Abstract
Purpose
Glucagon-like peptide 1 (GLP-1) is an incretin hormone that appears to play a major role in the control of food intake. The aim of this investigation was to evaluate and quantify the association of circulating GLP-1 concentration with ad libitum total calorie and macronutrient intake.
Methods
One-hundred fifteen individuals (72 men) aged 35 ± 10 years were admitted for an inpatient study investigating the determinants of energy intake. Ad libitum food intake was assessed during 3 days using a reproducible vending machine paradigm. Fasting plasma GLP-1 concentrations were measured on the morning of the first day and on the morning of the fourth day after ad libitum feeding.
Results
Plasma GLP-1 concentrations increased by 14% after 3 days of ad libitum food intake. Individuals overate on average 139 ± 45% of weight-maintaining energy needs. Fasting plasma GLP-1 on day 1 was negatively associated with carbohydrate intake (r=−0.2, p=0.03) and with daily energy intake from low fat-high simple sugar (r=−0.22, p=0.016).
Conclusion
Higher plasma GLP-1 concentrations prior to ad libitum food intake were associated with lower carbohydrate intake and lower simple sugar ingestion, indicating a possible role of the GLP-1 in the reward pathway regulating simple sugar intake.
Keywords: Glucagon-like peptide 1, ad libitum food intake, carbohydrates intake
Introduction
A chronic positive energy balance is responsible for weight gain leading to obesity and its associated co-morbidities [1]. Physiological, environmental and genetic factors play an important role as determinants of weight gain [2]. Physiological effectors of weight gain include neuronal and humoral signals from peripheral organs, such as the pancreas, gut, and adipose tissue which carry information about satiety and hunger to the CNS and are involved in the regulation of food intake. Glucagon like peptide 1 (GLP-1), an incretin hormone released from the L cells of the intestinal mucosa after meal ingestion, may modulate food intake by conveying meal-related information to the brain, with no substantial effect on energy metabolism [3]. Pharmacologic doses of GLP-1 decreased food intake in rodents [4] and in humans [5] and GLP-1 increases satiety and reduces gastric emptying [6]. Additionally, GLP-1 contributes to postprandial glucose regulation by improving meal related insulin production and secretion from pancreas [7] suppressing glucagon secretion in a glucose dependent manner [8]. Subjects with type 2 diabetes and obesity have been reported to have a lower fasting GLP-1 concentration compared to healthy volunteers [9]. Regardless of diabetes status, infusion of GLP-1 decreased food intake in subjects with and without obesity [10, 11].
In humans, GLP-1 secretion responds to positive energy balance acting as a compensatory or possibly a protective mechanism to reduce appetite and increase satiety. To date, only few studies have investigated the link between circulating GLP-1 concentrations and overfeeding in humans and the results have been conflicting. For instance, previous studies have shown no change in GLP-1 concentration in response to short term overfeeding [12, 13], whereas another study found a significant increase in circulating GLP-1 after 7 days of overfeeding diet with 70% above weight maintaining calories [14].
We sought to investigate the relationship between objectively measured ad libitum food intake and circulating plasma GLP-1 concentrations. The aims of the present study were to evaluate whether fasting GLP-1 concentrations predict subsequent food or macronutrient intake over a 3-day period of ad libitum food intake and whether the degree of overconsumption is related to changes in fasting GLP-1 concentrations.
Methods
Participants and study design
The volunteers in this analysis were part of a larger inpatient study investigating determinants of food intake (Clinical Trials # NCT00342732) [15] conducted between 2003 and 2015 at the Obesity and Diabetes Clinical Research Center of the NIDDK/NIH in Phoenix, AZ. The protocol was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Out of 250 subjects who completed the study, 115 (age < 55 years with BMI 30.3 ± 9.5) had available blood samples as well as measurements of ad libitum food intake. Due to interruption of the study between 2005 and 2009, we have two subsets of blood samples from individuals (before 2005 and after 2009).
Prior to admission all subjects signed written and informed consent. Based on medical history, physical examination and laboratory testing, all subjects had no evidence of medical diagnoses other than obesity and/or impaired glucose tolerance, were non-smokers, and were not currently taking medications.
On the day of admission to our metabolic unit, subjects were fed a standard weight maintaining diet (50% carbohydrates, 30% fat and 20% protein) for 3 days prior to any metabolic testing. For each individual, weight maintaining energy needs (WMEN) were calculated based on body weight and gender as previously demonstrated [16]. Glucose tolerance was assessed using a 75-g oral glucose tolerance test according to the American Diabetes Association criteria [17] and only data from individuals without diabetes were analyzed. Body composition (percentage of body fat, PFAT) was assessed using dual energy x-ray absorptiometry (DPX-1; Lunar Radiation Corp, Madison, Wisconsin) to calculate body fat mass (FM) and fat free mass (FFM).
Following four days of initial testing, ad libitum food intake was assessed for three days (described in detail below) (Supplemental, Figure S1). Blood samples were drawn at 530AM on day 7 of the study before starting the 3-day ad libitum vending machine paradigm (day 1 of ad libitum period) as well as on the discharge day (named day 4, after completion of 3-day ad libitum period). True overnight fasting measures can only be considered in the first blood draw due to the unlimited free access to food during the vending machine paradigm for the 3 consecutive days. Total GLP-1 was measured by immunoassay kit (version 2) from MSD (Rockville MD). The intra-assay CV was 3.32% and the inter-assay CV was 5.01%. Fasting insulin concentrations were assessed by using an automated immunoenzymometric assay (Tosoh Bioscience Inc., Tessenderlo, Belgium).
