Abstract
The bone morphogenetic proteins (BMPs) belong to the same superfamily as related to transforming growth factor (TGF)β, growth and differentiation factors (GDFs), and activins. They were initially described as inducers of bone formation but are now known to be involved in morphogenetic activities and cell differentiation throughout the body, including the development of adipose tissue and adipogenic differentiation. BMP4 and BMP7 are the most studied BMPs in adipose tissue, with major roles in white and brown adipogenesis, respectively, but other BMPs such as BMP2, BMP6, and BMP8b as well as some inhibitors and modulators have been shown to also affect adipogenesis. It has become ever more important to understand adipose regulation, including the BMP pathways, in light of the strong links between obesity and metabolic and cardiovascular disease. In this review, we summarize the available information on BMP signaling in adipose tissue using preferentially articles that have appeared in the last decade, which together demonstrate the importance of BMP signaling in adipose biology.
Keywords: Bone morphogenetic protein (BMP), adipogenesis, obesity
INTRODUCTION
Obesity and overweight have reached pandemic levels in the U.S.; 35.0% of men and 40.4% of women were obese in 2013-14 1. If the current trend continues, it is estimated that 38% of the world population will be overweight and 20% will be obese by 2030 2, and 85% of the adults in the U.S. will be either overweight or obese 3. The societal and individual burden of obesity increases significantly if we also take into account the strong links between obesity and metabolic and cardiovascular disease 4, 5.
Obesity results from an imbalance between intake and expenditure of energy 4, 5. White adipose tissue (WAT) is responsible for the storage of energy 6 and has a relevant role in the production of adipokines and hormones 7. WAT consists of white adipocytes containing big lipid droplets that displace the nuclei and other organelles to the periphery of the cells. Brown adipose tissue (BAT), on the other hand, consists of brown adipocytes containing multiple small lipid droplets. BAT is a highly vascularized tissue that dissipates energy by generating heat, potentially contributing to the elimination of excess of calories 6, 8. A third kind of adipocytes are referred to as “beige” or “brite” (brown-in-white) adipocytes 9 in that they are able to transition from white-like to brown-like adipogenesis, a process referred to as “browning” that occurs in response to specific stimuli such as beta-agonists 10-12.
White and brown adipogenesis are regulated by numerous signaling pathways, which have been extensively reviewed in recent publications 13-16. The bone morphogenetic proteins (BMPs) and their modulators are relatively late additions to the field of adipose regulation. The BMPs belong to the same superfamily of growth factors as growth/differentiation factors (GDFs), transforming growth factor (TGF) beta, activins, nodal and anti-Müllerian hormone 17, 18. The BMPs were first discovered because of their ability to induce ectopic bone formation in soft tissue by Marshall Urist 19 and were later purified by Wang et al. and cloned by Wozney et al. 20, 21. BMP signaling is critical in many developmental processes such as the determination of the body axis, germ layer specification, organ development and cell differentiation 22-25. BMP signaling relies on binding of BMPs to type I and II BMP receptors (Figure 1), which triggers an array of intracellular mediators including the canonical SMAD pathway 26. The BMPs and GDFs activate the SMAD1/5/8 pathway though the Type I receptors, i.e. activin receptor-like kinases (ALK)1, 2, 3, or 6, in combination with the BMP receptor type II, or the activin receptor type-2A or −2B 22-26. In this review, we summarize the available information on BMP signaling in adipose tissue, which together demonstrate the importance of the BMP signaling pathways in adipose biology.
Figure 1: Schematic overview of the BMP signaling system and associated transcription factors relevant for adipogenesis.
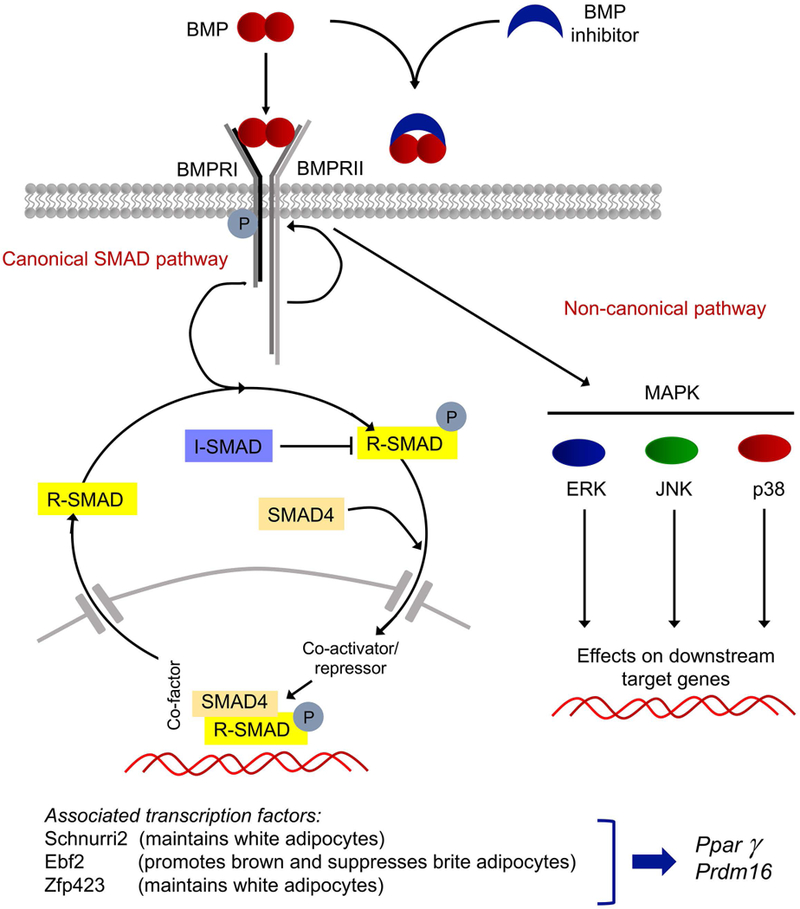
Top, BMP dimers bind to a complex consisting of BMPRI and BMPRII, or are sequestered by BMP inhibitors. In canonical BMP signaling, the type II receptor phosphorylates (activates) the type I receptor, which in turn activates regulatory SMAD1/5/8. The R-SMADs combine with SMAD4, a co-SMAD, and enters the nucleus to regulate gene expression together with the appropriate co-activators and repressors. The R-SMADs can also be inhibited by the I-SMADs. In non-canonical BMP signaling, MAPK, ERK, JNK, and p38 are activated. Bottom, transcription factors known to affect adipocyte differentiation. BMP, bone morphogenetic protein; BMPRI, BMP type I receptor; BMPRII, BMP type II receptor; R-SMAD, regulatory SMAD; I-SMAD, inhibitory SMAD; MAPK, mitogen-activated protein kinase; ERK, extracellular signal-regulated kinase; JNK, Jun N-terminal kinase; p38, p38 mitogen-activated protein kinase; Ebf2, early B-cell factor 2; Zfp423, zinc finger protein 423; Pparγ, peroxisome proliferator-activated receptor γ; Prdm16, PR domain containing 16.
Bone Morphogenetic Proteins
The BMPs that have been linked to adipogenesis so far belong to four BMP-subgroups 26, BMP2/4, BMP9/10, BMP5-8, and BMP3. The information that is available pertains mostly to individual BMP signaling components (Table I, Figure 2), and less to potentially overlapping effects and mutual regulation of BMPs, BMP modulators and receptors.
