Abstract
Aims/hypothesis
We examined the hypothesis that elevation in urinary albumin creatinine ratio (ACR) in adolescents with type 1 diabetes is associated with abnormal retinal vascular geometry (RVG) phenotypes.
Methods
A cross-sectional study at baseline of the relationship between ACR within the normoalbuminuric range and RVG in 963 adolescents aged 14.4 ± 1.6 years with type 1 diabetes (median duration 6.5 years) screened for participation in AdDIT. A validated algorithm was used to categorise log10 ACR into tertiles: upper tertile ACR was defined as ‘high-risk’ for future albuminuria and the lower two tertiles were deemed ‘low-risk’. RVG analysis, using a semi-automated computer program, determined retinal vascular calibres (standard and extended zones) and tortuosity. RVG measures were analysed continuously and categorically (in quintiles: Q1–Q5) for associations with log10 ACR and ACR risk groups.
Results
Greater log10 ACR was associated with narrower vessel calibres and greater tortuosity. The high-risk group was more likely to have extended zone vessel calibres in the lowest quintile (arteriolar Q1 vs Q2–Q5: OR 1.67 [95% CI 1.17, 2.38] and venular OR 1.39 [0.98, 1.99]) and tortuosity in the highest quintile (Q5 vs Q1–Q4: arteriolar OR 2.05 [1.44, 2.92] and venular OR 2.38 [1.67, 3.40]). The effects of retinal vascular calibres and tortuosity were additive such that the participants with the narrowest and most tortuous vessels were more likely to be in the high-risk group (OR 3.32 [1.84, 5.96]). These effects were independent of duration, blood pressure, BMI and blood glucose control.
Conclusions/interpretation
Higher ACR in adolescents is associated with narrower and more tortuous retinal vessels. Therefore, RVG phenotypes may serve to identify populations at high risk of diabetes complications during adolescence and well before onset of clinical diabetes complications.
Keywords: AdDIT, Adolescents, Diabetic retinopathy, Microvascular complications, Nephropathy, Retinal vascular geometry, Type 1 diabetes
Introduction
Despite significant advances in the management of type 1 diabetes, premature cardiovascular mortality has not been significantly ameliorated, with most of the increase in cardiovascular risk explained by diabetic nephropathy [1]. An effective method of identifying individuals who will develop severe microvascular complications, including diabetic nephropathy, remains elusive. While urinary albumin excretion measurement, a functional measure, remains the favoured method for identifying and monitoring persons who develop diabetic nephropathy, it is clear that not all individuals develop albuminuria or proteinuria before progressing to end-stage renal disease [2–4]. Furthermore, persons diagnosed with diabetes during childhood may have well over a decade of diabetes duration before transition to adult care. However, children and adolescents do not typically receive treatment during this prolonged period, as clinically overt complications are infrequent and hypertension uncommon. Nonetheless, evidence suggests that growth and puberty are critical periods that accelerate the systemic endotheliopathy leading to microvascular complications [5, 6].
The retina offers a unique opportunity to visualise and study the vasculature (especially the microvasculature) in vivo. Previous retinal vascular geometry (RVG) studies considered the proximal branches of central retinal vessels only [7]. We recently demonstrated the adverse effect of glycaemic burden on more peripheral retinal vessel calibres in youth with type 1 diabetes [8] and postulate that the earliest adverse phenotypes will be apparent in the peripheral retina. In adult studies of cardiovascular disease, arteriolar narrowing has been associated with higher blood pressure and greater cardiovascular risk, while venular changes have been separately associated with proteinuria.
Evidence suggests an inherent predisposition to the development of diabetes micro- and macrovascular complications in a proportion of the population [9, 10]. Identifying persons at high risk at the earliest possible time may allow for a preventive intervention strategy ideally during early life. There is, however, a paucity of data in paediatric populations with type 1 diabetes prior to onset of clinical diabetes complications, arguably a period when the greatest benefit from intervention may be derived and complications prevented rather than ameliorated. The Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT; http://isrctn.com registration number 91419926) is the first interventional study in a group of normoalbuminuric adolescents with type 1 diabetes determined to be at high risk of developing kidney disease based on their urinary albumin creatinine ratio (ACR) [11].
In this study, we examine the cross-sectional associations at baseline between urinary ACR and RVG measures in adolescents with type 1 diabetes screened for participation in AdDIT. We hypothesised that higher urinary ACR is associated with an adverse RVG phenotype involving both arterioles and venules in the peripheral retina.
Methods
Study population
A cross-sectional study was carried out at baseline of a multinational cohort comprising 1041 normoalbuminuric adolescents with type 1 diabetes, screened for eligibility to the AdDIT, with mean age 14.4 years, median diabetes duration 6.5 years and HbA1c 69 mmol/mol (8.5%). Data for the current study were collected at the study baseline visit prior to, or within 6 months of, trial commencement. We present the baseline data only. Treatment outcomes and unmasking of treatment allocation are beyond the scope of this paper. Briefly, in the multicentre trial across the UK, Australia and Canada, adolescents considered to be at ‘high-risk’ of diabetic nephropathy were randomised to an ACE inhibitor (ACE-I) and/or statin (HMG-CoA reductase inhibitor) or placebo [12], while the ‘low-risk’ cohort was followed as a natural history observational cohort.
This study conformed to the Declaration of Helsinki and was approved by The Cambridge University Research Ethics Committee and the local ethics committees of each participating centre. Written informed consent was obtained from the parents and assent from all study participants.