Ad libitum food intake
Measurement of ad libitum food intake was assessed over 3 days using a computerized vending machine paradigm which has been shown to be highly reproducible and valid as previously described [18]. On the day of the admission, a food selection questionnaire was provided to each volunteer to assess food preferences. Subjects were asked to rate each item using a 9-point Likert scale (1=dislike extremely, 5=neutral, 9=like extremely). 40 different foods given an intermediate rating were used to stock the vending machines for ad libitum food intake. Subjects were given free access to the computer-operated vending machine system for 3 days. Volunteers had 23.5h ad libitum access to select food items. All food was weighed prior to placement in the vending machines and returned food leftovers were also weighed to determine actual intake. The CBORD Professional Diet Analyzer Program (CBORD, Inc., Ithaca, NY, USA) and the Food Processor database (ESHA version 10.0.0, ESHA Research, Salem, OR, USA) were used to calculate the daily total and individual macronutrient kilocalories consumed. The recorded measurement of eating time during the 3 days of vending machine paradigm was able to identify night eaters, defined as individuals who ate after 1100 PM during the day 3 of ad libitum food intake. In these volunteers with nighttime eating episodes, GLP-1 measurements collected the morning (530 AM) on day 4 may be altered since they were not in a true fasting state.
The average of total ad libitum food intake over 3 days vending period was taken and expressed as total kcals eaten daily. Similar calculations were performed for the ratio to the WMEN prior to the 3-day vending period and for each macronutrient intake.
Macronutrient Categories
Six groups of food were categorized from each food on the vending machine questionnaire, based on the content of the macronutrient as a percentage of the total energy intake. Food categories were identified as low in fat (<20% kcal) or high in fat (≥45% kcal). Furthermore, food groups were categorized as high in protein (≥13% kcal), high in complex carbohydrates (≥30% kcal), high in simple sugars (≥30% kcal). From these classifications, six different groups were formed: low-fat/high-simple sugar (LF/HSS), low-fat/high-complex carbohydrate (LF/HCC), low-fat/high-protein (LF/HP), high-fat/high-complex carbohydrate (HF/HCC), high-fat/high-protein (HF/HP) and high-fat/high-simple sugar (HF/HSS) [19].
Statistical analysis
Analyses were performed using SAS software (SAS 9.3, Enterprise guide version 5.1; SAS Institute, Cary, NC). Data are expressed as means ± standard deviations. Normally distributed data are expressed as mean ± SD, and skewed data are presented as medians with 95% CI. Correlations between normally distributed and skewed variables were assessed with Pearson and Spearman correlation coefficients, respectively.
Fasting plasma GLP-1 and insulin data were log10 transformed to meet the assumptions of linear regression (i.e., homoscedasticity and normal distribution of residuals). Student t test or 1-way analysis of variance were used to assess the differences between groups. Due to the differences in time and conditions of sample storage, fasting GLP-1 at baseline (pre-ad libitum food intake period) was adjusted for cohort (before 2005 and after 2009).
To assess determinants of fasting plasma GLP-1 prior to ad libitum food intake, we used a multivariate linear regression model including age, sex, FM, FFM, and race.
Glucose area under the curve (AUC) and insulin AUC were calculated using the trapezoidal method. A linear model was used to correlate the log fasting GLP-1 prior to 3-day ad libitum energy intake with glucose AUC and insulin AUC (adjusting for fasting glucose during oral glucose tolerance test). Similar analyses were performed to evaluate whether the fasting GLP-1 response on day 1 to glucose and insulin was different between ethnicities.
Change in GLP-1 concentrations was defined as the difference between morning GLP-1 measured on day 4 (after 3-day ad libitum food intake period) and GLP-1 measured on day 1 (prior to ad libitum food intake period).
Linear regression analysis was used to calculate residuals of total ad libitum food intake after adjustments for age, sex, FFM, and FM. Similar analyses were performed to calculate residuals of individual macronutrient intakes and for the different food groups. Linear regression models were used to evaluate the relationships between fasting GLP-1 prior to ad libitum intake and residuals of total ad libitum food intake, macronutrients (fat, carbohydrate, or protein) intake and different food groups after adjustment for age, gender, FM, FFM. Similar linear regression models were used to correlate change in plasma GLP-1 concentrations (before and after ad libitum period) and residuals of ad libitum food intake and macronutrients.
Results
Baseline characteristic
General and anthropometric characteristics for all subjects are reported in Table 1. On average, volunteers were young (34.9 ± 10.4 yrs), were overweight (30.1±6.9 kg/m2), and the expected differences in body composition between males and females were observed. Twenty-seven out of 115 individuals had at least one episode of nighttime eating during the last day of the ad libitum period, characterized as food intake between 2300 at 0500. There was no significance difference between nighttime eaters and non-nighttime eaters in age, weight or body composition (FM, FFM and percent of body fat). In the analysis of change in GLP-1, there was no difference in the results if nighttime eaters were excluded from the analysis. Night eaters were excluded from the analysis when examining the relationship between the last day (day 3) of the ad libitum period and plasma GLP-1 on day 4.