TABLE 1:
Proposed functions of BMP components.
| Type | Component | Proposed Functions |
|---|---|---|
| BMPs | BMP2 | • Linked to WAT (27, 28) (h,m) • May have metabolic effects (28) (m) • Adipogenic effects vary in different progenitor cells (h,m) |
| BMP4 | • Essential for white adipogenesis (45, 46) (m), but is important UCP1 induction in BAT (57) (h) • Increases with cold exposure (58) (m) • Promotes adipocyte differentiation (51-55) (h, m) |
|
| BMP6 | • Increase in BAT with cold exposure (58) (m) • Induces brown-like characteristics in progenitor cells (63) (m,c) |
|
| BMP7 | • Essential for brown adipogenesis (63, 71) (m) • Stimulates adipose “browning” (72) (h) • Possible effect on food intake (66, 70) (m) |
|
| BMP8b | • Increases with cold exposure and high fat diet (58, 74) (m) | |
| BMP3 | • Stimulates proliferation of progenitor cells (75) (m) | |
| BMP14 | • Adipocyte-specific overexpression decreases body fat (80) (m) | |
| Inhibitors | Gremlin | • Silencing of Gremlin-1 promotes adipogenic differentiation and brown-like characteristics in progenitor cells (55, 86) (h,m) |
| Noggin | • Contradictory results | |
| MGP | • Strongly expressed in pre-adipocytes (94) (h) | |
| Follistatin | • Induced during brown differentiation and cold exposure (96, 97) (m) | |
| BAMBI | • Negative regulation of adipogenesis (107) (m) | |
| Chordin-like | • Overexpression protects against diet-induced obesity (109) (m) | |
| Follistatin-like | • Decreases during adipogenic induction (110) (m) • Increased in tissue of obese mice and serum of obese individuals (111) (h,m) • Gene deletion reduces body fat (112) (m) |
|
| Receptors | BMPR1A (ALK3) | • Higher adipose expression in obese individuals (113) (h) • Deletion in brown precursors led to less BAT (65) (m) • Overexpression, together with that of BMPR1B, promotes adipogenesis (114) (m) |
| BMPR2 | • Increased in adipose tissue of obese patients (115) (h) | |
| ActRIIB | • Blockade activates BAT function (117) (m) |
Abbreviations: h, human; m, mouse; WAT, white adipose tissue; BAT, brown adipose tissue; BMP, bone morphogenetic protein; MGP, matrix Gla protein; BAMBI; BMP and activin membrane bound inhibitor; BMPR, BMP receptor; ActR, activin receptor, UCP1, uncoupling protein 1.
Figure 2: Schematic summary of BMP action on white and brown adipocytes based on current knowledge.
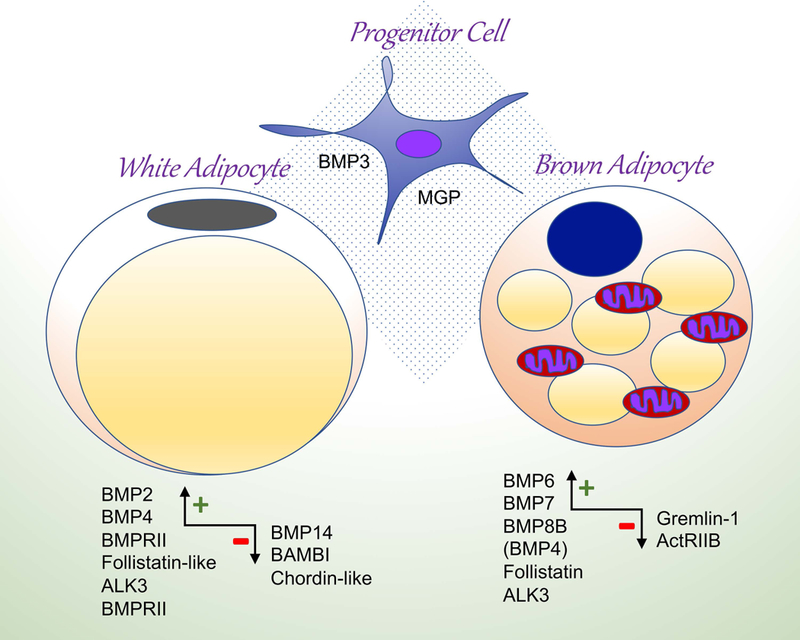
The + and - signs indicate largely stimulating and inhibitory action, respectively. ActRIIB, activin type IIB receptor; ALK, activin receptor-like kinase; BAMBI, BMP and activin membrane-bound inhibitor; BMP, bone morphogenetic protein; BMPRII, BMP type II receptor; MGP, matrix Gla protein.
Bone morphogenetic protein 2 (BMP2)
BMP2 is expressed in human adipose tissue, at higher levels in visceral than subcutaneous WAT, and in overweight and obese individuals than in lean individuals 27. However, the role of BMP2 remains somewhat ambiguous. Mouse studies have implicated BMP2 in white adipogenesis, in that ablation of Schnurri2, a gene targeted and activated by BMP2, reduced white but not brown adipose mass 28. The Schnurri2 ablation also resulted in an increase of blood insulin and a decrease of blood glucose 28, which suggest that BMP2 has metabolic effects. In addition, BMP2 also targets Zfp423 29, responsible for the maintenance of the characteristics of white adipocytes and the suppression of brite activation 30.
Numerous studies have examined the effects of BMP2 on adipogenic differentiation in various adipose progenitor cells. C3H10T1/2 transfected with BMP2 preferentially differentiated into osteoblast-like cells, although a large number of adipocytes were also obtained 31. However, in adipogenic medium, BMP2 preferentially induced adipogenic differentiation 32 suggesting that the effect of BMP2 may be dependent on context and type of delivery. The induction of adipogenesis in C3H10T1/2 cells by BMP2 takes place trough activation of peroxisome proliferator-activated receptor (PPAR)-γ via SMAD and p38MAPK signaling 33.
In adipose-derived stromal cells, BMP2 supports both adipogenic and osteogenic differentiation. In one study, addition of BMP2 to human adipose-derived stromal cells inhibited osteogenic differentiation in cells from six out of eight donors but enhanced it in cells from the remaining two donors 34. Similar inconsistencies were also seen in other studies. Two studies showed that BMP2 was unable to induce osteogenesis in adipose-derived stromal cells 35, 36 whereas a third study showed strong osteogenic effects 37. Possible contributing factors to these inconsistencies may be variability in the source of BMP2 and biological activity.
The results in human mesenchymal cells (hMSCs) were more pro-adipogenic. A combination of BMP2 and 3-isomethyl-1-buthylxanthine (IBMX) induced a 6-fold increase in adiponectin expression 38 even though the effect in human cells was also influenced by the donor 39. In hMSC from bone marrow cultured in adipogenic medium, treatment with BMP2 increased peroxisome proliferator-activated receptor (PPAR)γ activation, regardless of the origin of the cells 40. Similarly, in 3T3-L1 adipocytes, BMP2 enhanced insulin-mediated uptake of glucose through PPARγ and glucose transporter type 4 (GLUT4) 41. When combined with insulin-like growth factor, BMP2 was even able to induce adipocyte differentiation in tendon stem cells 42.
