC50 values (M) ±SEM for norepinephrine, serotonin, ergotamine, and tall fescue seed extract in umbilical and uterine arteries collected from ewes fed endophyte-infected or endophyte-free seed from day 35 to day 133 of gestation or switched on day 861
| Myograph treatment | Seed treatment2 | P-value3 | |||
|---|---|---|---|---|---|
| E–E– | E+E– | E–E+ | E+E+ | ||
| Umbilical artery4 | |||||
| Serotonin | 4.37 ± 0.11 | 4.35 ± 0.11 | 4.33 ± 0.12 | 4.14 ± 0.12 | 0.52 |
| Ergotamine | 5.56 ± 0.11 | 5.55 ± 0.11 | 5.34 ± 0.14 | 5.59 ± 0.11 | 0.56 |
| Ergovaline5 | 5.25 ± 0.08 | 5.33 ± 0.08 | 5.09 ± 0.08 | 5.16 ± 0.08 | 0.19 |
| Uterine artery | |||||
| Norepinephrine | 4.24 ± 0.12 | 4.45 ± 0.13 | 4.48 ± 0.13 | 4.42 ± 0.12 | 0.53 |
| Serotonin | 4.24 ± 0.24 | 4.34 ± 0.24 | 4.51 ± 0.36 | 4.83 ± 0.28 | 0.45 |
| Ergotamine | 5.62a ± 0.16 | 5.46a ± 0.16 | 4.67b ± 0.16 | 5.19a ± 0.16 | 0.002 |
| Ergovaline5 | 4.88 ± 0.24 | 5.01 ± 0.24 | 5.27 ± 0.28 | 5.27 ± 0.28 | 0.65 |
1Used the three parameter nonlinear equation to fit line to data. Fifty percent of the effective concentration (EC50) is a measure of the potency of an agonist and is the concentration where 50% of the contractile response is achieved.
2Endophyte-infected (E+) and endophyte-free (E–) tall fescue seed.
3The probability of seed treatment differing with row.
4The EC50 values for norepinephrine response in umbilical artery were not obtained due to a lack of convergence because of negligible responses to this agonist.
5A tall fescue seed extract was used to supply ergovaline and the extract was diluted based on ergovaline concentrations.
abMeans within a row not sharing like superscripts differ (P < 0.05).
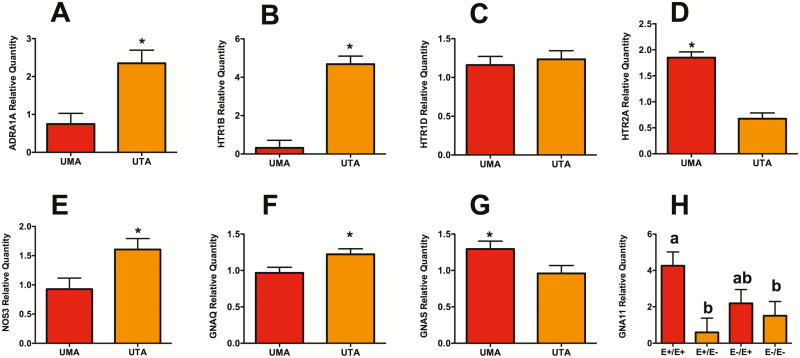
There was no interaction of seed treatment × blood vessel type (P > 0.14) in the assessment of genes potentially involved in vascular smooth muscle contraction. Except for HTR1D and GNA11, there was an effect of vessel type (P < 0.04), but no effect of seed treatment on the remaining genes evaluated (P > 0.24; Figure 5). There were greater expressions of ADRA1A, HTR1B, NOS3, and GNAQ in uterine artery isolates compared to umbilical artery (P < 0.05). Conversely, expression levels of HTR2A and GNAS were greater in umbilical artery isolates than in uterine artery (P < 0.05). For the G protein GNA11, there was an effect of seed treatment (P = 0.01), where ewes receiving E+/E+ had greater expression than either group of ewes receiving E– at the end of gestation (P < 0.05; Figure 5H).
Figure 5.