Table 1.
Demographic and anthropometric measures of the study group.
| Whole study group (n=115) |
Men (n=72) |
Women (n=43) |
P* | Night eaters (n=27) |
|
|---|---|---|---|---|---|
| Ethnicity | 10 AA, 42 W, 9 H, 47 NA, O 9 |
5 AA, 27 W, 6 H, 27 NA, 7 O |
5 AA, 13 W, 3 H, 20 NA, 2 O |
0.5 | 4 AA, 12 W, 1 H, 10 NA |
| Sex (F/M) | 43/72 | 72 | 43 | ||
| Age (years) | 34.9 ± 10.4 | 34.8 ± 10.7 | 34.5 ± 10.0 | 0.8 | 31.0 ± 10.5 |
| Body weight (kg) | 86.9 ± 20.6 | 89.0 ± 19.5 | 83.6 ± 22.1 | 0.1 | 82.3 ± 16.7 |
| BMI (kg/m²) | 30.1 ± 6.9 | 28.8 ± 6.1 | 32.2 ± 8.0 | 0.04 | 28.1 ± 6.7 |
| FFM (kg) | 59.8 ± 12.5 | 64.8 ± 10.5 | 50.5 ± 10.3 | <.0001 | 59.2 ± 9.1 |
| FM (kg) | 27.1 ± 12.2 | 23.9 ± 10.7 | 33.0 ± 12.9 | <0.001 | 23.1 ± 12.0 |
| Body fat (%) | 30.2 ± 8.9 | 25.8 ± 6.9 | 38.3 ± 6.8 | 0.0002 | 26.7 ± 10.6 |
| Fasting glucose (mg/dL) |
93.1 ± 6.5 | 92.5 ± 7.4 | 95.8 ± 8.4 | 0.7 | 89.2 ± 5.9 |
| 2-h glucose (mg/dL) | 127.6 ± 29.2 | 121.5 ± 28.8 | 131.9 ± 27.5 | 0.07 | 124.7 ± 27.4 |
| Fasting GLP-1 pre (pg/mL)1 |
14.4 (12.9–16.0) | 15.4 (13.4–17.3) | 12.9 (10.3–15.5) | 0.1 | 13.27 (10.2–16.4) |
| Fasting GLP-1 post (pg/mL)2 |
16.8 (15.1–18.5) | 18.2 (15.9–20.5) | 14.6 (12.2–17.0) | 0.03 | 17.4 (14.7–20.1) |
| Fasting OGTT Insulin3 |
11.7 (9.8–13.7) | 11.6 (8.6–14.4) | 12.1 (9.9–14.2) | 0.8 | 9.5 (6.7–12.3) |
| Fasting insulin pre (mU/L)1 |
11.9 (9.9–13.8) | 11.5 (8.7–14.2) | 12.7 (10.3–15.0) | 0.3 | 10.0 (4.5–13.5) |
| Fasting insulin post (mU/L)2 |
15.7 (11.9–19.4) | 14.9 (11.2–18.7) | 17.1 (8.6–25.5) | 0.4 | 22.0 (6.5–37.7) |
Table 1. Data are presented as the mean ± SD, unless otherwise indicated. AA, African American; H, Hispanic; NA, Native American; W, white: O, other
P values are for differences between male/female groups as determined by Student t test.
Hormones concentration was measured in fasting state (530 AM) on day 7 before starting the day ad libitum vending machine paradigm (day 1 of ad libitum period).
Hormones concentration was measured in fasting state (530 AM) on day 10 (discharge day) after completion of 3-day ad libitum period.
Fasting insulin concentration was measured in fasting state during OGTT.
Data are log expressed with mean ± 95% confidence limits.
Fasting GLP-1 concentrations and body composition
Mean plasma GLP-1 concentrations on day 1 and day 4 were 12.5 pg/ml (CI 95%: 11.4 to 13.8) and 14.7 pg/ml (CI 95%: 13.3 to 16.2), respectively. The fasting GLP-1 concentration on day 1 was not correlated with body weight (p = 0.13), FFM (p = 0.16), FM (p = 0.29), PFAT (p = 0.5) or BMI (p = 0.23) after adjustment for differences in individual storage time. Additionally, the pre-ad libitum feeding GLP-1 concentrations did not differ significantly by race and sex.
Glucose regulation and association with GLP-1 concentrations
After adjustment for all covariates (age, sex, percentage of body fat and cohorts), fasting GLP-1 concentration before the ad libitum period was associated with glucose AUC (r = 0.2, p = 0.01) and with fasting insulin concentrations (r = 0.5 p = 0.03). Fasting GLP-1 concentration was also associated with insulin AUC after further adjustment for glucose AUC (r = 0.54 p = 0.002) during OGTT and with fasting insulin measured on day 1 ad libitum period (r = 0.33, p = 0.009). In those with normal glucose regulation (n = 91), we did not find any correlation between fasting insulin and 2 h insulin and morning GLP-1 when we stratified by race (Native Americans, Caucasian and other races).
