Abstract
The threshold for amyloid positivity by visual assessment on PET has been validated by comparison to amyloid load measured histopathologically and biochemically at post mortem. As such, it is now feasible to use qualitative visual assessment of amyloid positivity as an in-vivo gold standard to determine those factors which can modify the quantitative threshold for amyloid positivity. We calculated quantitative amyloid load, measured as Standardized Uptake Value Ratios (SUVRs) using [18-F]florbetaben PET scans, for 159 Hispanic and non-Hispanic participants, who had been classified clinically as Cognitively Normal (CN), Mild Cognitive Impairment (MCI) or Dementia (DEM). PET scans were visually rated as amyloid positive (A+) or negative (A-), and these judgments were used as the gold standard with which to determine (using ROC analyses) the SUVR threshold for amyloid positivity considering factors such as age, ethnicity (Hispanic versus non-Hispanic), gender, cognitive status, and apolipoprotein E ε4 carrier status. Visually rated scans were A+ for 11% of CN, 39.0% of MCI and 70% of DEM participants. The optimal SUVR threshold for A+ among all participants was 1.42 (sensitivity = 94%; specificity = 92.5%), but this quantitative threshold was higher among E4 carriers (SUVR = 1.52) than non-carriers (SUVR = 1.31). While mean SUVRs did not differ between Hispanic and non-Hispanic participants;, a statistically significant interaction term indicated that the effect of E4 carrier status on amyloid load was greater among non-Hispanics than Hispanics. Visual assessment, as the gold standard for A+, facilitates determination of the effects of various factors on quantitative thresholds for amyloid positivity. A continuous relationship was found between amyloid load and global cognitive scores, suggesting that any calculated threshold for the whole group, or a subgroup, is artefactual and that the lowest calculated threshold may be optimal for the purposes of early diagnosis and intervention.
Keywords: Amyloid, APOE, Threshold, Cognition, Hispanic, SUVR
Highlights
-
•
Demographic factors did not affect the threshold for amyloid positivity.
-
•
Cognitive status did not affect this threshold for amyloid positivity.
-
•
APOE4 carriers had a higher threshold for amyloid positivity than non-carriers.
-
•
Among APOE4 carriers, non-Hispanics had higher amyloid load than non- Hispanics.
-
•
There was a continuous relationship between amyloid load and cognitive status.
1. Introduction
Among cognitively normal (CN) individuals, the frequency of amyloid positivity increases progressively with advancing age (Aizenstein et al., 2008; Wolk and Klunk, 2009). Fewer than 5% of participants 50 to 60 years, 10% of those 60 to 70 years, 25% of those 70 to 80 years, and >50% of those 80–90 years of age have been found to be amyloid positive (A+) on Amyloid Positron Emission Tomography (Aß-PET). Amyloid positivity is also associated with greater cognitive impairment (Johnson et al., 2013). Among cognitively healthy elderly individuals, those destined to become A+ may begin to have progressive increases in amyloid load as much as 12 to 20 years before reaching the threshold which is known as a positive amyloid scan (Perani et al., 2014; Rowe et al., 2010; Villemagne et al., 2011). APOEε4 genotype carriers (APOEε4+) have higher amyloid loads than non-carriers, at all ages and at all levels of cognitive impairment (Li et al., 2017; Rowe et al., 2007). Further, individuals with mild cognitive impairment (MCI) and dementia (DEM) have higher amyloid tracer retention than cognitively normal (CN) individuals, primarily because a higher proportion of cognitively impaired individuals are likely to have Alzheimer's disease (AD) and to be APOEε4+ as well (Jack et al., 2013). Sex has not been found to have a significant effect on amyloid load (Murphy et al., 2013; Schmidt et al., 2015). The risk for AD associated with APOEε4 varies across ethnic groups with Whites Americans showing a higher risk compared to Hispanics (Campos et al., 2013) and African Americans (Tang et al., 1998). This association has been inconsistent in ethnic minority groups. While the number of copies of the e4 allele in some studies has not been associated with risk or age-of onset of LOAD (Evans et al., 2003;, Murrell et al., 2006; Tycko et al., 2004), other studies have observed such effect (Graff-Radford et al., 2002).
Qualitative methods for determining amyloid positivity on amyloid PET scans are based on an expert reader's ability to make accurate binary visual assessments (presence or absence) of the amyloid binding tracer in the cerebral cortex, in one or more brain regions. These binary visual ratings have high inter-rater reliability and have been validated by demonstration at postmortem; there is a high correspondence between amyloid burden in PET scans and histopathological and biochemical evidence of amyloid plaques at autopsy (Ikonomovic et al., 2008). Quantitative methods for establishing amyloid positivity require the determination of a threshold level of amyloid load antemortem, which corresponds to the presence and density of amyloid plaques assessed histopathologically at autopsy (Johnson et al., 2013). In the case of the PET amyloid ligand, [18-F] florbetaben, the semi-quantitative threshold for amyloid positivity, validated histopathologically in patients with terminal diseases, has been reported to be 1.48, measured in standardized uptake value ratios (SUVR) normalized to the gray matter of the cerebellum (Sabri et al., 2015).
More recently, Aß-PET scans, visually rated as A+ or A-, have been used as a “gold standard” of amyloid positivity, instead of histopathological validation of amyloid positivity. All of the currently available amyloid tracers have received approval from governmental regulatory bodies only after histopathological validation at autopsy. The use of “imaging validation” of amyloid positivity eliminates the requirement for performing Aß-PET scanning on terminal patients and following them to autopsy and facilitates the determination of the threshold of amyloid positivity in much greater numbers of living subjects, of varying ages, ethnicities and levels of cognitive impairment, including in cognitively normal individuals (Yeo et al., 2015). Using imaging validation, and the cerebellar gray matter as the reference region, the optimal threshold for amyloid positivity for [18-F] florbetaben amyloid PET scans among a group of cognitively normal and AD patients, was determined to be an SUVR of 1.43 (Bullich et al., 2017).
In this study we used the “imaging validation” of amyloid positivity (i.e., a binary visual reading as the gold standard) as described by Bullich et al. (2017), on [18-F] florbetaben PET scans, so as to calculate the optimal quantitative SUVR threshold that distinguished A+ from A- scans. The study was conducted using data obtained from participants in the 1Florida Alzheimer's Disease Research Center (1Florida ADRC) who were cognitively normal, as well as patients impaired to varying degrees, as seen in a typical clinical practice. Our primary goal was to determine the quantitative threshold of amyloid positivity considering factors such as age, ethnicity (Hispanic versus non-Hispanic), sex, severity of cognitive impairment, and APOE genotype. Ethnic groups, other than non-Hispanic Whites at risk of AD, are genetically understudied (Reitz and Mayeux, 2014) and this study contributes to closing this gap.
2. Materials and methods
2.1. Recruitment, clinical evaluations, and diagnoses
Participants (N = 159) were recruited into the 1Florida Alzheimer's Disease Research Center (1Florida ADRC) longitudinal study between mid-2015 and the end of 2017. This study was approved by the Mount Sinai Medical Center IRB and all participants and their study partners provided informed consent. The participants were between the ages of 50 and 90 years, and had completed an extensive medical, neurological, psychiatric and neuropsychological evaluation, including all the elements required by the National Alzheimer's Coordinating Center (NACC) Uniform Data Set, version 3.0 (Beekly et al., 2004, Beekly et al., 2007). The Clinical Dementia Rating Scale (CDR) (Morris, 1993) was administered by an experienced geriatric psychiatrist (MG), who was blinded to the neuropsychological test results. Ethnicity of the participants was determined using self-report (National Research Council, 2004) using this question: “Does the subject report being of Hispanic/Latino ethnicity (i.e., having origins from a mainly Spanish-speaking Latin American country), regardless of race.”
