Abstract
Although schizophrenia is considered a brain disorder, the role of brain organization for symptomatic improvement remains inadequately defined. We investigated the relationship between baseline brain morphology, resting-state network connectivity and clinical response after 24-weeks of antipsychotic treatment in patients with schizophrenia (n=95) using integrated multivariate analyses. There was no significant association between clinical response and measures of cortical thickness (r=0.37, p=0.98) and subcortical volume (r=0.56, p=0.15). By contrast, we identified a strong mode of covariation linking functional network connectivity to clinical response (r=0.70; p=0.04), and particularly to improvement in positive (weight=0.62) and anxious/depressive symptoms (weight=0.49). Higher internal cohesiveness of the default mode network was the single most important positive predictor. Key negative predictors involved the functional cohesiveness of central executive subnetworks anchored in the frontoparietal cortices and subcortical regions (including the thalamus and striatum) and the inter-network integration between the default mode and sensorimotor networks. The present findings establish links between clinical response and the functional organization of brain networks involved both in perception and in spontaneous and goal-directed cognition, thereby advancing our understanding of the pathophysiology of schizophrenia.
Introduction
Schizophrenia features amongst the leading causes of disability worldwide1 because it is associated with chronicity,2,3 psychosocial disadvantage4,5 and reduced life expectancy6,7. The five years following the first psychotic episode are considered a critical period as the long-term pattern of the disease course is typically established during this time8,9. Prior research on the prognostic significance of clinical and demographic features has highlighted the importance of symptomatic improvement in response to antipsychotic treatment both in clinical trials and in observational studies10–13. Although schizophrenia is reliably associated with alterations in brain structure14–16 and functional connectivity14,17,18 the relevance of brain organization for symptomatic response is poorly understood. Magnetic resonance imaging (MRI) studies have produced encouraging results in terms of identifying prognostic biomarkers in other brain disorders, such as epilepsy19 and Alzheimer’s disease20 and this evidence motivates research efforts to identify brain imaging predictors of treatment response in schizophrenia.
The neuroimaging literature thus far has been encouraging but inconclusive. Despite negative results21–24, several structural (sMRI) studies have linked poor treatment response to reductions in gyrification25 in global26, prefrontal27 and temporal28 cortical gray matter, and in parahippocampal29,30 and striatal volumes31. The functional (fMRI) literature is silent about the role of large-scale brain networks since the few available studies have each examined the connectivity of specific regions of interest. Specifically, Nejad and colleagues32 reported that baseline frontoparietal connectivity during a working-memory task distinguished patients with persistent and non-persistent negative symptoms after 6 months of antipsychotic treatment. Kraguljac and colleagues33 found that higher baseline hippocampal connectivity with the dorsal anterior cingulate, caudate, and auditory cortex coupled with lower connectivity with the lingual gyrus predicted response after 6 weeks of antipsychotic treatment. Finally, Sarpal and colleagues34 showed that antipsychotic response at 12 weeks was associated with lower striatal connectivity with the anterior cingulate and medial prefrontal cortex and higher striatal connectivity with posterior brain regions.
There are at least four challenges to be addressed in moving the field forward. First, previous studies divided patients into subgroups using various definitions of response derived from consensus statements (e.g., Remission in Schizophrenia Working Group35). Here we adopt a dimensional rather than a dichotomous approach because regardless of the specific definition, these categorical subgroups do not have meaningful differences in symptom severity and do not resonate with the experience of patients and carers36. Second, we expand previous literature by considering the entire spectrum of psychopathology in schizophrenia, which includes depressive/anxious symptoms and agitation/disorganization37 in addition to positive and negative symptoms. This dimensional symptom structure is present at the first psychotic episode and is longitudinally stable37 (further details in supplemental information (SI)). Therefore, inclusion of all four-symptom dimensions should be integral to any attempts to relate neuroimaging measures to clinical improvement. Third, our analyses also consider body mass index (BMI) and IQ, that represent robust indices of cardiometabolic health38 and cognition39,40, respectively, are strongly associated with brain organization41 and with symptom severity42,43. Inclusion of these key variables when modeling the relationship of neuroimaging features to clinical response increases confidence that the findings reflect schizophrenia–related neurobiological processes. Fourth, we extend the scope of enquiry beyond testing regionally specific hypotheses is critical for the detection of functional connectivity predictors of clinical response that are not a priori predicted.
Here, we acquired sMRI and resting-state functional (rs-fMRI) neuroimaging data from patients at the early stages of schizophrenia prior to initiation of antipsychotic treatment. Clinical response was assessed in terms of change in positive, negative, anxious/depressive and agitation/disorganization symptoms 24 weeks later. IQ and BMI were also assessed as part of the clinical dataset. We used sparse Canonical Correlation Analyses (sCCA)44 to investigate the underlying relationship between the neuroimaging and clinical datasets as this multivariate method is ideally suited for predicting one dataset from the other while accounting for correlations between variables45,46. Our aim of this data-driven approach was to identify previously undetected associations between whole-brain structural and functional neuroimaging features and clinical response following treatment. When considering preexisting evidence our working hypotheses were that measures of cortical thickness and of functional integration between cortical and subcortical regions would emerge as the most significant predictors of symptomatic change.