Retinal photography and measurements
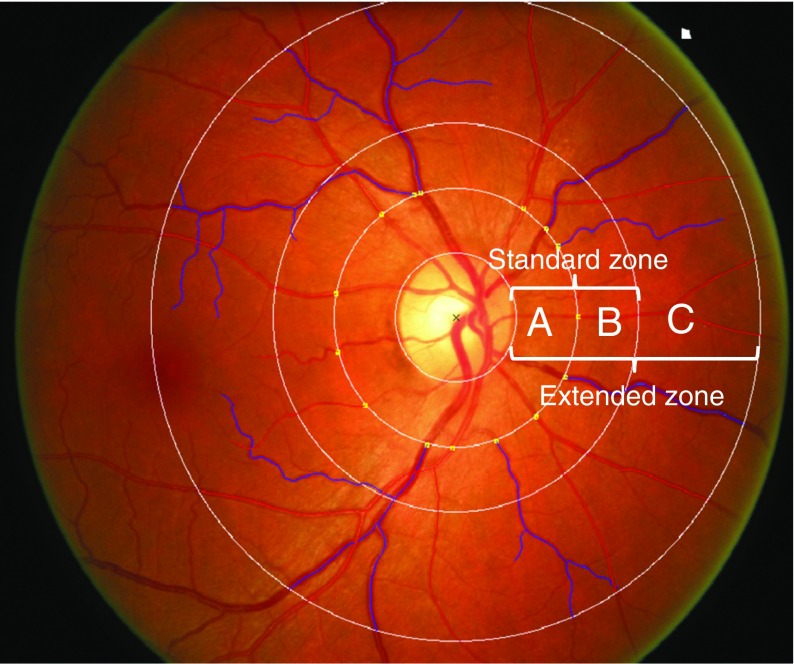
Of the 1041 participants, 982 (94%) had full clinical data and standardised digital two-field retinal photographs from both eyes taken at baseline (central optic disc field and macula field). There were no clinically or biochemically significant differences between those with and without retinal photographs. We excluded those with ungradable photographs due to either poor quality of retinal photographs (n = 14, 1.4%) or for whom fewer than four of the largest vessels could be traced by the computer-assisted program in either eye (n = 5, 0.5%). Thus, 963 (92%) participants were included in analyses for this report. RVG was assessed using SIVA (Singapore I Vessel Assessment, Singapore, Republic of Singapore), a semi-automated computer-assisted image program. A single trained grader, masked to participants’ identities, applied the program to each retinal photograph, to measure retinal microvascular geometric variables within a concentric zone between the optic disc margin and two optic disc diameters away from the optic disc margin. The grader allowed the software to detect the centre of the optic disc and divided the region into three subzones surrounding the optic disc, zones A, B, C, corresponding to 0.5, 1.0 and 2.0 optic disc diameters away from the optic disc margin, respectively (Fig. 1). Once the optic disc and the three concentric subzones were appropriately located, the grader executed the program to trace all vessels. The grader ensured that all arterioles and venules were correctly identified. The software combined the individual measurements into summary indices of vessel calibres: the six largest vessels were used to calculate the central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE) in the standard zone B; the mean width of all measurable arterioles in the extended zone (exMWa) and mean width of all measurable venules in the extended zone (exMWv) (out to zone C) were calculated and arteriolar curvature tortuosity (CTa) and venular curvature tortuosity (CTv) were calculated as measures of vessel undulation, as previously described [13].
Fig. 1.
Retinal photograph taken at baseline, showing central optic disc field and macula field. Retinal zones according to SIVA: standard zone (A+B), extended zone (A + B + C)
Renal function measures
Centralised assessment of all urine samples was performed at The WellChild laboratory at The Evelina Children’s Hospital, London, UK. Samples were stored at −70°C prior to shipping. Urine albumin was measured using laser immunonephelometry (Siemens BN Prospec; Siemens Healthcare, Erlangen, Germany) and, for concentrations <2.1 mg/l, by an ELISA [11]. Urine creatinine was measured using a chromatographic stable isotope dilution electrospray MS–MS method. For each participant, the two time point log10 ACR measures, each based on three consecutive early morning samples at two separate visits, were averaged on the log10 ACR scale and the average residual calculated using age, sex and duration and the coefficients from the previously described linear regression model in the Oxford Regional Prospective Study (ORPS) cohort [14]. High-risk ACR was defined as being in the highest tertile, within the normoalbuminuric range, according to a standardised ACR that predicted 85% of adolescents with type 1 diabetes who later developed microalbuminuria and 100% who developed clinical proteinuria in the ORPS [15]. The upper ACR tertile (high-risk group) was assigned to residual value >1.2, the middle ACR tertile to values of 0.8–1.2 and lower ACR tertile to values <0.8. For this study, the lower two tertiles were combined for analysis as the low-risk group [11].
The eGFR was calculated using the following formula: eGFR (ml min−1 1.73 m−2) = 42 × height (cm) / plasma creatinine (μmol/l) [12]. Hyperfiltration was defined as eGFR >135 ml min−1 1.73 m−2 [16].
Other variables
HbA1c was analysed locally at each centre, using DCCT aligned methods (HbA1c Variant analyser [Bio-Rad Laboratories, Hercules, CA, USA], Adams Arkray [Kyoto, Japan], Vantage analyser [Siemens Diagnostics, Camberley, UK] or DCA 2000, [Siemens Diagnostics, Tarrytown, NY, USA]). Lipid profile measurements (cholesterol, HDL-cholesterol, LDL-cholesterol, triacylglycerol) were measured using routine laboratory methods.
The standard deviation score (SDS) was calculated for height, weight and BMI according to the least-mean squares method [17]. Blood pressure was measured using an Omran M6 blood pressure monitor (Hoofddorp, the Netherlands) and/or Dinamap monitor (Tampa, FL, USA) using an appropriate sized cuff. Age- and sex-related percentiles and SDS for systolic blood pressure (SBP) and diastolic blood pressure (DBP) were calculated according to published standards [18].