There is only one study proposing a possible relationship of BMP2 with brown adipogenesis. Salisbury et al. 43 suggested involvement of BMP2 in the activation and expansion of progenitors of brown adipocyte-like cells in the perineurium of peripheral nerves. Injection of BMP2 stimulated an increase of noradrenalin that in turn resulted in an increase of cells expressing the beta3 adrenergic receptor that migrated from the nerves and then transitioned through a brown-like adipogenic state. Finally, BMP2 was found to form heterodimers with BMP7, which promoted the phosphorylation of the SMAD1/5/8 without affecting the MAPK pathway 44 although it is unclear whether these heterodimer affects adipogenesis.
Bone Morphogenetic Protein 4 (BMP4)
Overall, BMP4 is mostly known to be associated with WAT in mice. BMP4 induces the transcription of Zfp423 29, which favors stability of white adipocytes and prevents the activation of brite 30. Qian et al. demonstrated that overexpression of BMP4 in adipose tissue, as mediated by a Bmp4 transgene, led to a general reduction of visceral WAT and adipocyte size 45. The investigators observed a decrease in the expression of brown adipocyte developmental- and mitochondrial-related genes in BAT, whereas the expression increased in WAT 45. However, these results conflict somewhat with those of Modica et al. 46 who reported that overexpression of BMP4 in the inter-scapular BAT, as mediated by an adenovirus containing the Bmp4 gene, promoted a brown-to-white adipogenic shift by impairing the acquisition of functional thermogenesis and decreasing free fatty acid oxidation and oxygen consumption. The lipid droplets in BAT were larger and the expression of uncoupling protein 1 (UCP1) was lower, even after 24 hours of cold exposure, in mice injected with Bmp4-containing virus compared to control mice 46. The apparent contradiction between the studies may be explained by the fact that the transgene used by Qian et al. 45 was regulated by the promoter of the Fatty acid-binding protein 4 (Fabp4) gene (also known as Adipocyte protein 2, aP2), which is also expressed in cells other than adipocytes 47, 48, whereas Modica et al. 46 used the Adiponectin promoter. It is also likely that the effect of BMP4 is influenced by the dose, location in the tissues, and fat depot 45, 49.
BMP4 may be involved in the insulin response since deletion of adipose Bmp4 in mice results in reduced insulin sensitivity along with increased adiposity 45. Furthermore, correlations between high serum levels of BMP4 and high degrees of diabetes, adiposity and insulin sensitivity have been reported in patients 46. Similar results were obtained in two models of insulin resistance in mice (db/db and high fat fed), where the serum levels of BMP4 increased in parallel with the serum insulin levels 50.
Several studies have shown that BMP4 regulates adipogenesis in vitro even though the patterns of induction seem to differ between cell models. Suenaga et al. 51 reported that expression of BMP4 increases in 3T3-L1 preadipocytes before they differentiate into adipocytes, and that the increase in BMP4 is required for the differentiation to occur. However, committed A33 preadipocytes expressed BMP4 in parallel with activation of SMAD1/5/8 during proliferation, whereas BMP4 expression diminished when they reached confluence 52. When BMP4 was blocked by Noggin, the committed proliferating A33 cells lost their preadipocyte phenotype 52, further indicating a need of BMP4 to maintain commitment.
In uncommitted C3H10T1/2 cells, however, BMP4 expression increased when the cells reached confluency 52 and treatment with BMP4 induced adipogenic commitment. However, certain threshold concentration of BMP4 were required: 10 ng/ml showed no effect, whereas both 50 ng/ml and 100 ng/ml resulted in adipocyte differentiation 53. Induction of BMP4 has also been reported in primary human preadipocytes undergoing differentiation 54. It is believed that adipogenic commitment caused by BMP4 is mediated by the nuclear entry of the zinc finger protein (ZNF)423 transcription factor, which leads to PPARγ induction 55. Interestingly, BMP4 causes the loss of the platelet-derived growth factor receptor (PDGFR)β at the same time that adipose markers appear in both pericytes and C3H10T1/2 cells 56, suggesting that BMP4 might play a role in connecting the vascular system to the adipose tissue.
The role of BMP4 in BAT is less well studied but has been reported to induce UCP1 expression in human adipose stromal cells (ASCs) when combined with BMP7 57. Despite its strong association with whitening, BMP4 expression in BAT increases the response to cold exposure 58. Brown preadipocytes treated with BMP4 have reduced expression of mitochondrial genes, and decreased oxygen consumption and basal lipolysis, which results in larger lipid droplets 46. BMP4 is able to form heterodimers with BMP7, which reportedly have higher activity in bone than the correspondent homodimers 59 but their effects in white and brown adipogenesis remain to be studied. Finally, BMP4 activity may be subject to gender differences since estrogen depletion upregulates BMP4 in adipose tissue and BMP4 stabilizes the estrogen receptor α and estrogen signaling in WAT 60.
Bone Morphogenetic Protein 6 (BMP6)
There is only limited research available on the role of BMP6 in adipogenesis, and the results are mixed. BMP6 is highly homologous with BMP7 but displays a 20-fold higher affinity to ALK3 (also known as BMP receptor IA, BMPRIA) than BMP7 and a stronger resistance to inhibition by Noggin 61. Under cold conditions, the expression of BMP6 was shown to increase in BAT 58. In addition, BMP6 was able to convert C2C12 murine precursor cells into adipocytes under proadipogenic conditions with induction of BAT markers, resulting in a bioenergetics profile similar to that of brown adipocytes 62. In brown preadipocytes, however, BMP6 increased lipid accumulation but did not increase expression of brown adipose genes 63. In intramuscular preadipocytes from chicken, BMP6 expression was enhanced during adipogenic differentiation 64, and in 3T3-L1 adipocytes, BMP6 increased expression of PPARγ, which enhanced GLUT4 and insulin-mediated uptake of glucose 41.
Bone Morphogenetic Protein 7 (BMP7)
BMP7 is the BMP that is most strongly linked to brown adipogenesis. Gene deletion of Bmp7 in mice reduced BAT at birth with up to 70% 63. Interestingly, lack of ALK3 (BMPRIA), which mediates BMP7 signaling, also results in impaired cervical BAT formation due to alterations in the progenitor cells 65. Furthermore, lean C57BL6/J mice that were fed a high fat diet and treated with BMP7 via subcutaneous osmotic minipumps for 4 weeks at 21°C showed an increase in BAT volume and UCP1 expression 66. There was also a dose-dependent increase in fat oxidation and energy expenditure 66. Two different mouse models of obesity, db/db mice and C57BL/6J mice fed with high fat diet, that were treated with intraperitoneal injections of BMP7 for a month showed a reduction in body weight and body fat as well as improved glucose levels and reduced inflammation, when compared to their respective controls 50
There is evidence that BMP7 controls, through SMAD signaling, both Zfp423 and Ebf2, which have different effects on adipogenesis 67-69. The proposed model of BMP function has two parts: in the first part, Zfp423 binds to Ebf2 and acts as a transcriptional co-repressor of crucial Ebf2-target genes, such as Prdm16, resulting in the suppression of the overall thermogenic gene program in white adipocytes30. In the second part, BMP7 prompts a Smad1/4 interaction with Zfp423, which results in the activation of Pparγ expression and adipogenesis. This way, Zfp423 is sequestered from Ebf2, permitting Ebf2 to drive the thermogenic gene program of BAT 30.