A standard neuropsychological battery was administered in the preferred language to self-identified Hispanic and White non-Hispanic participants, by a Spanish/English bilingual psychometrician who was blinded to the clinical evaluation and the CDR score. This neuropsychological protocol included the following tests,: (1) the Hopkins Verbal Learning Test-Revised (HVLT-R: Benedict et al., 1998), (2) delayed recall from the Logical Memory subtest of the National Alzheimer's Coordinating Center Uniform Dataset Neuropsychological Battery (Beekly et al., 2007), (3) Category Fluency (Lucas et al., 1998), (4) the Block Design subtest of the Wechsler Adult Intelligence Scales-Fourth Edition (Wechsler, 2008), (5) Parts A and B of the Trail Making Test (Reitan, 1955) and (6) the Folstein Mini-Mental State Exam (Folstein et al., 1975). Translated and standardized Spanish versions of all tests were used with the corresponding age and education normative data (Arango-Lasprilla et al., 2015; Acevedo et al., 2009; Peña-Casanova et al., 2009).
2.2. Diagnostic procedures
Cognitive Diagnoses followed the NACC D1 classification protocol, which includes Cognitively Normal (CN), amnestic and non-amnestic Mild Cognitive Impairment (MCI), and Dementia (DEM). Using the above Consensus diagnosis criteria, 159 participants were classified as follows: CN (n = 47; mean age = 70.3 ± 6.1 years; mean MMSE = 29.1 ± 1.2), amnestic or non-amnestic MCI (n = 75; mean age = 72.6 ± 7.0 years, mean MMSE = 27.0 ± 2.4), and DEM (n = 37; mean age 72.4 ± 9.5 years; mean MMSE = 22.1 ± 4.6) (Table 1). All participants included in this study also underwent structural 3-D volumetric magnetic resonance imaging (MRI) and an Aß-PET scan, using [18-F] florbetaben.
Table 1.
Demographics, Cognitive Scores, APOE, SUVR and Hippocampal Volumes for CN, MCI and DEM Subjects.
| CN (n = 47) | MCI (n = 75) | Dementia (n = 37) | F Statistic or X2 | Eta-Square or Cramer's V | |
|---|---|---|---|---|---|
| Age | 70.3 ± 6.1 | 72.6 ± 7.0 | 72.4 ± 9.5 | 1.6 | 0.0199 |
| Education | 16.5 ± 3.1a | 14. ±3.3b | 15.4 ± 3.5a,b | 4.7⁎ | 0.0565 |
| Female (%) | 31(66%) | 42 (56%) | 20 (54%) | 1.6 | 0.0994 |
| Hispanic by self-report (%) | 27 (57%) | 46 (61%) | 21 (57%) | 0.3 | 0.0429 |
| MMSE Score | 29.1 ± 1.2a | 27.0 ± 2.4b | 22.1 ± 4.6c | 62.9⁎⁎⁎ | 0.4482 |
| APOEε4+ve (%) | 9 (23%) | 31 (47%) | 13 (42%) | 6.3⁎ | 0.2106 |
| Mean SUVR | 1.22 ± 0.16a | 1.40 ± 0.27b | 1.60 ± 0.35c | 21.5⁎⁎⁎ | 0.2162 |
| Mean SUVR for A− | 1.18 ± 0.08a | 1.24 ± 0.14a | 1.17 ± 0.16a | 3.69⁎ | 0.0715 |
| Mean SUVR for A+ | 1.58 ± 0.21a | 1.66 ± 0.23a | 1.79 ± 0.22a | 3.26⁎ | 0.1027 |
| Amyloid + Visual Read (%) | 5 (11%) | 29 (39%) | 26 (70%) | 31.4⁎⁎⁎ | 0.4443 |
Significant group differences tests: ANOVA for continuous variables and Chi-square tests for categorical values; significance level is 0.05 (*p < .05; **p < .01; ***p < .001); 2) Means with different alphabetic superscripts are statistically significant by the post-hoc Tukey Honestly Significant Difference (HSD) Test; 3) Mean SUV for frontal, parietal, temporal, cingulate, normalized to cerebellar gray matter.
A+ = Amyloid Positive by visual read; A− = Amyloid negative by visual read.
2.3. MRI
MRI scans were performed using a Siemens Skyra 3 T MRI scanner at Mount Sinai Medical Center, Miami Beach. MRI scans were evaluated by visual inspection as well as with T2 weighted FLAIR (5 mm thick sequential axial slices), and a 3D T1 weighted volumetric magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (which provides high tissue contrast and high spatial resolution with whole brain coverage). FreeSurfer Version 5.3 software was used to obtain parcellation of regional brain volumes and cortical thickness at 1.0 mm isotropic resolution (http://surfer.nmr.mgh.harvard.edu) for co-registration with amyloid PET scans.
2.4. Radiotracer production for amyloid imaging
The full radiosynthesis of [18-F] florbetaben was performed at an FDA approved manufacturing facility following Good Manufacturing Practice (Neuraceq©).
2.5. PET scan imaging
A 3D Hoffmann brain phantom was used to establish a standardized acquisition and reconstruction method. Participants were infused with [18-F] florbetaben 300 MBQ over a 3 min period. Scanning commenced 70 min after the infusion for a duration of 20 min on a Siemens Biograph 16 PET/CT scanner operating in 3D mode (55 slices/frame, 3 mm slice thickness 128 × 128 matrix). The PET data were reconstructed into a 128 × 128 × 63 (axial) matrix with voxel dimensions of 0.21 × 0.21 × 0.24 cm. Reconstruction was performed using manufacturer-supplied software and included corrections for attenuation, scatter, random coincidences and dead time. Images for regional analyses were processed using Fourier analysis followed by direct Fourier reconstruction. Images were smoothed with a 3 mm Hann filter. Following reconstruction, image sets were inspected and, if necessary, corrected for inter-frame motion. Images were obtained from the top of the head to the top of the neck and computed tomography (CT) data were employed for initial attenuation correction and image reconstruction in the sagittal, axial and coronal planes.
2.6. Qualitative/visual assessment of PET scans
All Aß-PET scans were read initially by an independent, trained radiologist, who was not otherwise involved in this study, and a trained and experienced reader (RD) both of whom were blinded to the cognitive and clinical diagnosis, using a methodology similar to that described by Seibly (Seibyl et al., 2016). Images were displayed using a reader-adjustable gray scale to provide optimal discrimination of the cerebellar gray matter from white matter. Subsequently, all the Aß-PET scan slices were viewed using this gray scale adjustment. There was initial disagreement, which was resolved by inclusion of an additional reader and majority decision, on readings for three participants, all diagnosed clinically as CN. Tracer uptake was assessed in six cortical regions (orbitofrontal, frontal, parietal, lateral temporal, occipital and precuneus/posterior cingulate cortex, combining values from the left and right hemispheres) using the regional cortical tracer uptake (RCTU) system (Bullich et al., 2017). A final dichotomous (A+ versus A-) diagnosis was made by each reader. Inter-rater reliability was assessed on 95 PET scans (20% were from the cognitively normal group, 53% from MCI subjects, and 27% from dementia subjects). Among the amyloid PET scans, 41 were read as A+ and 54 as A-. The agreement between the two readers was 93.2% for positive scans and 100% for negative scans.
