Abstract
Background:
Pathogenic mutations in genes mediating homologous recombination (HR) DNA repair are present in 20-30% of men with metastatic castrate-resistant prostate cancer (mCRPC). Radium-223 is a bone-seeking α-emitter that induces double-strand DNA breaks, thereby killing cancer cells in the bone microenvironment.
Objective:
To evaluate the potential impact of germline or somatic HR-deficiency (HRD) mutations on radium-223 efficacy in bone mCRPC.
Design, setting, and participants:
This is a retrospective single-institution study. Medical records of 190 mCRPC patients for whom germline and/or somatic DNA sequencing data were available were reviewed. Of these patients, 28 had received standard-of-care radium-223 at Johns Hopkins between February 2013 and February 2018.
Outcome measurements and statistical analysis:
Alkaline phosphatase (ALP) responses and time-to-ALP-progression were the coprimary endpoints. Prostate-specific antigen (PSA) responses, overall survival (OS), and time to next systemic therapy were also evaluated.
Results and limitations:
Of the 28 patients included, 10 men (35.7%) had a germline/somatic HRD mutation (three in BRCA2, and one each in ATM, ATR, CHEK2, FANCG, FANCI, FANCL, and PALB2) and 18 (64.3%) did not. Men with HRD mutations (HRD+) had numerically lower ages (66 vs 73 yr, p = 0.25), more soft-tissue metastases (50% vs 38%, p = 0.43), and higher baseline ALP levels (130 vs 108 U/l, p = 0.84). Compared with HRD(−) men, HRD(+) patients showed greater ALP responses (80% vs 38%, p = 0.04), longer time to ALP progression (median10.4 vs 5.8 mo, hazard ratio [HR] 6.4, p = 0.005), and a trend toward longer OS (median 36.9 vs 19.0 mo, HR 3.3, p = 0.11). PSA responses (0% vs 0%, p > 0.99) and time to next systemic therapy (HR 1.5, p = 0.39) were similar between the two groups. Results are limited by the retrospective nature of the analysis and the small sample size.
Conclusions:
In this exploratory study, bone-metastatic mCRPC patients with inactivating HRD mutations demonstrated significantly improved ALP responses and time to ALP progression. These results should motivate prospective validation of the “synthetic lethality” hypothesis between HRD mutations and radium-223 activity.
Keywords: Cancer, DNA repair, Germline, Mutations, Prostate cancer, Radium-223, Somatic
Patient summary:
In this report, we retrospectively examined outcomes to metastatic prostate cancer in patients with and without DNA repair mutations who received radium-223, a therapy that kills cancer cells by causing direct DNA damage. Our study suggested that patients who have inherited or acquired DNA repair gene mutations derived greater benefit from radium-223 when compared with patients without these mutations. We concluded that radium-223 might have an important role in this setting; however, prospective studies are needed to confirm whether DNA repair mutations truly make radium-223 work better or not.
1. Introduction
Prostate cancer (PCa) is a heterogeneous disease at the clinical, pathological, and molecular levels. Based on genetic abnormalities, especially in genes that control mechanisms of DNA repair, new attempts to classify the different molecular subgroups of this disease have been made [1]. In recent years, the critical importance of DNA repair defects, especially in the homologous recombination (HR) pathway as well as the mismatch repair pathway, has been demonstrated in both germline and somatic lineages, and has prognostic and therapeutic implications [1,2]. The genes responsible for the HR pathway, particularly BRCA1, BRCA2, CHEK2, ATM, RAD51D, and PALB2, play a crucial role in repairing double-strand (ds) DNA breaks [3]. Defects in some of these genes have been associated with an increased risk of PCa development and disease aggressiveness [4,5].
Germline mutations in DNA repair genes are present in 8-12% of metastatic PCa [1,2], whereas the previously estimated prevalence was 4-5% in localized disease [6,7]. In addition, somatic aberrations in genes responsible for DNA repair are seen in 20-25% of PCa patients [1]. Different studies have confirmed that together both germline and somatic HR-deficiency (HRD) pathogenic mutations are seen in up to one-third of patients with metastatic castration- resistant prostate cancer (mCRPC) [1,8], strengthening not only the high prevalence of these mutations but also their role as prognostic [9-12] and predictive biomarkers [8,13].