Relative expression levels of genes associated with aspects of vascular smooth muscle contraction and relaxation. None of the genes evaluated had a significant blood vessel type × seed treatment interaction, thus main effects for vessel type or seed treatment were presented. Adrenergic receptor (A) ADRA1A; serotonin receptors (HTR), (B) HTR1B, (C) HTR1D, and (D) HTR2A; endothelial nitric oxide synthase (E) NOS3; and G proteins (F) GNAQ, (G) GNAS, and (H) GNA11, and isolated from umbilical artery (UMA) and uterine artery (UTA). Expression levels not containing the same superscript or indicated by an asterisk differ by P < 0.05.
DISCUSSION
The premise of this study was to contribute to an understanding of how ergot alkaloid exposure during gestation results in reduced fetal growth observed by Duckett et al. (2014) by assessing vascular contractility and genes associated with vasoactivity. The animal model was established by feeding toxic endophyte-infected tall fescue seed at 1.77 mg ergovaline ⋅ hd−1 ⋅ d−1 and this was effective at inducing toxicosis in pregnant ewes. Specifically, serum prolactin concentrations and urinary alkaloids for this study were reported by Britt et al. (2019). Urinary ergot alkaloid excretion was consistently 10-fold greater in ewes that received E+ seed, whereas serum prolactin concentrations were lower in ewes that received E+ compared to E– seed. As hallmarks of fescue toxicosis, the significant effect of feeding E+ seed on these variables confirmed the successful induction of fescue toxicosis during gestation.
It was hypothesized that the presence of ergot alkaloids would cause localized vasoconstriction in reproductive vasculature that may restrict blood flow and cause or contribute to the reductions in fetal growth. Poole et al. (2018) reported in vivo reductions in the diameter of ovarian and uterine arteries in heifers that received E+ seed for 63 d. Similarly, vascular dimensions of E+/E+ treated ewes in the current study were consistently smaller than E–/E– ewes. Additionally, exposure to ergot alkaloids early in gestation (prior to day 86; E+/E– and E+/E+) resulted in a decrease in uterine artery wall thickness, but not in late gestation (E–/E+). Conversely, exposure to ergot alkaloids in late gestation decrease uterine artery outside diameter regardless of treatment prior to day 86. Thus, the timing of ergot alkaloid exposure may influence the morphology of the uterine artery as it develops to support the gravid uterus.
Ergovaline has been shown to cause contraction of bovine uterine and umbilical arteries (Dyer, 1993). Previous work using similar isolated blood vessel techniques as done in the current study, but with other vascular models collected from animals on an ergot alkaloid treatment consistently demonstrate reductions in contractility in the E+ treated animals. Specifically, the vasoactivity of gut vasculature (Egert et al., 2014; Jia et al., 2015; Trotta et al., 2018) and peripheral vasculature (Klotz et al., 2012, 2016) is reduced in blood vessels that have been exposed to ergot alkaloids both in vivo and in vitro. This observation persisted in the current study with ewes receiving E– seed at the time of collection producing significantly greater umbilical and uterine artery contractile responses to ergovaline and serotonin compared to those ewes receiving E+ seed. This trend was also apparent for the contractile response to increasing ergotamine, although not significant for the umbilical artery. Klotz et al. (2016) reported that vascular recovery from ergot alkaloid exposure requires 35 to 42 d. Sufficient time had passed from the treatment switch at day 86 of gestation for the effects of ergot alkaloids to clear from vasculature in the E+/E– ewes and negative effects in the E–/E+ ewes to become established. Thus, differences between E–/E– compared with E+/E– and E+/E+ compared with E–/E+ did not persist to the time of collection on day 133.
The physiology behind the reduction in contractility that is repeatedly observed in experiments where livestock are treated with E+ tall fescue is not fully understood. A potential explanation is a change in the expression of genes associated with vasoactivity and the rationale in the current study was to select genes encoding receptors, intracellular signaling proteins, and enzymes associated with vasoconstriction and vasorelaxation from the ovine genome. This permitted the evaluation of whether expression of these genes changed due to the exposure to ergot alkaloids and to determine whether altered gene expression was contributing to the change in vasoactivity observed at the tissue level in the myograph. Although the results did not directly implicate a broad change in gene expression contributing to the observed decrease in vasoactivity associated with ergot alkaloid exposure, there were some observations worthy of discussion and are included with vasoactivity discussion below.