Materials and Method
Study Design
Patients within the first 5 years of being diagnosed with schizophrenia based on the criteria of the Diagnostic and Statistical Manual of Mental disorders, Fifth Edition (DSM-5)47 were recruited via clinician referrals from the psychiatric services of the Mount Sinai Health System at the Icahn School of Medicine at Mount Sinai (ISMMS), New York. Clinicians were asked to refer patients who were willing to re-start antipsychotic treatment following a minimum 2-week medication free period based on patients’ self-report. Patients were screened to exclude individuals with IQ<70 and those with treatment refractory illness, history of head trauma or loss of consciousness, pervasive developmental disorder, lifetime history of DSM-5 substance use disorder, medical or neurological disorders that could cause psychiatric symptoms and contraindications to MRI. Eligible patients were enrolled prior to restarting antipsychotic treatment. Brain MRI scans were acquired as soon as patients could tolerate this examination to ensure minimal exposure to antipsychotic medications. Patients were evaluated clinically on the day of scanning and then 24 weeks later. The study was approved by the institutional review board of the ISMMS; all participants provided written informed consent prior to study enrolment.
Clinical Assessment
Enrollment only: The patients’ diagnostic status was confirmed with the Structured Clinical Interview for DSM-548. Current IQ was obtained with the Wechsler Abbreviated Scale of Intelligence (WASI-II)49. IQ was used as an index of overall cognitive ability because it correlates with domain-specific cognitive tests39 and accounts for most of the variability in cognitive function40. In the context of schizophrenia, IQ is also associated with variability in the clinical profile and brain structure of patients41,50.
Day of scanning and at 24-week follow-up: Psychopathology was assessed using the 24-item Brief Psychiatric Rating Scale (BPRS)51 because it can reliably assess positive and negative symptoms, agitation/disorganization and anxiety/depression52 (Supplementary Table 1). Medication type and dose were recorded in all patients and supplemented with information from their medical records. The daily antipsychotic dose was converted to chlorpromazine equivalents (CPZE)53. The BMI was included as a general measure of cardiometabolic health38 with known associations with symptom severity42 and brain structure in patients with psychosis41.
Treatment and Evaluation of Clinical Outcome
The choice and dosing of the antipsychotic agent was based on the judgment of the patients’ treating psychiatrists since differences in the effectiveness of individual antipsychotics are small54,55 and appropriate treatment decisions crucially depend on patients’ history, preference and circumstances. During the inter-assessment interval, patients received routine clinical care which consisted of outpatient appointments with their treating psychiatrist. These appointments involved informal supportive therapy and monitoring of mental state, adverse events and adherence. Patients were seen weekly for the first three weeks post-treatment initiation. Thereafter, planned appointments occurred depending on individual patient’s needs and preferences. Adherence was monitored via self-report during appointments and though the Mount Sinai electronic prescription records (also in SI). None received psychological or electroconvulsive therapy. Additional support was provided by social workers in connection to disability benefits and accommodation. The primary outcome was the change in the BPRS subscale scores for positive, negative, anxious/depressive and agitation/disorganization symptoms between initial (T1) and 24-week follow-up assessment (T2) calculated as ; positive and negative values respectively represent symptomatic improvement or worsening. We adopted a dimensional approach to symptomatic change rather than dichotomous remission/non-remission categorizations for the following reasons: (i) although there are several criteria for remission there is still no consensus on the cut-offs that define remission in schizophrenia; (ii) it is questionable whether clinically defined remitted and non-remitted patients (regardless of the criteria applied) represent biologically distinct groups; (iii) although there is some evidence that treatment resistant patients may have a distinct neurobiological profile such patients were excluded here by design. In support of these statements we conducted supplemental analyses detailed in SI.
Neuroimaging
MRI Acquisition and Processing:
We acquired high-resolution structural (sMRI) and resting-state functional MRI (rs-fMRI) data on the same 3T Skyra scanner (Siemens Medical Systems, Erlangen, Germany) using identical protocols for all participants. Comprehensive quality control (including head motion) was carried out on all neuroimaging datasets using previously published quality control procedures. Details of the image acquisition, preprocessing and quality assurance are provided in the Supplement Information (SI).
Cortical reconstruction and volumetric segmentation:
In each individual sMRI dataset, we used FreeSurfer image analysis suite (v.5.3.0) (http://surfer.nmr.mgh.harvard.edu/) to derive 64 cortical thickness measures and 18 subcortical volumetric measures from the Desikan atlas56(details in SI and Supplementary Figure 1).
