Abstract
Both aging and obesity are associated with increased levels of pro-inflammatory metabolites, while weight reduction is associated with improvements in inflammatory status. However, few studies have explored the response of key inflammatory markers to the combined settings of weight reduction in an aging population. There are also few studies that have investigated the potential impact of diet composition on inflammatory marker responses. In the MEASUR-UP trial, we evaluated changes in baseline levels of inflammatory markers with post-study levels for a traditional weight loss control group versus a group with generous, balanced protein intake. In this 6-month randomized controlled trial (RCT), older (≥60 yrs) adults with obesity (BMI ≥30 kg/m2) and Short Physical Performance Battery (SPPB) score of 4–10 were randomly assigned to either a traditional weight loss regimen, (Control, n=14) or one with higher protein intake (≥30 g) at each meal (Protein, n=25). All participants were prescribed a hypo-caloric diet and attended weekly support and education groups and weigh-ins. Protein participants consumed ≥30 g of high quality protein/meal, including lean and extra lean beef provided to them for two of the three meals per day. Protein intakes were 0.8 and 1.2 g/kg/day for Control and Protein, respectively. Adiponectin, leptin, C-reactive protein (hs-CRP), tumor necrosis factor-α (TNF- α), interleukin-1 (IL-1), IL-6, IL-8, serum amyloid A (SAA), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and glycated serum protein (GSP) levels were measured at 0 and 6-month time points. At the 6-month endpoint, there was significant weight loss and decrease in BMI in both the Control (−4.8 ± 8.2 kg; −2.3 ± 2.4 kg/m2; P=0.05) and Protein (−8.7 ± 7.4 kg; −2.9 ± 2.3 kg/m2; P<0.0001) groups. SPPB scores improved in both arms, with a superior functional response in Protein (P<0.05). Body fat (%) at baseline was positively correlated with leptin, hs-CRP, VCAM-1, ICAM-1, and GSP. Several markers of inflammation responded to the Protein group: leptin (P<0.001), hs-CRP (P<0.01), and ICAM-1 (P<0.01) were decreased and adiponectin increased (P<0.01). There were no significant changes in any inflammatory markers in the Control arm. In the between group comparison, only adiponectin trended towards a group difference (more improvement in Protein; P<0.07). Our findings in the MEASUR-UP trial show that a weight loss diet with enhanced protein intake is comparable to an adequate protein diet in terms of weight loss success and that it can lead to improvements in inflammatory status, specifically for adiponectin, leptin, hs-CRP, and ICAM-1. These findings are important given current recommendations for higher protein intakes in older adults and justify additional study of the inflammatory impact of an enhanced protein diet. (ClinicalTrials.gov identifier: NCT01715753)
Keywords: Inflammation, protein intake, weight reduction, obesity, older adults
INTRODUCTION
Aging and Inflammation
Older adults account for about 15% of the US population, and by 2030 this number will rise to roughly 20% (1). The aging process is characterized by low-grade systematic inflammation that occurs even in the absence of any infection. More recently, the term “inflammaging” (2) has been used to describe this hallmark of aging. That chronic inflammation is associated with aging is widely accepted; however, the etiology of this inflammation is still unknown. It has been suggested that age-related inflammation is potentially due to a number of causes including, increases in adiposity and changes in body composition with age (3), cellular senescence (4), immunosenescence (4), changes in gut microbiota (4), coagulation system activation (4), and accumulation of damaged macromolecules and cells (4). In older adults, inflammation shows a strong correlation with insulin resistance, heart disease, type II diabetes, cancer, osteoporosis, and macular degeneration, as well as neurological conditions such as Alzheimer’s and Parkinson’s Disease (4). The extensive impact that inflammation has on aging has led to an increased interest (5) in not only gaining a better understanding of this relationship, but also understanding ways to reduce the negative impact of increased inflammation in older adult populations.
Obesity and Inflammation
In the United States, the prevalence of obesity has reached unprecedented levels, including a rate of 38.5% in adults 60 and older (6). Not only are there major health risks posed by obesity, but adults with obesity also have physical deficits. Their physical function is impaired, not only because of decreased mobility, but also because of chronic inflammation (7, 8). Individuals with obesity have a larger adipose stores and thus heightened production and release of inflammatory cytokines, thus giving obesity the categorization of being an inflammatory disease (9, 10). Adiposity level is well known to be correlated with levels of a number of metabolic inflammatory markers, specifically: leptin, C-reactive protein (hs-CRP), serum amyloid A (SAA), tumor necrosis factor (TNF- α), interleukin-1 (IL-1), IL-6, and IL-8 in morbidly obese individuals (11–13). An additional defining characteristic of obesity is low serum levels of adiponectin, a hormone involved in glucose regulation. Conversely, leptin is found in much larger concentrations in individuals with obesity, and is directly correlated with fat mass (14, 15). Leptin has also been shown to induce the expression of adhesion molecules, such as vascular cell adhesion molecules and intercellular adhesion molecules. The cellular adhesion molecules primarily involved in regulating the inflammatory response are VCAM-1 and ICAM-1. VCAM-1 is identified at higher levels when there is an immune response against disease, thus promoting tissue inflammation (16). It has also been directly correlated with obesity (17). IL-6 is one of the more studied interleukins associated with obesity, has been found at higher levels in adipose tissue of individuals with obesity. IL-1 and IL-8 are two more interleukins closely associated with the inflammatory response. A newer marker, acute-phase protein SAA, is an apolipoprotein associated with lipoproteins, prompting researchers to look at a possible link between SAA and obesity. Several studies have found positive associations between SAA levels and BMI, as well as decreases in SAA levels when obesity reduction occurs (18). Obesity, particularly in the older adult population, continues to be a considerable public health concern because the associated chronic, underlying inflammation also increases the risk of age-related disease (19), and leads to progressive decline in muscle quality (20).