2.7. Quantitative assessment of PET scans
The florbetaben PET/CT scans, including the outline of the skull, were co-registered linearly (i.e., trilinear interpolation) with 12 degrees of freedom, onto the volumetric MRI scan using a T1-weighted (MP-RAGE) (Smith et al., 2004; Lizarraga et al., 2018). Region-of-interest (ROI) boundaries were defined manually using the structural MRI for anatomical reference, and criteria that have been proven to provide highly reproducible outcomes (Desikan et al., 2006). This registration process ensured that the florbetaben PET/CT image had the same accurate segmentation and parcellation as in the MRI scan. Atrophy correction was not used because of the additional noise-error added to quantification of regional counts in the PET images.
The average activity was calculated in the ROIs corresponding to cerebellar gray matter and cerebral cortical regions. A composite SUVR was calculated by the ratio of the mean volume-weighted SUVR of 5 cortical regions (frontal, temporal, parietal, anterior and posterior cingulate cortex regions, each region summed from left and right hemispheres) to the cerebellar gray matter (Rowe et al., 2008).
2.8. APOE genotyping
Samples were genotyped for the APOE E2, E3 and E4 alleles using predesigned TaqMan SNP Genotyping Assays for SNPs rs7412 and rs429358 (Thermo Fisher Scientific, Massachusetts, USA) on the QuantStudio 7 Flex Real-Time PCR system (Applied Biosystems, California, USA) following the manufacturer's protocol.
2.9. Statistical analyses
Group differences in demographic measures among the diagnostic categories were compared using both t-tests anda series of one-way analyses of variance (ANOVA) and chi-square tests. Statistically significant results (p < .05) from the ANOVA analyses were followed by post-hoc analyses using Tukey's Honestly Significant Difference (HSD) test. Measures of effect size were calculated for means (eta-square) and proportions (Cramer's V). Receiving Operator Characteristic Curve (ROC) analyses were performed to determine the optimal cut-offs for florbetaben SUVR positivity that best discriminated between A+ and A- groups identified via amyloid scan visual reads. Strength of discrimination was assessed via the area under the ROC curve (AUC). Youden's criterion (sensitivity + specificity – 1) was used to identify the optimal threshold of amyloid positivity to best discriminate between groups (Youden, 1950). The 95% confidence interval for the Youden's index was generated by 1000 bootstrap samples using a bias-corrected and accelerated method. Correlation coefficient matrices were constructed to examine the association of cognitive tests scores with SUVR and hippocampal volumes. All statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.), except for ROC analyses, which were conducted via MedCalc (MedCalc Software, version 18.2.1, Ostend, Belgium). Statistical significance was inferred by a two-tailed p-value of <0.05.
3. Results
Amyloid PET, MRI scans, neuropsychological tests and APOE genotyping were obtained for all 159 subjects. Demographic, selected neuropsychological and APOE genotyping data for CN, MCI and DEM are provided in Table 1. As indicated, ANOVA tests did not reveal any statistically significant differences in age, gender or percentage of individuals who were Hispanic. However, the CN group had higher levels of educational attainment relative to the MCI group. There were monotonic decreases in both Mini Mental State Exam (MMSE) scores and hippocampal volume across CN, MCI and dementia participants and corresponding increases in SUVR and the percentage of subjects with A+ PET scans. Visual reads for amyloid positivity for all 159 participants was 39%, increasing from 11% for CN, to 39% for MCI and 70% for DEM (χ2 = 31.4; p < .0001; Cramer's V = 0.4443). The frequency of APOEε4+ carrier status was 23% in the CN group compared to 47% in the MCI group and to 41% in the DEM group (χ2 = 6.3; p = .01; Cramer's V = 0.2106).
Comparing Hispanic to non-Hispanic participants (Table 2), there were no differences in mean age, proportion of CN, MCI and DEM subjects, mean MMSE scores, frequencies of APOEε4+ carrier status, A+ visual reads, mean SUVRs, and hippocampal volumes (as a percentage of intracranial volumes). However, Hispanic participants reported fewer years of education than non-Hispanics, and had a higher proportion of females. As expected, most Hispanic participants were tested in Spanish, whereas all non-Hispanics were tested in English.
Table 2.
Demographics, Cognitive Scores, APOE, SUVR and Hippocampal Volumes for Hispanics and Non-Hispanics.
| Hispanics (n = 94) | Non-Hispanics (n = 65) | t-Test or χ2 | Eta-square or Cramer's V | |
|---|---|---|---|---|
| Age | 71.1 ± 7.4 | 73.0 ± 7.3 | 1.6 | 0.0157 |
| Education | 14.6 ± 3.3 | 16.4 ± 3.2 | 3.3⁎⁎ | 0.0660 |
| Female (%) | 63 (67%) | 30 (46%) | 6.9⁎⁎ | 0.2082 |
| Test Language | 26 (28%) | 65 (100%) | 106.2⁎⁎⁎ | 0.7188 |
| English (%)/Spanish (%) | 68 (72%) | 0 (0%) | ||
| Cognitive Dx | 27 / 46 / 27 | 20 / 29 / 16 | 0.3 | 0.0429 |
| CN(%)/MCI(%)/DEM (%) | 29%/49%/22% | 31%/45% /25% | ||
| MMSE Score | 26.3 ± 4.0 | 26.8 ± 3.6 | 0.9 | 0.0047 |
| APOEε4 + ve (%) | 30/83 (36%) | 23/53 (43%) | 0.7 | 0.0725 |
| SUVRa | 1.38 ± 0.27 | 1.42 ± 0.33 | 0.8 | 0.0039 |
| Amyloid Visual Read (% positive) | 35 (37%) | 25 (38%) | 0.02 | −0.0124 |
| Hippocampal Volumeb | 5.4 ± 1.0 | 5.1 ± 1.3 | 1.9 | 0.0234 |
⁎Significant group differences test: t-Tests for continuous variables and Chi-square test for categorical values; significance level is 0.05 by default (*p < .05; **p < .01; ***p < .001).
Mean Standard Uptake Value Ratio (SUVR) for frontal, parietal, temporal, cingulate, normalized to gray cerebellum.
Right + left hippocampal volumes/ Intra Cranial Volume (×10−3).
Optimal SUVR thresholds determined by Youden's criteria, for the entire sample was 1.42 (sensitivity = 94%; specificity = 92.5%) (Table 3). Among Hispanics and non-Hispanics the mean SUVRs and percentage of A+ by visual reads were not significantly different, and optimal thresholds for amyloid positivity were similar between the groups. Among younger participants (<70 years) the mean SUVR was 1.36 ± 0.28 and visual reads were A+ in 29%. Among older participants (≥70 years), the mean SUVR was 1.42 ± 0.31 (t = 1.35; p = .18) and visual reads were A+ in 43% (χ2 = 3.28; p = .07). The optimal thresholds for amyloid positivity among older (SUVR =1.42) and younger (SUVR = 1.37) participants were not different using the 95% confidence interval of Youden's index. Similarly, among participants with MMSE scores ≥27, the optimal threshold for amyloid positivity was 1.34, which was not different from that for participants with MMSE scores <27, who had an optimal threshold of 1.36.
Table 3.