Computation of Resting-state Connectivity:
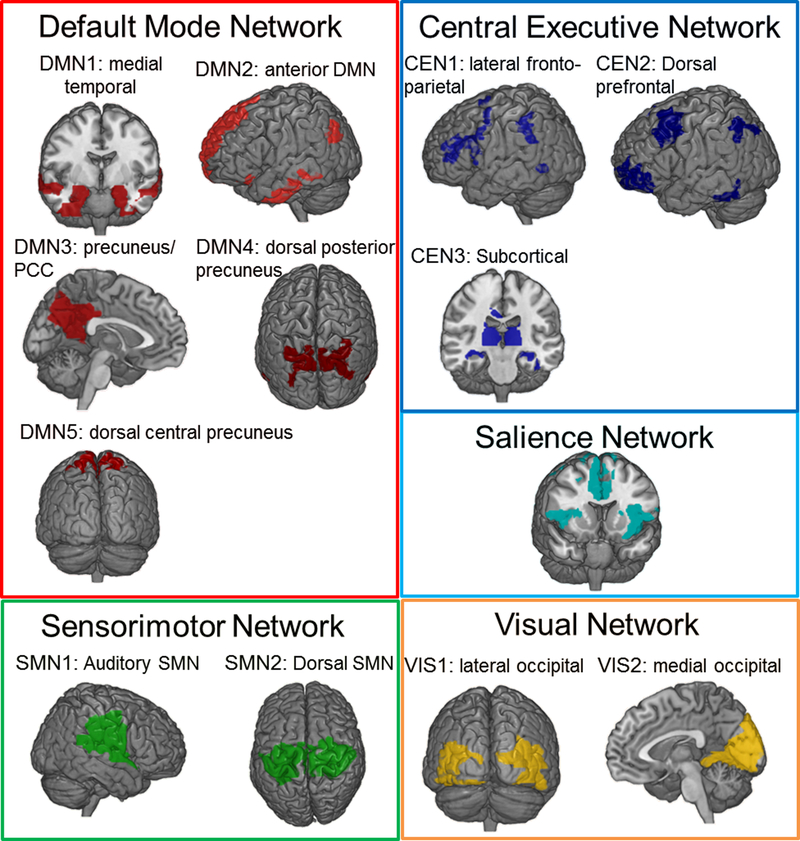
We used previously established and reproducible templates57,58 based data from 496 participants of the Human Connectome Project (www.humanconnectomeproject.com) to partition the functional connectome into the 5 most replicated resting-state networks (RSNs) and their subdivisions: default mode (DMN; 5 subnetworks), salience (SAL), central executive (CEN; 3 subnetworks), sensorimotor (SMN; 2 subnetworks) and visual (VIS; 2 subnetworks) (Figure 1, Supplementary Table 2). This partition was based on averaged regional blood oxygen-level-dependent (BOLD) time series from each of 638 brain regions chosen to maximize the likelihood of detecting subtle effects at the level of subnetworks as per Zalesky et al.59 and Crossley et al.60. In each participant, Fisher Z-transformed Pearson’s correlation coefficients were computed to calculate functional connectivity within (i.e., cohesiveness) and between (i.e., integration) subnetworks. Cohesiveness was computed as the average correlation of each voxel’s BOLD signal time series with every other gray matter voxel within the subnetwork. Integration was computed as the correlation between the average time-series of each pair of subnetworks. These computations yielded 91 connectivity measures.
Figure 1: Spatial Distribution of the Resting-State Networks and Subnetworks.
Details of the regions comprising each resting-state subnetwork are provided in Supplementary Table 3.
Statistical Analyses
We used paired t-tests to examine differences in symptom severity, BMI and antipsychotic dose between initial and 24-week assessments. We used univariate correlations and curve estimation to determine the zero-order associations between clinical and neuroimaging features (Supplementary Figure 2). We conducted sCCA to determine the covariation patterns between clinical features (clinical dataset) with sMRI (sMRI dataset) and with rs-fMRI (rs-fMRI dataset) neuroimaging measures. The clinical dataset comprised sex, IQ and change in the each of the BPRS subscale scores and in BMI and antipsychotic dose (Supplementary Table 1). The sCCA can be considered as a generalization of multiple linear regression and aims to identify those linear combinations (pairs of canonical variates or otherwise modes) of variables in each dataset that best express the maximal correlation (i.e., canonical correlation) between the two datasets. The weight of association between a variable of one dataset to the variate of the other dataset can be conceptually construed as an extension of a correlation coefficient (additional details in SI). All sCCAs were implemented in MatlabR2015b using an in-house script according to our previously published work41,61. Age and the interval between T1 and T2 were then regressed out of the change scores. Continuous variables were z-standardized prior to being entered into the sCCA. The sMRI dataset comprised 82 variables corresponding to measures of cortical thickness and subcortical volume (Supplementary Figure 1). The rs-fMRI dataset comprised 91 variables corresponding to measures of RSN cohesiveness and integration. Age was regressed out of the sMRI and fMRI variables before they were z-standardized and entered in the sCCA.
For each sCCA, we (a) computed the sparse parameters for range of candidate values from to 0.1×√p (high sparsity) to 1×√p (low sparsity) at 0.1 increments (where p is the number of features in that view of the data) and fitted the resulting models; (b) selected the optimal sparse criteria combination based on the parameters that corresponded to the values of the model that maximized the sCCA correlation value; (c) determined the optimal sCCA model and established its significance at p<0.05 using permutations (n=10,000). The p-value was defined as the number of permutations that resulted in a higher correlation than the original data divided by the total number of permutations. Thus the p-value is explicitly corrected for multiple testing as it is compared against the null distribution of maximal correlation values across all estimated sCCAs. For each permutation, we tested all sparsity criteria combinations as for the original data and then extracted the sCCA correlation with the highest coefficient among the tested options, independently of whether this combination was the same as in the original data. In this way we ensured that we did not underestimate the chance of a permutation achieving the same or higher value than the original data. If the sCCA was significant, we reported the canonical correlation weight of each variable.
Reliability analyses
We confirmed that the results reported were not influenced by the choice of functional partition, by repeating the analyses using measures of functional cohesiveness and integration extracted from publicly available RSN masks. These analyses are detailed in the SI.
Supplemental categorical analyses
Although we adopted a dimensional approach for our core analyses, we also tested the usefulness of categorical definitions of remission as we detail in SI. Specifically we investigated (a) whether we could identify differences in neuroimaging features when patients were categorized into remitted and non-remitted using the three most widely used criteria, and (b) whether we could identify clusters of patients based on their imaging features that differed in terms of their remission status.
Results
Clinical Features
Ninety-five patients were initially enrolled and 19 were excluded from the final analyses (7 because poor quality scans and 12 because they declined the follow-up interview). The demographic and clinical characteristics of the final sample are shown in Table 1. The majority of the patients were started on second generation antipsychotics [most commonly risperidone/paliperidone (64%) and olanzapine (26%)] while 12 patients (15.8%) were prescribed first generation agents [most commonly haloperidol (58%) and fluphenazine (41%)]. Overall, there was a significant reduction in all symptom dimensions at the follow-up assessment (Table 1). There were no significant differences in any clinical or demographic information (i.e., age, disease duration, age at onset, BMI, medication change, IQ, change in symptoms) between men and women (all p>0.07 at an uncorrected threshold). Although we adopted a dimensional approach, here we also report rates of remission based on the Remission in Schizophrenia Working Group (RSWG) criteria35 to allow comparisons of the clinical data with other studies that have used dichotomous categorizations. Consistent with the rates reported in prior literature62, 50 (65.8%) patients were remitted based on RSWG criteria; as expected there was significant overlap in the distribution of symptom scores between remitted and non-remitted patients (Supplementary Figure 2).
