Abstract
BACKGROUND
Prefrontal subregions, including the ventromedial prefrontal cortex (VMPFC), dorsomedial prefrontal cortex (DMPFC), and dorsolateral prefrontal cortex (DLFPC), are differentially implicated in the pathophysiology of PTSD, though few existing studies have examined subregional differences in resting state functional connectivity (rsFC). We hypothesized that PTSD would involve weaker positive rsFC between VMPFC, DMPFC, and other default mode network (DMN) regions, and increased negative rsFC between DLPFC and posterior DMN regions. Additionally, we hypothesized that prefrontal regions exhibiting group differences in rsFC would be characterized by alterations in cortical thickness.
METHODS
Participants included 36 healthy controls (HC), 30 trauma-exposed controls (TENC), and 21 individuals with current DSM-IV PTSD resulting from community-acquired trauma. Participants completed the Clinician Administered PTSD Scale, questionnaires (Childhood Trauma Questionnaire, Adverse Childhood Events, Life Events Checklist, Beck Depression Inventory), structural neuroimaging, and resting-state fMRI. RSFC of DLPFC, VMPFC, and DMPFC seeds was evaluated in SPM12 and CONN. Cortical thickness for regions with significant rsFC findings was assessed using FreeSurfer.
RESULTS
Relative to both HC and TENC participants, PTSD patients showed increased negative rsFC between the DLPFC and a region of precuneus. This finding was associated with increased overall symptom severity, but not with trauma load or childhood trauma exposure. In addition, greater negative DLPFC-precuneus connectivity was associated with greater bilateral precuneus thickness.
CONCLUSIONS
Given participation of precuneus subregions in the central executive network, increased anticorrelation between right DLPFC and precuneus in this sample may reflect increased opposition between anterior and posterior central executive network hubs in PTSD.
Keywords: posttraumatic stress disorder, prefrontal cortex, resting state, functional connectivity, precuneus, cortical thickness
Introduction
Early task-based functional neuroimaging studies in posttraumatic stress disorder (PTSD) established the critical role of medial prefrontal cortex hypoactivity in insufficient inhibition of hyperresponsive amygdala output (1, 2), resulting in hyperarousal and re-experiencing symptoms. While these relationships are well-established during affective processing tasks, alterations in resting state functional connectivity (rsFC) in PTSD have also received increasing attention. RSFC measures the coherence of brain region activity in the absence of explicit task-related demands, and in healthy individuals is correlated with neural activity during task performance (3), as well as important functional outcomes, including emotional perception and regulation (4) and cognitive performance (5, 6).
Many rsFC studies in PTSD have focused on within- and cross-network coherence in three major resting state networks, consistent with Menon’s (2011) triple network model articulating how aberrant interactions between three large-scale resting state networks contribute to psychopathology. According to Menon’s model and spatial organization of these networks, the first is the default mode network (DMN), with central hubs in the posterior cingulate cortex (PCC) and medial prefrontal cortex; this network is active in the absence of explicit task demands and is associated with functions including self-referential processing (8, 9). The second is the salience network (or “salience and emotional network”: SEN), with hubs in the dorsal anterior cingulate cortex (dACC) and anterior insula, involved in allocating cognitive resources to other large-scale networks on the basis of salient internal or external events (10, 11). Of note, in this model, the SEN also includes the amygdala and the substantia nigra/ventral tegmental area (7, 12). The third key network is the central executive network (CEN), with hubs in the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex, involved in working memory and execution of goal-directed behavior (11, 13).
Extensive focus on these core hubs within the PTSD rsFC literature, however, may result in insufficient attention to regionally nuanced findings. This may be particularly critical for dysfunction of the prefrontal cortex, for which there is evidence that collapsing across functionally differentiated subregions may obscure important results.For example, recent work in healthy adults indicates that the frontal lobe component of the DMN subdivides into a ventromedial prefrontal cortex (VMPFC) component (functionally connected to medial temporal lobe regions and playing a role in learning and memory) and a dorsomedial prefrontal cortex (DMPFC) component (involved in mentalizing and self-reflection) (14). Differential contributions of DMPFC and VMPFC to fear and anxiety broadly (15, 16) and to PTSD specifically (17, 18) have been identified, but few existing resting-state studies in PTSD examine connectivity from these prefrontal subregions separately. Given the functional roles of these subregions in processes impaired in PTSD – including the role of the VMPFC in fear extinction learning and recall (19, 20) and reward valuation (21), and of the DMPFC in mentalizing (22) – targeting these regions separately for resting-state analyses could be critical. Additionally, PTSD rsFC studies examining medial PFC connectivity often do not concurrently probe alterations in lateral PFC connectivity. Evaluating medial and lateral PFC connectivity in the same sample is particularly important because of their potential interactions. PTSD has been associated with dorsolateral prefrontal cortex (DLPFC) hypoactivation during cognitive reappraisal (23) and executive control tasks (24), but there are relatively few studies of intrinsic rsFC of the DLPFC in PTSD. Below, we summarize the existing literature on dysfunction in prefrontal rsFC from three prefrontal regions implicated in cognitive and emotional dysfunction in PTSD: the VMPFC, DMPFC, and DLPFC.
