Abstract
Randomized, controlled trials (RCTs) show intentional weight loss improves body composition and physical function in older adults; however, the long-term benefits (and risks) are unknown. We conducted a pilot study to assess the feasibility of recalling prior RCT participants to examine the long-term effects of intentional weight loss on body composition and physical function. A weighted, random sample of 60 older adults who were randomized to caloric restriction plus exercise (CR+EX) or exercise (EX) only in 5 prior RCTs (mean age at randomization, 67.3 years; 69% women, 80% white) were invited to participate. Follow-up was obtained on 89% (42 clinic visits, 10 phone interviews, 1 death) an average of 3.5 years (range, 2.2-5.8 years) after RCT completion. Despite greater weight, fat and lean mass loss during the RCT (mean difference in change (95% CI): −4.19 (−7.52,−0.86), −2.75 (−5.10,−0.40), and −2.32 (−3.69,−0.95) kg, respectively) in those randomized to CR+EX, long-term changes in weight (2.05 (−2.35,6.45) kg) and body composition (1.80 (−1.56,5.17) and 0.03 (−2.20,2.26) kg for fat and lean mass, respectively) from baseline and physical function at long-term follow-up (mean difference in 400-m walk and SPPB (95% CI): 23.2 (−19.3,65.6) sec and −0.03 (−1.02,0.96) points, respectively) were similar in CR+EX and EX only. Although improvements in weight and body composition following intentional weight loss may not be sustained long-term, physical function does not appear to be negatively impacted. A larger study is needed to confirm these results.
Keywords: Intentional weight loss, body composition, physical function
INTRODUCTION
Aging is associated with declines in muscle mass, strength, and physical performance, which lead to disability and loss of independence.1 Obesity, present in over one-third of older adults 2, exacerbates age-related declines in muscle mass3,4 and physical function,5 and is also associated with poorer health outcomes and quality of life in this age group.5–7 Moreover, excess adipose tissue contributes to the increased risk of several chronic diseases that are associated with aging, including osteoarthritis, type 2 diabetes, and coronary heart disease.8,9 These adverse health consequences of obesity in older adults highlight the need to identify effective treatments with sustained benefits in this population.
Clinical trials of obesity treatment in older adults show that diet-induced weight loss, particularly when combined with exercise, improves body composition and physical function at least in the short-term (e.g., immediately following treatment).10–20 However, limited data currently exist on whether these health benefits are sustained over time. We showed that randomization to a weight loss intervention does not increase the risk of mortality in this age group;21–23 but the long-term effects of intentional weight loss on physical function and morbidity in older adults has not been studied. One small (n=16) pilot study in older adults found that weight remained below baseline and improvements in physical function were maintained 30 months after completion of a weight loss intervention.24 In middle aged and older adults with type 2 diabetes from the Look AHEAD trial, individuals randomized to a long-term (~10 years) intensive lifestyle intervention designed to achieve weight loss through caloric restriction and increased physical activity had better physical function approximately 11 years post-randomization compared to those randomized to a diabetes support and education control.25 However, given that weight loss may worsen age-related loss of muscle mass and bone density, the overall safety and long-term risks and/or benefits of intentional weight loss in this population remain controversial.26–28
Despite evidence that older adults with obesity can successfully lose weight, most individuals are not successful at long-term maintenance of weight loss;29,30 and, if regained weight is disproportionately fat as observed in both lifestyle interventions31,32 and observational studies,33,34 overall body composition may be worse following weight loss and subsequent weight regain. It remains unknown whether long-term improvements in body composition and physical function persist in older adults after intentional weight loss, particularly if weight regain occurs. The challenge in addressing this question is the lack of long-term follow-up data to examine whether the health benefits of prior intentional weight loss persist over time. The primary objective of this pilot study was to access the feasibility of recalling participants who had been in prior randomized, controlled trials (RCTs) involving a weight loss intervention consisting of caloric restriction and exercise and to begin to determine the long-term effects of intentional weight loss on body composition and physical function in older adults.
METHODS
Study sample
From November 2013 through May 2014 we contacted a weighted (i.e., probability of recall approximately proportional to study size), random sample of older adults (n=60) who had been randomized to a weight loss intervention (5-18 months duration) consisting of caloric restriction and exercise (CR+EX) or to the exercise intervention alone (no weight loss control; EX only) in one of 5 RCTs previously conducted at Wake Forest University (n=854): INFINITE,20 I’M FIT,16 SECRET,17 IDEA,15 and CLIP.13 The inclusion/exclusion criteria and main study results for the five trials included in this pilot study have been published.13,15–17,20 All participants were older, overweight or obese, and sedentary (see Table 1). The diet interventions were either behavioral-focused with group/individual meetings with a registered dietitian and/or nutrition interventionist (3 studies)13,15,16 and/or provided hypocaloric daily meals or meal replacements (4 studies)15–17,20 (see Supplemental Table). The exercise interventions consisted of aerobic training (3 studies),13,17,20 resistance training (one study)16 or a combination of aerobic and resistance training (one study)15 on 3 or 4 days per week for 20 to 60 minutes duration. Participants were informed that the aim of this pilot study was to provide information on the long-term changes in health and physical function after participation in an exercise study to minimize response bias among those who may have regained weight. Individuals who agreed to participate in the study were scheduled for an in-person clinic visit or a telephone interview (for those who were unable or unwilling to come for a clinic visit). The study was approved by the Wake Forest School of Medicine Institutional Review Board, and all participants provided written (clinic visit) or verbal (telephone interview) informed consent.