Radium-223 is an alpha-particle-emitting bone-targeted therapy that demonstrated consistent improvement in pain [14,15] and overall survival (OS) in patients with mCRPC harboring bone disease [16]. Alpha particles emitted at the site of disease have high linear energy transfer, resulting in the deposition of energy in the immediate vicinity of the radionuclide’s decay. This highly localized radiotherapy selectively targets the bone microenvironment and metastatic tumor cells, causing unrepairable dsDNA breaks [17], resulting in potent but locally restricted cytotoxic effects [18]. By the mechanism of synthetic lethality, tumors with defects in mechanisms of DNA repair are theoretically more susceptible to therapies that cause DNA damage, such as ds breaks [19,20]. Therefore, the present study hypothesized that patients who harbor germline and/or somatic HRD mutations may have a greater clinical benefit from radium-223, due to dsDNA breaks going unrepaired because of an underlying HRD in the tumor cells [21]. To this end, we performed a retrospective study to test this biological hypothesis.
2. Patients and methods
Patients with mCRPC who received radium-223 over a 5-yr period (between February 2013 and February 2018) and who were being seen at the Johns Hopkins Hospital formed the study population. These consecutive patients were offered somatic and/or germline genomic panel testing for clinical purposes, using different commercially available (Foundation One, Personal Genome Diagnostics, Color Genomics, Invitae) and in-house next-generation DNA sequencing platforms. This was an unselected patient cohort; patients were not selected for radium-223 treatment based on prior knowledge of HRD mutation status. The Johns Hopkins University Institutional Review Board and the Human Research Ethics Committee approved this retrospective study.
Demographic, histopathological, and clinical characteristics of all patients were collected. We interrogated for the presence or absence of pathogenic or likely pathogenic somatic and/or germline HRD mutations, and classified patients into mutation-positive (HRD+) and mutation-negative (HRD-) groups. The coprimary clinical endpoints were alkaline phosphatase (ALP) response (defined as a decline of ≥30% from baseline within 12 wk) and time to ALP progression (defined as an increase in ALP level of ≥25% from baseline in patients with no decrease from baseline or an increase of ≥25% above the nadir in patients with an initial decrease from baseline). Prostate-specific antigen (PSA) response rate (a decline of ≥50% from baseline within 12 wk), OS, and time to next systemic therapy were also assessed. In order for patients to be evaluable for ALP response rates and PSA response rates, a minimum of 12 wk of ALP/PSA data were required following initiation of radium-223 treatment (all patients met this criterion for both parameters).
The sample size was opportunistically selected (based on cross referencing of our clinical genomics database and our radium-223 pharmacy database) and was not based on prospective hypothesis testing. A two-sided Fisher’s exact test was used to compare proportions for categorical baseline variables (eg, Gleason score, baseline Eastern Cooperative Oncology Group [ECOG] status, proportion of patients with visceral or soft tissue disease, and proportion of patients who received a previous taxane) between HRD+ and HRD- patients. The Mann-Whitney U test was used to compare distributions of age, number of radium-223 doses received, baseline pain score, and PSA and ALP between the two groups. All statistical tests were two sided, with statistical significance set at p ≤ 0.05. Since this study was hypothesis generating, we did not perform Bonferroni corrections for multiple comparisons. Kaplan-Meier curves were used to visualize time-to-event data. Hazard ratios, associated 95% confidence intervals (CIs), and differences between groups were calculated with the use of a Cox proportional-hazards model.