Association of ad libitum food and macronutrient intake with GLP-1 concentrations
Energy intake data are described in Table 2. Average 3-day ad libitum energy intake was 3849±1431 Kcal/d (range: 1273 to 9544 Kcal/d) and 138.7 ± 45.4 % expressed as %WMEN. Fasting day 1 GLP-1 concentrations (prior to ad libitum period and adjusted for cohort) were not associated with residuals of total food intake (r = −0.15, p = 0.12, Figure 1A), residuals of fat intake (r = −0.06, p = 0.5, Figure 1C) or residuals of protein intake (r = −0.05, p = 0.5, Figure 1D), but was negatively associated with residuals of carbohydrate intake (r = −0.2, p = 0.03, Figure 1B). Consistent with this finding, day 1 fasting GLP-1 concentrations were negatively associated with daily energy intake from LF/HSS (r = − 0.22, p = 0.016, Figure 2A) but not with any of the other groups, LF/HCC, LF/HP, HF/HP, HF/HSS and HF/HCC (all p >0.3, Figure 2 B-C-D-E-F, respectively). Results were similar after adjustment for total daily energy intake. Similarly, no changes in GLP-1 results were observed after further adjustment for fasting insulin measured on day 1 (first day of ad libitum period). There was no association between morning plasma GLP1 concentrations (measured on day 1 and defined as baseline) and residuals of food intake (p = 0.56).
Table 2.
Ad Libitum Food Intake Measures of the Study group
| Whole study group (n=115 |
Men (n=72) |
Women (n=43) |
p | Night eaters (n=27) |
p | |
|---|---|---|---|---|---|---|
| Computerized vending machine system | ||||||
|
Total energy intake (Kcal/day) |
3849.3 ± 1431 | 4272.7 ± 1395.6 | 3130.5 ± 1195.3 | <0.001 | 4270.6 ± 1327.9 | 0.2 |
|
Total energy intake (% WMEN) |
138.7 ± 45.4 | 149.8 ± 43.2 | 119.9 ± 44.0 | <0.001 | 155.9 ± 44.4 | 0.08 |
| CHO intake (kcal/day) | 495.9 ± 176.3 | 551.4 ± 168.3 | 401.7 ± 148.7 | <0.001 | 550.8 ± 159.8 | 0.1 |
| FAT intake (kcal/day) | 162.79± 75.0 | 180.6 ± 76.4 | 132.9 ± 62.6 | <0.001 | 131.8 ± 38.9 | 0.3 |
| PRO intake (kcal/day) | 117.1 ± 43.7 | 127.9 ± 42.8 | 98.8 ± 39.2 | <0.001 | 179.6 ± 73.8 | 0.1 |
| HF/HCC (kcal/day) | 473.3 ± 400.3 | 545.8 ± 461.9 | 351.9 ± 223.9 | 0.003 | 499.4 ± 379.8 | 0.7 |
| HF/HP (kcal/day) | 1054.2 ± 589 | 1201.5 ± 603.6 | 807.7 ± 475.6 | 0.002 | 1139.4 ± 665.3 | 0.2 |
| HF/HSS (kcal/day) | 713.6 ± 535.3 | 774 ± 483.6 | 612.5 ± 604.7 | 0.14 | 898 ± 682.9 | 0.4 |
| LF/HCC (kcal/day) | 307.5 ± 193.9 | 323.9 ± 201.8 | 280.1 ± 178.7 | 0.2 | 265.6 ± 167 | 0.2 |
| LF/HP (kcal/day) | 437.5 ± 210.9 | 475.5 ± 197.1 | 374 ± 220. | 0.01 | 523.1 ± 221.1 | 0.2 |
| LF/HSS (kcal/day) | 683.3 ± 327.4 | 773.6 ± 311.6 | 532.1 ± 299.2 | <0.001 | 761.4 ± 355.1 | 0.1 |
Data are presented as mean±SD, unless otherwise indicated.
Ad libitum food intake measures are reported as the average of three days on the vending machines. HF=High Fat; HCC=High Complex Carbohydrate; HP=High Protein; HSS=High Simple Sugar; LF=Low Fat
Figure 1.
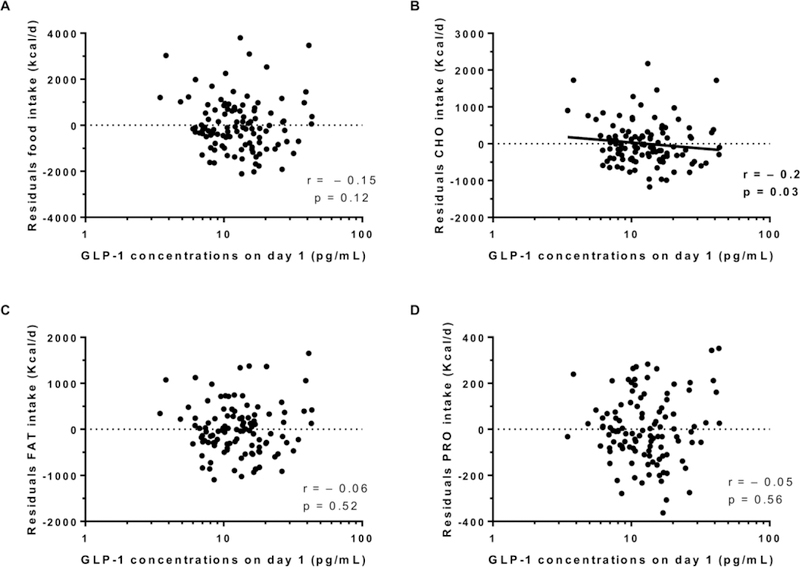
Relationship between morning GLP-1 (day 1) and residuals of total food intake (A, carbohydrates intake (B), fat intake (C) and protein intake (D). The total ad libitum food intake during the 3-day vending period is expressed as the average over 3 days in Kcal per day. In each panel, the Pearson’s correlation coefficient (r) is reported along with its significance (p). All correlations were adjusted for storage time. The plasma GLP-1 is expressed in logarithmic scale.
