Abstract
Gardenia jasminoides Ellis, which belongs to the Rubiaceae family, is a widely used traditional Chinese medicine. Although effect of Gardenia jasminoides Ellis has been widely reported, its anti-inflammatory role in intestinal mucosal injury induced by LPS remains unclear. In the present study, we investigated the effects of decoction extracted from Gardenia jasminoides on the morphology and intestinal antioxidant capacity of duodenum induced by LPS in mice. Further analysis was carried out in the expression of inflammatory and anti-inflammatory cytokines. Nuclear factor-kappa B (NF-κB) was determined by Western blot. Gardenia jasminoides water extract was qualitative analyzed by high-performance liquid chromatography coupled with electro spray ionization quadrupole time-of-flight mass spectrometry. The results showed that Gardenia decoction markedly inhibited the LPS-induced Tumor necrosis factor (TNF)-α, Interleukin (IL)-6, IL-8, and IL-1 production. It was also observed that Gardenia decoction attenuated duodenum histopathology changes in the mouse models. Furthermore, Gardenia decoction inhibited the expression of NF-κB in LPS stimulated mouse duodenum. These results suggest that Gardenia decoction exerts an anti-inflammatory and antioxidant property by up-regulating the activities of the total antioxidant capacity (T-AOC), the total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-Px). Gardenia decoction is highly effective in inhibiting intestinal mucosal damage and may be a promising potential therapeutic reagent for intestinal mucosal damage treatment.
Keywords: Gardenia jasminoides, decoction, Lipopolysaccharide, cytokine, NfκB
Introduction
The intestine plays a crucial role in digesting and absorbing nutrients, balancing microbiota, protecting immunological functions and serves as a barrier against harmful pathogens and antigens (Vancamelbeke and Vermeire, 2017). Lipopolysaccharide (LPS/endotoxin), the major constituent of the outer membrane of gram-negative bacteria, is a common trigger of intestinal mucosal injury. The LPS-induced cytokine release leads to the pathophysiologic derangement associated with intestinal mucosal injury. Many cellular signals that are activated by Gram-negative bacteria are contributed to LPS. Not only does LPS trigger inflammatory responses, but it also activates pro-apoptotic signals in macrophages, endothelial cells, epithelial cells and immune cells (Ma et al., 2012; Plociennikowska et al., 2015). NF-κB plays a critical role in immune and inflammatory responses. It has been known to be present in most cell types and many of the inflammatory proteins expressed are regulated by NF-κB (Hayden and Ghosh, 2014).
Despite a growing understanding of the pathophysiology of intestinal mucosal damage, its molecular regulatory mechanisms in induction of cytokines expression and activation/recruitment of inflammatory in intestinal mucosal damage remain elusive. However, there is a need for innovative anti-inflammatory therapeutic protocols and some previous studies have shown that intestinal mucosal damage is associated with persistent activation of NF-κB (Medicherla et al., 2015). Therefore, the remedial measures of anti-inflammatory properties based on the NF-κB signaling pathway may be a potentially useful option. Likewise, growing evidence suggests that numerous components of Chinese medicinal herbs exert excellent anti-inflammatory effects through the negative regulation of NF-κB signaling pathway (Shen et al., 2018).
Gardenia jasminoides, an evergreen tree that belongs to the Rubiaceae family, is cultivated in multiple areas in China, with a Chinese name of Zhi Zi. It grows in many temperate regions and has fragrant white flowers (Ma et al., 2017). It is not only used as natural yellow dyes for many years (Chen et al., 2017; Ma et al., 2017), but also has various biological activities, such as antidiabetic (Wu et al., 2009), anti-inflammatory (Oliveira et al., 2017), anti-depression (Tao et al., 2014), and antioxidant properties (Guo et al., 2014), and improvement of the quality of sleep (Zhang et al., 2017). It is commonly used in traditional Chinese medicine. However, there are not so many reports studies focusing on the decoction of Gardenia jasminoides. Some studies showed that Geniposide inhibited Lipopolysaccharide-induced Apoptosis (Song et al., 2014). It is still not elucidated whether oral administration of Gardenia jasminoides decoction (GD) could provide a protective effect during intestinal mucosal injury and what is the underlying mechanism. The current study, we investigated the preventive effect of GD in LPS induced experimental intestinal mucosal injury in mice.
Materials and Methods
Reagents
The main reagents and antibodies used in our experiments are as follows: Antibodies recognizing NF-κB p65 (10745-1-AP) was purchased from Proteintech and β-actin was from Cell Signaling (Beverly, MA, USA). Secondary antibody was from Biosynthesis (Beijing, China). LPS from Escherichia coli 055:B5 was obtained from Sigma (St. Louis, MO, USA). LPS was suspended in physiological saline and stored as a 20 mg/ml stock. Animals were weighed before injecting LPS and the LPS of each animal was diluted to an appropriate dose. Dilute solution prior to injection were into normal saline.
Animals
Animal protocols were approved by Heilongjiang Bayi Agricultural University's Institutional Animal Care and Use Committee. A total of 50 male ICR mice (22–25 g body weight) were purchased from the Animal Experiment Center of HARBIN MEDICAL UNIVERSITY (Daqing, China). All animals were kept in the temperature controlled room with 12 h dark/light cycles and maintained under specific-pathogen-free conditions and were given a standard mice diet and tap water for 1 week before experiments.
Preparation of G. jasminoides Decoction
Gardenia jasminoides were purchased from Fu Rui Bang Chinese Medicine Co., Ltd. (Daqing, China). The general preparation procedure of G. Jasminoides decoction (GD) is as follows (Qin et al., 2015; Yu et al., 2015; Cui et al., 2017). Briefly, 100 g G. jasminoides Fruit were extracted by refluxing with water (1:10, w/v) for 2 h following sonication for 30 min, and then the extraction solutions were combined to be filtered and concentrated to 100 mL under reduced pressure. The concentrations of the residues were 1 g/mL for G. jasminoides fruit (Tao et al., 2014). Finally, the concentration be adjusted to the required with distilled water for intragastrical administration. After being autoclaved at 100°C for 20 min, the stock solution was stored at 4°C.
LC/MS Analysis
The samples were thawed at room temperature, 100 μL of them was then transferred into Centrifuge Tubes (1.5 mL) by pipette. All samples were extracted with 300 μL of methanol, and 10 μL of internal standard (3 mg/mL, DL-o-Chlorophenylalanine) was added. The samples were then ultra-sonicated at 4 K Hz on ice bath for 30 min. The samples were vortexes for 30 s, and centrifuged at 12,000 rpm and 4°C for 15 min. Two hundred microliter of supernatant was transferred to vial for LC-MS analysis. Analysis platform: LC-MS (Thermo, Ultimate 3000LC, Orbitrap Elite) Column: Waters ACQUITYUPLC HSS T3column (2.1 mm × 100 mm, 1.8 μm) Chromatographic separation conditions: Column temperature: 40°C; Flow rate: 0.3 mL/min; Mobile phase A: water + 0.1% formic acid; Mobile phase B: acetonitrile + 0.1% formic acid; Injection volume: 4 μL; Automatic injector temperature: 4°C. The data was performed feature extraction and preprocessed with Compound Discoverer software (Thermo), and then normalized and edited into two-dimensional data matrix by excel 2010 software, including Retention time(RT), Compound Molecular Weight (comp MW), Observations (samples) and peak intensity.