3. Results
3.1. Baseline genomic and clinical characteristics
Between February 15, 2013 and February 15, 2018, a total of 190 mCRPC patients agreed to undergo clinical-grade somatic and/or germline genetic testing using the next-generation DNA sequencing platforms listed above. All germline testing was performed from saliva. Somatic testing involved a mix of primary tumor testing as well as from metastatic biopsies. Of these 190 mCRPC cases, 28 patients had received radium-223 at some point during their treatment course, forming our study population. No patients had received either PARP inhibitors or platinum agents prior to radium. Among these 28 men, pathogenic or likely pathogenic HRD mutations were identified in 10 patients (36%), while 18 men (64%) did not harbor any HRD mutations. Pathogenic alterations were defined a priori as those that resulted in protein- truncating defects (nonsense mutations, frameshift insertions or deletions, and splice site mutations at the conserved splice donor and acceptor sites) or missense mutations that were denoted as pathogenic in the ClinVar database. The HR genes of interest included in this study were BAP1, BARD1, BRAP, BRCA1, BRCA2, BRIP1, CDK12, CHEK1, CHEK2, FANCA, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, GEN1, NBN, PALB2, RAD51, RAD51B, RAD51C, RAD51D, and RAD54L; this list of 25 genes was also specified a priori. Table 1 summarizes the 10 pathogenic HRD mutations found in these patients. A complete list of mutations, including those in genes other than DNA repair genes, is listed in Supplementary Table 1.
Table 1 –
List of pathogenic homologous recombination deficiency (HRD) mutations
| Sample ID | Gene | Origin of mutation | Amino acid change | Nucleotide change | Mutation mechanism | Type of analysis |
|---|---|---|---|---|---|---|
| 02 | BRCA2 | Germline | D3095E | c.9285C>G | Missense | Germline + somatic |
| 03 | BRCA2 | Somatic | E1646Qfs*23 | c.4936_4939delGAAA | Frameshift deletion | Germline + somatic |
| 14 | CHEK2 | Somatic | R519X* | c.1555C>T | Nonsense | Germline + somatic |
| 15 | ATM | Somatic | E2014X* | c.6040G>T | Nonsense | Germline + somatic |
| 18 | ATR | Germline | - | c.2634-1G>A | Splicing | Germline only |
| 19 | FANCI | Germline | K808X* | c.A2422T | Nonsense | Germline only |
| 20 | FANCL | Somatic | T372Nfs*4 | c.A2422T | Frameshift insertion | Germline + somatic |
| 29 | PALB2 | Somatic | - | c.212-2A>G | Splicing | Germline + somatic |
| 31 | FANCG | Germline | L53Afs*4 | c.156insG | Frameshift insertion | Germline + somatic |
| 32 | BRCA2 | Somatic | S3147Cfs*2 | c.9435_9436delGT | Frameshift deletion | Germline + somatic |
Demographic, clinical, and pathological characteristics of our patients are shown in Table 2. Despite some numeric differences between HRD+ and HRD- patients, no statistically significant differences were seen between the two groups. Patients with deleterious HRD mutations had a trend toward younger ages at the time of radium-223 use (66 vs 73 yr, p = 0.25), higher Gleason sums (Gleason ≥8 in 80% vs 66%, p = 0.44), more visceral and soft tissue disease (50% vs 38%, p = 0.43), and higher baseline ALP levels (130 vs 108 U/l, p = 0.84). All patients had an ECOG score of 0-1 when they started radium-223 treatment, and there was no difference in the number of cycles of the drug between the two groups (five vs five cycles, p > 0.99).