Table 1.
Descriptive characteristics of the 5 randomized, controlled trials included in the pilot study.
| INFINITE (N=180) | I’M FIT (N=126) | SECRET (N=51) | IDEA (N=302) | CLIP (N=195) | |
|---|---|---|---|---|---|
| Enrollment period | 10/2009 – 8/2014 | 5/2010 – 12/2013 | 1/2009 – 9/2012 | 10/2006 – 6/2011 | 1/2005 – 4/2010 |
| Sample size | |||||
| EX only, N | 61 | 63 | 26 | 150 | 97 |
| CR+EX, N | 119 | 63 | 25 | 152 | 98 |
| Age, mean (SD), years | 69.1 (3.5) | 69.9 (3.7) | 67.4 (5.5) | 65.5 (6.2) | 67.0 (4.8) |
| Age range, years | 65-79 | 65-79 | 60-82 | 55-85 | 60-79 |
| Female gender, % | 76.3% | 55.0% | 80.6% | 66.2% | 72.0% |
| White race, % | 75.6% | 89.2% | 56.6% | 82.7% | 80.2% |
| BMI, mean (SD), kg/m2 | 34.4 (3.4) | 31.0 (2.4) | 40.4 (7.0) | 33.6 (3.7) | 33.7 (4.4) |
| BMI range, kg/m2 | 30-45 | 27-35 | ≥30 | ≥27 | ≥30 |
| Intervention | AT vs CR+AT | RT vs CR+RT | AT vs CR+AT | AT/RT vs CR+AT/RT | AT vs CR+AT |
| Weight loss goal | 5-11% | 8-10% | 10-15% | 10-15% | 7-10% |
| Achieved weight loss | |||||
| EX only, % | −1.5% | −0.2% | −3.1% | −2.0% | −1.0% |
| CR+EX, % | −9.0% | −5.7% | −9.7% | −11.4% | −7.7% |
| Intervention duration | 5 months | 5 months | 5 months | 18 months* | 18 months* |
| Number recalled (EX only / CR+EX) | 13 (7 / 6) | 8 (4 / 4) | 5 (3 / 2) | 20 (11 / 9) | 14 (8 / 6) |
| Age, mean (SD), years | 68.1 (2.3) | 69.1 (2.8) | 68.2 (5.3) | 65.3 (4.7) | 66.6 (4.8) |
| Female gender, % | 61.5% | 87.5% | 80.0% | 70.0% | 50.0% |
| White race, % | 84.6% | 100% | 60.0% | 95.0% | 71.4% |
Intensive weight loss phase during the first 6 months; continued weight loss, if safe (<20% between 6 and 12 months and <30% after 12 months in IDEA; BMI >20 kg/m2 in CLIP), and/or weight maintenance during the second 12 months.
Abbreviations: EX, exercise; CR, caloric restriction; AT, aerobic training; RT, resistance training; SD, standard deviation.
Body weight and composition
Body mass was measured in kilograms (kg) on a standard calibrated scale, and height was measured in centimeters using a stadiometer. Body mass index was calculated as body mass in kg divided by height in meters squared. Fat and whole body and appendicular lean tissue mass was measured by dual-energy X-ray absorptiometry (DXA; Hologic Delphi QDR) at baseline, immediately following the intervention, and at the long-term follow-up visit in all 5 trials. Bone mineral content was subtracted from the total lean mass to determine total non-bone lean mass. Appendicular lean mass was calculated as the sum of lean mass in arms and legs, assuming that all non-fat and non-bone tissue is skeletal muscle. Skeletal muscle index was calculated as appendicular lean mass divided by height in meters squared.35
Physical function
At the long-term follow-up visit, a walking-based test of exercise tolerance and aerobic fitness over 400 meters was administered.36 The course was 20-m long marked by cones at each end. Participants were instructed to complete 10 laps of the 20-m course as quickly as possible at a pace they could maintain; the time to complete the walk was recorded in seconds. Encouragement was given in a standardized fashion every lap. All participants in the CR+EX group were able to complete the 400-m walk; however, one participant in the EX only group was not able to complete the 400-m walk. Three of the 5 trials also included the 400-m walk test at baseline and immediately following intervention (CLIP, I’M FIT, and INFINITE).13,16,20
Lower-extremity physical function was assessed at the long-term follow-up visit using the Short Physical Performance Battery (SPPB), which includes progressively more challenging standing balance tasks held for 10 seconds each (side-by-side, tandem and semi-tandem), a 4-m walk to assess usual gait speed, and time to complete 5 repeated chair stands.37 Each of the three performance measures is assigned a score ranging from 0 (inability to do the test) to 4 (the highest level of performance) and summed to create an SPPB summary score ranging from 0 to 12 (best). All of the trials (albeit only in a subset in IDEA) included the SPPB at baseline and immediately following intervention. The Short Physical Performance Battery was modestly expanded (expanded Physical Performance Battery, PPBexp) at the long-term follow-up visit to minimize ceiling effects of the SPPB.38 The PPBexp increases the holding time of the standing balance tasks to 30 seconds and adds a single leg stand and a narrow walk test of balance (walking at usual pace within lines of tape spaced 20 cm apart). The PPBexp component scores are calculated as the ratio of observed performance to the best possible performance and summed to provide a continuous score ranging from 0 to 4, with higher scores indicative of better performance.