Figure 2.
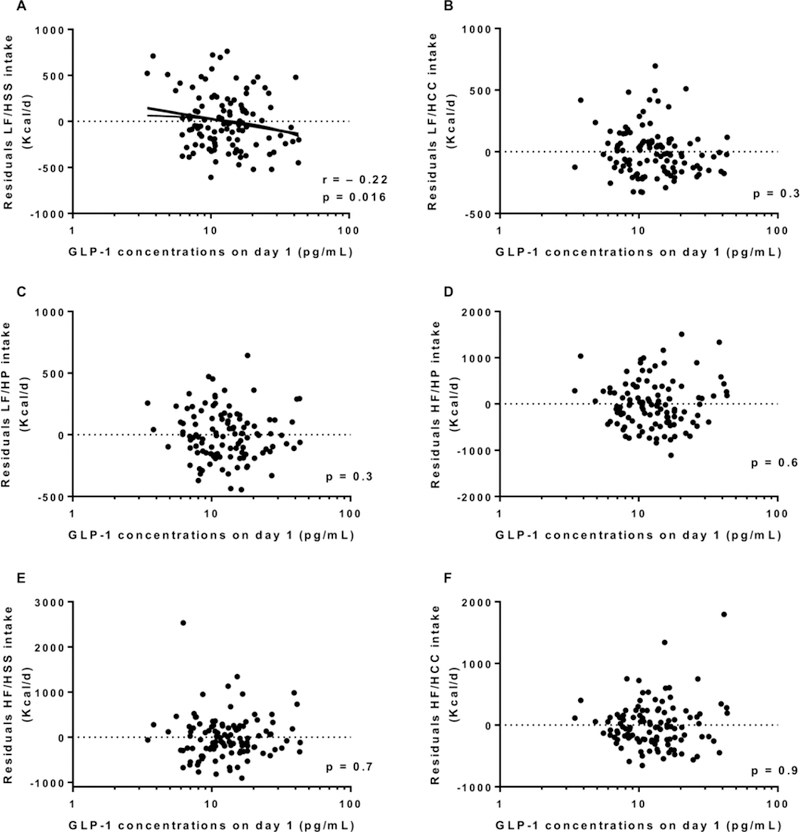
Relationship between day 1 GLP-1 and 3-day daily average of energy intake from the LF/HSS food group (A), the LF/HCC food group (B), the LF/HP food group (C), the HF/HP food group (D), the HF/HSS food group (E) and the HF/HCC food group (F).
GLP-1 concentrations increased by 1.8 pg/ml (CI 95%: 0.4 to 3.3, p = 0.01, Figure 3) after 3 days of ad libitum food intake (an increase of nearly 14 %) with no difference according to gender (p=0.7) and ethnicity (p=0.2). We observed a positive correlation between change in GLP-1 concentrations with the residuals of total food intake (r = 0.25, p = 0.002, Figure 4A), carbohydrate intake (r = 0.21, p = 0.0006, Figure 4B), protein intake (r = 0.24, p = 0.012, Figure 4C) and fat intake (r = 0.22, p = 0.017, Figure 4D). Similar results were observed after adjustment for the change in fasting insulin from day 1 to day 3 of ad libitum period. No associations were found between change in GLP-1 concentrations and daily energy intake from the 6 food groups. There was no association between ad libitum food intake on day 3 and the morning plasma GLP-1 measured on day 4 (p = 0.09).
Figure 3. Change in plasma GLP-1 concentrations pre- and post-ad libitum food intake.
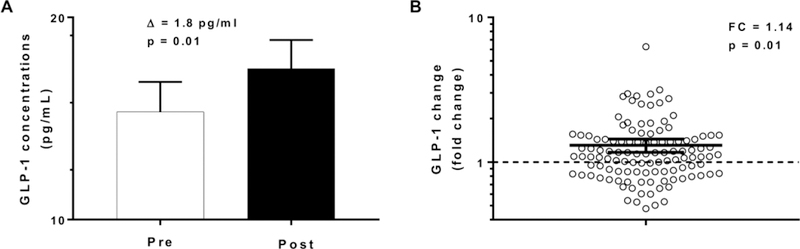
Panel A, the white bar represents GLP-1 concentrations on day 1 (PRE, prior to 3-day ad libitum period) and the black bar represents GLP-1 concentrations on day 4 (POST, after 3-day ad libitum period). The βs indicate the absolute values of the change in GLP-1 concentrations between pre and post ad libitum food intake.
Panel B, fold change in GLP-1 from day 4 to day 1. The dotted line represents, in a logarithmic scale, the separation between individuals who increase GLP-1 concentration (above the line) and individuals who decrease GLP-1 (below the line).
The night eaters (n=27) were excluded from this analysis
Error bars represent the % confidence interval of the mean.
Figure 4.
