Abstract
Background: Lhermitte-Duclos disease (LDD) stems from the development of a rare benign lesion of uncertain pathogenesis that distorts the normal cerebellar laminar cytoarchitecture. We explored the lesion's appearance on conventional magnetic resonance imaging (MRI) combined with susceptibility-weighted imaging, diffusion-weighted imaging, perfusion imaging, or arterial spin labeling. Although many cases of LDD have been previously reported in the literature, the radiologic-pathologic correlation has been described in only a few of these cases. To the best of our knowledge, this is the first case report to provide detailed information about the radiologic-pathologic correlation of LDD. Case Report: A 48-year-old woman presented with left facial tics, occipital headache, and dizziness for 1 month. MRI revealed a left cerebellar lesion with hypointensity on T1-weighted images. On T2-weighted images, the mass was hyperintense with tigroid appearance due to alternating high and normal signal intensities. High signal intensity was noted on fluid-attenuated inversion recovery images. Magnetic resonance spectroscopy indicated decreased level of choline (Cho), N-acetyl aspartate, and myoinositol with elevated level of lactate on the affected side. The lesion showed a bright signal on diffusion-weighted images, whereas apparent diffusion coefficient mapping revealed no disturbance of diffusion. The pathology of the excised lesion was consistent with LDD. Conclusion: MRI with advanced techniques can provide not only preoperative diagnosis but also better pathologic correlation.
Keywords: Lhermitte-Duclos disease, Radiologic-pathologic correlation, MRI
Abbreviations: ASL, arterial spin labeling; Cho, choline; CS, Cowden syndrome; CT, computed tomography; DWI, diffusion-weighted imaging; EMA, epithelial membrane antigen; FLAIR, fluid-attenuated inversion recovery images; GFAP, glial fibrillary acidic protein; LDD, Lhermitte-Duclos disease; MI, myoinositol; MR, magnetic resonance; MRI, magnetic resonance image; MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate; NCV, nerve conduction velocity; PTEN, phosphatase and tensin homologue; SWI, susceptibility-weighted imaging
Introduction
Lhermitte-Duclos disease (LDD) is a disorder due to a rare benign lesion of uncertain pathogenesis. Also known as dysplastic gangliocytoma of the cerebellum, it is characterized by distortion of the normal cerebellar laminar cytoarchitecture. On magnetic resonance images (MRI), the lesion appears as a nonenhancing mass in the cerebellar hemisphere and has a striated pattern. MRI has helped clinicians to understand the pathologic and metabolic changes occurring in these lesions. In this report, we explored LDD lesion appearance on susceptibility-weighted imaging (SWI), diffusion-weighted imaging (DWI), perfusion imaging and arterial spin labeling combined with conventional MRI. Although many cases of LDD have been reported in the medical literature, the radiologic-pathologic correlation has been described in only a few of these cases. To the best of our knowledge, this is the first case report that provides detailed information about the radiologic-pathologic correlation in LDD.
Case report
A 48-year-old woman presented with new-onset left facial tics, occipital headache, and dizziness for 1 month. Neurologic examination revealed no evidence of focal sensory or motor deficit and no cerebellar dysfunction. A facial nerve conduction velocity study was done for left facial tics and revealed decreased amplitude. Subsequently, computed tomography (CT) demonstrated a hypodense left cerebellar mass with focal calcification. MRI revealed a left cerebellar lesion with hypointensity on T1-weighted images (repetition time msec/echo time msec, 1790/3). On T2-weighted images (3850/88), the mass was predominantly hyperintense, with the alternate high- and normal signal intensity giving it a tigroid appearance (Fig. 1). The signal intensity of the lesion was higher than that of the cerebellar white matter on fluid-attenuated inversion recovery images (7000/118). There was bright signal within the mass on DWI, whereas apparent diffusion coefficient (ADC) mapping revealed no disturbance of diffusion. SWI demonstrated thin hairy blood vessels, possibly veins, which ran deep in between the thick folia. MR perfusion imaging using arterial spin labeling showed relative increase in perfusion (Fig. 2). Magnetic resonance spectroscopy (MRS) indicated a decrease in the levels of choline (Cho), N-acetyl aspartate, and myoinositol with elevated level of lactate on the affected side. The characteristic pattern of these morphologic features on CT and MR images afforded us the opportunity to diagnose LDD preoperatively. The patient underwent complete resection of the lesion.
