INTRODUCTION
Thyroid cancer is a common endocrine malignancy, which histologically includes follicular epithelial cell-derived thyroid cancer and para-follicular C cell-derived medullary thyroid cancer (MTC).1 The former consists of papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), and anaplastic thyroid cancer (ATC). PTC and FTC are the most common thyroid cancer, accounting for 80–90% and 5–10% of all thyroid malignancies, respectively. ATC and MTC are uncommon, each accounting for 2–3% of thyroid malignancies. PTC is further classified into several variants, including most commonly conventional PTC (CPTC), followed by follicular-variant PTC (FVPTC), tall-cell PTC (TCPTC) and a few other rare variants.1–3
PTC and FTC are differentiated thyroid cancer (DTC), which are generally indolent clinically with a low overall mortality but significant recurrence rate with the current treatments. Disease recurrence of thyroid cancer is associated with increased risk of patient morbidity and mortality. ATC can develop from preexisting DTC or de novo. Although ATC is uncommon, it is the most aggressive type of thyroid cancer, with a rapid lethality. There is also poorly differentiated thyroid cancer (PDTC), which can develop from DTC and has an intermediate clinical aggressiveness between DTC and ATC. PTC of tumor ≤1.0 cm is uniquely defined as papillary thyroid microcarcinoma (PTMC),4 which has an excellent clinical outcome in general but can be associated with poor prognosis and even mortality in some patients. The main goal in the clinical management of thyroid cancer is to prevent disease recurrence, patient morbidity and mortality while minimizing treatment-associated adverse consequences. Achievement of the optimal balance between therapeutic benefits and treatment complications rely on accurate risk stratification of thyroid cancer. This is currently pursued primarily based on the assessment of clinicopathological backgrounds, which has achieved considerably improved accuracy with comprehensive clinical approaches in recent years.5 Yet, controversies on how to optimally manage patients with thyroid cancer are still commonly encountered in clinical practice and in academic forums. The main challenge is that classical clinicopathological risk assessment is often inaccurate in thyroid cancer. An example is that clinically apparent low-risk thyroid cancer may turn out to be aggressive with poor outcomes, making clinical judgement and hence treatment decision making challenging. As a result, over-treatment of inherently low-risk thyroid cancer and under-treatment of potentially aggressive thyroid cancer are both common. Given the rapid increase in thyroid cancer incidence in recent decades and the currently large number of living patients with thyroid cancer,2,6 better risk stratification for more accurate management of thyroid cancer has become an even more important task than ever.
Molecular-based risk stratification of thyroid cancer has become an attractive strategy in guiding precision management of thyroid cancer in recent years.7 This is practically particularly important and promising for DTC given its high incidence2,6 and well-known prognostic molecular markers,1,7–14 which will be the main focus of the present discussion.
MAJOR ONCOGENIC GENETIC ALTERATOINS IN THYROID CANCER
Among the many genetic alterations in thyroid cancer, the prognostically most promising are oncogenic mutations, most prominently BRAF V600E and TERT promoter mutations and a few others. 1,7–14 The occurrence of these mutations has an excellent concordance between the primary DTC and the matched metastatic tumor, consistent with their role in the progression of thyroid cancer.15
1). BRAF V600E Mutation in Thyroid Cancer
The BRAF V600E mutation was initially found to exist in thyroid cancer in 2003,16–21 which occurred with a prevalence of about 45–50% in PTC, 25–30% in ATC, and none in FTC and benign thyroid neoplasm.13 This is the most common activating point mutation in the BRAF gene, resulting in a valine-to-glutamic acid change in the BRAF protein, causing constitutive activation of the BRAF protein kinase and hence oncogenic activation of the MAP kinase pathway.22 Over the last 15 years, numerous studies have devoted to the characterization of the prognostic value of this mutation.1,7,9,10,12–14,23 In 2005, a Johns Hopkins study for the first time demonstrated the association of BRAF V600E with poor clinical outcomes of PTC, including increased disease recurrence and radioactive iodine (RAI) refractoriness of recurrent tumors, in addition to aggressive pathological behaviors, such as extrathyroidal invasion and lymph node metastasis.24 Subsequent studies widely confirmed these findings although inconsistent studies were sometimes also reported.14,25 A multicenter study on 2,099 cases of PTC demonstrated that BRAF V600E had an independent prognostic value for the recurrence of PTC.26 A separate multicenter study on 1,849 patients with PTC showed a strong association between BRAF V600E and PTC-specific mortality.27 Overall, these and other studies demonstrate an important oncogenic role of BRAF V600E in the progression and aggressiveness of PTC.
2). TERT Promoter Mutations in Thyroid Cancer
The telomerase reverse transcriptase (TERT) was initially identified and characterized with a fundamental function to maintain the integrity of chromosomes by adding telomeres to their ends in early 1980’s.28,29 TERT is now widely known to also promote various cancer-hallmark cellular and molecular activities.30 These functions of TERT drive cell immortality and oncogenesis. Two somatic mutations, chr5:1,295,228C>T and chr5:1,295,250C>T (termed here as C228T and C250T, respectively), in the promoter of the TERT gene were found in melanoma31,32 and other cancers, including thyroid cancer.33 Both TERT promoter mutations are predicted to generate a consensus binding site for E-twenty-six (ETS) transcription factors and confer the TERT promoter increased transcriptional activities.31,32 GABPA, an ETS transcriptional factor, was demonstrated to selectively bind the mutant promoter of the TERT gene and promote the expression of TERT.34 Indeed, TERT promoter mutations were found to be associated with increased expression of mRNA and protein of TERT and telomere length.35 TERT C228T is far more common than TERT C250T in thyroid cancer and the two collectively occur in 10–15% DTC, 40–45% PDTC and ATC, and virtually none in benign thyroid neoplasm.8,11 TERT promoter mutations have been widely found to be associated with aggressive tumor behaviors and poor clinical outcomes of thyroid cancer; it occurs particularly common in aggressive DTC, PDTC, and ATC and is associated with increased recurrence and mortality of DTC.15,33,36–45 These studies suggest a strong oncogenic role of TERT promoter mutations in the development of aggressiveness of thyroid cancer.