Norepinephrine additions were used as an evaluation for the presence of adrenergic receptors and the effect that the seed treatment had on those receptor populations. The contractile response of uterine vessels to increasing concentrations of norepinephrine was different than other compound and vessel combinations because there was no real separation by seed treatment or detectable suppression of contractile response by the alkaloid treatments. Vasoconstriction in the ovine uterine artery and umbilical vein has been shown to be mediated by α1-adrenergic receptors (Isla and Dyer, 1990; Zhang and Dyer, 1991), but there is no information regarding ovine umbilical artery. Oliver et al. (1998) demonstrated an elevated α2-adrenergic vasoactivity, but not α1-adrenergic vasoactivity in lateral saphenous veins biopsied from steers grazing E+ tall fescue. In the current study, the gene expression of α1A-adrenergic receptor was evaluated and like Oliver et al. (1998) was not affected by seed treatment. However, the expression of ADRA1A was significantly greater in uterine artery than umbilical artery where there was no observed norepinephrine-induced contractility.
The neurotransmitter serotonin serves in various capacities throughout the body, but in the case of the current study, the function associated with vascular smooth muscle contractility was of interest. Like norepinephrine, serotonin was used as an indicator of serotonin receptor presence and as a measure of how the seed treatment affected vasoactivity of these receptors. Prior work using rodent models has shown that the serotonin receptor 5HT2A and to a lesser extent 5HT1B/1D are responsible for ergot alkaloid-derived vasoconstriction (Schöning et al., 2001). While the current study only evaluated the vasoactivity of serotonin and not a more selective agonist, there was a large difference in activity not only across seed treatments, but across vessel type. Specifically, the vasoactivity of serotonin was 100-fold greater in the umbilical artery than in the uterine artery. This observation was also observed with the ergotamine and ergovaline additions. Zhang and Dyer (1990) demonstrated that serotonin receptor 5HT2 mediates contraction of ovine umbilical arteries. In addition to the contractility data, current transcriptomic expression of HTR2A was greater in umbilical than uterine arteries, whereas HTR1B was greater in the uterine artery. Interestingly, the expression of GNA11, a G-protein involved with HTR2A signaling, had greater expression in E+ treated ewes. This could be a consequence of the continuous signaling associated with ergot alkaloid exposed serotonin receptors (Unett et al., 2013) and the mechanism that results in ergot alkaloid-produced prolonged vasoconstriction (Pesqueira et al. 2014; Klotz et al., 2016). Furthermore, there was no effect on NOS3 (endothelial nitric oxide synthase) as expression of this gene involved in vasorelaxation was not influenced by seed treatment.
As the predominantly produced ergot alkaloid by the Claviceps purpurea fungus, the negative effects that maternal exposure to ergotamine can have during gestation has been evaluated in humans (Hughes et al., 1988; Bánhidy et al., 2007), goats (Engeland et al., 1998), and sheep (Greatorex and Mantle, 1974) with results that range from low birthweights to death. Ergotamine was chosen as a representative of the ergopeptine class of ergot alkaloids to use in the myograph vascular bioassay as a comparison to the tall fescue seed extract used in the current study. The extract was the only available source of ergovaline and was of interest as the predominantly produced ergot alkaloid in toxic endophyte-infected tall fescue (Yates and Powell, 1988). Like the responses to serotonin, both ergotamine and ergovaline were 100-fold more vasoactive in the umbilical artery compared to the uterine artery. Previous work with nonpregnant equine uterine arteries also reported low vasoactivity of ergotamine (Klotz and McDowell, 2017). As described for serotonin, this contractile response is driven by serotonin receptor 5HT2A mediating vasoconstriction in the umbilical artery. The vasoactivity of both ergovaline and ergotamine are reduced with ketanserin, a 5HT2A antagonist (Klotz et al., 2013). Recent work has shown that 5HT2A-derived vasoconstriction is substantially diminished in ergovaline-exposed gut blood vessels (Trotta et al., 2018).