Grouping and Treatment
In experiments, animals were randomly divided into five groups: the normal control group, the LPS group, and GD high-dose, medium-dose and low-dose groups.GD-treated groups were given G. jasminoides decoction by intragastric administration once daily for 3 d. The normal control group and the LPS group were orally administered with double distilled water. One hour after the oral administration on 3 d, the control group received intraperitoneal injection of normal saline, while the other group received intraperitoneal injection of LPS (Escherichia coli 055:B5, 5 mg/kg; Sigma). At 20 h after the injection of LPS, all of the mice were sacrificed and their duodenum tissues were collected. The blood samples were centrifuged at 5,000 rpm for 10 min, and were subsequently stored at −80°C before analysis.
Estimation of Cytokine Levels
Serum levels of various cytokines were estimated by enzyme-linked immunosorbent assay (Boster, Wu han, China). All analyses were conducted as described by the manufacturer.
Duodenum Morphology
Part of the intestinal wall of the duodenum was prepared for histological examination by fixing in 4% formaldehyde-buffered solution, embedding in paraffin, and sectioning. The tissues were then embedded in paraffin and cut into 5 μm sections used for H&E staining (Cui et al., 2018). Villous height and the associated crypt depth were evaluated as described by Nabuurs et al. (1993) and Greig and Cowles (2017).
Determination of Antioxidant Index in the Duodenum
To evaluate the provident-antioxidant balance in the duodenum, we determined total antioxidant capacity (T-AOC), the total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-Px) activities (Nanjing jiancheng Bioengineering institute). The method was describe as Fang et al. (2016). The duodenum samples were thawed, weighed, and homogenized (1:10, wt/vol) in 9 volumes of ice-cold physiologic saline. The homogenates were centrifuged at 3,000 × g for 10 min at 4°C, the supernatants collected and enzyme activities analyzed.
Western Blot
The proteins were extracted from frozen intestinal tissues with an extract kit according to the manufacturer's protocol (cat. no. P0028; Beyotime Institute of Biotechnology, Haimen, China). Protein concentration was determined using a BCA assay. Equal amounts (50 μg per lane) of protein were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 100 v for 3 h electrophoretic ally transferred to nitrocellulose/polyvinyl lidenedifluoride membranes (Pierce Biotechnology, Rockford, IL/Bio-Rad Laboratories, Hercules, CA), and blocked for 1 h in phosphatebuffered saline containing Tween 20 (0.1%) and non-fat milk (5%). The membranes were incubated overnight at 4°C with rabbit anti-mouse polyclonal antibodies to NF-κB (1:1,000 dilution), β-actin (1:2,000 dilution). After washing for three times with Tris-buffered saline containing Tween-20, the membranes were incubated with the corresponding goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000 dilutions) for 1 h at room temperature. Band intensities were measured using Image J software (National Institutes of Health).
Data Analysis
All the experimental values obtained were expressed as mean ± SD. One-way ANOVA showed significant differences among multiple group comparisons. Analysis was performed with the software SPSS version 16.0 (SPSS Inc., USA). P < 0.05 was considered significant. Statistical significance was calculated by use of Student's t-test (two-group comparison).
Results
Characteristics of Compounds From the Herbal Formula GD
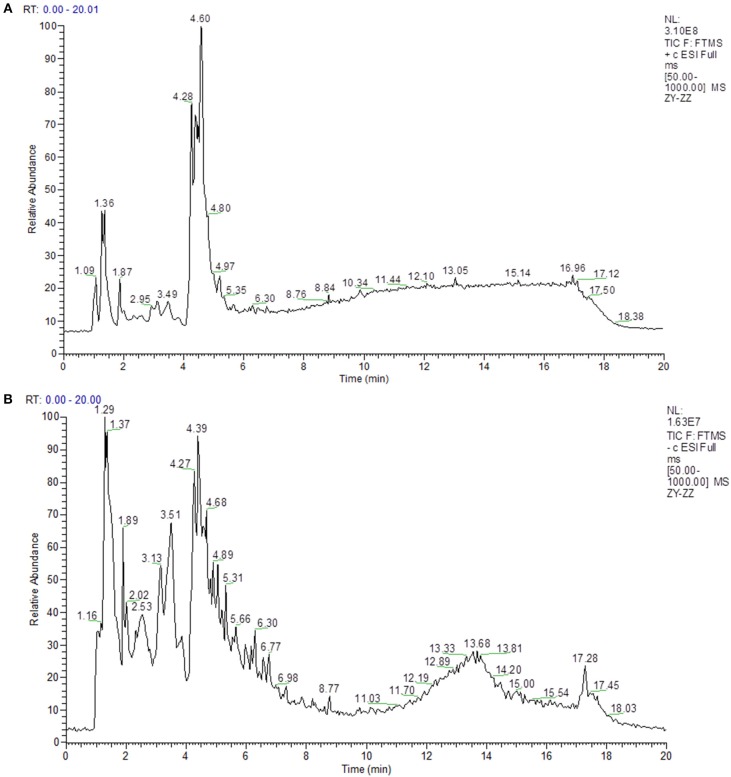
In this study, LC-MS analysis was performed in negative and positive ion modes to obtain complete information about the chemical constitution of GD. The peak MS spectrum has been presented in Figure 1. All constituents were full spectrum identified based on the accurate mass and network database Metlin. The identified compounds are shown in Tables 1,2.
Figure 1.
The Total Ion Chromatogram of GD. (A) The Total Ion Chromatogram of GD (ESI+). (ESI+) represents the positive ion detection mode, in which the mass analyzer scans only positive charged ions and filters out negative charged ions to obtain positive charged ions information during the detection process. (B) The Total Ion Chromatogram of GD (ESI–). (ESI–) denotes the negative ion detection mode, in which the mass analyzer scans only negative charged ions and filters out positive charged ions, thus obtaining the information of negative charged ions.
Table 1.