Table 1:
Study sample characteristics at the initial (T1) and the 24-week follow-up (T2) assessments
| Patients with Schizophrenia N=76 |
||
|---|---|---|
| Age (years) | 26.9 (7.0) | |
| Age of onset (years) | 21.6 (5.1) | |
| Sex (% Female) | 19 (25.0%) | |
| IQ | 95.0 (15.1) | |
| T1 | T2 | |
| BMI | 26.7 (6.3) | 27 (6) |
| BPRS Total Score* | 50.2 (18.6) | 36.9 (14.2) |
| BPRS Positive Symptoms* | 12.1 (6.2) | 7.7 (5.1) |
| BPRS Negative Symptoms* | 6.9 (3.7) | 5.4 (3.0) |
| BPRS Anxiety/Depression* | 9.3 (5.5) | 6.6 (3.7) |
| BPRS Agitation/Disorganization* | 7.6 (4.3) | 6.2 (3.1) |
| Antipsychotic dose (in CPZE) | 256 (266) | 280 (317) |
Continuous data are shown as mean (standard deviation); Categorical variables are shown as number of cases (percentage, %); BMI: Body Mass Index; BPRS: 24-Item Brief Psychotic Rating Scale, in the Brief Psychiatric Rating Scale each item is rated from 1 (absent) to 7 (extremely severe); CPZE: Chlorpromazine equivalents; IQ: Intelligence Quotient;
paired t-tests, p-value<0.001. Variables are defined in Supplementary Table 1.
Brain structural predictors of clinical response
sCCA models that considered all the structural data simultaneously (r=0.54, p=0.3) and models that were restricted to either subcortical (r=0.56, p=0.15) or cortical measures (r=0.37, p=0.98) were not significant. This pattern was also seen at the level of univariate correlations between clinical and sMRI measures were small (r values) not significant after correction for multiple comparisons (Supplementary Figure 3A and B).
Brain functional connectivity predictors of clinical response
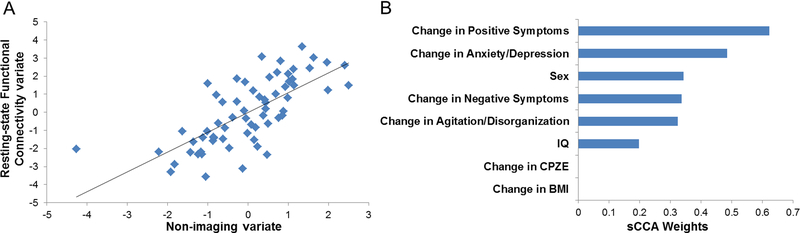
Only the first mode of the sCCA of the clinical and rs-fMRI datasets was significant (r=0.70, p=0.04) (Figure 2A). The weight of the association with imaging variate was highest for positive symptoms (weight=0.62) followed by anxiety/depression (weight=0.49), negative symptoms (weight=0.33) and agitation/disorganization (weight=0.32) (Figure 2B). The weights for BMI or antipsychotic dose were zero. The association between clinical variables and the rs-fMRI variate was higher for women (Supplementary Figure 4); however, removal of sex did not change the overall results. We focus on variables with at least moderate absolute weights (≥0.20, Figure 3) and we provide details of all the weights of association between the rs-fMRI measures and the clinical variate in Supplementary Table 4. Positive weights indicate that the corresponding variables are positive predictors while negative weights denote negative predictors. Higher functional integration of DMN subnetworks (DMN1 with DMN4 and DMN5), and particularly higher connectivity between medial temporal regions and the dorsal precuneus (PCu), emerged as the main positive predictor of response (Figure 3). The negative predictors comprised features of cross-network integration and within-network cohesiveness. Key negative predictors consisted of the connectivity between the DMN3 and SMN2 (i.e., functional connectivity between the PCu/posterior cingulate cortex and sensorimotor cortices), between the DMN5 and SAL (i.e., functional connectivity between the PCu and regions of the salience network that mainly involve the insula and anterior cingulate cortex) and between the CEN1 and the VIS1 (i.e., functional connectivity between the lateral frontoparietal and lateral visual cortices) (Figure 3). Key functional cohesiveness predictors implicated the CEN1 (comprising frontoparietal regions) and CEN3 (comprising the thalamus, striatum and posterior hippocampus).
Figure 2: Sparse Canonical Correlation Analysis of the Rs-fMRI and the Clinical Datasets.
(A) Single significant canonical mode linked the rs-fMRI measures and the clinical features (r=0.70, p=0.04). (B) Weights of each clinical variable in the sparse canonical correlation analysis; rs-fMRI=resting state functional magnetic resonance imaging
Figure 3: Brain Functional Predictors of Symptomatic Response in Schizophrenia.