Lower extremity muscle strength and power were assessed at the long-term follow-up visit. Knee extensor strength was measured using an isokinetic dynamometer (Biodex) at a speed of 60° per second with the participant sitting and the hips and knee flexed at 90°. Start and stop angles were set at 90° and 30°. All testing was performed on both legs unless contraindicated (e.g., hip or knee replacement or acute injury) and then only the eligible leg was used. Participants with bilateral knee replacements were excluded from testing (3 participants in the CR+EX group and 5 participants in the EX only group). Participants were asked to extend the knee and push as hard as possible against the resistance pad. Strength was expressed as peak torque in Newton-meters (Nm). Lower extremity muscle power was measured using the Nottingham Power Rig. Participants sit in a chair and unilaterally depress a foot lever attached to a flywheel as hard and as fast as they can. Power output, derived from the acceleration of the flywheel, was recorded in watts. All participants in the CR+EX group were able to do the leg power test; however, one participant in the EX only group was not.
Statistical analyses
Descriptive statistics (means, SD, proportions) were obtained by study for the originally enrolled participants and for those selected for inclusion in this pilot. Linear contrasts from general linear models for repeated outcomes were used to calculate mean change (95% CI) between time points within intervention groups (CR+EX and EX only) and differences in change between intervention groups. An unstructured covariance matrix was used to account for correlation between repeated measures and a factor for study was included in each model. Where measures only existed at the long-term follow-up, a general linear model was used adjusting for study. Since this was a pilot study, our analyses focused on feasibility of recall, estimating means (SD), and longitudinal change (95% CI); hypothesis tests were not conducted on the pilot sample.39 Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Participant characteristics
Of the 60 participants who were contacted, 42 (70%) agreed to complete a clinic visit for this study and 10 (17%) completed a phone interview. Five participants refused participation; two participants could not be located/contacted; and one participant had died. Recalled participants were similar in age at baseline to that of the original trials; however, the gender and racial distribution differs from that of the original trials (see Table 1). The mean (SD) time interval between the end of participation in the prior weight loss RCT and the follow-up assessment was 3.5 (1.0) years (range, 2.2-5.8 years). Here we report body composition and physical function data from those participants who completed the clinic visit (n=42).
The baseline (prior to beginning the RCT) participant characteristics of the study sample by randomized group assignment are presented in Table 2. The mean age of the sample at randomization was 67 years (range, 59-77 years) with over 70% age 65 years or older at baseline, and the majority were female, white, and well educated. At baseline, approximately 60% had hypertension, 30% had hyperlipidemia, 20% had a history of cardiovascular disease, and 14% had diabetes.
Table 2.
Baseline characteristics of participants who attended the in-person clinic visit at long-term follow-up
| EX Only (N=21) | CR+EX (N=21) | |
|---|---|---|
| Age, mean (SD), years | 68.2 (4.6) | 65.8 (3.9) |
| Gender | ||
| Female, N (%) | 15 (71.4%) | 14 (66.7%) |
| Male, N (%) | 6 (28.6%) | 7 (33.3%) |
| Race | ||
| White, N (%) | 17 (81.0%) | 19 (90.5%) |
| African American, N (%) | 4 (19.0%) | 2 (9.5%) |
| Education | ||
| High school, N (%) | 2 (9.5%) | 2 (9.5%) |
| College, N (%) | 14 (66.7%) | 17 (81.0%) |
| Post graduate, N (%) | 5 (23.8%) | 2 (9.5%) |
| Comorbid illness, N (%) | ||
| Hypertension | 13 (61.9%) | 12 (57.1%) |
| Hyperlipidemia | 5 (23.8%) | 7 (33.3%) |
| Cardiovascular disease | 3 (14.3%) | 5 (23.8%) |
| Type 2 Diabetes | 1 (4.8%) | 5 (23.8%) |
| Weight, mean (SD), kg | 86.3 (17.4) | 88.6 (16.0) |
| Height, mean (SD), m | 1.65 (0.10) | 1.65 (0.09) |
| BMI, mean (SD), kg/m2 | 31.6 (4.7) | 32.3 (4.0) |
Abbreviations: EX, exercise; CR, caloric restriction; SD, standard deviation; BMI, body mass index.
Body weight and composition
Table 3 shows the body weight and composition of both groups at baseline, after intervention, and at long-term follow-up, change in body weight and composition from baseline by group, and differences in change in body weight and composition from baseline between groups. Body weight and BMI were lower after intervention in both groups, with almost double the weight loss and BMI change in the CR+EX group compared to the EX only group. Participants in both groups maintained a lower body weight at the long-term follow-up despite an average of 3.5 years of no active intervention. Fat mass (absolute and percent) was lower after intervention in both groups; with absolute fat mass loss being approximately 2 times greater after intervention in the CR+EX group compared to the EX only group. However, absolute fat mass was estimated to be within 1 kg of baseline at the long-term follow-up in both groups and percent fat mass was 2.5% higher at long-term follow-up than at baseline in the CR+EX group and 1.2% higher in the EX only group. Total and appendicular lean mass and skeletal muscle index were lower after intervention in the CR+EX group, with little change from baseline estimated in the EX only group. Changes from baseline at long-term follow-up in total and appendicular lean mass and skeletal muscle index were similar to changes seen after the intervention in the CR+EX group, but were somewhat greater at long-term follow-up compared to after the intervention in the EX only group. Lean mass as a percentage increased from baseline in both groups after intervention, but was lower than at baseline in the CR+EX group at long-term follow-up.