Chemical components identified from GD by high-performance liquid chromatography-electrospray ionization/mass spectrometry (ESI+).
| Name | Rt (min) | Molecular weight | CAS | Content (ng/μL) |
|---|---|---|---|---|
| L-Phenylalanine | 2.983 | 165.0785 | 63-91-2 | 79.79 |
| L-Arginine | 1.201 | 174.111 | 74-79-3 | 6.23 |
| L-Tyrosine | 1.397 | 181.0732 | 60-18-4 | 6.58 |
| L-Glutamate | 1.313 | 147.0525 | 56-86-0 | 1.72 |
| L-Isoleucine | 2.072 | 131.0941 | 61-90-5 | 28.07 |
| L-Lysine | 1.16 | 146.1049 | 56-87-1 | 0.21 |
| L-Proline | 1.341 | 115.0628 | 147-85-3 | 3.61 |
| Pyroglutamic acid | 1.98 | 129.042 | 98-79-3 | 7.89 |
| Ferulic acid | 4.69 | 194.0573 | 1135-24-6 | 18.63 |
| Sinapic acid | 4.66 | 224.0675 | 530-59-6 | 43.02 |
| Styrene | 6.038 | 104.0621 | 100-42-5 | 0.39 |
| Chorismic acid | 3.906 | 226.0474 | 617-12-9 | 4.24 |
| m-Coumaric acid | 4.64 | 164.0467 | 588-30-7 | 78.65 |
| 1,2,3-Trihydroxybenzene | 3.232 | 126.0311 | 533-73-3 | 0.78 |
| Caffeic Acid | 4.48 | 180.0415 | 4607-41-4 | 3.03 |
| Thymol | 4.451 | 150.1038 | 89-83-8 | 15.89 |
| Adenosine | 1.965 | 267.0958 | 58-61-7 | 60.13 |
| Adenine | 1.957 | 135.0546 | 73-24-5 | 0.06 |
| Guanosine | 1.96 | 283.0908 | 118-00-3 | 3.02 |
| Guanine | 1.441 | 151.0487 | 73-40-5 | 1.98 |
| cAMP | 1.473 | 329.0502 | 60-92-4 | 11.12 |
| Quercetin 3-galactoside | 4.491 | 464.0946 | 482-36-0 | 3.94 |
| Arcapillin | 5.282 | 360.0832 | NA | 9.50 |
| Glyceollin | 5.928 | 338.1144 | NA | 0.02 |
| Isorhamnetin | 4.954 | 316.0571 | 480-19-3 | 0.65 |
| Malvidin | 5.267 | 330.0727 | 643-84-5 | 2.13 |
| Naringenin | 5.177 | 272.0675 | 480-41-1 | 0.07 |
| Quercetin | 4.988 | 302.0415 | 117-39-5 | 1.27 |
| Quercetin 3-(3-p-coumaroylglucoside) | 4.653 | 610.1301 | 76211-70-6 | 0.12 |
| Rhamnetin | 5.528 | 316.0572 | 480-19-3 | 0.03 |
| Taxifolin | 4.405 | 304.0572 | 480-18-2 | 0.09 |
| Cyanidin 3-O-rutinoside | 4.334 | 594.1558 | 28338-59-2 | 0.29 |
| Diosmetin | 5.179 | 300.0621 | 520-34-3 | 0.51 |
| Eriodictyol | 4.51 | 288.0621 | 552-58-9 | 0.11 |
| Genistein | 4.478 | 270.0516 | 446-72-0 | 0.15 |
| Genistin | 4.476 | 432.1037 | 529-59-9 | 0.30 |
| Luteolin | 4.806 | 286.0465 | 491-70-3 | 1.53 |
| Pelargonidin 3-O-(6-O-malonyl-β-D-glucoside) | 4.525 | 518.1035 | 165070-68-8 | 0.08 |
| Pelargonidin 3-O-rutinoside | 4.389 | 578.1612 | NA | 0.72 |
| Petunidin 3-O-glucoside | 4.537 | 478.1092 | 6988-81-4 | 0.09 |
| Quercitrin | 4.503 | 448.0987 | 522-12-3 | 1.07 |
| Sakuranin | 4.532 | 448.1351 | NA | 0.10 |
| Scutellarein 5-glucuronide | 4.501 | 462.0778 | NA | 0.25 |
| Naringin | 4.482 | 580.1763 | 10236-47-2 | 0.64 |
| Gallocatechin | 1.388 | 306.0707 | NA | 4.92 |
| Peonidin 3-rhamnoside 5-glucoside | 13.76 | 609.1748 | 53859-11-3 | 0.26 |
| Hesperetin | 4.538 | 302.0778 | 520-33-2 | 0.58 |
| 2-Hexyl-3-phenyl-2-propenal | 5.773 | 216.1506 | 101-86-0 | 0.98 |
| DL-pipecolic acid | 1.925 | 129.0785 | 535-75-1 | 3.26 |
| Hydroquinidine | 4.963 | 326.1984 | 1435-55-8 | 0.03 |
| Hypoxanthine | 1.963 | 136.0379 | 68-94-0 | 0.25 |
| Trigonelline | 1.584 | 137.0471 | 535-83-1 | 0.63 |
| Xanthosine | 4.474 | 284.0787 | 146-80-5 | 0.20 |
| Caffeine | 4.413 | 194.0837 | 58-08-2 | 0.10 |
| D-Mannitol | 1.231 | 182.0785 | 69-65-8 | 0.72 |
| a-L-Rhamnose | 1.239 | 164.0679 | 6014-42-2 | 0.83 |
| Gibberellin A53 | 5.402 | 348.1923 | NA | 0.08 |
| Glutinosone | 5.699 | 220.1455 | 55051-94-0 | 0.37 |
| Plaunol B | 4.789 | 356.1247 | 69749-00-4 | 2.28 |
| Quillaic acid | 6.58 | 486.3329 | 631-01-6 | 8.02 |
| Genipin | 4.406 | 226.083 | 6902-77-8 | 12.45 |
| Medicagenic acid | 6.215 | 502.327 | 599-07-5 | 5.55 |
| p-Cymene | 4.894 | 134.1089 | NA | 0.56 |
| Pantothenic Acid | 3.524 | 219.1103 | 137-08-6 | 59.57 |
| Pyridoxine | 2.326 | 169.0736 | 65-23-6 | 1.04 |
| Pyridoxal | 3.258 | 167.0579 | 66-72-8 | 0.09 |
| Niacin | 5.633 | 123.0314 | 59-67-6 | 0.09 |
| Niacinamide | 1.985 | 122.0473 | 98-92-0 | 7.07 |
| Palmitic amide | 9.57 | 255.2558 | 629-54-9 | 8.89 |
| 13Z-Docosenamide | 13.06 | 337.3334 | 112-84-5 | 20.22 |
| Oleamide | 9.873 | 281.2709 | 301-02-0 | 34.54 |
| Stearamide | 12.982 | 283.2865 | 124-26-5 | 1.67 |
| Coumarin | 5.111 | 146.0362 | 91-64-5 | 1.58 |
| 3 Hydroxycoumarin | 3.902 | 162.0309 | 939-19-5 | 10.58 |
| Scopoletin | 4.766 | 192.0414 | NA | 3.25 |
| Benzoic acid | 4.7 | 122.0362 | 65-85-0 | 0.50 |
| α-ketoisovaleric acid | 1.86 | 116.0469 | 759-05-7 | 1.07 |
| Succinic acid | 1.957 | 118.0273 | 110-15-6 | 14.87 |
| nandrolone | 5.468 | 274.1923 | 434-22-0 | 1.36 |
| α-Linolenic Acid | 7.357 | 278.224 | 463-40-1 | 2.15 |
| Butyric acid | 1.866 | 88.0521 | 107-92-6 | 2.26 |
| LysoPC (16:0) | 7.257 | 495.3313 | NA | 9.12 |
| MG (0:0/18:3/0:0) | 6.214 | 352.2602 | NA | 1.28 |
| Indoleacrylic acid | 4.278 | 187.0625 | 1204-06-4 | 25.29 |
| Methyl cinnamate | 3.805 | 162.0675 | 103-26-4 | 15.33 |
| 5-Hydroxy-L-tryptophan | 2.276 | 220.0845 | 4350-09-8 | 7.11 |
| Indoleacetaldehyde | 2.371 | 159.0681 | NA | 0.98 |
| Acetylcholine | 2.005 | 145.1099 | 51-84-3 | 0.04 |
| Cinnamic acid | 3.612 | 148.0521 | 621-82-9 | 5.94 |
| Gingerol | 5.765 | 294.182 | 58253-27-3 | 1.93 |
| Hippuric acid | 4.356 | 179.0576 | 495-69-2 | 0.28 |
| Jasmolone | 5.898 | 180.1144 | 54383-66-3 | 5.56 |
| (-)-Jasmonic acid | 5.713 | 210.1247 | 6894-38-8 | 0.04 |
| Indole | 4.301 | 117.0573 | 120-72-9 | 19.62 |
| Methyl jasmonate | 4.519 | 224.1403 | 39924-52-2 | 12.53 |
| Phenylacetic acid | 4.746 | 136.0518 | 103-82-2 | 2.22 |
| Acetophenone | 4.403 | 120.0568 | 98-86-2 | 148.13 |
| Choline | 9.289 | 103.0991 | 62-49-7 | 2.23 |
| Tropic acid | 4.458 | 166.065 | 552-63-6 | 0.28 |
Table 2.
