ABSTRACT
Background
Multiple studies have indicated that formula-fed infants show a different growth trajectory compared with breastfed infants. The observed growth rates are suggested to be linked to higher postprandial levels of branched chain amino acids (BCAAs) and insulin related to differences in protein quality.
Objective
We evaluated the effects of milk protein denaturation and milk protein composition on postprandial plasma and hormone concentrations.
Methods
Neonatal piglets were bolus-fed randomly, in an incomplete crossover design, 2 of 3 milk protein solutions: native whey protein isolate (NWPI), denatured whey protein isolate (DWPI), or protein base ingredient, comprising whey and casein (PBI). Postprandial plasma amino acids (AAs), insulin, glucagon-like peptide 1, glucose, and paracetamol concentrations were assayed. Plasma responses were fitted with a model of first-order absorption with linear elimination.
Results
DWPI (91% denatured protein) compared with NWPI (91% native protein) showed lower essential amino acids (EAAs) (∼10%) and BCAA (13–19%) concentrations in the first 30–60 min. However, total amino acid (TAA) concentration per time-point and area under the curve (AUC), as well as EAA and BCAA AUC were not different. PBI induced a ∼30% lower postprandial insulin spike than NWPI, yet plasma TAA concentration at several time-points and AUC was higher in PBI than in NWPI. The TAA rate constant for absorption (ka) was twofold higher in PBI than in NWPI. Plasma BCAA levels from 60 to 180 min and AUC were higher in PBI than in NWPI. Plasma EAA concentrations and AUCs in PBI and NWPI were not different.
Conclusions
Denaturation of WPI had a minimal effect on postprandial plasma AA concentration. The differences between PBI and NWPI were partly explained by the difference in AA composition, but more likely differences in protein digestion and absorption kinetics. We conclude that modifying protein composition, but not denaturation, of milk protein solutions impacts the postprandial amino acid availability in neonatal piglets.
Keywords: whey protein, insulin, amino acids, infant formula, human milk, neonatal piglets
Introduction
Breastfeeding is widely recognized as the best nutrition for infants during the first year of life, providing many benefits to the growing infant (1, 2). Infants who are exclusively fed human milk have decreased rates of infectious and allergic diseases, respiratory disorders, and obesity (2–9). In cases where breastfeeding is not possible, high-quality formulas that fulfill the complex nutrient requirements of the infant must be available as an alternative (4).
The protein content of human milk gradually declines over the course of lactation. Most commercially available infant formulas contain a higher protein content (g/L) than human milk to meet nutritional requirements for infants of all ages (10). The higher protein concentration in infant formula has been suggested to program the metabolism toward overweight and obesity in later life, known as the early protein hypothesis (5, 7). The proposed mechanism of the early protein hypothesis is that increased protein intake leads to increased levels of insulin and insulin-like growth factor 1 (IGF-1)-releasing amino acids (AAs). In particular, the branched chain amino acids (BCAAs) leucine (Leu), valine (Val), and isoleucine (Ile), the aromatic amino acids (AAAs) phenylalanine (Phe) and tyrosine (Tyr), and other essential amino acids (EAAs) such as lysine (Lys) are insulinogenic AA. High levels of insulin and IGF-1 can cause weight gain and adipogenesis, potentially leading to obesity (5, 6).
It has been shown in preterm infants that the postprandial EAA and BCAA peaks after human milk ingestion are lower (∼30% and ∼25% respectively) and occur later (∼30 and ∼15 min respectively) than with bovine protein-based infant formula when fed a similar protein quantity (11). Studies that compared the gastric emptying kinetics of human milk to infant formula generally find a similar or slower half-emptying time for human milk (12), suggesting protein digestion and absorption kinetics are dominant factors in the differences observed in the postprandial time course in preterm infants.
Protein digestion kinetics have been shown to be influenced by several parameters. First, in vitro digestion studies have shown a differential impact of heat treatment on milk protein hydrolysis rate. Dupont et al. observed that after heat sterilization (120°C, 10 min) of milk, some regions in casein AA chains became more resistant to enzymatic hydrolysis, whereas the hydrolysis kinetics of intact β-lactoglobulin, a major whey protein, was increased under in vitro infant gastro-duodenal conditions (13). Possibly, the heat treatment decreased the amount of native whey protein. Denatured (unfolded) proteins are expected to have faster hydrolysis kinetics than native (still folded) proteins (14). Others have shown that indeed heat treatment (90°C, 120 min) accelerates β-lactoglobulin intact protein hydrolysis kinetics. In particular, high-molecular-weight non-native aggregates were shown to be hydrolyzed faster under in vitro gastric conditions. On the other hand, non-native dimers of β-lactoglobulin were formed as a result of heating and were particularly resistant to intact protein hydrolysis (15). Second, it is known that milk protein hydrolysis is protein-type-dependent, i.e., intact bovine caseins appear to be hydrolyzed faster than bovine whey proteins in vitro (16). However, in vivo, a lower postprandial AA peak upon casein ingestion than upon whey ingestion has been observed, which is attributed to the physicochemical behavior of casein (i.e., clotting) under the acidic gastric conditions causing slower gastric emptying (17–19).
We aimed to investigate the effect of different protein processing and composition on the postprandial plasma responses in vivo. We hypothesized that denaturing whey protein by heating, as well as changing protein composition by including casein, would increase protein hydrolysis kinetics, leading to faster digestion and absorption, and eventually to a higher postprandial plasma AA and insulin peak. We tested this hypothesis in neonatal piglets because they are a well-established animal model of the human infant with respect to body size, gastrointestinal physiology, and AA metabolism.
Materials and Methods
Test proteins
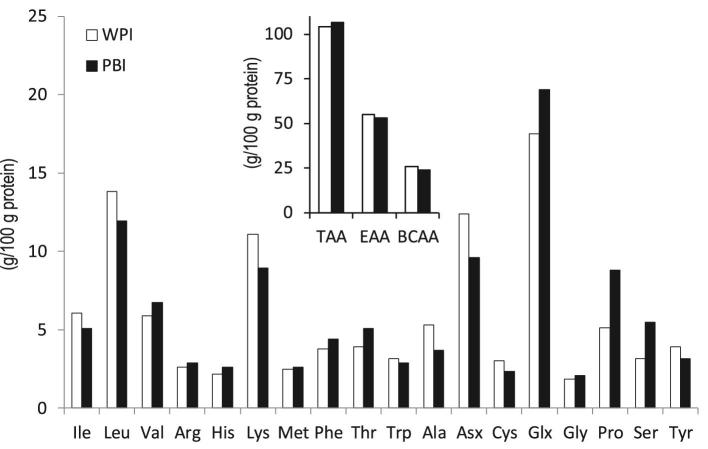
Whey protein isolate (WPI; BiPro™) was obtained from Davisco Foods International Inc. The protein content of the WPI powder was 92% (w/w) as determined by the Dumas method (20) (N × 6.25). The protein composition of WPI was analyzed using reverse-phase high-pressure liquid chromatography (RP-HPLC) as previously described (21) (Table 1). Bovine serum albumin (BSA) and immunoglobulins (IGs) cannot be quantified using the RP-HPLC method. However, the supplier of WPI indicates BSA and immunoglobulins (IGs) are present, and these proteins could account for the remaining 14% other protein. A protein-based ingredient (PBI) for use in infant milk formula was prepared on pilot factory scale using cold membrane filtration as described elsewhere (22). The composition of PBI powder was 72.7% (w/w) protein as determined by the Dumas method (N × 6.25). The PBI powder contained 21.9% (w/w) lactose as determined by high-performance liquid chromatography with refractive index detection after preclean-up with solid-phase extraction C18 cartridge (Eurofins CLF Specialized Nutrition Testing Services). PBI protein composition included 30% β-casein as determined by RP-HPLC (21) (Table 1). The reducing SDS-PAGE analysis reported by McCarthy et al. (22) shows proteins or polypeptide chains that suggest BSA and IGs are present in PBI as well. The 12% by RP-HPLC unidentified protein in PBI could therefore be BSA and IGs as well. The AA compositions of WPI and PBI were determined using ultra performance liquid chromatography after acid hydrolysis as described previously (23) (Figure 1).