3). Genetic Duet of BRAF V600E and TERT Promoter Mutations in Thyroid Cancer
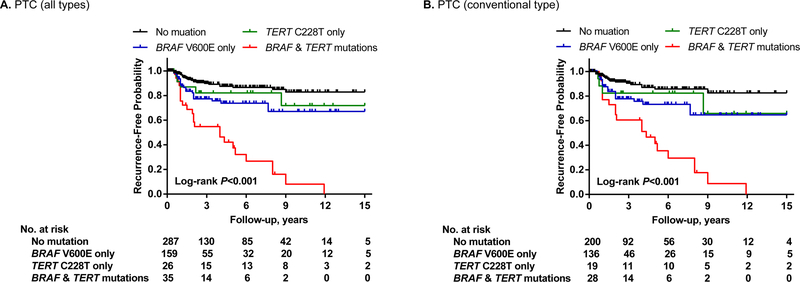
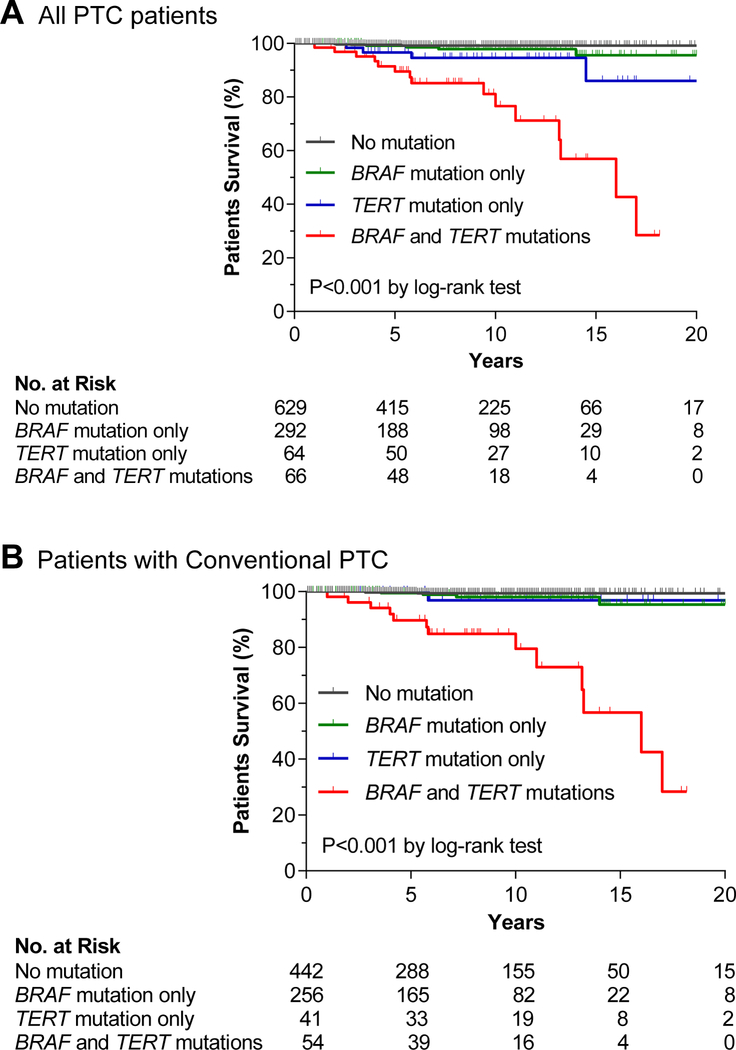
The initial 2013 study reporting TERT promoter mutations in thyroid cancer also reported an interesting association between BRAF V600E and TERT promoter mutations in PTC.33 This phenomenon has been widely confirmed in many other studies in both primary PTC and metastatic PTC.8,11,15 The prevalence of the genetic duet of BRAF V600E and TERT promoter mutations in primary PTC was 7.7 % (145/1892) on average.11 Several studies also reported an association between TERT promoter mutations and the BRAF V600E mutation in melanoma,31,46 suggesting that this is a general phenomenon in human cancer. Given the known oncogenic role of BRAF V600E and TERT promoter mutations each in thyroid cancer, it was speculated and demonstrated that this mutation duet was a robust genetic background for the most severe aggressiveness of PTC, hence predicting the worst clinicopathological outcomes of PTC.45 In this study, when the cohort was divided into four groups—no mutation, BRAF V600E, TERT promoter mutations, and the genetic duet of coexisting mutations, each mutation alone had a modest adverse effect while the mutation duet had a robust effect on the poor clinicopathological outcomes of PTC, including extrathyroidal invasion, lymph node metastasis, distant metastasis, and disease recurrence (Figure 1).45 This pattern of the effects of the genetic duet of BRAF V600E and TERT promoter mutations was similarly observed with PTC-related patient mortality,44 which was fully confirmed in an expanded large study on 1,051 patients with PTC, showing a robust synergistic adverse effect of the genetic duet on PTC-specific patient survival; the mortality was virtually zero in the absence of either mutation, slightly increased with either mutation alone, and robustly increased with the genetic duet (Figure 2).39 Many subsequent studies demonstrated a similarly robust synergistic adverse effect of the genetic duet of BRAF V600E and TERT promoter mutations on clinicopathological outcomes of thyroid cancer.15,37,42,47,48 This remarkable clinical finding of the genetic duet was beautifully explained by a novel molecular mechanism described recently.49 In this mechanism, the BRAF V600E constitutively activates the MAP kinase pathway, causing the phosphorylation and activation of FOS, which, as a novel transcriptional factor for the GABPB gene, binds and activates the promoter of GABPB and up-regulates its expression. GABPB, at elevated level, drives the formation of GABPA/GABPB complex, which in turn selectively binds and activates the mutant TERT promoter and robustly promotes the expression of TERT, conferring strong TERT oncogenicity. This represents a robust molecular mechanism underpinning the synergism between the BRAF V600E and TERT promoter mutations in driving the aggressiveness and poor clinical outcomes of thyroid cancer.