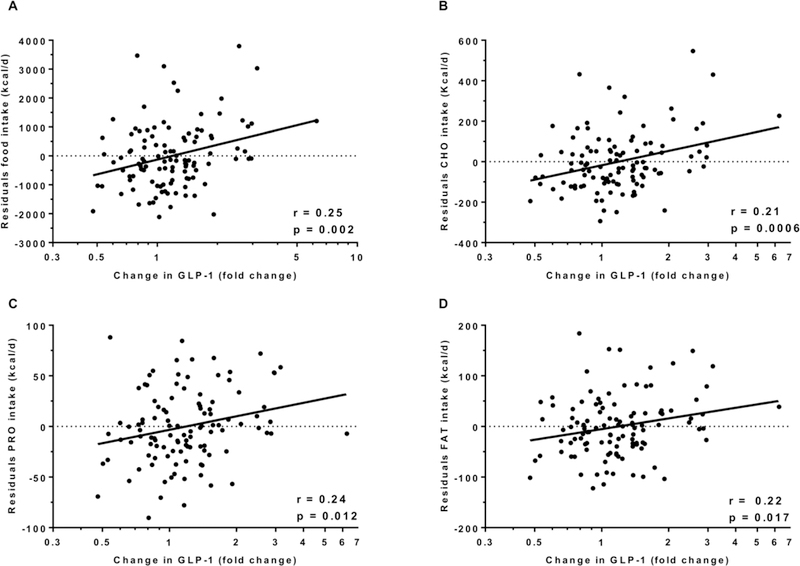
Change in GLP-1 and total food intake (A), carbohydrates intake (B), protein intake (C) and fat intake (D).
In each panel, the Pearson’s correlation coefficient (r) is reported along with its significance (p).
The night eaters on day 3 (n=27) were excluded from this analysis
Discussion
In the current study, we investigated whether fasting GLP-1 concentrations was associated with subsequent ad libitum total energy and macronutrient intake and the response of circulating GLP-1 concentrations during this ad libitum period in 115 non-diabetic individuals. We found that fasting GLP-1 concentration prior to ad libitum food intake was a negative predictor of carbohydrate intake and was also negatively associated with daily energy intake from foods categorized as high simple sugar-low fat but did not predict either the total daily food intake or the fat and protein intake. Fasting GLP-1 concentrations also increased in association with degree of overfeeding across all macronutrient groups.
Previous studies have demonstrated an effect of GLP-1 receptor agonists on energy intake [10, 20] through both central and peripheral mechanisms in rats and humans [21, 22]. Our findings that GLP-1 was negatively associated with carbohydrate and LF/HSS intake and not total energy intake indicates a possible different mechanism by which GLP-1 regulates intake and may be explained by the potential role of GLP-1 in food reward and satiety. Recently, the gut-brain axis has been identified as a possible mediator of satiety and food reward [23]. Lower responsiveness to palatable food in reward regions might lead to overeating to attempt to compensate for the relative hypostimulation of these regions [24]. GLP-1 receptor agonists potently decreased the intake of palatable carbohydrates in lean and obese mice due to a hypothesized role of GLP-1 in the food reward system [25]. In line with this finding, we observed that higher circulating GLP-1 concentrations predict lower ingestion of carbohydrates, mainly high simple sugars, suggesting GLP-1 as possible mediator in the food reward system.
Hypothalamic and brainstem nuclei have been identified as GLP-1 targets for anorexic signals [26, 27]. However, GLP-1 receptors have also been identified in mesolimbic areas, such as the ventral tegmental area with its dopaminergic projections to the striatum and in the nucleus accumbens, both of which are implicated in reward behavior [28–30]. Thus, GLP-1 may convey signals from the gut about the nutritional status of the body to the brain [30, 31].
Another possible mechanism is GLP1 signaling mediation sweet taste perception [32] and that sweet taste receptors may play an important role in both food intake and glucose regulation. Sweet taste receptors (TRC), found in the oral cavity [33] and in the gastrointestinal tract [34], are activated by sugars, convey signals to the brain via sensory afferent neurons [35] and are involved in the central processing of food reward [36]. Brain centers may receive signals from the gastrointestinal tract via the vagal nerve coordinating with satiety hormones, such as GLP-1, to “alert” the digestive system for incoming carbohydrate intake [37]. Our results might suggest a possible role for GLP-1 in the reward system by increasing “sensitivity” to reward circuitry of carbohydrate intake and, hypothetically, this heightened sensitivity may occur via simple sugars ingestion leading to GLP-1 secretion and activation of neuronal afferent fiber.
In our study GLP-1 concentrations significantly increased by 14% after 3-days of ad libitum intake in which the subjects ate almost 40% more than their weight maintaining energy needs. This was not due to an effect of energy intake on the last day (day 3) of the ad libitum period on the day 4 GLP-1 concentrations, thus indicating a sustained effect of overeating.
GLP-1 concentrations are known to increase in the context of single mixed meal studies but the relationship between circulating GLP-1 concentrations and long term food intake in humans is controversial. We have found a more pronounced effect of energy intake on GLP-1 changes than was previously reported. Moreover, we have shown that this increase is directly proportional to the degree of overfeeding using a validated reproducible ad libitum paradigm [18].