TABLE 1.
Protein composition of the whey protein isolate and protein-based ingredient protein preparations tested
| Whey protein isolate | Protein-based ingredient | |
|---|---|---|
| Protein, g/100 g total protein | ||
| α-Lactalbumin | 18 | 13 |
| β-Lactoglobulin | 68 | 45 |
| αs1-Casein | n.d.1 | n.d. |
| β-Casein | n.d. | 30 |
| κ-Casein | n.d. | n.d. |
| Others | 14 | 12 |
1n.d., not detectable.
FIGURE 1.
Mean AA composition of the WPI and PBI protein ingredients used to prepare the test solutions as measured in duplicate. PBI, protein-based ingredient; WPI, whey protein isolate; Asx, sum of Asp and Asn; Glx, sum of Glu and Gln; Cys, sum of cysteine and cystine; EAA (essential amino acids), sum of Leu, Val, Ile, Phe, Met, Arg, Trp, His, Thr, and Lys; TAA (total amino acids), sum of EAA, Tyr, Ser, Glx, Pro, Gly Asx, and Ala; BCAA, sum of Ile, Leu, and Val. Cys was not included in TAA because it could not be quantified in the plasma samples. Asx and Glx are presented as Asn and Gln are converted to Asp and Glu, respectively, during the acid hydrolysis.
Animals
Twenty term, vaginally delivered piglets (domestic pigs, species Sus scrofa domesticus) were obtained from an approved local swine farm and transported to the Children's Nutrition Research Center (CNRC) at 7 d after birth. Piglets were allowed 4 d to acclimate to CNRC animal facility housing while being fed a sow milk formula diet (Litterlife, Merrick's). Piglets were derived from a mixture of Duroc, Hampshire, Yorkshire, and Landrace breeds. Piglets were housed individually in stainless steel cages (dimensions 30 cm width × 60 cm height × 60 cm depth) allowing for nose contact with neighboring cage piglets at 31–32°C with a 12-h light (0600–1800)/dark cycle. Piglets were provided clean blanket bedding and cage enrichment, and monitored for rectal body temperature and overall clinical health daily by investigators and attending veterinary staff. After acclimation, piglets were surgically implanted with a 20-gauge silicone catheter in the jugular vein and a 6 French polyethylene orogastric feeding tube using aseptic, sterile procedures under general anesthesia with 1–2% isoflurane. The catheters were tunneled subcutaneously, exteriorized dorsally between the shoulders, and attached to tether and harness apparatus fitted on the piglets to allow freedom of movement around the cages. Piglets received sustained release buprenorphine (0.3 mg/mL) at the time of surgery for 24–48 h for pain management. The piglets recovered from surgery for 4 d, were fed formula (40 mL/kg; Litterlife, Merrick's) every 4 h, and received a jugular infusion of normal saline (2 mL/h) to maintain catheter patency. This rate of formula feeding provided a daily volume of 240 mL and 12.5 g of protein per kilogram of body weight. Protein solutions were administered through orogastric catheters, which were placed to ensure that piglets received the full amount of test solution in a time-controlled manner. The study protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council) (24).
Preparation of protein test solutions
All protein test solutions were mixed in batches, using predetermined amounts of water, test protein, and lactose in powdered form to contain a protein level as found in sow milk (25, 26) 6.25% (w/w) and 1.88% (w/w) lactose in total. Solutions were stirred until all powder had dissolved. The pH was adjusted to 7.1 by addition of 1 M NaOH. Heat treatment and production of denatured whey protein isolate (DWPI): 500-mL glass bottles containing 200 mL native whey protein isolate (NWPI) were placed in a shaking water bath at 83°C for 20 min, with the water level higher than the level of protein test solution in the bottle. Temperature was monitored and reached a maximum of 80°C after 10 min; the effective heat treatment was therefore 10 min at 80°C. Following heat treatment, bottles were placed in a room temperature water bath for 40 min before placing in a 4°C refrigerator overnight. All test solutions were prepared fresh the day before administration to pigs and stored at 4°C. The morning of the test protein solution feeding, paracetamol was mixed with each test protein solution such that each pig received 60 mL/kg of test protein solution containing 3.75 g of protein and 0.02 g/kg paracetamol per unit body weight in the bolus given orogastrically; this ensured a constant dose of protein and paracetamol relative to body weight. The dose of paracetamol was chosen based on previous reports in pigs (27).
Characterization of protein test solutions
The degree of whey protein denaturation in the protein test solutions was determined by precipitation of denatured protein at pH 4.6 by addition of HCl, followed by centrifugation (15,000 × g) and N analysis of the supernatant by the Dumas method (28). The soluble protein proportion in the protein test solutions was determined by the Dumas method on the supernatant after centrifugation (15,000 × g) at pH 7.1. To assess the extent of Maillardation, total amino groups were quantified before and after heating using OPA analysis as described previously (29).
Experimental design
Study design followed an incomplete Latin square model where 20 pigs were randomly assigned to receive 2 of the 3 test proteins in random order such that each test protein is replicated (n = 13). We used the incomplete Latin square to limit the feeding to only 2 of the 3 test proteins in order to minimize the amount of blood collected from each pig and to complete the study protocol in less than 1 week given the rapid growth and development of neonatal pigs. In the days between the test protein feeding treatments, pigs were fed sow milk formula in 4 feedings per day. During the study, 1 animal experienced mild diarrhea, and after review of the data, this animal was excluded from of the analysis. Thus, 13 animals received PBI (n = 13), 13 animals received NWPI (n = 13), and 12 animals received DWPI (n = 12), with a mean body weight (g) ± SD at the time of study of 3336 ± 223, 3393 ± 250, and 3276 ± 322, respectively. On study day 5, following an overnight fast (∼8 h), the piglets were randomly assigned to receive a 60 mL/kg body weight bolus of 1 of 3 protein test solutions via the orogastric tube. All staff were blinded to the group assignments until the end of the study, and protein test solutions were prepared by a single person at each test period. A baseline blood sample was taken before the bolus, and additional blood samples were taken at 15, 30, 45, 60, 90, 120, 150, 180, and 240 min after protein solution administration. Blood samples were collected in EDTA tubes and immediately placed on ice. Samples were then centrifuged (10 min, 4000 × g at 4°C), plasma was transferred to vials, and stored at −80°C until analysis. On study day 8, the above protocol was repeated, with each piglet receiving a second, different protein test solution. On completion of blood sampling on day 8, piglets were euthanized with an intravenous injection (1 mL/4.5 kg) of a commercially available euthanasia solution (Beauthanasia; Merck Animal Health).
Plasma sample analysis
Plasma levels of glucose, insulin, paracetamol, glucagon-like peptide 1 (GLP-1), and AA were measured for each piglet at each sampling point. Glucose was measured with a glucose oxidase kit (Infinity Glucose Oxidase Liquid Stable Reagent; Thermo Scientific). To assess gastric emptying kinetics, paracetamol was measured with an enzymatic assay (Paracetamol enzyme assay Kit; Cambridge Life Sciences). Insulin was measured using a radioimmunoassay (Porcine Insulin RIA; Millipore). GLP-1 was measured with an ELISA (Glucagon-Like Peptide-1 [Active] ELISA; Millipore). Individual plasma free AA concentrations were determined by reverse-phase HPLC, without prior acid hydrolysis, of their phenylisothiocyanate derivatives, as described previously (30). In this method, cysteine and cystine cannot be quantified accurately.