However, the effect of BMP7 on body weight might not be limited to its role in the induction and maintenance of BAT. Two studies have suggested that it affects food intake, although with some contradictions. Townsend et al. 70 observed that administration of a BMP7-containing adenovirus to diet-induced obese mice reduced their food intake and consequently the weight of the mice. It was estimated that 75% of the weight reduction was due to decreased food intake and 25% to increased BAT activation. On the other hand, Boon et al. 66 described that mice treated with BMP7 via subcutaneous osmotic mini-pumps for 4 weeks increased their food intake. The contradictory effects may be due to differences in BMP7 administration or the duration or the dose of the respective treatments. Another possibility is that the increase in BAT leads to an increase in energy dissipation, which in turn enhances the need for energy and stimulates food intake. Together, the findings point to a possible role of BMP7 in the regulation of food intake although further research is needed to support this.
In vitro studies also supported a role of BMP7 in brown adipogenic differentiation. Tseng et al. 63 showed that brown preadipocytes enhanced expression of UCP1 and mitochondrial biogenesis in response to BMP7 more than to any other BMP. In addition, BMP7 induced commitment of mesenchymal stem cells to the brown adipogenic lineage 63, and increased mitochondrial activity in mature brown adipocytes by increasing fatty acid uptake and oxidation, without increasing the number of mitochondria 71. BMP7 may play a role in the browning of WAT, as suggested by the finding that BMP7 increases the expression of brown and beige markers as well as metabolic activity in human ASCs 72. In C3H10T1/2 cells, BMP7 also coordinates a panel of insulin signaling components, and is able to rescue brown adipogenesis in cells with defective insulin signaling 73.
Bone Morphogenetic Protein 8B (BMP8B)
BMP8b was recently found to be preferentially expressed in BAT in mice 74, unlike the BMP8A isoform, which was enriched in WAT. The expression of BMP8B increased after high fat diet or cold (4°C) exposure 58, 74. It was also expressed in the hypothalamus where it acted as a thermogenic protein together with AMP-activated protein kinase in key hypothalamic nuclei 74. Mice with Bmp8b gene deletion exhibited altered BAT with enlarged lipid droplets, impaired thermogenesis and susceptibility to diet-induced obesity 74. Interestingly, the adipose expression of BMP8B showed gender differences. BAT of females had higher BMP8B expression than that of males, and males injected with diethylstilbestrol (estrogen analog) exhibited an increase in body weight and BMP8B expression 58.
Bone Morphogenetic Protein 3 (BMP3)
Despite being a BMP, the action of BMP3 is mediated in a way resembling that of TGFβ. It binds to activin receptor type IIB (ActRIIB), which, together with ALK4, activates the SMAD2/3 pathway 75. In vivo, increased BMP3 expression has been associated with the C57BL/6J mice that gained the most weight in response to a high fat diet 76. However, in C3H10T1/2 or 3T3-L1 cells, BMP3 stimulated proliferation rather than adipogenic differentiation 75. BMP3 has also been identified as a key factor in a model of maternal protein restriction model of visceral adiposity 77.
Bone Morphogenetic Protein 3b (BMP3b)
BMP3b (also referred to as GDF10) increased in the mesenteric adipose tissue of mice with diet-induced obesity 78 and transgenic mice with adipose overexpression of BMP3b (as directed by the aP2-promoter) appeared to be protected against diet-induced obesity 79.
This would be consistent with the findings in 3T3-L1 cells that BMP3b suppresses adipogenesis, and that BMP3b is expressed at higher levels in preadipocytes than in mature adipocytes 78. Thus, it may function as feedback mechanism to limit adipogenesis in abdominal obesity 78.
Bone Morphogenetic Protein 14 (BMP14)
BMP14 (also referred to as GDF5) is higher in inter-scapular BAT than in any kind of WAT in mice. Adipose-specific Bmp14-transgenic mice driven by the aP2 promoter has less adipose tissue and smaller adipocytes than control mice, despite no difference in food intake 80. Furthermore, blood glucose levels were reduced in the Bmp14 transgenic mice compared to controls, while oxygen consumption and energy expenditure were increased 80. Conversely, dominant-negative GDF5 mutant mice fed a high fat diet gained more weight than controls while oxygen consumption and energy expenditure decreased 80. In vitro, BMP14 increased progressively in 3T3-L1 preadipocytes during adipogenic induction 81, and promoted brown adipogenesis in cells from BAT and subcutaneous WAT, but not from visceral WAT 80.
Inhibitors and Modulators of BMP Signaling
A limited number of studies have examined the possible roles of BMP inhibitors and modulators in adipogenic differentiation and adipose metabolism, leaving this field largely open for future investigations. The available information suggests that the modulators of BMP signaling plays a relevant role in the adipose tissue, with differences in their effects on WAT and BAT (Table I, Figure 2).
Gremlin
There are two isoforms of Gremlin. Gremlin-1 is the most studied isoform, and has been shown to bind BMP2, BMP4, and BMP7 82, 83. Both isoforms were upregulated by BMP4 46 and might have a limiting effect on adipogenesis. Gremlin-1 was more expressed in omental fat than in its subcutaneous counterpart 84 but increased in the subcutaneous adipose tissue of individuals with hypertrophic obesity 55. In these individuals, its expression correlated positively with that of BMP4 in whole adipose tissue and in individual adipocytes 55. The disappearance of Gremlin-1 promoted adipogenic differentiation in vitro. In 3T3-L1 cells undergoing adipogenesis, Gremlin-1 expression gradually disappeared over a few days 85 and when Gremlin-1 was silenced using siRNA in human preadipocytes, PPARγ was greatly induced 55. Furthermore, expression of the brown adipocyte markers ZIC1 and UCP1 and mitochondrial content increased with the silencing of Gremlin-1 55.
The other isoform, Gremlin-2, was reduced in the epididymal adipose tissue of C57BL/6 mice subjected to high fat diet, as well as in ob/ob mice and db/db mice 86. Gremlin-2 also declined in 3T3-L1 cells upon adipogenic induction, and cells overexpressing Gremlin-2 accumulated less lipids than control cells, whereas knock-down of Gremlin-2 increased the adipogenic potential 86.
Noggin
In vitro experiments have shown that Noggin binds a wide range of BMPs, including at least BMP2, BMP4, BMP5, BMP6, BMP7, BMP13 and BMP14 87. In addition, its expression was induced by BMP4 signaling 46, potentially establishing feedback regulation. The few studies available suggest that the effect on adipogenic differentiation of Noggin varies from cell to cell. In bone marrow mesenchymal stem cells (MSCs), Noggin treatment promoted adipogenesis 88, whereas in human stromal cells, Noggin had an inhibitory effect on adipogenic differentiation if added prior to full differentiation 54. It has also been found that expression of Noggin is reduced or eliminated in differentiated cells, whether they are adipocytes or cardiac/skeletal myocytes 55, 89, which suggests that modulation by Noggin may be most important during the early cell differentiation. Elevated plasma levels of Noggin levels have been reported in spontaneously obese mice and patients with body mass index (BMI)>27 88 although the mechanistic connection between circulating Noggin and the adipose tissue remains to be clarified.