This study was approved by the Human Research Ethics Committees of each participating centre. Informed consent was obtained from participants and their families.
Statistics
Descriptive data are summarised as mean ± SD for parametric data and median (interquartile range [IQR]) for non-parametric data. Urinary ACR was log10 transformed for analysis and ACR groups were expressed as categorical variables (high-risk [upper tertile] vs low-risk [the two lower tertiles]). Thresholds were explored and quintiles deemed the optimal category for analysis. RVG measures were analysed as both continuous and categorical variables (quintiles). Mean differences in retinal vascular measures between high-risk and low-risk groups were determined using independent samples t tests for normally distributed variables or Mann–Whitney U tests for non-parametric variables. A χ2 test was used to compare the likelihood of being in the high-risk ACR group depending on retinal vascular measure quintiles.
Associations between log10 ACR, log10 eGFR and retinal vascular measures as continuous variables were examined using linear regression models. Binary logistic regression was used to explore threshold effects of retinal vascular measures, arriving at quintiles as the optimal grouping as predictors of high-risk and low-risk ACR groups. For vessel calibres, Q2–Q5 were combined into a single category for analysis. For tortuosity Q1–Q4, which were not statistically significantly different, were combined into a single category for analysis. Univariable regression analyses were carried out and multivariable regression models built using variables that were significant in univariable analysis, including HbA1c, BMI, SBP, lipids and duration. To test our proposed hypotheses that both arteriolar and venular components of the microvascular system contribute to the risk of microvascular dysfunction, we created two compound binary variables: (1) exMWaQ1 & CTvQ5 (the lowest exMWa quintiles and highest CTv quintile) and (2) exMWaQ1 & exMWvQ1 (the lowest quintiles of exMWa and exMWv). All statistical analyses were conducted using IBM SPSS version 22, Armonk, NY, USA.
Results
Participant characteristics
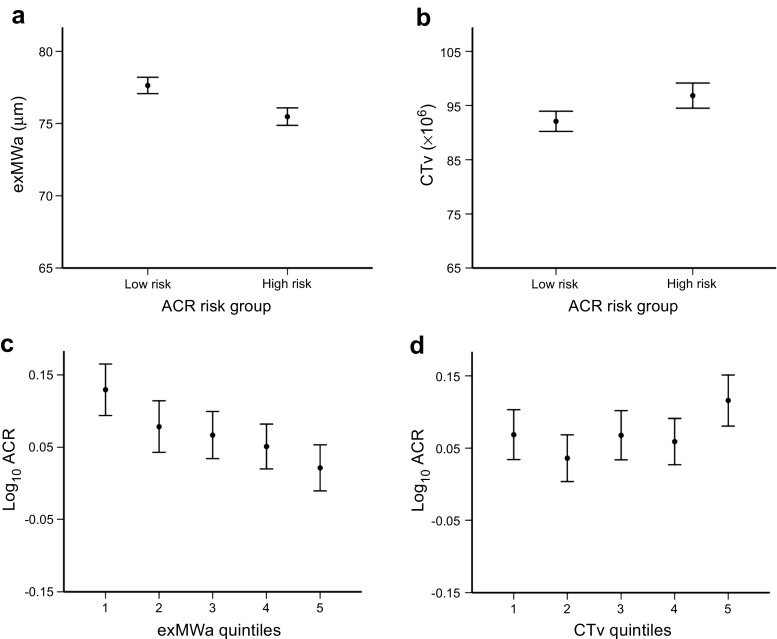
Characteristics of participants in the high-risk and low-risk groups are shown in Table 1. Adolescents in the high-risk group were younger, with shorter diabetes duration, lower BMI, higher SBP SDS and higher eGFR than those in the low-risk group. There were no significant differences in sex distribution, HbA1c or lipid profiles between the two groups. Participants in the high-risk ACR group had narrower arteriolar and venular calibres in both standard (CRAE, CRVE) and extended zones (exMWa, exMWv) (Fig. 2) as well as more tortuous vessels (CTa and CTv) compared with those in the low-risk group.
Table 1.
Clinical characteristics and retinal vascular measures by ACR risk group
| Characteristic | Low-risk (n=517) |
High-risk (n=446) |
p value |
|---|---|---|---|
| Male sex, n (%) | 276 (53) | 237 (53) | 0.9 |
| Age, years | 14.5 ± 1.6 | 14.2 ± 1.6 | 0.001 |
| Diabetes duration, years | 7.4 (4.9–10.2) | 5.4 (3.6–8.1) | <0.001 |
| Height, cm | 165.0 ± 10.4 | 163.6 ± 10.6 | 0.049 |
| Weight, kg | 61.8 ± 13.6 | 58.4 ± 13.4 | <0.001 |
| BMI, kg/m2 | 21.9 (19.7–24.6) | 20.9 (18.8–23.5) | <0.001 |
| BMI SDS | 0.95 ± 1.00 | 0.72 ± 1.05 | 0.001 |
| Waist, cm | 76.0 ± 10.0 | 74.3 ± 9.2 | 0.01 |
| Waist:height | 0.46 ± 0.06 | 0.45 ± 0.05 | 0.07 |
| SBP, mmHg | 115.6 ± 11.3 | 116.5 ± 12.0 | 0.3 |
| SBP SDS | −0.14 ± 1.05 | 0.03 ± 1.13 | 0.02 |
| DBP, mmHg | 65.3 ± 8.1 | 66.1 ± 8.1 | 0.2 |
| DBP SDS | 0.90 ± 0.92 | 1.01 ± 0.93 | 0.1 |
| Smoking, proportion (%) | 5/522 (1.0) | 3/399 (0.8) | 0.6 |
| HbA1c, mmol/mol | 68 ± 14 | 69 ± 16 | 0.3 |
| HbA1c, % | 8.4 ± 1.3 | 8.5 ± 1.4 | 0.3 |
| Cholesterol, mmol/l | 4.40 ± 0.86 | 4.38 ± 0.84 | 0.7 |
| eGFR, ml min−1 1.73 m−2 | 122 ± 21 | 129 ± 24 | <0.001 |
| RVG | |||
| CRAE, μm | 154.2 ± 12.3 | 151.7 ± 12.3 | 0.002 |
| CRVE, μm | 217.8 ± 17.5 | 215.6 ± 17.7 | 0.045 |
| exMWa, μm | 77.6 ± 6.6 | 75.5 ± 6.6 | <0.001 |
| exMWv, μm | 88.8 ± 7.4 | 87.0 ± 7.9 | 0.001 |
| CTa ×106 | 96.1 ± 1.3 | 99.7 ± 1.4 | 0.035 |
| CTv ×106 | 89.8 ± 1.2 | 93.7 ± 1.3 | 0.006 |
Data are presented as mean ± SD for parametric data, median (IQR) for non-parametric data and n (%) or proportion (%) for discrete data
Fig. 2.