VMPFC
Many previous reports of rsFC in PTSD have focused on a medial prefrontal region located in the VMPFC (25) that overlaps with the prototypical DMN but may also extend into the so-called affective network (26). Reduced connectivity between the VMPFC and DMN regions (including PCC and right superior parietal lobule) has been demonstrated in individuals with PTSD compared with healthy controls (27). In addition to this hypoconnectivity between areas of VMPFC and DMN regions, PTSD may involve abnormal cross-network coupling of VMPFC with salience network regions including the dACC (18). Relatedly, rsFC between VMPFC and the amygdala has been implicated in PTSD; across a mixed diagnostic sample of patients with depression and PTSD, anxiety was associated with hyperconnectivity between the VMPFC and the amygdala (28). Interestingly, healthy adults with greater connectivity between VMPFC and regions of the salience and central executive networks (i.e., greater between-network connection strength) show worse attentional performance (poor distractor suppression), a cognitive deficit seen in PTSD (29). From a neurodevelopmental perspective, there is evidence that, unlike in adult PTSD, pediatric PTSD may be associated with hyperconnectivity between the VMPFC and other DMN regions (30). To summarize, the existing literature suggests that PTSD involves hypoconnectivity between VMPFC and DMN as well as hyperconnectivity between VMPFC and SEN regions, though the developmental timing of PTSD onset may influence this pattern.
DMPFC
The DMPFC is a region of the DMN that is functionally involved in mentalizing and self-reflection (14, 31). In PTSD, DMPFC hyperconnectivity with the basolateral amygdala has been demonstrated (32), perhaps reflecting intrusion of amygdala-driven fear-related content during self-reflective thought. Increased negative connectivity between the amygdala/hippocampus and the DMPFC occurs in the context of increasing childhood trauma exposure and combat exposure, while increasing positive amygdala/hippocampus to DMPFC connectivity scales with overall PTSD symptom severity (33). In a previous rsFC study, we found that increased right DMPFC-nucleus accumbens functional connectivity was associated with greater anhedonia in trauma-exposed adults (34), consistent with the perspective that DMPFC hyperconnectivity with regions outside the DMN may contribute to dimensional features of trauma-related psychopathology. Together, these findings suggest that DMPFC hyperconnectivity may be an important feature in PTSD and may be related to symptoms including alterations in reward processing.
DLPFC
Overall, prior PTSD rsFC literature on VMPFC and DMPFC connectivity suggests that PTSD is associated with hyperconnectivity between these midline regions and regions outside the DMN, including SEN components. In contrast, studies of DLPFC connectivity in PTSD have generally demonstrated that PTSD involves hypoconnectivity between DLPFC and SEN regions (35), including reduced rsFC between the DLPFC and the anterior insula (36). Similarly, in a transdiagnostic sample of individuals with depression or PTSD, depressive symptom severity was associated with hypoconnectivity between the DLPFC and the amygdala (28). This hypoconnectivity is presumed to reflect insufficient goal-directed cognitive regulation of affective and attentional processes. PTSD also is associated with decreased connectivity between lateral PFC and DMN regions (37); for instance, re-experiencing symptom severity is associated with lower ‘degree’ (i.e., weaker network influence) between the right DLPFC and the right hippocampus (38). Consistent with the hypothesis that PTSD involves hypoconnectivity between DLPFC and DMN regions, successful treatment via mindfulness-based exposure therapy and associated reduction in avoidant and hyperarousal symptoms is associated with increased rsFC between DLPFC and DMN nodes including the PCC (39). While only a few studies to date have examined DLPFC-DMN rsFC in PTSD, the existing findings suggest that PTSD may involve altered DLPFC modulation of DMN circuits involved in autobiographical memory. This stands in contrast to the bulk of findings in major depressive disorder which suggest hyperconnectivity (namely, reduced anticorrelations in activity) between frontal control network regions (including DLPFC) and DMN (40).
Limitations of the existing rsFC prefrontal literature
Most of the existing prefrontal rsFC literature in PTSD has focused on PTSD patients with combat trauma and has been conducted in predominantly male samples (e.g. ≥ 85% male) (18, 27, 29, 33, 36-39). Studies that include women have tended to focus on single-incident, non-interpersonal traumas such as natural disasters (41-43). One study that did include females was conducted in adolescents and demonstrated strikingly different findings than existing studies in adult samples, i.e., no indication that PTSD involves hypoconnectivity within the DMN (30). While the authors primarily interpreted their divergent findings in terms of possible differences between pediatric-onset and adult-onset PTSD, it is also possible that the difference in sex distribution of the samples may have contributed.
Linking structural morphometry and functional connectivity
Establishing relationships between alterations in structure and function is an important step in bridging the structural and functional psychopathology neuroimaging literature. There is evidence that regional prefrontal group differences in cortical thicknesses are associated with alterations in rsFC in MDD (44), and with altered resting state activity in PTSD (45). These and other findings (46) suggest possible associations between altered cortical thickness and rsFC, though to our knowledge there have not been previous investigations linking rsFC and cortical thickness within a PTSD sample.