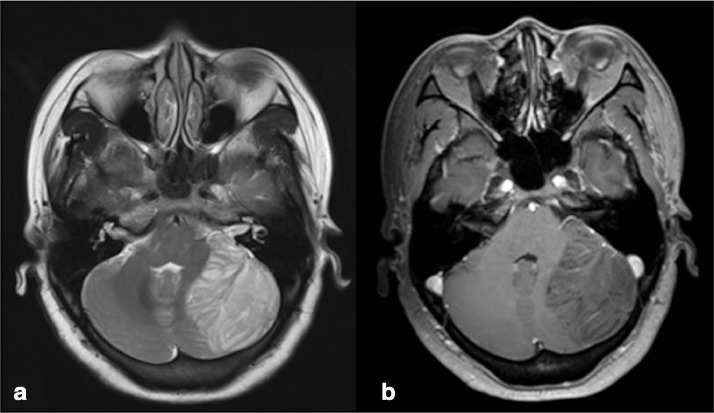
Fig. 1.
Left cerebellar hemisphere. (a) Axial T2-weighted image reveals a heterogeneously high-signal mass whose laminated architecture is characteristic of LDD. (b) A contrast-enhanced axial T1-weighted image shows limited patchy enhancement within the lesion.
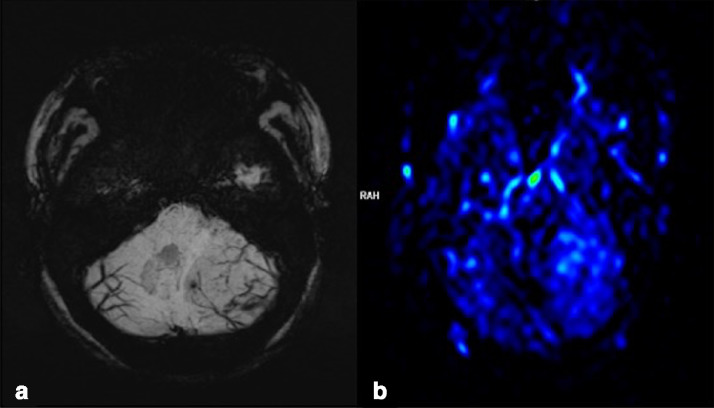
Fig. 2.
Left cerebellar hemisphere. (a) Susceptibility-weighted imaging (SWI) reveals numerous veins running between the folia. (b) Perfusion-weighted imaging using an arterial spin labeling technique shows increase in perfusion.
Swelling of the left side of the cerebellum with compression of the contralateral side was found operatively. The tumor was gray whitish in color, soft in consistency, and moderately vascular. The tumor mass originated from cerebellar tissue and had an unclear margin. Histopathologic examination revealed a relatively preserved cerebellar architecture with abundant dysplastic ganglion cells of different sizes. There were ectatic vessels with focal calcification. Neither mitosis nor necrosis was seen. Immunohistochemistry was positive for glial fibrillary acidic protein and synaptophysin, and negative for epithelial membrane antigen. Phosphatase and tensin homologue expression was weak in dysplastic ganglion cells. Ki-67 labeling index was less than 1% (Fig. 3).
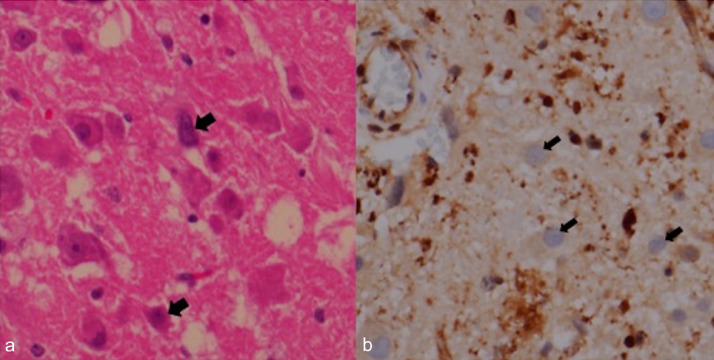
Fig. 3.
Surgically resected specimen. (a) Photomicrograph (original magnification 400×) reveals numerous dysplastic ganglion cells (thick arrows) (b) PTEN stain shows loss of PTEN expression in dysplastic ganglion cells (thin arrows).
The patient made a good postoperative recovery. No clinical signs of Cowden syndrome were detected, and no LDD or Cowden syndrome was in her family history. Furthermore, to screen for harmatomas and malignancies, CT of the chest and abdomen was ordered and it detected a small pulmonary nodule, which remained stable for more than 1 year. At the time of this report, she was under follow-up care.