Obesity, Aging, and Function
Because both obesity (pathological) and aging (physiological) are associated with increased inflammation and insulin resistance and impaired metabolic control, their co-occurrence is a double threat to health, resulting in metabolic and inflammatory dysfunction (21). Furthermore, both obesity and the aging process lead to compromised muscle function through multiple mechanisms. Sarcopenia, also known as muscle wasting, is a progressive loss of muscle mass/strength that occurs almost universally with aging (22). There is a substantial loss of lean muscle mass, from about 50% of total body weight in young adults to about 25% at 75–80 yrs (23). Older adults also experience a decline in muscle strength and power, slower movement, and an increased risk for falls (24). Although they utilize different mechanisms, age-related loss of physical function and deterioration of muscle quality also occurs in older adults with obesity. This combination of sarcopenia and obesity markedly accelerates functional decline. Muscle quality is even further reduced due to the accumulation of lipids between muscles associated with greater adiposity. Chronic activation of the innate immune system causes persistent low-grade inflammation in older adults with obesity, which leads to muscle depletion by enhancing protein breakdown and impairing myogenesis in parallel (7, 25). This impairment is further exacerbated by obesity reducing the ability to be physically active, and thus leading to progressive muscle atrophy due to disuse.
Intentional Weight Loss in Older Adults with Obesity
Obesity reduction has been associated with improved inflammatory status. However, weight reduction is difficult to achieve in adults of all ages (26, 27) and it is especially challenging to treat in older adults, who have lower basal metabolic rates, calorie requirements, and rates of physical activity (28–30). Moreover, efforts to reduce body weight in older adults with obesity raise concerns about the concomitant loss of lean body mass (31). Previous studies have found that sarcopenia is exacerbated by weight loss and as such, researchers have been investigating the best approach to combat obesity while limiting the amount of muscle (lean) mass lost (32). A higher protein intake during obesity reduction could help circumvent loss of muscle mass and strength. Protein intakes exceeding the RDA level (>0.8 g/kg) are linked with better preservation of lean mass (33, 34) and new findings from the Framingham study have confirmed the benefits of high quality (animal) protein for protection of appendicular lean mass (35), preservation of grip strength (36) and decreased risk of function decline in the long-term (37). While both higher protein intake and decreased inflammation are known to benefit muscle, the direct impact of higher protein intake on whole body inflammatory state is a matter of active study and some disagreement in the literature (38–40), due in part to the limited number of published trials. Only a few studies have explored the response of inflammatory markers to enhanced protein intake during obesity reduction (40–42) and we could not find any such studies focusing on older adult participants. The purpose of this analysis of secondary outcomes from a randomized controlled trial of protein enhanced weight loss in physically frail older adults with obesity was to evaluate the responses of a wide array of traditional and novel biomarkers of inflammation, namely adiponectin, leptin, IL-1, IL-6, IL-8, hs-CRP, TNF, ICAM, VCAM, SAA and GSP, to this intervention.
METHODS
Study design and participants
This is an analysis of secondary outcomes from the ‘‘The Measuring Eating, Activity and Strength: Understanding the Response-Using Protein” (MEASUR-UP) study, a randomized controlled trial that compared a protein-enhanced diet to a traditional weight loss regimen in frail older adults with obesity during a 6-month intervention. The study design and methods (32), and primary results from the intent-to-treat parent study have been previously reported and published (25). The study was approved by Duke University School of Medicine Institutional Review Board and written consent was obtained from all study participants. Briefly, physically frail men and women aged ≥60 years with obesity (BMI ≥30 kg/m2) living in or near Durham, North Carolina, were enrolled. Physical frailty at baseline was qualified by having a score of 4–10 on the Short Physical Performance Battery (43). Sixty-seven participants were randomly assigned in a 1:2 ratio to a traditional (Control) weight loss regimen or one with higher protein intake (≥30 g) at each meal (Protein). All participants were prescribed a hypo-caloric diet designed to achieve a target of 10% body weight loss. Participants were weighed weekly and attended weekly group support sessions led by Registered Dietitians.
The present analyses were performed on 39 frail, older (≥60 years) men and women with obesity (BMI ≥30 kg/m2), who participated in the MEASUR-UP trial and who had blood samples available at baseline and endpoint to analyze for inflammatory biomarkers (Control n=14; Protein n=25). This includes 6 participants whose intervention lasted 3 months instead of 6.
Intervention
A supervised 6-month weight loss treatment program was prescribed to both the Control and Protein groups and delivered by Registered Dietitians (Interventionists). Interventionists prescribed a 500 kcal deficit diet and provided individualized kcal prescriptions and meal plans to achieve a weight 10% weight loss goal. The macronutrient distribution for the Control group was 15% protein, 30% fat, 55% carbohydrate and their protein intake met the Recommended Dietary Allowance of 0.8 g/kg body weight. Protein group macronutrient distribution was 30% protein, 30% fat, 40% carbohydrate and protein intake was 1.2 g/kg body weight. Additionally, the Protein meal plans included ≥30 grams of lean, high-quality protein three times a day. Protein participants were provided two ≥30 gram portions of prepared, frozen or chilled, lean and extra lean beef (ground sirloin, deli roast beef, and flank steak) daily and were counseled on ways to achieve 30 grams of lean high quality protein at the third daily meal. Both groups participated in weekly group meetings led by Interventionists that provided education, counseling, peer support, and supervised weekly weigh-ins.