Figure 1. Impacts of BRAF V600E or TERT promoter mutation alone or their coexistence on disease-free survival of patients with papillary thyroid cancer (PTC).
Kaplan-Meier analyses of 507 patients with PTC were performed. Panel A shows the analysis results of patients with PTC of all types and Panel B shows the analysis results of patients with conventional-variant PTC only. Four groups of patients were included in each panel, including patients with neither mutation (black line), TERT mutation only (green line), BRAF V600E mutation only (blue line), and the genetic duet of the two coexisting mutations (red line). Adapted from Xing et al. J Clin Oncol. 2014;32(25):2718–26 (Reference 45) with permission.
Figure 2. Impacts of BRAF V600E or TERT promoter mutation alone or their coexistence on disease-specific survival of patients with papillary thyroid cancer (PTC).
Kaplan-Meier analyses of 1,051 patients with PTC were performed. Panels A and B show the analysis results of patients with PTC of all types and only patients with conventional-variant PTC, respectively. In each panel, the patients were divided into four genotype groups— no mutation (black line), BRAF V600E only (green line), TERT promoter mutation only (blue line), and genetic duet of the two coexisting mutations (red line). Adapted from Liu R et al. JAMA Oncol. 2017;3(2):202– 208 (Reference 39) with permission.
4). RAS Mutation and Its Genetic Duet with TERT Promoter Mutation in Thyroid Cancer
RAS mutations are common in follicular thyroid neoplasms, occurring in about 20–25% follicular thyroid adenoma, 30–45% FVPTC, 30–45% FTC, 20–40% PDTC and ATC, and rarely in CPTC and virtually none in TCPTC.1 A significant association between RAS and TERT promoter mutations in thyroid cancer was reported in a number of studies, particularly in FTC and FVPTC.15,42,48 This genetic duet of RAS and TERT promoter mutations was also shown to be associated with poor clinical outcomes of thyroid cancer, including robustly increased disease recurrence and patient mortality.15,42,48 Some previous studies showed an association between RAS mutation and poor clinicopathological outcomes in FTC50 and PDTC.51,52 It is possible that this effect of RAS mutation was actually the effect of the genetic duet of RAS and TERT promoter mutations since RAS mutation alone often occurs in benign follicular adenoma and low-risk DTC. TERT promoter mutations are fairly common in FTC and very common in PDTC and ATC.8,11,15 The genetic duet of RAS and TERT promoter mutations is thus probably common and constitutes an important genetic background for the aggressiveness of these cancers. In fact, the duet of RAS and TERT promoter mutations were shown to be highly concordant between primary and matched metastatic FTC and associated with ominous clinical outcomes.15 The molecular mechanism for this synergism between RAS and TERT promoter mutations in driving thyroid cancer aggressiveness remain to be elucidated, but it likely involves also the MAP kinase/ROS/GABP/TERT pathway used by the duet of BRAF V600E and TERT promoter mutations since RAS can also activate the MAP kinase pathway albeit less robustly. As RAS and BRAF mutations are mutually exclusive,1,13 their synergism with TERT promoter mutations represents a complementary mechanism of the two mutations to each other in driving the aggressiveness of thyroid cancer.
5). Other Oncogenic Genetic Alterations in Thyroid Cancer
There are some other oncogenic genetic alterations that play a role in thyroid cancer aggressiveness and may potentially have prognostic values. Among them are TP53, EIF1A and β-catenin mutations, which occur commonly in PDTC and ATC, but very rarely in DTC.53–60 This distribution pattern suggests that these genetic alterations are involved in the development of aggressiveness of thyroid cancer and their occurrence, if ever, in DTC likely has a strong prognostic power for poor prognosis. However, the rareness of these mutations in DTC suggests that their clinical utility in the risk stratification of DTC is practically limited.
Genomic and large-scale genetic studies in recent years have unveiled overwhelming new findings in thyroid cancer.54,59,60 Large-scale epigenetic studies have similarly uncovered tremendous molecular information in thyroid cancer, such as aberrant miRNA and methylation.23,54,61 These are now bringing in a potentially exciting opportunity for discovering new prognostic molecular markers in thyroid cancer. Nevertheless, the biological functions of the new genomic/genetic/epigenetic molecular changes in thyroid cancer are largely undefined. Unlike BRAF V600E and TERT promoter mutations, most of these new molecular markers have limited clinical prognostic potential in thyroid cancer. Some of the common genetic rearrangements, such as RET/PTC and PAX8/PPARγ, have no apparent impact on the development of aggressiveness of thyroid cancer and thus have a limited prognostic value.1,13
CLINICAL APPLICATION OF PROGNOSTIC GENETIC MARKERS IN THYROID CANCER
Given the strong association of some of the oncogenic genetic markers, particularly BRAF V600E and TERT promoter mutations, with poor clinical outcomes and their relatively common occurrence, their prognostic application in thyroid cancer has robust clinical utility. However, depending on the genetic type and clinical setting, these mutations do not necessarily always herald ominous outcomes of thyroid cancer. Therefore, their prognostic use should not be indiscriminative but should be tailored to meet individualized need in specific clinical settings. A good utility of prognostic genetic markers is that they can be “tiebreaker” when a physician is faced with ambiguity from conventional clinicopathological risk assessment in treatment decision making for thyroid cancer. In this context, it is also important to emphasize that the high negative predictive values (NPVs) of these genetic markers for poor prognosis of thyroid cancer make a negative genetic test equally valuable. The following specific clinical settings exemplify the clinical application of prognostic genetic markers, particularly BRAF V600E and TERT promoter mutations, in thyroid cancer.