Discussion
A rare cerebellar malformation of uncertain etiology and pathogenesis, LDD was first described in 1920. Since then [1], at least 230 cases have been reported in the medical literature. Most frequently, LDD presents in the third and fourth decades of life [1], but it can manifest anywhere from birth [2] to the sixth decade [3]. There is no obvious sex preponderance [4]. According to the literature, the duration of symptoms ranges from a few months to more than 10 years [5], [6]. Characteristic symptoms include cranial nerve palsies, unsteadiness of gait, ataxia, and sudden neurologic deterioration because of acute or chronic hydrocephalus. Signs and symptoms of increased intracranial pressure, such as headaches, nausea and vomiting, papilledema, mental disturbances, and loss of consciousness occur in a later stage of the disease and are caused by the progressive mass effect of the growing tumor [1]. More unusual symptoms associated with dysplastic cerebellar gangliocytoma are isolated orthostatic hypotension [7], tinnitus [8], and psychiatric disturbances [9]. Furthermore, there is an association of dysplastic cerebellar gangliocytoma with multiple hamartoma-neoplasia complex (Cowden syndrome). Cowden syndrome is an autosomal dominant hereditary multiple hamartoma-neoplasia complex, often presenting with systemic hamartomas and neoplasms of the breast, thyroid, genitourinary tract, and endometrium [1].
The typical neuroimaging features of dysplastic gangliocytoma stem from indolent growth reflecting the generally benign biological behavior of these lesions [10]. On CT, LDD is usually seen as a well-defined isodense- to hypodense mass in the posterior cranial fossa with no contrast enhancement. Its tigroid appearance on MRI may also be seen on high-resolution fine-slice CT through the posterior fossa. Calcifications may be present [11]. Depending on the tumor's size, a mass effect with displacement of the fourth ventricle and obstructive hydrocephalus may occur. Conventional MR findings in our case are in accordance with other published cases of LDD.
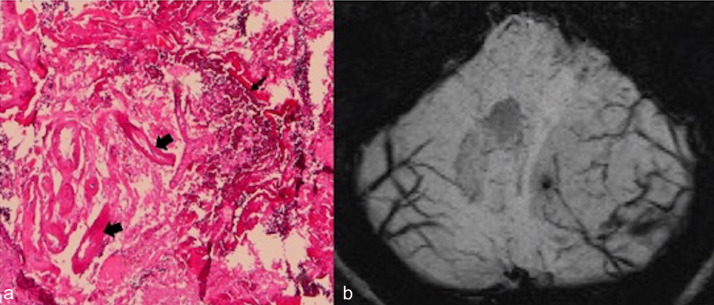
Kulkantrakorn et al have described the MRI correlates of dysplastic-hypertrophied cerebellar folia, which are histopathologic features in this condition [12]. The central core of T1 hypointensity and T2 hyperintensity corresponds to the thinned white matter, widened granular cell layer, and the inner portions of the dysplastic molecular layer. The outer thinner layer on MR images (T1 isointense, T2 iso- to hypointense) can be attributed to the outer molecular layer and the leptomeninges (Fig. 4). They also showed that this layer is associated with abnormal vessels and areas of calcification [13]. In our patient, the veins surrounding the thickened folia were demonstrated well by SWI. This can be correlated with a pathologic feature of the disease, ectatic vessels (Fig. 5). Owing to the T2 effect, the abnormally thickened folia in LDD were characterized by slightly higher signal intensities on DWI, whereas ADC mapping showed no disturbance of water diffusion. The signals on DWI and ADC maps depend on cell density, low extracellular water content, and enhancement of the tumor [14]. Profusion of dysplastic cortical neurons in LDD, a thickening of the molecular layer, the loss of Purkinje cells, and thinning of medullary white matter may be responsible for the findings on DWI. There was no contrast enhancement in our case on MR images, whereas perfusion-weighted imaging showed increased perfusion compared to adjacent cerebellar tissue. Lack of MR contrast enhancement is emphasized as an important MR diagnostic criterion of the disease, but according to Spaargaren et al [15] contrast enhancement may be due to venous proliferation. Venous proliferation may also explain the large draining veins that are seen on angiography and as flow voids on MRI. The absence of contrast enhancement suggests that there is no significant blood-brain barrier damage and no extracellular edema [10]. Hyperperfusion without enhancement correlates closely to the histopathologic observation of numerous dilated thin-walled blood vessels [14].