There are no published reports that describe permeability of the placentome to ergot alkaloids in sheep. There is work in other species that demonstrates the ability of ergot alkaloids to cross the placental barrier. Indänpään-Heikkilä and Schoolar (1969) demonstrated transplacental passage of [14C]LSD that was given intravenously to mice during the final week of pregnancy. Five minutes following infusion, the label was detected in the fetus. Leist and Grauwiler (1973) looked at the intravenous infusion of the structurally more complex ergopeptide alkaloid [3H]ergotamine in rats. Female rats received infusions of ergotamine 14 d following breeding and radioactivity levels were three-fold higher in uterus and placenta than in blood. Further, radioactivity was detected in amniotic fluid and fetal tissues (Leist and Grauwiler, 1973). While ergot alkaloid transport across the placentome was not directly measured in the current study, the evidence of suppressed vasoactivity that suggests that there are vascular effects occurring on the fetal side that can only be a result of transport across the placentome. These observations combined with the reported decreased birth weights in lambs (Britt et al. 2019) from the same ewes that provided the blood vessels used in this study make a strong case for impaired blood flow causing the negative effects in lambs.
In conclusion, this is the first direct assessment of ergot alkaloid vasoactivity on gravid ovine reproductive vasculature. While ergot alkaloids were vasoactive in both uterine and umbilical arteries, the vasoactivity was much greater on the fetal side of the placentome. The effect of feeding toxic endophyte-infected seed resulted in a suppression of vasoactivity on both the maternal and fetal sides. This suppression of vasoactivity associated with ergot alkaloid exposure combined with the sensitivity of the umbilical artery are indications of vasoconstriction occurring on the fetal side. Future work should evaluate strategies to mitigate the ergot alkaloid-induced intra-uterine growth restriction in livestock that cannot be removed from toxic pastures during critical periods such as gestation.
ACKNOWLEDGEMENTS
The authors would like to extend their gratitude to Adam J. Barnes of the Forage-Animal Production Research Unit (Lexington, KY) for his technical expertise in the laboratory and for agreeing to travel to Clemson to complete the study.
Conflict of interest statement. There is no conflict of interest.
Footnotes
This research was supported by USDA Agriculture and Food Research Initiative, Competitive Grant No. 2015-67015-23218. Mention of trade name, proprietary product of specified equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be available. USDA is an equal opportunity provider and employer.
LITERATURE CITED
- Applied Biosystems 2010. Applied biosystems StepOne and StepOnePlus real-time PCR systems: relative standard curve and comparative CT experiments. 4376785 Rev. F. Foster City, CA. [Google Scholar]
- Bánhidy F., Acs N., Puhó E., and Czeizel A. E.. 2007. Ergotamine treatment during pregnancy and a higher rate of low birthweight and preterm birth. Br. J. Clin. Pharmacol. 64:510–516. doi:10.1111/j.1365-2125.2007.02901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt J. L., Greene M. A., Bridges W. C., Klotz J. L., Aiken G. E., Andrae J. G., Pratt S. L., Long N. M., Schrick F. N., Strickland J. R., et al. 2019. Impact of feeding endophyte-infected tall fescue seed containing ergot alkaloids to ewes during mid-and/or late gestation on uteroplacental sufficiency and fetal growth. J. Anim. Sci. (Submitted). [Google Scholar]
- Chomczynski P., and Sacchi N.. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159. doi:10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Duckett S. K., Andrae J. G., and Pratt S. L.. 2014. Exposure to ergot alkaloids during gestation reduces fetal growth in sheep. Front. Chem. 2:68. doi:10.3389/fchem.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. C. 1993. Evidence that ergovaline acts on serotonin receptors. Life Sci. 53:PL223–PL228. [DOI] [PubMed] [Google Scholar]