Data analysis
BCAAs were calculated as a sum of Ile, Leu, and Val. Total essential amino acids EAAs were calculated as a sum of 10 AA, which are the BCAAs mentioned above and Arg, His, Phe, Lys, Met, Thr, and Trp. Arg was included because it is considered essential for piglets (31). Total amino acid (TAA) was calculated as a sum of 19 proteinogenic AA, which are the EAAs mentioned above and Asn, Asp, Ala, Glu, Gln, Gly, Pro, Ser, and Tyr.
Plasma concentrations were analyzed by generalized estimating equations with exchangeable correlation structure, and robust estimation was used in order to account for repeated measures (IBM SPSS Statistics). Pig was the subject factor, and study period was included in both the model comparing treatments with respect to AUC and the model comparing treatments across time. The AUC was calculated using incremental trapezoidal integration. Pairwise comparison among treatments was made when indicated by a significant treatment effect and treatment comparisons at specific time points done only when indicated by a significant treatment × time interaction.
Plasma paracetamol TAA, EAA, BCAA, and individual EAA concentrations were fitted with a model describing a first-order absorption of a single dose with linear elimination (equation 1).
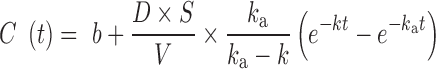 |
(1) |
In this equation C(t) (µM) is the concentration of AA or paracetamol as function of postprandial time t (min), D is the administered dose (µmol), V is the total blood volume (L), ka is the rate constant for absorption (min−1), and k is the rate constant for elimination (min−1). The standard model does not include effects of splanchnic extraction and systemic protein breakdown. Hence, for AA, D was multiplied by a factor S that accounts for the proportion of the administered dose that is not extracted by the splanchnic mass, plus the effect of systemic protein breakdown inhibition. The value of S was based on the obtained highest coefficient of determination (R2) and lowest %CV in ka and k after fitting, which was at a value of 0.15. To account for the basal plasma AA concentrations a constant b was included as well in the equation. For V, a value of 100 mL/kg body weight was used (32). Solver function in Microsoft Excel was used to calculate the values for ka, k, and b that resulted in the lowest sum of squares of residuals, as well as to calculate the concentration maximum (Cmax) and time to reach Cmax (tCmax) using the equation with the calculated ka, k, and b. Calculated fitting parameters were analyzed using general linear model univariate ANOVA with pig and study period included as covariates in the model (IBM SPSS Statistics).
Power analysis indicated that, based on our prior experience with these measurements, and between-animal variation of up to 25–30%, our statistical power calculations indicate 12 pigs/group are necessary to detect, with a type I error of 0.05 and a power of 0.80, a difference of 5–10% in various endpoints of AA concentrations. As mentioned above in study design, we started with a total of 20 pigs but excluded 1 animal from the study, so the final analysis included the following group sizes: PBI (n = 13), NWPI (n = 13), and DWPI (n = 12). Pairwise comparisons among treatments were made with Bonferroni correction. Differences with a probability of <0.05 were considered significant and are indicated in the tables and figures by symbols. We used symbols to denote the significance of pairwise comparisons to simplify presentation rather than show multiple letters on graphs.
Results
Characterization of protein test solutions
Protein in NWPI was 100% soluble, 91% native, and 9% denatured; protein in DWPI was also 100% soluble, but only 9% was native and 91% denatured. Protein in PBI was 97% soluble, and 73% of the protein was soluble at pH 4.6. Because 30% of the protein in PBI is β-casein (Table 1), which precipitates at pH 4.6, the whey protein fraction is considered to be 100% native. Assessment of degree of Maillardation by total free amino groups analysis showed no difference between NWPI and DWPI (52 ± 1.6 mM and 56 ± 5.4 mM, respectively).
Postprandial plasma responses
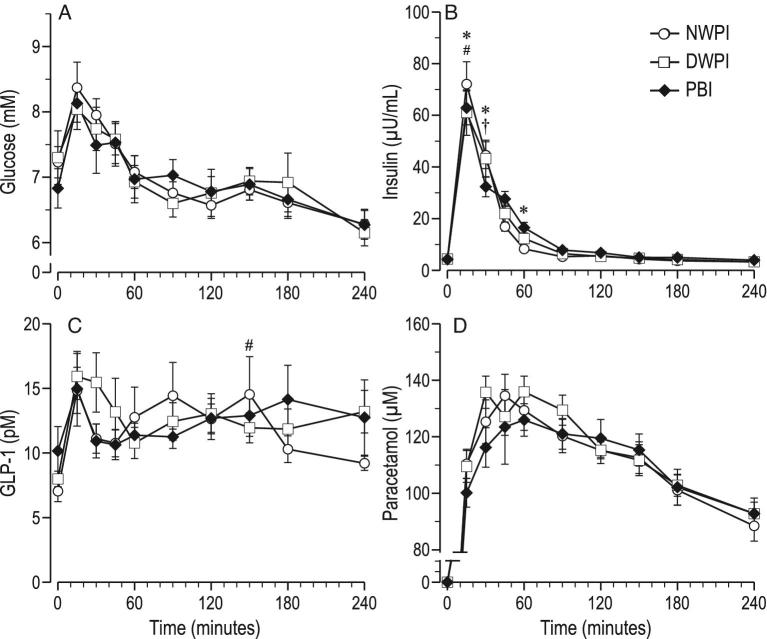
No significant differences were noted in postprandial plasma concentrations of paracetamol, glucose and GLP-1 among the 3 protein test solutions (Figure 2A, C, and D). Fitting parameters for plasma paracetamol concentration were also not significantly different among the 3 protein test solutions (Table 2). Significant differences were noted in postprandial plasma concentrations of insulin, TAA, EAA, BCAA, and most individual AA among administration of the 3 protein test solutions. The largest differences were present in the first hour after feeding the protein test solutions.
FIGURE 2.
Time course of postprandial plasma concentrations from baseline up to 4 h postadministration (mean ± SEM, n = 12–13); (A) glucose, (B) insulin, (C) GLP-1, (D) paracetamol; significant effect of time postadministration in all parameters (P < 0.01). #significant difference between NWPI and DWPI; *significant difference between PBI and NWPI (treatment × time, P < 0.05); †significant difference between PBI and DWPI (treatment × time, P < 0.05). GLP-1, glucagon-like peptide 1; PBI, protein-based ingredient; NWPI, native whey protein isolate; DWPI, denatured whey protein isolate.
TABLE 2.