SUVRs and Visual Reads by APOE Genotype, Ethnicity, Age and Cognitive Status.
| Mean SUVR (±SD) | Amyloid+ Visual Read (%) | ROC-AUC (95% CI) | Optimal Threshold for Amyloid+ | Sensitivity for Amyloid+ | Specificity for Amyloid+ | |
|---|---|---|---|---|---|---|
| Hispanic | 1.38 ± 0.27 | 37% | 0.974 (0.947–1.000) | 1.42 | 94.3 | 93.2% |
| Non-Hispanic | 1.42 ± 0.33 | 38% | 0.948 (0.873–1.000) | 1.38 | 96% | 90% |
| Age (<70 years) | 1.36 ± 0.28 | 29% | 0.985 (0.963–1.000) | 1.37 | 100% | 93.2% |
| Age (≥70 years) | 1.42 ± 0.31 | 43% | 0.957 (0.9109–1.000) | 1.42 | 92.9% | 90.9% |
| MMSE (≥27) | 1.29 ± 0.21 | 20% | 0.958 (0.917–1.000) | 1.34 | 95% | 98.3% |
| MMSE (<27) | 1.58 ± 0.34 | 67% | 0.961 (0.910–1.000) | 1.36 | 97.4% | 89.5% |
Amyloid + = Amyloid positivity; MMSE = Mini-Mental State Examination Scores; ROC-AUC = Receiving operator characteristic (curves)- area under the curve; SUVR = Standardized uptake value ratios.
Table 4 lists (1) Youden's optimal thresholds for the CN, MCI and DEM groups, with corresponding 95% CI and sensitivities and specificities; (2) AUC for the ROCs with corresponding standard errors and 95% CI for the AUCs. These data show high sensitivity and specificity of the three ROCs, although the CI for AUC in the MCI group was fairly wide and fell outside the limits of the other two groups. The mean, bootstrapped optimal thresholds and confidence intervals showed that: (1) mean thresholds for the CN and DEM groups did not differ; (2) mean thresholds for the CN group fell marginally below the confidence intervals for the MCI group; (3) the MCI mean threshold fell within the CN and DEM confidence intervals, suggesting that the three mean optimal thresholds were not reliably different.
Table 4.
Youden based SUVR threshold for different patient groups with various degree of cognitive impairment and APOEε4 carrier status.
| Group | Youden-based SUVR threshold | 95% BCa lower bound | 95% BCa upper bound | Sensi-tivity | Speci-ficity | AUC | SE | 95% Lower bound | 95% Upper bound |
|---|---|---|---|---|---|---|---|---|---|
| CN | 1.31 | 1.28 | 1.49 | 1.00 | 0.98 | 0.990 | 0.011 | 0.907 | 1.000 |
| MCI | 1.41 | 1.28 | 1.47 | 0.93 | 0.87 | 0.925 | 0.040 | 0.840 | 0.973 |
| DEM | 1.25 | 1.18 | 1.50 | 1.00 | 0.91 | 0.990 | 0.012 | 0.886 | 1.000 |
| APOEε4- | 1.31 | 1.20 | 1.41 | 0.94 | 0.91 | 0.959 | 0.023 | 0.891 | 0.990 |
| APOEε4+ | 1.52 | 1.49 | 1.52 | 0.88 | 1.00 | 0.988 | 0.010 | 0.911 | 1.000 |
APOEε4 = apolipoprotein Eε4 carrier status; CN = Cognitively normal; MCI = Mild cognitive impairment; DEM = dementia; BCa = bootstrapped (1000 iterations, random number seed 978), bias corrected and accelerated 95% confidence intervals; AUC = Area under the Receiver Operating Characteristic Curve; SE = standard error of AUC.
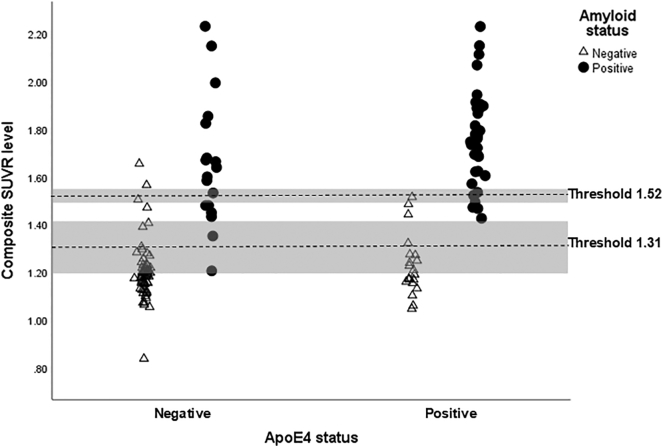
Using the same methodology, 1000 bootstrapped samples were generated for comparing APOEε4+ and ε4- groups in Table 4. Visual reads were A+ in 62% of APOE ε4+ and 24% of APOEε4- participants (χ2 = 22.7; p < .0001); APOEε4+ subjects also had a higher mean composite SUVR (1.56 ± 0.32 versus 1.30 ± 0.25; t = 5.37, p < .0001). The optimal threshold for the mean value of APOEε4- participants was outside the bootstrapped confidence limits for APOEε4+ participants, and the optimal threshold for APOEε4+ participants was outside the bootstrapped confidence limits for APOEε4- participants, thereby confirming a statistical difference in the optimal thresholds. Fig. 2 shows the distribution of composite SUVR scores by APOEε4 carrier status and binary visual read status, with the optimal thresholds and confidence intervals superimposed.
Fig. 2.
The figure show the distribution of composite SUVR scores by APOEε4 carrier status and binary visual read status. Optimal SUVR thresholds (shown as dashed lines) to discriminate amyloid positive and amyloid negative, as determined by visual reads, were computed using Youden's criterion for ApoE ε4 positive and negative subjects (Youden, 1950). The 95% confidence intervals for the Youden's index (shown as shaded regions) were generated by 1000 bootstrap samples using a bias-corrected and accelerated method. Optimal thresholds and confidence intervals are superimposed.
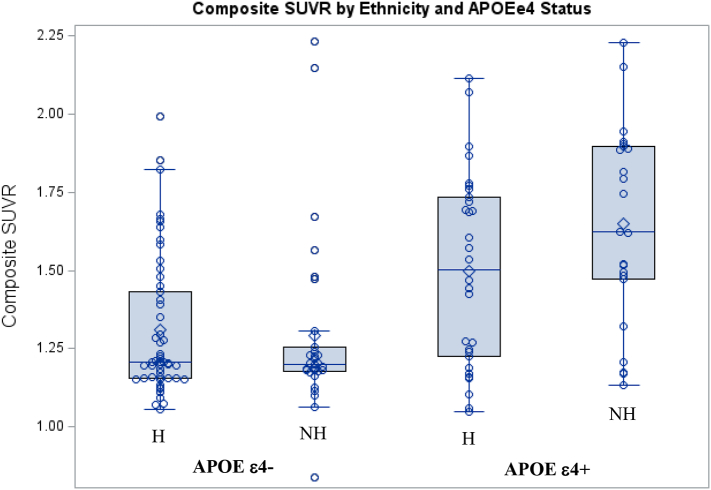
Using a multiple regression analysis, with mean SUVR as the dependent variable, and age, ethnicity, MMSE scores and APOE genotype as the independent variables (Table 5), MMSE score (p = .001) and APOE genotype (p = .001) predicted SUVR (i.e., lower MMSE scores and ApoE4+ status predicted higher SUVR), but there were no significant effects observed in relation to age or ethnicity. However, a significant interaction between ethnicity and APOE genotype was identified, such that SUVRs were higher among Non-Hispanics than Hispanics who were ApoE4+, but lower among non-Hispanics than Hispanics who were ApoE4- (Table 5; Fig. 1).
Table 5.