Prefrontal effects of PTSD versus early life stress (ELS)
One additional feature that is not frequently evaluated in studies of rsFC in combat populations is the possible contribution of effects related to prior childhood/adolescent adversity or trauma exposure. ELS exposure is associated with alterations in prefrontal rsFC, including increased negative connectivity between the DLPFC and precuneus / inferior parietal lobule (47). This increased anticorrelation between DLPFC and precuneus may reflect increased dissociation between DMN and CEN in the context of ELS: the opposite of the pattern described above for PTSD. Similarly, childhood maltreatment severity is associated with increased anticorrelation between SEN seeds (amygdala, hippocampus) and VMPFC, DMPFC, and DLPFC (33). Increased negative rsFC between the amygdala and the DLPFC also was identified in women exposed to threat-related ELS (48). The increased DLPFC anticorrelation has been characterized as a possible compensatory mechanism involving increased voluntary emotional regulation (33). However, participants in these studies did not have PTSD related to childhood abuse. Therefore, it is unclear whether increased prefrontal anticorrelations are a ‘scar’ related to ELS exposure or a feature associated with resilience to that exposure. These findings also contrast with those described above in non-ELS samples, which generally show DLPFC hypoconnectivity in the context of PTSD. The effect of stress/trauma exposure on prefrontal rsFC may depend on the developmental timing of the stress exposure; for instance, childhood/adolescent trauma exposure may lead to heightened differentiation between DMN and CEN, while adult trauma exposure may lead to a loss of cross-network differentiation.
Summary and hypotheses
To summarize, the VMPFC, DMPFC, and DLPFC are differentially implicated in cognitive and emotional functioning in PTSD. The existing rsFC literature suggests that the VMPFC and DMPFC may demonstrate hypoconnectivity with DMN regions and hyperconnectivity with SEN regions including the amygdala; DMPFC hyperconnectivity may scale with trauma load and dimensional features such as anhedonia. In contrast, the existing PTSD rsFC literature suggests that the DLPFC may demonstrate hypoconnectivity with SEN regions (amygdala, anterior insula) as well as hypoconnectivity with the DMN. Importantly, these patterns have primarily been established in combat samples, and extension to community-based samples (including those with a higher proportion of women) is a critical next step. Community-based PTSD samples tend to include balanced sex distributions, as well as individuals with childhood trauma exposure. Based on the existing prefrontal rsFC literature, we hypothesized that, relative to both trauma-exposed controls (TENC) and healthy, non-trauma exposed controls (HC), individuals with PTSD would demonstrate reduced within-network connectivity from VMPFC and DMPFC seeds to other DMN regions and increased cross-network connectivity from VMPFC and DMPFC seeds to salience network regions. Based on the adult PTSD literature in combat samples, we anticipated hypoconnectivity between DLPFC and DMN regions, though in this sample of individuals with significant childhood trauma, we also considered the possibility of increased negative connectivity between DLPFC and posterior DMN regions. Finally, we hypothesized that prefrontal regions demonstrating group differences in rsFC would also demonstrate alterations in cortical thickness, consistent with prior demonstrations of associations between cortical thickness and rsFC.
Methods and Materials
Participants
Eighty-seven participants (36 HC, 30 TENC, 21 current PTSD) were included (demographics: Table 1). Our laboratory previously published findings related to rsFC of the nucleus accumbens in relation to anhedonia in the TENC and PTSD participants (34). Participants were recruited from the Boston area via advertisements. Inclusion criteria included right-handedness, age 20-50, and trauma exposure appropriate to group (for detailed inclusion/exclusion criteria, see Supplement). During the interview, all TENC participants endorsed having experienced at least one event meeting DSM-IV PTSD criterion A, while HC participants reported no history of events meeting criterion A. Participants provided written informed consent, and study procedures were approved by the Partners Human Research Committee (PHRC).
Table 1.
Demographics and clinical variables: group characteristics
| HC (n = 36) | TENC (n = 30) | PTSD (n = 21) | Group difference | |
|---|---|---|---|---|
| Age | 32.81 (9.03) | 30.99 (7.91) | 34.11 (6.94) | F = 0.942, ns |
| Sex | 19F, 17M | 18F, 12M | 12F, 9M | X2 = 0.838, ns |
| CAPS re-exp. | -- | 2.43 (3.78) | 14.67 (7.00) | t = −7.30, p < 0.001* |
| CAPS avoidance | -- | 3.37 (5.20) | 27.57 (8.94) | t = −11.16, p < 0.001* |
| CAPS hyperarousal | -- | 2.37 (3.72) | 17.43 (6.42) | t = −9.68, p < 0.001* |
| CAPS total | -- | 8.17 (9.87) | 59.67 (18.75) | t = −11.52, p < 0.001* |
| CTQ emotional abuse3,5 | 6.14 (1.76) | 9.45 (4.04) | 14.48 (7.43) | F = 19.62, p < 0.001* |
| CTQ physical abuse2 | 5.64 (1.05) | 8.20 (4.00) | 10.05 (4.78) | F = 13.71, p < 0.001* |
| CTQ sexual abuse1,5 | 5.25 (1.50) | 7.14 (4.44) | 9.05 (6.26) | F = 5.74, p = 0.007* |
| CTQ emotional neglect2 | 7.78 (3.16) | 11.10 (4.85) | 14.14 (5.99) | F = 12.87, p < 0.001* |
| CTQ physical neglect3 | 5.44 (0.10) | 6.73 (2.16) | 9.14 (3.57) | F = 14.02, p < 0.001* |
| ACE total2 | 0.63 (0.81) | 3.00 (1.89) | 4.67 (2.85) | F = 36.81, p < 0.001* |
| LEC total3 | 2.61 (2.63) | 6.27 (2.99) | 9.10 (3.56) | F = 30.85, p < 0.001 |
| BDI total3,4 | 0.64 (1.22) | 6.47 (7.02) | 20.30 (11.28) | F = 39.03, p < 0.001* |
| Motion (# flagged scans) | 6.44 (5.84) | 7.20 (5.71) | 6.57 (5.52) | F = 0.154, ns |
Notes: CAPS: Clinician Administered PTSD Scale; CTQ: Childhood Trauma Questionnaire; ACE: Adverse Childhood Experiences scale; LEC: Life Experiences Checklist; BDI: Beck Depression Inventory
HC < PTSD, no significant differences between TENC and HC or PTSD
HC < PTSD, HC < TENC, no significant differences between TENC and PTSD
HC < TENC < PTSD
For BDI, 1 missing data point in PTSD group (n = 20)
For CTQ, 1 missing data point in TENC group (n = 29)
tests adjusted for non-homogeneity of variances: e.g. Welch test for ANOVA; Games-Howell post-hoc.
