Abstract
Despite the increasing attention to early-life adversity and its long-term consequences on health, behavior, and the etiology of neurodevelopmental disorders, our understanding of the adaptations and interventions that promote resiliency and rescue against such insults are underexplored. Specifically, investigations of the perinatal period often focus on negative events/outcomes. In contrast, positive experiences (i.e. enrichment/parental care//healthy nutrition) favorably influence development of the nervous and endocrine systems. Moreover, some stressors result in adaptations and demonstrations of later-life resiliency. This review explores the underlying mechanisms of neuroplasticity that follow some of these early-life experiences and translates them into ideas for interventions in pediatric settings. The emerging role of the gut microbiome in mediating stress susceptibility is also discussed. Since many negative outcomes of early experiences are known, it is time to identify mechanisms and mediators that promote resiliency against them. These range from enrichment, quality parental care, dietary interventions and those that target the gut microbiota.
Keywords: resiliency, early life adversity, parental care, enrichment, microbiome, translational research
Introduction
Adverse experiences in early life can severely and profoundly reorganize the brain, leading to changes in function that can be related to mental health outcomes. The far-reaching impact of early life adversity on health indicators, as for instance highlighted in the foundational research of the Adverse Childhood Experiences Study (ACES; Felitti et al., 1998; Anda et al., 2002; Edwards et al., 2003), has underscored the importance of research focusing on early developmental periods. The focus on these early time periods is important, not only for understanding the mechanisms by which stress affects the brain, but for developing protective interventions that may be feasibly implemented into either prenatal and/or pediatric settings.
The maternal-placental-fetal interface is an important route whereby environmental stressors can impact the developmental trajectory of the fetus (Entringer et al., 2015). The prenatal period is particularly critical to brain development as it is a stage where fundamental processes such as neurogenesis, migration, apoptosis, synaptogenesis, gliogenesis and myelination take place (Estes & McAlister, 2016; Workman et al., 2013; Salmaso et al., 2014). Important indicators of immune system development, such as hematopoiesis and proliferation/migration of immune stem cells, are also observed (Simon et al., 2015; Weiskopf et al., 2016). The early postnatal stage is another sensitive period, marked by major events such as the continuation of neurodevelopmental processes (e.g. synaptogenesis, gliogenesis) and growth, experience-dependent synaptic pruning, bacterial colonization of the gut, and functional maturation of innate and adaptive immunity (Belkaid & Hand, 2014; Estes & McAlister, 2016; Salmaso et al., 2014; Simon et al., 2015; Workman et al., 2013).
The rapid formation and dynamic shaping of the brain is particularly vulnerable during these early periods. This is primarily evidenced by experimental animal data demonstrating that adverse experiences such as deprivation (e.g. nutritional, caregiver neglect), threat (e.g. social, physical stressors), and injury or illness have long term influences on brain development and functioning (see Morgane et al., 1993; Alamy & Bengelloun, 2012; Meaney et al., 1996; Plotsky & Meaney, 1993; Kaiser & Sachser, 2005; Schmitz et al., 2002; Figure 1). One of the first laboratory demonstrations showing that early experience can impact later life outcomes used a daily brief maternal separation procedure (Levine et al., 1956). Moreover, several of the first papers published in the original issue of Developmental Psychobiology focused on the effects of early life experience, such as handling (Levine 1968; Denenberg et al. 1968; Altman et al., 1968; Thoman et al., 1968) on later outcomes. In fact, the very first article in the original issue, by Victor Denenberg and colleagues (1968), addressed how early life stress (ELS) can “program life histories”. Since that time, several models of ELS have evolved including protocols of social isolation, chronic mild stress, restraint stress, and administration of drugs or viral/bacterial mimetics (Blaze et al., 2017; Lorenz, 1972; Kehoe et al., 1995; Rangon et al., 2007; Abdul Aziz, et al., 2012; Baker et al., 2008; Weinberg, 1992; Schmitz et al., 2002; Maccari et al., 2003; Meyer et al., 2008; Fatemi et al., 2002; Borrell et al., 2002; Yan & Kentner, 2017; Connors et al., 2014), as well as models using more prolonged maternal separation periods (reviewed in Maccari et al., 2017; Tractenberg et al., 2016; Lomanowska et al., 2017; Lomanowska & Melo, 2016), or limited bedding as a model of ELS (Gilles et al., 1996; Heun-Johnson & Levitt, 2016; Walker et al., 2017; Doherty et al., 2016; Blaze & Roth, 2017). Each of these models can affect maternal care, which may mediate the alterations in neurodevelopment, thus highlighting the challenge of understanding the impact of early life experiences. Importantly, mammals are believed to be somewhat protected from stress and increased levels of glucocorticoids during a critical period in early life, termed the stress hyporesponsive period, during which the hypothalamic pituitary adrenal (HPA) axis is relatively unresponsive to certain stressors (Shapiro et al., 1962, Sapolsky and Meaney 1986). However, this dampening of the stress response may depend on the presence of the mother (Stanton et al., 1987; van Oers et al., 1998; Lomanowska et al., 2011) and the amount of ELS studies showing negative long-term effects suggest that this downregulation during the stress hyporesponsive period does not protect neonates from all stressors.
Figure 1. Adverse Early Life Experiences.
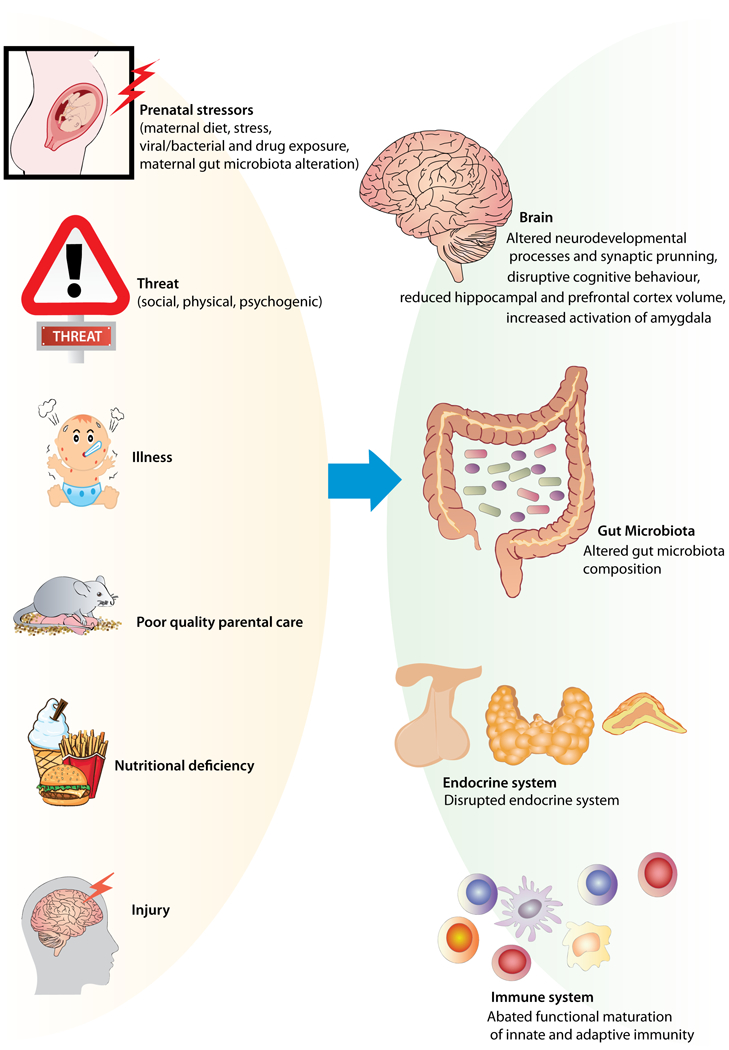
Early life adverse experiences such as deprivation (e.g. nutritional, caregiver neglect), threat (e.g. social, physical stressors), and injury or illness have long term influences on development and functioning.
The culmination of data from studies using these animal models have informed recent clinical research questions, which have in turn identified translational corollaries between basic and human neuroscience research. For example, some of the affective and cognitive disruptions that occur in offspring, following exposure to psychosocial stressors in utero, are expressed in humans (Van Den Berg et al., 2004; Tarabulsy et al., 2014; Kashan et al., 2008; Markham et al., 2011) and other animals (Vallée et al., 1997; Lordi et al., 2000; Estanislau & Morato, 2005) in a similar manner. Moreover, several brain correlates can be found between humans and animals following ELS exposure including smaller hippocampal (Ahmed-Leitao et al., 2016; Woon & Hedges, 2008; Coe et al., 2003; Mandyam et al., 2008; Mychasiuk et al., 2011) and prefrontal cortex (Anderson et al., 2 008; Harmelen et al., 2010; Mychasiuk et al., 2011) volumes in later life. An additional neural marker affected by ELS is the observation of greater amygdala activation in both humans and mice in response to a perceived threat (Malter Cohen et al., 2013).
Importantly, the biobehavioral changes that take place following ELS exposure may not always lead down a path of psychopathology but may instead be indicative of an adaptation of the organism to its predicted future environment (Bateson et al., 2014). This is particularly evident given the fact that not everyone who experiences stressful events in childhood develops mental illness. Moreover, since many negative outcomes of early experiences are known, it is time to begin identifying mechanisms that promote resiliency against them. In this review, we provide a summary of research in humans as well as in animals suggesting a role for the brain, gut, genes, and environment in promoting resiliency against early life adversity (Figure 2). We will explore the underlying mechanisms of neuroplasticity that follow these early life experiences and translate them into feasible ideas for interventions in pediatric settings. Further, we will discuss how sex may play a role in vulnerability or protection against certain stressors. We propose that by using translational animal models that more closely resemble the human situation, we can better explore ways to improve behavioral and neuroendocrine outcomes after ELS and determine the underlying mechanisms of these adaptations.
Figure 2. Factors Promoting Resilience.
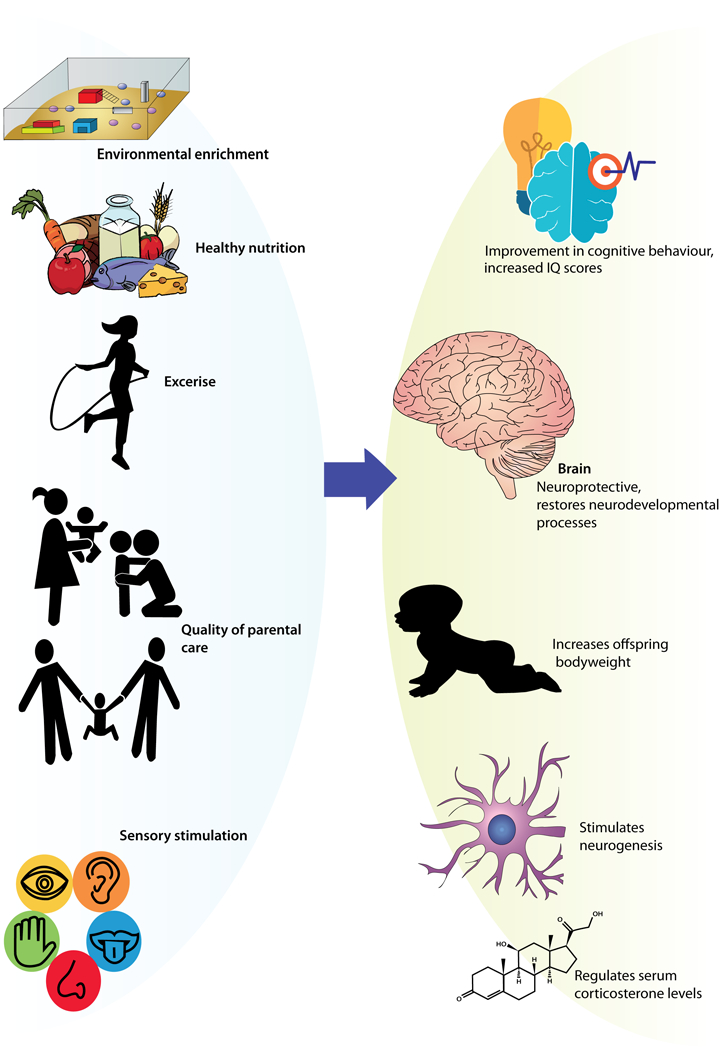
Positive early life experiences such as enrichment, quality parental care, and a healthy diet can promote healthy development and adaptive responses to stressors, leading to resilience.
Harnessing the Environment to Promote Resiliency to Early Life Adversity
One of the first observations of experience-dependent neural plasticity took place in the 1950’s when Rosenzweig and colleagues noticed differences in cortical acetylcholinesterase (AChE) activity as a function of the type of spatial maze rats had been tested in. Further experiments demonstrated that animals that had undergone behavioral testing had significantly different cortical AChE activity than those that remained untested (Rosenzweig et al., 1960; Rosenzweig, 2007). These initial observations of environmental remodeling were the impetus for the now famous environmental enrichment (EE) studies, which are classically recognized as providing direct support of plasticity in not only adult brain chemistry, but brain anatomy too (Rosenzweig et al., 1962; Bennett et al., 1964; Diamond et al., 1964).
Although there is no standardized form of EE, increased complexity in either physical or social stimulation comprise its primary requisites; maintaining the novelty factor of such stimulation may be viewed as an added benefit and on its own, novelty has been shown to be fundamental to the enrichment experience for at least some sensory modalities (Veyrac et al., 2009). In the animal laboratory, enhanced opportunities for activity and group engagement help to facilitate naturalistic species typical behaviors. That said, an important caveat is that enrichment preferences and associated benefits are both species and sex dependent (Lin et al., 2011; Simpson et al., 2012; Kentner et al., 2018; Wurbel and Garner, 2007; Welberg et al., 2006; Bessinis et al., 2013). Unfortunately, there is a paucity of research that evaluates the effects of EE on both sexes (Simpson & Kelley, 2011), and very few comparative studies have been done on other species compared to rodents. Of the work that has been completed, it is clear that male and female rodents are differentially effected by EE housing. Specifically, while both sexes demonstrated increased levels of prosocial engagement following adolescent exposure to EE, only male rats showed elevated gene expression of hippocampal corticotropin-releasing hormone receptor 2 (Kentner et al., 2018). In contrast, life-long EE increased social engagement in female, but not male, rats (Connors et al., 2015) and was associated with a sex-dependent neuroendocrine responsivity to acute and chronic stressors (Welberg et al., 2006). Moreover, female mice reared in EE have been reported to show higher anxiety-like behaviors compared to males (Lin et al., 2011). Importantly, adding shelters and other types of physical enrichment devices to group-caged male mice leads to territorial aggression and elevated stress responses in subordinate animals (Würbel and Garner, 2007; McQuaid et al., 2012; Howerton et al., 2008). Still, the success of EE protocols to promote synaptic structural changes (e.g. increased spine density, dendritic branch length/complexity; see Mychasiuk et al., 2014; Restivo et al., 2005; Greenough et al., 1985), to augment the expression of plasticity-related markers (e.g. postsynaptic density protein 95, synaptophysin, insulin like growth factor (IGF), brain derived neurotrophic factor (BDNF); Rampon et al., 2000; Ickes et al., 2000; Frick et al., 2003;Nithianantharajah et al., 2004; Baldini et al., 2013; Chatterjee et al., 2007), and to induce long-term potentiation, long-term depression, and metaplasticity (Bushler & Manahan-Vaughan, 2012; Artola et al., 2006) have reinforced the view of environmental stimulation as a potential therapeutic intervention in a number of clinical settings. This is particularly underscored by evidence that these central changes are accompanied by functional improvements such as enhanced learning and memory in animals of both sexes (Frick et al., 2003; Novkovic et al., 2015; Kempernann et al., 1997) and the amelioration of symptoms associated with several animal models of disease (Nithianantharajah et al., 2008; van Dellen et al., 2000; Lazarov et al., 2005; Levi et al., 2003; Faherty et al., 2005; Maegele et al., 2005; Young et al., 1999; Ohlsson & Johansson, 1995; Biernaskie and Corbett, 2001). For example, rats housed in EE either prior to or following a focal stroke had better neurological functioning (e.g. motor and cognitive) compared to those housed in standard laboratory conditions (Ohlsson & Johansson, 1995; Johansson, 2004; Gobbo & O’Mara, 2004).