Curve-fitting values for plasma amino acids and paracetamol after feeding1
| Native whey protein isolate | Denatured whey protein isolate | Protein-based ingredient | |
|---|---|---|---|
| Total amino acids | |||
| ka, ×10−3 | 1.81 ± 0.186a | 1.62 ± 0.232a | 3.31 ± 0.276b |
| k, ×10−3 | 27.2 ± 3.6 | 24.5 ± 1.61 | 29.9 ± 4.78 |
| b, μM | 4418 ± 221.7 | 4265 ± 235.7 | 3770 ± 180.7 |
| R2 | 0.726 ± 0.061 | 0.655 ± 0.058 | 0.802 ± 0.033 |
| Cmax, μM | 7657 ± 242a | 7450 ± 447a | 9010 ± 340b |
| tCmax, min | 120.4 ± 10.9 | 125.3 ± 6.3a | 94.9 ± 8.5b |
| Essential amino acids | |||
| ka, ×10−3 | 1.87 ± 0.241a | 1.93 ± 0.288a | 3.58 ± 0.281b |
| k, ×10−3 | 21.4 ± 1.8 | 19.9 ± 1.68 | 25.8 ± 3.95 |
| b, μM | 1832 ± 178.9a | 1544 ± 156.2 | 1358 ± 132.9b |
| R2 | 0.728 ± 0.069 | 0.704 ± 0.058 | 0.829 ± 0.037 |
| Cmax, μM | 3723.5 ± 150.4 | 3638.3 ± 287.0 | 4255.3 ± 255.8 |
| tCmax, min | 138.8 ± 13.6 | 145.1 ± 16.6a | 99.8 ± 8.4b |
| Branched chain amino acids | |||
| ka, ×10−3 | 2.72 ± 0.428 | 1.76 ± 0.287a | 3.62 ± 0.312b |
| k, ×10−3 | 35.8 ± 6.1 | 25.9 ± 2.49 | 33.8 ± 6.26 |
| b, μM | 344 ± 62.7 | 350 ± 36.9 | 286 ± 38.4 |
| R2 | 0.761 ± 0.036 | 0.721 ± 0.042 | 0.824 ± 0.034 |
| Cmax, μM | 1222.9 ± 52.2 | 1129.7 ± 101.3a | 1469.9 ± 82.7b |
| tCmax, min | 94.0 ± 9.9 | 127.6 ± 16.9 | 91.0 ± 10.4 |
| Paracetamol | |||
| ka, ×10−3 | 3.89 ± 0.182 | 3.90 ± 0.207 | 3.31 ± 0.180 |
| k, ×10−3 | 49.4 ± 1.9 | 48.4 ± 2.56 | 44.2 ± 2.10 |
| R2 | 0.872 ± 0.023 | 0.849 ± 0.054 | 0.863 ± 0.019 |
| Cmax, μM | 140.3 ± 5.2 | 143.7 ± 7.4 | 134.8 ± 6.3 |
| tCmax, min | 56.8 ± 2.0 | 58.4 ± 3.2 | 64.9 ± 2.7 |
1Values are means ± SEMs (n = 12–13). Different superscript letters indicate significant differences at P < 0.05. b, fasting basal concentration; Cmax, peak maximum concentration; k, rate constant for elimination; ka, rate constant for absorption; R2, coefficient of determination; tCmax, time at peak concentration.
Effect of denaturation
DWPI induced a ∼20% lower plasma insulin spike than NWPI at 15 min (Figure 2B), but the plasma insulin AUC was not significantly different in DWPI compared with NWPI (Table 3).
TABLE 3.
Area-under-the-curve values for plasma insulin and amino acids during the 4 h after feeding1
| Native whey protein isolate | Denatured whey protein isolate | Protein-based ingredient | |
|---|---|---|---|
| Insulin, mU/mL × min | 3.13 ± 0.23 | 3.06 ± 0.29 | 3.06 ± 0.19 |
| Total amino acids, mM × min | 1736.38 ± 52.28a | 1692.36 ± 89.02a | 1928.16 ± 70.06b |
| Essential amino acids, mM × min | 815.45 ± 33.19 | 786.71 ± 59.33 | 875.65 ± 51.35 |
| Branched chain amino acids, mM × min | 254.31 ± 9.11a | 238.55 ± 17.60a | 289.84 ± 13.76b |
| Ile, mM × min | 64.91 ± 2.82 | 63.60 ± 4.07 | 65.67 ± 4.69 |
| Leu, mM × min | 103.94 ± 5.00 | 97.01 ± 8.24 | 107.41 ± 7.13 |
| Val, mM × min | 85.79 ± 3.50a | 77.41 ± 6.04a | 116.92 ± 3.74b |
| Arg, mM × min | 31.31 ± 3.33a | 32.25 ± 3.48a | 38.36 ± 2.92b |
| His, mM × min | 22.26 ± 2.62a | 30.02 ± 3.83 | 33.91 ± 3.04b |
| Met, mM × min | 48.75 ± 2.45a | 40.53 ± 2.65b | 57.89 ± 2.53c |
| Phe, mM × min | 18.72 ± 1.18a | 23.82 ± 2.21b | 32.43 ± 1.09c |
| Trp, mM × min | 19.99 ± 1.42a | 17.32 ± 1.89 | 16.51 ± 1.24b |
| Gln, mM × min | 150.69 ± 6.08 | 140.81 ± 6.33a | 161.13 ± 7.72b |
| Glu, mM × min | 39.17 ± 2.21 | 37.07 ± 2.84a | 42.92 ± 2.57b |
| Pro, mM × min | 81.44 ± 2.56a | 83.00 ± 3.88a | 141.83 ± 5.50b |
1Values are means ± SEMs (n = 12–13). Different superscript letters indicate significant differences at P < 0.05.
Plasma TAA concentrations per time point were not significantly different between NWPI and DWPI (Figure 3A), nor were the plasma TAA AUC and the plasma TAA fitting parameters (Table 2). Plasma EAA concentrations per time point were significantly lower (∼10%) in DWPI than upon NWPI at 15 and 30 min (Figure 3B), but the plasma EAA AUC was not significantly different, nor were the plasma EAA fitting parameters. Plasma BCAA concentrations per time point were significantly lower (13–19%) upon DWPI than upon NWPI from 15 to 60 min (Figure 3C). However, the plasma BCAA AUC was not significantly different, nor were the plasma BCAA fitting parameters upon DWPI compared with upon NWPI.
FIGURE 3.
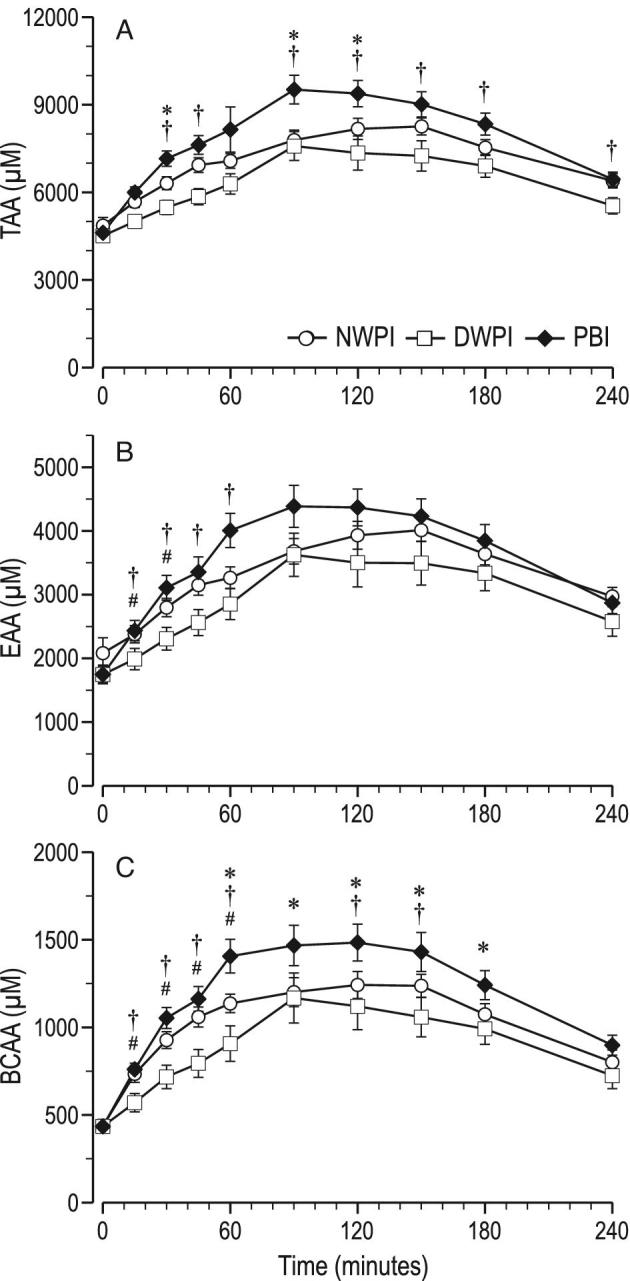
Time course of postprandial plasma summed AA concentrations from baseline up to 4 h postadministration (mean ± SEM, n = 12–13); (A) TAA, (B) EAA, and (C) BCAA; significant effect of time postadministration in all parameters (P < 0.01); significant effect of treatment (P < 0.05) for BCAA; #significant difference between NWPI and DWPI (treatment × time, P < 0.05); *significant difference between PBI and NWPI (treatment × time, P < 0.05); †significant difference between PBI and DWPI (treatment × time, P < 0.05). AA, amino acid; EAA, essential amino acids; TAA, total amino acids; PBI, protein-based ingredient; NWPI, native whey protein isolate; DWPI, denatured whey protein isolate.