Assessments
Inflammatory markers
Fasting blood samples were collected using standard procedures at baseline (n=59) and completion of intervention (n=39) by a Registered Nurse. Blood serum was analyzed for biomarkers of inflammation by Dr. Virginia Kraus’ laboratory in the Duke Molecular Physiology Institute. Adiponectin assessment was by MSD Human Adiponectin Kit (#K151BXC-1) and leptin by MSD Human Leptin Kit (#K151BYC-1). The MSD Human Proinflammatory 4-plex (#K15052D) was used to quantify IL-1β, IL-6, IL-8, and, TNF-α. For hs-CRP, SAA, ICAM-1, and VCAM-1 we used the MSD Human Vascular Injury Panel 2 (#K15198D) and for glycosylated serum proteins (GSP) we used the Glycated Serum Protein Assay (Diazyme#DZ112B). For these assays, intra-assay CVs were <7.7% and inter-assay CVs were <11.3%.
Body Composition and Function
Body weight, lean mass, and fat mass were measured in the fasting state using the BOD POD™ air displacement plethysmography method (Life Measurement Inc, Concord, CA). Height was recorded to the nearest 0.5 cm using a stadiometer. Physical function was measured using the Short Physical Performance Battery, a widely used functional assessment developed for use in older adults; it includes three component domains (balance, strength, and gait speed) that yield a total score of 0 (poor) to 12 (high) (43).
Dietary intake
Three-day food intake records (2 week days and 1 weekend day) were collected at baseline and at the endpoint of the intervention. Food records were translated to food codes by Registered Dietitians and analyzed using Food Processor Nutrition Analysis Software (ESHA Research, Salem, OR) for total calories and distribution of macronutrients (protein, carbohydrates, and fat).
Statistical analysis
All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary NC). Descriptive statistics were calculated for baseline characteristics. Spearman correlation coefficients were computed for baseline inflammatory markers and baseline body weight, body fat (%), function (SPPB), and age. For a small subset of participants (6: n=2 Protein; n=4 Control) who had assessments at three-months but not six-months, the three-month data was carried forward and used as the six-month measure. For each outcome of interest, change scores were created using baseline and six-month data. Inflammatory marker levels were log transformed to better approximate normality to satisfy linearity assumptions. Two-Sample t-test and paired t-test were used to determine changes between and within groups. Data were then back-transformed according to intervention group for ease of interpretation.
RESULTS
Baseline and Descriptive characteristics
As outlined in the consort diagram (Figure 1), 67 participants were randomized into either Control or Protein group. The data presented in this analysis is from a subset of 39 participants who completed the intervention and had blood serum available for analysis of inflammatory biomarkers (Control n=14 and Protein n=25). Baseline characteristics by group and for the total subset are shown in Table 1. The average age of study participants was 68.3 ± 5.6 and the population was mostly female (79.5%) and predominantly white (69.2%). Additionally, 21% of participants reported CVD, 16% reported Type 2 Diabetes and 71% reported hypertension. Participants had a mean BMI of 37.0 ± 6.8 kg/m2 (Class II obesity). At baseline, there were significant correlations between percent body fat and these inflammatory markers: leptin (r=0.739, P<0.001), hs-CRP (r=0.351, P=0.031), ICAM (r=0.552, P<0.001), and VCAM-1 (r=0.475, P=0.003) (data not shown).
Figure 1.
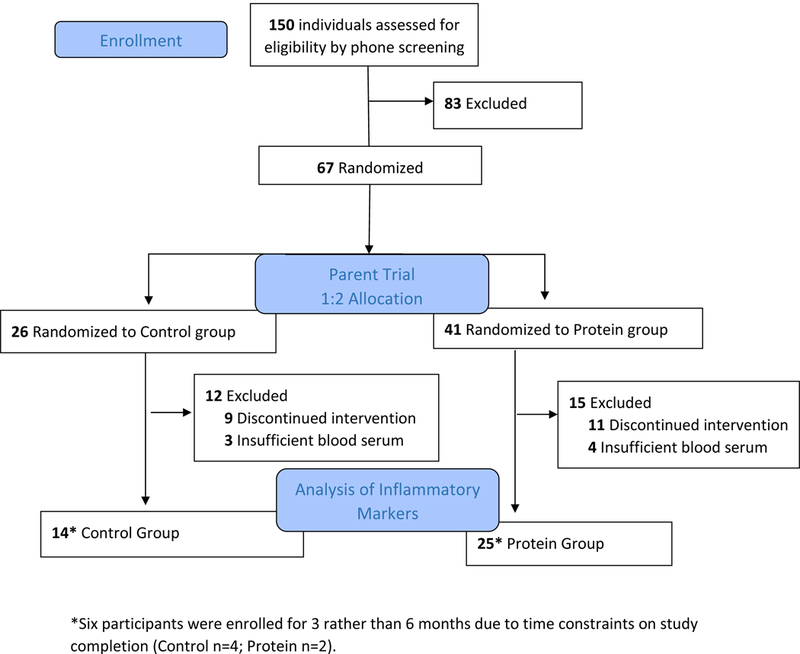
Consort Diagram
Table 1:
Descriptive Characteristics of Participants
| Control (n=14) Mean ± SD, n (%) |
Protein (n=25) Mean ± SD, n (%) |
Total (N=39) Mean ± SD, n(%) |
|
|---|---|---|---|
| Age (y) | 68.8 ± 6.1 | 68.0 ± 5.5 | 68.3 ± 5.6 |
| Body weight (kg) | 101.7 ± 16.1 | 106.6 ± 24.5 | 105.0 ± 21.9 |
| BMI (kg/m2) | 36.0 ± 5.3 | 37.5 ± 7.5 | 37.0 ± 6.8 |
| Female (%) | 11 (78.6) | 20 (80.0) | 31 (79.5) |
| Race | |||
| Black (%) | 3 (21.4) | 8 (32.0) | 11 (28.2) |
| White (%) | 11 (78.6) | 16 (64.0) | 27 (69.2) |
| GFR (mL/min/1.73 m2)*† | 74.8 ± 19.4 | 80.3 ± 14.6 | 78.4 ± 16.4 |
| Cardiovascular Disease | 1 (7.1) | 7 (29.2) | 8 (20.5) |
| Type 2 Diabetes | 1 (7.1) | 5 (20.8) | 6 (15.8) |
| Hypertension | 11 (78.6) | 16 (66.7) | 27 (71.0) |
Notes: BMI = Body mass index; GFR = glomerular filtration rate
GFR was calculated as (mL/min/1.73 m2) = 175×(Scr)-1.154×(Age)-0.203×(0.742 female)×(1.212 Black).
