Abstract
Background
Studies on the applications of bone transport using the Ilizarov method for osteosarcoma (OS) patients with surgical resection and neoadjuvant chemotherapy are rare.
Methods
A retrospective analysis was conducted in 10 patients with limb OS receiving limb-salvage treatment by Ilizarov method from 2007 to 2012 in our hospital. The general information, treatment outcomes and follow-up data of the patients were collected.
Results
The mean length of the transported fragment and the mean transport distance of the affected limb were both 14 cm. The mean time in the external fixator was 34.2 ± 11.2 months (16–47 months) and the mean external fixation index (EFI) was 75 days/cm. The mean follow-up time was 68.6 ± 26.6 months (37–103 months). Seven patients underwent additional operations to treat the postoperative complications, and the mean number of operation was 1.7 times. Only one patient underwent amputation due to tumor relapse and all patients survived without tumor. The limb-salvage rate was 90%. At the time of external fixator removal, the ASAMI-bone score was good in 66.7% of patients and the ASAMI-function score was fair in 66.7% of cases. The mean MSTS score was 18.6 ± 3.2 (n = 9). At 10 months after fixator removal, both the ASAMI-bone score and ASAMI-function score were both excellent in 80% and good in 20% cases, and the mean MSTS score was further improved to 27.2 ± 1.11 (n = 5).
Conclusion
Bone transport using the Ilizarov method can achieve good therapeutic effectiveness in the limb-salvage treatment for OS patients with neoadjuvant chemotherapy as long as the complications can be timely recognized and well managed.
Keywords: Osteosarcoma (OS), Ilizarov method, Limb salvage, Limb reconstruction, Neoadjuvant chemotherapy
1. Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumor, characterized by the production of osteoid or immature bone by the malignant cells [1]. The 5-year survival rate of patients with local OS has been improved to 70–80% [2], [3]. Neoadjuvant chemotherapy combined with surgical resection is now the standard treatment for OS. The limb-salvage treatment can dramatically improve the quality of life and has gradually become one of the important treatment for OS [4]. With recent advances in neoadjuvant chemotherapy, the current limb-salvage rate of OS can reach up to 90% [5]. Limb-salvage treatments for OS include tumor prosthesis, vascularized fibula graft, and allograft, but there are still various complications, such as prosthesis-related infections, long-term loosening and disunion of transplanted bone [6], [7], [8], [9].
Ilizarov method is an external fixation technique used in orthopedic surgery and can achieve skeletal reconstruction by lengthening patient's own limbs [10]. Ilizarov method has been widely employed in a variety of treatments for limb deformity, bone defect and limb shortening [4]. In 1998, Ozaki et al. adopted Ilizarov method for bone transport in limb-salvage treatment following bone tumor resection for Ewing's sarcoma and OS [11]. However, the incidence of complications (such as pin tract infection, unconnected bone, refracture, bone malformation and transform death) was high and bone formation was poor and slow. Multiple operations were conducted after initial surgery and the survival time of most patients was relatively short [11]. The complications considerably reduce the limb-salvage effect of Ilizarov method for bone transport, which may be attributed to the technical limitations and inadequate doses of chemotherapy.
The neoadjuvant chemotherapy has markedly promoted the survival rate and limb-salvage rate in OS patients [5], [12], and is able to make the tumor shrink, edema disappears, and ossification of the tumor surface, providing a better local environment for surgery and following treatment. Therefore it may have the potential to improve the therapeutic effect of Ilizarov method for OS patients. However, current studies on the applications of Ilizarov method for OS patients following surgical resection and neoadjuvant chemotherapy are extremely rare [13], [14], [15]. Our hospital started to adopt the bone transport using the Ilizarov method in the limb-salvage treatment of bone defect in OS patients following tumor resection and neoadjuvant chemotherapy in 2007. This retrospective study aimed to evaluate the safety and effectiveness of bone transport using the Ilizarov method in limb-salvage treatment for OS following tumor resection and neoadjuvant chemotherapy.
2. Material and methods
2.1. Patients
A total of 10 consecutive OS patients receiving tumor resection and subsequent skeletal reconstruction and bone transport by the Ilizarov method in our hospital from December 2007 to December 2012 were included in this study. The criteria for Ilizarov method for bone transport were: (1) patient was pathologically diagnosed with II-B stage of OS without distal metastasis, which was estimated to have a longer survival time; (2) the tumor was located in the middle of the long bone. After extensive resection of the tumor, there was room for the fastening nails; (3) patients with a normal skin condition; (4) patients and the family relatives were willing to undergo surgery and the following treatment with Ilizarov external fixator for a long time. This study was approved by the institutional review board of our hospital and performed in accordance with the guideline of the Declaration of Helsinki. Written informed consent was obtained from each patient.