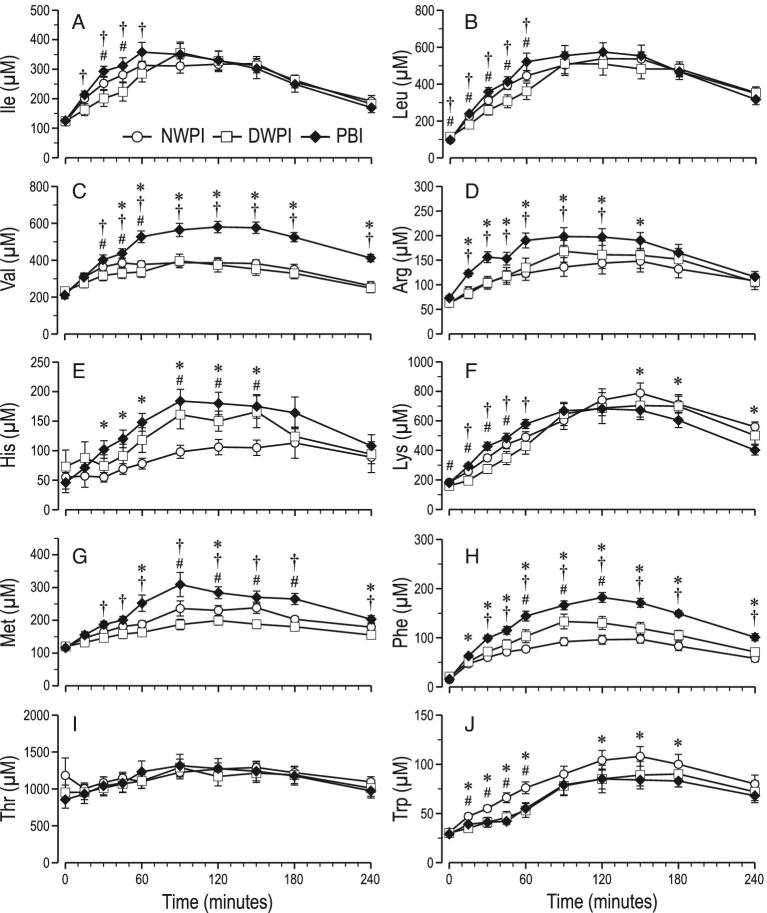
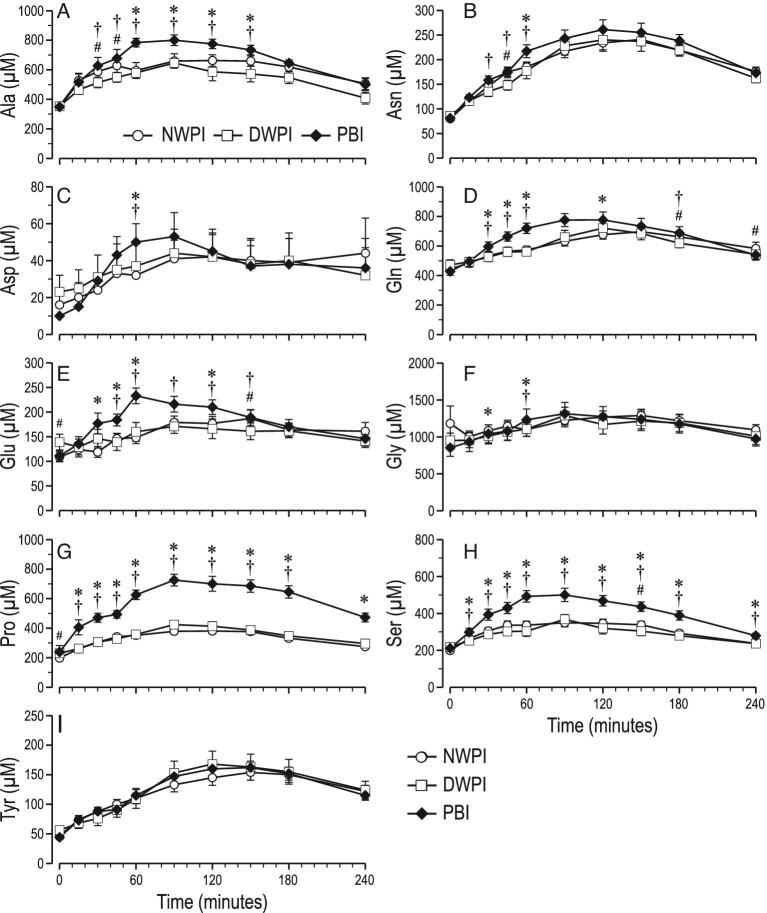
In the DWPI group, plasma Ile at 30 and 45 min, Leu from 0 to 60 min, Val from 30 to 60 min, Lys from 0 up to 45 min, Met from 90 up to 180 min and Trp from 15 up to 60 min was lower than in NWPI (Figure 4). However, only the plasma Met AUC was lower in DWPI than in NWPI. In contrast, in the DWPI group, His from 90 to 180 min and Phe from 60 to 120 min was higher than in NWPI. However, only the plasma Phe AUC was significantly higher in DWPI than in NWPI. Plasma Arg and Thr concentrations in the DWPI group were not different from NWPI. The only significantly different fitting parameter found for plasma individual EAA was the rate constant for absorption ka for Val, which was 1.7-fold higher in DWPI than in NWPI (Supplemental Table 1). In the DWPI group, plasma at Ala 30 and 45 min and Asn at 45 min were lower than in NWPI (Figure 5). No significant differences in Asp, Glu, Gln, Gly, Pro, Ser, and Tyr plasma concentrations were found between DWPI and NWPI. Plasma Orn and Tau were also higher in PBI than in DWPI (Supplemental Figure 1).
FIGURE 4.
Time course of postprandial plasma concentrations of individual essential amino acids from baseline up to 4 h postadministration (means ± SEMs, n = 12–13); (A) Ile, (B) Leu, (C) Val, (D) Arg, (E) His, (F) Lys (G), Met (H), Phe (I), Thr, (J) Trp; significant effect of time postadministration in all parameters (P < 0.01); significant effect of treatment (P < 0.05) for Val, Arg, His, Met, Trp, Phe. #significant difference between NWPI and DWPI; *significant difference between PBI and NWPI (treatment × time, P < 0.05); †significant difference between PBI and DWPI (treatment × time, P < 0.05). PBI, protein-based ingredient; NWPI, native whey protein isolate; DWPI, denatured whey protein isolate
FIGURE 5.