- Egert A. M., Kim D. H., Schrick F. N., Harmon D. L., and Klotz J. L.. 2014. Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature. J. Anim. Sci. 92:1768–1779. doi:10.2527/jas.2013-7141. [DOI] [PubMed] [Google Scholar]
- Engeland I. V., Andresen Ø., Ropstad E., Kindahl H., Waldeland H., Daskin A., and Eik L. O.. 1998. Effect of fungal alkaloidson the development and pregnancy and endocrine foetal-placental function in the goat. Anim. Reprod. Sci. 52:289–302. [DOI] [PubMed] [Google Scholar]
- Ferrell C. L., and Reynolds L. P.. 1992. Uterine and umbilical blood flows and net nutrient uptake by fetuses and uteroplacental tissues of cows gravid with either single or twin fetuses. J. Anim. Sci. 70:426–433. [DOI] [PubMed] [Google Scholar]
- Foote A. P., Harmon D. L., Brown K. R., Strickland J. R., McLeod K. R., Bush L. P., and Klotz J. L.. 2012. Constriction of bovine vasculature caused by endophyte-infected tall fescue seed extract is similar to pure ergovaline. J. Anim. Sci. 90:1603–1609. doi:10.2527/jas.2011-4513. [DOI] [PubMed] [Google Scholar]
- Ford S. P. 1995. Control of blood flow to the gravid uterus of domestic livestock species. J. Anim. Sci. 73:1852–1860. [DOI] [PubMed] [Google Scholar]
- Greatorex J. C., and Mantle P. G.. 1974. Effect or rye ergot on the pregnant sheep. J. Reprod. Fertil. 37:33–41. [DOI] [PubMed] [Google Scholar]
- Hellemans J., Mortier G., De Paepe A., Speleman F., and Vandesompele J.. 2007. Qbase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8:R19. doi:10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H. E., and Goldstein D. A.. 1988. Birth defects following maternal exposure to ergotamine, beta blockers, and caffeine. J. Med. Genet. 25:396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indänpään-Heikkilä J. E., and Schoolar J. C.. 1969. LSD: Autoradiographic study of the placental transfer and tissue distribution in mice. Science. 164:1295–1297. [DOI] [PubMed] [Google Scholar]
- Isla M., and Dyer D. C.. 1990. Characterization of alpha-adrenoceptors in the late pregnant ovine uterine artery. Eur. J. Pharmacol. 178:321–331. [DOI] [PubMed] [Google Scholar]
- Ji H., Fannin F., Klotz J., and Bush L.. 2014. Tall fescue seed extraction and partial purification of ergot alkaloids. Front. Chem. 2:110. doi:10.3389/fchem.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Harmon D. L., Flythe M. D., and Klotz J. L.. 2015. Interaction of isoflavones and endophyte-infected tall fescue seed extract on vasoactivity of bovine mesenteric vasculature. Front. Nutr. 2:32. doi:10.3389/fnut.2015.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L. 2015. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins (Basel). 7:2801–2821. doi:10.3390/toxins7082801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Bussard J. R., Foote A. P., Harmon D. L., Goff B. M., Schrick F. N., and Strickland J. R.. 2016. Vasoactivity and vasoconstriction changes in cattle related to time off toxic endophyte-infected tall fescue. Toxins (Basel). 8(271):1–19. doi:10.3390/toxins8100271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Egert-McLean A. M., Schrick F. N., Chattopadhyay N., and Harmon D. L.. 2018. Effects of grazing different ergovaline concentrations on vasoactivity of bovine lateral saphenous vein. J. Anim. Sci. 96:3022–3030. doi:10.1093/jas/sky163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Johnson J. M., Brown K. R., Bush L. P., and Strickland J. R.. 2013. Antagonism of lateral saphenous vein serotonin receptors from steers grazing endophyte-free, wild-type, or novel endophyte-infected tall fescue. J. Anim. Sci. 91:4492–4500. doi:10.2527/jas.2012-5896. [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Brown K. R., Xue Y., Matthews J. C., Boling J. A., Burris W. R., Bush L. P., and Strickland J. R.. 2012. Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 90:682–693. doi:10.2527/jas.2011-4323. [DOI] [PubMed] [Google Scholar]