Clinical symptom and trauma questionnaires
PTSD symptom ratings: CAPS-DX.
Total current DSM-IV PTSD severity scores and subscale scores for reexperiencing, avoidance, and hyperarousal were generated via structured interview using the Clinician Administered PTSD Scale, Current and Lifetime Version (CAPS-DX (49)).
Childhood trauma exposure: CTQ and ACE.
The Childhood Trauma Questionnaire (CTQ (50)) is a 28-item self-report measure of childhood trauma exposure, including emotional abuse and neglect, physical abuse and neglect, and sexual abuse. The Adverse Childhood Experiences scale (ACE (51)) is a 10-item self-report measure of childhood abuse, neglect, and stressful life experiences.
Trauma load: LEC.
The Life Experiences Checklist (LEC (52)) was used as a measure of trauma load (number of personally experienced plus personally witnessed lifetime events).
Depression: BDI.
The Beck Depression Inventory, version 1A (53) total score was used as a measure of depression severity.
MR image acquisition and analysis
Full details of image acquisition and preprocessing are described in our prior manuscript (34) and available in the Supplement. Briefly, scans were acquired on a 3T Siemens Tim Trio scanner (Siemens, Erlangen, Germany), using a 32-channel head coil. Structural T1-weighted MPRAGE images were collected over 128 sagittal slices (TR/TE/flip angle = 2530 ms/ 3.31 ms/ 7 degrees, 256 × 256 matrix), voxel size = 1.3 × 1.0 × 1.3 mm3. An eyes-open resting state scan was acquired; 180 T2-weighted echoplanar images were collected over 34 transverse interleaved slices (TR/TE/flip angle = 2000 ms/ 30 ms/ 90 degrees), voxel size = 3.5 × 3.5 × 3.5 mm, FOV = 224 mm. SPM12 (revision number 6225) was used for data preprocessing in MATLAB R2014a. Standard processing steps were performed in CONN (version 15.h), including denoising via CompCor and band-pass filtering (0.008-0.09 Hz).
Resting state analysis
To define the peak coordinates for the DLPFC, DMPFC, and VMPFC ROIs, we drew from the normative cognitive neuroscience literature. Following Baumgartner et al. (2011), we used the following peak coordinates: DLPFC (peak for a control-related region in BA 9: x = ±39, y = 37, z = 26); DMPFC (peak for a mentalizing-related region: −3, 48, 30); posterior VMPFC (peak for a reward valuation-related region: 2, 41, −6). Using FSL, spheres of 5-mm radius were dilated at these peak coordinates and binarized (Supplementary Figure S1). Pearson correlations between the time course of each seed (left and right DLPFC, DMPFC, VMPFC) and the time course of every other voxel in the brain were computed and transformed using Fisher’s Z. For the second-level analysis, first-level maps were entered into whole brain regression analyses. For each seed, we performed an ANCOVA to test for a main effect of group (PTSD, TENC, HC), controlling for age and sex. Results of this F test were thresholded at p < 0.001 (uncorrected height threshold), p < 0.05, FDR-corrected cluster-size threshold. To correct for multiple comparisons (analyses initiated from four seeds), results were considered significant at Bonferroni-corrected p < 0.0125 (0.05/4). A whole-brain analysis was performed from each seed in order to allow for the possibility of significant results outside the canonical rsFC networks (DMN, SEN, CEN). Connectivity values (Fisher’s r to Z) were extracted from significant F-test clusters for correlations with symptom and trauma scores.
Cortical thickness
FreeSurfer (version 5.3.0) was used for cortical surface reconstruction using a standard processing pipeline. Cortical thickness measurements were taken by averaging the distance from the pial surface to the gray-white interface. FreeSurfer segmentations were manually inspected and edited by the first author in accordance with recommended guidelines. Cortical thickness of regions demonstrating significant group differences in the resting state analyses was derived (see Supplement for ROI derivation).