Table 2 –
Baseline demographic, clinical, and pathological characteristics of our patient cohort, according to HRD status
| Patient characteristics |
|||
|---|---|---|---|
| HRD(+) | HRD(-) | p value | |
| (N = 10) | (N = 18) | ||
| Median age in years (Q1-Q3) | 66 (60-69) | 73 (64-75) | 0.28 |
| Number of radium-223 doses received | 5.5 | 5.5 | 0.50 |
| Median number (Q1-Q3) | (5-6) | (2-6) | |
| Gleason sum at diagnosis, % (N) | |||
| ≤7 | 10 (1) | 33 (6) | 0.44 |
| ≥8 | 80 (8) | 67 (12) | |
| Not reported | 10 (1) | 0 (0) | |
| ECOG status at time of radium-223 | |||
| 0-1 | 100% (10) | 100% (18) | >0.00 |
| ≥2 | 0% (0) | 0% (0) | |
| Bone pain score at time of radium-223 | 2 | 1 | 0.58 |
| Median (Q1-Q3) | (0-3) | (0-2.5) | |
| Presence of any soft-tissue disease at time of radium-223 | 50% (5) | 39% (7) | 0.43 |
| Previous taxane use | 50% (5) | 44% (8) | 0.54 |
| Baseline PSA level (ng/ml) | 77.1 | 71.6 | 1.00 |
| Median (Q1-Q3) | (7.9-236.0) | (18.4-162.9) | |
| Baseline alkaline phosphatase (U/l) | 130 | 108.5 | 0.70 |
| Median (Q1-Q3) | (85-194) | (72-185) | |
ECOG = Eastern Cooperative Oncology Group; HRD = homologous recombination deficiency; PSA = prostate-specific antigen.
Tests used were the two-sided Fisher’s exact test and Mann-Whitney test.
3.2. Efficacy of radium-223 in patients with and without HRD mutations
Overall, 53% (15/28) of all patients had a decline of ≥30% in ALP within 12 wk, meeting the cutoff for an ALP response. Patients with an HRD mutation (HRD+ men) had a statistically significant improvement in ALP response rates compared with HRD- patients (80% vs 38%, p = 0.04; Table 3). Despite some marginal reductions in PSA levels seen in 18% (5/28) of all patients, no individual had a PSA decline of ≥50% from baseline within 12 wk. The relationship between ALP response and PSA response according to HRD status is depicted in the waterfall plots in Figures 1 and 2, respectively. In addition, of those individuals who had elevated ALP levels at baseline, all patients with HRD mutations (five of five patients) had normalization of ALP after starting radium-223 compared with only one-third (three of nine patients) of HRD- patients (100% vs 33%, p = 0.03; Table 3).
Table 3 –
PSA and ALP responses in HRD(+) and HRD(-) patients
| HRD(+) | HRD(-) | p value | |
|---|---|---|---|
| N = 10 | N = 18 | ||
| PSA (≥50%) response | 0% (0) | 0% (0) | 1.00 |
| ALP (≥30%) response | 80% (8) | 39% (7) | 0.04 |
| Patients with ALP normalization (if baseline ALP was elevated) | 100% (5) | 33% (3) | 0.03 |
ALP = alkaline phosphatase; HRD = homologous recombination deficiency; PSA = prostate-specific antigen.
Response rate is defined as a decrease in PSA of ≥50% and in ALP of ≥30% from baseline within 12 wk.
Fig. 1 –
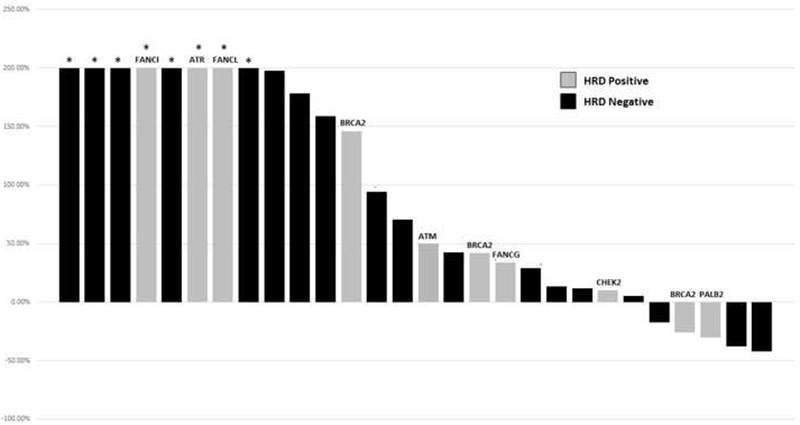
Waterfall plot of best PSA response within 12 wk, by HRD status. HRD = homologous recombination deficiency; PSA = prostate-specific antigen. * Indicates truncated bars at >+200%.