Linear regression using mean SUVR as dependent variable.
| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Corrected Model | 5 | 0.839 | 15.7 | 0.000 |
| Intercept | 1 | 3.67 | 68.8 | 0.000 |
| Age | 1 | 0.073 | 1.4 | 0.245 |
| MMSE | 1 | 1.35 | 25.4 | 0.000 |
| APOEε4 +/− | 1 | 1.68 | 31.5 | 0.000 |
| Ethnicity | 1 | 0.031 | 0.6 | 0.451 |
| APOE*Ethnicity | 1 | 0.359 | 6.7 | 0.011 |
APOE = apolipoprotein E; MMSE = Mini-Mental State Examination scores; Ethnicity (Hispanic versus Non-Hispanic).
Fig. 1.
Composite cortical SUVR shown for Hispanic (H) and Non-Hispanic (NH) participants who are APOE ε4- and APOE ε4+. The box and whisker plots are overlaid with data values (open circles). The boxes show the median (a line in the middle), the upper and lower interquartiles (Q3 and Q1), the upper fence (Q3 + 1.5 x Inter quartile range [IQR]) and the lower fence (Q1 – 1.5xIQR). There was a significant interaction between Hispanic ethnicity and APOEε4 carrier status (F1,132 = 4.79; p = .03).
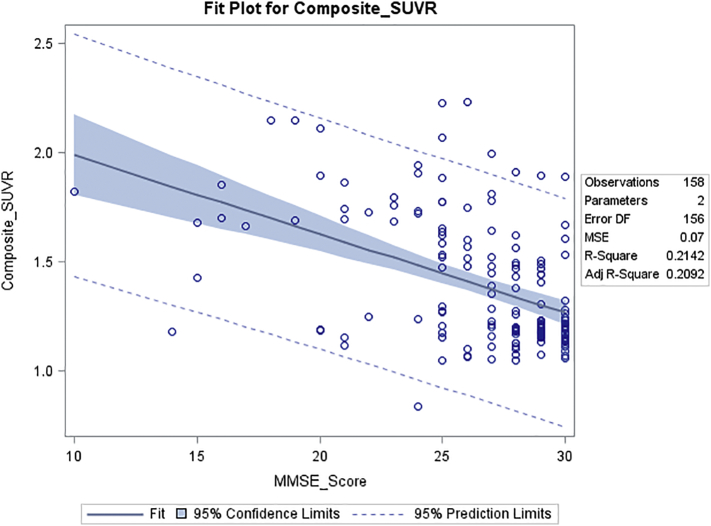
Composite SUVR correlated significantly with scores on the MMSE (r = −0.46; p < .0001) (Fig. 3), Category Fluency test (r = −0.36; p < .0001), HVLT-delayed (r = −0.38; p < .0001) and Trails B (r = 0.38; p < .0001).
Fig. 3.
Correlation of composite SUVR with MMSE Score. Composite SUVR was calculated as the ratio of the mean SUV of 5 cortical regions (frontal, temporal, parietal, anterior and posterior cingulate cortex regions, each region summed from left and right hemispheres) to the cerebellar gray matter SUV. The regression model fit and 95% confidence limits are plotted and model statistics are shown. The correlation of composite SUVR to MMSE was r = −0.46, p < .0001.
4. Discussion
The determination of amyloid positivity or negativity on Aß-PET scans by qualitative or quantitative methods has important implications for determining diagnosis and prognosis. In a review of several studies Pontecorvo and Mintun (2011) concluded that “the threshold for detection of amyloid on the Aß-PET scan appears close to the levels of neuropathology typical for a diagnosis of AD”. In the Alzheimer's Disease Neuroimaging (ADNI) Study, amyloid positive individuals were twice as likely as amyloid negative individuals to have worsening on CDR scores over a 4-year period (Donohue et al., 2017). It is therefore important for clinical purposes, as well as to determine eligibility for clinical trials, to be able to identify an accurate and consistent threshold level of amyloid load in the brain which can distinguish individuals who are A+ from those who are A-.
The threshold for amyloid positivity has major diagnostic and prognostic implications and has been studied extensively in the last several years. Villeneuve et al. (2015), described several different methods to obtain thresholds for amyloid positivity, each yielding different thresholds. Methodological as well as biological factors, may contribute to this variability in thresholds. As stated by Villeneuve et al. (2015), “amyloid-β deposition occurs on a continuum; at present there is no clear a priori way to separate individuals who have pathologically relevant amyloid-β deposition from those who do not. Nevertheless, there are important reasons to consider categorical classification of individual subjects. Classification of individuals as amyloid ‘positive’ or ‘negative’ is relevant for clinical diagnosis, for inclusion of subjects in anti-amyloid therapeutic trials, and for distinguishing amyloid-β-dependent and amyloid-β-independent changes in cognition and in brain structure and function”. Very recently Farrell et al. (Neurology 2018) showed, in longitudinal imaging studies, that the rate of change in amyloid load in the “subthreshold range” has greater biological significance, as evidenced by the impact on performance on memory tests, than any threshold based on cross-sectional data.
The gold standard which has been used to obtain FDA approval of amyloid PET ligands has been the visual read, referenced to pathological findings at autopsy. The study by Sabri et al. (2015) demonstrated that in four brain regions (middle frontal gyrus, occipital cortex, anterior cingulate gyrus and posterior cingulate/precuneus regions), which are required for the histopathological diagnosis of AD using CERAD criteria, there was a high degree of correspondence between the visual reads of amyloid positive PET scans using [18-F] Florbetaben as the ligand, and the presence of moderate to frequent neuritic plaques, as opposed to no or sparse neuritic plaques. The sensitivity of this correspondence was in the range of 81.8% to 90%, and the specificity was in the range of 85.7% to 95%. Bullich et al. (2017) used these findings to justify an “Optimized classification of 18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment”. We have also used the findings by Sabri et al. to provide the justification for using the visual read as a gold standard for amyloid positivity/negativity. There are certain limitations which apply to the study by Sabri et al., which would also be applicable to other comparisons of in vivo ß-amyloid PET imaging with post-mortem histopathology [22,23]. These limitations include the fact that end-stage individuals have medical illnesses, which could affect the pharmacokinetics and dynamics of the ligand uptake in the brain, as well as pronounced brain atrophy, which makes visual assessment of the PET scans challenging. These limitations negatively bias the association between the PET and histopathology data. However, these limitations, cited by Sabri et al., do not apply to the current study, which includes participants who were either cognitively normal, had MCI or mild dementia. Hence, we would argue that our approach, which uses binary visual reads (amyloid positive versus negative), comes closer to a gold standard for determining amyloid positivity, than was the case in the Sabri et al. and similar studies, with respect to the correspondence of the PET data to the frequency of amyloid plaques in the brain.
Using binary visual ratings of [18-F] florbetaben Aß-PET scans as the gold standard, we found an optimal quantitative threshold for amyloid positivity (SUVR = 1.42) (approximately 31 to 33 in centiloid units; Schwarz et al., 2018) for the entire cohort of 159 participants. This threshold, using cerebellar gray matter as the reference region, was similar to the threshold obtained for amyloid positivity (SUVR = 1.43) in a prior study (Bullich et al., 2017), which also used visual rating of PET scans as the gold standard. In comparison, the threshold for amyloid positivity for [18-F] Florbetaben obtained by Sabri et al. (2015) was 1.48, using post mortem histopathology as the gold standard and antemortem PET scans in non-demented and demented cases, many of who were severely cognitively impaired. This somewhat higher SUVR threshold for florbetaben may be related to the inclusion of individuals with severe dementia who were terminal at the time they underwent amyloid PET scans (Sabri et al., 2015).