1). General Consideration of Clinical Application of High-risk Prognostic Genetic Markers
From the above discussion on the robust oncogenic role of the high-risk genetic alterations in the aggressiveness of thyroid cancer, including high patient mortality, it is reasonable to recommend that their existence in DTC should generally favor more aggressive treatment. These include the genetic duet of BRAF V600E and TERT promoter mutations, the genetic duet of RAS and TERT promoter mutations, and the rare TP53, EIF1A and β-catenin mutations. Specifically, it is reasonable to recommend total thyroidectomy for all patients with DTC harboring such genetic markers, even in cases of PTMC. Therapeutic and prophylactic neck dissections and RAI ablation will be usually reasonable in such patients. As only 7.7% of PTC on average harbor the genetic duet of BRAF V600E and TERT promoter mutations,11 if guided by this genetic duet, less than 10% of patients with PTC overall will be treated relatively aggressively. As high-risk genetic alterations are very rare in PTMC, only a small number of patients with PTMC will be treated aggressively based on them. In PTC >1.0–2.0 cm, particularly tumors >2.0 cm, prophylactic central neck dissection may be reasonable given the fact that large tumors are associated with increased disease recurrence62 and the high-risk genetic alterations are often associated with RAI refractoriness of recurrent disease,1,24,63 thus making initial elimination of metastatic lymph nodes particularly important. Patients with FTC harboring high-risk genetic alterations, such as TP53, EIF1A, and β-catenin mutations as well as the genetic duet of RAS and TERT promoter mutations, similarly favor aggressive treatments.
2). Clinical Application of BRAF V600E in Solitary Intrathyroidal PTC
It is generally acceptable to treat clinically apparent invasive/metastatic thyroid cancer with relatively aggressive approaches, such as total thyroidectomy and, in appropriate settings, therapeutic/prophylactic neck dissections and radioiodine ablation. However, how to treat intrathyroidal PTC—PTC without extrathyroidal invasion, lymph node metastasis, and distant metastasis—is not completely agreeable among physicians. The current American Thyroid Association (ATA) guidelines recommend lobectomy as an alternative option to total thyroidectomy for solitary intrathyroidal PTC (SI-PTC) of 1–4 cm in size.5 This recommendation is somehow controversial as it may not be a straightforward effort to decide lobectomy versus total thyroidectomy in an individual patient as such apparently clinically low-risk thyroid cancer may not uniformly turn out to be free of poor clinical outcomes. A recent multicenter study shed promise on tackling this dilemma using BRAF V600E as a prognostic genetic marker to guide better risk stratification in such patients.62 In this study, the recurrence rates of SI-PTC >1.0 cm, particularly tumors >2.0 cm, were 20–30% versus recurrence rates of only 2–3% in matched wild-type BRAF SI-PTC, with NPVs of BRAF V600E for recurrence being 97–98% or 100% if only structural recurrence was considered. Remarkably, this was true even in SI-PTC >4.0 cm. For intrathyroidal PTMC, recurrence rates were both low in BRAF V600E patients and wild-type BRAF patients, being only 1–2%. Given these findings, decision making on SI-PTC can now be more accurate if BRAF status is included in prognostic consideration: it is reasonable to treat wild-type BRAF SI-PTC of any tumor size, even tumor >4.0 cm, with thyroid lobectomy; BRAF V600E-positive SI-PTC >1.0 cm, particularly tumors >2.0 cm, should be treated aggressively—with total thyroidectomy and prophylactic neck dissection, followed by RAI ablation if indicated. BRAF mutation-positive SI-PTC >1.0 cm and ≤4.0 cm account for 23.1% and mutation-positive SI-PTC >2.0cm and ≤4.0 cm account for 8.3% of all cases of SI-PTC. Thus, only a small minority of SI-PTC need to be treated aggressively. Given the low recurrence rates, SI-PTC <2.0 cm, particularly tumors <1.0 cm (i.e., low-risk PTMC), may be treated with lobectomy regardless of the BRAF status. With this BRAF status-guided strategy, the vast majority of SI-PTC patients can be treated with thyroid lobectomy only, which is associated with fewer surgical complications and a good chance of patient staying euthyroid, obviating the need for life-long thyroid hormone replacement.