Table 3.
Effect of randomization to caloric restriction on body weight and composition from baseline to long-term follow-up
| EX Only | CR+EX | (CR+EX) – EX Only | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n=21) | After Intervention (n=21) | After long-term follow-up (n=21) | Baseline (n=21) | After Intervention (n=18-21) | After long-term follow-up (n=21) | Difference in Changes from Baseline to After Intervention | Difference in Changes from Baseline to Long-term Follow-up | |
| Weight, kg | ||||||||
| Mean (SD) | 91.6 (17.1) | 88.1 (16.7) | 86.3 (17.4) | 91.8 (14.8) | 84.1 (16.2) | 88.6 (16.0) | ||
| Change from baseline (95% CI) a | −3.50 (−5.85, −1.15) | −5.27 (−8.38, −2.16) | −7.69 (−10.0, −5.34) | −3.22 (−6.33, −0.11) | −4.19 (−7.52, −0.86) | 2.05 (−2.35, 6.45) | ||
| BMI (kg/m2) | ||||||||
| Mean (SD) | 33.1 (4.1) | 31.9 (4.4) | 31.6 (4.7) | 33.2 (3.8) | 30.4 (4.4) | 32.3 (4.0) | ||
| Change from baseline (95% CI) | −1.22 (−2.09, −0.36) | −1.51 (−2.56, −0.45) | −2.85 (−3.71, −1.98) | −0.98 (−2.03, 0.08) | −1.62 (−2.85, −0.40) | 0.53 (−0.96, 2.02) | ||
| Fat mass (kg) | ||||||||
| Mean (SD) | 38.7 (8.2) | 36.4 (8.9) | 37.7 (9.0) | 36.0 (10.0) | 29.6 (10.9) | 36.8 (9.4) | ||
| Change from baseline (95% CI) | −2.30 (−3.92, −0.69) | −1.02 (−3.39, 1.36) | −5.06 (−6.77, −3.35) | 0.79 (−1.59, 3.17) | −2.75 (−5.10, −0.40) | 1.80 (−1.56, 5.17) | ||
| Percent fat mass (%) | ||||||||
| Mean (SD) | 42.3 (5.5) | 41.0 (6.5) | 43.4 (5.7) | 39.1 (8.5) | 36.1 (10.1) | 41.6 (7.4) | ||
| Change from baseline (95% CI) | −1.26 (−2.40, −0.13) | 1.18 (−0.29, 2.65) | −2.51 (−3.72, −1.30) | 2.52 (1.05, 3.99) | −1.25 (−2.91, 0.42) | 1.34 (−0.74, 3.42) | ||
| Lean mass (kg) | ||||||||
| Mean (SD) | 50.8 (12.2) | 50.0 (11.9) | 46.4 (10.8) | 53.9 (11.4) | 49.9 (11.1) | 49.6 (11.1) | ||
| Change from baseline (95% CI) | −0.78 (−1.71, 0.15) | −4.36 (−5.94, −2.78) | −3.10 (−4.10, −2.10) | −4.33 (−5.91, −2.75) | −2.32 (−3.69, −0.95) | 0.03 (−2.20, 2.26) | ||
| Percent lean mass (%) | ||||||||
| Mean (SD) | 55.2 (5.3) | 56.7 (6.4) | 53.8 (5.6) | 58.7 (8.2) | 61.9 (10.0) | 55.9 (7.3) | ||
| Change from baseline (95% CI) | 1.49 ( 0.36, 2.62) | −1.36 (−2.87, 0.15) | 2.67 ( 1.47, 3.87) | −2.79 (−4.30, −1.28) | 1.18 (−0.47, 2.83) | −1.43 (−3.56, 0.70) | ||
| Appendicular lean mass (kg) | ||||||||
| Mean (SD) | 21.5 (6.0) | 21.2 (5.8) | 19.4 (5.2) | 22.9 (6.0) | 21.0 (5.5) | 20.8 (5.3) | ||
| Change from baseline (95% CI) | −0.30 (−0.75, 0.15) | −2.03 (−2.81, −1.24) | −1.67 (−2.15, −1.19) | −2.14 (−2.92, −1.35) | −1.37 (−2.03, −0.71) | −0.11 (−1.22, 1.00) | ||
| Skeletal muscle index (aLM/Ht2) | ||||||||
| Mean (SD) | 7.7 (1.3) | 7.6 (1.2) | 7.0 (1.1) | 8.2 (1.5) | 7.6 (1.3) | 7.4 (1.4) | ||
| Change from baseline (95% CI) | −0.10 (−0.26, 0.06) | −0.71 (−0.96, −0.46) | −0.60 (−0.77, −0.43) | −0.76 (−1.01, −0.50) | −0.50 (−0.74, −0.27) | −0.04 (−0.40, 0.31) | ||
Change and 95% CI calculated from adjusted least squares means (adjusted for study) from general linear model for repeatedly measured outcomes using an unstructured covariance matrix to account for correlation within persons. Sample sizes vary as 3 individuals didn’t have DXA done at the end of intervention.