Visual ratings of amyloid positivity, which was used in this study as the gold standard against which to determine the threshold of amyloid positivity, is a direct, accessible, and non-quantitative method of identifying the presence or absence of amyloid plaques in the brain. In the current study the visual reading of amyloid positivity required that at least one region in the brain had an area of gray matter uptake equal to or greater in intensity than the adjacent white matter uptake (Bullich et al., 2017). Among patients with dementia, visual readings of Aß-PET scans are generally reported to show pronounced uptake in many brain regions and are largely dichotomous in distribution, i.e., clearly positive or negative (Jack et al., 2013; Jack et al., 2010), whereas, among patients with MCI, Aß-PET scans are generally read as moderately positive (RCTU score of 2) in a limited number or only one region (Wolk and Klunk, 2009), consistent with the greater heterogeneity expected in the underlying neuropathology. There is an important distinction between visual reading of amyloid positivity and quantitative assessment, using SUVRs, of amyloid positivity. Amyloid positivity using visual reading is based on the distribution of amyloid binding in various cortical regions and not amyloid load in the brain,
In contrast, quantitative PET measures of amyloid load (SUVRs) are obtained by measurement and averaging of tracer counts across preselected regions of the brain, such as the frontal, superior temporal, parietal/precuneus cortices and the anterior and posterior cingulate regions.
A quantitative (SUVR) threshold for amyloid positivity, is calculated for a particular ligand, so as to optimally distinguish the presence of amyloid plaques (Thal phase 3 or greater) from the absence of amyloid plaques (Thal et al., 2015). A quantitative threshold is likely to be less sensitive than a visual rating method, which allows for determination of amyloid positivity by identifying only one unilateral brain region (Grothe et al., 2017) to be moderately positive (Mountz et al., 2015). While amyloid load is greater among participants who have amyloid positive visual reads, as compared to amyloid negative reads (Table 1), the visual read itself is based on qualitative criteria and is not directly comparable to the amyloid load in various brain regions.
Demographic, genetic, and methodological factors may account for differences in estimates of amyloid positivity in different studies (Chetelat et al., 2013; McDonough, 2017). In this study, we found the frequencies of amyloid positivity by visual rating to be numerically higher in older participants versus younger, in the cognitively more impaired versus less impaired, and in those who were APOEε4+ versus APOEε4- (Table 3 and Fig. 2). Our findings of positive amyloid scans among 11% of CN, 39% of MCI and 70% of DEM subjects are within the range of 0–47% for CN subjects, 37–72% for MCI, and 68–100% for AD reported by Chetelat et al. (2013). We did not find any differences between Hispanic and non-Hispanic participants, in the frequency of visually rated positive scans (Table 3). Across all demographic groups and diagnoses (CN, MCI or dementia) the amyloid load, independent of the visual reading of amyloid positivity or negativity, was found to be greater among APOE4+ than E4- participants, as is evidenced by the mean SUVRs for APOE4+ and E4- participants (Table 3).
Among our participants there was no significant difference in APOEε4 frequency between Hispanics and White non-Hispanics (Table 2). Contrary to our results, lower APOEε4 frequencies and lower risk for Alzheimer's disease associated with APOEε4 genotype among Hispanics, as compared to white non-Hispanics have been previously reported (Campos et al., 2013), which may possibly be explained by the fact that participants in the study by Campos et al. were Mexican Hispanics, whereas our Hispanic participants are primarily from such countries as Cuba, Colombia and Venezuela.
We did find an interaction between ethnicity and APOE genotype, such that APOEε4+ non-Hispanic subjects tended to have higher SUVRs than APOEε4+ Hispanics, whereas APOEε4- Hispanics tended to have higher SUVRs than non-Hispanics (Table 4 and Fig. 1). Genetic studies on Hispanics suggest that the effect of APOEε4 on AD risk may be less than that in Caucasians (Farrer et al., 1997; Tang et al., 1998; Ertekin-Taner, 2007). Our findings of a weaker influence of APOEε4 on Abeta load in Hispanics, combined with the reportedly greater prevalence and risk for AD among Hispanics, as compared to white non-Hispanics (Alzheimer's Association, 2018) appears to be consistent with these earlier genetic data, and may suggest that there is a greater risk for clinical dementia by non-APOEε4 genetic or non-genetic factors than that for non-Hispanic Caucasians.
Few studies have investigated thresholds for amyloid positivity in subgroups of participants classified by demographic or biological characteristics, due to limitations imposed by requiring validation of amyloid content in the brain by histopathology. We did not find differences in the optimal quantitative threshold for amyloid positivity as a function of age, sex, ethnicity (Hispanics versus non-Hispanic), or in relation to cognitive status of the group. However, the threshold for amyloid positivity was found to be substantially lower among APOEε4- (SUVR = 1.31) than APOEε4+ participants (SUVR = 1.52).
It is well known that APOEε4+ status, older age and cognitive impairment are associated with higher average SUVRs on Aß-PET and lower cerebrospinal fluid Aβ protein levels (Head et al., 2012; Li et al., 2017; Reiman et al., 1955). In a histopathological study of non-demented individuals, APOEε4 carriers had greater amyloid load than APOEε4 non-carriers, even though there was no difference between the two groups in AD pathology (neuritic plaques and neurofibrillary tangles) (Caselli et al., 2010). Among APOEε4+ carriers, the increased risk for developing AD, as well for developing AD at an earlier age, appears to be related to earlier and greater Aβ accumulation, in the brain (Liu et al., 2013). Cognitively normal APOEε4+ carriers have been found, on average, to become A+ at 56 years of age, whereas APOEε4 non-carriers become amyloid positive on average, at about 76 years of age (Fleisher et al., 2013). It is likely that our findings of a higher optimal threshold for amyloid positivity among APOEε4 carriers is related to the association of APOEε4 carrier status on amyloid load, independent of the effect of coexisting AD, greater age, more impaired cognitive status and ethnicity. In fact, the higher amyloid load among APOEε4 carriers, as compared to non-APOEε4 carriers appears to be superimposed on the effects of other factors associated with increased amyloid load, such as aging and cognitive impairment (Li et al., 2017; Rowe et al., 2007).
In the present investigation, quantitative measures (SUVRs) show a continuous increase of amyloid load in relation to cognitive test performance (Fig. 3), spanning the range from CN to mild to moderate dementia, with SUVRs ranging from clearly A- to the higher levels seen in AD. In longitudinal studies using amyloid imaging, the increase in amyloid load in CN elderly has been about 1% per year (Villain et al., 2012), with about 3% of CN subjects progressing from A- to A+ per year, with a higher progression rate (7%) in APOEε4 carriers (Vlassenko et al., 2011). A relationship between cognitive performance and amyloid load across the spectrum of CN and cognitively impaired individuals has been reported previously, and is detectable well before the threshold of amyloid positivity is reached (Pike et al., 2007; Chetelat et al., 2010). In their study, Chetelat et al. (2010) demonstrated a strong relationship between measures of cortical and hippocampal atrophy and β-amyloid deposition very early in the disease process (i.e., even among those with subjective memory complaints). A strong relationship between impaired episodic memory performance and amyloid tracer retention has been reported recently in elderly normal subjects, using highly sensitive memory tests (Loewenstein et al., 2016; Loewenstein et al., 2015). Other AD biomarkers (such as regional brain atrophy and regional cerebral glucose metabolism) were also found to correlate with gradually increasing amyloid load in cognitively healthy elderly individuals, over a decade or more prior to reaching the threshold for amyloid positivity (Insel et al., 2016). These findings suggest that any categorical cut point to discriminate between A+ and A- cases must be arbitrary, and dependent on the population being studied.