Time course of postprandial plasma concentrations of individual nonessential amino acids from baseline up to 4 h postadministration (mean ± SEM, n = 12–13); (A) Ala, (B) Asn, (C) Asp, (D) Gln, (E) Glu, (F) Gly, (G) Pro, (H) Ser, (I) Tyr; significant effect of time postadministration in all parameters (P < 0.01); significant effect of treatment (P < 0.05) for Ala, Ser, Pro, Gln, Glu. #significant difference between NWPI and DWPI (treatment × time, P < 0.05); *significant difference between PBI and NWPI (treatment × time, P < 0.05); †significant difference between PBI and DWPI (treatment × time, P < 0.05). PBI, protein-based ingredient; NWPI, native whey protein isolate; DWPI, denatured whey protein isolate.
Effect of protein composition
PBI induced a lower plasma insulin concentration than NWPI at 15 and 30 min, whereas it induced a higher insulin concentration at 60 min. The plasma insulin AUC was not significantly different.
Plasma TAA concentrations were significantly higher in PBI than in NWPI at 30, 90, and 120 min, and the plasma TAA AUC was significantly higher in PBI than in NWPI. The TAA content (g/100 g protein) of PBI was ∼1.3% higher than that of WPI (Figure 1). The fitting parameter ka for TAA was almost twofold higher, and the calculated TAA Cmax was also higher in PBI than NWPI. Plasma EAA concentrations and AUCs in PBI were higher, but not significantly different from NWPI (Figure 3B). The EAA content (g/100 g protein) of PBI was ∼4% lower than that of NWPI. Concomitantly, the fitting parameter ka for EAA was almost twofold higher in PBI than in NWPI. Plasma BCAA concentrations were higher in PBI than in NWPI from 60 to 180 min, and the plasma BCAA AUC was approximately 14% higher in PBI than in NWPI. The BCAA content (g/100 g protein) of PBI was ∼8% lower than that of NWPI. There were no significant differences found in the fitting parameters of plasma BCAA concentration between PBI and NWPI.
In the PBI group, plasma Val from 45 to 240 min, Arg from 15 to 150 min, His from 45 to 150 min, Met at 60, 90, and 240 min, and Phe from 15 to 240 min were higher than for NWPI. The plasma Val, Arg His, Met, and Phe AUCs were also higher in PBI than in NWPI, 38, 22, 52, 19 and 73%, respectively. PBI Val, Arg, His, Met, and Phe content (g/100 g protein) was higher than that of NWPI. The fitting parameter ka was higher only for His and Lys upon PBI compared with NWPI. Plasma Cmax was calculated to be higher for Val, Arg, and Phe in PBI than in NWPI. In addition, a higher k and lower tCmax for Lys were found in PBI than in NWPI. In contrast, in PBI, plasma Trp from 15 to 60 min and Lys from 150 to 240 min vs. NWPI were lower, also the plasma Trp AUC was 21% lower in PBI than in NWPI. Plasma Leu and Thr concentration and AUC were not different between PBI and NWPI. PBI Leu content (g/100 g protein) was less than that of NWPI. Concomitantly fitting parameter ka of Leu was higher in PBI than in NWPI. In PBI, plasma Ser from 15 to 240 min, Gln and Glu from 30 to 60 and at 120 min, Pro from 15 to 240 min, Gly at 30 and 60 min, Asp and Asn at 60 min, and Ala from 60 to 150 min were higher than NWPI. However, only the plasma Pro AUC was significantly higher (74%) in PBI than in NWPI. No significant differences in Tyr plasma concentration between PBI and NWPI were found.
Differences in postprandial plasma responses between PBI and DWPI generally followed the same pattern as between PBI and NWPI. Plasma insulin concentration was lower at 30 min (Figure 2B), but plasma insulin AUC was not significantly different between PBI and DWPI. Plasma TAA concentrations from 30 to 240 min and AUC were higher in PBI than DWPI, except at 60 min (Figure 3A). The fitting parameter ka for TAA was almost twofold higher, and the calculated Cmax was ∼21-fold higher in PBI than in DWPI. Plasma EAA concentrations in PBI were higher than in DWPI from 15 to 60 min, but the plasma EAA AUC was not significantly different. The fitting parameter ka for EAA was almost twofold higher in PBI than in DWPI. Plasma BCAA concentrations in PBI were also higher than in DWPI from 15 to 150 min with the exception of 90 min (Figure 3C), and the plasma BCAA AUC was approximately 22% higher in PBI than in DWPI. The fitting parameter ka for BCAA was twofold higher, and a 30% higher plasma Cmax was calculated from the fitted curves in PBI than DWPI. In the PBI group, plasma Ile from 45 to 60, Leu from 0 to 60 min, Val from 45 to 240 min, Arg from 15 to 120 min, Lys from 15 to 60 min, Met from 45 to 240 min, and Phe from 45 to 240 was higher than in DWPI. The plasma Val, Arg, Met, and Phe AUCs were also higher in PBI than in DWPI, 51, 19, 42, and 36%, respectively. The fitting parameter ka was found to be higher only for Leu and Lys in PBI than in DWPI. Plasma Val, Met, and Phe Cmax were calculated to be higher in PBI than in DWPI.
Discussion
Increased concentrations of protein in infant formula compared with human milk have been linked to overweight and obesity risk in children. The early protein hypothesis that has been postulated to explain these differences in growth rate is mechanistically linked to high insulin and IGF-1 levels that are released due to increased plasma AA concentrations. In this study, our aim was to investigate whether changing milk protein denaturation or composition would affect postprandial plasma AA and insulin concentrations. We found that the difference in protein composition significantly affected postprandial plasma responses (PBI compared with NWPI). Although PBI resulted in a lower plasma insulin spike within 15 min of feeding, the plasma concentrations of most AA and BCAA were higher with PBI than with NWPI. The plasma insulin and GLP-1 plasma AUC were not different between the 3 protein test solutions. In contrast, the plasma AUC of BCAA was ∼14% higher in PBI, even though the BCAA content of PBI was ∼8% lower than for WPI. Because BCAAs, especially Leu and also Arg, are known to promote insulin secretion (33), our finding of a lower plasma insulin peak in PBI was not expected. The largest increase in plasma concentration among the BCAA in PBI was for Val, which is a weak insulin secretagogue compared to Leu and Ile (34, 35). Unexpectedly, we found little effect of denaturation (DWPI compared with NWPI) which runs counter to our hypothesis that denatured proteins, as compared to native proteins, lead to faster absorption of digestion products and increased plasma AA concentrations. This finding is consistent with Barbe et al. (36), who observed no significant differences in plasma leucine between unheated and heated (90°C for 10 min) milk protein (containing 80% casein) in mini pigs.
During multiple time points in the postprandial period, the plasma concentrations of several AAs were consistently higher with the PBI than with NWPI/DWPI solutions resulting in a higher plasma AUC for these AAs. We did not expect this result, given that the EAA content of NWPI/DWPI test solutions was higher than for PBI. Moreover, by curve fitting, we found that the rate constant for absorption (ka) was higher, and the tCmax was earlier for EAA in the PBI group. The total BCAA content of NWPI/DWPI was slightly higher than for PBI, yet Val was more enriched in PBI. Given these differences in BCAA composition, we found that the time course of plasma BCAA levels and plasma AUC were both greater in PBI, especially for Val. This was reflected by the higher ka of BCAA and Leu for PBI than for DWPI. We suggest that the observed differences in plasma postprandial AA kinetics, especially BCAA, between the test protein groups are therefore likely related to protein digestion and absorption kinetics in addition to AA composition alone.