Consistent with our results, Wadden et al [14] showed an increase in GLP-1 concentrations in a cohort of 72 males after a 7-day overfeeding period, but others have not demonstrated this effect. After five days of high-fat diet overfeeding, fasting GLP-1 concentrations did not change [13] and no significant changes in GLP-1 levels were found after an overfeeding liquid diet in lean males [38]. In a previous study from our group [39], we also did not observe any changes in GLP-1 concentrations after the ad libitum period in a smaller sample size (n=30). In the current study, however, we were able to more precisely quantify GLP-1 response in a larger cohort, demonstrating an important effect of ad libitum intake on change in GLP-1.
GLP-1 is the most potent incretin hormone involved in the regulation of glucose stimulated-insulin secretion and its differential secretion may play a role in the reported racial differences in glucose regulation after a meal ingestion. Previous groups have reported racial differences, especially in regard to the interaction between GLP-1 and glucose regulation [40–42]. However, in our study, after stratifying by race, we did not observe any association between incretin secretion and glucose dependent insulin secretion.
One of the strengths of the present study is the large cohort of individuals with a variety of ages and ethnicities, making this investigation one of the larger studies to examine circulating GLP-1 as a predictor of and in response to short term ad libitum intake in humans. Furthermore, no prior studies have shown an association between the degree of change in circulating GLP-1 concentrations and ad libitum food intake. We were also able to exclude night eaters from the analysis, due to the precise measurement of eating time recorded by vending machine system, yet still measure the effect of GLP-1 post ad libitum feeding.
However, several limitations must be acknowledged. Due to the very short half-life of active GLP-1 and due to its rapid degradation, we measured total GLP-1, which gives an indication of the secretion from intestinal cells. However, active GLP-1 has been previously shown to positively correlate with total GLP-1 [43]. Additionally, during the ad libitum food intake period on the vending machines, some volunteers had at least one episode of nighttime eating and, thus, the morning GLP-1 measured on day 4 was not have been drawn in “fasting” state for these individuals. Yet, because of the precise timing measurements of the vending machine paradigm, we were able to exclude those volunteers when necessary. Unfortunately, we did not have assessments of levels of hunger or satiety during the ad libitum period, which might be helpful to further understand the relationship between GLP-1 and reward mechanisms of palatable food.
Conclusion
In conclusion, we investigated whether fasting plasma GLP-1 predicted energy intake and its response to short term ad libitum food intake in 115 individuals without type 2 diabetes. Fasting GLP-1 concentrations measured prior to ad libitum period was a negatively associated with carbohydrate intake and percentage of LF/HSS foods. We also observed a significant increase in GLP-1 concentrations after 3 days of ad libitum overeating. Our results indicate that GLP-1 may have a role in central reward processing limiting the desire to ingest processed high carbohydrate foods while also serving as a protective mechanism against ongoing overeating following energy intake excess.
Supplementary Material
Acknowledgment
The authors thank the volunteers who participated in our studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations. Also, the authors thank the metabolic kitchen stuff. This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors have nothing to disclose.
Funding. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
ClinicalTrials.gov identifier: NCT00342732
Abbreviations:
- GLP-1
Glucagon-like peptide 1
- FM
fat mass
- FFM
fat free mass
- OGTT
oral glucose tolerance test
- DXA
dual energy X-ray absorptiometry
- CNS
central nervous system
- LF
low fat
- HSS
high simple sugar
- HF
high fat
- HCC
high complex carbohydrate
- HP
low fat/high protein
Footnotes
Disclosure statement: The authors have nothing to disclose
Conflict of interest. The authors have no conflict of interest to declare.
Prior presentation. The study has not been published previously in abstract form or manuscript.
References
- [1].Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. New England Journal of Medicine 2011; 364: 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ravussin E, Bogardus C. Energy balance and weight regulation: genetics versus environment. British Journal of Nutrition 2000; 83: S17–S20 [DOI] [PubMed] [Google Scholar]
- [3].Poggiogalle E, Donini L, Chiesa C, et al. Does endogenous GLP-1 affect resting energy expenditure and fuel selection in overweight and obese adults? Journal of endocrinological investigation 2018; 41: 439–445 [DOI] [PubMed] [Google Scholar]
- [4].Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY 3–36 and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal– brainstem–hypothalamic pathway. Brain research 2005; 1044: 127–131 [DOI] [PubMed] [Google Scholar]
- [5].Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. Journal of Clinical Investigation 1998; 101: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Holst JJ. The physiology of glucagon-like peptide 1. Physiological reviews 2007; 87: 1409–1439 [DOI] [PubMed] [Google Scholar]
- [7].Kreymann B, Ghatei M, Williams G, Bloom S. Glucagon-like peptide-1 7–36: a physiological incretin in man. The Lancet 1987; 330: 1300–1304 [DOI] [PubMed] [Google Scholar]
- [8].De Heer J, Rasmussen C, Coy D, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 2008; 51: 2263. [DOI] [PubMed] [Google Scholar]
- [9].Alssema M, Rijkelijkhuizen JM, Holst JJ, et al. Preserved GLP-1 and exaggerated GIP secretion in type 2 diabetes and relationships with triglycerides and ALT. European journal of endocrinology 2013; 169: 421–430 [DOI] [PubMed] [Google Scholar]