Results
Group differences in rsFC
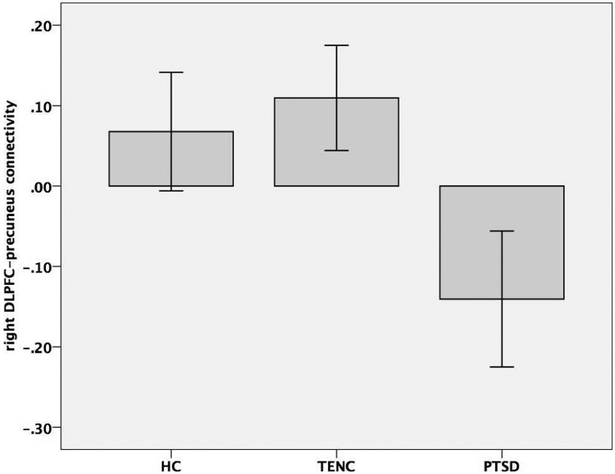
There were no significant diagnostic group differences in rsFC from the DMPFC, VMPFC, or left DLPFC seeds. In contrast, there was a significant group difference in rsFC from the right DLPFC seed to a region in the bilateral precuneus (Figure 1: 212 voxels, cluster p-FDR = 0.000064). Subsequent pairwise comparison analyses showed lower rsFC values (increased negative rsFC) in the PTSD group compared to both the HC and TENC groups, with no significant difference between the HC and TENC groups (Figure 2).
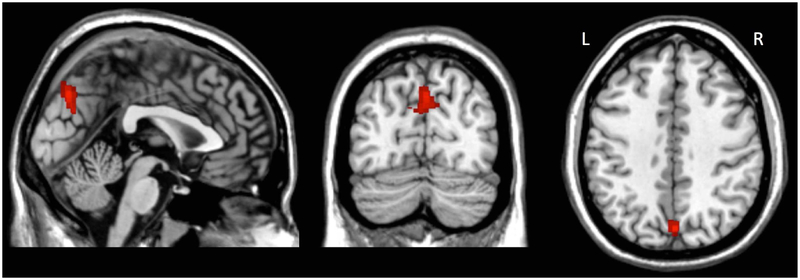
Figure 1.
Region in precuneus with significant group difference in right DLPFC resting state functional connectivity. k = 212 voxels, p-FDR = 0.000064, peak coordinates = (+8, −72, +32).
Figure 2.
Group comparison of right DLPFC-precuneus rsFC values. Note: rsFC values in this graph are raw (not corrected for age and sex). Error bars: 95% confidence interval.
Correlations with cortical thickness
Across the whole sample, controlling for age and sex, greater negative DLPFC-precuneus connectivity was associated with greater precuneus thickness on the right, partial r(83) = −0.218, p = 0.045, and on the left, partial r(83) = −0.223, p = 0.040. There were no group differences in precuneus thickness on the right, F(2,80) = 0.016, p = 0.984, or the left, F(2, 80) = 0.146, p = 0.864; or in DLPFC thickness on the right, F(2,80) = 0.265, p = 0.768, or the left, F(2,80) = 0.085, p = 0.918. DLPFC thickness was not significantly correlated with DLPFC-precuneus rsFC.
Relationships with core clinical outcomes (CAPS scores)
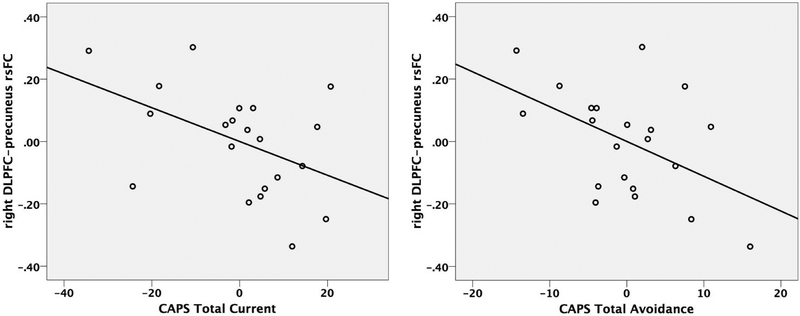
Within the PTSD group, controlling for age and sex, greater negative DLPFC-precuneus connectivity was associated with greater total CAPS scores, partial r(17) = −0.463, p = 0.046, and greater CAPS avoidance symptoms, partial r(17)= −0.485, p = 0.035 (Figure 3; see also Supplementary Table S1 for partial correlations between right DLPFC-precuneus rsFC and symptom scores within the PTSD sample).
Figure 3.
Partial correlation of CAPS total symptom scores (left) and CAPS avoidance scores (right) with right DLPFC-precuneus rsFC in PTSD group, controlling for age and sex.
Relationships with childhood trauma exposure, trauma load, and depression
As shown in Table 1, both the PTSD and TENC groups reported elevated exposure to childhood adverse events compared to the HC group using the self-report measures. Correlations within the PTSD group were not significant for the relationship between DLPFC-precuneus connectivity and ACE total scores, partial r(17) = 0.021, p = 0.931, or CTQ subscale scores, partial rs(17) = −0.325 to 0.154, p = 0.175 to 0.739. In this PTSD sample, there also was no significant relationship between childhood trauma load and current PTSD symptoms: the association was nonsignificant between CAPS total scores and either ACE total scores, r(19) = −0.185, p = 0.449, or CTQ subscale scores, rs(19) = −0.150 to 0.221, p = 0.336 to 0.940. DLPFC-precuneus rsFC was also not related to lifetime trauma load (LEC) within the PTSD sample, partial r(17) = 0.010, p = 0.968. The relationship between rsFC and depression (BDI score) did not reach significance, partial r(16) = −0.252, p = 0.313 (with one participant missing data on the BDI).