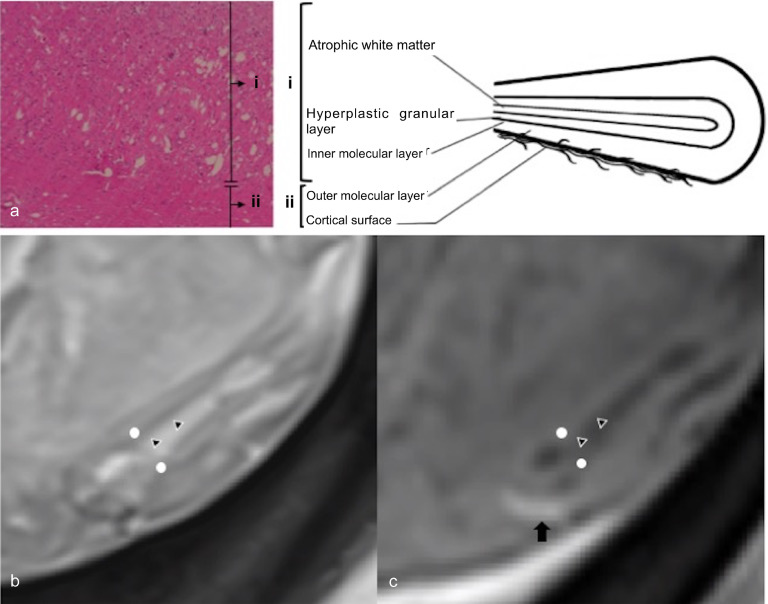
Fig. 4.
Radiopathologic correlation of LDD. (a) Photomicrograph of the surgically resected specimen (original magnification 40×) and schematic diagram of cerebellum folia, (b). Axial T2-weighted image, (c) Contrast-enhanced axial T1-weighted image. The thinned white matter, widened granular cell layer and the inner portion of the dysplastic molecular layer, which is marked as area i in a, corresponds to central core of T1 hypointensity and T2 hyperintensity on MRI (marked by triangles). The outer molecular layer and the leptomeninges, which are marked as area ii in a, corresponds to the outer thinner layer on MR images (T1 isointense, T2 iso to hypointense) (marked by white dots). An enhanced vessel runs on the cortical surface, marked by an arrow.
Fig. 5.
Surgically resected specimen. (a) Photomicrograph (original magnification 40×) shows ectatic vessels (thick arrows) and calcification (thin arrow). (b) Axial susceptibility-weighted imaging (SWI) reveals veins running between the folia.
Decreased N-acetyl aspartate and increased lactate, but not increased levels of lipids, are characteristic features of LDD. Low cell membrane turnover in this highly metabolically active lesion is suggested by reduced Cho level [13], [14], increased glycolysis, by the presence of lactate, and non-necrotic nature, by the absence of lipids [14]. These findings together with imaging findings point to the relatively benign nature of this lesion. Verheggen et al [16] concluded that the decreased Cho/Cr ratio observed in 1 case of bilateral LDD was in strict contrast to observations in cerebral tumors. Lactate, normally undetectable in brain, accumulates in cysts, necrotic tissue, or within active tumors due to the high rate of glycolysis (glucose to lactate) within tumors of all types, including aerobic tumors. We assumed that the elevated lactate level was due to an abnormally high rate of glycolysis in LDD, rather than to the high lactate level in cystic or necrotic components of the LDD lesion. In non-necrotic areas of the LDD lesion, the histopathologic findings support our hypothesis that elevated lactate is not indicative of cell death. Therefore, our MRS results in LDD, in part, contrast with those reported by Verheggen et al [16], who showed no change in lactate level. It has been suggested that LDD can be diagnosed from its typical neuroimaging features.
Advancement in neurosurgical techniques has made surgical resection the mainstream therapy for these tumors. However, the main technical problem during surgery is the absence of a clear margin between the tumor and normal brain tissue. Postoperative cerebellar mutism due to extensive tumor resection was reported in 1 LDD patient. Intraoperative MRI and high-definition fiber tractography have been proposed as guidance tools to increase the accuracy of resection [17].
Conclusions
The LDD lesion is an extremely rare benign cerebellar mass that can be diagnosed from its unique neuroimaging features. Although the exact pathophysiological explanation for the signal characteristics of LDD in DWI/perfusion-weighted imaging, SWI, and MRS remains unclear, the combination of MRI with advanced imaging modalities used in our case was shown to provide not only preoperative diagnosis but also better correlation with LDD pathology and additional information about LDD pathophysiology.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2019.03.020.