While the calculation of the optimal threshold for amyloid positivity is independent from the amyloid load itself, the difference in thresholds reflects the fact that APOE4+ individuals are more likely to have greatly elevated levels. In fact, across all demographic groups and diagnoses (CN, MCI or dementia) the amyloid load, independent of the visual reading of amyloid positivity or negativity, was found to be greater among APOE4+ than E4- participants, as is evidenced by the mean SUVRs for APOE4+ and E4- participants (Table 3). Our findings demonstrate that the quantitative threshold (SUVR) level for amyloid positivity is influenced by APOEε4 carrier status, which could provide evidence for using different thresholds based on an individual's APOEε4 carrier status. This point is clearly illustrated in Fig. 2, which shows the distribution of cases which are E4 + and E4 according to whether they were found to be amyloid positive or negative by visual reads. The lack of overlap in the 95% confidence intervals between the calculated thresholds for E4+ and E4- cases is also clearly demonstrated in the figure. However, given that there is a continuous relationship, from a CN state to dementia, between SUVR, cognitive measures and, presumably, biomarkers of neurodegeneration, any calculated threshold for the whole group, or a subgroup, is likely to be artefactual. The results of studies by Chetelat et al. (2010), Villeneuve et al. (2015) and Insel et al. (2016) strongly suggest the biological impact of amyloid load are present well before the quantitative threshold of amyloid positivity is reached. In this study the optimal SUVR threshold for amyloid positivity for all 159 participants in this study (florbetaben SUVR = 1.42) expressed in centiloid units, is 30.25. The conversion for the optimal threshold for APOE4- participants (florbetaben SUVR = 1.31) is 18 centiloid units. As such, it would seem appropriate to use the lowest level of amyloid load that corresponds to a positive visual read, as the threshold for amyloid positivity, regardless of the E4 status. Which happens to be very close to the threshold of 19.0 in centiloid units for amyloid positivity/negativity, as suggested by Jack Jr. et al. (2017).
The strengths of this study include the diversity of subjects- and inclusion of age, ethnicity, severity of cognitive impairment and APOEε4 carrier status in the analysis. In addition, the use of visual assessment as the gold standard for amyloid positivity is a strength, which avoids the limitations of being dependent on autopsy studies to serve as the gold standard for amyloid positivity. These added strengths allow for larger numbers of patients, who are younger, less impaired and more diverse to also be studied. One limitation in this study is the relatively modest number of subjects included in the study thus far, considering the number of interactions and variables included in the analyses. Longitudinal analyses of this cohort will be important for elucidating the relationship between different amyloid thresholds and the impact on rate of progression to more impaired cognitive states.
In conclusion, we found that although mean SUVRs were influenced by age, cognitive status and APOEε4 carrier status, only APOEε4 carrier status had an impact on the optimal threshold for amyloid positivity. Nevertheless, we provide a rationale for using the lowest optimal threshold that can be identified for a particular ligand, for classification of A+ or A-, given that brain amyloid accumulation appears to be a continuous process.
Acknowledgments
Acknowledgements
This research was supported by the National Institute of Aging Grant number 5 P50 AG0477266021 Florida Alzheimer's Disease Research Center (Todd Golde, PI); National Science Foundation grant CNS-1532061 (Malek Adjouadi, PI).
Dr. Shea's overseas training was sponsored by the 2017/2018 Hospital Authority Corporate Scholarship Program, Hong Kong.
Conflict of interest/disclosure statement
Dr. DeKosky reports serving on scientific advisory boards for Amgen, Biogen, and Cognition Therapeutics.
Dr. Duara has received consulting fees from Medical Learning Group for the Speaker's Bureau for Eli Lilly and Company.
Dr. De Santi is an employee of Life Molecular Imaging Inc.
References
- Acevedo A., Krueger K.R., Navarro E., Ortiz F., Manly J.J., Padilla-Vélez M.M., Weintraub S., López O.L., Mungas D. The Spanish translation and adaptation of the uniform data set of the National Institute on Aging Alzheimer's Disease Centers. Alzheimer Dis. Assoc. Disord. 2009;23(2):102. doi: 10.1097/WAD.0b013e318193e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein H.J., Nebes R.D., Saxton J.A. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association 2018 Alzheimer's disease facts and figures. Alzheimers Dement. 2018;14(3):367–429. [Google Scholar]
- Arango-Lasprilla J.C., Rivera D., Garza M.T., Saracho C.P., Rodriguez W., Rodríguez-Agudelo Y., Aguayo A., Schebela S., Luna M., Longoni M., Martínez C. Hopkins verbal learning test–revised: normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. 2015;37(4):699–718. doi: 10.3233/NRE-151286. [DOI] [PubMed] [Google Scholar]
- Beekly D.L., Ramos E.M., van Belle G. The National Alzheimer's Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis. Assoc. Disord. 2004;18(4):270–277. [PubMed] [Google Scholar]
- Beekly D.L., Ramos E.M., Lee W.W. NIA Alzheimer's Disease Centers. (2007). The national Alzheimer's coordinating center (NACC) database: the uniform data set. Alzheimer Dis. Assoc. Disord. 2007;21(3):249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- Benedict R.H.B., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test – revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- Bullich S., Seibyl J., Catafau A.M. Optimized classification of 18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. NeuroImage. 2017;15:325–332. doi: 10.1016/j.nicl.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M., Edland S.D., Peavy G.M. Exploratory study of apolipoprotein E ε4 genotype and risk of Alzheimer's disease in Mexican Hispanics. J. Am. Geriatr. Soc. 2013;61(6):1038–1040. doi: 10.1111/jgs.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli R.J., Walker D., Sue L., Sabbagh M., Beach T. Neurosci. Lett. 2010;473(3):168–171. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G., Villemagne V.L., Bourgeat P. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann. Neurol. 2010;67(3):317–324. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- Chetelat G., La Joie R., Villain N., Perrotin A., de La Sayette V., Eustache F., Vandenberghe R. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer's disease. Neuroimage Clin. 2013;2:356–365. doi: 10.1016/j.nicl.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Donohue M.C., Sperling R.A., Petersen R., Sun C.K., Weiner M.W., Aisen P.S., Alzheimer's Disease Neuroimaging Initiative Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA. 2017;317(22):2305–2316. doi: 10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertekin-Taner N. Genetics of Alzheimer's disease: a centennial review. Neurol. Clin. 2007;25(3):611–667. doi: 10.1016/j.ncl.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.A., Bennett D.A., Wilson R.S. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch. Neurol. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- Farrer L., Cupples L.A., Haines J.L. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Fleisher A.S., Chen K., Liu X. Apolipoprotein E epsilon4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol. Aging. 2013;34(1):1–12. doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., McHugh, Folstein S.E., McHugh P.R. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatric Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Graff-Radford N.R., Green R.C., Go R.C. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch. Neurol. 2002;59(4):594–600. doi: 10.1001/archneur.59.4.594. [DOI] [PubMed] [Google Scholar]