Associations between RVG measures and ACR. (a, b) RVG measures by ACR risk groups. Retinal arteriolar calibre in the extended zone was narrower (a, p<0.001) and tortuosity of venules was greater (b, p=0.005) in the high-risk group than in the low-risk group. (c, d) Continuous association between log10 ACR by RVG measure quintiles. Data for all panels are presented as mean and 95% CIs. p values represent ANOVA for whole-group analysis. p<0.001 in (c); p=0.02 in (d)
Linear regression
In univariable analysis, log10 ACR was inversely associated with BMI SDS (β −0.024 [95% CI −0.038, −0.009], p = 0.002) and diabetes duration (β −0.017 [95% CI −0.021, −0.012], p < 0.001) but not with HbA1c (β 0.0004 [95% CI −0.0006, 0.0014], p = 0.5), SBP SDS (β 0.010 [95% CI −0.004, 0.024], p = 0.1) or cholesterol (β −0.009 [95% CI −0.027, 0.008], p = 0.3).
Log10 ACR was inversely associated with the calibres of the six largest arterioles (CRAE) in extended zones (exMWa and exMWv) but not the calibres of the six largest venules (CRVE). The compound variables (exMWaQ1 & CTvQ5; exMWaQ1 & exMWvQ1) showed an additive effect on log10 ACR (Table 2).
Table 2.
RVG measures are associated with urinary albumin excretion
| RVG measure | Linear regression: outcome log10 ACR | Logistic regression: outcome high-risk ACR group | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable model | Multivariable modela | Univariate model | Multivariable modelb | |||||
| β (95% CI) | p value | β (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| RVG calibrec | ||||||||
| CRAE (μm) | −0.002 (−0.003, −0.001) | 0.004 | −0.001 (−0.002, 0.0003) | 0.1 | 1.20 (0.89, 1.65) | 0.3 | 1.04 (0.73, 1.49) | 0.8 |
| CRVE (μm) | −0.001 (−0.001, 0.0003) | 0.2 | 0.0001 (−0.001, 0.001) | 0.8 | 1.27 (0.92, 1.74) | 0.1 | 0.99 (0.70, 1.41) | 0.95 |
| exMWa (μm) | −0.006 (−0.008, −0.003) | <0.001 | −0.004 (−0.006, −0.002) | 0.001 | 1.96 (1.42, 2.71) | <0.001 | 1.67 (1.17, 2.38) | 0.005 |
| exMWv (μm) | −0.003 (−0.004, −0.001) | 0.01 | −0.001 (−0.003, 0.0008) | 0.3 | 1.77 (1.29, 2.44) | <0.001 | 1.39 (0.98, 1.99) | 0.07 |
| RVG tortuosityd | ||||||||
| Loge CTa ×106 | 0.048 (−0.007, 0.103) | 0.09 | 0.079 (0.02, 0.13) | 0.005 | 1.78 (1.30, 2.46) | <0.001 | 2.05 (1.44, 2.92) | <0.001 |
| Loge CTv ×106 | 0.067 (0.004, 0.13) | 0.03 | 0.096 (0.03, 0.16) | 0.003 | 2.15 (1.56, 2.98) | <0.001 | 2.38 (1.67, 3.40) | <0.001 |
| Combined RVG models | ||||||||
| exMWaQ1 & CTvQ5 vs otherse | 0.12 (0.06, 0.17) | <0.001 | 0.11 (0.05, 0.17) | <0.001 | 3.42 (1.96, 5.97) | <0.001 | 3.32 (1.84, 5.96) | <0.001 |
| exMWaQ1 & exMWvQ1 vs othersf | 0.08 (0.03, 0.13) | 0.002 | 0.05 (−0.01, 0.10) | 0.09 | 2.24 (1.43, 3.50) | <0.001 | 1.77 (1.08, 2.90) | 0.02 |
Each row represents a separate model. Outcome variable for linear regression modelling was log10 ACR. HbA1c, sex, lipids and SBP SDS were not significant in univariable models. Outcome variable for logistic regression modelling was high-risk ACR group. HbA1c, sex and lipids were not significant in univariable models
aMultivariable models are adjusted for BMI SDS and duration
bMultivariable models are adjusted for BMI SDS, duration and SBP SDS
cLogistic regression outcome is shown for Q1 vs Q2–Q5
dLogistic regression outcome is shown for Q5 vs Q1–Q4
eLogistic regression outcome is shown for exMWaQ1 & CTvQ5 vs the others
fLogistic regression outcome is shown for exMWaQ1 & exMWvQ1 vs the others
In multivariable models for each RVG variable, log10 ACR was associated with narrower arteriolar calibres across extended zones (exMWa) and greater arteriolar and venular tortuosity (CTa and CTv). There was a similar trend for venular calibres (exMWv) but this did not reach statistical significance. The compound variable exMWaQ1 & CTvQ5 remained significant with an additive effect on log10 ACR. The associations remained significant after adjusting for BMI SDS and diabetes duration. The compound variable exMWaQ1 & exMWvQ1 showed a similar trend but did not reach statistical significance (Table 2).