3). Clinical Application of BRAF V600E in Clinically Low-risk PTMC
It is widely agreeable that clinically aggressive PTMC should be generally treated as for large PTC. Controversy exists, however, on how to treat clinically low-risk PTMC— PTMC without extrathyroidal extension, lymph node metastasis, distant metastasis and other aggressive features. Most physicians currently favor surgery as a preferred treatment option for such PTMC, usually lobectomy.5 In recent years, non-surgical active surveillance has become an alternative option acceptable to some physicians for clinically low-risk PTMC.5 This is primarily based on several prospective Japanese studies showing that clinically low-risk PTMC in the vast majority of patients remained indolent without serious clinical consequence after active surveillance for an average of 5–6 years.64–66 Yet, it has been well known that a small percentage of cases of PTMC present with significant tumor aggressiveness and even patient mortality and all large PTC have grown from PTMC. A major challenge is that no clinical features can reliably differentiate the relatively small number of patients with PTMC with disease destined to progress from the larger population of patients harboring inherently indolent PTMCs. Thus, there is concern on whether indiscriminate active surveillance of all clinically low-risk PTMC, particularly for long term (e.g., decades), is a reasonable strategy as serious disease progression, such as metastasis, may occur even if tumor size may remain relatively stable.
Knowledge of the BRAF status may now help facilitate decision making on such PTMC. As discussed above, mortality risk is extremely low in wild-type BRAF PTC in general regardless of tumor size and even lower in clinically low-risk PTMC. Even recurrence rate is extremely low in clinically low-risk wild-type BRAF PTC, including PTMC.62 Thus, active surveillance for clinically low-risk wild-type BRAF PTMC seems to be reasonable and has the advantage of avoiding surgical complications and preserving normal thyroid functions. Given the lack of specific long-term prospective data, it is less clear, however, whether long-term active surveillance is reasonable for clinically low-risk but BRAF V600E-positive PTMC. The recent study on SI-PTC showed no significant impact of BRAF V600E on clinical outcomes of clinically low-risk PTMC treated with thyroidectomy.62 It is possible, however, that because surgical removal of PTMC at an early stage before BRAF V600E had sufficient time to exert adverse effects, there were no serious clinical consequences, even recurrence. The outcomes of increase in tumor size may potentially be problematic in the presence of BRAF V600E. As discussed above, in clinically low-risk SI-PTC, BRAF V600E was associated with robustly increased recurrence in tumors >1.0 cm, particularly tumors >2.0 cm, even after total thyroidectomy.62 Theoretically, if giving sufficient time, BRAF V600E may cause consequences, such as metastasis. Currently, there is no consensus on what is the appropriate size PTMC can be allowed to grow to before triggering thyroidectomy during active surveillance. If allowing BRAF V600E-positive but clinically apparent low-risk PTMC to grow to >2.0 cm, there may be a substantially increased risk of serious consequence, such as incurable RAI-refractory metastatic disease and even perhaps mortality. In contrast, some increase in tumor size (e.g., >3 mm), which could trigger a decision to operate PTMC in patients under currently suggested active surveillance, 64–66 may not be as worrisome as currently believed if BRAF mutation is absent.
There is a well-known synergism between BRAF V600E and invasive tumor behaviors (e.g., extrathyroidal invasion, lymph node metastasis and distant metastasis) in adversely affecting PTC-related mortality.27 Thus, surgical treatment rather than active surveillance of BRAF V600E-positive and clinically low-risk PTMC before the mutation has sufficient time to exert adverse actions seems to be reasonable. In such case, however, thyroid lobectomy may be just sufficient at this early stage of the disease when BRAF V600E does not exert adverse actions yet.62 This seems to be practical as only a small minority of thyroid cancers are clinically low-risk PTMC harboring BRAF V600E mutation.62 As discussed in the early section above, clinically low-risk PTMC, if ever found to harbor high-risk genetic alterations (e.g., duets of BRAF V600E/RAS and TERT promoter mutations, TP53, EIF1A and β-catenin mutations, etc), should be most reasonably treated relatively aggressively (e.g., total thyroidectomy).
4). Clinical Application of Prognostic Genetic Markers in FTC
Unlike PTC in which the prognostic value of BRAF V600E has been well characterized, molecular prognostication has been less well defined in FTC. Nevertheless, a few genetic markers are prognostically promising for FTC. RAS mutation was previously reported to be associated with aggressive behaviors of FTC.50 RAS and TERT promoter mutations are both common in FTC and there is a significant association between them.11,15,42,48 In analogy with the robust prognostic value of the genetic duet of BRAF V600E and TERT promoter mutations in PTC, it is possible that the genetic duet of RAS and TERT promoter mutations may have an important prognostic value in FTC. In fact, this has been well demonstrated in DTC, including FTC, in several studies.15,42,48 Therefore, the duet of RAS and TERT promoter mutations is an important genetic background and prognostic genetic pattern for poor clinical outcomes of FTC. It is thus reasonable to aggressively treat FTC harboring the genetic duet of RAS and TERT promoter mutations, including, for example, total thyroidectomy and RAI ablation. Other high-risk genetic alterations, such as TP53, EIF1A and β-catenin mutations, if every found, should be treated as important genetic predictors for poor clinical outcomes of FTC and favor aggressive treatment.