Thoughts on the translational relevance of animal models of therapy, including environmental enrichment, have been considered for decades; following the eighth Annual Winter Conference on Brain Research in 1976, an edited volume called Environments as Therapy for Brain Dysfunction was published in a series hosted by Advances in Behavioral Biology (Walsh et al., 1976). The chapters explored the potential for environmental manipulations to treat brain injury, hyperthyroidism, and sensory deprivation. Since this time, EE has demonstrated some success in clinical populations, such as those undergoing stroke recovery. For instance, a prospective nonrandomized block design intervention study has shown that patients exposed to enrichment have increased physical activity levels and a reduced amount of time spent alone (Janssen et al., 2014). The typical experience of an inpatient stroke survivor is that of inactivity and reduced social contact; in one study, patients were reported to be alone more than 60% of the time while spending more than 50% of their day in bed (Bernhardt et al., 2004). In contrast, during the first series of feasibility studies evaluating EE (Janssen et al., 2012; Janssen et al., 2014; White et al., 2015), patients were given access to a computer with internet connection, various reading materials, audio books, music, jigsaw puzzles and board games. Moreover, they were provided with opportunities for additional social interactions including group recreational activities (Nintendo Wii gaming) and a dining area for meals. Behavior was evaluated for 48 hours, sampled across 16 days using a behavioral mapping tool (e.g. checklists comprised of predetermined categories to track patient activity, location, and social engagement). In addition to the positive investigator reports (Janssen et al., 2014), patients also described increased motor, cognitive, and sensory stimulation, reduced boredom, increased feelings of personal control and a higher number of social interactions (White et al., 2015), all variables integral to stress reduction which is important given that stress can exacerbate the adverse consequences of brain injury (Bohnen et al., 2009; Gouvier et al., 1992; Jin et al., 2010). More recently, the implementation of EE into acute stroke units has been associated with reductions in time to hospital discharge and adverse events (as defined by established definitions, see Goldfarb, 2012); Rosenbergen et al., 2017). Additionally, inpatients in a tertiary neurorehabilitation unit who were exposed to EE reported reduced stress and depression and improved mobility in a randomized controlled trial (Khan et al., 2016). Taken together, these data suggest that enhanced enrichment opportunities significantly increase the quality of life and function of brain injury survivors, supporting its use and feasibility in clinical settings. Still, research evaluating the optimization of clinical EE is in its infancy and additional studies are required to evaluate the potential of EE to promote functional recovery, and reduce morbidity, mortality and stress. Moreover, the research community will need to develop a more coherent consensus of what constitutes clinical EE in humans.
Animal studies can help illuminate the underlying mechanisms involved in EE’s capacity to improve symptoms following stress, including those experienced in early life (see Figure 2). Indeed, environmental complexity is rehabilitative in animal models of ELS such that it reverses the synaptic and behavioral consequences of parental neglect that are observed in adulthood when animals are placed into enrichment either at, or following, weaning (Bredy et al., 2004; Bredy et al., 2003; Vivinetto et al., 2013). This remediating benefit of environmental stimulation also extends to animal models of neonatal brain injury (Schwartz, 1964; Komitova et al., 2013; Schuch et al., 2016; Gibb et al., 2014), fetal alcohol syndrome (Rema & Ebner, 1999) and exposure to antiadrenergic hypertensive drugs (Ryan & Pappas, 1990), inflammatory mimetics (Kentner et al., 2016), and maternal physical stress (Laviola et al., 2004; Morley-Fletcher et al., 2003). However, its success in reversing the effects of ELS are not universal (when animals are placed into EE in later life), nor are its benefits robust for all measures (Brummelte et al., 2008; Mychasiuk et al., 2014; Kentner et al., 2016; Buschert et al., 2016). This suggests that earlier exposure to EE may be more effective due to a time-dependent factor (e.g. the existence of critical periods).
Enhanced environmental stimulation, in the form of EE, has been shown to affect the structure and underlying plasticity of the neonatal rodent brain (Malkasian & Diamond, 1971; He et al., 2010), suggesting that EE has potential translational utility in early development. As might be predicted, EE exposure during the early prenatal and/or neonatal periods is neuroprotective (Connors et al., 2014; MacRae et al., 2015; Welberg et al., 2006; Cymerblit-Sabba et al., 2013; Brummelte et al., 2007; Pedrini Schuch et al., 2016). This is important because animal studies that investigate prevention have largely focused on pharmacotherapies rather than environmental interventions. While pharmacotherapies can be advantageous in the treatment of some neuropsychiatric disorders, they often have several limitations including the cost of development, accessibility, specificity and tolerability (Berk, 2012; Hyman, 2012; Munos, 2013). Moreover, pharmacological treatments during childhood or later adolescence may have unknown long-term consequences, as the brain has not fully matured, and may be more vulnerable to non-specific effects of the drug treatment (Rodriguez-Porcel et al., 2011). There has been a decline in new drug development based on a variety of factors including, most importantly, a lack of understanding of the underlying mechanisms of many neuropsychiatric diseases (Berk, 2012; Hyman, 2012; Munos, 2013). Research examining the benefits of environmental interventions and the mechanisms underlying the success of early EE will not only help to uncover the etiology of disease but may also lead to the discovery of effective novel therapeutics. Specifically, investigating the mechanisms of EE may reveal molecular targets for other agents that mimic or enhance environmental stimulation (termed “environmentalmimetics”; Nithianantharajah, & Hannan, 2006). Finally, recent randomized controlled clinical trials have demonstrated the efficacy of EE as a therapy for autism spectrum disorder (Arnoff et al., 2016; Woo et al., 2015; Woo & Leon, 2013), highlighting its translational feasibility and acceptability in clinical practice for young populations.
The initial use of EE in pediatric settings was inspired by basic science (Reynolds et al., 2010; Woo et al., 2015; Schneider et al., 2006). Observations that autism-like symptoms induced by prenatal valproic acid exposure in rats (Schneider & Przewlocki, 2005; Schneider et al., 2006) were attenuated following placement into EE led to the development of a sensory enrichment therapy protocol for children with autism spectrum disorder (Woo & Leon, 2013; Woo et al., 2015; Arnoff et al., 2016). In humans, this clinical intervention is comprised of sensorimotor exercises that simultaneously stimulate multiple systems (e.g. olfaction, tactile, visual, balance, motor). In one randomized clinical trial, parents were provided with kits and written instructions for implementing the EE sensorimotor exercises, which increased in difficulty across the 6-month intervention period. Each session was approximately 15–30 minutes in duration (made up of 4–7 exercises) and were scheduled to take place twice a day. Overall, autism severity, as evaluated with the Childhood Autism Rating Scale, improved significantly in the enriched group compared to the standard care group (e.g. general behavioral therapies). Moreover, sensorimotor enrichment was associated with improved Leiter International Performance Scale–Revised scores (a measure of objective nonverbal cognitive abilities), significant increases in IQ, Reynell receptive language scores, and improvements in sensory reactivity (Woo & Leon, 2013; Woo et al., 2015). Using similar forms of sensorimotor stimulation, there is now an emerging body of clinical work supporting the use of EE in young patients with Down syndrome, cerebral palsy, and Rett syndrome (Purpura et al., 2014; Morgan et al., 2013; Morgan et al., 2015; Downs et al., 2018). While there is some initial evidence that EE exposure changes the expression of IGF and BDNF in both humans and animals (Downs et al., 2018; Field et al., 2008; Landi et al., 2009; Baldini et al., 2013), the underlying mechanisms and necessary/sufficient components of the EE protocols that promote clinical benefit are not fully understood.
The benefits of enrichment interventions may be mediated by changes in parent-child dynamics. In alignment with others using rodent models (Welberg et al., 2006; Korgan et al., 2016), we have shown that early life EE changes the quality of maternal care (Connors et al., 2015), which is well recognized to affect the physiology and behavior of offspring (Francis et al., 1999). The passive observation and evaluation of rodent nursing postures are one common way to appraise the quality of maternal care. For example, investigators can score the frequency of ‘active’ high-arched back nursing postures which allows for pups to easily access the teats to feed, compared to the more restrictive low-arched back/prone (or blanket) nursing in which the dam lays over her pups. In our hands, EE dams engaged in fewer nursing episodes and spent less time in contact with the nest. In contrast, standard housed dams spent more time on the nest in close proximity with their pups; they were also more often observed displaying low arched-back nursing. Additionally, standard housed dams could often be seen eating and drinking without ever leaving the nest while EE mothers frequently left to eat, sleep, and play in the extra space allotted to them in the larger cages. Intuitively, this may seem as though the EE dams were neglectful, however, when EE dams were nursing, they were more likely to demonstrate a high-arched back posture; Moreover, their pup licking frequency was comparable to standard housed dams even with less time spent on the nest, which suggested they were more efficient mothers. Importantly, EE offspring demonstrated better behavioral adaptability in response to their test environment (e.g. novel vs familiar contexts) and had central features suggestive of better stress regulation (e.g. elevated GLUR1 and GABA concentrations in the prefrontal cortex) compared to standard housed offspring (Connors et al., 2015). In the wild, rat dams must leave their nests in order to forage and defend. This limits their duration of direct contact with offspring similarly to what we observe with EE dams, a naturalistic separation model that appears to shape offspring stress resiliency and adaptability. Traditional standard laboratory housing instead may promote ‘helicopter parenting’, in which a dam is continually in contact with her offspring, as she has nowhere else to go. The translational value of this classic housing condition, associated with more time on the nest and increased nursing episodes, must be considered as it may result in pup overfeeding and/or changes in species typical thermoregulation, both understood to affect development (Jans & Woodside, 1990; Stern & Lonstein, 1996; Smith & Spencer, 2012).
Paternal enrichment experience also affects offspring (Yeshurun et al., 2017; Mashoodh et al., 2012). Prenatally housing rodent fathers in EE is associated with increased offspring body weight (Korgan et al., 2018; Yeshurun et al., 2017; Mashoodh et al., 2012), a modest reduction in latency to demonstrate immobility in the forced swim test, and sex specific elevations in serum corticosterone (Yeshurun et al., 2017). Specific transgenerational benefits of EE exposed fathers have been reported for offspring in rodent models of epilepsy via reduced seizure frequency (Dezsi et al., 2016) and maternal separation, as indicated by a mitigation of anxiety-like behavior (Gapp et al., 2016). Overall, these basic research findings underscore the importance of environmental factors in mediating parental contributions to offspring outcomes through behavioral and potentially epigenetic mechanisms (Yeshurun & Hannan, 2017; described in the next section). This is important to note given that the clinical application of EE in pediatric settings is typically administered by parents (Purpura et al., 2014; Morgan et al., 2013; Morgan et al., 2015; Downs et al., 2018; Woo & Leon, 2013; Woo et al., 2015) and not surprisingly, at least for children with autism, therapy is more likely to be beneficial if parents are adherent to the intervention procedures (Aronoff et al., 2016). At this point it is unclear how the parental driven EE interventions affect the parent-child relationship, or even functioning of the caregiver (e.g. changes in anxiety, stress or depression scores, attachment), highlighting an underexplored area for future research.
Typically, the clinical use of EE is comprised of several different combinations of sensorimotor stimulation tasks which may overburden caregivers, reducing adherence, and consequently clinical benefit. Therefore, the development of targeted task/deficit specific interventions should be the goal, in terms of reducing cost (e.g. time, money, personnel) and increasing feasibility/acceptability. Of the limited work that has been done, evaluating the enhancement of individual sensory modalities in isolation, much has focused on tactile stimulation (e.g. Kangaroo care, described below), with less of a focus on auditory and olfactory interventions. In the neonatal intensive care unit (NICU), some studies have used auditory stimulation in the form of music, maternal voice, or heart beat sounds (Pineda et al., 2017), with evidence suggesting that the former is associated with improved infant feeding, and that it may alleviate parental stress (Loewy et al., 2015). Maternal odor stimulation has also been correlated with improvements in oral feeding (reduced transition time from gavage to breast feeding), in addition to a shorter time to hospital discharge (Yildiz et al., 2011). Recently, we have conducted some work where neonatal rat pups received either tactile brushing (using a paintbrush to mimic maternal licking of the pup; adapted from Lovic et al., 2006; 2013), or exposure to either auditory (a simulated lactating rat dam heart beat), or olfactory (a series of aroma therapy scents) stimulation during the maternal separation period of a rodent nosocomial infection model of the NICU. This work suggests that olfactory (not just specific to maternal odors) and auditory, rather than tactile, stimulation applied in isolation are effective in mitigating disturbances in plasma corticosterone levels following ELS, while all three sensory enhancements individually elevated hippocampal glucocorticoid receptor expression. Moreover, sensory enrichment was effective in counteracting the accelerated loss of the rooting reflex, precocious puberty, and disruptions in ultrasonic vocalizations as precipitated by ELS exposure (Kentner et al., 2018). Combined with data showing that early life tactile stimulation can itself be neuroprotective against ELS (Gonzalez et al., 2001; Lovic et al., 2006; Barrett et al., 2015), this work suggests that multimodal sensory interventions are not always necessary, as singular sensory interventions can be sufficient (Figure 2).
Relatedly, many EE animal housing conditions are confounded by access to running wheels; in these rearing conditions, the neuroprotective benefits of physical activity cannot be dissociated from the social or novel factors of the enriched experience. Research shows that physical exercise and EE can result in different physiological and behavioral outcomes (Rogers et al., 2016; Lambert et al., 2005). Notably, when both types of interventions lead to similar endpoints, the underlying mechanisms by which they function may differ. For example, both exercise and EE promote neurogenesis, but the former intervention increases cell proliferation while the latter increases cell survival (Olson et al., 2006), suggesting that for some intervention plans, multimodal stimulation may offer additional benefit or may even be essential (Figure 2). When comparing between the potency of EE, physical activity, and even formal training, enriched environments have been identified as the most efficacious in promoting recovery in animal models of brain injury, although combining EE with other treatments may result in additive effects (Will et al., 2004). These findings have major implications for our understanding of experience-dependent neuroplasticity and underscore the necessity of developing targeted task/deficit interventions.
Finally, with respect to the efficacy of EE following ELS, scientists may wish to consider whether observed improvements are due to a ‘true’ recovery of the circuits that underlie a specific function, or if they are instead the result of compensatory processes (Rosenzweig, 2007). Whether this is important or not may depend on one’s personal scientific outlook, but either way, the pathways by which the environment can help promote recovery cannot be disregarded.