Normal GFR values were defined as ≥60 mL/min/1.73 m2.
Intervention and Inflammatory Markers
Intervention effects on weight loss, body composition, and physical function in the MEASUR-UP trial have been previously reported (25). Results in this subsample followed the same pattern. Both groups lost weight (P<0.05) and reduced their BMI (P<0.01) (Table 2). Physical function, measured by SPPB, improved in both groups, with significantly greater improvement in Protein arm (P=0.028) (Table 2).
Table 2.
Baseline and Change scores at 6 Months for Body Composition, Function, and Inflammatory Markers by Treatment Group
| Control (N=14) Mean ± SD |
P value | Protein (N=25) Mean ± SD |
P value |
P value Control vs. Protein |
|
|---|---|---|---|---|---|
| Body Composition and Physical Function | |||||
| Body Weight, kg | |||||
| Baseline, mean ± SD | 101.7 ± 16.1 | 106.6 ± 24.5 | |||
| Change at 6 months, mean ± SD | −4.8 ± 8.2 | 0.05 | −8.4 ± 7.1 | <0.001 | 0.17 |
| Fat Mass, kg | |||||
| Baseline, mean ± SD | 45.4 ± 18.9 | 51.7 ± 18.7 | |||
| Change at 6 months, mean ± SD | −3.7 ± 6.8 | 0.06 | −7.5 ± 6.4 | <0.001 | 0.09 |
| Fat Mass, % | |||||
| Baseline, mean ± SD | 47.1 ± 8.6 | 47.7 ± 8.3 | |||
| Change at 6 months, mean ± SD | −1.5 ± 3.7 | 0.17 | −3.6 ± 3.0 | <0.001 | 0.07 |
| Lean Mass, kg | |||||
| Baseline, mean ± SD | 49.4 ± 16.5 | 55.0 ± 11.4 | |||
| Change at 6 months, mean ± SD | −1.0 ± 2.8 | 0.21 | −0.9 ± 1.6 | 0.009 | 0.91 |
| BMI, kg/m2 | |||||
| Baseline, mean ± SD | 36.0 ± 5.3 | 37.5 ± 7.5 | |||
| Change at 6 months, mean ± SD | −2.3 ± 2.4 | 0.008 | −2.9 ± 2.3 | <0.001 | 0.45 |
| SPPB, Total Score | |||||
| Baseline, mean ± SD | 8.9 ± 1.1 | 8.2 ± 0.5 | |||
| Change at 6 months, mean ± SD | 0.9 ± 1.6 | 0.07 | 2.4 ± 1.9 | <0.001 | 0.028 |
| Inflammatory Markers* | |||||
| Adiponectin, ng/ml | |||||
| Baseline, mean ± SD | 21236.4 ± 8729.87 | 20007.3 ± 16990.33 | |||
| Change at 6 months, mean ± SD | 1619.1 ± 5121.5 | 0.71 | 0.12 ± 0.19 | 0.004 | 0.074 |
| Leptin, ng/ml | |||||
| Baseline, mean ± SD | 96.2 ± 108.0 | 60.6 ± 45.1 | |||
| Change at 6 months, mean ± SD | −41.0 ± 85.5 | 0.16 | −15.4 ± 24.9 | 0.001 | 0.70 |
| IL-1, pg/ml | |||||
| Baseline, mean ± SD | 0.02 ± 0.02 | 0.05 ± 0.10 | |||
| Change at 6 months, mean ± SD | −0.005 ± 0.02 | 0.26 | −0.002 ± 0.16 | 0.89 | 0.97 |
| IL-6, pg/ml | |||||
| Baseline, mean ± SD | 4.5 ± 12.3 | 1.3 ± 0.9 | |||
| Change at 6 months, mean ± SD | −2.9 ± 10.2 | 0.34 | 0.1 ± 0.6 | 0.32 | 0.14 |
| IL-8, pg/ml | |||||
| Baseline, mean ± SD | 14.1 ± 5.0 | 22.5 ± 29.3 | |||
| Change at 6 months, mean ± SD | 1.8 ± 6.2 | 0.31 | −2.3 ± 8.4 | 0.62 | 0.23 |
| TNF-α, pg/ml | |||||
| Baseline, mean ± SD | 2.4 ± 0.6 | 2.6 ± 0.6 | |||
| Change at 6 months, mean ± SD | 0.2 ± 0.5 | 0.10 | 0.1 ± 0.7 | 0.92 | 0.21 |
| CRP, ng/ml | |||||
| Baseline, mean ± SD | 6078.8 ± 6029.2 | 7556.2 ± 11157.4 | |||
| Change at 6 months, mean ± SD | −941.2 ± 7022.5 | 0.54 | −2437.8 ± 4709.3 | 0.005 | 0.34 |
| SAA, ng/ml | |||||
| Baseline, mean ± SD | 12117.4 ± 23020.4 | 15647.4 ± 33769.0 | |||
| Change at 6 months, mean ± SD | −6715.5 ± 23442.9 | 0.35 | −8150.3 ± 33956.4 | 0.22 | 0.94 |
| ICAM-1, ng/ml | |||||
| Baseline, mean ± SD | 504.4 ± 112.9 | 513.9 ± 114.8 | |||
| Change at 6 months, mean ± SD | −21.4 ± 0.1 | 0.27 | −53.0 ± 77.0 | 0.001 | 0.11 |
| VCAM-1, ng/ml | |||||
| Baseline, mean ± SD | 654.5 ± 178.3 | 649.3 ± 179.5 | |||
| Change at 6 months, mean ± SD | 6.7 ± 55.0 | 0.69 | −22.3 ± 66.4 | 0.07 | 0.14 |