Abbreviations: EX, exercise; CR, caloric restriction; BMI, body mass index; aLM, appendicular lean mass; Ht, height; CI, confidence interval.
The CR+EX group lost a greater percentage of their initial body weight during the intervention compared to the EX only group (8.7% vs 3.8%; Figure 1). During the period after intervention until long-term follow-up, the CR+EX group regained an average of 5.8±6.9% of their post-intervention body weight, compared to an average loss of 1.9±7.5% of post-intervention body weight in the EX only group. However, there was large variation among individuals in each group. The relative loss of both fat and lean mass was greater during the intervention in the CR+EX group compared to the EX only group (see Figure 1). However, fat mass increased while lean mass continued to decrease in both groups after intervention until long-term follow-up, with somewhat greater percentage increases in fat mass in the CR+EX compared to the EX only group, and percentage decreases in lean mass in the EX only being somewhat greater compared to the CR+EX group.
Figure 1.
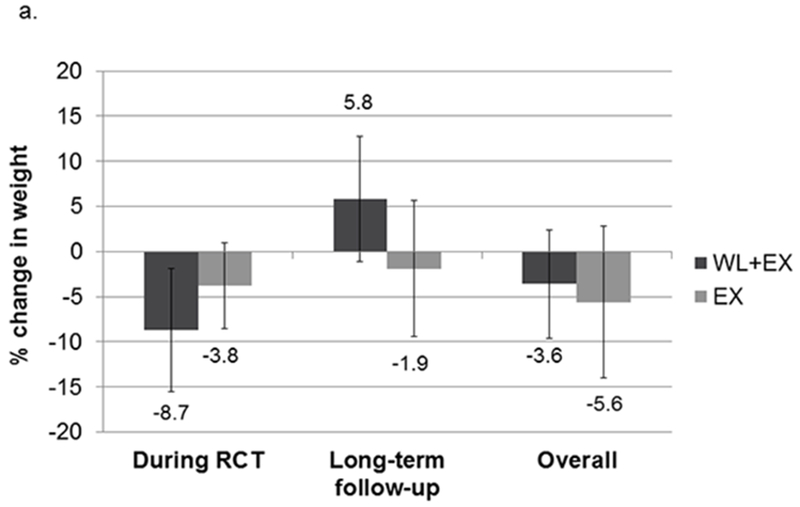
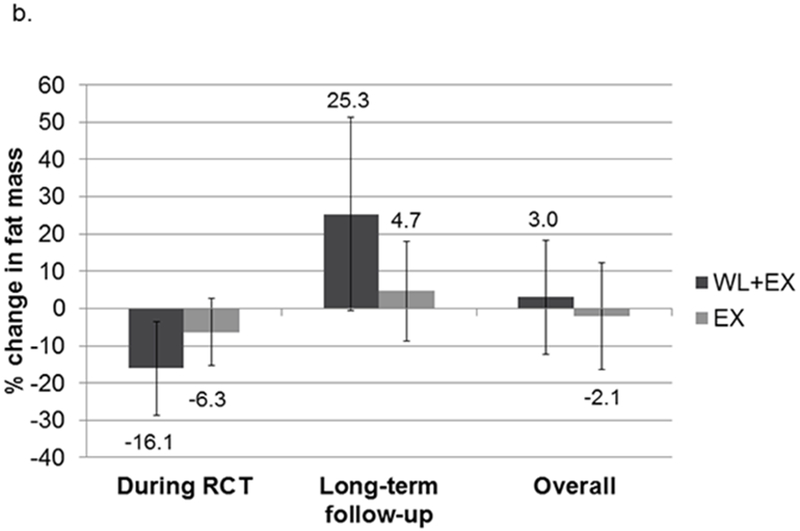
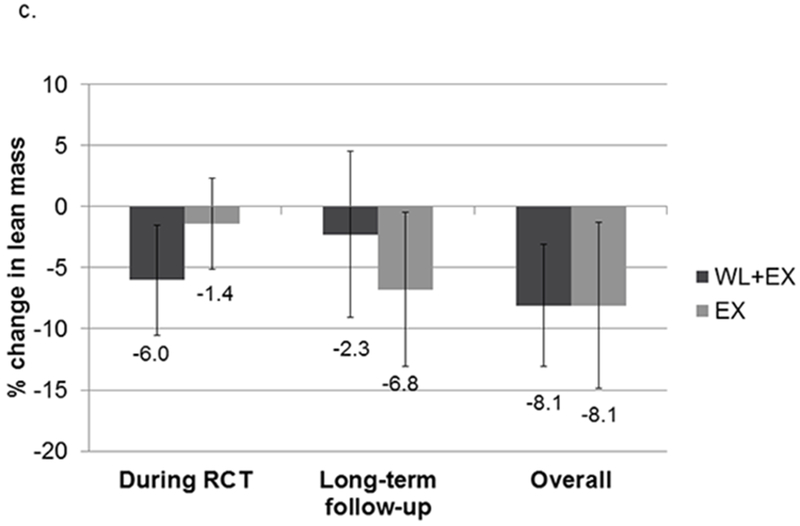
Relative change in weight (a), fat (b) and lean (c) mass during intervention, after intervention, and overall. Mean (SD). Abbreviations: EX, exercise; WL, weight loss.