A Mother’s Touch: The Science behind the Magic
It has long been known to midwives and neonatal care providers that a ‘mother’s touch’ can have beneficial (“magic”) effects for an infant. We know from Harlow’s early attachment studies in rhesus monkeys how important maternal care and touch are for healthy development. In fact, Bowlby once compared the importance of the mother to essential neurotrophic factors during critical periods of tissue development (Bowlby, 1951). Bowlby’s evolutionary theory of attachment (Ainsworth, 1973; Bowlby, 1958) replaced the behavioral theory that was centered around food as the reason for the infant to form an attachment (Dollard and Miller 1950). It was based on his own and others’ clinical observations of emotional and physical stunting of orphaned and hospitalized infants (Spitz, 1945), Harlow’s seminal experiments confirming that infant monkeys required social contact to develop properly (Harlow, 1965), and on studies of imprinting in birds (Bolhuis and Honey, 1998; Lorenz, 1970). Harlow’s studies showed that a ‘cloth mother’ was preferred for comfort even if she was not the source of milk, highlighting the importance of the social factor rather than food for forming an attachment (Meyer et al., 1975). His studies also confirmed that there are critical periods for attachments to form and that even brief periods of social contact with siblings, not just a mother, can produce relatively normal behavior in adulthood, already hinting at the amazing resilience of brain against early-life deprivation. We know that there are critical windows and certain stages for human infants to form secure social attachments (NICHD Early Care Research Network, 2006; Schaffer and Emerson, 1964; Waters et al., 2000). However, it is exceptionally difficult to really study the effects of parental deprivation in a human population, as it is challenging to define or quantify what an “appropriate amount of quality parental care” is. One way to approach this issue is by investigating children that grew up in an orphanage and experienced institutional deprivation as a form of reduced parental care (Figure 1). Studies on this form of ELS revealed that impoverished care was associated with emotional and cognitive deficits (Korosi and Baram, 2009). Intriguingly, institutionalized children display altered neuroanatomical features such as decreased total grey and white matter volumes but increased amygdala volumes (Mehta et al., 2009; Tottenham et al., 2010). In line with this, Gee et al., (Gee et al., 2013) also found changes in amygdala-medial prefrontal cortex (mPFC) connections that were associated with an increased maturation pattern of this network in institutionalized children. Further, children in foster care who had experienced maltreatment had lower full-scale IQ scores compared to a matched control group (Viezel et al., 2015). These results suggest that parental care deprivation leads to changes in neuronal volumes and connectivity that may reflect the adaptation to this early challenging environment for these children. Importantly, some of the negative effects were partially reversible by fostering the children (Hodel et al., 2015; McLaughlin et al., 2012; Nelson et al., 2007; Zeanah et al., 2009). However, for non-institutionalized children, there are many contributing factors besides caregiver interaction and attachment security in their early environment that make it challenging to study the effects of parental care in humans. For instance, socioeconomic and family environment over time, as well as maternal distress and degree of social support can predict behavioral and linguistic development in children (Miceli et al., 2000; Resnick et al., 1990). And though attachment security is relatively stable over decades, it can be influenced by dramatic experiences during childhood (Waters et al., 2000). Surprisingly, despite half a century of research on attachment and maternal care, including care provided by fathers (Kentner et al., 2010), we are still far from fully understanding the underlying mechanisms of how responsive and sensitive parenting affect offspring, providing them with the best possible development and adaptation to their environment (Figure 2). However, animal research has identified several neural circuits and mechanisms that help explain the effect of parental care and attachment on the healthy biobehavioral development of offspring.
Early work by Denenberg, Levine, Rosenberg and others, some of it published in the first issue of Developmental Psychobiology about 50 years ago, confirmed the importance of maternal care and early-life experiences in mice and rats (Altman, 1968; Denenberg, 1968; Thoman, 1968). These and other early studies investigated the effects of early handling or maternal separation on stress response and/or behavioral and emotional outcome later in life and found that maternal care had a tremendous impact on the adult phenotype (Bayart et al., 1990; Denenberg and Bell, 1960; Denenberg et al., 1962; Denenberg, 1999; Hennessy et al., 1980; Joffe et al., 1972; Levine, 1967; Levine et al., 1988; Rosenberg et al., 1970). Notably, these early studies already established that increases in maternal licking and grooming can have a beneficial effect on the adult phenotype and promote resilience to early-life stress. In Levine’s seminal studies, neonatal rats were separated from the mothers for brief, intermittent periods and exposed to foot-shocks that he believed would result in increased emotionality later in life, based on Freud’s theory. To their surprise, rats instead showed diminished emotional instability and improved stress responsivity as adults.(Levine et al., 1956; Levine, 1962). This effect was later attributed to the increase in maternal care and mother pup interaction upon reunion (Brown et al., 1977; Denenberg, 1999; Pryce et al., 2001; Smotherman et al., 1977a; Sullivan and Holman, 2010). For instance, in 1977, William Smotherman and colleagues (Smotherman et al., 1977a; Smotherman et al., 1977b) showed that the dam’s HPA responsiveness to the separation mediated the dam’s behavior in response to the pup’s stress experience, which in turn could help explain the differential outcome after early manipulations of the pups. However, adaptations to ELS in other species (i.e. monkeys) seem to be less dependent on subsequent maternal care (Lyons et al., 2010), which suggests that the associations between ELS, maternal care and later vulnerability or resilience to stress is complex. We will discuss this relationship in more detail in the following section and talk about how we can use high quality maternal care to improve health outcomes after early adversity in humans.
As mentioned earlier, in humans it may be difficult to disentangle the effect of parental care from other socioeconomic and developmental factors (Figure 1). Further, it is difficult to define or characterize high or low quality paternal care in humans and thus there is a lack of data on the consequences of the varying degrees of quality of parental care, but studies on orphaned, neglected or mistreated children may provide a proxy for the consequences that ELS can have in human children, though these situations may be on the extreme end of possible variations in parental care. Though many studies have suggested an association between maternal care and brain development in humans, only few studies provide actual evidence for such a relationship. Luby et al., (Luby et al., 2012; Luby et al., 2016) showed that higher levels of early maternal support predicted increased hippocampal volume and growth in healthy children at school-age. Further, Lupien et al. (Lupien et al., 2011) found an association between maternal depressive symptoms and amygdala volume in a small study of 10-year old children. Maternal depression is frequently associated with reduced mother-infant interaction, bonding and attachment (Brummelte and Galea, 2016) and it is assumed that some of the negative outcomes of exposed offspring is due to the alteration in maternal care in depressed mothers (Field, 2010). For instance, it is believed that externalizing problems and poor cognitive skills in children of depressed mothers are partly due to the effects of depression on maternal behaviors (Brummelte and Galea, 2016), as depressed mothers tend to breastfeed less (Dennis and McQueen, 2009), have a reduced likelihood of having secure attachments with their infants (Benoit, 2004) and generally exhibit more negative and less affectionate behaviors towards their infants (Ferber, 2004; Field, 2010; Lovejoy et al., 2000). However, infants of depressed mothers may already have different developmental trajectories due to the exposure to maternal depression and altered hormonal milieus during pregnancy and through breast milk (Brummelte and Galea, 2016). An emerging literature on paternal depression is further suggesting that the mental health of fathers may also be associated with poorer outcomes in their children, including anxiety and negative affect (Sweeny et al., 2016).
Another example of a reduced parental care situation in humans that is less dramatic than neglected or orphanage children, is that of infants being hospitalized for a prolonged period of time. And though there are several studies linking early hospitalization experiences to reduced bonding and even altered brain development (Brummelte et al., 2012; Fernandez Medina et al., 2017; Smith et al., 2011), less is known about the role of parental care on this negative outcome. In support of the idea that some of the negative consequences may be mediated through reduced parental care in these situations, studies aiming to increase parental touch through skin-to-skin (kangaroo) care have consistently shown positive and protective effects in neonates (for review see Bailey, 2012; Hall and Kirsten, 2008). This underlines the importance of parental involvement for building resilience in the offspring.
Considering the limitations of investigating the effect of parental care in humans, rodent models are a great tool to shed some light on the underlying mechanisms and the contribution of maternal care on developmental outcomes. There have been many animal models that investigated the effects of maternal separation or maternal stress on the outcome of the offspring since Levine’s early studies (reviewed in Maccari et al., 2017; Tractenberg et al., 2016; Gilles et al., 1996; Heun-Johnson & Levitt, 2016; Walker et al., 2017; Doherty et al., 2016; Blaze & Roth, 2017). There are also several mammalian models of biparental care (e.g. Prairie vole, California mouse, Mongolian gerbil) in which the effects of paternal behavior can be evaluated. In general, the animal literature indicates that the neural correlates related to paternal care are similar to those involved in maternal nurturing and that the presence of the father is associated with increased offspring body weight and survival rates (see Kentner et al., 2010). Importantly, the length of the maternal separation and type of maternal stressor matters significantly as short periods of separation can increase maternal corticosterone levels and stimulate care, thus resulting in resilience of the offspring to early-life or later-life stressors. In contrast, prolonged periods of separation typically result in negative consequences. In line with this, rat dams housed in EE spend less time in contact with the nest compared to standard housed animals (Connors et al., 2015). While EE dams engaged in fewer nursing episodes overall, they displayed higher frequencies of active or ‘better quality’ nursing behaviors (e.g. high-arched back nursing). Overall, this reduced time with the nest was associated with positive outcomes in the offspring in that they demonstrated more adaptive-like responses to their environment.
Investigating the changes in the brain of the offspring exposed to various parental care manipulations may help to determine why certain stressors result in resilience and others in vulnerability to later-life challenges. Prolonged maternal separation such as 3–24 h during the neonatal period can result in deficits in cognitive and behavioral tasks and in a decrease in hippocampal volume, a decrease in prefrontal cortex and motor cortex thickness, a decrease in fiber densities and an increase in apoptotic cells in various cortices, hippocampus, and cerebellar cortex in adult animals (Aksic et al., 2013; Zhang et al., 2002;Huot et al., 2002;Aisa et al., 2007; Daniels et al., 2004; Lee et al., 2007). These neuroanatomical and behavioral impairments are likely due to increased glucocorticoid levels in response to the maternal separation (Avishai-Eliner et al., 1995; Veenema and Neumann, 2009). Interestingly, the specific molecular changes in the brain are dependent on the duration of the separation as well as whether it was recurrent or not, though variable results have been reported even for the same stressor. For instance, after a single 24h maternal separation, some studies found reduced CRH expression in the PVN, while others found no change in expression levels (for review see: (Korosi and Baram, 2009)). The question remains why the same or similar stressors result in apparently negative outcomes in some studies, but resilience (no change) in others. Identifying the key methodological differences between these studies may help us shed some light on why some animals are more resistant to early-life stressors than others. Besides species, strain, sex, and age of the animal during separation, one crucial difference between studies could be the time of measurement of the outcome variable. For instance, for CRH expression levels, Schmidt et al., (2004) showed that maternal separation induced a cascade of sequential changes at various levels of the HPA axis, with the main activation occurring 4–8h after maternal separation. Thus, timing could be very essential and single measurements may not reflect dynamic alterations in the stress response. Further, litter size, early housing conditions and the circumstances during the separation (such as temperature, olfactory cues, or nutrition status of the pups) that may vary between studies could also contribute to differential outcomes. Importantly, recurrent separation influences maternal care upon reunion, thus adding another layer of complexity to studying the effect of quality vs. quantity of maternal care in separation studies (Korosi and Baram, 2009).
Tallie Baram and her laboratory developed a more translational model of maternal stress and reduced maternal care that is based on reduced bedding. Not having sufficient nesting material available before and after birth is a considerable stressor for the dam and can result in fragmented and frequently shifting maternal behaviors and thus chronic ELS for the offspring (Ivy et al., 2008; Rice et al., 2008; Walker et al., 2017). This approach has been adopted in many other labs since then and we now have a pretty good understanding of how some of the consequences are mediated through maternal and infant HPA axis activation. Most notably, we see changes in CRH-expressing neurons and CRH mRNA levels in hippocampal regions that persist into adulthood of exposed offspring that could help explain the cognitive deficits in several behavioral tests that these animals display (for review see: (Walker et al., 2017). Interestingly, female offspring seem more resilient to this type of early-life stressor than males and several research groups are currently trying to unravel the mechanisms behind this phenomenon. For instance, Tania Roth’s laboratory found sex-dependent methylation patterns of the BDNF gene in various brain regions, suggesting that this important neurotrophic factor, that plays a role in closing of sensitive periods, may be important in mediating resilience or vulnerability to the scarcity model in males and females (Blaze et al., 2013; Roth et al., 2014). However, other molecular and behavioral deficits following the reduced bedding model are found in both sexes, emphasizing the fact that more research is needed to understand potentially different susceptibility to this type of ELS in males and females.
These results from the limited bedding model are in line with work of Regina Sullivan and colleagues who studied early-life stress exposure and the importance of sensitive periods and the presence of the dam (Moriceau et al., 2010; Sullivan and Holman, 2010). Their seminal work showed how pups learn fear and/or attachment during a sensitive period of early life. Specifically, while pups are still in the sensitive period of attachment formation they do not learn or exhibit a “normal” fear reaction in response to pain (foot shock). This inability of pups to exhibit avoidance learning with pain-odor pairings (i.e. pups approach an odor even if it is paired with a painful stimulus during sensitive period) is believed to promote attachment formation (Ades et al., 2006; Roth and Sullivan, 2005; Roth et al., 2013; Sullivan and Holman, 2010). In other words, pups will learn to attach to a dam even if they are (sometimes) mistreated by her. This makes sense from an evolutionary perspective as some pain cannot be avoided (e.g. if the dam is moving around in her nest and stepping on pups) and it would explain why pups may be resilient to certain types of stressors early in life as long as the dam is present. In fact, it has been shown that pups have an active stress system when the dam is away but a buffered stress response when the dam is present (Moriceau et al., 2004; Raineki et al., 2010). Further, it was revealed that this buffering was mediated through blocking norepinephrine release in the PVN when the dam is present (Sullivan and Holman, 2010). These findings further help to shed light on the stress hyporesponsive period and why certain stressors can still elicit a corticosterone increase in pups while most stressors cannot during the sensitive period. Indeed, if the sensory and olfactory clues provided by the mothers are dampening the pups corticosterone levels (Stanton et al., 1987; van Oers et al., 1998), it is not surprising that prolonged maternal separation is one of the stressors that can still elicit a rise in corticosterone during the stress hyporesponsive period (Sullivan and Holman, 2010).
Models of ELS suggest that the dam may have a huge impact on determining whether a neonatal experience may result in resilient or vulnerable offspring later in life. In line with this, Michael Meaney and colleagues work has beautifully demonstrated that increased maternal care (i.e. licking and grooming) can rescue the effects of early adversity and produces a different adult phenotype compared to low maternal care (Champagne and Meaney, 2001; Liu et al., 1997; Meaney and Szyf, 2005; Weaver et al., 2004). Dams were selected based on their natural variation in the amount of time they spent licking and grooming their pups to establish a line of “high licking and grooming (LG)” and “low LG” dams. Not surprisingly, the amount of maternal licking and grooming that the pups received was associated with the pups’ stress response as adults. In particular, offspring of high LG dams had a damped corticosterone response to stress that was in line with reduced CRH expression in the hypothalamus, and reduced methylation of the glucocorticoid receptor gene which in turn correlated with increased expression of the receptor in the hippocampus (Champagne et al., 2008; Champagne and Meaney, 2001; Francis et al., 1999). Further, adult offspring of high LG dams displayed an increased spine density, dendritic branching and enhanced LTP in hippocampal neurons that was in line with enhanced cognitive performance compared to offspring from low LG dams (Champagne et al., 2008; Liu et al., 2000). This suggests that improved maternal care can have a beneficial impact on offspring’s long-term behavioral and cognitive outcomes.