| GSP, ng/ml | |||||
| Baseline, mean ± SD | 425.2 ± 113.7 | 429.6 ± 162.9 | |||
| Change at 6 months, mean ± SD | 38.5 ± 83.9 | 0.11 | −2.5 ± 93.5 | 0.46 | 0.11 |
P-values are based on log-transformed change scores
Table 2 shows the baseline means and change scores for all inflammatory biomarkers in each intervention group between baseline and end of intervention. There were no significant between-group differences; however, there was a trend towards greater improvements in adiponectin in Protein group compared to Control (P=0.07). Within the Protein group, improvements in inflammation included, increased adiponectin (P=0.004) and decreased leptin (P<0.001), hs-CRP (P=0.005), ICAM-1 (P=0.001), and VCAM-1 (P=0.07). No significant with-in group changes were detected in the Control group.
As illustrated in Table 3, both Control and Protein significantly reduced their calorie intakes during the intervention (P<0.01). The Protein group significantly increased protein intake from 0.89 to 1.22 g/kg bw per day (P<0.01), while there was no significant change from baseline for the Control group (P=0.28).
Table 3.
Baseline means and change scores at 6 months for calorie and macronutrient intakes by treatment group
| Control (n=14) Mean ± SD |
P value | Protein (n=25) Mean ± SD |
P value |
P value Control vs. Protein |
|
|---|---|---|---|---|---|
| Total Energy, kcal | |||||
| Baseline | 1942.6 ± 427.5 | 2064.8± 854.6 | |||
| Change at 6 months | −544.2 ± 430.2 | 0.001 | −518.6 ± 759.7 | 0.003 | 0.91 |
| Protein Intake, g/d | |||||
| Baseline | 83.8 ± 16.4 | 91.6 ± 27.8 | |||
| Change at 6 months | −11.7 ± 19.4 | 0.042 | 24.4 ± 37.6 | 0.004 | 0.002 |
| Average Protein Intake, g/kg of body weight/day | |||||
| Baseline | 0.83 ± 0.21 | 0.89 ± 0.32 | |||
| Change at 6 months | −0.07 ± 0.22 | 0.28 | 0.33 ± 0.38 | 0.001 | 0.002 |
| Protein Intake, % of total kcal | |||||
| Baseline | 18 ± 4 | 19 ± 7 | |||
| Change at 6 months | 3 ± 2 | 0.001 | 11 ± 7 | <0.001 | 0.001 |
| Carbohydrate Intake, g/d | |||||
| Baseline | 217.4 ± 61.9 | 220.1 ± 102.5 | |||
| Change at 6 months | −46.7 ± 68.7 | 0.025 | −71.4 ± 91.6 | 0.001 | 0.39 |
| Carbohydrate Intake, % of total kcal | |||||
| Baseline | 44 ± 7 | 43 ± 8 | |||
| Change at 6 months | 4 ± 9 | 0.09 | −4 ± 12 | 0.11 | 0.029 |
| Fat Intake, g/d | |||||
| Baseline | 83.3 ± 25.9 | 88.4 ± 46.6 | |||
| Change at 6 months | −34.1 ± 22.0 | <0.001 | −34.5 ± 44.5 | 0.001 | 0.97 |
| Fat Intake, % of total kcal | |||||
| Baseline | 38 ± 5 | 37 ± 8 | |||
| Change at 6 months | −6 ± 9 | 0.016 | −6 ± 10 | 0.006 | 0.92 |
| Saturated Fat Intake, g/d | |||||
| Baseline | 27.4 ± 10.5 | 26.7 ± 14.5 | |||
| Change at 6 months | −11.8 ± 10.2 | 0.001 | −7.4 ± 15.5 | 0.029 | 0.35 |
Changes in Inflammatory Status in the Combined Sample
Given that there were no significant differences in intervention responses between Protein and Control (other than a trend for adiponectin to be more improved in Protein), we repeated the analysis with the groups combined. Results for this analysis showed that, between baseline and endpoint, adiponectin increased (P=0.006), while Leptin (P<0.001), hs-CRP (P=0.014), and ICAM-1 (P<0.001) decreased. Spearman correlations were then performed between inflammatory marker change scores and fat mass change scores. Change in fat mass was directly correlated with change in leptin levels (r=0.618, P<0.001) and SAA levels (r=0.384, P=0.017) and inversely correlated with levels of adiponectin (r=−0.331, P=0.042).