Appendix. Supplementary materials
References
- 1.Nowak D.A., Trost H.A. Lhermitte-Duclos disease (dysplastic cerebellar gangliocytoma): a malformation, hamartoma or neoplasm? Acta Neurol Scand. 2002;105:137–145. doi: 10.1034/j.1600-0404.2002.1r127.x. [DOI] [PubMed] [Google Scholar]
- 2.Roessmann U., Wongmongkolrit T. Dysplastic gangliocytoma of cerebellum in a newborn. Case report. J Neurosurg. 1984;60:845–847. doi: 10.3171/jns.1984.60.4.0845. [DOI] [PubMed] [Google Scholar]
- 3.Gessaga E.C. Lhermitte-Duclos disease (diffuse hypertrophy of the cerebellum). Report of two cases. Neurosurg Rev. 1980;3(2):151–158. doi: 10.1007/BF01644067. [DOI] [PubMed] [Google Scholar]
- 4.Vinchon M., Blond S., Lejeune J.P., Krivosik I., Fossati P., Assaker R. Association of Lhermitte-Duclos and Cowden disease: report of a new case and review of the literature. J Neurol Neurosurg Psychiatry. 1994;57:699–704. doi: 10.1136/jnnp.57.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambler M., Pogacar S., Sidman R. Lhermitte-Duclos disease (granule cell hypertrophy of the cerebellum) pathological analysis of the first familial cases. J Neuropathol Exp Neurol. 1969;28:622–647. doi: 10.1097/00005072-196910000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Leech R.W., Christoferson L.A., Gilbertson R.L. Dysplastic gangliocytoma (Lhermitte-Duclos disease) of the cerebellum. Case report. J Neurosurg. 1977;47:609–612. doi: 10.3171/jns.1977.47.4.0609. [DOI] [PubMed] [Google Scholar]
- 7.Ruchoux M.M., Gray F., Gherardi R., Schaeffer A., Comoy J., Poirier J. Orthostatic hypotension from a cerebellar gangliocytoma (Lhermitte-Duclos disease). Case report. J Neurosurg. 1986;65:245–248. doi: 10.3171/jns.1986.65.2.0245. [DOI] [PubMed] [Google Scholar]
- 8.Lobo C.J., Mehan R., Murugasu E., Laitt R.D. Tinnitus as the presenting symptom in a case of Lhermitte-Duclos disease. J Laryngol Otol. 1999;113:464–465. doi: 10.1017/s0022215100144226. [DOI] [PubMed] [Google Scholar]
- 9.Otheman Y., Aalouane R., Aarab C., Rammouz I. A case report of Lhermitte-Duclos disease revealed by psychiatric disturbances. Ann Gen Psychiatry. 2017;16:24. doi: 10.1186/s12991-017-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moonis G., Ibrahim M., Melhem E.R. Diffusion-weighted MRI in Lhermitte-Duclos disease: report of two cases. Neuroradiology. 2004;46:351–354. doi: 10.1007/s00234-004-1190-6. [DOI] [PubMed] [Google Scholar]
- 11.Klisch J., Juengling F., Spreer J., Koch D., Thiel T., Buchert M. Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. Am J Neuroradiol. 2001;22:824–830. [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkantrakorn K., Awwad E.E., Levy B., Selhorst J.B., Cole H.O., Leake D. MRI in Lhermitte-Duclos disease. Neurology. 1997;48:725–731. doi: 10.1212/wnl.48.3.725. [DOI] [PubMed] [Google Scholar]
- 13.Thomas B., Krishnamoorthy T., Radhakrishnan V.V., Kesavadas C. Advanced MR imaging in Lhermitte-Duclos disease: moving closer to pathology and pathophysiology. Neuroradiology. 2007;49:733–738. doi: 10.1007/s00234-007-0241-1. [DOI] [PubMed] [Google Scholar]
- 14.Klisch J., Juengling F., Spreer J., Thiel T., Büchert M., Arnold S. Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. Am J Neuroradiol. 2001;22:824–830. [PMC free article] [PubMed] [Google Scholar]
- 15.Spaargaren L., Cras P., Bomhof M.A., Lie S.T., de Barsy A.M., Croese P.H. Contrast enhancement in Lhermitte-Duclos disease of the cerebellum: correlation of imaging with neuropathology in two cases. Neuroradiology. 2003;45:381–385. doi: 10.1007/s00234-003-0984-2. [DOI] [PubMed] [Google Scholar]
- 16.Verheggen R., Bruhn H., Schroder B.U., Frahm J., Markakis E. Lhermitte-Duclos disease: a critical appraisal of different radiologic methods. Eur J Radiol. 1994;19:21–24. doi: 10.1016/0720-048x(94)00552-n. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes-Cabral D.T., Zenonos G.A., Hamilton R.L., Panesar S.S., Fernandez-Miranda J.C. High-definition fiber tractography in the evaluation and surgical planning of Lhermitte-Duclos disease: a case report. World Neurosurg. 2016;92:587.e589. doi: 10.1016/j.wneu.2016.04.128. 587.e513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.