In line with the results from low LG vs. high LG dams is data obtained from artificial rearing studies that use ‘licking-like’ stimulation using stroking with brushes or other devices (Lomanowska and Melo, 2016). The “pup-in-a-cup” model that was originally based on early nutrition studies has since been used by several labs to study the effects of continuous isolation and social deprivation on brain and behavioral development of the pup (e.g. Hall et al., 1999; Chatterjee et al., 2007; Lovic and Fleming, 2015; Lomanowska et al, 2017; de Medeiros et al., 2009). Artificial upbringing results in several modifications in behavior of male and female animals in adulthood as well as changes to neuroanatomical markers, electrophysiological properties and HPA axis functioning (for an excellent review on the artificial rearing model see Lomanowska and Melo 2016). However, the fact that these artificially-reared pups are still able to engage in relatively normal sexual, parental and social behaviors as adults, highlights the amazing capacity of the brain to adapt to ELS. An advantage of this model is that it allows to manipulate social stimuli by adding maternal or littermate contact or stroking to mimic maternal licking and grooming. This licking-like stimulation is able to rescue some of the negative consequences of artificial rearing (Chatterjee 2007; Gonzalez et al., 2001; Lovic and Fleming 2004) and this further strengthens the idea that (maternal-like) touch and stimulation early in life are important contributors to a healthy brain development.
Interestingly, Weaver et al (2005) also found that some effects of low LG maternal care were partly reversible in adulthood, though they achieved this improvement through epigenetic manipulations such as methyl supplementation or a histone deacetylase inhibitor given in adulthood. In line with this, epigenetic factors are increasingly recognized to play a crucial role in the relationship between early life experiences and later psychopathological outcome (Roth and Sweatt, 2011, Brummelte et al., 2017). Animal studies established a link between increased methylation and decreased expression of the glucocorticoid receptor after early adversity such as reduced maternal care (for review see: Szyf et al., 2005, 2007) which is in line with human data from McGowan and colleagues (2009) that revealed a link between childhood abuse and increased methylation and decreased transcription of the glucocorticoid receptor gene in human brain tissue obtained from suicide victims. Further, maternal mood, maternal stress and other childhood adversities have been associated with alterations in methylation levels of the glucocorticoid receptor and other relevant genes in human blood or saliva (Oberlander et al., 2008 Essex et al., 2013; Caldji et al., 2011; Beach et al., 2010; Kang et al., 2013), but less is known about epigenetic changes in the brain in response to variations in maternal care in humans.
In rhesus monkeys, being reared in a nursery resulted in increased methylation of the serotonin transporter gene (SLC6A4) compared to monkey being reared with their mothers (Kinnally et al., 2010). Interestingly, Murgatroyd and colleagues found that maternal stroking of the infant (self-reported) during the first week of life could rescue the increased methylation of the glucocorticoid receptor gene that was observed in saliva of infants exposed to postnatal depression (low prenatal but high postnatal depression scores). This again underlines the importance of touch for improving resilience in infants exposed to early-adversity. Importantly, methylation levels may differ by genotype, and genetic variations in itself can predict the risk for vulnerability to later-life stressors, thus adding another level of complexity when trying to understand the pathways to resilience or sensitivity after early-life adverse experiences (Brummelte et al. 2017). For excellent reviews on the role of genetic predispositions and gene-environment interactions in mediating resilience or vulnerability to ELS see: Lomanowska & Melo, 2016; Bowes and Jaffe 2013, Feder et al., 2009, Daskalakis et al., 2013.
As mentioned earlier, and similar to the effects of exposure to high LG, early handling or brief maternal separation have also been shown to result in a blunted stress response in adulthood (Sanchez et al., 2001; Schmidt et al., 2002). This reduced stress response was again associated with reduced CRH expression in the hypothalamus and increased GR in the hippocampus (Meaney et al., 1996; Avishai-Eliner et al., 2001), suggesting a similar pattern of alteration in the brain due to different manipulations that all seem to result in resilience. Further, early handling also led to improved cognitive function and reduced risk for depressive-like behavior (Dinces et al., 2014; Meaney et al., 1991; Reeb-Sutherland and Tang, 2012; Spivey et al., 2011; Tang, 2001), suggesting that the extra stimulation early in life leads to a more resilient phenotype that is based on alterations in HPA axis functioning.
Interestingly, though offspring of low LG dams have usually poor behavioral outcomes compared to high LG dams (Weaver et al., 2004), the offspring of low LG dams seemed to ‘outperform’ offspring from high LG under stressful conditions in adulthood (Champagne et al., 2008). This suggests that the quality of maternal care may transmit information about the quality of the environment to the offspring, possibly through epigenetic modifications. Under low resource conditions, a dam may spend less time licking and grooming and more time finding food, which could lead to HPA axis programming in the offspring that will make them better prepared/adapted for survival under challenging conditions (Mooney-Leber and Brummelte, 2017). These results further support the idea that maternal care can “program” the HPA axis of the offspring to be best prepared for the existing environment. In other words, a more reactive HPA axis may actually be advantageous in a stressful (low resource) environment, thus low LG dams are providing their offspring with the necessary resilience to a challenging adult life. Further, a high fat diet given to dams with limited bedding has been shown to prevent some of the negative consequences in the offspring (Walker et al., 2017). This also strengthens the idea that the offspring are programmed by the mother’s nutrition and behavior to be best prepared for a given environment. If there is not one clear ‘signal’ (i.e. reduced bedding and high fat diet are a ‘mismatch’ and suggest opposite qualities of the environment), the consequences for the offspring are more complex and may reflect adaptations in various directions. In line with this, Schmidt and colleagues (Santarelli et al., 2017) tested this mismatch theory by placing mice in either a supportive or aversive early environment followed by either a supportive (social housing) or aversive (chronic social defeat stress) adult environment and found that an early stressful environment did indeed result in better coping skills for a challenging adult environment. These kind of studies can help us understand why certain forms of ELS may provide resilience and protection against a stressful adult environment. As mentioned earlier, early manipulations to the dam, pups (handling) or the environment also usually lead to changes in the maternal care which in turn can contribute to observed alterations in biobehavioral development, adding another layer of complexity to trying to understand the impact of early life stress and maternal care alterations.
Investigations assessing the ability of ways to directly increase or decrease maternal care to better understand the mediating impact of maternal licking and grooming on resilience to early-life adversity are currently ongoing (Mooney-Leber and Brummelte, 2017). In particular, approaches to directly increase maternal licking and grooming without the need to select dams for this behavior or handling the pups are being assessed. Better understanding of the influence of maternal care on long-term phenotype development may help to translate findings about what promotes resilience into interventions for human mothers and their children at risk (Lomanowska & Melo, 2016; Lomanowska et al., 2017).
The underlying mechanisms of inducing and maintaining maternal care behaviors have been described in detail elsewhere (Bridges, 2015; Brunton and Russell, 2008; Fleming et al., 1987). Obviously, many steroid hormones are involved in the complex communication between body and brain to prepare the female body for motherhood. Not surprisingly therefore, glucocorticoids, estrogens, progesterone and oxytocin have all been found to play a role in maternal care in mammals (Fleming et al., 1987; Fleming et al., 1997b; Glynn et al., 2016; Rosenblatt, 1980). For instance, the estradiol to progesterone ratio during gestation has been linked to attachment and sensitivity of a mother in the postpartum period (Fleming et al., 1997a). Further, healthy postpartum mothers with higher plasma cortisol levels engaged in more affectionate mother-infant interaction with their infants compared to women with lower cortisol levels (Fleming et al., 1987) (Fleming et al., 1997b). In line with this, higher cortisol levels in first-time mothers were associated with better recognition and higher attraction to their own baby’s body odor compared to foreign infant odors and showed more affectionate contact with their baby in a mother-infant interaction session (Fleming et al., 1997b). In animals, manipulating steroid hormone levels can alter parental behavior in various ways (for recent review see: Bridges, 2015; Siegel and Rosenblatt, 1975; Terkel and Rosenblatt, 1972), which suggests that altering hormone levels in humans may be a potential pathway for improving maternal behaviors in at-risk cases, such as severely depressed mothers, in the future.
Importantly, if altered hormonal milieus are responsible for changes in maternal care, then they may also contribute directly or indirectly to alterations in the development of the offspring. This underlines the complexity of studying the impact of early-life experiences as it is impossible to disentangle these interactions in humans. One simple approach to elevate levels of maternal care in humans for hospitalized infants, and premature babies in particular, is through Kangaroo care. This increased skin-to-skin contact between the neonate and mother has been linked to positive outcomes which range from typical brain development to improved cognitive functioning (for review see Bailey, 2012; Hall and Kirsten, 2008; Johnston et al., 2003; Feldman et al., 2014; Scher et al., 2009; Welch et al., 2017). However, not much is known about how skin-to-skin care produces resilience and improvements in at-risk infants, such as premature babies. Importantly, more positive outcomes are often associated with more days of Kangaroo care (Bera et al., 2014). Initial physiological changes seen after Kangaroo care include improvements in body temperature, respiration rate, heart rate, and oxygen saturation as well as reduced cortisol levels (Mooncey et al., 1997) (Bera et al., 2014) that may contribute to the long-term developmental outcomes. Interestingly, Kangaroo care also functions as an acute analgesic for painful procedures (Johnson et al., 2003), and the effects are in line with Regina Sullivan’s and others work in rodents showing that pain-and stress responses are diminished by the presence or contact with the mother (Blass et al., 1995; Stanton et al., 1987). The calming effect of parental care in rodents and humans is believed to be mediated through the HPA axis and CRF signaling though many other downstream effects such a downregulation of glutamatergic signaling may also be involved (Mooney-Leber and Brummelte 2017). Further, studies revealing sex differences in the outcome of the offspring after reduced maternal care suggest that hormones such as estrogens and oxytocin may also play a role in mediating the effect (He, 2018). Unfortunately, improving the quality of parental care or increasing the time caregivers can spend with their at-risk children is not always easy as it means tackling the reason behind the altered parental behaviors. For instance, if maternal/paternal depression is the underlying reason for reduced caregiver-infant interactions, interventions should also address the caregiver’s mental illness, but depressive symptoms are not always easily improved. Further, parents of preterm infants may be limited in regards to the amount of time they can spend in the NICU depending on infant’s length of stay, distance from the hospital and whether there are siblings to take care of at home (Reynolds et al., 2013;Latva et al., 2007). But overall, creating ways to improve parental care and involvement seem to be promising avenues to build resilience and protection against early-life stress and translational animal models may help to further elucidate the underlying mechanisms behind the magic of a ‘mother’s touch’.
The best way to a child’s heart is through their gut
Within the past decade, what is perhaps at first thought an unlikely factor, the gut microbiota, has emerged as a key regulator of the stress response especially in early life (Foster et al. 2017; Moloney et al 2014; Gur et al., 2015; O’Mahony et al., 2017; De Weerth 2018). Although it has been known since the 1800s that the gut and brain communicate to maintain homeostasis, advances in sequencing technologies have facilitated the realization that this gut-brain axis is modulated by the trillions of microbes, mainly bacteria, but also archaea, yeasts, helminth parasites, viruses, and protozoa together within the gut which outnumber human cells in the body (Sender et al., 2016). For the most part, in humans and other mammals, colonization of the infant gut begins at birth, when delivery through the birth canal exposes the infant to its mother’s vaginal microbiota, thus initiating a critical maternal influence over the offspring’s lifelong microbial signature (Mueller et al., 2015). Microbiota composition influences almost every aspect of the organism’s physiology throughout its life including brain health and stress responses. Moreover, factors that influence this composition have thus the potential to impact neurodevelopment and stress sensitivity (Borre et al., 2015; Sampson and Mazmanian, 2015; Kundu et al., 2017).
In a seminal study, Sudo and colleagues (2004) demonstrated that mice which lack microbes (germ-free) have an exaggerated HPA axis response to an acute stressor which could be reversed by colonization with a specific Bifidobacteria species. This was followed by a number of key germ free studies showing that bacteria are required for normal brain development (Neufeld et al., 2011; Diaz Heijtz et al., 2011; Clarke et al., 2013;) as well as brain function in adulthood (Stilling et al., 2015; Lucyinski et al). Indeed, key processes such as neurogenesis (Ogbonnaya et al., 2015), myelination (Hoban et al. 2016), blood brain barrier integrity (Brainiste et al., 2015) and microglia activation (Erny et al., 2015; Thion et al., 2018) are perturbed in germ free mice. Changes in hippocampal, amydgala and prefrontal cortex microRNAs are also observed in these animals (Hoban et al., 2016; 2017). This may be of interest to stress resilience neurobiology given the increasing attention that microRNAs have received in mediating stress resilience (Gururajan et al., 2017). Functionally, in addition to increased activation of HPA axis these changes translate to alterations in anxiety-related behavior, social behavior, fear learning and increased visceral pain (see (Luczynski et al., 2016a) for review). Antibiotic exposure is another strategy to assess the impact of the microbiota on brain and behavior. Early-life antibiotic administration in rat pups leads to altered central nervous system pathways, particularly those associated with pain signaling from the gut via the spinal cord (O’Mahony et al., 2014). Moreover, early life penicillin treatment in mice has lasting effects on gut microbiota, increases cytokine expression in frontal cortex, modifies blood-brain barrier integrity and alters behaviour (Leclerque et al., 2017).
Although the relationship between stress and microbiota composition dates back many decades, to when Tannock and Savage (1974) noted that ‘stressed mice showed dramatic reductions in these populations of lactobacilli’, it was a further two decades until Bailey and Coe published in Developmental Psychobiology that maternal separation decreased fecal Lactobacillus in rhesus monkeys three days post-separation (Bailey and Coe, 1999). In rodents, early life maternal separation disrupts the offspring’s microbiota (O’Mahony et al., 2009). More recently mouse studies have shown that the presence of gut microbiota may not be essential for the ability of maternal separation to alter stress-related HPA axis activity, but it is necessary to alter the development of anxiety- and depressive-like behaviors (De Palma et al., 2015 Specifically, germ free mice were sensitive to the enhanced corticosterone response to early life stress but not the behavioral changes. Additionally, prenatal stress (Figure 1) affects the composition of the maternal microbiome at genus level and thus affects the microbial signature that is subsequently transferred to the offspring in rats (Golubeva et al., 2015) and mice (Gur, 2017) and even humans (Zijlmans et al., 2015). Together these data show that the microbiome may be a key link between the intrauterine environment and adult behavioral changes. In addition, chronic stress in either adolescence (Dunphy-Doherty, 2018) or adulthood has been shown to modify the microbiome composition coupled with changes in gut barrier function, immunity and behaviour (Bailey, 2011; Galley et al 2014; Bharwani et al 2016; Burokas et al., 2017; Partrick et al., 2018).
Recently, fecal microbiota transplantation approaches have been utilized to demonstrate that stress-related microbiota composition plays a causal role in behavioral changes. The transfer of stress-prone Balb/C microbiota to control germ free Swiss Webster mice has been shown to increase anxiety-related behavior, whereas transfer in the opposite direction reduced anxiety-related behavior (Bercik et al., 2011). This clearly shows that the microbiota of Swiss Webster mice harbors the ability to produce factors important for resilience whereas that of the Balb/C is able to confer susceptibility to anxiety-provoking situations. A link between disease-related microbiota and behavior was also recently demonstrated, where fecal microbiota transplantation from depressed patients to microbiota-depleted rats increased anhedonia and anxiety-like behaviors (Kelly et al., 2016). Efforts are also being made to correlate early life microbiota with infant brain and behavior. In toddlers (18–27 months old), a higher level of extraversion was associated with greater phylogenetic diversity in the microbiome (Christian et al, 2015). Moreover, the microbiome at one year old has been shown to correlate with cognitive function at two years of age with higher alpha diversity associated with lower scores on the overall composite score, visual reception scale, and expressive language scale (Carlson et al., 2018). Interestingly, at an imaging level, no correlation was shown between microbiota composition and fMRI measures in this study. On the other hand a recent porcine magnetic resonance spectroscopy study demonstrated that fecal Ruminococcus levels predicted brain N-acetylaspartate concentrations in 30 day old pigs, an effect that co-varied with serum cortisol levels (Mudd et al., 2017).