Discussion
In this community-based sample of adults, adults with PTSD were characterized by increased negative rsFC between the right DLPFC and a bilateral precuneus cluster, compared to both TENC and HC subjects. There were no significant differences between trauma-exposed and healthy controls. Within the PTSD group, increased negative right DLPFC-precuneus rsFC was associated with greater overall current PTSD symptom scores and with higher current avoidance symptoms, but was unrelated to the extent of childhood adverse event exposure. In contrast, there were no significant group differences in rsFC seeded from the left DLPFC, VMPFC, or DMPFC.
Interpretation of the right DLPFC-precuneus rsFC finding is complicated because the participation of the precuneus in resting state networks is multifaceted. While the precuneus has been described as playing a role in the DMN in some contexts (especially in conjunction with the PCC), in other contexts it has been characterized as part of the fronto-parietal network or CEN (55). In fact, there is evidence that the posterior DMN hub is limited to the PCC and does not extend to the precuneus (56, 57). This may be related to differential functional connectivity across precuneus subregions; while anterior and posterior precuneus are functionally connected to sensorimotor and visual regions respectively, the central precuneus is a cognitive/associative region functionally connected to the DLPFC, DMPFC, and lateral inferior parietal cortex (57).
The present finding is squarely in the precuneus and is not close to the PCC (Supplementary Figure S2), raising the possibility that it localizes to a subregion participating in the CEN as opposed to the DMN. In this case, increased negative rsFC between the right DLPFC and precuneus in PTSD would reflect increased opposition between the anterior and posterior CEN nodes. To confirm this interpretation, we examined placement of our finding relative to an established 17-network model of resting state networks (26). This analysis confirmed that our result primarily involves voxels that participate in cognitive control as opposed to default mode networks (see Supplement). In PTSD, increasing “fractionation” of the CEN into anterior-posterior subnetworks may have functional implications; for example, hyperactivity among posterior sensory/perceptual integration systems may correspond with decreased activity in goal-oriented PFC regions, consistent with previous documentation of precuneus hyperactivity and DLPFC hypoactivity in PTSD (55). If the precuneus is instead conceptualized as part of the DMN, then the present finding would reflect increased opposition between the DMN and CEN. In this case, our finding would be consistent with a recent analysis demonstrating that successful PTSD treatment via exposure therapy (and associated reduction in avoidant and hyperarousal symptoms) is linked to increased rsFC between DLPFC and DMN nodes including the PCC (39).
Additionally, in this sample increased right DLPFC-precuneus negative rsFC was associated with increased precuneus cortical thickness, which could occur in the context of precuneus hyperactivity producing excessive DLPFC inhibition. Precuneus thickness but not DLPFC thickness was correlated with DLPFC-precuneus connectivity. Although our cross-sectional data cannot speak to the timing of how this pattern might emerge, one possibility is that individuals with greater precuneus cortical thickness (perhaps associated with well-documented precuneus hyperactivity in PTSD (58)) might be more vulnerable to disruption of precuneus-DLPFC rsFC following trauma exposure. From a clinical perspective, although cognitive performance was not examined in this sample, the observed increased negative rsFC between DLPFC and precuneus may contribute to well-established working memory deficits in PTSD (59).
In this study we have identified the same rsFC pattern as was demonstrated in a sample without psychopathology but with ELS (47). Our identification of this pattern in a sample with PTSD suggests that the observed difference in DLPFC-parietal connectivity is not a feature associated with resilience. Because the vast majority of our PTSD participants did report childhood trauma exposure, we are not able to differentiate specific effects related to the timing of trauma exposure (during childhood versus adulthood). Increased DLPFC-precuneus negative rsFC may thus be a feature that arises in the context of childhood/adolescent trauma exposure, though in this sample the rsFC finding did not scale with childhood trauma load.
We did not find group differences in DMPFC or VMPFC rsFC; this could be related to our use of a whole-brain search strategy with particularly stringent statistical thresholds. Alternatively, this may be related to the seed selection in this analysis; investigating other targeted regions of DMPFC or VMPFC as seeds may reveal other functional circuits that are disrupted in PTSD (e.g., see Miller et al. 2017 for a positive finding for group differences in VMPFC connectivity with different seed placement). The DLPFC itself (like the VMPFC and DMPFC) is a large and heterogeneous region composed of functionally distinct subregions (60); analyzing differential patterns of rsFC across DLPFC subregions in PTSD is a goal for future research.
One limitation of the rsFC literature broadly, including the present study, concerns whether the resting state “task” elicits equivalent behavior across diagnostic groups. To the extent that PTSD involves hyper-attention to internal bodily signals, it is possible that the actual state sampled during resting state reflects reduced engagement in mind-wandering (and hyperengagement in attention to body sensations), which may partially account for existing rsFC findings. Addressing this possibility via pseudorandom sampling for self-report of mental activity during resting state fMRI paradigms (61) may be an important direction for future research. An additional limitation is that, given the cross-sectional nature of the study design, it is unclear whether the present findings reflect an effect of PTSD pathology or a risk factor for developing PTSD.