The key compositional difference between PBI and NWPI/DWPI was that PBI contained β-casein. The fast and slow protein concept as postulated by Boirie is based on the observations that postprandial plasma AA peak height appears to be protein-type-dependent (17). For example, whey protein ingestion results in a higher postprandial and earlier AA peak than casein protein ingestion. This slower postprandial AA peak with casein than with whey protein has been attributed to the slower gastric emptying rate due to the distinct physicochemical behavior (i.e., clotting) of the proteins under the acidic gastric conditions (18). In vitro investigation of the gastric clotting behavior using a method described earlier (37) showed that PBI, in contrast to similar preparations containing α-, β-, and κ-caseins, did not form particles larger than 1 mm (data not shown). This indicates that for gastric clotting to form particles that could be contained by gastric pyloric sieving (i.e., larger than 1 mm [38]), a mixture of caseins may be needed. In our current study, the presence of β-casein in the PBI did not alter gastric emptying as assessed by plasma paracetamol appearance, suggesting that indeed a “slowing” effect of β-casein on gastric emptying did not occur. Paracetamol is a water-soluble marker and may therefore not reflect emptying of solidified material. However, a slowing of gastric emptying would not explain the higher plasma AA concentrations, because peaks were higher in PBI than in NWPI. Therefore, it is more likely to be due to the rates of digestion and absorption of AAs in the small intestine.
Previously, a complex 13 compartmental model was built to study postprandial nitrogen metabolism. This model demonstrated that intestinal absorption kinetics are affected by protein quantity and quality intake, and this in turn impacts first-pass splanchnic catabolism and peripheral availability of dietary AAs (39). In this study, we used a pharmacokinetic model to fit postprandial plasma AA concentrations. Thus, because we did not isotopically label the AAs in the test proteins, we cannot discriminate between AAs appearing in the plasma derived from the test protein and whole body tissue proteolysis. However, with the adaptations to the model (i.e., inclusion of a term for basal concentrations, and a determined mean fraction of uptake), we were able to gain insights in the relation between protein (and AA) composition on postprandial plasma responses by determining ka. The fraction of absorption (S) that gave the best fit of our experimental data was 0.15, meaning the sum of splanchnic extraction and inhibition of systemic protein breakdown is estimated to be 85%; this is in line with what has been found experimentally. Others have experimentally shown that in neonatal piglets, the splanchnic extraction of BCAA could amount to 44% (40), whereas we have previously found that the portal-drained viscera can account for use of up to 90% of dietary AAs (41).
One potential limitation of this study is that the piglets were not fed a complete meal. The test solutions contained only protein and lactose. We aimed to investigate the effects of the protein with as few potential confounders as possible, to understand the mechanism. However, feeding these same proteins as part of a complete meal (i.e., including lipids and higher levels of lactose) or in larger, single bolus feedings may impact the results and warrants further study.
In summary, we conclude that a protein milk composition including β-casein (PBI) did not significantly delay gastric emptying or show slower digestion kinetics than with a whey protein isolate. The PBI protein mixture did result in a lower postprandial insulin peak, but higher postprandial concentrations of BCAA and several individual AAs than with NWPI/DWPI. The differences observed between PBI and NWPI can be partly explained by the difference in AA composition, but we suggest that differences in protein digestion and absorption kinetics are also involved. Furthermore, we conclude that under the used conditions, almost complete whey protein denaturation (91% denatured compared with 91% native) does not result in faster digestion and absorption as reflected in postprandial plasma responses. Curve fitting using pharmacokinetic models can be helpful in gaining insights in the mechanism underlying differences in postprandial responses of protein test solutions with different protein and AA composition by distinguishing between the effects of AA content (D) and kinetics of digestion and absorption (ka).
Supplementary Material
Acknowledgments
We thank Joseph Thomas Ryan and Cian Hickey for the preparation of the PBI, and Greg Guthrie, Liwei Cui, Sen Lin, Lee Call, and Dennis Kunichoff for their assistance with the animal protocol and surgical procedures. The authors’ responsibilities were as follows—DGB, BS, and EA: designed the research; BS and RJW-J: conducted the research; EA, BJMvdH, and IBR: provided essential materials; EA, RJW-J, PAW, DGB, and OS: analyzed the data and performed the statistical analysis; RJW-J, DGB, EA, and BJMvdH: wrote the paper; DGB and EA: had primary responsibility for the design, writing, and final content; and all authors: read and approved the final manuscript.
Notes
This work was supported in part by federal funds from the USDA, Agricultural Research Service under Cooperative Agreement Number 3092-51000-060-01, and grants from Nutricia Research and RJW was supported by NIH Grant OD014808.
Author disclosures: EA, BJMvdH, and IBR, employees of Nutricia Research; DGB, received grant support for this study from Nutricia Research. RJW-J, BS, O'BS, and PAW, no conflicts of interest.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AA, amino acid; AAA, aromatic amino acid; AUC, area under the curve; BCAA, branched chain amino acid; BSA, bovine serum albumin; Cmax, concentration maximum; DWPI, denatured whey protein isolate; EAA, essential amino acid; GLP-1, glucagon-like peptide 1; IG, immunoglobulin; IGF-1, insulin-like growth factor 1; NWPI, native whey protein isolate; PBI, protein-based ingredient; RP-HPLC, reverse-phase high-pressure liquid chromatography; TAA, total amino acid; tCmax, time to reach concentration maximum; WPI, whey protein isolate.
References
- 1. Lee FE, Edmunds LS, Cong X, Sekhobo JP. Trends in breastfeeding among infants enrolled in the Special Supplemental Nutrition Program for Women, Infants and Children—New York, 2002–2015. Morb Mortal Wkly Rep 2017;66:610–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luque V, Closa-Monasterolo R, Escribano J, Ferré N. Early programming by protein intake: The effect of protein on adiposity development and the growth and functionality of vital organs. Nutr Metab Insights 2015;8(Suppl 1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prell C, Koletzko B.. Breastfeeding and complementary feeding. Dtsch Arztebl Int 2016;113(25):435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Le Huerou-Luron I, Blat S, Boudry G. Breast- v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr Res Rev 2010;23(1):23–36. [DOI] [PubMed] [Google Scholar]
- 5. Koletzko B, Demmelmair H, Grote V, Prell C, Weber M. High protein intake in young children and increased weight gain and obesity risk. Am J Clin Nutr 2016;103(2):303–4. [DOI] [PubMed] [Google Scholar]
- 6. Koletzko B, Brands B, Poston L, Godfrey K, Demmelmair H; Early Nutrition Project. Early nutrition programming of long-term health. Proc Nutr Soc 2012;71(3):371–8. [DOI] [PubMed] [Google Scholar]