Fig. 2 –
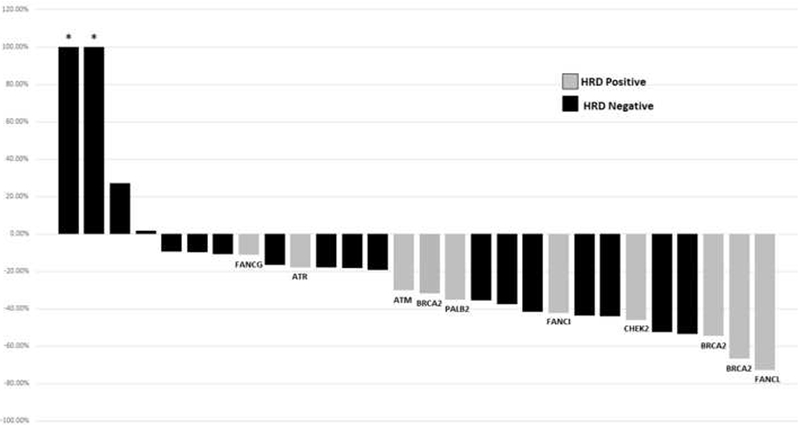
Waterfall plot of best alkaline phosphatase response within 12 wk, by HRD status. HRD = homologous recombination deficiency; PSA = prostate-specific antigen. * Indicates truncated bars at >+100%.
All the primary and secondary endpoints favored patients with HRD mutations, although not all associations were statistically significant. Compared with HRD- patients, HRD+ men had significantly prolonged time to ALP progression (median 10.4 vs. 5.8 mo; hazard ratio [HR] 6.4, 95% CI, 1.5-28.9; p = 0.005; Fig. 3). Time to the next systemic therapy was also numerically longer in HRD+ compared with HRD- patients (median 9.7 vs 7.2 mo; HR 1.5, 95% CI, 0.5-5.3; p = 0.39; Fig. 4). Finally, median OS was 36.9 versus 19.0 mo in patients with versus without HRD mutations (HR 3.3, 95% CI, 0.7-15.6; p = 0.11; Fig. 5).
Fig. 3 –
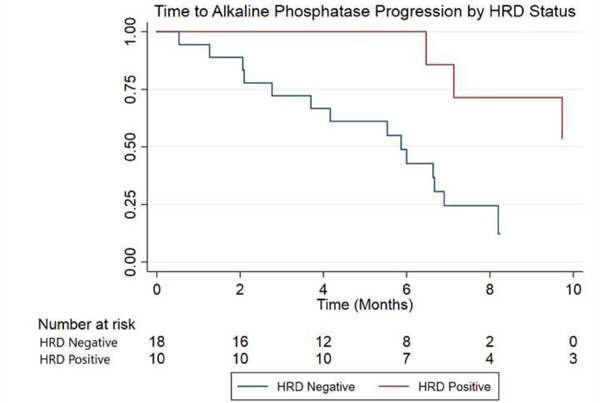
Kaplan-Meier curve for time to ALP progression, by HRD status (the x-axis is truncated at 10 mo). ALP = alkaline phosphatase; HRD = homologous recombination deficiency.
Fig. 4 –
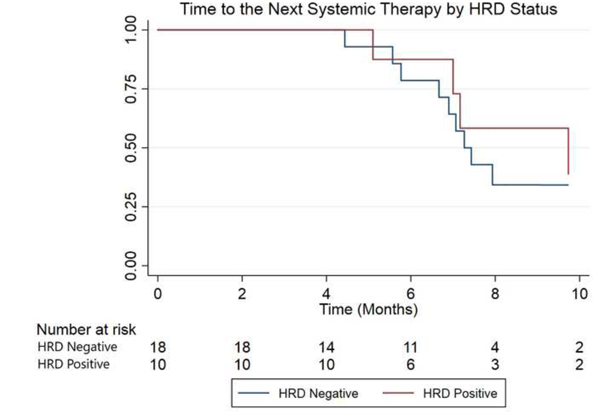
Kaplan-Meier curve for time to the next systemic therapy, by HRD status (the x-axis is truncated at 10 mo). HRD = homologous recombination deficiency.