To summarize, in this community-based sample, adults with PTSD demonstrated increased negative DLPFC-precuneus rsFC relative to both trauma-exposed and non-trauma-exposed control participants. This abnormality scaled with PTSD symptomatology but not with early life trauma load. Further consideration of these regions as potential targets for intervention may be warranted.
Supplementary Material
ACKNOWLEDGMENTS
Funding: This work was supported by the National Institute of Mental Health (5R01MH096987 [IMR]). DAP was partially supported by R37 MH068376 and R01 MH095809. EAO was partially supported by K23 MH112873.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: All authors declare no biomedical financial interests or potential conflicts of interest related to the present work.
FINANCIAL DISCLOSURES
EAO reports receiving research funding from the National Institute of Mental Health, the Brain & Behavior Research Foundation, and from McLean Hospital.
RHK reports receiving research funding from the National Institute of Mental Health, the Brain & Behavior Research Foundation, from McLean Hospital, and from University of Colorado Boulder.
DAP reports receiving research funding from the National Institute of Mental Health and the Dana Foundation. Over the past three years, he received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehreinger Ingelheim, Posit Science, and Takeda for activities unrelated to the present study.
SLR reports receiving research funding from NIMH and The US Army. He is employed by McLean Hospital/Partners Healthcare. He serves on a VA Research Advisory Committee on Gulf War Illness. He provides unpaid Board service for a number of non-profit organizations, including ADAA and Project 375. He is also paid as the secretary of SOBP. He receives royalty payments from Oxford University Press and American Psychiatric Press Inc.
IMR reports receiving research funding from the NIMH, the Brain & Behavior Research Foundation, and McLean Hospital. Over the past two years, she also has received consulting fees from Aptinyx Inc for activities unrelated to the present work.
REFERENCES
- 1.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. (2004): Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 61: 168–76. [DOI] [PubMed] [Google Scholar]
- 2.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. (2005): A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 62: 273–81. [DOI] [PubMed] [Google Scholar]
- 3.Mennes M, Kelly C, Zuo X, Di Martino A, Biswal BB, Castellanos FX, Milham MP (2010): Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 50: 1690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killgore WDS, Smith R, Olson EA, Weber M, Rauch SL, Nickerson LD (2017): Emotional intelligence is associated with connectivity within and between resting state networks. Soc Cogn Affect Neurosci 12: 1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song M, Zhou Y, Li J, Liu Y, Tian L, Yu C, Jiang T (2008): Brain spontaneous functional connectivity and intelligence. Neuroimage. 41: 1168–76. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Liu Z, Rolls ET, Chen Q, Yao Y, Yang W, et al. (2018): Verbal creativity correlates with the temporal variability of brain networks during the resting state. Cereb Cortex. . doi: 10.1093/cercor/bhy010. [DOI] [PubMed] [Google Scholar]
- 7.Menon V (2011): Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15: 483–506. [DOI] [PubMed] [Google Scholar]
- 8.Davey CG, Pujol J, Harrison BJ (2016): Mapping the self in the brain’s default mode network. Neuroimage. 132: 390–397. [DOI] [PubMed] [Google Scholar]
- 9.Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 100: 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon V, Uddin LQ (2010): Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs RH, Barba A, Gowins JR, Klumpp H, Jenkins LM, Mickey BJ, et al. (2016): Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol Med 46: 1055–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 105: 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional-anatomic fractionation of the brain’s default network. Neuron. 65: 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Åhs F, Kragel PA, Zielinski DJ, Brady R, LaBar KS (2015): Medial prefrontal pathways for the contextual regulation of extinguished fear in humans. Neuroimage. 122: 262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011): Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 21: 1667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grupe DW, Wielgosz J, Davidson RJ, Nitschke JB (2016): Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychol Med. 46: 1885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M (2017): Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biol psychiatry Cogn Neurosci neuroimaging. 2: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL (2005): Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 102: 10706–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004): Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 43: 897–905. [DOI] [PubMed] [Google Scholar]
- 21.Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M (2015): Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev 51: 189–204. [DOI] [PubMed] [Google Scholar]
- 22.Plana I, Lavoie M-A, Battaglia M, Achim AM (2014): A meta-analysis and scoping review of social cognition performance in social phobia, posttraumatic stress disorder and other anxiety disorders. J Anxiety Disord 28:169–77. [DOI] [PubMed] [Google Scholar]
- 23.Rabinak CA, MacNamara A, Kennedy AE, Angstadt M, Stein MB, Liberzon I, Phan KL (2014): Focal and aberrant prefrontal engagement during emotion regulation in veterans with posttraumatic stress disorder. Depress Anxiety. 31: 851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morey RA, Petty CM, Cooper DA, Labar KS, McCarthy G (2008): Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res 162: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I (2012): Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med 74: 904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiGangi JA, Tadayyon A, Fitzgerald DA, Rabinak CA, Kennedy A, Klumpp H, et al. (2016): Reduced default mode network connectivity following combat trauma. Neurosci Lett 615: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satterthwaite TD, Cook PA, Bruce SE, Conway C, Mikkelsen E, Satchell E, et al. (2016): Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol Psychiatry. 21: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole VN, Robinson ME, Singleton O, DeGutis J, Milberg WP, McGlinchey RE, et al. (2016): Intrinsic functional connectivity predicts individual differences in distractibility. Neuropsychologia. 86: 176–182. [DOI] [PubMed] [Google Scholar]