Chemical components identified from GD by high-performance liquid chromatography-electrospray ionization/mass spectrometry (ESI–).
| Name | Rt (min) | Molecular weight | CAS | Content (ng/μL) |
|---|---|---|---|---|
| L-Isoleucine | 2.06 | 131.09469 | 61-90-5 | 69.85 |
| L-Phenylalanine | 2.933 | 165.07893 | 63-91-2 | 1577.95 |
| Pyroglutamic acid | 1.991 | 129.04272 | 98-79-3 | 130.58 |
| L-Cystine | 4.179 | 240.02653 | 56-89-3 | 29.79 |
| Chlorogenic Acid | 4.127 | 354.09478 | 327-97-9 | 6.83 |
| ferulic acid | 4.705 | 194.0574 | 1135-24-6 | 67.37 |
| Sinapic acid | 4.68 | 224.06787 | 530-59-6 | 1442.50 |
| 1,2,3-Trihydroxybenzene | 3.154 | 126.03172 | 533-73-3 | 406.95 |
| Caffeic Acid | 3.013 | 180.04208 | 4607-41-4 | 13.88 |
| Gallic acid | 3.708 | 170.02138 | 149-91-7 | 75.26 |
| Gentisic acid | 3.623 | 154.0266 | 490-79-9 | 418.77 |
| Shikimic acid | 1.836 | 174.05273 | 138-59-0 | 1125.65 |
| Homogentisic acid | 3.694 | 168.04204 | 451-13-8 | 92.70 |
| m-Coumaric acid | 4.65 | 164.04712 | 588-30-7 | 85.87 |
| Syringic acid | 2.887 | 198.05249 | 530-57-4 | 259.47 |
| Salicylic acid | 4.496 | 138.03141 | 69-72-7 | 186.01 |
| Uridine | 2.02 | 244.06907 | 58-96-8 | 29.12 |
| Inosine | 1.276 | 268.07889 | 58-63-9 | 1744.91 |
| IMP | 4.452 | 348.04661 | 131-99-7 | 5.67 |
| cAMP | 1.971 | 329.05183 | 60-92-4 | 12.55 |
| Diosmetin | 5.179 | 300.06245 | 520-34-3 | 10.54 |
| Genistein | 4.566 | 270.05208 | 446-72-0 | 5.29 |
| Malvidin | 5.272 | 330.07307 | 643-84-5 | 49.08 |
| Naringenin | 5.185 | 272.06776 | 480-41-1 | 5.08 |
| Quercetin | 5.038 | 302.04179 | 117-39-5 | 151.07 |
| Cyanidin 3-O-rutinoside | 4.326 | 594.15626 | 28338-59-2 | 215.56 |
| Isorhamnetin | 4.948 | 316.05741 | 480-19-3 | 28.09 |
| Luteolin | 4.861 | 286.04682 | 491-70-3 | 98.57 |
| Pelargonidin 3-O-rutinoside | 4.944 | 578.16133 | NA | 7.12 |
| Petunidin 3-O-glucoside | 4.585 | 478.10942 | 6988-81-4 | 12.44 |
| Quercitrin | 4.555 | 448.09913 | 522-12-3 | 54.73 |
| Dihydromyricetin | 4.479 | 320.05192 | 27200-12-0 | 5.70 |
| Eriodictyol | 4.523 | 288.06209 | 552-58-9 | 6.25 |
| Naringin | 4.499 | 580.17667 | 10236-47-2 | 497.90 |
| Quercetin 3-(3-p-coumaroylglucoside) | 4.67 | 610.12941 | 76211-70-6 | 5.09 |
| Quercetin 3-galactoside | 4.519 | 464.09335 | 482-36-0 | 355.12 |
| Scutellarein 5-glucuronide | 4.502 | 462.07786 | NA | 26.79 |
| Taxifolin | 4.43 | 304.05702 | 480-18-2 | 5.79 |
| Rutin | 4.428 | 610.14931 | 153-18-4 | 869.84 |
| Hesperetin | 4.523 | 302.07789 | 520-33-2 | 7.26 |
| Purine | 1.299 | 120.04223 | 120-73-0 | 2218.84 |
| 2-Furoic acid | 1.439 | 112.01615 | 88-14-2 | 5378.42 |
| Caffeine | 4.492 | 194.08423 | 58-08-2 | 8.44 |
| D-Glucarate | 1.543 | 210.03737 | 87-73-0 | 1339.49 |
| D-Glucuronic acid | 1.264 | 194.04247 | 6556-12-3 | 465.85 |
| Glutaric acid | 1.311 | 132.04226 | 110-94-1 | 14780.91 |
| L-Xylulose | 1.458 | 150.05294 | 527-50-4 | 77.69 |
| D-Mannitol | 1.265 | 182.07878 | 69-65-8 | 3230.88 |
| Gluconic acid | 1.299 | 196.058 | 526-95-4 | 15723.38 |
| α-D-Glucose | 1.307 | 180.06317 | 492-62-6 | 2610.07 |
| α,α-Trehalose | 1.738 | 342.1154 | 57-50-1 | 694.30 |
| Raffinose | 4.067 | 504.16731 | 512-69-6 | 9.04 |
| Genipin | 4.414 | 226.08368 | 6902-77-8 | 1182.88 |
| Gibberellin A12 | 8.093 | 332.19787 | NA | 7.66 |
| Medicagenic acid | 6.193 | 502.32825 | 599-07-5 | 5112.01 |
| Quillaic acid | 6.564 | 486.33328 | 631-01-6 | 2459.37 |
| Rishitin | 7.443 | 222.16141 | 18178-54-6 | 495.43 |
| Gibberellin A17 | 4.924 | 378.1664 | 18411-79-5 | 48.05 |
| Gibberellin A36 | 5.465 | 362.17181 | NA | 56.04 |
| Ganoderic acid H | 17.348 | 572.2945 | 98665-19-1 | 391.13 |