Physical function
Physical function measured at the long-term follow-up is shown in Table 4. As this is a small pilot, confidence intervals on between group differences in 400-m fast walk time, usual gait speed over 4-meters, SPPB, expanded PPB, muscle strength and power at long-term follow-up are quite wide. In a subset who had 400-m walk (n=23) and SPPB (n=31) measured at baseline and immediately following the intervention, 400-m walk time was improved after intervention (mean change (95% CI): −13.1 (−32.8, 6.65) and −21.8 (−40.7, −2.88) sec, respectively) but had returned to baseline at the long-term follow-up (mean change (95% CI): −0.93 (−17.2, 15.30) and −2.23 (−17.8, 13.31) sec, respectively) in both the CR+EX and the EX only groups while SPPB improved after intervention (mean change (95% CI): 1.05 (0.44, 1.77) points) and the improvement was maintained at long-term follow-up (mean change (95% CI): 0.84 (0.22, 1.46) points) in the EX only group but not in the CR+EX group (mean change (95% CI): 0.23 (−0.44, 0.91) and 0.45 (−0.23, 1.13) points after intervention and at long-term follow-up, respectively). Differences in change from baseline to after intervention and baseline to long-term follow-up for 400-m walk and SPPB between the CR+EX and the EX only groups were small (mean (95% CI): 8.69 (−18.6, 36.0) sec and −0.81 (−1.72, 0.09) points after intervention; 1.30 (−21.1, 23.7) sec and −0.39 (−1.31, 0.52) points at long-term follow-up).
Table 4.
Effect of randomization to caloric restriction on measures of physical function at long-term follow-up
| EX Only (N=21) | CR+EX (N=21) | Differencea of Means (95% CI) | |
|---|---|---|---|
| 400-m walk (sec) | 346.4 (80.9) | 324.3 (51.2) | 23.2 (−19.3, 65.6) |
| SPPB summary score (0-12) | 10.8 (1.5) | 10.8 (1.6) | −0.03 (−1.02, 0.96) |
| Expanded PPB score (0-4) | 2.19 (0.48) | 2.31 (0.46) | −0.11 (−0.41, 0.19) |
| 4-m gait speed walk (m/sec) | 1.01 (0.18) | 1.06 (0.19) | −0.04 (−0.16, 0.08) |
| Lower extremity muscle strength (Nm) | 94.8 (39.2) | 83.4 (31.9) | 11.07 (−16.40, 38.53)b |
| Lower extremity muscle power (Watts) | 106.2 (44.2) | 96.3 (40.9) | 10.37 (−19.95, 40.68)b |
Mean (SD)
Differences of means for all but 400-m walk and SPPB obtained from general linear model, adjusting for study. Differences for 400-m walk and SPPB obtained from repeated measures general linear model, adjusting for study, and accounting for covariance using an unstructured covariance matrix.
Results for the differences of means obtained from general linear model, adjusting for study and gender, for lower extremity muscle strength and power are as follows: muscle strength, 9.14 (−11.61-29.88); muscle power, 6.72 (−19.83, 33.26).
Abbreviations: EX, exercise; CR, caloric restriction; sec, seconds; SPPB, Short Physical Performance Battery; PPB, Physical Performance Battery; Nm, Newton-meters; SD, standard deviation.
DISCUSSION
This pilot study shows the feasibility of recalling prior study participants who had been randomized to a weight loss intervention consisting of caloric restriction plus exercise or an exercise only intervention for a follow-up assessment of their health and function up to 5.8 years after participation in an RCT. Overall, we achieved 87% follow-up, with 42 out of 60 individuals completing a clinic visit and 10 completing a phone interview, an average of 3.5 years after RCT completion. Despite significant weight loss during the RCT and weight regain during long-term follow-up in those randomized to caloric restriction plus exercise, these preliminary data indicate that long-term changes in relative weight, fat and lean mass change from baseline may be similar compared to those randomized to exercise only. Additionally, even though weight was regained following completion of the RCTs, physical function at long-term follow-up appeared to be similar among those randomized to caloric restriction plus exercise compared to exercise only.
Weight loss via caloric restriction, particularly when combined with exercise, is effective in reducing total body and fat mass in older adults, at least in the short term.11,14–17,19,20,40,41 However, weight regain following intentional weight loss is common,29,30 and may negatively influence body composition in older adults by promoting sarcopenic obesity. In a small pilot study in frail older adults, Waters et al. showed that although approximately 7% weight loss was maintained 18 months after participating in a 1-year weight loss intervention, almost half of the total fat mass had been regained while lost lean mass was not recovered.24 However, only the first 26 participants out of 52 randomized to weight loss were included in this small pilot and only 16 returned for follow-up 18 months after completing the intervention, potentially resulting in selection bias. Additionally, their pilot study lacked a “no weight loss” control group as participants who were randomized to the control or exercise only groups were not included.