- Klotz, J. L. and K. J. McDowell. 2017. Tall fescue ergot alkaloids are vasoactive in equine vasculature. J. Anim. Sci. 95:5151–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist K. H., and Grauwiler J.. 1973. Transplacental passage of 3H-ergotamine in the rat, and the determination of the intra-amniotic embryotoxicity of ergotamine. Experientia (Basel). 29:764. [Google Scholar]
- Lyons P. C., Plattner R. D., and Bacon C. W.. 1986. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 232:487–489. [DOI] [PubMed] [Google Scholar]
- Oliver J. W., Strickland J. R., Waller J. C., Fribourg H. A., Linnabary R. D., and Abney L. K.. 1998. Endophytic fungal toxin effect on adrenergic receptors in lateral saphenous veins (cranial branch) of cattle grazing tall fescue. J. Anim. Sci. 76:2853–2856. [DOI] [PubMed] [Google Scholar]
- Pesqueira A., Harmon D. L., Branco A. F., and Klotz J. L.. 2014. Bovine lateral saphenous veins exposed to ergopeptine alkaloids do not relax. J. Anim. Sci. 92:1213–1218. doi:10.2527/jas.2013-7142. [DOI] [PubMed] [Google Scholar]
- Poole D. H., Lyons S. E., Poole R. K., and Poore M. H.. 2018. Ergot alkaloids induce vasoconstriction of bovine uterine and ovarian blood vessels. J. Anim. Sci. 96:4812–4822. doi:10.1093/jas/sky328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. K., and Thompson F. N. Jr. 1992. Effects of fescue toxicosis on reproduction in livestock. J. Anim. Sci. 70:1594–1603. [DOI] [PubMed] [Google Scholar]
- Schöning C., Flieger M., and Pertz H. H.. 2001. Complex interaction of ergovaline with 5-HT2A, 5-HT1B/1D, and alpha1 receptors in isolated arteries of rat and guinea pig. J. Anim. Sci. 79:2202–2209. [DOI] [PubMed] [Google Scholar]
- Strickland J. R., Looper M. L., Matthews J. C., Rosenkrans C. F. Jr, Flythe M. D., and Brown K. R.. 2011. Board-invited review: St. Anthony’s fire in livestock: causes, mechanisms, and potential solutions. J. Anim. Sci. 89:1603–1626. doi:10.2527/jas.2010-3478. [DOI] [PubMed] [Google Scholar]
- Trotta R. J., Harmon D. L., and Klotz J. L.. 2018. Interaction of ergovaline with serotonin receptor 5-HT2A in bovine ruminal and mesenteric vasculature. J. Anim. Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., and Speleman F.. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unett D. J., Gatlin J., Anthony T. L., Buzard D. J., Chang S., Chen C., Chen X., Dang H. T., Frazer J., Le M. K., et al. 2013. Kinetics of 5-HT2B receptor signaling: profound agonist-dependent effects on signaling onset and duration. J. Pharmacol. Exp. Ther. 347:645–659. doi:10.1124/jpet.113.207670. [DOI] [PubMed] [Google Scholar]
- Wilbanks S. K., Kojima C. J., Britt J. L., Schrick F. N., and Duckett S. K.. 2018. Does genotype play a role in resistance to fescue toxicosis in ovine? Sm. Rum. Res. (Submitted). [Google Scholar]
- Wu G., Bazer F. W., Wallace J. M., and Spencer T. E.. 2006. Board-invited review: intrauterine growth retardation: implications for the animal sciences. J. Anim. Sci. 84:2316–2337. doi:10.2527/jas.2006-156. [DOI] [PubMed] [Google Scholar]
- Yates S. G., and Powell R. G.. 1988. Analysis of ergopeptine alkaloids in endophyte-infected tall fescue. J. Agric. Food Chem. 36:337–340. doi:10.1021/jf00080a023 [Google Scholar]
- Zhang L., and Dyer D. C.. 1991. Characterization of alpha-adrenoceptors mediating contraction in isolated ovine umbilical vein. Eur. J. Pharmacol. 197:63–67. [DOI] [PubMed] [Google Scholar]
- Zhang L. B, and Dyer D. C.. 1990. Characterization of serotonergic receptors mediating contraction of ovine umbilical artery. J. Pharmacol. Exp. Ther. 255:233–239. [PubMed] [Google Scholar]