Logistic regression
In univariable analysis, high-risk ACR was associated with shorter diabetes duration (OR 0.87 [95% CI 0.84, 0.91], p < 0.001), younger age (OR 0.87 [95% CI 0.80, 0.94], p = 0.001), lower BMI SDS (OR 0.80 [95% CI 0.70, 0.91], p = 0.001) and higher SBP SDS (OR 1.14 [95% CI 1.01, 1.29], p < 0.001), while its association with HbA1c (OR 1.005 [95% CI 0.996, 1.014], p = 0.2) was not significant.
In multivariable analysis, high-risk ACR was associated with the lowest quintile for calibres in the extended zone (exMWa and exMWv, Q1 vs Q2–Q5) and the highest quintile for vessel tortuosity (CTa and CTv, Q5 vs Q1–Q4). The compound variables (exMWaQ1 & CTvQ5; exMWaQ1 & exMWvQ1) showed an additive effect for high-risk ACR (Table 2). These findings remained significant after adjusting for BMI SDS, duration and blood pressure SDS.
eGFR was not significantly associated with RVG variables, either continuously or categorically. No associations were found between hyperfiltration and RVG.
Discussion
In this study we demonstrate that higher urinary ACR in adolescents with type 1 diabetes is associated with a specific RVG phenotype even before onset of clinical complications. Higher ACR was associated with narrower retinal vascular calibres and increased vessel tortuosity even within the normoalbuminuric range.
Our study adds significant new knowledge to this field. First, we provide detailed and extensive analysis over a wider area of the retina (extended zone), measuring not only vessel calibres but also vessel tortuosity. Second, we report that extended zone calibres, both arteriolar and venular (exMWa and exMWv, respectively) rather than CRAE and CRVE, were more robust predictors of log10 ACR. Narrower retinal arterioles (CRAE) in the standard zone have previously been associated with vascular dysfunction and adverse cardiovascular events [19–21]; here, we describe a novel association with vessel calibres in the extended zone. In our study, participants with the narrowest retinal calibres in the extended zone were twice as likely to be in the high-risk ACR group. This pattern suggests that inappropriate microvascular vasoconstriction occurs in the high-risk ACR group, compounding the neuroretinal ischaemia and pro-inflammatory state present in the diabetes milieu early in the disease process. Since the microvasculature provides the greatest contribution to systemic vascular resistance, we postulate that persistent inappropriate arteriolar vasoconstriction will in due course result in systemic blood pressure elevation [21] contributing to shear-stress on the vessel wall and accelerating microvascular pathology. This is in keeping with our observation that the high-risk group had higher SBP SDS, albeit within the normal range. Narrower venules in the setting of inadequate perfusion are also likely to represent dysfunctional autoregulation, which together with higher systemic blood pressure may increase hydrostatic vessel damage. There are clear mechanistic prospects such as hyperglycaemia leading to a downregulation of Ca2+-activated K+ (BK) channels that are important in vasodilatation [22]. Such risk may be mediated through inherited individual differences in sensitivity and response to similar glycaemic exposure [23].
We also demonstrate that greater tortuosity was associated with higher log10 ACR. Notably, the highest quintile of venular tortuosity was associated with greatest probability of being in the high-risk ACR group. We previously demonstrated that greater tortuosity was associated with increased risk of retinopathy [24] and renal dysfunction in our clinic population [25, 26]. We now present the first multinational cohort supporting these findings. We speculate that increased tortuosity may be the result of a maladaptive compensatory increase in vessel density (or early neovascularisation), to improve neuroretinal perfusion, in a predisposed population.
Third, we provide evidence supporting a unifying haemodynamic model integrating afferent (arteriolar) and efferent (venular) components of the microcirculation whereby both components contribute to risk in an additive manner. We report an adverse retinal phenotype comprising narrower vessel calibres and greater tortuousity that was associated with a significantly greater likelihood of a person being in the high-risk ACR group than when either of these measures were used alone.
We observed that individuals in the high-risk ACR group were younger, had shorter diabetes duration and higher blood pressure but no difference in glycaemic control compared with the low-risk group. Thus, our data suggest the high-risk ACR group, screened through ACR, may indeed be a population predisposed to earlier onset of complications and likely to benefit from earlier intervention. Current evidence supports both genetic and metabolic mechanisms that protect against and predispose to diabetes complications [9, 10, 27]. However, a clinically measurable and reproducible biomarker associated with such risk has been elusive. The Renin Angiotensin System Study (RASS), which examined young adults with type 1 diabetes and normoalbuminuria, found that central retinal vascular calibres were associated with histological renal glomerular indices and their progression [7]. This was observed despite there being no functional differences in albumin excretion between the intervention (renin–angiotensin system blockade) and placebo groups, thus highlighting the relevance of the retinal microvasculature as an early biomarker of diabetic nephropathy. Retinal vascular phenotypes may reflect such genetic and metabolic predispositions and thus may serve to identify persons at high risk of future complications. In addition, retinal vascular phenotypes in the extended zones may be more responsive to intervention, including ACE-I and statin therapy and more intensive glycaemic control, early in the disease process.