5). Application of Prognostic Genetic Markers in Predicting Radioiodine Refractoriness of Thyroid Cancer
Since it was first reported in 2005 that BRAF V600E was associated with recurrence of PTC and RAI refractoriness and hence incurability of recurrent PTC,24 numerous studies have confirmed this finding and demonstrated impaired or even absent expression of the thyroid iodide-metabolizing genes—genes for thyroid-stimulating hormone receptor (TSHR), sodium/iodide symporter, thyroperoxidase, thyroglobulin (Tg), thyroid transcription factor 1, and PAX8 transcription factor—in PTC harboring BRAF V600E.1,7,13,14 A direct functional link between BRAF V600E and the impairment of thyroid iodide-metabolizing gene expression was the demonstration that introduced expression of BRAF V600E in normal thyroid cells caused silencing of thyroid iodide-metabolizing genes; cessation of the expression of BRAF V600E or inhibition of the BRAF/MAP kinase pathway could restore the expression of thyroid genes.67 This was later recapitulated in animal models68 and even human patients—treatment of patients carrying RAI-refractory PTC with an inhibitor of the MAP kinase pathway could restore RAI avidity of the tumor.69 Recent studies demonstrated that TERT promoter mutations may also be associated with impairment or loss of RAI avidity and, in fact, the genetic duet of BRAF V600E and TERT promoter mutations was most robustly associated with RAI refractoriness of metastatic PTC.63 Pediatric PTC is generally highly curable with RAI treatment, consistent with its low prevalence of BRAF V600E13 and very rare occurrence of TERT promoter mutations.70 Since BRAF V600E is associate with increased risk of RAI refractoriness of recurrent disease of PTC, mostly recurrent metastatic lymph nodes, a positive test for BRAF V600E, particularly the genetic duet of BRAF V600E and TERT promoter mutations, favors thorough therapeutic neck dissection and, in appropriate cases, prophylactic neck dissection, to minimize the risk of the development of RAI-refractory recurrent disease. Prophylactic neck dissection is particularly applicable to BRAF mutation-positive PTC >2.0 cm as discussed in the above sections. However, even with impairment of RAI avidity, BRAF V600E-positive PTC still should be treated with RAI ablation when clinically indicated as RAI avidity loss is often partial. Also, as the mutation-positive cancer has an increased risk for disease recurrence and even mortality, patients need to be effectively monitored in the follow-up surveillance following total thyroidectomy. Thus, reliable specific Tg test is particularly important, which is made possible with RAI ablation of normal thyroid tissues. It is not clear at this time whether a higher dose of RAI may be beneficial to BRAF V600E-positive PTC given the impairment of RAI avidity in such cancer.
6). Application of Prognostic Genetic Markers in DTC Already with Clinically Apparent Aggressiveness
Genetic markers may even be prognostically useful in DTC that already shows clinically apparent aggressiveness, such as extrathyroidal invasion, lymph nodes metastasis and distant metastasis. Even though aggressive initial treatment is generally needed in such patients, the outcomes from the current standard treatments may be different depending on the genotype of BRAF and TERT in the tumor. In the absence of BRAF V600E and TERT promoter mutations, the mortality of PTC, particularly CPTC and FVPTC, is extremely low, while, in contrast, the genetic duet of BRAF V600E and TERT promoter mutations was associated with robustly high mortality in a manner independent of conventional clinicopathological risk factors.39 The genetic duet of RAS and TERT promoter mutations has similar prognostic value.15,42,48 Also, as discussed above, these mutations are strongly associated with loss of RAI avidity and hence often treatment failure of recurrent disease. Thus, even the initial clinical presentation of the tumor aggressiveness is similar in patients, the disease prognosis can be different in such patients depending on whether there are these mutations. Therefore, these genetic markers still have values in assisting prognostic evaluation of clinically apparent aggressive DTC. Existence of these genetic markers implies increased risk of therapeutic failure of the current treatment and emphasizes the importance of complete eradication of the disease in the initial treatment and the need for subsequent vigilant active surveillance for disease recurrence.
7). Potential Prognostic Value of Genetic Markers in ATC and PDTC
Although ATC is generally an extremely aggressive cancer, its aggressiveness seems to be differentiable by certain genetic patterns. For example, TERT promoter mutation-positive ATC was found to be more commonly associated with distant metastasis,43 which is likely to be translated into accelerated mortality. The genetic duet of BRAF V600E and TERT promoter mutations are likely to represent a genetic background for more aggressiveness of ATC. RAS mutation was reported to be associated with increased aggressiveness of PDTC.51,52 It is likely that this effect of RAS mutation originates from the genetic duet of RAS and TERT promoter mutations. Therapeutically, BRAF V600E-positive ATC has recently been demonstrated to have a remarkable response to the combination treatment with BRAF V600E and MEK inhibitors,71 promoting an expedited approval by FDA of the combination use of the BRAF V600E inhibitor dabarafenib and the MEK inhibitor trametinib for the treatment of BRAF V600E-positive ATC. Thus, testing of certain genetic markers in ATC may have both prognostic and predictive values. This may likely become true also for PTC.
8). Special Consideration of RAS Mutation in Several Clinical Settings
As RAS mutations can occur in both benign and malignant thyroid neoplasms, its value as a diagnostic and prognostic marker by itself had been uncertain until recently.72 It has been recently demonstrated that long-term follow-up of cytologically benign but RAS mutation-positive thyroid nodules had no clinical consequence and RAS mutation alone in DTC was usually associated with low-risk disease with excellent clinical outcomes.73 As discussed above, it is the genetic duet of RAS and TERT promoter mutations that is robustly associated with poor clinical outcomes of DTC.15,42,48 Given these and other studies, the following recommendations as recently proposed72 may be reasonable to consider for thyroid neoplasms that are negative for other high-risk genetic markers (e.g., TERT promoter mutation) unless clinically suggested otherwise: 1) Cytolologically benign but RAS mutation-positive thyroid nodules can be non-surgically managed with long-term surveillance; 2) RAS mutation-positive thyroid nodules with cytological atypia of undetermined significance or follicular neoplasm may be treated surgically with limited extent (e.g., lobectomy); 3) Clinically low-risk but RAS mutation-positive DTC can be generally treated with limited extent (e.g., lobectomy).