To date, a clear relationship between microbiota composition and stress resilience has received less attention. Ongoing studies are investigating if basal microbiome composition could predict stress susceptibility in adult mice (Gururajan and Cryan unpublished). Similar approaches have been undertaken in Syrian hamsters, a useful model to study social stress due to the fact that they rapidly form dominance hierarchies. A single exposure to social stress induces changes in the gut microbiota that increases following repeated exposures (Partick et al., 2018). Interestingly, this study found no association between the effects of social stress-induced changes in the microbiota and HPA axis measures although the composition of the microbiome was different between hamsters that were dominant or submissive. Future studies must focus on whether the microbiome in early life provides a mechanism for stress susceptibility or resilience both in rodents and humans.
There is growing evidence that targeted manipulations of the microbiota might confer protection to the brain to ameliorate the negative effects of stress during vulnerable developmental periods. To this end, several studies have shown that diets that modify the microbiota (Figure 2), prebiotics, and probiotics can reduce stress-related behavior and HPA activation (Sarkar et al., 2016). Prebiotics are non-digestible food ingredients that promote growth of commensal bacteria. Prebiotics including the oligosaccharides 3’sialyllactose, 6’sialyllactose, polydextrose, fructooligosaccharides & galactooligosaccharides reverse the behavioral, neurochemincal and immune-related effects of chronic stress in rodents (Tarr et al., 2015; Burokas et al.2017; Thompson et al., 2017). Interestingly, prebiotic administration of bimuno-galactooligosaccharides reduced salivary cortisol awakening response in healthy individuals (Schmidt et al., 2015). The reversal of stress effects by probiotics has also been demonstrated. For example, administration of Lactobacillus helveticus NS8 to Sprague Dawley rats improved stress-induced behavior deficits and attenuated the stress-induced levels of corticosterone (Liang et al., 2015) whereas L. rhamnosus JB-1 attenuated anxiety and stress in Balb/C mice (Bravo et al., 2011). Similarly, administration of L. heleveticus R0052 and Bifidobacterium longum R0175 prevented stress-induced changes in neurogenesis, gut barrier integrity, and stress-reactivity (Ait-Belgnaoui et al., 2012). L. rhamnosus GG in combination with polydextrose reverses early-life stress-induced effects on anxiety behavior in the open field, learning in the Morris Water Maze & hippocampal GABA receptor mRNA (McVey Neufeld et al., 2017). Interestingly, the microbiome can be targeted to improve maternal mental health which will have knock-on effects on infant development. A recent double-blind placebo-controlled study with L. rhamnosus during pregnancy and lactation significantly reduced post-partum anxiety and depression (Slykerman et al., 2017).
Taken together these data highlight the importance of the microbiome in early brain development and in regulating the stress response. Future studies must investigate the exact mechanistic relationship that the microbiome has in mediating stress resilience. Moreover, there is a need to assess the impact of environmental enrichment on the microbiome in early life. Interestingly there is a growing interest on the effects of enrichment and exercise on the microbiome in adulthood (Fuller et al., 2018; Mika et al., 2016; Cronin et al., 2018). Moreover, In addition, targeting the microbiome in early life or even prenatally may offer a novel approach to chart the appropriate trajectory for children at risk for early childhood adversity.
Conclusions
Establishing relevant translational animal models is crucial for advancing our understanding of developmental psychobiology in general, and identifying successful interventions in particular. Moreover, a better understanding of how these early interventions prevent negative outcomes will help to improve the well-being of both caretakers and children.
Acknowledgments
The authors would like to express their gratitude to Dr. Kiran Sandhu for designing the figures. JFC is funded by Science Foundation Ireland, Grant/Award Number: SFI/12/RC/2273; ACK receives financial support from the National Institute of Mental Health under Award Number R15MH114035. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the financial supporters. The authors confirm no conflict of interest.
References
- Abdul Aziz NH, Kendall DA, Pardon MC (2012). Prenatal exposure to chronic mild stress increases corticosterone levels in the amniotic fluid and induces cognitive deficits in female offspring, improved by treatment with the antidepressant drug amitriptyline. Behavioural Brain Research, 231, 29–39. [DOI] [PubMed] [Google Scholar]
- Ades LC, et al. (2006). FBN1, TGFBR1, and the Marfan-craniosynostosis/mental retardation disorders revisited. American Journal of Medical Genetics Part A. 140, 1047–1058. [DOI] [PubMed] [Google Scholar]
- Ainsworth MDS (1973). The development of infant-mother attachment In: Review of child development research. Vol. 3, C.B.R. H, ed.. University of Chicago Press, Chicago, pp. 1–94. [Google Scholar]
- Aisa B, et al. , (2007). Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology, 32, 256–66. [DOI] [PubMed] [Google Scholar]
- Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. (2012). Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology, 37(11):1885–95. [DOI] [PubMed] [Google Scholar]
- Aksic M, et al. (2013). Long-term effects of the maternal deprivation on the volume and number of neurons in the rat neocortex and hippocampus. Acta Neurobiol Exp (Wars), 73, 394–403. [DOI] [PubMed] [Google Scholar]
- Alamy M, Bengelloun WA (2012). Malnutrition and brain development: an analysis of the effects of inadequate diet during different stages of life in rat. Neuroscience and Biobehavioral Reviews, 36, 1463–1480. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD, Anderson W (1968). Effects of infantile handling on morphological development of the rat brain: an exploratory study. Developmental Psychobiology, 1, 10–20. [Google Scholar]
- Ahmed-Leitao F, Spies G, van den Heuvel L, Seedat S (2016). Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: a systematic review. Psychiatry Research, 256, 33–43. [DOI] [PubMed] [Google Scholar]
- Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, Williamson DF (2002). Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatric Services, 53, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. Journal of Neuropsychiatry Clinical Neuroscience, 20, 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff E, Hillyer R, Leon M (2016). Environmental enrichment therapy for autism: outcomes with increased access. Neural Plasticity, e2734915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A, Von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, Spruijt BM (2006). Long lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. European Journal of Neuroscience, 23, 261–272. [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, et al. (1995). Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neuroscince Letters, 192, 49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, et al. (2001). Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology, 142, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P (2016). Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology, 63, 217–27. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M (2011). Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behavior and Immunity, 25, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Coe CL (1999). Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol, 35, 146–155. [PubMed] [Google Scholar]
- Bailey S (2012). Kangaroo mother care. Br J Hosp Med (Lond), 73, 278–81. [DOI] [PubMed] [Google Scholar]
- Baker SL, Chebli M, Rees S, LeMarec N, Godbout R, Bielajew C (2008). Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Research, 1213, 98–110. [DOI] [PubMed] [Google Scholar]
- Baldini S, Restani L, Baroncelli L, Coltelli M, Franco R, Cenni MC, Maffei L, Berardi N (2013). Enriched early life experiences reduce adult anxiety-like behavior in rats: a role for insulin-like growth factor 1. The Journal of Neuroscience, 33, 11715–11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Arambula SE, Young LJ (2015). The oxytocin system promotes resilience for the effects of neonatal isolation on adult social attachment in female prairie voles. Translational Psychiatry, 5, e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Gluckman P, Hanson M (2014). The biology of developmental plasticity and the predictive adaptive response hypothesis. Journal of Physiology, 11, 2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayart F et al. (1990). Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta). Behavioral Neuroscience. 104, 98–107. [PubMed] [Google Scholar]
- Beach SRH, Brody GH, Todorov AA, Gunter TD, Philibert RA (2010) Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. Am J Med Genet B Neuropsychiatr Genet 153B:710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW (2014). Role of the microbiota in immunity and inflammation. Cell, 157, 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit D (2004). Infant-parent attachment: Definition, types, antecedents, measurement and outcome. Paediatr Child Health, 9, 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR (1964). Chemical and anatomical plasticity of brain. Science, 3644, 610–619. [DOI] [PubMed] [Google Scholar]
- Bera A, et al. (2014). Effect of kangaroo mother care on vital physiological parameters of the low birth weight newborn. Indian J Community Med, 39, 245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology, 141, 599–609, 609.e1–3. [DOI] [PubMed] [Google Scholar]
- Berk M (2012). Pathways to new drug discovery in neuropsychiatry. BMC Medicine, 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt J, Dewey H, Thrift A, Donnan G (2004). Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke, 35, 1005–1009. [DOI] [PubMed] [Google Scholar]
- Bessinis DP, Dalla C, Kokras N, Pitychoutis PM, Papadopoulou-Daifoti Z (2013). Sex-dependent neurochemical effects of environmental enrichment in the visual system. Neuroscience, 254, 130–140. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D (2001). Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. The Journal of Neuroscience, 21, 5272–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass EM, et al. (1995). Mother as shield: differential effects of contact and nursing on pain responsivity in infant rats--evidence for nonopioid mediation. Behav Neurosci, 109, 342–53. [DOI] [PubMed] [Google Scholar]
- Blaze J, Scheuing L, Roth TL (2013). Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Dev Neurosci, 35, 306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, Asok A, Borrelli K, Tulbert C, Bollinger J, Ronca AE, Roth TL (2017). Interuterine exposure to maternal stress alters Bdnf IV DNA methylation and telomere length in the brain of adult rat offspring. International Journal of Developmental Neuroscience, 62, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, Roth TL (2017). Caregiver maltreatment causes altered neuronal DNA methylation in female rodents. Developmental and Psychopathology, 29, 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Honey RC (1998). Imprinting, learning and development: from behaviour to brain and back. Trends Neurosci, 21, 306–11. [DOI] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. (2014). Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med, 20, 509–18. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, Guaza C (2002). Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology, 26, 204–215. [DOI] [PubMed] [Google Scholar]
- Bowes L, Jaffee SR (2013). Biology, genes and resilience: toward a multidisciplinary approach. Trauma, Violence Abuse, 14, 195–208. [DOI] [PubMed] [Google Scholar]
- Bowlby J (1951). Maternal care and mental health. Vol. (Serial No. 2), ed. World Health Organization Monograph. [PMC free article] [PubMed] [Google Scholar]
- Bowlby J (1958). The nature of the child’s tie to his mother. Int J Psychoanal, 39, 350–73. [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med, 6, 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A, 108,16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ (2004). Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. European Journal of Neuroscience, 20, 1355–1362. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ (2003). Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience, 118, 571–576. [DOI] [PubMed] [Google Scholar]
- Bridges RS (2015). Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol, 36C, 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CP, Smotherman WP, Levine S, 1977. Interaction-induced reduction in differential maternal responsiveness: an effect of cue-reduction or behavior? Dev Psychobiol, 10, 273–80. [DOI] [PubMed] [Google Scholar]
- Brummelte S, et al. (2012). Procedural pain and brain development in premature newborns. Ann Neurol, 71, 385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Galea LA (2016). Postpartum depression: Etiology, treatment and consequences for maternal care. Horm Behav, 77, 153–66. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Grund T, Moll GH, Teuchert-Noodt G, Dawirs RR. (2008). Environmental enrichment has no effect on the development of dopaminergic and GABAergic fibers during methylphenidate treatment of early traumatized gerbils. Journal of Negative Results in Biomedicine, 7:2, doi: 10.1186/1477-5751-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Neddens J, Teuchert-Noodt G. (2007). Alteration in the GABAergic network of the prefrontal cortex in a potential animal model of psychosis. Journal of Neural Transmission, 114, 539–547. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TF (2017). Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience. 342:212–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA (2008). The expectant brain: adapting for motherhood. Nat Rev Neurosci. 9, 11–25. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Jolles J, Twijnstra A, Mellink R, Sulon J (1992). Coping styles, cortisol reactivity, and performance in a vigilance task of patients with persistent postconcussive symptoms after a mild head injury. Int J Neurosci, 64, 97–105. [DOI] [PubMed] [Google Scholar]
- Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. (2017). Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry, 82, 472–487. [DOI] [PubMed] [Google Scholar]
- Buschler A, Manahan-Vaughan D (2012). Brief environmental enrichment elicits metaplasticity of hippocampal synaptic potentiation in vivo. Frontiers in Behavioral Neuroscience, 6, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschert J, Sakalem ME, Saffari R, Hohoff C, Rothermundt M, Arolt V, Zhang Q, Ambree O (2016). Prenatal immune activation in mice blocks the effects of environmental enrichment on exploratory behavior and micoglia density. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 67, 10–20. [DOI] [PubMed] [Google Scholar]
- Caldji C, Hellstrom IC, Zhang TY, Diorio J, Meaney MJ (2011) Environmental regulation of the neural epigenome. FEBS Lett 585:2049–2058. [DOI] [PubMed] [Google Scholar]
- Champagne DL, et al. , (2008). Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 28, 6037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ, 2001. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 133, 287–302. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Chatterjee-Chakraborty M, Rees S, Cauchi J, de Medeiros CB, Fleming AS. (2007). Maternal isolation alters the expression of neural proteins during development: ‘Stroking’ stimulation reverses these effects. Brain Res, 1158, 11–27. [DOI] [PubMed] [Google Scholar]
- Cong X, Ludington-Hoe SM, Walsh S, 2011. Randomized crossover trial of kangaroo care to reduce biobehavioral pain responses in preterm infants: a pilot study. Biol Res Nurs. 13, 204–16. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E (2003). Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biological Psychiatry, 54, 1025–1034. [DOI] [PubMed] [Google Scholar]
- Connors EJ, Shaik AN, Migliore MM, Kentner AC (2014). Environmental enrichment mitigates the sex-specific effects of gestational inflammation on social engagement and the hypothalamic pituitary adrenal axis-feedback system. Brain, Behavior, and Immunity, 42, 178–190. [DOI] [PubMed] [Google Scholar]
- Connors EJ, Migliore MM, Pillsbury SL, Shaik AN, Kentner AC (2014). Environmental enrichment models a naturalistic form of maternal separation and shapes the anxiety response patterns of offspring. Psychoneuroendocrinology, 52, 153–167. [DOI] [PubMed] [Google Scholar]
- Christian LM, Galley JD, Hade EM, Schoppe-Sullivan S, Kamp Dush C, Bailey MT. Gut microbiome composition is associated with temperament during early childhood. Brain Behav Immun. 2015. March;45:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, O’Mahony SM, Dinan TG, Cryan JF. Priming for health: gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatr. 2014. August;103(8):812–9. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013. June;18(6):666–73. [DOI] [PubMed] [Google Scholar]
- Cronin O, Barton W, Skuse P, Penney NC, Garcia-Perez I, Murphy EF, Woods T, Nugent H, Fanning A, Melgar S, Falvey EC, Holmes E, Cotter PD, O’Sullivan O, Molloy MG, Shanahan F. (2018). A Prospective Metagenomic and Metabolomic Analysis of the Impact of Exercise and/or Whey Protein Supplementation on the Gut Microbiome of Sedentary Adults. mSystems, 3, e00044–18. doi: 10.1128/mSystems.00044-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012. October;13(10):701–12. [DOI] [PubMed] [Google Scholar]
- Cymerblit-Sabba A, Lasri T, Gruper M, Aga-Mizrachi S, Zubedat S, Avital A (2013). Prenatal enriched environment improves emotional and attentional reactivity to adulthood stress. Behavioural Brain Research, 241, 185–190. [DOI] [PubMed] [Google Scholar]