The strengths of our study include the multinational collaboration and large sample size, standardised retinal photography images and single-centre grading of retinal images masked to clinical and biochemical participant data. However, these are the baseline data and therefore cross-sectional in nature. The longitudinal follow-up from AdDIT will provide invaluable insights into the mechanisms of diabetes complications and potential benefits of early ‘pre-complications’ intervention in paediatric cohorts. In addition to their role as potential biomarkers for risk stratification of future diabetes complications, RVG measures may provide an opportunity to quantify the effect of intervention on the microvasculature in vivo. Furthermore, these RVG quantification techniques may be applied to other previously conducted interventional study cohorts such as RASS and the DCCT. Long-term follow-up of such cohorts may determine the potential to shift the current screening paradigm from one of detection and treatment of diabetes complications to one of identification of persons at risk and prevention of microvascular complications.
In conclusion, we demonstrate that urinary ACR is associated with RVG variables prior to the onset of clinical complications in adolescents with type 1 diabetes. Higher ACR and greater likelihood of being in the high-risk ACR group were associated with narrower and more tortuous retinal vessels, particularly in the extended zones. RVG may assist in identifying individuals at high risk of complications early in the disease process and may provide useful biomarkers by which to quantify response to diabetes therapy.
Acknowledgements
The authors thank all the participants and their families for their involvement in this study. We also thank the Trial Steering Committee, which consisted of Sally Marshall (Chair, Newcastle University, Newcastle, UK), Jane Armitage (University of Oxford, Oxford, UK), Polly Bingley (University of Bristol, Bristol, UK), William Van't Hoff (Great Ormond Street Hospital, London, UK), David Dunger (University of Cambridge, Cambridge, UK), R. Neil Dalton (King's College London, London, UK), Denis Daneman (University of Toronto, Toronto, ON, Canada), Andrew Neil (University of Oxford, Oxford, UK), John Deanfield (University College London, London, UK), Tim Jones (University of Western Australia, Perth, WA, Australia) and Kim Donaghue (University of Sydney, Sydney, NSW, Australia). We thank all the AdDIT centres and research nurses as well as the English National Diabetic Retinopathy Screening Program, especially P. Scanlon and H. Lipinski (Department of Ophthalmology, University of Oxford, UK) for their advice and support, and the Cambridge NIHR Biomedical Research Centre (BRC), Cambridge, UK. We thank A. Pryke, J. Cusumano and T. Jopling (Institute of Endocrinology and Diabetes, The Children’s Hospital at Westmead, NSW, Australia) for data compilation. We thank the study coordinators and research nurses: in Australia, B. Sheil, C. Czank and J. Dart (Dept of Endocrinology, Princess Margaret Hospital, Perth, WA, Australia), N. D’Silva, J. Nesbit and J. Wilson (Department of Paediatric Endocrinology, Mater Children’s Hospital, Brisbane, QLD, Australia), M. Krieg and T. Kelly (Dept of Endocrinology, Women’s and Children’s Hospital, Adelaide, SA, Australia) and N. Jackson and C. Bingley (Dept of Endocrinology, Royal Children’s Hospital, Melbourne, VIC, Australia). In the UK and Canada, our research collaborators and research nurses: A. Murray (South Tees Hospitals NHS Foundation Trust, Middlesbrough, UK), A. Kempa, L. Dudgeon and J. Spimpolo (Northampton General Hospital, Northampton, UK), C. Cleaver (Stoke Mandeville Hospital, Aylesbury, UK), C. Fish (Bolton NHS Foundation Trust, Bolton, UK), C. Megson, J. Bowen-Morris and L. Bunton (Oxford University Hospitals NHS Foundation Trust, Oxford, UK), C. O’Brien and S. Chapman (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK), E. Thomson and P. Woodsford (The Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle, UK), F. Riley and J. Hassler-Hurst (Ipswich Hospitals NHS Trust, Ipswich, UK), H. Roper and L. Fear (Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, UK), I. Newham (Birmingham Children’s Hospital, Birmingham, UK), J. Exall (The Leeds Teaching Hospitals NHS Trust, Leeds, UK), L. Swart (Royal Manchester Children’s Hospital, Manchester, UK), N. Pemberton (Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan, UK), S. Dymond (University Hospitals Bristol NHS Foundation Trust, Bristol, UK), S. Bennett (Stockport NHS Foundation Trust, Stockport, UK) and Y. Elia (Hospital for Sick Children, Toronto, ON, Canada). We thank and acknowledge all participating AdDIT investigators listed in the Appendix.
Some of the data from this study were presented in abstract form and as a poster at the ADA 76th Scientific Sessions, 2016, New Orleans, USA.
Contribution statement
KCD is the guarantor of this work, and takes full responsibility for the contents of the article. PZBA performed data analysis and wrote the manuscript. DBD, TWJ and KCD were responsible for study design and review and editing of the manuscript. LABH performed RVG grading and critically reviewed the manuscript. PZBA, TYW, AC, MEC, EAD, JJC, FJC, FHM and RND were all involved in data generation and collection and critically reviewed/edited the manuscript. All the named authors gave final approval for publication of the manuscript.