Fig. 5 –
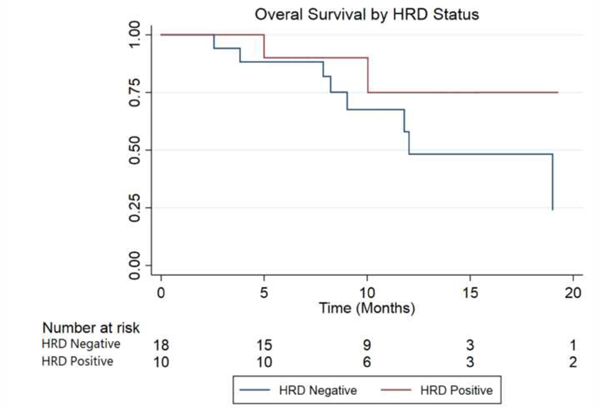
Kaplan-Meier curve for overall survival, by HRD status (the x-axis is truncated at 20 mo). HRD = homologous recombination deficiency.
4. Discussion
The findings of our hypothesis-generating study support the theoretical rationale that tumors harboring HRD mutations may be more sensitive to therapies that cause direct damage to DNA, such as radium-223. In the pivotal phase III ALSYMPCA study [16], which compared radium-223 versus placebo plus best supportive care, ALP response rates (≥30% declines from baseline) were found in 47% versus 3% of patients (p < 0.001). In our study, the overall ALP response rate in the unselected population broadly mirrored this estimate, but the ALP response rate was considerably higher in patients with HRD mutations (80% vs 38%, p = 0.04); there was also a difference in the proportion of patients who normalized their ALP level with radium-223 treatment depending on HRD status (100% vs 33%, p = 0.03). Furthermore, the median time to ALP progression in our study was significantly longer in HRD+ patients (10.4 vs 5.8 mo, p = 0.005) compared with HRD- patients. This time to delay of ALP progression in the HRD+ group also appears to be greater than that in the pivotal phase III trial (which showed 7.4 vs 3.8 mo until ALP progression for radium-223 and placebo, respectively) [16], although direct comparisons cannot be made. Finally, HRD+ patients had numerically longer OS compared with HRD- men, although this analysis was clearly underpowered to demonstrate a statistical improvement.
Our study primarily used ALP endpoints to evaluate the hypothesis that patients with HRD might have a greater benefit from radium-223 than those with HR-proficient tumors. Why was such an emphasis placed on ALP-based endpoint? This is because there are data indicating that within patients with mCRPC and bone metastasis who receive chemotherapy, serum ALP responses may be prognostic for OS independently of PSA changes [22]. In addition, in an exploratory analysis of the ALSYMPCA trial [23], it was demonstrated that patients with ≥30% ALP declines had a 55% relative reduction in the risk of death compared with patients who did not have ALP declines [23]. Finally, focusing on ALP-related endpoints seemed to be reasonable in the context of a bone-targeting therapy. Taken together, these data suggest that improvements in ALP response rates and prolongation of ALP progression (as found in the HRD+ men in our study) are reasonable intermediate endpoints to evaluate the clinical efficacy of radium-223.