DISCUSSION
This study is the first to explore the impact of a balanced, higher protein weight loss diet (two of three meals as lean or extra lean beef) compared to a traditional weight loss diet on markers of inflammation in physically frail, older adults with obesity. Generous protein intakes, particularly from high quality protein sources, stimulate muscle protein synthesis and reduce muscle protein breakdown in older adults. Diets higher in protein have been shown to improve weight loss and weight maintenance by increasing satiety hormones, peptide YY and glucagon-like peptide (44), increasing postprandial thermogenesis (45, 46) and maintain resting energy expenditure (45–47). Furthermore, short-term studies have shown preservation of lean mass with higher protein hypocaloric diets as compared to those with protein at the recommended dietary allowance level (48). For older adults with obesity, who are at greater risk of losing lean muscle mass while undergoing intentional weight loss, these findings are particularly relevant and in agreement with our findings for improved function in our Protein treatment group.
In response to equivocal findings in the literature (38, 40, 49) on the inflammatory impact of meat protein and recognizing there is very limited study of higher protein weight loss diets in older adults, our analysis focused specifically on the effect of a higher protein (predominantly lean beef) diet during obesity reduction on markers of inflammatory status in physically frail older adults. We found that this diet did not have a negative impact on inflammatory markers; there were essentially no group differences between Control and Protein group outcomes for inflammatory biomarkers. Additionally, there were several beneficial within-group changes in the Protein group, for adiponectin, leptin, hs-CRP, ICAM-1, and VCAM-1. The absence of a detrimental effect of protein on inflammation agrees with several other studies. A systematic review of five pooled studies showed no difference in inflammation (hs-CRP) between high and low protein diets of middle age (median age of 45) men and women (39). Additionally, Hodgson et al. (49), found no difference in markers of inflammation or oxidative damage when lean red meat replaced carbohydrates in an isoenergetic diet consumed by adults (mean age 58.6±8.6 years). The within-group improvements we found with higher protein intakes are supported by a recently published study investigating higher protein intake plus resistance training (38). In this trial by Daly et al. (38), women 60 to 90 years old of age were provided two servings of lean red meat daily for 6 days per week during a 4-month RCT. Compared to an isocaloric control diet (plus resistance training), the higher protein group had greater reductions in IL-6, and significant within-group reductions in TNF-α (38).
With regards to the influence of higher protein intake during weight loss, our findings are consistent with other studies in middle-aged populations. In the POUNDS LOST study of overweight and obese adults aged 30 to 70, Nicklas et al. (41) reported significant improvements in hs-CRP in diets with average and high protein with no difference between groups. Similarly, in a 3-month weight loss intervention in overweight and obese women (mean age 42 years), Aadbakht et al. (42) found no difference between control (protein, carbohydrate, and fat: 15%, 55%, and 30% respectively) and high protein (protein, carbohydrate, and fat: 25%, 45% and 30%, respectively) diet groups with regards to inflammatory impact. In contrast to these reports and our own findings, Lopez-Legarrea et al. (40) conducted an 8-week weight loss intervention (the RESMENA project) and found that a control diet (15% of energy from protein) had significantly greater improvements in inflammatory scores than a higher protein diet (30% of energy from protein) diet. A sub-analysis showed that diets higher in animal protein and meat protein were positively associated with inflammation. The reason for the discrepancy between the RESMENA findings and ours is not clear, but it could relate to differences in diet composition. Much of the protein consumed in the MEASUR-UP study was provided directly to participants and it was always lean or extra lean. In fact, despite a considerable increase in protein content, fat intakes were decreased from 37 to 31% in the MEASUR-UP Protein group (P<0.01) and their saturated fat intake was also reduced (P<0.05), whereas fat intake in the RESMENA study protein group was 37%. It is also possible that the difference in participant age between the MEASUR-UP (mean age = 68 yr) and RESMENA (mean age = 50 yr) trials played a role in the response to protein. Additional studies with larger numbers of older adults with obesity are needed to conclusively describe the impact of higher protein intakes from animal protein sources on indicators of inflammation in this high risk population.
Our findings for the combined results of Control plus Protein participants in the MEASUR-UP trial are important additions to the literature. Between baseline and end of intervention, we documented important improvements in adiponectin, leptin, hs-CRP, and ICAM-1 and a correlation between changes in fat mass and improvements in adiponectin and SAA levels. While not unexpected based on findings in younger populations, these results confirm the benefits of obesity reduction to inflammatory status in older adults, whether they are consuming traditional or higher levels of protein (50–52).