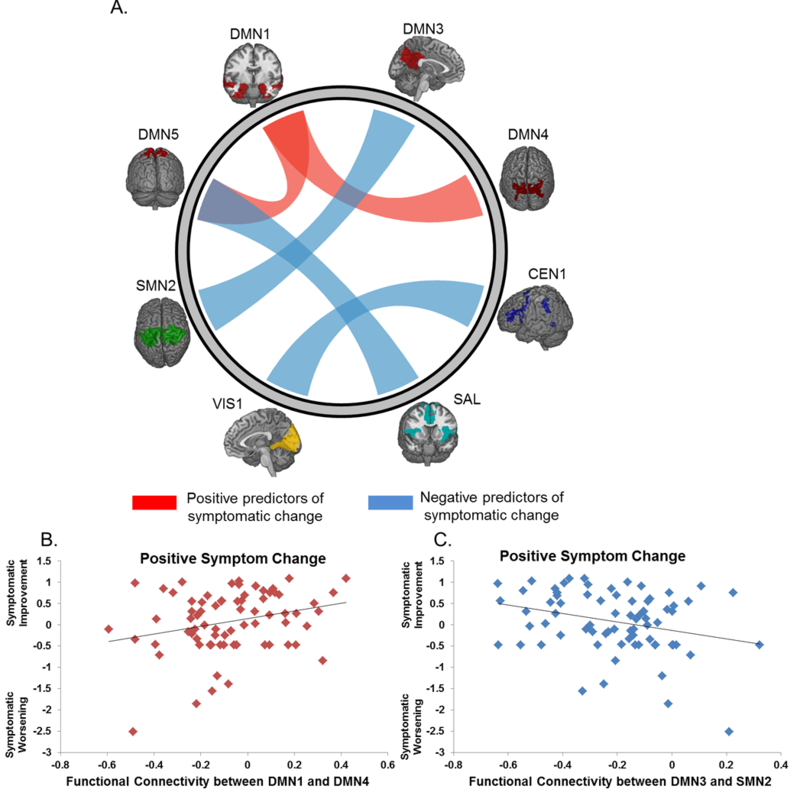
(A) Links depict the canonical weights of the association between the clinical variate and the connectivity of the subnetworks depicted; Red links represent positive predictors, Blue links represent negative predictors. Panels (B) and (C) provide illustrative examples of the associations between functional integration and improvement in positive symptoms. Improvement in positive symptoms was chosen because it had the highest canonical weight to the rs-fMRI variate. DMN1=medial temporal default mode subnetwork; DMN3=anterior precuneus/posterior cingulate cortex; DMN4= dorsal posterior precuneus; SMN2=sensorimotor network, dorsal subnetwork; rs-fMRI=resting state functional magnetic resonance imaging; details of the spatial distribution of the networks are provided in Figure 1 and Supplementary Table 3.
Supplemental Results
As detailed in SI, adopting a categorical approach to data analysis did not yield statistically significant results suggesting that remission and non-remission are more likely to represent a continuum rather than biological distinct states.
Discussion
We investigated the role of baseline sMRI and rs-fMRI measures as predictors of symptomatic improvement in response to antipsychotic treatment in the early course of schizophrenia. The present results establish the importance of system-level functional brain organization and clinical response. Specifically, the cohesiveness of the DMN and CEN and their cross-network integration with the salience, somatosensory and visual networks emerged as key predictors of short-term symptomatic change in schizophrenia. Moreover, our supplemental analyses argue against the notion that remission can be predicted by distinct functional connectivity features and suggest that short-term symptomatic change in schizophrenia is dimensionally linked to alterations in functional connectivity.
These findings can be best understood within the context of the developmental trajectories and organizational principles of the human connectome. At the highest level, the functional connectome of healthy adults is intrinsically organized into two anticorrelated large-scale neural systems: the DMN which comprises midline brain regions associated with unconstrained mental activity and a neural system preferentially engaged by goal-directed cognition and sensorimotor processes58,63–66. The latter is functionally distinguished into higher-order networks, notably the CEN (concerned with maintenance and manipulation of goal-directed mental operations) and the SAL (involved in switching activity between the DMN to CEN) and in lower-order sensory and motor networks63,64. Transition from childhood to adulthood typically is associated with changes in the intrinsic functional connectivity of these networks. For the DMN this entails greater intra-network cohesiveness and greater inter-network integration with the higher-order networks58,67. Gu et al.58 proposed that this pattern of connectivity allows the DMN to function as cohesive driver of intrinsic brain function that supports a range of mental operations relating mostly to self-referential processes and spontaneous thought66,68. The development of critical DMN connectivity may be disrupted in schizophrenia based on previous reports of reduced functional cohesiveness between DMN nodes and particularly the hippocampus17, coupled with hyperconnectivty of the posterior DMN with brain regions outside the network69. In the present study, higher intranetwork cohesiveness and lower cross-network connectivity of the DMN were associated with symptomatic improvement (Figure 3). These findings complement previous observations and indicate that the functional properties of the DMN may play a pivotal role both in disease expression and in treatment response in schizophrenia.
In higher-order networks typical development is associated with decreased intra-network cohesiveness58 indicating that in the adult brain the nodes of these networks show inherently divergent activity profiles, that likely reflect their engagement in numerous and diverse cognitive operations. The present results implicate mainly the CEN which is known to support multiple aspects of cognitive and attentional control and in goal-directed selection of stimuli and responses63. Increased cohesiveness of the CEN has been associated with positive psychotic symptoms in schizophrenia70 and lower baseline cohesiveness within the subcortical subnetwork of the CEN has been shown to predict antipsychotic response34. In accordance with these reports, we found that higher cohesiveness with the frontoparietal and subcortical subnetworks of the CEN was a negative predictor of response.
Based on the multivariate analyses employed here, the predictive value of sMRI features for clinical response was not significant. Nevertheless, the univariate contrasts between responders and non-responders (described in the SI), although not statistical significant in the context of the multiple comparisons undertaken, implicated the hippocampus bilaterally, and thus align with previous literature29,30.