- Grothe M.J., Barthel H., Sepulcre J., Dyrba M., Sabri O., Teipel S.J. Alzheimer's Disease Neuroimaging Initiative. In vivo staging of regional amyloid deposition. Neurology. 2017;89(20):2031–2038. doi: 10.1212/WNL.0000000000004643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D., Bugg J.M., Goate A.M. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch. Neurol. 2012;69(5):636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic M.D., Klunk W.E., Abrahamson E.E. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131(Pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P.S., Mattsson N., Mackin R.S. Accelerating rates of cognitive decline and imaging markers associated with β-amyloid pathology. Neurology. 2016;86(20):1887–1896. doi: 10.1212/WNL.0000000000002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Jr., Wisteb H.J., Weigand S.D., Therneau T.M., Lowe V.J., Knopman D.S., Gunter J.L., Senjem M.L., Jones D.T., Kantarcia K., Machuld M.M., Mielkeb M.M., Robert R.O., Vemuria P., Reyes D.A., Petersen R.C. Defining imaging biomarker cut-points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205–216. doi: 10.1016/j.jalz.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Wiste H.J., Vemuri P. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133(11):3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R., Knopman D.S., Jagust W.J. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.A., Minoshima S., Bohnen N.I. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement. 2013;9(1) doi: 10.1016/j.jalz.2013.01.002. (e-1-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Loewenstein D.A., Duara R., Cabrerizo M., Barker W., Adjouadi M., Alzheimer's Disease Neuroimaging Initiative The relationship of brain amyloid load and APOE status to regional cortical thinning and cognition in the ADNI cohort. J. Alzheimers Dis. 2017;59(4):1269–1282. doi: 10.3233/JAD-170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga G., Li C., Cabrerizo M., Barker W., Loewenstein D.A., Duara R., Adjouadi M. A neuroimaging web services Interface as a cyber physical system for medical imaging and data Management in Brain Research: design study. JMIR Med. Inform. 2018;6(2):e26. doi: 10.2196/medinform.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein D.A., Greig M.T., Curiel R. Proactive semantic interference is associated with total and regional abnormal amyloid load in non-demented community-dwelling elders: a preliminary study. Am. J. Geriatric Psychiatry. 2015;23(12):1276–1279. doi: 10.1016/j.jagp.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein D.A., Curiel R.E., Greig M.T. A novel cognitive stress test for the detection of preclinical Alzheimer's disease: discriminative properties and relation to amyloid load. Am. J. Geriatric Psychiatry. 2016;24(10):804–813. doi: 10.1016/j.jagp.2016.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J.A., Ivnik R.J., Smith G.E., Bohac D.L., Tangalos E.G., Graff-Radford N.R., Petersen R.C. Mayo's older Americans normative studies: category fluency norms. J. Clin. Exp. Neuropsychol. 1998;20(2):194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- McDonough I.M. Beta-amyloid and cortical thickness reveal racial disparities in preclinical Alzheimer's disease. NeuroImage. 2017;16:659–667. doi: 10.1016/j.nicl.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mountz J.M., Laymon C.M., Cohen A.D. Comparison of qualitative and quantitative imaging characteristics of [11C]PiB and [18F]flutemetamol in normal control and Alzheimer's subjects. Neuroimage Clin. 2015;9:592–598. doi: 10.1016/j.nicl.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.R., Landau S.M., Choudhury K.R. Mapping the effects of ApoE4, age and cognitive status on 18F-florbetapir PET measured regional cortical patterns of beta-amyloid density and growth. Neuroimage. 2013;78:474–480. doi: 10.1016/j.neuroimage.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell J.R., Price B., Lane K.A. Association of apolipoprotein E genotype and Alzheimer disease in African Americans. Arch. Neurol. 2006;63(3):431–434. doi: 10.1001/archneur.63.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . The National Academies Press; Washington, DC: 2004. Eliminating Health Disparities: Measurement and Data Needs. [PubMed] [Google Scholar]
- Peña-Casanova J., Quiñones-Ubeda S., Quintana-Aparicio M. Neuronorma study team. Spanish multicenter normative studies (neuronorma project): norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch. Clin. Neuropsychol. 2009;24(4):321–341. doi: 10.1093/arclin/acp038. [DOI] [PubMed] [Google Scholar]
- Perani D., Schillaci O., Padovani A. A survey of FDG- and amyloid-PET imaging in dementia and GRADE analysis. Biomed. Res. Int. 2014 doi: 10.1155/2014/785039. (Article ID 785039, 22 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike K.E., Savage G., Villemagne V.L. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. Pt 11. [DOI] [PubMed] [Google Scholar]
- Pontecorvo M.J., Mintun M.A. PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer's disease. Alzheimers Res. Ther. 2011;3(2):11. doi: 10.1186/alzrt70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman E.M., Chen K., Liu X. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 1955;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R.M. The relation of the trail making test to organic brain damage. J. Consult. Psychol. 1955;19(5):393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- Reitz C., Mayeux R. Genetics of Alzheimer's disease in Caribbean Hispanic and African American populations. Biol. Psychiatry. 2014;75(7):534–541. doi: 10.1016/j.biopsych.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe C.C., Ng S., Ackermann U. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- Rowe C.C., Ackerman U., Browne W. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7(2):129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- Rowe C.C., Ellis K.A., Rimajova M. Amyloid imaging results from the Australian imaging, biomarkers and lifestyle (AIBL) study of aging. Neurobiol. Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Sabri O., Seibyl J., Rowe C., Barthel H. Beta-amyloid imaging with florbetaben. Clin. Transl. Imaging. 2015;3(1):13–26. doi: 10.1007/s40336-015-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Chiao P., Klein G. The influence of biological and technical factors on quantitative analysis of amyloid PET: points to consider and recommendations for controlling variability in longitudinal data. Alzheimers Dement. 2015;11(9):1050–1068. doi: 10.1016/j.jalz.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Schwarz C.G., Tosakulwong N., Senjem M.L., Gunter J.L., Therneau T.M., Vemuri P., Lowe V.J., Jack C.R., Jr. Considerations for performing level-2 centiloid transformations for amyloid PET SUVR values. Sci. Rep. 2018;8:7421. doi: 10.1038/s41598-018-25459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibyl J., Catafau A.M., Barthel H. Impact of training method on the robustness of the visual assessment of 18F-florbetaben PET scans: results from a phase-3 study. J. Nucl. Med. 2016;57(6):900–906. doi: 10.2967/jnumed.115.161927. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tang M.X., Stern Y., Marder K. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- Thal D.R., Beach T.G., Zanette M. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer's disease: specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement. 2015;11(8):975–985. doi: 10.1016/j.jalz.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Tycko B., Lee J.H., Ciappa A. APOE and APOC1 promoter polymorphisms and the risk of Alzheimer disease in African American and Caribbean Hispanic individuals. Arch. Neurol. 2004;61(9):1434–1439. doi: 10.1001/archneur.61.9.1434. [DOI] [PubMed] [Google Scholar]
- Villain N., Chételat G., Grassiot B. Regional dynamics of amyloid-beta deposition in healthy elderly, mild cognitive impairment and Alzheimer's disease: a voxelwise PiB-PET longitudinal study. Brain. 2012;135:2126–2139. doi: 10.1093/brain/aws125. Pt 7. [DOI] [PubMed] [Google Scholar]
- Villemagne V.L., Ong K., Mulligan R.S. Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J. Nucl. Med. 2011;52(8):1210–1217. doi: 10.2967/jnumed.111.089730. [DOI] [PubMed] [Google Scholar]
- Villeneuve S., Rabinovici G.D., Cohn-Sheehy B.I. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. Pt 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko A.G., Mintun M.A., Xiong C. Amyloid-Beta plaque growth in cognitively normal adults: longitudinal PIB data. Ann. Neurol. 2011;70(5):857–861. doi: 10.1002/ana.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. PsychCorp; United States: 2008. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) [Google Scholar]
- Wolk D.A., Klunk W. Update on amyloid imaging: from healthy aging to Alzheimer's disease. Curr. Neurol. Neurosci. Rep. 2009;9(5):345–352. doi: 10.1007/s11910-009-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]