- Daniels WM, et al. , 2004. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 19, 3–14. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Bagot RC, Parker KJ, Vinkers CH, de Kloet ER (2013). The three-hit concept of vulnerability and resilience: toward and understanding of adaptation to early life adversity outcome. Psychoneuroendocrinology, 38, 1858–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH, Bell RW, 1960. Critical periods for the effects of infantile experience on adult learning. Science. 131, 227–8. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Ottinger DR, Stephens MW, 1962. Effects of maternal factors upon growth and behavior of the rat. Child Dev. 33, 65–71. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, 1999. Commentary: is maternal stimulation the mediator of the handling effect in infancy? Dev Psychobiol. 34, 1–3. [DOI] [PubMed] [Google Scholar]
- Denenberg VH K. GG; Rosenberg KM; Schell SF, 1968. Programming life histories: An experimental design and initial results Developmental Psychobiology. 1, 3–9. [Google Scholar]
- Denenberg VH, Karas GG, Rosenberg KM, Schell SF (1968). Programming life histories: an experimental design and initial results. Developmental Psychobiology, 1, 3–9. [Google Scholar]
- Dennis CL, McQueen K, 2009. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 123, e736–51. [DOI] [PubMed] [Google Scholar]
- Dinces SM, et al. , 2014. Enhancing offspring hypothalamic-pituitary-adrenal (HPA) regulation via systematic novelty exposure: the influence of maternal HPA function. Front Behav Neurosci. 8, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Blennerhassett P, Lu J, Deng Y, Park AJ, Green W, Denou E, Silva MA, Santacruz A, Sanz Y, Surette MG, Verdu EF, Collins SM, Bercik P. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat Commun. 2015. July 28;6:7735. [DOI] [PubMed] [Google Scholar]
- de Medeiros CB, Fleming AS, Johnston CC, Walker CD. (2009). Artificial rearing of rat pups reveals the beneficial effects of mother care on neonatal inflammation and adult sensitivity to pain. Pediatr Res, 66, 272–277. doi: 10.1203/PDR.0b013e3181b1be06. [DOI] [PubMed] [Google Scholar]
- de Weerth C Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev. 2017. December;83:458–471. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014. February;19(2):146–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezsi G, Ozturk E, Salzberg MR, Morris M, O’Brien TJ, Jones NC. (2016). Environmental enrichment imparts disease-modifying and transgenerational effects on genetically-determined epilepsy and anxiety. Neurobiol Dis, 93, 129–36. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Krech D, Rosenzweig MR (1964). The effects of an enriched environment on the histology of the rat cerebral cortex. The Journal of Comparative Neurology, 123, 111–120. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011. February 15;108(7):3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J Physiol. 2017. January 15;595(2):489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TS, Forster A, Roth TL (2016). Global and gene specific DNA methylation alterations in the adolescent amygdala and hippocampus in an animal model of caregiver maltreatment. Behavioural Brain Research, 298, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J, Rodger J, Li C, Tan X, Wong K, de Klerk N, Leonard H (2018). Environmental enrichment intervention for Rett syndrome: an individually randomized stepped wedge trial. Orphanet Journal of Rare Diseases, 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy-Doherty F, O’Mahony SM, Peterson VL, O’Sullivan O, Crispie F, Cotter PD, Wigmore P, King MV, Cryan JF, Fone KCF. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav Immun. 2018. February;68:261–273. [DOI] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Anda RF, Felitti VJ. Experiencing multiple forms of childhood maltreatment and adult mental health in community respondents: results from the Adverse Childhood Experiences (ACE) Study. American Journal of Psychiatry. 2003;160(8):1453–1460. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Hertzman C, Lam LL, Armstrong JM, Neumann SM, Kobor MS (2013) Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Dev 84:58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etringer S, Buss C, Wadhwa PD (2015). Prenatal stress, development, health and disease risk: a psychobiological perspective – 2015 Curt Richter Award Paper. Psychoneuroendocrinology, 62, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estanislau C, Morato S (2005). Prenatal stress produces more behavioral alterations than maternal separation in the elevated plus-maze and in the elevated T-maze. Behavioral Brain Research, 163, 70–77. [DOI] [PubMed] [Google Scholar]
- Estes ML, McAllister AK (2016). Maternal immune activation: implications for neuropsychiatric disorders. Science, 353, 6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faherty CJ, Shepard KR, Herasimtschuk A, Smeyne RJ (2005). Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Molecular Brain Research, 134, 170–179. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R (2002). Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cellular and Molecular Neurobiology, 22, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience, 10, 446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Rosenthal Z, Eidelman AI, 2014. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 75, 56–64. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventative Medicine, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Ferber SG, 2004. The nature of touch in mothers experiencing maternity blues: the contribution of parity. Early Hum Dev. 79, 65–75. [DOI] [PubMed] [Google Scholar]
- Fernandez Medina IM, et al. , 2017. Bonding in neonatal intensive care units: Experiences of extremely preterm infants’ mothers. Women Birth. [DOI] [PubMed] [Google Scholar]
- Field T, 2010. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. 33, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Dieter JN, Kumar A, Schanberg S, Kuhn C (2008). Insulin and insulin-like growth factor-1 increased in preterm neonates following massage therapy. Journal of Developmental and Behavioral Pediatrics, 29, 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Anderson V, 1987. Hormonal and attitudinal correlates of maternal behavior during the early postpartum period. Dev Psychol. 26, 137–143. [Google Scholar]
- Fleming AS, et al. , 1997a. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm Behav. 31, 145–58. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Steiner M, Corter C, 1997b. Cortisol, hedonics, and maternal responsiveness in human mothers. Horm Behav. 32, 85–98. [DOI] [PubMed] [Google Scholar]
- Francis DD, et al. , 1999. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 46, 1153–66. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ (1999). Maternal care, gene expression, and the development of individual differences in stress reactivity. Annals of the New York Academy of Sciences, 896, 66—84. [DOI] [PubMed] [Google Scholar]
- Frick KM & Fernandez SM (2003). Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiology of Aging, 24, 615–626. [DOI] [PubMed] [Google Scholar]
- Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017. March 19;7:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller AK, Bice BD, Venancio AR, Crowley OM, Staab AM, Georges SJ, Hidalgo JR, Warncke AV, Angus-Hill ML. (2018). A Method to Define the Effects of Environmental Enrichment on Colon Microbiome Biodiversity in a Mouse Colon Tumor Model. J Vis Exp. 28;(132). doi: 10.3791/57182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapp K, Bohacek J, Grossmann J, Brunner AM, Manuella F, Nanni P, Mansuy IM (2016). Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology, 41, 2749–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, et al. , 2013. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 110, 15638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb RL, Gonzalez CLR, Kolb B (2014). Prenatal enrichment and recovery from perinatal cortical damage: effects of maternal complex housing. Frontiers in Behavioral Neuroscience, 8, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, and Baram TZ (1996). Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr. Neurol.15,114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, et al. , 2016. Gestational hormone profiles predict human maternal behavior at 1-year postpartum. Horm Behav. 85, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Hwang H-MF, Gorman C (1995). Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proceedings of the National Academy of Sciences of the United States of America, 82, 4549–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo OL, O’Mara SM (2004). Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemia. Behavioural Brain Research, 152, 231–241. [DOI] [PubMed] [Google Scholar]
- Goldfarb N (2012). Adverse event terminology. J Clin Res Best Prac, 8, 1–17. [Google Scholar]
- Golubeva AV, Crampton S, Desbonnet L, Edge D, O’Sullivan O, Lomasney KW, Zhdanov AV, Crispie F, Moloney RD, Borre YE, Cotter PD, Hyland NP, O’Halloran KD, Dinan TG, O’Keeffe GW, Cryan JF. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology. 2015. October;60:58–74. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS (2001). Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Developmental Psychobiology, 38, 11–32. [DOI] [PubMed] [Google Scholar]
- Gouvier WD, Cubmic B, Jones G, Brantley P, Cutlip Q (1992). Postconcussion symptoms and daily stress in normal and head-injured college populations. Arch Clin Neuropsychol, 7, 193–211. [PubMed] [Google Scholar]
- Gur TL, Shay L, Palkar AV, Fisher S, Varaljay VA, Dowd S, Bailey MT. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav Immun. 2017. August;64:50–58. [DOI] [PubMed] [Google Scholar]
- Gur TL, Worly BL, Bailey MT. Stress and the commensal microbiota: importance in parturition and infant neurodevelopment. Front Psychiatry. 2015. February 2;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan A; Dinan TG; Cryan JF True grit: the role of neuronal microRNAs as mediators of stress resilience Current Opinion in Behavioral Sciences 14, 9–18 2017. [Google Scholar]
- Hall D, Kirsten G, 2008. Kangaroo Mother Care--a review. Transfus Med. 18, 77–82. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW (1999). Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse, 32, 37–43. [DOI] [PubMed] [Google Scholar]
- Harlow H.H. M, 1965. The affectional system In: Behavior of nonhuman primates. Vol. 2, A.H. Schrier H; Stollnitz F, ed.^eds. Academic Press, New York, pp. 287–334. [Google Scholar]
- van Harmelen A-L, van Tol M-J, van der Wee NJA, Veltman DJ, Aleman A, Spinhoven P, … Elzinga BM (2010). Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biological Psychiatry, 68, 832–838. [DOI] [PubMed] [Google Scholar]
- He S, Ma J, Liu N, Yu X (2010). Early enriched environment promotes neonatal GABAergic neurotransmission and accelerates synapse maturation. The Journal of Neuroscience, 30, 7910–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, et al. , 1980. Maternal behavior, pup vocalizations, and pup temperature changes following handling in mice of 2 inbred strains. Dev Psychobiol. 13, 573–84. [DOI] [PubMed] [Google Scholar]
- Heun-Johnson H, Levitt P (2016). Early-life stress paradigm transiently alters maternal behavior, dam-pup interactions, and offspring vocalizations in mice. Frontiers in Behavioral Neuroscience, 10, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Moloney G, Shanahan F, Dinan TG, Clarke G, Cryan JF. The microbiome regulates amygdala-dependent fear recall. Mol Psychiatry. 2017. May 16. doi: 10.1038/mp.2017.100. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, M Moloney G, Moloney RD, Shanahan F, Dinan TG, Cryan JF, Clarke G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome. 2017. August 25;5(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016. April 5;6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, et al. , 2015. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 105, 112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton CL, Garner JP, Mench JA (2008) Effects of a running wheel-igloo enrichment on aggression, hierarchy linearity, and stereotypy in group-housed male CD-1 (ICR) mice. Appl Anim Behav Sci, 115, 90–103. [Google Scholar]
- Huot RL, et al. , 2002. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 950, 52–63. [DOI] [PubMed] [Google Scholar]
- Hyman SE. (2012). Revolution Stalled Sci Transl Med., 4, 155cm11. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm A-C (2000). Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Experimental Neurology, 164, 45–52. [DOI] [PubMed] [Google Scholar]
- Ivy AS, et al. , 2008. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 154, 1132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans JE, Woodside BC (1990). Nest temperature: effects on maternal behavior, pup development, and interactions with handling. Developmental Psychobiology, 23, 519–534. [DOI] [PubMed] [Google Scholar]
- Janssen H, Ada L, Bernhardt J, McElduff P, Pollack M, Nilsson M, Spratt NJ (2014). An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed-rehabilitation unit: a pilot non-randomized controlled trial. Disability and Rehabilitation, 36, 255–262. [DOI] [PubMed] [Google Scholar]
- Janssen H, Ada L, Karayanidis F, Drysdale K, McElduff P, Pollack M, White J, Nilsson M, Bernhardt J, Spratt NJ (2012). Translating the use of an enriched environment poststroke from bench to bedside: study design and protocol used to test the feasibility of environmental enrichment on stroke patients in rehabilitation. International Journal of Stroke, 7, 5210526. [DOI] [PubMed] [Google Scholar]
- Jin Z, Wu J, Oh SY, Kim KW, Shin BS (2010). The effect of stress on stroke recovery in a photothrombotic stroke animal model. Brain Res, 1363, 191–197. [DOI] [PubMed] [Google Scholar]
- Johansson BB. (2004). Functional and cellular effects of environmental enrichment after experimental brain infarcts. Restorative Neurology and Neuroscience, 22,163–174. [PubMed] [Google Scholar]
- Joffe JM, Milkovic K, Levine S, 1972. Effects of changes in maternal pituitary-adrenal function on behavior of rat offspring. Physiol Behav. 8, 425–30. [DOI] [PubMed] [Google Scholar]
- Johnston CC, et al. , 2003. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 157, 1084–8. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Sachser N (2005). The effects of prenatal social stress on behavior: mechanisms and function. Neuroscience and Biobehavioral Reviews, 29, 283–294. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim JM, Stewart R, Kim SY, Bae KY, Kim SW, Shin IS, Shin MG, Yoon JS (2013) Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog Neuropsychopharmacol Biol Psychiatry 44:23–28. [DOI] [PubMed] [Google Scholar]
- Kashan AS, Abel KM, McNamee R, Pederson MG, Webb RT, Baker PN, Kenny LC, Mortensen PB (2008). Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry, 65,146–152. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Hoffman JH, Austin-LaFrance RJ, Bronzino JD (1995). Neonatal isolation enhances hippocampal dentate response to tetanization in freely moving juvenile male rats. Experimental Neurology, 136, 89–97. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016. November;82:109–18. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature, 386, 493–495. [DOI] [PubMed] [Google Scholar]
- Kentner AC (2015). Neuroprotection from early-life adversity: considerations for environmental enrichment. Neural Regeneration Research, 10, 1545–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, Khoury A, Lima Queiroz E, MacRae M (2016). Environmental enrichment rescues the effects of early life inflammation on markers of synaptic transmission and plasticity. Brain, Behavior, and Immunity, 57, 151–160. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Abizaid A, Bielajew C (2010). Modeling Dad: animal models of paternal behavior. Neuroscience & Biobehavioral Reviews, 34, 438–451. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Lima E, Migliore MM, Shin J, Scalia S (2018). Complex environmental rearing enhances social salience and affects hippocampal corticotropin releasing hormone receptor expression in a sex-specific manner. Neuroscience, 369, 399–411. [DOI] [PubMed] [Google Scholar]
- Kentner AC, Scalia S, Shin J, Migliore MM, Rondón-Ortiz AN (2018). Targeted sensory enrichment interventions protect against behavioral and neuroendocrine consequences of early life stress. Psychoneuroendocrinology. 98, 74-85. [DOI] [PubMed] [Google Scholar]
- Khan F, Amatya B, Elmalik A, Lowe M, Ng L, Reid I, Galea MP (2016). An enriched environmental programme during inpatient neuro-rehabilitation: A randomized controlled trial. J. Rehabil. Med. 48, 417–25. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Capitanio JP, Leibel R, Deng L, LeDuc C, Haghighi F, Mann JJ (2010) Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes Brain Behav 9:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Xenos D, Salmaso N, Tran KM, Brand T, Schwartz ML, Ment L, Vaccarino FM (2013). Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. The Journal of Neuroscience, 33, 13375–13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgan AC, O’Leary E, Bauer J, Fortier A, Weaver ICG, Perrot TS (2016). Effects of paternal predation risk and rearing environment on maternal investment and development of defensive responses in the offspring. eNeuro, 3, e0231–16.2016 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgan AC, O’Leary E, King JL, Weaver ICG, Perrot TS (2018). Effects of paternal high-fat diet and rearing environment on maternal investment and development of defensive responses in the offspring. Psychoneuroendocrinology, 91, 20–30. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ, 2009. The pathways from mother’s love to baby’s future. Front Behav Neurosci. 3, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Blacher E, Elinav E, Pettersson S. Our Gut Microbiome: The Evolving Inner Self. Cell. 2017. December 14;171(7):1481–1493. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM (2005). Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiology of Learning and Memory, 83, 206–216. [DOI] [PubMed] [Google Scholar]