| Geranyl diphosphate | 4.391 | 314.06284 | 763-10-0 | 9.75 |
| Pantothenic Acid | 3.485 | 219.1103 | 137-08-6 | 1709.46 |
| Riboflavin | 4.246 | 376.1359 | 83-88-5 | 416.48 |
| Sulfuric acid | 1.575 | 97.96744 | 7664-93-9 | 5308.81 |
| Phosphoric acid | 1.471 | 97.97696 | 7664-38-2 | 473.05 |
| Benzoic acid | 4.717 | 122.03673 | 65-85-0 | 171.79 |
| Citric acid | 1.446 | 192.02674 | 77-92-9 | 56678.22 |
| Lactic acid | 2.959 | 90.0318 | 50-21-5 | 37.83 |
| Pyruvate | 1.45 | 88.01615 | 127-17-3 | 1368.82 |
| Hexadecanedioic acid | 5.656 | 286.21382 | NA | 37.39 |
| Quinic acid | 4.373 | 192.06302 | 77-95-2 | 1839.95 |
| Aconitic acid | 2 | 174.0164 | 499-12-7 | 1098.77 |
| Itaconic acid | 2.512 | 130.02669 | 97-65-4 | 344.08 |
| Maleic acid | 1.996 | 116.01102 | 110-16-7 | 192.83 |
| Malic acid | 1.879 | 134.02155 | 6915-15-7 | 13050.35 |
| Oxoglutaric acid | 1.487 | 146.02162 | 328-50-7 | 1208.40 |
| Succinic acid | 2.072 | 118.02664 | 110-15-6 | 8700.38 |
| Glyceric acid | 1.354 | 106.02678 | 473-81-4 | 223.93 |
| Nandrolone | 5.46 | 274.19264 | 434-22-0 | 7.66 |
| α-Linolenic Acid | 7.321 | 278.22397 | 463-40-1 | 140.85 |
| LysoPC(15:0) | 7.22 | 481.31539 | NA | 978.13 |
| Traumatic Acid | 5.273 | 228.13561 | 6402-36-4 | 66.26 |
| Acetophenone | 4.646 | 120.05742 | 98-86-2 | 42.39 |
| Citramalic acid | 1.499 | 148.03727 | 2306-22-1 | 1513.97 |
| Mevalonic acid | 3.028 | 148.07363 | 150-97-0 | 44.67 |
| Phenylacetic acid | 4.741 | 136.05243 | 103-82-2 | 150.15 |
| (-)-Jasmonic acid | 5.711 | 210.12533 | 6894-38-8 | 5.62 |
| Malonic acid | 1.474 | 104.0111 | 141-82-2 | 304.15 |
| Xanthoxin | 6.275 | 250.15644 | 8066-07-07 | 8.61 |
| Gentisin | 4.621 | 258.05214 | 437-50-3 | 38.04 |
| Tropic acid | 4.432 | 166.06257 | 552-63-6 | 341.15 |
| Xanthoxic acid | 9.992 | 266.15443 | NA | 39.28 |
Serum Concentrations of Cytokine
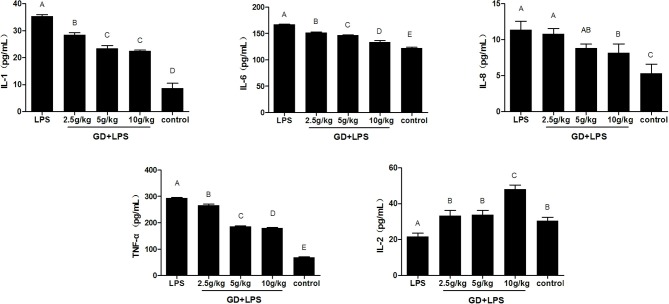
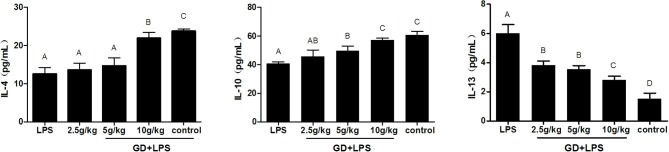
Compared with the control group, the concentration of inflammatory cytokine IL-1, IL-6, IL-8, and TNF-α in the LPS model group were significantly higher (P < 0.05). The level of IL-1, IL-6, IL-8, and TNF-α in the low, medium and high dose GD-treated group were significantly lower than that of the LPS group with a dose-dependent manner (P < 0.05). IL-2 was contrary to the changes of other inflammatory cytokines (Figure 2). In addition, compared with the control group, the concentration of anti-inflammatory cytokine IL-4, and IL-10 were significantly reduced in the LPS model group in the serum (P < 0.05). Compared with the LPS model group, the level of IL-4 and IL-10 in the low, medium and high dose GD-treated group were gradually increased with a dose-dependent manner and significant difference (P < 0.05). However, IL-13 was contrary to the changes of other anti-inflammatory cytokines (Figure 3).
Figure 2.
Effects of GD on the production of inflammatory cytokines in the serum. The data are expressed as the mean ± SD (n = 10 per treatment group). The values with same superscript letters between groups are of no significant difference (P > 0.05), those with same letters are of significant difference (P < 0.05). Interleukin (IL)−1, IL-2, IL-6, IL-8, and Tumor necrosis factor (TNF)-α.
Figure 3.
Effects of GD on the production of anti-inflammatory cytokines in the serum. The data are expressed as the mean ± SD (n = 10 per treatment group). The values with same superscript letters between groups are of no significant difference (P > 0.05), those with different letters are of significant difference (P < 0.05). Interleukin (IL)−4, IL-10, and IL-13.