- [10].Verdich C, Flint A, Gutzwiller J-P, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. The Journal of Clinical Endocrinology & Metabolism 2001; 86: 4382–4389 [DOI] [PubMed] [Google Scholar]
- [11].Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. The Lancet 2002; 359: 824–830 [DOI] [PubMed] [Google Scholar]
- [12].Parry SA, Smith JR, Corbett TR, Woods RM, Hulston CJ. Short-term, high-fat overfeeding impairs glycaemic control but does not alter gut hormone responses to a mixed meal tolerance test in healthy, normal-weight individuals. British Journal of Nutrition 2017; 117: 48–55 [DOI] [PubMed] [Google Scholar]
- [13].Brøns C, Jensen CB, Storgaard H, et al. Impact of short‐term high‐fat feeding on glucose and insulin metabolism in young healthy men. The Journal of physiology 2009; 587: 2387–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wadden D, Cahill F, Amini P, et al. Circulating glucagon-like peptide-1 increases in response to short-term overfeeding in men. Nutrition & metabolism 2013; 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Piaggi P, Thearle MS, Krakoff J, Votruba SB. Higher daily energy expenditure and respiratory quotient, rather than fat-free mass, independently determine greater ad libitum overeating. The Journal of Clinical Endocrinology & Metabolism 2015; 100: 3011–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. The American journal of clinical nutrition 1991; 53: 1368–1371 [DOI] [PubMed] [Google Scholar]
- [17].Gavin JR III, Alberti K, Davidson MB, DeFronzo RA. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care 1997; 20: 1183. [DOI] [PubMed] [Google Scholar]
- [18].Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. The American journal of clinical nutrition 2010; 91: 343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiology & behavior 1998; 63: 919–928 [DOI] [PubMed] [Google Scholar]
- [20].Basolo A, Burkholder J, Osgood K, et al. Exenatide has a Pronounced Effect on Energy Intake but not Energy Expenditure in Non-Diabetic Subjects with Obesity: A Randomized, Double-blind, Placebo-Controlled Trial. Metabolism 2018: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analogue, liraglutide. International journal of obesity (2005) 2012; 36: 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Näslund E, Barkeling B, King N, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. International Journal of Obesity & Related Metabolic Disorders 1999; 23: [DOI] [PubMed] [Google Scholar]
- [23].Zanchi D, Depoorter A, Egloff L, et al. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neuroscience & Biobehavioral Reviews 2017; 80: 457–475 [DOI] [PubMed] [Google Scholar]
- [24].Wang G-J, Volkow ND, Thanos PK, Fowler JS. Imaging of brain dopamine pathways: implications for understanding obesity. Journal of addiction medicine. 2009; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pritchett CE, Hajnal A. Glucagon‐Like Peptide‐1 Regulation of Carbohydrate Intake Is Differentially Affected by Obesogenic Diets. Obesity 2012; 20: 313–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sandoval DA, Bagnol D, Woods SC, D’alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 2008; 57: 2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 2009; 150: 2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Merchenthaler I, Lane M, Shughrue P. Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. Journal of Comparative Neurology 1999; 403: 261–280 [DOI] [PubMed] [Google Scholar]
- [29].Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PloS one 2015; 10: e0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. Journal of Neuroscience 2012; 32: 4812–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hayes MR, Kanoski SE, De Jonghe BC, et al. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2011; 301: R1479–R1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shin YK, Martin B, Golden E, et al. Modulation of taste sensitivity by GLP‐1 signaling. Journal of neurochemistry 2008; 106: 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr 2007; 27: 389–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Young RL. Sensing via intestinal sweet taste pathways. Frontiers in neuroscience 2011; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sclafani A Sweet taste signaling in the gut. Proceedings of the National Academy of Sciences 2007; 104: 14887–14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zheng H, Berthoud H- R. Eating for pleasure or calories. Current opinion in pharmacology 2007;7: 607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Berthoud H-R, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol 2008; 59: 55–92 [DOI] [PubMed] [Google Scholar]
- [38].Brands M, Swat M, Lammers NM, et al. Effects of a hypercaloric diet on β‐cell responsivity in lean healthy men. Clinical endocrinology 2013; 78: 217–225 [DOI] [PubMed] [Google Scholar]
- [39].He J, Votruba S, Pomeroy J, Bonfiglio S, Krakoff J. Measurement of ad libitum food intake, physical activity, and sedentary time in response to overfeeding. PLoS One 2012; 7: e36225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Velasquez-Mieyer P, Cowan P, Umpierrez G, Lustig R, Cashion A, Burghen G. Racial differences in glucagon-like peptide-1 (GLP-1) concentrations and insulin dynamics during oral glucose tolerance test in obese subjects. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 2003; 27: 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yabe D, Kuroe A, Lee S, et al. Little enhancement of meal‐induced glucagon‐like peptide 1 secretion in Japanese: comparison of type 2 diabetes patients and healthy controls. Journal of diabetes investigation 2010; 1: 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sleddering MA, Bakker LE, Janssen LG, Meinders AE, Jazet IM. Higher insulin and glucagon-like peptide-1 (GLP-1) levels in healthy, young South Asians as compared to Caucasians during an oral glucose tolerance test. Metabolism 2014; 63: 226–232 [DOI] [PubMed] [Google Scholar]
- [43].Heijboer AC, Frans A, Lomecky M, Blankenstein MA. Analysis of glucagon-like peptide 1; what to measure? Clinica Chimica Acta 2011; 412: 1191–1194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