The present study has a number of strengths. To our knowledge, this is the first study to capture the association between neuroimaging measures and clinical improvement across the entire spectrum of psychopathology without being limited to positive symptoms. We used continuous measures of clinical improvement that represent the actual structure of the symptom scores (Supplementary Figure 2). We used a data-driven approach that is conducive to discovery and is more stringent that hypothesis-led analyses that are limited to specific brain regions or networks. We demonstrated the reliability of our main results using alternate brain functional parcellations.
In considering the study limitations, we note that currently available neuroimaging techniques include other modalities (such as diffusion weighted imaging) and other analytic methods (such as graph theory) that were not considered here. We chose to focus on sMRI and rs-fMRI as these represent the most commonly used neuroimaging approaches in psychosis and are also easier to implement in clinical settings because of their relative brevity and lack of requirement for active patient engagement. Sex differences have been reported in incidence, clinical characteristics and mental health service use in schizophrenia71,72. Our sample included a higher proportion of men which is aligned with findings regarding sex differences in service use in the USA72. The pattern of findings regarding the association between symptomatic change and resting-state functional connectivity appeared independent of sex, but studies with greater representation of women would be important for confirming our observations. We did not examine drug naïve patients but we ensured that antipsychotic exposure prior to the baseline scan was minimal. Although the daily antipsychotic dose did not correlate with any neuroimaging measure at follow-up, the effect of medication at baseline cannot be conclusively excluded. The results reported here are dependent on the severity of patients’ symptoms which varies across samples and within the same patient over time. Confirmation of the reproducibility of our findings requires replication in larger samples and in longitudinal studies.
Our study provides novel evidence of neuroimaging predictors of the short-term clinical outcome in schizophrenia. These neural network phenotypes can be further interrogated in future studies with regards to their relevance to genetic and molecular mechanisms. For example, the patterns of dysconnectivity observed point to testable hypotheses involving (among several possible alternatives) abnormalities in NMDA receptors and the GABA system73,74. The present findings also have the potential to inform cognitive neuroscientists and clinicians aiming to develop or refine neuromodulatory or psychological interventions that target neural networks most likely to be involved in clinical improvement75.
Supplementary Material
Acknowledgments:
This work was supported in part through the computational resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai. Dr. Frangou received support from the National Institutes of Health (R01 MH104284–01A1) and European Unit FP7 program (IMAGEMEND 602450; IMAging GEnetics for MENtal Disorders) projects. Dr. Moser received support from the Swiss National Science Foundation (P300PB_171584).
Footnotes
Supplementary information is available at MP’s website.
Financial Disclosures:
The authors report no conflict of interest.
References:
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380(9859): 2197–2223. [DOI] [PubMed] [Google Scholar]
- 2.Hafner H, an der Heiden W. The course of schizophrenia in the light of modern follow-up studies: the ABC and WHO studies. European archives of psychiatry and clinical neuroscience 1999; 249 Suppl 4: 14–26. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum B, Valbak K, Harder S, Knudsen P, Koster A, Lajer M et al. Treatment of patients with first-episode psychosis: two-year outcome data from the Danish National Schizophrenia Project. World psychiatry : official journal of the World Psychiatric Association 2006; 5(2): 100–103. [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey PD, Heaton RK, Carpenter WT Jr., Green MF, Gold JM, Schoenbaum M Functional impairment in people with schizophrenia: focus on employability and eligibility for disability compensation. Schizophr Res 2012; 140(1–3): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenheck RA, Estroff SE, Sint K, Lin H, Mueser KT, Robinson DG et al. Incomes and Outcomes: Social Security Disability Benefits in First-Episode Psychosis. The American journal of psychiatry 2017; 174(9): 886–894. [DOI] [PubMed] [Google Scholar]
- 6.Hayes JF, Marston L, Walters K, King MB, Osborn DPJ. Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. The British journal of psychiatry : the journal of mental science 2017; 211(3): 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of general psychiatry 2007; 64(10): 1123–1131. [DOI] [PubMed] [Google Scholar]
- 8.Davidson L, McGlashan TH. The varied outcomes of schizophrenia. Can J Psychiatry 1997; 42(1): 34–43. [DOI] [PubMed] [Google Scholar]
- 9.Heilbronner U, Samara M, Leucht S, Falkai P, Schulze TG. The Longitudinal Course of Schizophrenia Across the Lifespan: Clinical, Cognitive, and Neurobiological Aspects. Harv Rev Psychiatry 2016; 24(2): 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conus P, Cotton S, Schimmelmann BG, McGorry PD, Lambert M. Rates and predictors of 18-months remission in an epidemiological cohort of 661 patients with first-episode psychosis. Soc Psychiatry Psychiatr Epidemiol 2017. [DOI] [PubMed] [Google Scholar]
- 11.Koutsouleris N, Kahn RS, Chekroud AM, Leucht S, Falkai P, Wobrock T et al. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: a machine learning approach. Lancet Psychiatry 2016; 3(10): 935–946. [DOI] [PubMed] [Google Scholar]
- 12.Menezes NM, Arenovich T, Zipursky RB. A systematic review of longitudinal outcome studies of first-episode psychosis. Psychological medicine 2006; 36(10): 1349–1362. [DOI] [PubMed] [Google Scholar]