Matrix Gla protein (MGP)
MGP is best known for its ability to limit arterial calcification and vascular malformations, in part through inhibition of BMP2, BMP4 and BMP7 90-93. Nevertheless, microarray analysis in human adipocytes showed that MGP was strongly expressed in pre-adipocytes but decreased in the secretome during their differentiation to adipocytes 94. MGP expression was also depot-dependent, with higher expression in the omental than in the subcutaneous adipose tissue 95
Follistatin
Follistatin binds BMP2, BMP4, BMP6 and BMP7 and activin, and promotes BAT characteristics 96, 97. It was expressed in cold-induced BAT in mice 96, and during adipogenic differentiation of mouse brown preadipocytes and mouse embryonic fibroblasts (MEFs) 96. MEFs from mice with Follistatin gene deletion had decreased expression of the brown adipocyte markers UCP1 and PR domain containing 16 (PRDM16), which was reversed when Follistatin was added 96. Conversely, BAT from Follistatin transgenic mice had enhanced expression of UCP1 and PRDM16 and an increased amount of interscapular BAT 98. In addition, WAT from Follistatin transgenic mice showed an increase in brown/beige adipogenic markers 98. A study in obese and non-obese women showed that Follistatin mRNA levels in WAT were reduced in obesity but increased in subcutaneous WAT after weight loss 99.
Myostatin
Myostatin, also known as GDF8, blocks BMP7 activity in CH10T1/2 and 3T3-L1 cells by binding to its receptor, the activin type 2B receptor (ActRIIB), apart from having direct effects on SMAD2 and SMAD3 100, 101. The inactive form of myostatin, before its pro-peptide is removed, is also referred to as follistatin-like 3 102. Mice with Myostatin gene deletion exhibit less adipose tissue and higher muscular mass 103, whereas Myostatin transgenic mice have normal body composition with a reduction in adipocyte size 104. Specific deletion of myostatin in muscle decreased the fat mass and improved insulin sensitivity, whereas a similar deletion in adipose tissue reduced in serum free fatty acids but without change in body composition 102. Absence of Myostatin has also been linked to increases in expression of brown and brite markers and BMP7 105, 106.
BMP and Activin Membrane-Bound Inhibitor (BAMBI)
BAMBI is a membrane-bound modulator of paracrine factors that regulate adipogenesis such as TGFbeta, BMP and Wnt. It serves as a negative regulator of adipogenesis, and is downregulated in a mouse model of diet-induced obesity 107. Similarly, suppression of BAMBI in porcine preadipocytes promotes adipogenic differentiation 108.
Chordin-like
The Kielin/chordin-like protein inhibits both TGFβ and activin signals while enhancing BMP signaling. Kielin/chordin-like transgenic mice were protected from high fat diet-induced obesity, had higher body temperature (almost a full degree) and oxidized more glucose than control mice 109 These transgenic mice exhibited high levels of UCP1and PPARγ in the epididymal WAT 109.
Follistatin-like
Follistatin-like is distantly related to Follistatin 110. It was enriched in 3T3-L1 preadipocytes and decreased as adipogenesis induction occurs; conversely, it reappeared when adipocytes were dedifferentiated 110. A significant increase of follistatin-like was observed in adipose tissue of ob/ob mice as well as in the serum of overweight and obese individuals 111. Mice with deletion of Follistatin-like 3 had enhanced glucose tolerance and insulin sensitivity and their perigonadal visceral fat pad was reduced in size 112.
BMP receptors
ALK3 (BMPRIA)
ALK3 is known to bind and mediate signals from multiple BMPs 26. A study in a cohort of 941 type two diabetic patients and 944 non-diabetic controls showed that the its expression was higher in visceral than in subcutaneous fat, regardless of gender 113. When analyzed by weight, BMPR1A expression in overweight and obese individuals was higher than in lean subjects and increased in the adipose tissue of type 2 diabetics 113. ALK3 expression correlated directly with both BMI and percent body fat 113. In mice, specific deletion of Alk3 in cells expressing Myf5, a marker of brown adipocytes and the smooth muscle lineages, showed a strong reduction in constitutive BAT 65. The mice were born with reduced BAT mass, which remained significantly smaller into adulthood. The newborn mice exhibited reduced body temperature, whereas adults had an impaired response to cold temperatures 65. Overexpression of ALK3 and ALK6 (also known as BMPRIB) in C3H10T1/2 cells promoted adipogenic differentiation, even in the absence of changes in BMP2 and BMP4 114.
Type 2 receptors
BMP type 2 receptor (BMPR2) and Activin type 2B receptor (ActR2B)
A study in 930 patients with type 2 diabetes and 900 non-diabetic controls showed that BMPR2 expression was increased in the subcutaneous versus the visceral adipose tissue 115. Furthermore, the BMPR2 levels were increased in the visceral and subcutaneous adipose tissue of overweight and obese patients as compared to lean patients 115. The findings might be consistent with cellular findings that targeting BMPR2 negatively regulated porcine preadipocytes 116. In contrast, when mice were treated with antibodies against ActRIIB, the effect was selectively observed in the BAT. The BAT mass increased along with the brown adipocyte size, whereas no changes in WAT were reported 117. The treated mice were protected against hypothermia and had an increased oxygen consumption and CO2 release 117.
Unanswered questions and perspectives
So far, the available information suggests that BMP signaling has an important role in the adipose tissue. Some members of the BMP family seem to act preferentially in one type of adipose tissue and others may be relevant for specific fat depots. BMP2/4 appear to have links with WAT and the antagonists Gremlin and Noggin, whereas BMP6/7/8B and 14 connect with BAT and the antagonists Follistatin, Myostatin and Chordin-like.
Most studies have focused on one or two members of the BMP family but have failed to take into consideration the relationships that exist between many of the BMPs. For example, the details of the relationship between BMP4 and BMP7 remain unclear. As outlined above, both have been reported to act on brown adipocytes, whereas the single action of BMP4 seems specific for WAT. It is possible that BMP4/7 and BMP2/7 heterodimers play a role, that there is competition between the two BMPs for specific receptors, or that a temporal relationship exists between BMP4 and BMP7 where one of the BMPs has to “prime” the brown adipocytes prior to the action of the other.
The addition or removal of one BMP agonist or antagonist often affects the expression of other agonists or antagonists thereby upsetting the overall BMP activity. To fully evaluate the effect of an addition or removal, we would have to know what BMP balance would be optimal for each fat depot. The perfect BMP balance is likely affected by age, gender and adipose location and adds another dimension to BMP signaling that will require additional studies in mice and humans.
The BMP system might serve as an interface between adipose metabolism and diabetes. On one hand, diabetes induces BMP4 in the adipose tissue 118 and the vascular endothelium 119. On the other hand, transgenic and systemic BMP4 enhances glucose-stimulated insulin secretion and ameliorates glucose tolerance in mice through ALK3 signaling in beta cells 120. This might be a platform for crosstalk that could be used for modulation of diabetic complications in obesity. It would also be interesting to know if modulation of BMP signaling in the vascular endothelium affects adipocyte differentiation and metabolism.
The role of BMP inhibitors in obesity and obesity complications has not been well studied. Even though the BMP inhibitors regulate BMPs, the extent and circumstance of inhibition will depend on protein characteristics and the respective affinities for different BMPs and other binding partners, as well as the surrounding matrix and cellular localization. Considering all the “moving parts” in the BMP system, it may be necessary to develop a systems biology approach to account for the combined results of simultaneous actions. Such an approach might also be useful to identify and understand the relative importance of BMP target genes in adipose tissue.