SUMMARY
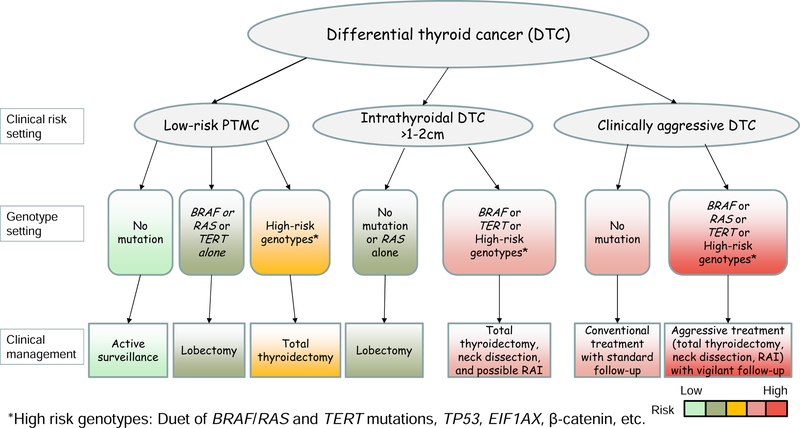
The value of prognostic genetic marker-based risk stratification and precision management of thyroid cancer is widely appreciated. Well characterized and clinically applicable prognostic genetic markers are best exemplified by BRAF V600E and TERT promoter mutations. The genetic duet of BRAF V600E/RAS and TERT promoter mutations has particularly robust prognostic power for poor clinical outcomes of DTC. The negative predictive values of these prognostic genetic markers are equally important clinically. Appropriate application of these prognostic markers can particularly improve the precision of management decision making for thyroid cancer in currently controversial areas, such as thyroid lobectomy versus total thyroidectomy, prophylactic neck discussion versus no neck dissection, RAI ablation versus no RAI ablation, and non-surgical active surveillance versus surgical treatment. A suggested approach for the clinical application of prognostic genetic markers in DTC is illustrated in Figure 3. In this approach, the best prognostic utility of the genetic markers in guiding the treatment of thyroid cancer can be achieved in a clinical risk level-based and genotype-individualized manner.
Figure 3. Summary of the clinical application of major prognostic genetic markers in thyroid cancer.
The diagram shows how thyroid cancer at different clinical risk levels in various clinical settings can be appropriately treated with the guidance of specific individual genotypes. DTC, differentiated thyroid cancer; PTMC, papillary thyroid microcarcinoma; RAI, radioactive iodine. *High-risk genotypes include the duet of BRAF V600E/RAS and TERT promoter mutations, TP53, EIF1A, β-catenin mutations, etc.
KEY POINTS.
-
1)
Controversies exist on how to optimally manage thyroid cancer as the prognosis is often uncertain based on clinical backgrounds.
-
2)
Prognostic genetic markers in thyroid cancer, exemplified by BRAF V600E and TERT promoter mutations, have been well characterized and widely appreciated.
-
3)
The genetic duet of BRAF V600E/RAS and TERT promoter mutations are most robust prognostic genetic patterns for poor prognosis of differentiated thyroid cancer.
-
4)
The high negative predictive values of the prognostic genetic markers are equally valuable.
-
5)
The best prognostic value of genetic markers in thyroid cancer is achieved through a clinical risk level-based and genotype-individualized manner.
Acknowledgments
Funding Support:
This work was supported by U.S.A. National Institutes of Health (NIH) grants R01CA215142 and R01CA189224 to M Xing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure:
Mingzhao Xing receives royalties as co-holder of a licensed USA patent related to BRAF V600E mutation in thyroid cancer.
REFERENCES
- 1.Xing M Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13(3):184–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer 2016;23(4):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi X, Liu R, Basolo F, et al. Differential Clinicopathological Risk and Prognosis of Major Papillary Thyroid Cancer Variants. J Clin Endocrinol Metab 2016;101(1):264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd RV, Osamura RY, Klöppel G, Rosai J, eds. WHO classification of tumours of endocrine organs (4th ed). Lyon, France: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 5.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 7.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013;381(9871):1058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alzahrani AS, Alsaadi R, Murugan AK, Sadiq BB. TERT Promoter Mutations in Thyroid Cancer. Horm Cancer 2016;7(3):165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Cruz AK, Vaish R, Vaidya A, et al. Molecular markers in well-differentiated thyroid cancer. Eur Arch Otorhinolaryngol 2018;275(6):1375–84. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Chen G, Sheng C, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a meta-analysis. Endocr Relat Cancer 2015;22(2):159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer 2016;23(3):R143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine (Baltimore) 2012;91(5):274–86. [DOI] [PubMed] [Google Scholar]
- 13.Xing M BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005;12(2):245–62. [DOI] [PubMed] [Google Scholar]
- 14.Xing M BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007;28(7):742–62. [DOI] [PubMed] [Google Scholar]
- 15.Sohn SY, Park WY, Shin HT, et al. Highly Concordant Key Genetic Alterations in Primary Tumors and Matched Distant Metastases in Differentiated Thyroid Cancer. Thyroid 2016;26(5):672–82. [DOI] [PubMed] [Google Scholar]
- 16.Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95(8):625–7. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene 2003;22(41):6455–7. [DOI] [PubMed] [Google Scholar]
- 18.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003;63(7):1454–7. [PubMed] [Google Scholar]
- 19.Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab 2003;88(9):4393–7. [DOI] [PubMed] [Google Scholar]
- 20.Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 2003;22(29):4578–80. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Quiros RM, Gattuso P, Ain KB, Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res 2003;63(15):4561–7. [PubMed] [Google Scholar]
- 22.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417(6892):949–54. [DOI] [PubMed] [Google Scholar]
- 23.de la Chapelle A, Jazdzewski K. MicroRNAs in thyroid cancer. J Clin Endocrinol Metab 2011;96(11):3326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 2005;90(12):6373–9. [DOI] [PubMed] [Google Scholar]
- 25.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65(3):364–8. [DOI] [PubMed] [Google Scholar]
- 26.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 2015;33(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013;309(14):1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985;43(2 Pt 1):405–13. [DOI] [PubMed] [Google Scholar]
- 29.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell 1982;29(1):245–55. [DOI] [PubMed] [Google Scholar]
- 30.Low KC, Tergaonkar V. Telomerase: central regulator of all of the hallmarks of cancer. Trends Biochem Sci 2013;38(9):426–34. [DOI] [PubMed] [Google Scholar]