Despite some recent case reports [24] and a case series [25] suggesting favorable responses to radium-223 in patients with HRD mutations, our study is the first (to our knowledge) that includes a control group of HRD- patients undergoing radium-223 treatment. Thus, this enabled us to compare outcomes in HRD+ versus HRD- men. Multiple recent studies have tried to assess the impact of DNA repair mutation status on response or resistance to standard-of-care therapies, with conflicting results. Some studies, for example, show no clear difference in prognosis according to HRD status with respect to taxane chemotherapies or novel AR-targeting therapies [26]. With respect to abiraterone and enzalutamide efficacy specifically, some studies have demonstrated a worse prognosis in men with HRD mutations [27], while others have suggested improved outcomes in the HR-deficient subsets [28]. However, the theoretical rationale for why an HR-deficient patient should respond better or worse to an AR- targeted therapy or a taxane chemotherapy appears weaker than that supporting the biological concept that an underlying HRD may produce a form of “synthetic lethality” in the setting of an alpha-particle-emitting dsDNA break-inducing agent.
If validated, our study results may impact clinical decisions, and aid therapy selection for radium-223 treatment and the evaluation of experimental alpha-particle emitters. With the wide availability of clinical-grade next-generation DNA sequencing panels, mutational profiles of many cancers (as well as their inherited backgrounds) are now increasingly being recognized. Notably, the recent National Comprehensive Cancer Network 2018 PCa guidelines [29] now recommend germline DNA testing for all men diagnosed with mCRPC, the same population in which radium-223 is indicated. Since multiple treatment options may be available to patients with bone-predominant mCRPC, knowing that a patient has a germline and/or somatic HRD mutation might make a clinician reach sooner for radium-223 in this context, perhaps saving other systemic therapies (eg, taxane chemotherapies) for later. Clearly, prospective validation of these hypothesis-generating results will be required before these findings become clinically actionable. Finally, these data may ignite interest in conducting dedicated clinical trials evaluating the use of radium-223 in biomarker-selected (ie, HR-deficient) mCRPC populations.
Our study has several limitations that should be considered when interpreting our results. First, this was a retrospective study and the sample size was not determined a priori using hypothesis testing; therefore, even though some strong associations may have been demonstrated, causal inferences cannot be made. Other inherent limitations of this retrospective study design include selection bias and information bias. We tried to mitigate selection bias by including consecutive patients who received radium-223 and had next- generation DNA sequencing data available. However, despite our study having a limited number of patients (which might cause limitations in the analysis, especially because of wide confidence intervals and nonsignificant p values), the study still met its primary endpoint, which was to demonstrate a greater benefit of radium-223 with respect to ALP endpoints in patients with HRD mutations. In particular, care should be taken when interpreting the Kaplan-Meier curves due to the very small number of patients per group (18 and 10 patients in the HRD- and HRD+ groups, respectively), resulting in very wide confidence intervals. Finally, due to the small sample size and the hypothesis-generating nature of this study, we were unable to control for potential discrepancies in baseline clinical factors. These results would now benefit from further prospective (or retrospective) validation, and must be considered hypothesis generating only and not definitive.
5. Conclusions
Our preliminary findings suggest that bone-metastatic mCRPC patients with germline and/or somatic mutations in HR-pathway genes may be associated with clinical benefit from radium- 223 (in terms of ALP responses, normalization of ALP, and the time to ALP progression) as well as potential prolongation of survival. The retrospective nature of our study and the limitations inherent to that design suggest that these provocative findings should be considered as hypothesis generating only at this time, but may spark dedicated trials investigating radium-223 in HR-deficient mCRPC patients.
Supplementary Material
Acknowledgments
Funding/Support and role of the sponsor: This work was partially supported by National Institutes of Health Grant P30 CA006973 (E.S.A.) and Department of Defense grant W81XWH-16-PCRP-CCRSA (E.S.A.).