Whether or not the possibility of BAT activation in humans to reduce body fat is feasible or effective still needs further evaluation. Theoretically, BAT activation would lead to an increase of energy expenditure and modify the balance between energy intake/expenditure. However, the exact volume of human BAT remains unknown and there are conflicting reports on whether or not BAT activation, even at its full capacity, would be enough to significantly affect energy expenditure 121. Attempts to activate BAT through adenoceptor agonists proved to be effective in mice, but did not yield similar results in clinical trials due to lack of bioavailability 122. Whether the direct use of BMPs and their inhibitors can be more effective in reducing body fat remains to be seen.
Once adipose BMP signaling is better understood, we would have a better perspective on what could be done in regards to BMP modulation as a treatment. It might be possible to create agonists and antagonists with select molecular properties that could target specific BMP components. Such molecular therapeutics might be used for systemic delivery via the blood stream, for local delivery through expression in the local endothelium, diffusion from depots in the adipose tissue, or transplantation of genetically modified stem cells or adipocytes. Since BMPs and their extracellular inhibitors have been implicated in gradients in tissue formation and development 123, it is possible that this principle could be mimicked for local treatments.
Thus, a full understanding of adipose BMP signaling would provide a window into adipose development and metabolism and allow for the development of new strategies targeting obesity and metabolic imbalance.
Acknowledgments:
Funding for this work was provided in part by NIH/NHLBI: grant number HL30568 (K.I.B.) and NIH/NHLBI: grant number HL81397 (K.I.B.).
Footnotes
Disclosures: Drs. Blázquez-Medela, Jumabay and Boström have nothing to disclose.
REFERENCES
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016; 315: 2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008; 32: 1431–7. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity. 2008; 16: 2323–30. [DOI] [PubMed] [Google Scholar]
- 4.Brondani LA, Assmann TS, Duarte GC, Gross JL, Canani LH, Crispim D. The role of the uncoupling protein 1 (UCP1) on the development of obesity and type 2 diabetes mellitus. Arq Bras Endocrinol Metabol. 2012; 56: 215–25. [DOI] [PubMed] [Google Scholar]
- 5.Malloy PJ, Feldman BJ. Cell-autonomous regulation of brown fat identity gene UCP1 by unliganded vitamin D receptor. Mol Endocrinol. 2013; 27: 1632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend KL, Tseng Y-H. Brown adipose tissue:Recent insight into development, metabolic function and therpeutic potential. Adipocyte. 2012; 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015; 36: 461–70. [DOI] [PubMed] [Google Scholar]
- 8.Birerdinc A, Jarrar M, Stotish T, Randhawa M, Baranova A. Manipulating molecular switches in brown adipocytes and their precursors: a therapeutic potential. Prog Lipid Res. 2013; 52: 51–61. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012; 150: 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himms-Hagen J, Cui J, Danforth E Jr. et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994; 266: R1371–82. [DOI] [PubMed] [Google Scholar]
- 11.Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev. 2012; 13 Suppl 2: 83–96. [DOI] [PubMed] [Google Scholar]
- 12.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001; 86: 1930–5. [DOI] [PubMed] [Google Scholar]
- 13.Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. 2012; 81: 715–36. [DOI] [PubMed] [Google Scholar]
- 14.Cohen P, Spiegelman BM. Cell biology of fat storage. Mol Biol Cell. 2016; 27: 2523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014; 76: 225–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiefer FW. The significance of beige and brown fat in humans. Endocr Connect. 2017; 6: R70–R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carreira AC, Alves GG, Zambuzzi WF, Sogayar MC, Granjeiro JM. Bone Morphogenetic Proteins: structure, biological function and therapeutic applications. Arch Biochem Biophys. 2014; 561: 64–73. [DOI] [PubMed] [Google Scholar]
- 18.Wang RN, Green J, Wang Z, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014; 1: 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971; 50: 1392–406. [DOI] [PubMed] [Google Scholar]
- 20.Wang EA, Rosen V, Cordes P, et al. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci U S A. 1988; 85: 9484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988; 242: 1528–34. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Miralles I, Schisler JC, Patterson C. New insights into bone morphogenetic protein signaling: focus on angiogenesis. Curr Opin Hematol. 2009; 16: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrell NW, Bloch DB, Ten Dijke P, et al. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol. 2016; 13: 106–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishinakamura R, Sakaguchi M. BMP signaling and its modifiers in kidney development. Pediatr Nephrol. 2014; 29: 681–6. [DOI] [PubMed] [Google Scholar]
- 25.Hegarty SV, O’Keeffe GW, Sullivan AM. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog Neurobiol. 2013; 109: 28–41. [DOI] [PubMed] [Google Scholar]
- 26.Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016; 12: 203–21. [DOI] [PubMed] [Google Scholar]
- 27.Guiu-Jurado E, Unthan M, Bohler N, et al. Bone morphogenetic protein 2 (BMP2) may contribute to partition of energy storage into visceral and subcutaneous fat depots. Obesity. 2016; 24: 2092–100. [DOI] [PubMed] [Google Scholar]
- 28.Jin W, Takagi T, Kanesashi SN, et al. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell. 2006; 10: 461–71. [DOI] [PubMed] [Google Scholar]
- 29.Gupta RK, Mepani RJ, Kleiner S, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012; 15: 230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao M, Ishibashi J, Kusminski CM, et al. Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab. 2016; 23: 1167–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahrens M, Ankenbauer T, Schroder D, Hollnagel A, Mayer H, Gross G. Expression of human bone morphogenetic proteins-2 or −4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993; 12: 871–80. [DOI] [PubMed] [Google Scholar]
- 32.Shin S, Seong JK, Bae YS. Ahnak stimulates BMP2-mediated adipocyte differentiation through Smad1 activation. Obesity. 2016; 24: 398–407. [DOI] [PubMed] [Google Scholar]
- 33.Hata K, Nishimura R, Ikeda F, et al. Differential roles of Smad1 and p38 kinase in regulation of peroxisome proliferator-activating receptor gamma during bone morphogenetic protein 2-induced adipogenesis. Mol Biol Cell. 2003; 14: 545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SY, Lee JH, Kim JY, Bae YC, Suh KT, Jung JS. BMP2 increases adipogenic differentiation in the presence of dexamethasone, which is inhibited by the treatment of TNF-alpha in human adipose tissue-derived stromal cells. Cell Physiol Biochem. 2014; 34: 1339–50. [DOI] [PubMed] [Google Scholar]
- 35.Cruz AC, Silva ML, Caon T, Simoes CM. Addition of bone morphogenetic protein type 2 to ascorbate and beta-glycerophosphate supplementation did not enhance osteogenic differentiation of human adipose-derived stem cells. J Appl Oral Sci. 2012; 20: 628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuk P, Chou YF, Mussano F, Benhaim P, Wu BM. Adipose-derived stem cells and BMP2: part 2. BMP2 may not influence the osteogenic fate of human adipose-derived stem cells. Connect Tissue Res. 2011; 52: 119–32. [DOI] [PubMed] [Google Scholar]
- 37.Panetta NJ, Gupta DM, Lee JK, Wan DC, Commons GW, Longaker MT. Human adipose-derived stromal cells respond to and elaborate bone morphogenetic protein-2 during in vitro osteogenic differentiation. Plast Reconstr Surg. 2010; 125: 483–93. [DOI] [PubMed] [Google Scholar]