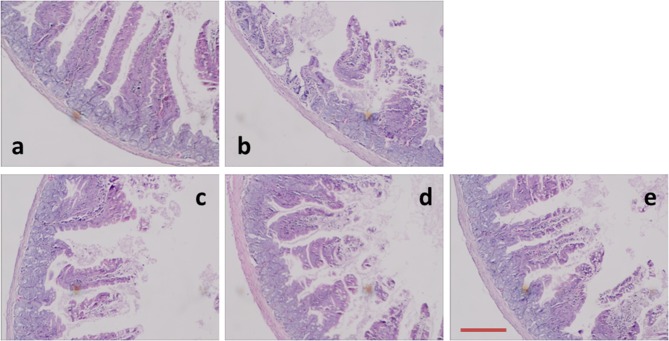
Histopathological Changes in Duodenum Tissue
The microscopic morphology was observed with HE staining. The pathological changes were obvious in the duodenum (Figure 4). Compared to the control animals, LPS-treated groups caused significant mucosal damage, and that is, epithelial shedding, villi fracturing, mucosal atrophy, edema and the villus had shortened. The length of duodenal villi in GD medium and high dose groups increased significantly compared with that in LPS group (P < 0.05), and the degree of intestinal mucosal injury was significantly lower than that in LPS group. Under the light microscope, the degree of duodenal mucosal injury was graded according to the standard (Chiu et al., 1970). The score of intestinal mucosal injury in each group was shown in Table 3. Compared with LPS group, the damaged level of intestinal mucous membrane was slighter than that of medium and high dose groups.
Figure 4.
Photomicrographs of mice duodenum tissues. (a) Control group, (b) LPS group, (c) GD low-dose group, (d) GD medium-dose group, and (e) GD high-dose group. Histological appearance of mice intestinal mucosa after hematoxylin and eosin (H&E) stain (original magnification 100×). Scale bars: 50 μm.
Table 3.
Effects of GD on the morphological structure of duodenum in the mice.
| Groups | VH | CD | V/C | Mucosal injury |
|---|---|---|---|---|
| LPS | 151.34 ± 15.26A | 74.75 ± 7.42A | 2.32 ± 0.52A | 4.32 ± 0.42A |
| Low | 175.25 ± 19.07A | 62.88 ± 12.30AB | 2.56 ± 0.70A | 3.97 ± 0.34A |
| medium | 213.00 ± 29.91B | 60.38 ± 6.22AB | 3.46 ± 1.46AB | 2.65 ± 0.24B |
| High | 246.5 ± 16.62B | 54.12 ± 3.25B | 4.36 ± 0.15BC | 1.23 ± 0.28C |
| Control | 272.53 ± 24.65B | 53.75 ± 2.84B | 5.47 ± 0.78C | 0.54 ± 0.02D |
The data are expressed as the mean ± SD (n = 10 per treatment group). VH (villus height), CD (crypt depth), and V/C (villus height/crypt depth). Grade of intestinal mucosal injury. Grade 0, normal mucosa; Grade 5, the most extensive denudation of mucosa. The difference between groups was significantly different in different capitals.
Histomorphological Analyses
As shown in Table 3, compared with the normal group, the Villus height (VH) and ratio of villus height to crypt depth (V/C) decreased significantly in LPS group. In addition, the VH decreased significantly in low dose group. The crypt depth (CD) was higher in the GD treatment group than that of control group, but lower than that of LPS group. The VH in high dose group decreased slightly, and the CD and V/C were not significantly different from those in normal group, which was significantly higher than that in LPS group.
Antioxidant Indicators in the Duodenum
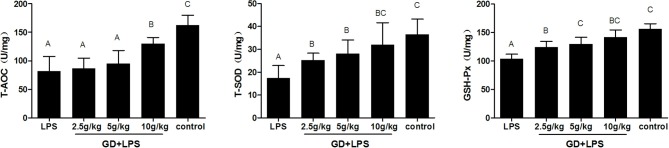
As the Figure 5 shows, compared with the control, the activities of the T-AOC, T-SOD, and GSH-Px significantly decreased in the LPS and low dose group, however, there was no significantly difference in high dose group. In addition, the content of T-AOC, T-SOD, and GSH-Px in each GD treatment group was higher than that in the LPS group. Moreover, in the process of the dose increased, the enzyme activity was significantly increased.
Figure 5.
Effects of GD on the antioxidant status of duodenum in the mice. The data are expressed as the mean ± SD (n = 10 per treatment group). The total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px). The values with same letters between groups are of no significant difference (P > 0.05), those with different letters are of significant difference (P < 0.05).
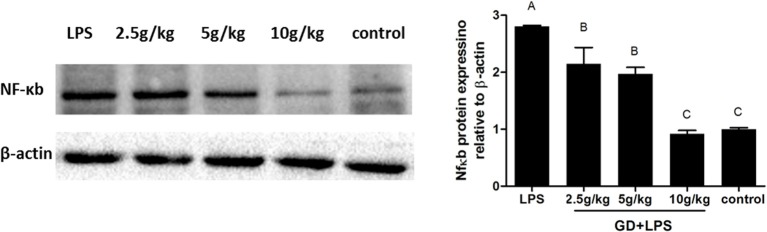
Effects of GD on NF-κB Expression
To evaluate the effects of GD on expression of NF-κB, the level of NF-κB in duodenum were assayed (Figure 6). In the GD treatment group and control group, the expression of NF-κB was lower significantly (P < 0.05) than in LPS group. In addition, in the GD low and medium dose groups the expression of NF-κB was higher significantly (P < 0.05) than in control group, however, there was no significantly difference in high dose group. In our present study, Western blot analysis revealed that GD can modify NF-κB activity.
Figure 6.
Effects of GD on NF-κB signaling pathway activation. Western blot analysis was used to assess the expression of NF-κB from each group and quantitated protein band intensities presented as β-actin normalized mean values. Representative western blot images and quantified expression levels are presented. Values are expressed as the mean ± standard deviation (n = 10). The values with same letters between groups are of no significant difference (P > 0.05), those with different letters are of significant difference (P < 0.05). NF-κB, nuclear factor-κB.
Discussion
Complete function of intestinal mucosa is essential for health and survival (Liu et al., 2008). As the main mediator between pathogen and intestinal tract, intestinal epithelial cells play an important role in the defense of pathogens (Qian et al., 2016). The function of intestinal epithelial cells depends on the homeostasis of intestinal mucosa. There is growing evidence that the balance between intestinal epithelial cells and the immune system maintains intestinal health (Maloy and Powrie, 2011). We studied whether GD could improve LPS-induced inflammation in mice. In the current experiment, we employed LPS as an inflammatory agent to establish a model of intestinal injury in mice. LPS challenge increased the level of TNF-α, IL-1, IL-6, and IL-8 in the serum (Figure 2). Importantly, GD reduced the concentrations of TNF-α, IL-1, IL-6, and IL-8 in the serum, compared to LPS-challenged mice. These findings indicate that the GD has beneficial effects in reducing intestinal mucosal inflammation. The level of IL-2 has a positive correlation with cellular immune function, and it can also improve the immune defense and immune repair ability of cells. Many studies have shown that the decrease of IL-2 can lead to cellular immune dysfunction (Boyman et al., 2015). In the GD treatment groups the level of IL-2 was significantly enhanced, indicating that the level of IL-2 increased the immune function of T cells, thus inhibiting the inflammation in the intestine. IL-10 has multiple functions such as immunomodulation and anti-inflammatory effects (da Silva et al., 2015). In this study, the expression level of IL 10 in the serum of the model group mice was lower than that of the control group, indicating that the expression level of IL-10 was negatively correlated with the degree of inflammation. The GD played a significant role in regulating IL-10 level, and increased with the increase of GD concentration (Figure 3). Importantly, significant correlation between NF-κB activity and concentrations of pro-inflammatory mediators was revealed in intestine (Zuo et al., 2013). The activation of NF-κB leads to production of pro-inflammatory molecules. Previous studies show that Gardenia jasminoides Ellis and Crocus sativus L could decrease NF-κB and inflammation (Xu et al., 2009). Our results showed that LPS significantly increased the expression of NF-κB, while blockade of GD significantly abolished these effects (Figure 5). GD significantly regulates the imbalance between pro-inflammatory and anti-inflammatory factors in the duodenum tissue of mice, down regulates the state of local immunoreaction and alleviates the damage of mucosal inflammation, which may be one of the mechanisms for the treatment of intestinal mucosal damage.