Our study has a number of strengths, including the randomized controlled trial design, the inclusion of a population often excluded from research studies (frail older adults with obesity), careful documentation of protein intake, a substantial and significant difference in protein intake between groups, and the measurement of a very wide array of traditional and novel markers of inflammation. The trial also has its limitations, the most important being the small sample size, which could account for the lack of within-group effects for the Control group and limited between-group differences. The generalizability of the findings may also be limited by the consumption of red meat twice daily. Although no detrimental changes were noted in the Protein group, the consumption of the same meat twice daily for 6 months would not be practical or necessary in community implementation of the enhanced protein diet.
In conclusion, we found that a weight loss diet with enhanced protein intake is comparable to an adequate protein diet in achieving weight loss and superior in terms of benefits to function. This diet caused no detrimental changes in inflammatory status; in fact, the Protein treatment was associated with beneficial changes in levels of adiponectin, leptin, HS-CRP, and ICAM-1. Given current recommendations for higher protein intakes in older adults, these findings strongly justify further study of the inflammatory impact of an enhanced protein weight loss diet in physical frail older adults with obesity. Given the magnitude of concern about elevated inflammatory status in older adults with obesity, the potential that lean high quality protein could reduce inflammation during weight reduction and the mechanism(s) responsible for this effect merit further exploration.
Acknowledgments
Funding: This study was funded by the Beef Checkoff and received additional support from the National Institutes of Health (5T32 AG000029 to KNPS), United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service Program (CDA-2/ IK2 RX002348), Pepper Center. These organizations had no role in the design or conduct of either the original study or this secondary analysis; collection, analysis, or interpretation of the data; or writing of this manuscript.
References
- 1.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States, Current Population Reports, P25–1140 U.S. Census Bureau, Washington, DC: 2014. [Google Scholar]
- 2.Franceschi C, Bonafe M, Valensin S, Olivieri F, Luca MD, Ottaviani E, et al. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Annals of the New York Academy of Sciences 2000;908(1):244–54. [DOI] [PubMed] [Google Scholar]
- 3.Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, et al. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol 2009;183(9):5948–56. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi C, Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. The Journals of Gerontology: Series A 2014;69(Suppl_1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 5.Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, et al. The role of inflammation in age-related disease. Aging (Albany NY) 2013;5(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costamagna D, Costelli P, Sampaolesi M, Penna F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediators Inflamm 2015;2015:805172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. Journal of the American Medical Directors Association 2011;12(4):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emanuela F, Grazia M, Marco de R, Maria Paola L, Giorgio F, Marco B. Inflammation as a Link between Obesity and Metabolic Syndrome. J Nutr Metab 2012;2012:476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol 2010;318(1–2):69–78. [DOI] [PubMed] [Google Scholar]
- 11.Poitou C, Coussieu C, Rouault C, Coupaye M, Cancello R, Bedel JF, et al. Serum amyloid A: a marker of adiposity-induced low-grade inflammation but not of metabolic status. Obesity (Silver Spring) 2006;14(2):309–18. [DOI] [PubMed] [Google Scholar]
- 12.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010;316(2):129–39. [DOI] [PubMed] [Google Scholar]
- 13.Frasca D, Blomberg BB, Paganelli R. Aging, Obesity, and Inflammatory Age-Related Diseases. Frontiers in Immunology 2017;8:1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paz-Filho G, Mastronardi C, Franco CB, Wang KB, Wong ML, Licinio J. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol 2012;56(9):597–607. [DOI] [PubMed] [Google Scholar]
- 15.van Dielen FMH, van’t Veer C, Schols AM, Soeters PB, Buurman WA, Greve JWM. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. International Journal Of Obesity 2001;25:1759. [DOI] [PubMed] [Google Scholar]
- 16.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular Cell Adhesion Molecule-1 Expression and Signaling During Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxidants & Redox Signaling 2011;15(6):1607–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isoppo de Souza C, Rosa DD, Ettrich B, Cibeira GH, Giacomazzi J, Tusset P, et al. Association of adipokines and adhesion molecules with indicators of obesity in women undergoing mammography screening. Nutrition & Metabolism 2012;9:97-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, He X, Shi X, Huang C, Liu J, Zhou S, et al. Association between serum amyloid A and obesity: a meta-analysis and systematic review. Inflamm Res 2010;59(5):323–34. [DOI] [PubMed] [Google Scholar]
- 19.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 2009;8(1):18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roubenoff R Sarcopenic Obesity: the Confluence of two Epidemics. Obes Res 2004;12(6):887–8. [DOI] [PubMed] [Google Scholar]
- 21.Pérez LM, Pareja-Galeano H, Sanchis-Gomar F, Emanuele E, Lucia A, Gálvez BG. ‘Adipaging’: ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. The Journal of Physiology 2016;594(12):3187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakuma K, Yamaguchi A. Molecular Mechanisms in Aging and Current Strategies to Counteract Sarcopenia. Curr Aging Sci 2010;3(2):90–101. [DOI] [PubMed] [Google Scholar]