- 38.van Zoelen EJ, Duarte I, Hendriks JM, van der Woning SP. TGFbeta-induced switch from adipogenic to osteogenic differentiation of human mesenchymal stem cells: identification of drug targets for prevention of fat cell differentiation. Stem Cell Res Ther. 2016; 7: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanhatupa S, Ojansivu M, Autio R, Juntunen M, Miettinen S. Bone Morphogenetic Protein-2 Induces Donor-Dependent Osteogenic and Adipogenic Differentiation in Human Adipose Stem Cells. Stem Cells Transl Med. 2015; 4: 1391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donoso O, Pino AM, Seitz G, Osses N, Rodriguez JP. Osteoporosis-associated alteration in the signalling status of BMP-2 in human MSCs under adipogenic conditions. J Cell Biochem. 2015; 116: 1267–77. [DOI] [PubMed] [Google Scholar]
- 41.Schreiber I, Dorpholz G, Ott CE, et al. BMPs as new insulin sensitizers: enhanced glucose uptake in mature 3T3-L1 adipocytes via PPARgamma and GLUT4 upregulation. Sci Rep. 2017; 7: 17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Chen L, Zhou Y, Liu X, Tang K. Insulin-like growth factor-1 and bone morphogenetic protein-2 jointly mediate prostaglandin E2-induced adipogenic differentiation of rat tendon stem cells. PLoS One. 2014; 9: e85469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salisbury EA, Lazard ZW, Ubogu EE, Davis AR, Olmsted-Davis EA. Transient brown adipocyte-like cells derive from peripheral nerve progenitors in response to bone morphogenetic protein 2. Stem Cells Transl Med. 2012; 1: 874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koh JT, Zhao Z, Wang Z, Lewis IS, Krebsbach PH, Franceschi RT. Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J Dent Res. 2008; 87: 845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian SW, Tang Y, Li X, et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci U S A. 2013; 110: E798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modica S, Straub LG, Balaz M, et al. Bmp4 Promotes a Brown to White-like Adipocyte Shift. Cell Rep. 2016; 16: 2243–58. [DOI] [PubMed] [Google Scholar]
- 47.Jeffery E, Berry R, Church CD, et al. Characterization of Cre recombinase models for the study of adipose tissue. Adipocyte. 2014; 3: 206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KY, Russell SJ, Ussar S, et al. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013; 62: 864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue R, Wan Y, Zhang S, Zhang Q, Ye H, Li Y. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am J Physiol Endocrinol Metab. 2014; 306: E363–72. [DOI] [PubMed] [Google Scholar]
- 50.Chattopadhyay T, Singh RR, Gupta S, Surolia A. Bone morphogenetic protein-7 (BMP-7) augments insulin sensitivity in mice with type II diabetes mellitus by potentiating PI3K/AKT pathway. Biofactors. 2017; 43: 195–209. [DOI] [PubMed] [Google Scholar]
- 51.Suenaga M, Kurosawa N, Asano H, et al. Bmp4 expressed in preadipocytes is required for the onset of adipocyte differentiation. Cytokine. 2013; 64: 138–45. [DOI] [PubMed] [Google Scholar]
- 52.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci U S A. 2006; 103: 13022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2004; 101: 9607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gustafson B, Smith U. The WNT inhibitor Dickkopf 1 and bone morphogenetic protein 4 rescue adipogenesis in hypertrophic obesity in humans. Diabetes. 2012; 61: 1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gustafson B, Hammarstedt A, Hedjazifar S, et al. BMP4 and BMP Antagonists Regulate Human White and Beige Adipogenesis. Diabetes. 2015; 64: 1670–81. [DOI] [PubMed] [Google Scholar]
- 56.Tang Y, Qian SW, Wu MY, et al. BMP4 mediates the interplay between adipogenesis and angiogenesis during expansion of subcutaneous white adipose tissue. J Mol Cell Biol. 2016; 8: 302–12. [DOI] [PubMed] [Google Scholar]
- 57.Elsen M, Raschke S, Tennagels N, et al. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am J Physiol Cell Physiol. 2014; 306: C431–40. [DOI] [PubMed] [Google Scholar]
- 58.Grefhorst A, van den Beukel JC, van Houten EL, Steenbergen J, Visser JA, Themmen AP. Estrogens increase expression of bone morphogenetic protein 8b in brown adipose tissue of mice. Biol Sex Differ. 2015; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian SW, Liu Y, Wang J, et al. BMP4 Cross-talks With Estrogen/ERalpha Signaling to Regulate Adiposity and Glucose Metabolism in Females. EBioMedicine. 2016; 11: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aono A, Hazama M, Notoya K, et al. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun. 1995; 210: 670–7. [DOI] [PubMed] [Google Scholar]
- 61.Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009; 20: 441–8. [DOI] [PubMed] [Google Scholar]
- 62.Sharma A, Huard C, Vernochet C, et al. Brown fat determination and development from muscle precursor cells by novel action of bone morphogenetic protein 6. PLoS One. 2014; 9: e92608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008; 454: 1000–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang T, Zhang X, Han K, et al. Analysis of long noncoding RNA and mRNA using RNA sequencing during the differentiation of intramuscular preadipocytes in chicken. PLoS One. 2017; 12: e0172389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulz TJ, Huang P, Huang TL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013; 495: 379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boon MR, van den Berg SA, Wang Y, et al. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One. 2013; 8: e74083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajakumari S, Wu J, Ishibashi J, et al. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013; 17: 562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang W, Kissig M, Rajakumari S, et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014; 111: 14466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stine RR, Shapira SN, Lim HW, et al. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol Metab. 2016; 5: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Townsend KL, Suzuki R, Huang TL, et al. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012; 26: 2187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Townsend KL, An D, Lynes MD, et al. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid Redox Signal. 2013; 19: 243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okla M, Ha JH, Temel RE, Chung S. BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes. Lipids. 2015; 50: 111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang H, Schulz TJ, Espinoza DO, et al. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010; 30: 4224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whittle AJ, Carobbio S, Martins L, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012; 149: 871–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart A, Guan H, Yang K. BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J Cell Physiol. 2010; 223: 658–66. [DOI] [PubMed] [Google Scholar]
- 76.Koza RA, Nikonova L, Hogan J, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006; 2: e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guan H, Arany E, van Beek JP, et al. Adipose tissue gene expression profiling reveals distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab. 2005; 288: E663–73. [DOI] [PubMed] [Google Scholar]
- 78.Hino J, Miyazawa T, Miyazato M, Kangawa K. Bone morphogenetic protein-3b (BMP-3b) is expressed in adipocytes and inhibits adipogenesis as a unique complex. Int J Obes. 2012; 36: 725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hino J, Nakatani M, Arai Y, et al. Overexpression of bone morphogenetic protein-3b (BMP-3b) in adipose tissues protects against high-fat diet-induced obesity. Int J Obes. 2017; 41: 483–88. [DOI] [PubMed] [Google Scholar]
- 80.Hinoi E, Nakamura Y, Takada S, et al. Growth differentiation factor-5 promotes brown adipogenesis in systemic energy expenditure. Diabetes. 2014; 63: 162–75. [DOI] [PubMed] [Google Scholar]