The complete structure of the small intestine is the physiological basis of its digestion and absorption function, and its morphological and structural changes directly affect the surface area of villi, thereby affecting the body's ability to absorb nutrients (Collins and Bhimji, 2017). VH, CD, and V/C can be regarded as a criterion to reflect the intestinal mucosal morphology and the absorption capacity of the small intestine (Greig and Cowles, 2017). VH and CD of intestinal mucosa are closely related to animal digestion. Detection of VH and CD can judge the degree of intestinal mucosal damage and the ability to repair (Dong et al., 2016). Thus, an increase in VH, V/C or decrease in the CD corresponds to an improvement in the digestion and absorption of nutrients (Hou et al., 2013). Accordingly, GD increased V/C and VH in the duodenum and decreased the VH, compared to the LPS mice. The result of serum metabolites (Figure 1) was also in agreement with the alteration of intestinal villus structure. These results indicated that GD has inhibitory effect on the intestinal mucosal damage in mice, and the inhibitory effect exhibits a dose-dependent manner.
HPLC analysis identified the main components-amino acids, organic acids, fatty acids, nucleosides, flavonoids and so on-included in GD. Geniposide, the major iridoid glycoside ingredient of gardenia herbs, has emerged as a novel multifunctional tissue-protective agent with antioxidant (Fu et al., 2012) and anti-inflammatory effects (Lee et al., 2009). SOD is an important enzyme system for scavenging oxygen free radicals, which has protective effect on cell damage. SOD can prevent the expansion of oxidation free radical chain reaction. It can be considered as an important line of oxygen free radical scavenging system in organism (Mansuroglu et al., 2015). GSH-PX catalyzes the redox reaction of the prototype GSH to the hydroperoxide, which can remove the harmful peroxide metabolites in the cells and block the lipid peroxidation chain reaction, thus protecting the membrane structure and function integrity of the cell (Gordeeva et al., 2015). The results of the experiment were that the content of T-AOC in GD treatment groups was higher than that of the model group, and there was a significant difference. The expression of T-SOD and GSH-Px in the GD treatment groups was significantly increased in the duodenum tissue and there was a significant difference (Figure 6). It is indicates that GD has the ability to protect the intestinal epithelial cells from oxygen free radical damage.
Conclusion
Gardenia jasminoides can promote tissue repair by inhibiting the expression of inflammatory factors, lowering the disease activity and deceasing intestinal mucosal damage. It could be important for intervening the cycle of inflammation associated with intestinal mucosal injury. Further studies of GD are necessary to develop a new effective plant-derived therapeutic modality for intestinal mucosal injury.
Author Contributions
YC and QW designated the study, collected and analyzed the data, and wrote the manuscript. MW and JJ contributed to data collection. RW supervised the study. All authors reviewed and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by China Postdoctoral Science Foundation (grant number 2017M620124; 2018T110320); Doctoral Program Foundation of Heilongjiang Bayi Agricultural University of China (grant number XDB-2016-10); Postdoctoral Program Foundation of Heilongjiang Bayi Agricultural University (grant number 601038); and Natural Science Foundation of Heilongjiang Province (grant number C201444).
References
- Boyman O., Kolios A. G., Raeber M. E. (2015). Modulation of T cell responses by IL-2 and IL-2 complexes. Clin. Exp. Rheumatol. 33, S54–57. 10.5167/uzh-123113 [DOI] [PubMed] [Google Scholar]
- Chen S., Zhao X., Sun P., Qian J., Shi Y., Wang R. (2017). Preventive effect of Gardenia jasminoides on HCl/ethanol induced gastric injury in mice. J. Pharmacol. Sci. 133, 1–8. 10.1016/j.jphs.2016.05.011 [DOI] [PubMed] [Google Scholar]
- Chiu C. J., Mcardle A. H., Brown R., Scott H. J., Gurd F. N. (1970). Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch. Surg. 101, 478–483. 10.1001/archsurg.1970.01340280030009 [DOI] [PubMed] [Google Scholar]
- Collins J. T., Bhimji S. S. (2017). Anatomy, Abdomen, Small Intestine. Treasure Island, FL: StatPearls. [Google Scholar]
- Cui Y., Sun R., Wang Q., Wang M. (2017). Hepatotoxicity induced by intragastrically administrated with Gardenia decoction in mice. Nat. Prod. Res. 31, 2824–2827. 10.1080/14786419.2017.1297934 [DOI] [PubMed] [Google Scholar]
- Cui Y., Wang Q., Sun R., Guo L., Wang M., Jia J., et al. (2018). Astragalus membranaceus (Fisch.) Bunge repairs intestinal mucosal injury induced by LPS in mice. BMC Complement. Altern. Med. 18:230. 10.1186/s12906-018-2298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva M. D., Bobinski F., Sato K. L., Kolker S. J., Sluka K. A., Santos A. R. (2015). IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol. Neurobiol. 51, 19–31. 10.1007/s12035-014-8790-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Yang C., Wang Z., Qin Z., Cao J., Chen Y. (2016). The injury of serotonin on intestinal epithelium cell renewal of weaned diarrhoea mice. Eur. J. Histochem. 60:2689. 10.4081/ejh.2016.2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang T., Jia G., Zhao H., Chen X., Tang J., Wang J., et al. (2016). Effects of spermine supplementation on the morphology, digestive enzyme activities, and antioxidant capacity of intestine in weaning rats. Anim. Nutr. 2, 370–375. 10.1016/j.aninu.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Liu B., Liu J., Liu Z., Liang D., Li F., et al. (2012). Geniposide, from Gardenia jasminoides Ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models. Int. Immunopharmacol. 14, 792–798. 10.1016/j.intimp.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Gordeeva A. E., Temnov A. A., Charnagalov A. A., Sharapov M. G., Fesenko E. E., Novoselov V. I. (2015). Protective effect of peroxiredoxin 6 in ischemia/reperfusion-induced damage of small intestine. Dig. Dis. Sci. 60, 3610–3619. 10.1007/s10620-015-3809-3 [DOI] [PubMed] [Google Scholar]