- 23.Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 2004;286(1):E92–101. [DOI] [PubMed] [Google Scholar]
- 24.Melton LJ 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc 2000;48(6):625–30. [PubMed] [Google Scholar]
- 25.Porter Starr KN, Pieper CR, Orenduff M, McDonald SR, Mauceri L, Payne ME, et al. Improved function with enhanced protein intake per meal: A pilot study of weight reduction in frail, obese older adults. J Gerontol A Biol Sci Med Sci 2016;71(10):1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon CP, Kumar A. Treatment of overweight and obesity: lifestyle, pharmacologic, and surgical options. Clin Cornerstone 2009;9(4):55–68; discussion 9–71. [DOI] [PubMed] [Google Scholar]
- 27.Ross R The challenge of obesity treatment: avoiding weight regain. CMAJ 2009;180(10):997–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzankoff SP, Norris AH. Effect of muscle mass decrease on age-related BMR changes. J Appl Physiol Respir Environ Exerc Physiol 1977;43(6):1001–6. [DOI] [PubMed] [Google Scholar]
- 29.Elia M, Ritz P, Stubbs RJ. Total energy expenditure in the elderly. Eur J Clin Nutr 2000;54 Suppl 3:S92–103. [DOI] [PubMed] [Google Scholar]
- 30.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005;82(5):923–34. [DOI] [PubMed] [Google Scholar]
- 31.Weinheimer EM, Sands LP, Campbell WW. A Systematic Review of the Separate and Combined Effects of Energy Restriction and Exercise on Fat-free Mass in Middle-aged and Older Adults: Implications for Sarcopenic Obesity. Nutr Rev 2010;68(7):375–88. [DOI] [PubMed] [Google Scholar]
- 32.McDonald SR, Porter Starr KN, Mauceri L, Orenduff M, Granville E, Ocampo C, et al. Meal-based enhancement of protein quality and quantity during weight loss in obese older adults with mobility limitations: Rationale and design for the MEASUR-UP trial. Contemp Clin Trials 2015;40:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87(1):150–5. [DOI] [PubMed] [Google Scholar]
- 34.Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased Consumption of Dairy Foods and Protein during Diet- and Exercise-Induced Weight Loss Promotes Fat Mass Loss and Lean Mass Gain in Overweight and Obese Premenopausal Women. J Nutr 2011;141(9):1626–34. Epub 2011 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahni S, Mangano KM, Hannan MT, Kiel DP, McLean RR. Higher Protein Intake Is Associated with Higher Lean Mass and Quadriceps Muscle Strength in Adult Men and Women. J Nutr 2015;145(7):1569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean RR, Mangano KM, Hannan MT, Kiel DP, Sahni S. Dietary Protein Intake Is Protective Against Loss of Grip Strength Among Older Adults in the Framingham Offspring Cohort. J Gerontol A Biol Sci Med Sci 2016;71(3):356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradlee ML, Mustafa J, Singer MR, Moore LL. High-Protein Foods and Physical Activity Protect Against Age-Related Muscle Loss and Functional Decline. J Gerontol A Biol Sci Med Sci 2017;73(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daly RM, O’Connell SL, Mundell NL, Grimes CA, Dunstan DW, Nowson CA. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am J Clin Nutr 2014;99(4):899–910. [DOI] [PubMed] [Google Scholar]
- 39.Santesso N, Akl EA, Bianchi M, Mente A, Mustafa R, Heels-Ansdell D, et al. Effects of higher-versus lower-protein diets on health outcomes: a systematic review and meta-analysis. European Journal of Clinical Nutrition 2012;66(7):780–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Legarrea P, de la Iglesia R, Abete I, Navas-Carretero S, Martinez JA, Zulet MA. The protein type within a hypocaloric diet affects obesity-related inflammation: the RESMENA project. Nutrition 2014;30(4):424–9. [DOI] [PubMed] [Google Scholar]
- 41.Nicklas JM, Sacks FM, Smith SR, LeBoff MS, Rood JC, Bray GA, et al. Effect of Dietary Composition of Weight Loss Diets on High Sensitivity C-Reactive Protein: The Randomized POUNDS LOST Trial. Obesity (Silver Spring, Md.) 2013;21(4):681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azadbakht L, Izadi V, Surkan PJ, Esmaillzadeh A. Effect of a High Protein Weight Loss Diet on Weight, High-Sensitivity C-Reactive Protein, and Cardiovascular Risk among Overweight and Obese Women: A Parallel Clinical Trial. Int J Endocrinol 2013;2013:971724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 44.Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. The American Journal of Clinical Nutrition 2013;97(5):980–9. [DOI] [PubMed] [Google Scholar]
- 45.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 2004;23(5):373–85. [DOI] [PubMed] [Google Scholar]
- 46.Eisenstein J, Roberts SB, Dallal G, Saltzman E. High-protein weight-loss diets: are they safe and do they work? A review of the experimental and epidemiologic data. Nutr Rev 2002;60(7 Pt 1):189–200. [DOI] [PubMed] [Google Scholar]
- 47.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;96(6):1281–98. [DOI] [PubMed] [Google Scholar]
- 48.Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, et al. The role of protein in weight loss and maintenance. The American Journal of Clinical Nutrition 2015;101(6):1320S–9S. [DOI] [PubMed] [Google Scholar]
- 49.Hodgson JM, Ward NC, Burke V, Beilin LJ, Puddey IB. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. J Nutr 2007;137(2):363–7. [DOI] [PubMed] [Google Scholar]
- 50.Weiss EP, Reeds DN, Ezekiel UR, Albert SG, Villareal DT. Circulating cytokines as determinants of weight loss-induced improvements in insulin sensitivity. Endocrine 2017;55(1):153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma W, Huang T, Zheng Y, Wang M, Bray GA, Sacks FM, et al. Weight-Loss Diets, Adiponectin, and Changes in Cardiometabolic Risk in the 2-Year POUNDS Lost Trial. J Clin Endocrinol Metab 2016;101(6):2415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Astrup A, Raben A, Geiker N. The role of higher protein diets in weight control and obesity-related comorbidities. Int J Obes (Lond) 2015;39(5):721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