- 81.Pei Z, Yang Y, Kiess W, Sun C, Luo F. Dynamic profile and adipogenic role of growth differentiation factor 5 (GDF5) in the differentiation of 3T3-L1 preadipocytes. Arch Biochem Biophys. 2014; 560: 27–35. [DOI] [PubMed] [Google Scholar]
- 82.Kisonaite M, Wang X, Hyvonen M. Structure of Gremlin-1 and analysis of its interaction with BMP-2. Biochem J. 2016; 473: 1593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wordinger RJ, Zode G, Clark AF. Focus on molecules: gremlin. Exp Eye Res. 2008; 87: 78–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoggard N, Cruickshank M, Moar KM, Bashir S, Mayer CD. Using gene expression to predict differences in the secretome of human omental vs. subcutaneous adipose tissue. Obesity. 2012; 20: 1158–67. [DOI] [PubMed] [Google Scholar]
- 85.Wade FM, Wakade C, Mahesh VB, Brann DW. Differential expression of the peripheral benzodiazepine receptor and gremlin during adipogenesis. Obes Res. 2005; 13: 818–22. [DOI] [PubMed] [Google Scholar]
- 86.Wu Q, Tang SG, Yuan ZM. Gremlin 2 inhibits adipocyte differentiation through activation of Wnt/beta-catenin signaling. Mol Med Rep. 2015; 12: 5891–6. [DOI] [PubMed] [Google Scholar]
- 87.Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011; 43: 478–81. [DOI] [PubMed] [Google Scholar]
- 88.Sawant A, Chanda D, Isayeva T, Tsuladze G, Garvey WT, Ponnazhagan S. Noggin is novel inducer of mesenchymal stem cell adipogenesis: implications for bone health and obesity. J Biol Chem. 2012; 287: 12241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawaguchi N, Nakao R, Yamaguchi M, Ogawa D, Matsuoka R. TGF-beta superfamily regulates a switch that mediates differentiation either into adipocytes or myocytes in left atrium derived pluripotent cells (LA-PCS). Biochem Biophys Res Commun. 2010; 396: 619–25. [DOI] [PubMed] [Google Scholar]
- 90.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997; 386: 78–81. [DOI] [PubMed] [Google Scholar]
- 91.Yao Y, Jumabay M, Wang A, Bostrom KI. Matrix Gla protein deficiency causes arteriovenous malformations in mice. J Clin Invest. 2011; 121: 2993–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Bostrom KI. A role for the endothelium in vascular calcification. Circ Res. 2013; 113: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malhotra R, Burke MF, Martyn T, et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PLoS One. 2015; 10: e0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mutch DM, Rouault C, Keophiphath M, Lacasa D, Clement K. Using gene expression to predict the secretome of differentiating human preadipocytes. Int J Obes. 2009; 33: 354–63. [DOI] [PubMed] [Google Scholar]
- 95.van Beek EA, Bakker AH, Kruyt PM, Hofker MH, Saris WH, Keijer J. Intra- and interindividual variation in gene expression in human adipose tissue. Pflugers Arch. 2007; 453: 851–61. [DOI] [PubMed] [Google Scholar]
- 96.Braga M, Reddy ST, Vergnes L, et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014; 55: 375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sidis Y, Tortoriello DV, Holmes WE, Pan Y, Keutmann HT, Schneyer AL. Follistatin-related protein and follistatin differentially neutralize endogenous vs. exogenous activin. Endocrinology. 2002; 143: 1613–24. [DOI] [PubMed] [Google Scholar]
- 98.Singh R, Braga M, Reddy ST, et al. Follistatin Targets Distinct Pathways To Promote Brown Adipocyte Characteristics in Brown and White Adipose Tissues. Endocrinology. 2017; 158: 1217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Flanagan JN, Linder K, Mejhert N, et al. Role of follistatin in promoting adipogenesis in women. J Clin Endocrinol Metab. 2009; 94: 3003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003; 23: 7230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sartori R, Gregorevic P, Sandri M. TGFbeta and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab. 2014; 25: 464–71. [DOI] [PubMed] [Google Scholar]
- 102.Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009; 4: e4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun. 2002; 291: 701–6. [DOI] [PubMed] [Google Scholar]
- 104.Feldman BJ, Streeper RS, Farese RV Jr., Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci U S A. 2006; 103: 15675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Braga M, Pervin S, Norris K, Bhasin S, Singh R. Inhibition of in vitro and in vivo brown fat differentiation program by myostatin. Obesity. 2013; 21: 1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ge X, Sathiakumar D, Lua BJ, Kukreti H, Lee M, McFarlane C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int J Obes. 2017; 41: 137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Luo X, Hutley LJ, Webster JA, et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI) as a potent negative regulator of adipogenesis and modulator of autocrine/paracrine adipogenic factors. Diabetes. 2012; 61: 124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mai Y, Zhang Z, Yang H, et al. BMP and activin membrane-bound inhibitor (BAMBI) inhibits the adipogenesis of porcine preadipocytes through Wnt/beta-catenin signaling pathway. Biochem Cell Biol. 2014; 92: 172–82. [DOI] [PubMed] [Google Scholar]
- 109.Soofi A, Wolf KI, Emont MP, et al. The kielin/chordin-like protein (KCP) attenuates high-fat diet-induced obesity and metabolic syndrome in mice. J Biol Chem. 2017; 292: 9051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu Y, Zhou S, Smas CM. Downregulated expression of the secreted glycoprotein follistatin-like 1 (Fstl1) is a robust hallmark of preadipocyte to adipocyte conversion. Mech Dev. 2010; 127: 183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan N, Sun H, Wang Y, et al. Follistatin-like 1: a potential mediator of inflammation in obesity. Mediators Inflamm. 2013; 2013: 752519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown ML, Bonomi L, Ungerleider N, et al. Follistatin and follistatin like-3 differentially regulate adiposity and glucose homeostasis. Obesity. 2011; 19: 1940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bottcher Y, Unbehauen H, Kloting N, et al. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes. 2009; 58: 2119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang H, Song TJ, Li X, et al. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009; 106: 12670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schleinitz D, Kloting N, Bottcher Y, et al. Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPR2) in the pathophysiology of obesity. PLoS One. 2011; 6: e16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu S, Sun G, Yuan B, et al. miR-375 negatively regulates porcine preadipocyte differentiation by targeting BMPR2. FEBS Lett. 2016; 590: 1417–27. [DOI] [PubMed] [Google Scholar]
- 117.Fournier B, Murray B, Gutzwiller S, et al. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol. 2012; 32: 2871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jumabay M, Moon JH, Yeerna H, Bostrom KI. Effect of Diabetes Mellitus on Adipocyte-Derived Stem Cells in Rat. J Cell Physiol. 2015; 230: 2821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of Vascular Bone Morphogenetic Protein Signaling in Diabetes Mellitus. Circ Res. 2010; 108: 446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007; 5: 207–19. [DOI] [PubMed] [Google Scholar]
- 121.Ruiz JR, Martinez-Tellez B, Sanchez-Delgado G, Osuna-Prieto FJ, Rensen PCN, Boon MR. Role of Human Brown Fat in Obesity, Metabolism and Cardiovascular Disease: Strategies to Turn Up the Heat. Prog Cardiovasc Dis. 2018; 61: 232–45. [DOI] [PubMed] [Google Scholar]
- 122.Mukherjee J, Baranwal A, Schade KN. Classification of Therapeutic and Experimental Drugs for Brown Adipose Tissue Activation: Potential Treatment Strategies for Diabetes and Obesity. Curr Diabetes Rev. 2016; 12: 414–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bier E, De Robertis EM. EMBRYO DEVELOPMENT. BMP gradients: A paradigm for morphogen-mediated developmental patterning. Science. 2015; 348: aaa5838. [DOI] [PubMed] [Google Scholar]