- Landi S, Ciucci F, Maffei L, Berardi N, Cenni MC (2009). Setting the pace for retinal development: environmental enrichment acts through insulin-like growth factor 1 and brain-derived neurotrophic factor. The Journal of Neuroscience, 29, 10809–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latva R, et al. , 2007. Visits by the family to the neonatal intensive care unit. Acta Paediatr. 96, 215–20. [DOI] [PubMed] [Google Scholar]
- Laviola G, Rea M, Morley-Fletcher S, Di Carlo S, Bacosi A, De Simone R, Bertini M, Pacifici R (2004). Beneficial effects of enriched environment on adolescent rats from stressed pregnancies. European Journal of Neuroscience, 20, 1655–1664. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang Y-P, Hairston IS, Korade-Mirnics Z, Lee VM-Y, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS (2005). Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell, 120, 701–713. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Mian FM, Stanisz AM, Bindels LB, Cambier E, Ben-Amram H, Koren O, Forsythe P, Bienenstock J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017. April 4;8:15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, et al. , 2007. Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neurosci Res. 58, 32–9. [DOI] [PubMed] [Google Scholar]
- Levi O, Jongen-Relo AL, Feldon J, Roses AD, Michaelson DM (2003). ApoE4 impairs hippocampal plasticity isoform-specifically and blocks the environmental stimulation of synaptogenesis and memory. Neurobiology of Disease, 13, 273–282. [DOI] [PubMed] [Google Scholar]
- Levine S (1968). Influence of infantile stimulation on the response to stress during preweaning development. Developmental Psychobiology, 1, 67–70. [Google Scholar]
- Levine S, Chevalier JA, Korchin SJ (1956). The effects of early handling and shock on later avoidance behavior. Journal of Personality, 24, 475–493. [DOI] [PubMed] [Google Scholar]
- Levine S, 1962. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 135, 795–6. [DOI] [PubMed] [Google Scholar]
- Levine S, 1967. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 156, 258–60. [DOI] [PubMed] [Google Scholar]
- Levine S, Stanton ME, Gutierrez YR, 1988. Maternal modulation of pituitary-adrenal activity during ontogeny. Adv Exp Med Biol. 245, 295–310. [DOI] [PubMed] [Google Scholar]
- Lin EJ, Choi E, Liu X, Martin A, During MJ (2011). Environmental enrichment exerts sex- specific effects on emotionality in C57BL/6J mice. Behavioural Brain Research, 216, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, et al. , 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 277, 1659–62. [DOI] [PubMed] [Google Scholar]
- Liu D, et al. , 2000. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 3, 799–806. [DOI] [PubMed] [Google Scholar]
- Loewy J (2015). NICU music therapy: song of kin as critical lullaby in research and practice. Ann NY Acad Sci, 1337, 178–185. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Boivin M, Hertzman C, Fleming AS (2017). Parenting begets parenting: A neurobiological perspective on early adversity and the transmission of parenting styles across generations. Neuroscience, 342, 120–139. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Chatterjee M, Steiner M, Kraemer GW (2011). Effects of motherless rearing on basal and stress-induced corticosterone secretion in rat pups. Stress, 16, 685–696. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Melo AI (2016). Deconstructing the function of maternal stimulation in offspring development: insights from the artificial rearing model in rats. Hormones and Behavior, 77, 224–236. [DOI] [PubMed] [Google Scholar]
- Lordi B, Patin V, Protais P, Mellier D, Caston J (2000). Chronic stress in pregnant rats: effects on growth rate, anxiety and memory capabilities of the offspring. International Journal of Psychophysiology, 37, 195–205. [DOI] [PubMed] [Google Scholar]
- Lorenz KZ, 1970. Companions as factors in the bird’s environment In: Studies in animal and human behavior. Vol., Lorenz KZ, ed.^eds. Harvard University Press, Cambridge. [Google Scholar]
- Lorenz r.J., (1972). Effects of differential preweaning social isolation on emotional reactivity and stress tolerance in the rat. Developmental Psychobiology, 5, 201–213. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS (2004). Artificially reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task--reversal of effects with maternal-like licking stimulation. Behavioral Brain Research, 148, 209–219. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS, Fletcher PJ (2006). Early life tactile stimulation changes adult rat responsiveness to amphetamine. Pharmacology, Biochemistry and Behavior, 84, 497–503. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS (2015). Propagation of maternal behavior across generations is associated with changes in non-maternal cognitive and behavioral processes. Behav Processes,117, 42–7. doi: 10.1016/j.beproc.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Lovic V, Belay H, Walker C-D, Burton CL, Meaney MJ, Sokolowski M, Fleming AS (2013). Early postnatal experience and DRD2 genotype affect dopamine receptor expression in the rat ventral striatum. Behavioural Brain Research, 237, 278–282. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, et al. , 2000. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 20, 561–92. [DOI] [PubMed] [Google Scholar]
- Luby JL, et al. , 2012. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc Natl Acad Sci U S A. 109, 2854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, et al. , 2016. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. Proc Natl Acad Sci U S A. 113, 5742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Tramullas M, Viola M, Shanahan F, Clarke G, O’Mahony S, Dinan TG, Cryan JF. Microbiota regulates visceral pain in the mouse. Elife. 2017. June 20;6 pii: e25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG, Cryan JF. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. 2016. November;44(9):2654–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int J Neuropsychopharmacol. 2016. August 12;19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, et al. , 2011. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 108, 14324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Schatzberg AF, 2010. Animal models of early life stress: implications for understanding resilience. Dev Psychobiol. 52, 616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae M, Macrina T, Khoury A, Migliore MM, Kentner AC (2015). Tracing the trajectory of behavioral impairments and oxidative stress in an animal model of neonatal inflammation. Neuroscience, 298, 455–466. [DOI] [PubMed] [Google Scholar]
- Malkasian DR, Diamond MC (1971). The effects of environmental manipulation on the morphology of the neonate rat brain. International Journal of Neuroscience, 2, 161–170. [DOI] [PubMed] [Google Scholar]
- Maegele M, Lippert-Gruener M, Ester-Bode T, Garbe J, Bouillon B, Neugebauer E, Klug N, Lefering R, Neiss EF, Angelov DN (2005). Multimodal early onset stimulation combined with enriched environment is associated with reduced CNS lesion volume and enhanced reversal of neuromotor dysfunction after traumatic brain injury in rats. European Journal of Neuroscience, 21, 2406–2418. [DOI] [PubMed] [Google Scholar]
- Maccari S, Polese D, Reynaert M-L, Amici T, Morley-Fletcher S, Fagioli F (2017). Early-life experiences and the development of adult diseases with a focus on mental illness: the human birth theory. Neuroscience, 342, 232–251. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O (2003). Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neuroscience & Biobehavioral Reviews, 27, 119–127. [DOI] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ (2013). Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Science USA, 110, 18274–18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam c.D., Crawford EF, Eisch AJ, Rivier CL, Richardson HN (2008). Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Developmental Neurobiology, 68, 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashoodh R, Franks B, Curley JP, Champagne FA (2012). Paternal social enrichment effects on maternal behavior and offspring growth. Proceedings of the National Academy of Sciences of the united States of America, 109, 17232–17238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey Neufeld KA, O’Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, Cryan JF. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr Neurosci. 2017. November 27:1–10. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, et al. , 2012. Attachment security as a mechanism linking foster care placement to improved mental health outcomes in previously institutionalized children. J Child Psychol Psychiatry. 53, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, Audet M-C, Anisman H (2012) Environmental enrichment in male CD-1 mice promotes aggressive behaviours and elevated corticosterone and brain norepinephrine activity in response to a mild stressor. Stress, 15, 354–360. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, et al. , 1991. The effects of neonatal handling on the development of the adrenocortical response to stress: implications for neuropathology and cognitive deficits in later life. Psychoneuroendocrinology. 16, 85–103. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM (1996). Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental Neuroscience, 18, 49–72. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, 2005. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 7, 103–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, et al. , 2009. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 50, 943–51. [DOI] [PubMed] [Google Scholar]
- Adv Neurobiol. 2015;10:219–48. doi: 10.1007/978-1-4939-1372-5_11. [DOI] [PubMed] [Google Scholar]
- Meyer JS, et al. , 1975. Behavioral and hormonal effects of attachment object separation in surrogate-peer-reared and mother-reared infant rhesus monkeys. Dev Psychobiol. 8, 425–35. [DOI] [PubMed] [Google Scholar]
- Miceli PJ, et al. , 2000. Brief report: birth status, medical complications, and social environment: individual differences in development of preterm, very low birth weight infants. J Pediatr Psychol. 25, 353–8. [DOI] [PubMed] [Google Scholar]
- Mika A, Rumian N, Loughridge AB, Fleshner M. (2016). Exercise and Prebiotics Produce Stress Resistance: Converging Impacts on Stress-Protective and Butyrate-Producing Gut Bacteria. Int Rev Neurobiol. 2016;131:165–191. doi: 10.1016/bs.irn.2016.08.004 [DOI] [PubMed] [Google Scholar]
- Mooncey S, et al. , 1997. The effect of mother-infant skin-to-skin contact on plasma cortisol and β-endorphin concentration in preterm newborns Infant behavior and development. 20, 553–557. [Google Scholar]
- Mooney-Leber SM, Brummelte S, 2017. Neonatal pain and reduced maternal care: Early-life stressors interacting to impact brain and behavioral development. Neuroscience. 342, 21–36. [DOI] [PubMed] [Google Scholar]
- Moriceau S, et al. , 2004. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 22, 415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Sullivan RM, 2010. Rodent model of infant attachment learning and stress. Dev Psychobiol. 52, 651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome. 2014. February;25(1–2):49–74 [DOI] [PubMed] [Google Scholar]
- Morgan C, Novak I, Badawi N (2013). Enriched environments and motor outcomes in cerebral palsy: systematic review and meta-analysis. Pediatrics, 132, e735–746. [DOI] [PubMed] [Google Scholar]
- Morgan C, Novak I, Dale RC, Badawi N (2015). Optimizing motor learning in infants at high risk of cerebral palsy: a pilot study. BMC Pediatrics, 15, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Diaz-Cintra S, Cintra L, Kemper T, Galler JR (1993). Prenatal malnutrition and development of the brain. Neuroscience and Biobehavioral Reviews, 17, 91–128. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G (2003). Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. European Journal of Neuroscience, 18, 3367–3374. [DOI] [PubMed] [Google Scholar]
- Munos BH (2013). Pharmaceutical Innovation Gets a Little Help from New Friends. Sci Transl Med, 5, 168ed1. [DOI] [PubMed] [Google Scholar]
- Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015. February;21(2):109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd AT, Berding K, Wang M, Donovan SM, Dilger RN. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes. 2017. November 2;8(6):589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Quinn JP, Sharp HM, Pickles A, Hill J. (2015). Effects of prenatal and postnatal depression, and maternal stroking, at the glucocorticoid receptor gene. Transl Psychiatry, 5, e560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Gibb R, Kolb B (2011). Prenatal bystander stress induces neuroanatomical changes in the prefrontal cortex and hippocampus of developing rat offspring. Brain Research, 1412, 55–62. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Bolb B (2014). Environmental enrichment alters structural brain plasticity of the adolescent brain but does not remediate the effects of prenatal nicotine exposure. Synapse, 68, 293–305. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J (2008). Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology, 33, 441–456. [DOI] [PubMed] [Google Scholar]
- Nelson CA 3rd, et al. , 2007. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 318, 1937–40. [DOI] [PubMed] [Google Scholar]
- NICHD Early Care Research Network, 2006. Infant-mother attachment classification: risk and protection in relation to changing maternal caregiving quality. Dev Psychol. 42, 38–58. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Barkus C, Murphy M, Hannan AJ (2008). Gene-environment interactions modulating cognitive function and molecular correlates of synaptic plasticity in Huntington’s disease transgenic mice. Neurobiology of Disease, 29, 490–504. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Levis H & Murphy M (2004). Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiology of Learning and Memory, 81, 200–210. [DOI] [PubMed] [Google Scholar]
- Novkovic T, Mittman T, Manahan-Vaughan D (2015). BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus, 25, 1–15. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM (2008) Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 3:97–106. [DOI] [PubMed] [Google Scholar]
- Olson a.K., Eadie BD, Ernst C, Christie BR (2006). Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus, 16, 250–260. [DOI] [PubMed] [Google Scholar]
- Ohlsson AL & Johansson BB (1995). Environment influences functional outcome of cerebral infarction in rats. Stroke, 26, 644–649. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Dinan TG, Cryan JF. Early-life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience. 2017. February 7;342:37–54. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, Marchesi JR, O’Toole PW, Dinan TG, Cryan JF. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience. 2014. September 26;277:885–901. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009. February 1;65(3):263–7. [DOI] [PubMed] [Google Scholar]
- Pedrini Schuch C, Diaz R, Deckmann I, Roja J, Deniz B, Pereira L (2016). Early environmental enrichment affects neurobehavioral development and prevents brain damage in rats submitted to neonatal hypoxia-ischemia. Neuroscience Letters, 617, 101–107. [DOI] [PubMed] [Google Scholar]
- Partrick KA, Chassaing B, Beach LQ, McCann KE, Gewirtz AT, Huhman KL. Acute and repeated exposure to social stress reduces gut microbiota diversity in Syrian hamsters. Behav Brain Res. 2018. February 21;345:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R, Guth R, Herring A, Reynolds L, Oberle S, Smith J (2017). Enhancing sensory experiences for very preterm infants in the NICU: an integrative review. Journal of Perinatology, 37, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ (1993). Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Research Molecular Brain Research, 18, 195–200. [DOI] [PubMed] [Google Scholar]
- Purpura G, Tinelli F, Bargagna S, Bozza M, Bastiani L, Cioni G (2014). Effect of early multisensory massage intervention on visual functions in infants with Down Syndrome. Early Human Development, 90, 809–813. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J, 2001. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol. 38, 239–51. [DOI] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang Y-P, Lockhard DJ, Schultz PG, Tsien JZ, Hu Y (2000). Effects of environmental enrichment on gene expression in the brain. Proceedings of the National Academy of Sciences of the United States of America, 97, 12880–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangon CM, Fortes S, Leliévre V, Leroux P, Plaisant F, Joubert C, Lanfumey L, Cohen-Salmon C, Gressens P (2007). Chronic mild stress during gestation worsens neonatal brain lesions in mice. The Journal of Neuroscience, 27, 7532–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, et al. , 2010. The neurobiology of infant maternal odor learning. Braz J Med Biol Res. 43, 914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Tang AC, 2012. Functional specificity in the modulation of novelty exposure effects by reliability of maternal care. Behav Brain Res. 226, 345–50. [DOI] [PubMed] [Google Scholar]
- Rema V, Ebner FF (1999). Effect of enriched environment rearing on impairments in cortical excitability and plasticity after prenatal alcohol exposure. Journal of Neuroscience, 19, 10993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick MB, et al. , 1990. Effects of birth weight and sociodemographic variables on mental development of neonatal intensive care unit survivors. Am J Obstet Gynecol. 162, 374–8. [DOI] [PubMed] [Google Scholar]