Abbreviations
- ACE-I
ACE inhibitor
- ACR
Albumin creatinine ratio
- AdDIT
Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial
- CRAE
Central retinal arteriolar equivalent
- CRVE
Central retinal venular equivalent
- CTa
Arteriolar curvature tortuosity
- CTv
Venular curvature tortuosity
- DBP
Diastolic blood pressure
- exMWa
Mean width of all measurable arterioles in the extended zone
- exMWv
Mean width of all measurable venules in the extended zone
- IQR
Interquartile range
- ORPS
Oxford Regional Prospective Study
- RASS
Renin Angiotensin System Study
- RVG
Retinal vascular geometry
- SBP
Systolic blood pressure
- SDS
Standard deviation score
Appendix
AdDIT investigators in Australia: Tim Jones (Princess Margaret Hospital for Children and University of Western Australia, Perth, WA, Australia), Kim Donaghue (The Children’s Hospital at Westmead and University of Sydney, Sydney, NSW, Australia), Maria Craig (The Children’s Hospital at Westmead, University of Sydney, and University of New South Wales, Sydney, NSW, Australia), Paul Benitez-Aguirre (The Children’s Hospital at Westmead and University of Sydney, Sydney, NSW, Australia), Fergus Cameron (Royal Children’s Hospital, Murdoch Children’s Research Institute and The University of Melbourne, Melbourne, VIC, Australia), Jennifer Couper (Women’s and Children’s Hospital and University of Adelaide, Adelaide, SA, Australia), Elizabeth Davis (Princess Margaret Hospital for Children and University of Western Australia, Perth, WA, Australia), Andrew Cotterill (University of Queensland, Brisbane, QLD, Australia), Bruce King (University of Newcastle, Newcastle, NSW, Australia), Charles Verge (Sydney Children’s Hospital and University of New South Wales, Sydney, NSW, Australia), Phil Bergman (Monash Children’s Hospital, Clayton, VIC, Australia), Christine Rodda (University of Melbourne, Melbourne, VIC, Australia).
AdDIT investigators in the UK: M. Loredana Marcovecchio (University of Cambridge, Cambridge, UK), John Deanfield (University College London, London, UK), Scott Chiesa (University College London, London, UK), Carlo Acerini (Addenbrooke’s Hospital and University of Cambridge, Cambridge, UK), Fran Ackland (Northampton General Hospital, Northampton, UK), Binu Anand (West Suffolk Hospital, NHS Foundation Trust, Bury St Edmunds, UK), Tim Barrett (Birmingham Children’s Hospital and University of Birmingham, Birmingham, UK), Virginia Birrell (James Cook Hospital, South Tees Hospitals NHS Foundation Trust, Middlesbrough, UK), Fiona Campbell (Leeds General Infirmary, The Leeds Teaching Hospitals NHS Trust, Leeds, UK), Marietta Charakida (King’s College London, London, UK), Tim Cheetham (Royal Victoria Infirmary, The Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University, Newcastle, UK), Chris Cooper (Stepping Hill Hospital, Stockport NHS Foundation Trust, Stockport, UK), Ian Doughty (Royal Manchester Children’s Hospital, Manchester, UK), Atanu Dutta (Stoke Mandeville Hospital, Aylesbury, UK), Julie Edge (John Radcliffe Hospital, Oxford, UK), Alastair Gray (University of Oxford, Oxford, UK), Julian Hamilton-Shield (University of Bristol and University Hospitals Bristol NHS Foundation Trust, Bristol, UK), James Heywood (University of Cambridge, Cambridge, UK), Nicola Leech (Newcastle General Hospital, The Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University, Newcastle, UK), Nick Mann (Royal Berkshire Hospital, Reading, UK), Richard Parker (University of Cambridge, Cambridge, UK), Gerry Rayman (Ipswich Hospitals NHS Trust, Ipswich, UK), Jonathon Mark Robinson (Royal Albert Edward Infirmary, Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan, UK), Michelle Russell-Taylor (Wycombe Hospital, Buckingham Healthcare NHS Trust, High Wycombe, UK), Vengudi Sankar (Royal Bolton Hospital, Bolton NHS Foundation Trust, Bolton, UK), Anne Smith (Northampton General Hospital, Northampton, UK), Nandu Thalange (Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, UK), Mark Wilson (University of Cambridge, Cambridge, UK), Chandan Yaliwal (Royal Berkshire Hospital, Reading, UK).
AdDIT investigators in Canada: Farid Mahmud (The Hospital for Sick Children and University of Toronto, Toronto, ON, Canada); Cheril Clarson (London Health Sciences Centre and Western University, London, ON, Canada); Jacqueline Curtis (The Hospital for Sick Children and University of Toronto, Toronto, ON, Canada), Denis Daneman, (The Hospital for Sick Children and University of Toronto, Toronto. ON, Canada), Etienne Sochett (The Hospital for Sick Children and University of Toronto, Toronto, ON, Canada).
Funding
This work was supported by the National Health and Medical Research Council of Australia (NHMRC 632521), JDRF (08-2007-902), Diabetes UK (DUK PO NO 2177 BDA:RD06/003341) and the British Heart Foundation.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to data currently being under embargo for publication but are available from the corresponding author on reasonable request.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
A list of AdDIT investigators is included in the Appendix and members of the Trial Steering Committee are listed in the Acknowledgements.