Financial disclosures: Emmanuel S. Antonarakis certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Emmanuel S. Antonarakis is a paid consultant/advisor to Janssen, Astellas, Sanofi, Dendreon, Medivation, ESSA, AstraZeneca, Clovis, and Merck; he has received research funding to his institution from Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai, Bristol Myers-Squibb, AstraZeneca, Clovis, and Merck; and he is the coinventor of a biomarker technology that has been licensed to Tokai and Qiagen. The remaining authors report no relevant conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Robinson D, Van Allen EM, Wu Y-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016;375:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature 2012;481:287–94. [DOI] [PubMed] [Google Scholar]
- [4].Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer 2011;105:1230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Leongamornlert D, Mahmud N, Tymrakiewicz M, et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer 2012;106:1697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. In press. 10.1016/j.eururo.2018.03.028 [DOI] [PubMed] [Google Scholar]
- [7].Romero-Laorden N, Castro E. Inherited mutations in DNA repair genes and cancer risk. Curr Probl Cancer 2017;41:251–64. [DOI] [PubMed] [Google Scholar]
- [8].Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol 2015;68:186–93. [DOI] [PubMed] [Google Scholar]
- [11].Na R, Zheng SL, Han M, et al. Germline mutations in ATM and BRCA1/2 distinguish risk for lethal and indolent prostate cancer and are associated with early age at death. Eur Urol 2017;71:740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murphy DG, Risbridger GP, Bristow RG, Sandhu S. The evolving narrative of DNA repair gene defects: distinguishing indolent from lethal prostate cancer. Eur Urol 2017;71:748–9. [DOI] [PubMed] [Google Scholar]
- [13].Mateo J, Boysen G, Barbieri CE, et al. DNA repair in prostate cancer: biology and clinical implications. Eur Urol 2017;71:417–25. [DOI] [PubMed] [Google Scholar]
- [14].Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone- refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 2007;8:587–94. [DOI] [PubMed] [Google Scholar]
- [15].Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014;15:1397–406. [DOI] [PubMed] [Google Scholar]
- [16].Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213–23. [DOI] [PubMed] [Google Scholar]
- [17].Ritter MA, Cleaver JE, Tobias CA. High-LET radiations induce a large proportion of non-rejoining DNA breaks. Nature 1977;266:653–5. [DOI] [PubMed] [Google Scholar]
- [18].Nilsson S, Larsen RH, Fossa SD, et al. First clinical experience with alpha-emitting radium- 223 in the treatment of skeletal metastases. Clin Cancer Res 2005;11:4451–9. [DOI] [PubMed] [Google Scholar]
- [19].Teply BA, Antonarakis ES. Treatment strategies for DNA repair-deficient prostate cancer. Expert Rev Clin Pharmacol 2017;10:889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McLornan DP, List A, Mufti GJ. Applying synthetic lethality for the selective targeting of cancer. N Engl J Med 2014;371:1725–35. [DOI] [PubMed] [Google Scholar]
- [21].Schweizer MT, Antonarakis ES. Prognostic and therapeutic implications of DNA repair gene mutations in advanced prostate cancer. Clin Adv Hematol Oncol 2017;15:785–95. [PubMed] [Google Scholar]
- [22].Sonpavde G, Pond GR, Berry WR, et al. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol Oncol 2012;30:607–13. [DOI] [PubMed] [Google Scholar]
- [23].Sartor O, Coleman RE, Nilsson S, et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol 2017;28:1090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Steinberger AE, Cotogno P, Ledet EM, Lewis B, Sartor O. Exceptional duration of radium- 223 in prostate cancer with a BRCA2 mutation. Clin Genitourin Cancer 2017;15:e69–71. [DOI] [PubMed] [Google Scholar]
- [25].Ramos JD, Mostaghel EA, Pritchard CC, Yu EY. DNA repair pathway alterations in metastatic castration-resistant prostate cancer responders to radium-223. Clin Genitourin Cancer 2018;16:106–10. [DOI] [PubMed] [Google Scholar]
- [26].Mateo J, Cheng HH, Beltran H, et al. Clinical outcome of prostate cancer patients with germline DNA repair mutations: retrospective analysis from an international study. Eur Urol 2018;73:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Annala M, Struss WJ, Warner EW, et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair-deficient prostate cancer. Eur Urol 2017;72:34–42. [DOI] [PubMed] [Google Scholar]
- [28].Hussain M, Daignault-Newton S, Twardowski PW, et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol 2018;36:991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].National Comprehensive Cancer Network. Genetic/familial high- risk assessment: breast and ovarian. 2018. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