- 30.Patriat R, Birn RM, Keding TJ, Herringa RJ (2016): Default-mode network abnormalities in pediatric posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 55: 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Overwalle F (2009): Social cognition and the brain: a meta-analysis. Hum Brain Mapp 30: 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown VM, LaBar KS, Haswell CC, Gold AL, Mid-Atlantic MIRECC Workgroup, McCarthy G, Morey RA (2014): Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 39: 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ (2014): Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety. 31: 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson EA, Kaiser RH, Pizzagalli DA, Rauch SL, Rosso IM (n.d.): Anhedonia in trauma-exposed individuals: functional connectivity and decision-making correlates. Biol Psychiatry Cogn Neurosci Neuroimaging. . doi: 10.1016/j.bpsc.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misaki M, Phillips R, Zotev V, Wong CK, Wurfel BE, Krueger F, et al. (2018): Connectome-wide investigation of altered resting-state functional connectivity in war veterans with and without posttraumatic stress disorder. NeuroImage Clin 17: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangaprakash D, Dretsch MN, Venkataraman A, Katz JS, Denney TS, Deshpande G (2018): Identifying disease foci from static and dynamic effective connectivity networks: Illustration in soldiers with trauma. Hum Brain Mapp 39: 264–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuveni I, Bonne O, Giesser R, Shragai T, Lazarovits G, Isserles M, et al. (2016): Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum Brain Mapp 37: 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spielberg JM, McGlinchey RE, Milberg WP, Salat DH (2015): Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry. 78: 210–6. [DOI] [PubMed] [Google Scholar]
- 39.King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, et al. (2016): Altered default mode network (DMN) resting state functional connectivity following a mindfulness-based exposure therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress Anxiety. 33: 289–299. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA psychiatry. 72: 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, et al. (2014): Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol Med 44: 1927–1936. [DOI] [PubMed] [Google Scholar]
- 42.Jin C, Jia H, Lanka P, Rangaprakash D, Li L, Liu T, et al. (2017): Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum Brain Mapp 38: 4479–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ke J, Chen F, Qi R, Xu Q, Zhong Y, Chen L, et al. (2016): Post-traumatic stress influences local and remote functional connectivity: a resting-state functional magnetic resonance imaging study. Brain Imaging Behav. 1–10. [DOI] [PubMed] [Google Scholar]
- 44.van Tol M-J, Li M, Metzger CD, Hailla N, Horn DI, Li W, et al. (2014): Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med 44: 2053–65. [DOI] [PubMed] [Google Scholar]
- 45.Bing X, Ming-Guo Q, Ye Z, Jing-Na Z, Min L, Han C, et al. (2013): Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 1490: 225–32. [DOI] [PubMed] [Google Scholar]
- 46.Park H, Park Y-H, Cha J, Seo SW, Na DL, Lee J-M (2017): Agreement between functional connectivity and cortical thickness-driven correlation maps of the medial frontal cortex. PLoS One. 12: e0171803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philip NS, Valentine TR, Sweet LH, Tyrka AR, Price LH, Carpenter LL (2014): Early life stress impacts dorsolateral prefrontal cortex functional connectivity in healthy adults: informing future studies of antidepressant treatments. J Psychiatr Res 52: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, et al. (2018): Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol Med 48: 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995): The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 8: 75–90. [DOI] [PubMed] [Google Scholar]
- 50.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. (1994): Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 151: 1132–6. [DOI] [PubMed] [Google Scholar]
- 51.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (1998): Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 14: 245–58. [DOI] [PubMed] [Google Scholar]
- 52.Gray MJ, Litz BT, Hsu JL, Lombardo TW (2004): Psychometric Properties of the Life Events Checklist. Assessment. 11: 330–341. [DOI] [PubMed] [Google Scholar]
- 53.Beck AT, Steer RA (1987): Manual for the Beck Depression Inventory, 1993 edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- 54.Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E (2011): Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat Neurosci 14: 1468–1474. [DOI] [PubMed] [Google Scholar]
- 55.Patel R, Spreng RN, Shin LM, Girard TA (2012): Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36: 2130–42. [DOI] [PubMed] [Google Scholar]
- 56.Buckner RL, Andrews-Hanna JR, Schacter DL (2008): The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- 57.Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. (2009): Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci 106: 20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel R, Spreng RN, Shin LM, Girard TA (2012): Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36: 2130–42. [DOI] [PubMed] [Google Scholar]
- 59.Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM, et al. (2015): A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull 141: 105–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, et al. (2013): Is there one DLPFC in cognitive action control? Evidence for heterogeneity from Co-activation-based parcellation. Cereb Cortex. 23:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scheibner HJ, Bogler C, Gleich T, Haynes J-D, Bermpohl F (2017): Internal and external attention and the default mode network. Neuroimage. 148: 381–389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.