- 13.Samara MT, Leucht C, Leeflang MM, Anghelescu IG, Chung YC, Crespo-Facorro B et al. Early Improvement As a Predictor of Later Response to Antipsychotics in Schizophrenia: A Diagnostic Test Review. The American journal of psychiatry 2015; 172(7): 617–629. [DOI] [PubMed] [Google Scholar]
- 14.Birur B, Kraguljac NV, Shelton RC, Lahti AC. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder-a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophr 2017; 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Molecular psychiatry 2016; 21(4): 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Erp TG, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 controls via the ENIGMA consortium. Biological psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophrenia bulletin 2018; 44(1): 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WH, Doucet GE, Leibu E, Frangou S. Resting-state network connectivity and metastability predict clinical symptoms in schizophrenia. Schizophr Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doucet G, Rider R, Taylor N, Skidmore C, Sharan A, Sperling M et al. Pre-surgery resting-state local graph-theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia 2015. [DOI] [PubMed] [Google Scholar]
- 20.Willette AA, Calhoun VD, Egan JM, Kapogiannis D, Alzheimers Disease Neuroimaging I. Prognostic classification of mild cognitive impairment and Alzheimer’s disease: MRI independent component analysis. Psychiatry research 2014; 224(2): 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D et al. Longitudinal study of brain morphology in first episode schizophrenia. Biological psychiatry 2001; 49(6): 487–499. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman J, Jody D, Geisler S, Alvir J, Loebel A, Szymanski S et al. Time course and biologic correlates of treatment response in first-episode schizophrenia. Archives of general psychiatry 1993; 50(5): 369–376. [DOI] [PubMed] [Google Scholar]
- 23.Molina V, Reig S, Sarramea F, Sanz J, Francisco Artaloytia J, Luque R et al. Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiatry research 2003; 124(3): 153–161. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwenhuis M, Schnack HG, van Haren NE, Lappin J, Morgan C, Reinders AA et al. Multi-center MRI prediction models: Predicting sex and illness course in first episode psychosis patients. NeuroImage 2017; 145(Pt B): 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palaniyappan L, Marques TR, Taylor H, Handley R, Mondelli V, Bonaccorso S et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA psychiatry 2013; 70(10): 1031–1040. [DOI] [PubMed] [Google Scholar]
- 26.Cahn W, van Haren NE, Hulshoff Pol HE, Schnack HG, Caspers E, Laponder DA et al. Brain volume changes in the first year of illness and 5-year outcome of schizophrenia. The British journal of psychiatry : the journal of mental science 2006; 189: 381–382. [DOI] [PubMed] [Google Scholar]
- 27.Prasad KM, Sahni SD, Rohm BR, Keshavan MS. Dorsolateral prefrontal cortex morphology and short-term outcome in first-episode schizophrenia. Psychiatry research 2005; 140(2): 147–155. [DOI] [PubMed] [Google Scholar]
- 28.Milev P, Ho BC, Arndt S, Nopoulos P, Andreasen NC. Initial magnetic resonance imaging volumetric brain measurements and outcome in schizophrenia: a prospective longitudinal study with 5-year follow-up. Biological psychiatry 2003; 54(6): 608–615. [DOI] [PubMed] [Google Scholar]
- 29.Bodnar M, Harvey PO, Malla AK, Joober R, Lepage M. The parahippocampal gyrus as a neural marker of early remission in first-episode psychosis: a voxel-based morphometry study. Clin Schizophr Relat Psychoses 2011; 4(4): 217–228. [DOI] [PubMed] [Google Scholar]
- 30.Bodnar M, Malla AK, Joober R, Lord C, Smith E, Pruessner J et al. Neural markers of early remission in first-episode schizophrenia: a volumetric neuroimaging study of the parahippocampus. Psychiatry research 2012; 201(1): 40–47. [DOI] [PubMed] [Google Scholar]
- 31.Molina V, Martin C, Ballesteros A, de Herrera AG, Hernandez-Tamames JA. Optimized voxel brain morphometry: association between brain volumes and the response to atypical antipsychotics. European archives of psychiatry and clinical neuroscience 2011; 261(6): 407–416. [DOI] [PubMed] [Google Scholar]
- 32.Nejad AB, Madsen KH, Ebdrup BH, Siebner HR, Rasmussen H, Aggernaes B et al. Neural markers of negative symptom outcomes in distributed working memory brain activity of antipsychotic-naive schizophrenia patients. Int J Neuropsychopharmacol 2013; 16(6): 1195–1204. [DOI] [PubMed] [Google Scholar]
- 33.Kraguljac NV, White DM, Hadley N, Hadley JA, Ver Hoef L, Davis E et al. Aberrant Hippocampal Connectivity in Unmedicated Patients With Schizophrenia and Effects of Antipsychotic Medication: A Longitudinal Resting State Functional MRI Study. Schizophrenia bulletin 2016; 42(4): 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M et al. Baseline Striatal Functional Connectivity as a Predictor of Response to Antipsychotic Drug Treatment. The American journal of psychiatry 2016; 173(1): 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreasen NC, Carpenter WT, Jr., Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. The American journal of psychiatry 2005; 162(3): 441–449. [DOI] [PubMed] [Google Scholar]
- 36.Karow A, Naber D, Lambert M, Moritz S, Initiative E. Remission as perceived by people with schizophrenia, family members and psychiatrists. European psychiatry : the journal of the Association of European Psychiatrists 2012; 27(6): 426–431. [DOI] [PubMed] [Google Scholar]
- 37.Russo M, Levine SZ, Demjaha A, Di Forti M, Bonaccorso S, Fearon P et al. Association between symptom dimensions and categorical diagnoses of psychosis: a cross-sectional and longitudinal investigation. Schizophrenia bulletin 2014; 40(1): 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esteghamati A, Khalilzadeh O, Anvari M, Ahadi MS, Abbasi M, Rashidi A. Metabolic syndrome and insulin resistance significantly correlate with body mass index. Arch Med Res 2008; 39(8): 803–808. [DOI] [PubMed] [Google Scholar]
- 39.Johnson W, Bouchard TJ Jr., Krueger RF, McGue M, Gottesman II. Just one g: consistent results from three test batteries. Intelligence 2004; 32: 95–107. [Google Scholar]