- Greig C. J., Cowles R. A. (2017). Muscarinic acetylcholine receptors participate in small intestinal mucosal homeostasis. J. Pediatr. Surg. 52, 1031–1034. 10.1016/j.jpedsurg.2017.03.037 [DOI] [PubMed] [Google Scholar]
- Guo Y. W., Zhao Z., Cheng Y. K., Wang D., Du S. Y., Lu Y. (2014). [Studies on release behavior of sustained release tablets of extracts of Gardenia by antioxidant activity]. Zhongguo Zhong Yao Za Zhi 39, 3274–3277. [PubMed] [Google Scholar]
- Hayden M. S., Ghosh S. (2014). Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 26, 253–266. 10.1016/j.smim.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Wang L., Yi D., Ding B., Yang Z., Li J., et al. (2013). N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45, 513–522. 10.1007/s00726-012-1295-x [DOI] [PubMed] [Google Scholar]
- Lee J. H., Lee D. U., Jeong C. S. (2009). Gardenia jasminoides Ellis ethanol extract and its constituents reduce the risks of gastritis and reverse gastric lesions in rats. Food Chem. Toxicol. 47, 1127–1131. 10.1016/j.fct.2009.01.037 [DOI] [PubMed] [Google Scholar]
- Liu Y., Huang J., Hou Y., Zhu H., Zhao S., Ding B., et al. (2008). Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 100, 552–560. 10.1017/S0007114508911612 [DOI] [PubMed] [Google Scholar]
- Ma C. Y., Shi G. Y., Shi C. S., Kao Y. C., Lin S. W., Wu H. L. (2012). Monocytic thrombomodulin triggers LPS- and gram-negative bacteria-induced inflammatory response. J. Immunol. 188, 6328–6337. 10.4049/jimmunol.1102266 [DOI] [PubMed] [Google Scholar]
- Ma W. W., Tao Y., Wang Y. Y., Peng I. F. (2017). Effects of Gardenia jasminoides extracts on cognition and innate immune response in an adult Drosophila model of Alzheimer's disease. Chin. J. Nat. Med. 15, 899–904. 10.1016/S1875-5364(18)30005-0 [DOI] [PubMed] [Google Scholar]
- Maloy K. J., Powrie F. (2011). Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306. 10.1038/nature10208 [DOI] [PubMed] [Google Scholar]
- Mansuroglu B., Derman S., Yaba A., Kizilbey K. (2015). Protective effect of chemically modified SOD on lipid peroxidation and antioxidant status in diabetic rats. Int. J. Biol. Macromol. 72, 79–87. 10.1016/j.ijbiomac.2014.07.039 [DOI] [PubMed] [Google Scholar]
- Medicherla K., Sahu B. D., Kuncha M., Kumar J. M., Sudhakar G., Sistla R. (2015). Oral administration of geraniol ameliorates acute experimental murine colitis by inhibiting pro-inflammatory cytokines and NF-kappaB signaling. Food Funct. 6, 2984–2995. 10.1039/C5FO00405E [DOI] [PubMed] [Google Scholar]
- Nabuurs M. J., Hoogendoorn A., Van Der Molen E. J., Van Osta A. L. (1993). Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in The Netherlands. Res. Vet. Sci. 55, 78–84. 10.1016/0034-5288(93)90038-H [DOI] [PubMed] [Google Scholar]
- Oliveira H., Cai X., Zhang Q., De Freitas V., Mateus N., He J., et al. (2017). Gastrointestinal absorption, antiproliferative and anti-inflammatory effect of the major carotenoids of Gardenia jasminoides Ellis on cancer cells. Food Funct. 8, 1672–1679. 10.1039/C7FO00091J [DOI] [PubMed] [Google Scholar]
- Plociennikowska A., Hromada-Judycka A., Borzecka K., Kwiatkowska K. (2015). Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 72, 557–581. 10.1007/s00018-014-1762-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X. X., Peng J. C., Xu A. T., Zhao D., Qiao Y. Q., Wang T. R., et al. (2016). Noncoding Transcribed Ultraconserved Region (T-UCR) uc.261 participates in intestinal mucosa barrier damage in Crohn's disease. Inflamm. Bowel Dis. 22, 2840–2852. 10.1097/MIB.0000000000000945 [DOI] [PubMed] [Google Scholar]
- Qin F. M., Liu B. L., Zhang Y., Zhou G. X. (2015). A new triterpenoid from the fruits of Gardenia jasminoides var. radicans Makino. Nat. Prod. Res. 29, 633–637. 10.1080/14786419.2014.980249 [DOI] [PubMed] [Google Scholar]
- Shen P., Zhang Z., He Y., Gu C., Zhu K., Li S., et al. (2018). Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 196, 69–76. 10.1016/j.lfs.2018.01.016 [DOI] [PubMed] [Google Scholar]
- Song X., Guo M., Wang T., Wang W., Cao Y., Zhang N. (2014). Geniposide inhibited lipopolysaccharide-induced apoptosis by modulating TLR4 and apoptosis-related factors in mouse mammary glands. Life Sci. 119, 9–17. 10.1016/j.lfs.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Tao W., Zhang H., Xue W., Ren L., Xia B., Zhou X., et al. (2014). Optimization of supercritical fluid extraction of oil from the fruit of Gardenia jasminoides and its antidepressant activity. Molecules 19, 19350–19360. 10.3390/molecules191219350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancamelbeke M., Vermeire S. (2017). The intestinal barrier: a fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 11, 821–834. 10.1080/17474124.2017.1343143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. Y., Wang G. F., Liu Z. Q., Rao J. J., Lu L., Xu W., et al. (2009). Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol. Sin. 30, 202–208. 10.1038/aps.2008.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. L., Li G., Ma H. P., Zhong H., Liu F., Ao G. Z. (2009). Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J. Agric. Food Chem. 57, 8325–8330. 10.1021/jf901752f [DOI] [PubMed] [Google Scholar]
- Yu S., Fu S., Liu B., Zhang Y., Zhou G. (2015). Two new quercetin glycoside derivatives from the fruits of Gardenia jasminoides var. radicans. Nat. Prod. Res. 29, 1336–1341. 10.1080/14786419.2014.1001389 [DOI] [PubMed] [Google Scholar]
- Zhang H., Lai Q., Li Y., Liu Y., Yang M. (2017). Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J. Ethnopharmacol. 196, 225–235. 10.1016/j.jep.2016.11.042 [DOI] [PubMed] [Google Scholar]
- Zuo D. C., Choi S., Shahi P. K., Kim M. Y., Park C. G., Kim Y. D., et al. (2013). Inhibition of pacemaker activity in interstitial cells of Cajal by LPS via NF-kappaB and MAP kinase. World J. Gastroenterol. 19, 1210–1218. 10.3748/wjg.v19.i8.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]