We previously showed in a subset of one of the included RCTs (I’M FIT) that although older adults who were randomized to caloric restriction plus resistance training regained some weight 18 months after completion of the 5-month RCT, their weight was still lower than at baseline; however, the regained weight was comprised disproportionately of fat mass.32 Furthermore, both those randomized to caloric restriction plus resistance training and those randomized to resistance training alone lost lean mass over the 18 month follow-up resulting in a greater risk for sarcopenic obesity among those randomized to caloric restriction. A limitation of this study was that participants were enrolled serially until the a priori sample size (n=24) was reached, potentially resulting in selection bias. In postmenopausal women, lost weight was, in general, regained, but weight was still lower than at baseline 1 year following a 5-month weight loss intervention; however, among women who regained ≥2 kg (84%), there was greater regain of fat relative to lean mass.31 This study was limited, however, by a lack of a no weight loss control group.
Body composition was also assessed in middle aged and older individuals with type 2 diabetes who were randomized to a long-term (~10 years) intensive lifestyle intervention designed to achieve weight loss through caloric restriction and increased physical activity or a diabetes support and education control over 8 years of follow-up in a subset of 4 of the 16 Look AHEAD sites.42 In those randomized to the long-term intensive lifestyle intervention, weight loss over the first year was comprised of both fat and lean mass; however individuals regained fat and continued to lose lean mass between years 1 and 8. Weight loss over 8 years of follow-up in those randomized to the diabetes support and education control group was comprised almost entirely of lean mass. However, those in the intensive lifestyle intervention had significantly lower total body and fat mass, but also lower lean mass, at 8-year follow-up than those in the control group. We observed similar changes in body composition in our pilot study with those randomized to caloric restriction plus exercise having greater total body, fat, and lean mass loses during the intervention; but much of the lost weight was disproportionately regained as fat mass approximately 3.5 years after completion of the intervention. In those randomized to exercise only, weight loss following the intervention was comprised mostly of lean mass.
Previous trials showing performance-based physical function benefits of weight loss interventions in older persons who were overweight or obese have been of shorter duration (5 to 18 months), with benefits observed immediately following the weight-reduced state.10,11,13–19,43,44 In a small pilot study, Waters et al. showed that improvements in physical function remained 18 months after participating in a 1-year weight loss intervention in frail older adults.24 After approximately 11 years of follow-up in Look AHEAD, middle aged and older adults with type 2 diabetes who had been randomized to a long-term intensive lifestyle intervention had significantly faster gait speed and better physical performance scores than those randomized to a diabetes support and education control.25 In our pilot study, gait speed, physical performance, and muscle strength and power were similar between older adults randomized to caloric restriction plus exercise compared to exercise only approximately 3.5 years after intervention. These pilot study results are encouraging, however, in that those randomized to weight loss plus exercise completed the 400-m walk an average of 23 seconds faster than the exercise only group and had a 0.04 m/sec faster usual gait speed on the 4-m walk at long-term follow-up – differences that are clinically meaningful.45,46 In the subset who had 400-m walk and SPPB measured during the intervention and at long-term follow-up, clinically meaningful improvements in SPPB remained approximately 3.5 years later in the exercise only group but not in the weight loss plus exercise group; however, improvements in 400-m walk time observed immediately following the intervention had returned to baseline in both groups at the long-term follow-up visit.
This pilot study has some notable strengths and limitations. Recall of participants approximately 3.5 years post-intervention was excellent, exceeding 80% of the original sample (70% agreed to a clinic visit and 17% agreed to a phone interview). The original interventions were successful in achieving weight loss in older individuals over 5 to 18 months. However, although a weighted, random sample was used to select former participants from the 5 RCTs, the sample size (n=60 out of 854 participants) was small and there is the possibility of differential follow-up. Nevertheless, this pilot study provided data on the feasibility, variances, and potential effects that may be observed for a larger study to recall participants from the original 5 trials that is currently underway (NCT03430115). This pilot study also did not include follow-up of a true control group (i.e., those not randomized to caloric restriction and/or exercise) which would allow control for the long-term effects of aging per se on weight, body composition, and physical function. Although the five RCTs achieved similar amounts of weight loss, there was variability in the dietary weight loss and exercise intervention approaches (i.e., behavioral-focused and/or meals/meal replacements provided; aerobic and/or resistance training). Physical function was only measured at baseline and immediately following the intervention in a subset of participants. Dietary and physical activity habits from the end of the intervention until the long-term follow-up were also not assessed.
In conclusion, despite clinically meaningful weight loss and concurrent favorable shifts in body composition immediately following caloric restriction plus exercise, improvements in body composition may not be sustained long-term relative to exercise alone. However, despite weight regain and concomitant increases in fat mass and decreases in lean mass, physical function did not appear to be negatively impacted in the caloric restriction plus exercise group. Replication of these findings in a larger study are needed. Identifying long-term weight loss maintenance strategies to prevent weight regain and the predisposition for regained weight to be composed disproportionately of fat mass in older adults is warranted.
Supplementary Material
TAKE AWAY POINTS.
Recalling prior RCT participants to examine the long-term effects of randomization to weight loss is feasible.
Weight regain, and concomitant increases in fat mass and decreases in lean mass, is observed in older adults who were previously randomized to an RCT involving caloric restriction and exercise.
Larger studies are needed to determine the long-term effects of weight loss on body composition, physical function, and overall health in older adults.
ACKNOWLEDGEMENTS
This work was supported by the following National Institutes of Health grants: R01 AG056418; R01 HL093713 (INFINITE); R01 AG020583 (I’M FIT); R37 AG018915 (SECRET); R01 AR052528 (IDEA); R01 HL076441 (CLIP); and the Wake Forest Pepper Center, P30 AG021332.