- 40.Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Molecular psychiatry 2015; 20(1): 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moser DA, Doucet GE, Lee WH, Rasgon A, Krinsky H, Leibu E et al. Multivariate associations among behavioral, clinical and multimodal imaging phenotypes in psychosis. JAMA psychiatry 2018; 75(4): 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermes E, Nasrallah H, Davis V, Meyer J, McEvoy J, Goff D et al. The association between weight change and symptom reduction in the CATIE schizophrenia trial. Schizophr Res 2011; 128(1–3): 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ventura J, Subotnik KL, Guzik LH, Hellemann GS, Gitlin MJ, Wood RC et al. Remission and recovery during the first outpatient year of the early course of schizophrenia. Schizophr Res 2011; 132(1): 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Witten DM, Tibshirani R, Hastie T. A penalized matrix decomposition, with applications to sparse principal components and canonical correlation analysis. Biostatistics 2009; 10(3): 515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breiman L, Friedman JH. Predicting multivariate responses in multiple linear regression. J R Stat Soc Series B Stat Methodol 1997; 59(1): 3–54. [Google Scholar]
- 46.Klami A, Virtanen S, Kaski S. Bayesian canonical correlation analysis. J Mac Learn Res 2013; 14: 965–1003. [Google Scholar]
- 47.Association AP. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing: Arlington, VA, 2013. [Google Scholar]
- 48.First MB, Williams JBW, Karg RS, Spitzer RL. Structured clinical interview for DSM-5, research version: Arlington, VA, 2015. [Google Scholar]
- 49.Wechsler D Wechsler Abbreviated Scale of Intelligence-2nd edition. NCS Pearson: San Antonio, TX, 2011. [Google Scholar]
- 50.Wells R, Swaminathan V, Sundram S, Weinberg D, Bruggemann J, Jacomb I et al. The impact of premorbid and current intellect in schizophrenia: cognitive, symptom, and functional outcomes. NPJ Schizophr 2015; 1: 15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale. International Journal of methods in psychiatric research 1993; 3: 221–244. [Google Scholar]
- 52.Kopelowicz A, Ventura J, Liberman RP, Mintz J. Consistency of Brief Psychiatric Rating Scale factor structure across a broad spectrum of schizophrenia patients. Psychopathology 2008; 41(2): 77–84. [DOI] [PubMed] [Google Scholar]
- 53.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. The American journal of psychiatry 2010; 167(6): 686–693. [DOI] [PubMed] [Google Scholar]
- 54.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382(9896): 951–962. [DOI] [PubMed] [Google Scholar]
- 55.Tiihonen J, Wahlbeck K, Lonnqvist J, Klaukka T, Ioannidis JP, Volavka J et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. Bmj 2006; 333(7561): 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006; 31(3): 968–980. [DOI] [PubMed] [Google Scholar]
- 57.Doucet GE, Rasgon N, McEwen BS, Micali N, Frangou S. Elevated Body Mass Index is Associated with Increased Integration and Reduced Cohesion of Sensory-Driven and Internally Guided Resting-State Functional Brain Networks. Cereb Cortex 2018; 28(3): 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC et al. Emergence of system roles in normative neurodevelopment. Proceedings of the National Academy of Sciences of the United States of America 2015; 112(44): 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zalesky A, Fornito A, Harding IH, Cocchi L, Yucel M, Pantelis C et al. Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage 2010; 50(3): 970–983. [DOI] [PubMed] [Google Scholar]
- 60.Crossley NA, Mechelli A, Vertes PE, Winton-Brown TT, Patel AX, Ginestet CE et al. Cognitive relevance of the community structure of the human brain functional coactivation network. Proceedings of the National Academy of Sciences of the United States of America 2013; 110(28): 11583–11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moser DA, Doucet GE, Ing A, Dima D, Schumann G, Bilder RM et al. An integrated brain-behavior model for working memory. Molecular psychiatry 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D. Remission in schizophrenia: validity, frequency, predictors, and patients’ perspective 5 years later. Dialogues Clin Neurosci 2010; 12(3): 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. Journal of cognitive neuroscience 2002; 14(3): 508–523. [DOI] [PubMed] [Google Scholar]
- 64.Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F et al. Brain activity at rest: a multiscale hierarchical functional organization. Journal of neurophysiology 2011; 105(6): 2753–2763. [DOI] [PubMed] [Google Scholar]
- 65.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America 2005; 102(27): 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raichle ME. The brain’s default mode network. Annual review of neuroscience 2015; 38: 433–447. [DOI] [PubMed] [Google Scholar]
- 67.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM et al. The maturing architecture of the brain’s default network. Proceedings of the National Academy of Sciences of the United States of America 2008; 105(10): 4028–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 2012; 18(3): 251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 2009; 106(4): 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krishnadas R, Ryali S, Chen T, Uddin LQ, Supekar K, Palaniyappan L et al. Resting state functional hyperconnectivity within a triple network model in paranoid schizophrenia. The Lancet 2014; 383: S65. [Google Scholar]
- 71.Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry 2010; 22(5): 417–428. [DOI] [PubMed] [Google Scholar]
- 72.Haynes VS, Zhu B, Stauffer VL, Kinon BJ, Stensland MD, Xu L et al. Long-term healthcare costs and functional outcomes associated with lack of remission in schizophrenia: a post-hoc analysis of a prospective observational study. BMC Psychiatry 2012; 12: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 2012; 109(41): 16720–16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Archives of neurology 2006; 63(10): 1372–1376. [DOI] [PubMed] [Google Scholar]
- 75.Keshavan MS, Vinogradov S, Rumsey J, Sherrill J, Wagner A. Cognitive training in mental disorders: update and future directions. The American journal of psychiatry 2014; 171(5): 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.