- 31.Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013;339(6122):959–61. [DOI] [PubMed] [Google Scholar]
- 32.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science 2013;339(6122):957–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer 2013;20(4):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell RJ, Rube HT, Kreig A, et al. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 2015;348(6238):1036–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borah S, Xi L, Zaug AJ, et al. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science 2015;347(6225):1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bu R, Siraj AK, Divya SP, et al. Telomerase reverse transcriptase mutations are independent predictor of disease-free survival in Middle Eastern papillary thyroid cancer. Int J Cancer 2018;142(10):2028–39. [DOI] [PubMed] [Google Scholar]
- 37.Jin L, Chen E, Dong S, et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: a study of 653 patients. Oncotarget 2016;7(14):18346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab 2013;98(9):E1562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol 2017;3(2):202–8. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Qu S, Liu R, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab 2014;99(6):E1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab 2014;99(5):E754–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen X, Liu R, Xing M. A six-genotype genetic prognostic model for papillary thyroid cancer. Endocr Relat Cancer 2017;24(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi X, Liu R, Qu S, et al. Association of TERT promoter mutation 1,295,228 C>T with BRAF V600E mutation, older patient age, and distant metastasis in anaplastic thyroid cancer. J Clin Endocrinol Metab 2015;100(4):E632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing M, Liu R, Bishop J. TERT promoter and BRAF mutations cooperatively promote papillary thyroid cancer-related mortality. Thyroid 2014;24(S1):A-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol 2014;32(25):2718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griewank KG, Murali R, Puig-Butille JA, et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst 2014;106(9):pii: dju246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rusinek D, Pfeifer A, Krajewska J, et al. Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients. Int J Mol Sci 2018;19(9):2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song YS, Lim JA, Choi H, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 2016;122(9):1370–9. [DOI] [PubMed] [Google Scholar]
- 49.Liu R, Zhang T, Zhu G, Xing M. Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer. Nat Commun 2018;9(1):579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fukahori M, Yoshida A, Hayashi H, et al. The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid 2012;22(7):683–9. [DOI] [PubMed] [Google Scholar]
- 51.Garcia-Rostan G, Zhao H, Camp RL, et al. ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 2003;21(17):3226–35. [DOI] [PubMed] [Google Scholar]
- 52.Volante M, Rapa I, Gandhi M, et al. RAS mutations are the predominant molecular alteration in poorly differentiated thyroid carcinomas and bear prognostic impact. J Clin Endocrinol Metab 2009;94(12):4735–41. [DOI] [PubMed] [Google Scholar]
- 53.Alzahrani AS, Murugan AK, Qasem E, et al. Absence of EIF1AX, PPM1D, and CHEK2 mutations reported in Thyroid Cancer Genome Atlas (TCGA) in a large series of thyroid cancer. Endocrine 2018. doi: 10.1007/s12020-018-1762-6 [Epub ahead of print] [DOI] [PubMed]
- 54.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014;159(3):676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donghi R, Longoni A, Pilotti S, Michieli P, Della Porta G, Pierotti MA. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest 1993;91(4):1753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang SH, Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest 1993;91(1):179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Rostan G, Camp RL, Herrero A, Carcangiu ML, Rimm DL, Tallini G. Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 2001;158(3):987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia-Rostan G, Tallini G, Herrero A, D’Aquila TG, Carcangiu ML, Rimm DL. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res 1999;59(8):1811–5. [PubMed] [Google Scholar]
- 59.Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016;126(3):1052–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pozdeyev N, Gay LM, Sokol ES, et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin Cancer Res 2018;24(13):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faam B, Ghaffari MA, Ghadiri A, Azizi F. Epigenetic modifications in human thyroid cancer. Biomed Rep 2015;3(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang Y, Qu S, Zhu G, et al. BRAF V600E Mutation-Assisted Risk Stratification of Solitary Intrathyroidal Papillary Thyroid Cancer for Precision Treatment. J Natl Cancer Inst 2018;110(4):362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X, Li J, Li X, et al. TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer. J Nucl Med 2017;58(2):258–65. [DOI] [PubMed] [Google Scholar]
- 64.Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34(1):28–35. [DOI] [PubMed] [Google Scholar]
- 65.Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 2010;34(6):1222–31. [DOI] [PubMed] [Google Scholar]
- 67.Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res 2007;13(4):1341–9. [DOI] [PubMed] [Google Scholar]
- 68.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest 2011;121(12):4700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368(7):623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alzahrani AS, Qasem E, Murugan AK, et al. Uncommon TERT Promoter Mutations in Pediatric Thyroid Cancer. Thyroid 2016;26(2):235–41. [DOI] [PubMed] [Google Scholar]
- 71.Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 2018;36(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xing M Clinical utility of RAS mutations in thyroid cancer: a blurred picture now emerging clearer. BMC Med 2016;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medici M, Kwong N, Angell TE, et al. The variable phenotype and low-risk nature of RAS-positive thyroid nodules. BMC Med 2015;13:184. [DOI] [PMC free article] [PubMed] [Google Scholar]