Contributor Information
David B. Dunger, Email: dbd25@cam.ac.uk
Kim C. Donaghue, Email: kim.donaghue@health.nsw.gov.au
The Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT):
Sally Marshall, Jane Armitage, Polly Bingley, William Van’t Hoff, David Dunger, R. Neil Dalton, Denis Daneman, Andrew Neil, John Deanfield, Tim Jones, and Kim Donaghue
References
- 1.Karalliedde J, Viberti G. Hypertension and microalbuminuria: risk factors for cardiovascular disease in diabetes. Curr Hypertens Rep. 2005;7:1–2. doi: 10.1007/s11906-005-0045-9. [DOI] [PubMed] [Google Scholar]
- 2.Lacquaniti A, Donato V, Pintaudi B, et al. ʻNormoalbuminuricʼ diabetic nephropathy: tubular damage and NGAL. Acta Diabetol. 2013;50:935–942. doi: 10.1007/s00592-013-0485-7. [DOI] [PubMed] [Google Scholar]
- 3.Mottl AK, Kwon KS, Mauer M, Mayer-Davis EJ, Hogan SL, Kshirsagar AV. Normoalbuminuric diabetic kidney disease in the U.S. population. J Diabetes Complicat. 2013;27:123–127. doi: 10.1016/j.jdiacomp.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molitch ME, Steffes M, Sun W, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33:1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho YH, Craig ME, Donaghue KC. Puberty as an accelerator for diabetes complications. Pediatr Diabetes. 2014;15:18–26. doi: 10.1111/pedi.12112. [DOI] [PubMed] [Google Scholar]
- 6.Dunger DB (1992) Diabetes in puberty. Arch Dis Child 67:569–570 [DOI] [PMC free article] [PubMed]
- 7.Klein R, Knudtson MD, Klein BE, et al. The relationship of retinal vessel diameter to changes in diabetic nephropathy structural variables in patients with type 1 diabetes. Diabetologia. 2010;53:1638–1646. doi: 10.1007/s00125-010-1763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velayutham V, Benitez-Aguirre PZ, Craig ME, et al. Innovative technology shows impact of glycaemic control on peripheral retinal vessels in adolescents with type 1 diabetes. Diabetologia. 2017;60:2103–2110. doi: 10.1007/s00125-017-4375-3. [DOI] [PubMed] [Google Scholar]
- 9.Alkayyali S, Lyssenko V. Genetics of diabetes complications. Mamm Genome. 2014;25:384–400. doi: 10.1007/s00335-014-9543-x. [DOI] [PubMed] [Google Scholar]
- 10.Boright AP, Paterson AD, Mirea L, et al. Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes. 2005;54:1238–1244. doi: 10.2337/diabetes.54.4.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcovecchio ML, Woodside J, Jones T, et al. Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT): urinary screening and baseline biochemical and cardiovascular assessments. Diabetes Care. 2014;37:805–813. doi: 10.2337/dc13-1634. [DOI] [PubMed] [Google Scholar]
- 12.The Adolescent type 1 Diabetes cardio-renal Intervention Trial Research Group (2009) Adolescent type 1 Diabetes Cardio-renal Intervention Trial (AdDIT). BMC pediatrics 9: 79 [DOI] [PMC free article] [PubMed]
- 13.Sasongko MB, Wang JJ, Donaghue KC, et al. Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care. 2010;33:1331–1336. doi: 10.2337/dc10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunger DB, Schwarze CP, Cooper JD, et al. Can we identify adolescents at high risk for nephropathy before the development of microalbuminuria? Diabet Med. 2007;24:131–136. doi: 10.1111/j.1464-5491.2006.02047.x. [DOI] [PubMed] [Google Scholar]
- 15.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ. 2008;336:697–701. doi: 10.1136/bmj.39478.378241.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherney DZ, Sochett EB. Evolution of renal hyperfiltration and arterial stiffness from adolescence into early adulthood in type 1 diabetes. Diabetes Care. 2011;34:1821–1826. doi: 10.2337/dc11-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 18.Task Force on Blood Pressure Control in Children Report of the Second Task Force on Blood Pressure Control in Children--1987. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 19.McGeechan K, Liew G, Macaskill P, et al. Risk prediction of coronary heart disease based on retinal vascular caliber (from the Atherosclerosis Risk In Communities [ARIC] Study) Am J Cardiol. 2008;102:58–63. doi: 10.1016/j.amjcard.2008.02.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy MS, Klein R, Janal MN. Relationship of retinal vessel caliber to cardiovascular disease and mortality in African Americans with type 1 diabetes mellitus. Arch Ophthalmol. 2012;130:561–567. doi: 10.1001/archophthalmol.2011.2725. [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Wai KL, McGeechan K, et al. Retinal vascular caliber and the development of hypertension: a meta-analysis of individual participant data. J Hypertens. 2014;32:207–215. doi: 10.1097/HJH.0b013e32836586f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGahon MK, Dash DP, Arora A, et al. Diabetes downregulates large-conductance Ca2+-activated potassium β1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Kateb H, Boright AP, Mirea L, et al. Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes. 2008;57:218–228. doi: 10.2337/db07-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benitez-Aguirre P, Craig ME, Sasongko MB, et al. Retinal vascular geometry predicts incident retinopathy in young people with type 1 diabetes: a prospective cohort study from adolescence. Diabetes Care. 2011;34:1622–1627. doi: 10.2337/dc10-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benitez-Aguirre PZ, Sasongko BM, Craig M, et al. Retinal vascular geometry predicts incident renal dysfunction in young people with type 1 diabetes. Diabetes Care. 2012;35:599–604. doi: 10.2337/dc11-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasongko MB, Wong TY, Donaghue KC, et al. Retinal arteriolar tortuosity is associated with retinopathy and early kidney dysfunction in type 1 diabetes. Am J Ophthalmol. 2012;153:176–183. doi: 10.1016/j.ajo.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Kao Y, Donaghue KC, Chan A, Bennetts BH, Knight J, Silink M. Paraoxonase gene cluster is a genetic marker for early microvascular complications in type 1 diabetes. Diabet Med. 2002;19:212–215. doi: 10.1046/j.1464-5491.2002.00660.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to data currently being under embargo for publication but are available from the corresponding author on reasonable request.