BIBLIOGRAPHY
- 1.Janssen I Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc 2006;54:56–62. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 3.Koster A, Visser M, Simonsick EM, et al. Association between fitness and changes in body composition and muscle strength. J Am Geriatr Soc 2010;58:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koster A, Ding J, Stenholm S, et al. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci 2011;66:888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen GL. Obesity and functional decline: epidemiology and geriatric consequences. Clin Geriatr Med 2005;21:677–87, v. [DOI] [PubMed] [Google Scholar]
- 6.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005;82:923–34. [DOI] [PubMed] [Google Scholar]
- 7.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev 2010;11:671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007;30:1562–6. [DOI] [PubMed] [Google Scholar]
- 9.Harris TB, Launer LJ, Madans J, Feldman JJ. Cohort study of effect of being overweight and change in weight on risk of coronary heart disease in old age. Bmj 1997;314:1791–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum 2004;50:1501–10. [DOI] [PubMed] [Google Scholar]
- 11.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch Intern Med 2006;166:860–6. [DOI] [PubMed] [Google Scholar]
- 12.Shah K, Wingkun NJ, Lambert CP, Villareal DT. Weight-loss therapy improves endurance capacity in obese older adults. J Am Geriatr Soc 2008;56:1157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rejeski WJ, Brubaker PH, Goff DC, Jr., et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med 2011;171:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr 2015;101:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rejeski WJ, Ambrosius WT, Burdette JH, Walkup MP, Marsh AP. Community Weight Loss to Combat Obesity and Disability in At-Risk Older Adults. J Gerontol A Biol Sci Med Sci 2017;72:1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med 2017;376:1943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicklas BJ, Brinkley TE, Houston DK, et al. Effects of caloric restriction on cardiorespiratory fitness, fatigue, and disability responses to aerobic exercise in older adults with obesity: A randomized controlled trial. J Gerontol A Biol Sci Med Sci 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shea MK, Houston DK, Nicklas BJ, et al. The effect of randomization to weight loss on total mortality in older overweight and obese adults: the ADAPT Study. J Gerontol A Biol Sci Med Sci 2010;65:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shea MK, Nicklas BJ, Houston DK, et al. The effect of intentional weight loss on all-cause mortality in older adults: results of a randomized controlled weight-loss trial. Am J Clin Nutr 2011;94:839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One 2015;10:e0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waters DL, Vawter R, Qualls C, Chode S, Armamento-Villareal R, Villareal DT. Long-term maintenance of weight loss after lifestyle intervention in frail, obese older adults. J Nutr Health Aging 2013;17:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houston DK, Neiberg RH, Miller ME, et al. Physical Function Following a Long-Term Lifestyle Intervention among Middle Aged and Older Adults with Type 2 Diabetes: the Look AHEAD Study. J Gerontol A Biol Sci Med Sci 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: a review of the controversy. Exp Gerontol 2013;48:1054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Normandin E, Houston DK, Nicklas BJ. Caloric restriction for treatment of geriatric obesity: Do the benefits outweigh the risks? Curr Nutr Rep 2015;4:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: Do benefits exceed potential risks? Experimental gerontology 2016;86:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82:222S–5S. [DOI] [PubMed] [Google Scholar]
- 30.Barte JC, ter Bogt NC, Bogers RP, et al. Maintenance of weight loss after lifestyle interventions for overweight and obesity, a systematic review. Obes Rev 2010;11:899–906. [DOI] [PubMed] [Google Scholar]
- 31.Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women? Am J Clin Nutr 2011;94:767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chmelo EA, Beavers DP, Lyles MF, Marsh AP, Nicklas BJ, Beavers KM. Legacy effects of short-term intentional weight loss on total body and thigh composition in overweight and obese older adults. Nutrition & diabetes 2016;6:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 2005;82:872–8. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Visser M, Tylavsky FA, et al. Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci 2010;65:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–63. [DOI] [PubMed] [Google Scholar]
- 36.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc 2001;49:1544–8. [DOI] [PubMed] [Google Scholar]
- 37.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol: Med Sci 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 38.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC Study. J Gerontol: Med Sci 2001;56A:M644–M9. [DOI] [PubMed] [Google Scholar]
- 39.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. Journal of psychiatric research 2011;45:626–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beavers KM, Beavers DP, Nesbit BA, et al. Effect of an 18-month physical activity and weight loss intervention on body composition in overweight and obese older adults. Obesity (Silver Spring) 2014;22:325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beavers KM, Ambrosius WT, Rejeski WJ, et al. Effect of Exercise Type During Intentional Weight Loss on Body Composition in Older Adults with Obesity. Obesity (Silver Spring) 2017;25:1823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pownall HJ, Bray GA, Wagenknecht LE, et al. Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2015;23:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci 2013;68:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter Starr KN, Pieper CF, Orenduff MC, et al. Improved Function With Enhanced Protein Intake per Meal: A Pilot Study of Weight Reduction in Frail, Obese Older Adults. J Gerontol A Biol Sci Med Sci 2016;71:1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–9. [DOI] [PubMed] [Google Scholar]
- 46.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging 2009;13:538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