- Reynolds LC, et al. , 2013. Parental presence and holding in the neonatal intensive care unit and associations with early neurobehavior. J Perinatol. 33, 636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S, Lane SJ, Richards L (2010). Using animal models of enriched environments to inform research on sensory integration intervention from the rehabilitation of neurodevelopmental disorders. Journal of Neurodevelopmental Disorders, 2, 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgoblo C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M (2005). Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America, 102, 11557–11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, et al. , 2008. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 149, 4892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Porcel F, Green D, Khatri N, Harris SS, May WL, Lin RC, Paul IA (2011). Neonatal exposure of rats to antidepressants affects behavioral reactions to novelty and social interactions in a manner analogous to autistic spectrum disorders. Anatomical Record, 294, 1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Vo U, Buret LS, Pang TY, Meiklejohn H, Zeleznikow-Johnston A, Churilov L, van den Buuse M, Hannan AJ, Renoir T (2016). Dissociating the therapeutic effects of environmental enrichment and exercise in a mouse model of anxiety with cognitive impairment. Translational Psychiatry, 6, e794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg KM, Denenberg VH, Zarrow MX, 1970. Mice (Mus musculus) reared with rat aunts: the role of rat-mouse contact in mediating behavioural and physiological changes in the mouse. Anim Behav. 18, 138–43. [DOI] [PubMed] [Google Scholar]
- Rosbergen CM, Grimley RS, Hayward KS, Walker KC, Rowley D, Campbell AM, McGufficke S, Robertson ST, Trinder J, Janssen H, Brauer SG (2017). Embedding an enriched environment in an acute stroke unit increases activity in people with stroke: a controlled before-after pilot study. Clinical Rehabilitation, 31, 1516–1528. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, 1980. Hormonal and nonhormonal regulation of maternal behavior: a theoretical survey. Reprod Nutr Dev. 20, 791–800. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR (2007). Modifications of brain circuits through experience In: Bermúdez-Rattoni F (ed). Neural Plasticity and Memory: From Genes to Brain Imaging. Boca Raton (FL): CRC Press/Taylor & Francis, Chapter 4, Frontiers in Neuroscience. [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL. (1960). A search for relations between brain chemistry and behavior. Psychological Bulletin, 57, 476–492. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC (1962). Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. Journal of Comparative and Physiological Psychology, 55, 429–437. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM, 2005. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 57, 823–31. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sweatt JD (2011) Annual Research Review: epigenetic mechanisms and environmental shaping of the brain during sensitive periods of development. J Child Psychol Psychiatry 52: 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, et al. , 2013. Neurobiology of secure infant attachment and attachment despite adversity: a mouse model. Genes Brain Behav. 12, 673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, et al. , 2014. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev Psychobiol. 56, 1755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CL, Pappas BA(1990). Prenatal exposure to antiadrenergic antihypertensive drugs: effects on neurobehavioral development and the behavioral consequences of enriched rearing. Neurotoxicology and Teratology, 12, 359–366. [DOI] [PubMed] [Google Scholar]
- Salmaso N, Tomasi S, Vaccarino FM (2014). Neurogenesis and maturation in neonatal brain injury. Clinics in Perinatology, 41, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015. May 13;17(5):565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM, 2001. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 13, 419–49. [DOI] [PubMed] [Google Scholar]
- Santarelli S, et al. , 2017. An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology. 78, 213–221. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, & Meaney MJ 1986. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res, 396(1), 64–76. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016. November;39(11):763–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer HR, Emerson PE, 1964. The Development of Social Attachments in Infancy. Monogr Soc Res Child Dev. 29, 1–77. [PubMed] [Google Scholar]
- Schapiro S, Geller E, Eiduson S 1962. Neonatal adrenal cortical response to stress and vasopressin. Proc Soc Exp Biol Med, 109, 937–941. [DOI] [PubMed] [Google Scholar]
- Scher MS, et al. , 2009. Neurophysiologic assessment of brain maturation after an 8-week trial of skin-to-skin contact on preterm infants. Clin Neurophysiol. 120, 1812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, et al. , 2002. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res. 139, 39–49. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Enthoven L, van Woezik JH, Levine S, de Kloet ER, Oitzl MS. (2004). The dynamics of the hypothalamic-pituitary-adrenal axis during maternal deprivation. J Neuroendocrinol, 16, 52–57. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology (Berl). 2015. May;232(10):1793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, & Przewlocki R (2005). Behavioral alterations in rats prenatally exposed to valproic acid: Animal model of autism. Neuro- psychopharmacology, 30, 80–89. [DOI] [PubMed] [Google Scholar]
- Schneider T, Turczak J, & Przewlocki R (2006). Environmental en- richment reverses behavioral alterations in rats prenatally exposed to valproic acid: Issues for a therapeutic approach in autism. Neuropsycho- pharmacology, 31, 36–46 [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA (2002). Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Molecular Psychiatry, 7, 810–813. [DOI] [PubMed] [Google Scholar]
- Schwartz S (1964). Effect of neonatal cortical lesions and early environmental factors on adult rat behavior. Journal of Comparative and Physiological Psychology, 57, 72–77. [DOI] [PubMed] [Google Scholar]
- Siegel HI, Rosenblatt JS, 1975. Progesterone inhibition of estrogen-induced maternal behavior in hysterectomized-ovariectomized virgin rats. Horm Behav. 6, 223–30. [DOI] [PubMed] [Google Scholar]
- Simon AK, Hollander GA, McMichael A (2015). Evolution of the immune system in humans from infancy to old age. Proceedings of the Royal Society B, 282 (1821): 20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Ryan C, Curley A, Mulcaire J, Kelly JP (2012). Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 37, 227–236. [DOI] [PubMed] [Google Scholar]
- Such c.P., Jeffers MS, Antonescu S, Nguemeni C, Gomez-Smith M, Pereira LO, Morshead c.M., Corbett D (2016). Enriched rehabilitation promotes motor recovery in rats exposed to neonatal hypoxia-ischemia. Behavioural Brain Research, 304, 42–50. [DOI] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016. January 28;164(3):337–40. [DOI] [PubMed] [Google Scholar]
- Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2017. August 2. doi: 10.1111/nyas.13416. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP (2011) The impact of environmental enrichment in laboratory rats-behavioural and neurochemical aspects. Behav Brain Res, 222, 246–264. [DOI] [PubMed] [Google Scholar]
- Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, Kang J, Rowden J, Stone P, Crane J, Stanley T, Abels P, Purdie G, Maude R, Mitchell EA; Probiotic in Pregnancy Study Group. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine. 2017. October;24:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, Vavasseur A, et al. (2011). Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 70, 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Spencer SJ (2012). Preweaning over- and underfeeding alters onset of puberty in the rat without affecting kisspeptin. Biology of Reproduction, 86, 145, 1–8. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Brown CP, Levine S, 1977a. Maternal responsiveness following differential pup treatment and mother-pup interactions. Horm Behav. 8, 242–53. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, et al. , 1977b. Maternal pituitary-adrenal responsiveness as a function of differential treatment of rat pups. Dev Psychobiol. 10, 113–22. [DOI] [PubMed] [Google Scholar]
- Spitz RA, 1945. Hospitalism; an inquiry into the genesis of psychiatric conditions in early childhood. Psychoanal Study Child. 1, 53–74. [PubMed] [Google Scholar]
- Spivey JM, et al. , 2011. Effects of maternal separation, early handling, and gonadal sex on regional metabolic capacity of the preweanling rat brain. Brain Res. 1367, 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton ME, Wallstrom J, Levine S, 1987. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev Psychobiol. 20, 131–45. [DOI] [PubMed] [Google Scholar]
- Stern JM, Lonstein JS (1996). Nursing behavior in rats is impaired in a small nestbos and with hyperthermic pups. Developmental Psychobiology, 29, 101–122. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, Dinan TG, Cryan JF. Microbes & neurodevelopment--Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015. November;50:209–220. doi: 10.1016/j.bbi.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ, 2010. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci Biobehav Rev. 34, 835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny S, MacBeth SS (2016). The effects of paternal depression on child and adolescent outcomes: a systematic review. Journal of Affective Disorders, 205, 44–59. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver ICG, Champagne FA, Diorio J, Meaney M (2005). Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Frontiers in Neuroendocrinology, 26, 139–162. [DOI] [PubMed] [Google Scholar]
- Zyf M, Weaver I, Meaney M (2007) Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol 24: 9–19. [DOI] [PubMed] [Google Scholar]
- Tang AC, 2001. Neonatal exposure to novel environment enhances hippocampal-dependent memory function during infancy and adulthood. Learn Mem. 8, 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974. March;9(3):591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabulsy GM, Pearson J, Vaillancourt-Morel MP et al. (2014) Meta-analytic findings of the relation between maternal prenatal stress and anxiety and child cognitive outcome. J Dev Behav Pediatr, 35, 38–43. [DOI] [PubMed] [Google Scholar]
- Tarr AJ, Galley JD, Fisher SE, Chichlowski M, Berg BM, Bailey MT. The prebiotics 3’Sialyllactose and 6’Sialyllactose diminish stressor-induced anxiety-like behavior and colonic microbiota alterations: Evidence for effects on the gut-brain axis. Brain Behav Immun. 2015. November;50:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS, 1972. Humoral factors underlying maternal behavior at parturition: corss transfusion between freely moving rats. J Comp Physiol Psychol. 80, 365–71. [DOI] [PubMed] [Google Scholar]
- Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo PM, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell. 2018. January 25;172(3):500–516.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoman EB, Levine S, Arnold WJ (1968). Effects of maternal deprivation and incubator rearing on adrenocortical activity in the adult rat. Developmental Psychobiology, 1, 21–23. [Google Scholar]
- Tractenberg SG, Levandowski ML, Araujo de Azeredo L, Orso R, Roithmann LG, Hoffmann ES, Brenhouse H, Grassi-Oliveira R (2016). An overview of maternal separation effects on behavioural outcomes in mice: evidence from a four-stage methodological systematic review. Neuroscience and Biobehavioral Reviews, 68, 489–503. [DOI] [PubMed] [Google Scholar]
- Tottenham N, et al. , 2010. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 13, 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S (1997). Prenatal stress induced high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. The Journal of Neuroscience, 17, 2626–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bergh BR, Marcoen A (2004) High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development, 75,1085–1097. [DOI] [PubMed] [Google Scholar]
- van Dellen A, Blakemore C, Deacon R, York D, Hannan AJ (2000). Delaying the onset of Huntington’s in mice. Nature, 404, 721–722. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, et al. , 1998. The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology. 139, 2838–46. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID, 2009. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 34, 463–7. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Sacquet J, Nguyen V, Marien M, Jourdan F, Didier A (2009). Novelty determines the effects of olfactory enrichment on memory through noradrenergic mechanisms. Neuropsychopharmacology, 34, 786–795. [DOI] [PubMed] [Google Scholar]
- Viezel KD, Freer BD, Lowell A, 2015. Cognitive Abilities of Maltreated Children. Psychology in the School. 52, 92–106. [Google Scholar]
- Vivinetto AL, Suárez MM, Rivarola MA (2013). Neurobiological effects of neonatal maternal separation and post-weaning environmental enrichment. Behavioral Brain Research, 240, 110–118. [DOI] [PubMed] [Google Scholar]
- Walker C-D, Bath KG, Joels M, Korosi A, Larauche M, Lucassen PJ, Morris MJ, Raineki C, Roth TL, Sullivan RM, Taché Y, Baram TZ (2017).Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress, 20, 1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Greenough WT (Eds.). (1976). Environments as Therapy for Brain Dysfunction. Plenum Press; New York: Volume 17 in Advances in Behavioral Biology. [Google Scholar]
- Walker CD, et al. , 2017. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 20, 421–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E, et al. , 2000. Attachment security in infancy and early adulthood: a twenty-year longitudinal study. Child Dev. 71, 684–9. [DOI] [PubMed] [Google Scholar]
- Weaver IC, et al. , 2004. Epigenetic programming by maternal behavior. Nat Neurosci. 7, 847–54. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M (2005). Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. Journal of Neuroscience, 25, 11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MG, et al. , 2017. Family nurture intervention in preterm infants increases early development of cortical activity and independence of regional power trajectories. Acta Paediatr. 106, 1952–1960. [DOI] [PubMed] [Google Scholar]
- Welberg L, Thrivikraman KV, Plotsky PM (2006). Combined pre-and postnatal environmental enrichment programs the HPA axis differentially in male and female rats. Psychoneuroendocrinology, 31, 553—564. [DOI] [PubMed] [Google Scholar]
- Weinberg J (1992). Prenatal ethanol effects: sex differences in offspring stress responsiveness. Alcohol, 9, 219–223. [DOI] [PubMed] [Google Scholar]
- Weiskopf K, Schnorr PJ, Pang WW, Chao MP, Chhabra A, Seita J, Feng M, Weissman IL (2016). Myeloid cell origins, differentiation, and clinical implications. Microbiology Spectrum, 4, doi: 10.1128/microbiolspec.MCHD-0031-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L, Thrivikraman KV, Plotsky PM (2006). Combined pre- and postnatal environmental enrichment programs the HPA axis differently in male and female rats. Psychoneuroendocrinology, 31, 5, 553–564. [DOI] [PubMed] [Google Scholar]
- White JH, Bartley E, Janssen H, Jordan LA, Spratt N (2015). Exploring stroke survivor experience of participation in an enriched environment: a qualitative study. Disability and Rehabilitation, 37, 593–600. [DOI] [PubMed] [Google Scholar]
- Will B, Kelche C, Rosenzweig MR (2004). Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training. Progress in Neurobiology, 72, 167–182. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW (2008). Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta analysis. Hippocampus, 18, 729–736. [DOI] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL (2013). Modeling transformations of neurodevelopmental sequences across mammalian species. The Journal of Neuroscience, 33, 7368–7383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CC, Leon M (2013). Environmental enrichment as an effective treatment for autism: a randomized controlled trial. Behavioral Neuroscience, 127, 487–497. [DOI] [PubMed] [Google Scholar]
- Woo CC, Donnelly JH, Steinberg-Epstein R, Leon M (2015). Environmental enrichment as a therapy for autism: a clinical trial replication and extension. Behavioral Neuroscience, 129, 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würbel H, Garner JP (2007). Refinement of rodent research through environmental enrichment and systematic randomization. National Centre for the Replacement, Refinement and Reduction of Animals in Research, 9, 1–9. [Google Scholar]
- Yan S, Kentner AC (2017). Mechanical allodynia corresponds to Oprm1 downregulation within the descending pain network of male and female rats exposed to neonatal immune challenge. Brain, Behavior, and Immunity, 63, 148–159. [DOI] [PubMed] [Google Scholar]
- Yeshurun S, Short AK, Bredy TW, Pang TY, Hannan AJ (2017). Paternal environmental enrichment transgenerationally alters affective behavioral and neuroendocrine phenotypes. Psychoneuroendocrinology, 77, 225–235. [DOI] [PubMed] [Google Scholar]
- Yeshurun S, Hannan AJ (in press). Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Molecular Psychiatry, http://www.nature.com/articles/s41380-018-0039-z [DOI] [PubMed]
- Yildiz A, Arikan D, Gözüm S, Taştekin A, Budancamanak I. (2011). The effect of the odor of breast milk on the time needed for transition from gavage to total oral feeding in preterm infants. Journal of Nursing Scholarship, 43, 265–273. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ (1999). Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nature Medicine, 5, 448–453. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, et al. , 2009. Institutional rearing and psychiatric disorders in Romanian preschool children. Am J Psychiatry. 166, 777–85. [DOI] [PubMed] [Google Scholar]
- Zhang LX, et al. , 2002. Maternal deprivation increases cell death in the infant rat brain. Brain Res Dev Brain Res. 133, 1–11. [DOI] [PubMed] [Google Scholar]
- Zijlmans MA, Korpela K, Riksen-Walraven JM, de Vos WM, de Weerth C. Maternal prenatal stress is associated with the infant intestinal microbiota. Psychoneuroendocrinology. 2015. March;53:233–45. [DOI] [PubMed] [Google Scholar]