- 7. Patro-Golab B, Zalewski BM, Kołodziej M, Kouwenhoven S, Poston L, Godfrey KM, Koletzko B, van Goudoever JB, Szajewska H. Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of overweight, obesity and body fat: A systematic review of systematic reviews. Obes Rev 2016;17(12):1245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mook-Kanamori DO, Durmuş B, Sovio U, Hofman A, Raat H, Steegers EA, Jarvelin MR, Jaddoe VW. Fetal and infant growth and the risk of obesity during early childhood: The Generation R Study. Eur J Endocrinol 2011;165(4):623–30. [DOI] [PubMed] [Google Scholar]
- 9. Damianidi L, Gruszfeld D, Verduci E, Vecchi F, Xhonneux A, Langhendries JP, Luque V, Theurich MA, Zaragoza-Jordana M, Koletzko B et al.. Protein intakes and their nutritional sources during the first 2 years of life: Secondary data evaluation from the European Childhood Obesity Project. Eur J Clin Nutr 2016;70(11):1291–7. [DOI] [PubMed] [Google Scholar]
- 10. Spalinger J, Nydegger A, Belli D, Furlano RI, Yan J, Tanguy J, Pecquet S, Destaillats F, Egli D, Steenhout P. Growth of infants fed formula with evolving nutrition composition: A single-arm non-inferiority study. Nutrients 2017;9(3)219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moro G, Minoli I, Boehm G, Georgi G, Jelinek J, Sawatzki G. Postprandial plasma amino acids in preterm infants: Influence of the protein source. Acta Paediatr 1999;88(8):885–9. [DOI] [PubMed] [Google Scholar]
- 12. Bourlieu C, Ménard O, Bouzerzour K, Mandalari G, Macierzanka A, Mackie AR, Dupont D. Specificity of infant digestive conditions: Some clues for developing relevant in vitro models. Crit Rev Food Sci Nutr 2014;54(11):1427–57. [DOI] [PubMed] [Google Scholar]
- 13. Dupont D, Mandalari G, Mollé D, Jardin J, Rolet-Répécaud O, Duboz G, Léonil J, Mills CE, Mackie AR. Food processing increases casein resistance to simulated infant digestion. Mol Nutr Food Res 2010;54(11):1677–89. [DOI] [PubMed] [Google Scholar]
- 14. Reddy IM, Kella NKD, Kinsella JE. Structural and conformational basis of the resistance of beta-lactoglobulin to peptic and chymotryptic digestion. J Agric Food Chem 1988;36(4):737–41. [Google Scholar]
- 15. Peram MR, Loveday SM, Ye A, Singh H. In vitro gastric digestion of heat-induced aggregates of beta-lactoglobulin. J Dairy Sci 2013;96(1):63–74. [DOI] [PubMed] [Google Scholar]
- 16. Lindberg T, Engberg S, Sjöberg LB, Lönnerdal B. In vitro digestion of proteins in human milk fortifiers and in preterm formula. J Pediatr Gastroenterol Nutr 1998;27(1):30–6. [DOI] [PubMed] [Google Scholar]
- 17. Bos C, Metges CC, Gaudichon C, Petzke KJ, Pueyo ME, Morens C, Everwand J, Benamouzig R, Tomé D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J Nutr 2003;133(5):1308–15. [DOI] [PubMed] [Google Scholar]
- 18. Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 1997;94(26):14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luiking YC, Abrahamse E, Ludwig T, Boirie Y, Verlaan S. Protein type and caloric density of protein supplements modulate postprandial amino acid profile through changes in gastrointestinal behaviour: A randomized trial. Clin Nutr 2016;35(1):48–58. [DOI] [PubMed] [Google Scholar]
- 20. Chibnall AC, Rees MW, Williams EF. The total nitrogen content of egg albumin and other proteins. Biochem J 1943;37(3):354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hinz K, Huppertz T, Kelly AL. Susceptibility of the individual caseins in reconstituted skim milk to cross-linking by transglutaminase: Influence of temperature, pH and mineral equilibria. J Dairy Res 2012;79(4):414–21. [DOI] [PubMed] [Google Scholar]
- 22. McCarthy NA, Wijayanti HB, Crowley SV, O'Mahony JA, Fenelon MA. Pilot-scale ceramic membrane filtration of skim milk for the production of a protein base ingredient for use in infant milk formula. Int Dairy J 2017;73:57–62. [Google Scholar]
- 23. Terrlink T, van Leeuwen PA, Houdijk A. Plasma amino acids determined by liquid chromatography within 17 minutes. Clin Chem 1994;40(2):245–9. [PubMed] [Google Scholar]
- 24. National Research Council. Guide for the care and use of laboratory animals. 8th ed.Washington (DC):The National Academies Press; 2011, 246. [Google Scholar]
- 25. Klobasa F, Werhahn E, Butler JE. Composition of sow milk during lactation. J Anim Sci 1987;64(5):1458–66. [DOI] [PubMed] [Google Scholar]
- 26. Daza A, Riopérez J, Centeno C.. Short communication. Changes in the composition of sows’ milk between days 5 to 26 of lactation. Span J Agric Res 2004;2(3):4. [Google Scholar]
- 27. Suenderhauf C, Tuffin G, Lorentsen H, Grimm HP, Flament C, Parrott N. Pharmacokinetics of paracetamol in Gottingen minipigs: In vivo studies and modeling to elucidate physiological determinants of absorption. Pharm Res 2014;31(10):2696–707. [DOI] [PubMed] [Google Scholar]
- 28. Delahaije RJ, Gruppen H, van Eijk van Boxtel EL, Cornacchia L, Wierenga PA. Controlling the ratio between native-like, non-native-like, and aggregated beta-lactoglobulin after heat treatment. J Agric Food Chem 2016;64(21):4362–70. [DOI] [PubMed] [Google Scholar]
- 29. Wierenga PA, Meinders MBJ, Egmond MR, Voragen FAGJ, de Jongh HHJ. Protein exposed hydrophobicity reduces the kinetic barrier for adsorption of ovalbumin to the air–water interface. Langmuir 2003;19(21):8964–70. [Google Scholar]
- 30. Stoll B, Chang X, Fan MZ, Reeds PJ, Burrin DG. Enteral nutrient intake level determines intestinal protein synthesis and accretion rates in neonatal pigs. Am J Physiol Gastrointest Liver Physiol 2000;279(2):G288–94. [DOI] [PubMed] [Google Scholar]
- 31. Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: The preterm pig as a model in pediatric gastroenterology. J Anim Sci 2013;91(10):4713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linderkamp O, Berg D, Betke K, Köferl F, Kriegel H, Riegel KP. Blood volume and hematocrit in various organs in newborn piglets. Pediatr Res 1980;14:1324. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Kobayashi H, Mawatari K, Sato J, Bajotto G, Kitaura Y, Shimomura Y. Effects of branched-chain amino acid supplementation on plasma concentrations of free amino acids, insulin, and energy substrates in young men. J Nutr Sci Vitaminol (Tokyo) 2011;57(1):114–7. [DOI] [PubMed] [Google Scholar]
- 34. Salehi A, Gunnerud U, Muhammed SJ, Ostman E, Holst JJ, Björck I, Rorsman P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on beta-cells. Nutr Metab (Lond) 2012;9(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans 2007;35(Pt 5):1180–6. [DOI] [PubMed] [Google Scholar]
- 36. Barbe F, Ménard O, Le Gouar Y, Buffière C, Famelart MH, Laroche B, Le Feunteun S, Dupont D, Rémond D. The heat treatment and the gelation are strong determinants of the kinetics of milk proteins digestion and of the peripheral availability of amino acids. Food Chem 2013;136(3–4):1203–12. [DOI] [PubMed] [Google Scholar]
- 37. van den Braak CC, Klebach M, Abrahamse E, Minor M, Hofman Z, Knol J, Ludwig T. A novel protein mixture containing vegetable proteins renders enteral nutrition products non-coagulating after in vitro gastric digestion. Clin Nutr 2013;32(5):765–71. [DOI] [PubMed] [Google Scholar]
- 38. Hellstrom PM, Gryback P, Jacobsson H. The physiology of gastric emptying. Best Pract Res Clin Anaesthesiol 2006;20(3):397–407. [DOI] [PubMed] [Google Scholar]
- 39. Fouillet H, Juillet B, Gaudichon C, Mariotti F, Tomé D, Bos C. Absorption kinetics are a key factor regulating postprandial protein metabolism in response to qualitative and quantitative variations in protein intake. Am J Physiol Regul Integr Comp Physiol 2009;297(6):R1691–705. [DOI] [PubMed] [Google Scholar]
- 40. Elango R, Pencharz PB, Ball RO. The branched-chain amino acid requirement of parenterally fed neonatal piglets is less than the enteral requirement. J Nutr 2002;132(10):3123–9. [DOI] [PubMed] [Google Scholar]
- 41. Stoll B, Burrin DG. Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers. J Anim Sci 2006;84(suppl_13):E60–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.