Patients’ demographic and clinical characteristics were summarized in Table 1. The median age at diagnosis for the 10 patients was 14 years (ranging from 11 to 55 years). The pathologic types of OS included 6 conventional OS, 2 chondroblastic, 1 teleangiectatic and 1 well-differential OS. As for the locations of OS, there were 7 cases in femur and 3 in tibia. Among the 9 patients undergoing chemotherapy, 7 patients achieved excellent response (≥90% tumor necrosis) while one case had a good response (70%–89%) and poor response (<70% tumor necrosis), respectively. Six patients had grade IV bone marrow suppression during pre- or post-chemotherapy.
Table 1.
Demographic and clinical characteristics.
| Case no. | Sex/Age | Subtypes | Tumor location | Biopsy | Treatment delaya (months) | First symptomb | Chemotherapy | Chemotherapy cyclesc | Alkaline phosphatase (U/L) before and after the treatment | Chemotherapy Response (Tumour necrosis rate) | Bone marrow suppressiond |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male/17 | Conventional | femur | Yes | 0.5 | P | I/D/A | 3 + 3 | 216/139 | >90% | M |
| 2 | Female/26 | Conventional | femur | Yes | 2 | P | I/M/A | 4 + 5 | 196/100 | >90% | S |
| 3 | Male/13 | Conventional | femur | Yes | 2 | P | I/M/A | 3 + 6 | 189/127 | >90% | M |
| 4 | Male/11 | Conventional | femur | Yes | 0.2 | P + S | I/A | 3 + 4 | 326/155 | <70% | S |
| 5 | Female/12 | Chondroblastic | tibia | Yes | 1 | P | I/M/A | 2 + 2 | 206/150 | 70%−90% | M |
| 6 | Male/15 | Conventional | tibia | Yes | 0.5 | P + S | I/M/A | 3 + 6 | 236/106 | >90% | S |
| 7 | Male/17 | Conventional | femur | Yes | 1 | P | I/M/A | 3 + 5 | 266/140 | >90% | S |
| 8 | Female/12 | Conventional | femur | Yes | 1.5 | P | I/M/A | 3 + 6 | 258/100 | >90% | S |
| 9 | Male/13 | Chondroblastic | femur | Yes | 1 | P | I/M/A | 3 + 6 | 199/151 | >90% | S |
| 10 | Female/51 | Well-differentiated | tibia | No | 30 | S | / | 0 | / | / | / |
Treatment delay due to waiting for the pathological results or a hospital bed.
First symptom: S-swelling; P-pain.
Session number of preoperative chemotherapy + session number of postoperative chemotherapy.
Chemotherapy: I, ifosfamide; M, methotrexate; D; Cisplatin; A, Adriamycin. ALK-Alkaline phosphatase: can be recorded as difference value before and after the treatment.
Bone marrow suppression was assessed as grade 0-IV according to WHO; grade IV is serious (S) and grade 0-III is M (mild).
2.2. Data collect and diagnosis
Medical records were retrospectively reviewed, and the demographics, histopathological data, imaging findings, treatment modalities and clinical outcomes were collected. All patients underwent either core needle or open biopsy for initial diagnosis, and subsequent surgical specimens were submitted for confirmation of the diagnosis. The subtypes of OS were classified into conventional, chondroblastic, fibroblastic, teleangiectatic, small cell and well-differential OS according to the criteria of the World Health Organization (WHO) classification. Tumor stage was assessed according to the Enneking classification. Imaging studies including computed tomography scan (CT), magnetic resonance imaging (MRI), bone radionuclide bone scan and positron emission computed tomography (PET) were used to define the location and extension of the primary tumor, and the presence of metastasis. Since 2009, CT scan of the chest was routinely included to evaluate lung metastases in our hospital.
2.3. Chemotherapy
All patients received pre-operative chemotherapy immediately after histologically confirmed diagnosis of OS. The dose and chemotherapy regimen were adjusted according to the response to the chemotherapy regimen and degree of myelosuppression. Response to preoperative chemotherapy was histopathologically assessed by the surgical specimen obtained after 3–4 cycles of preoperative chemotherapy. The chemotherapy response was accessed according to the rate of tumor necrosis after chemotherapy, which was graded as “excellent” (≥90% tumor necrosis), “good” (70%−89% tumor necrosis) or “poor” (<70% tumor necrosis). The postoperative chemotherapy was started at 2 weeks post-operation. Patients with good response to chemotherapy would receive additional 3–6 cycles of post-operative chemotherapy with the same protocol. For those with poor response, a modified post-operative chemotherapy (methotrexate was changed to cisplatin in patients younger than 30 years) with an increased dosage of chemotherapy regimen would be conducted for 6 cycles. The details of dosages and the protocols of chemotherapy were shown in Table 1. The preoperative chemotherapy usually took 12–15 weeks to complete, and the postoperative chemotherapy started at 2 weeks post-operation and completed at 21–24 weeks post-operation.
2.4. Surgical procedure
Surgery was performed after 3–4 weeks of preoperative chemotherapy. After the treatment courses of preoperative chemotherapy, MRI examination was conducted. The procedure of skeletal reconstruction and the following bone transport were as follows: (using single arm outrigger [Case 4] as an example, and the procedures of annular outrigger operation were the same):
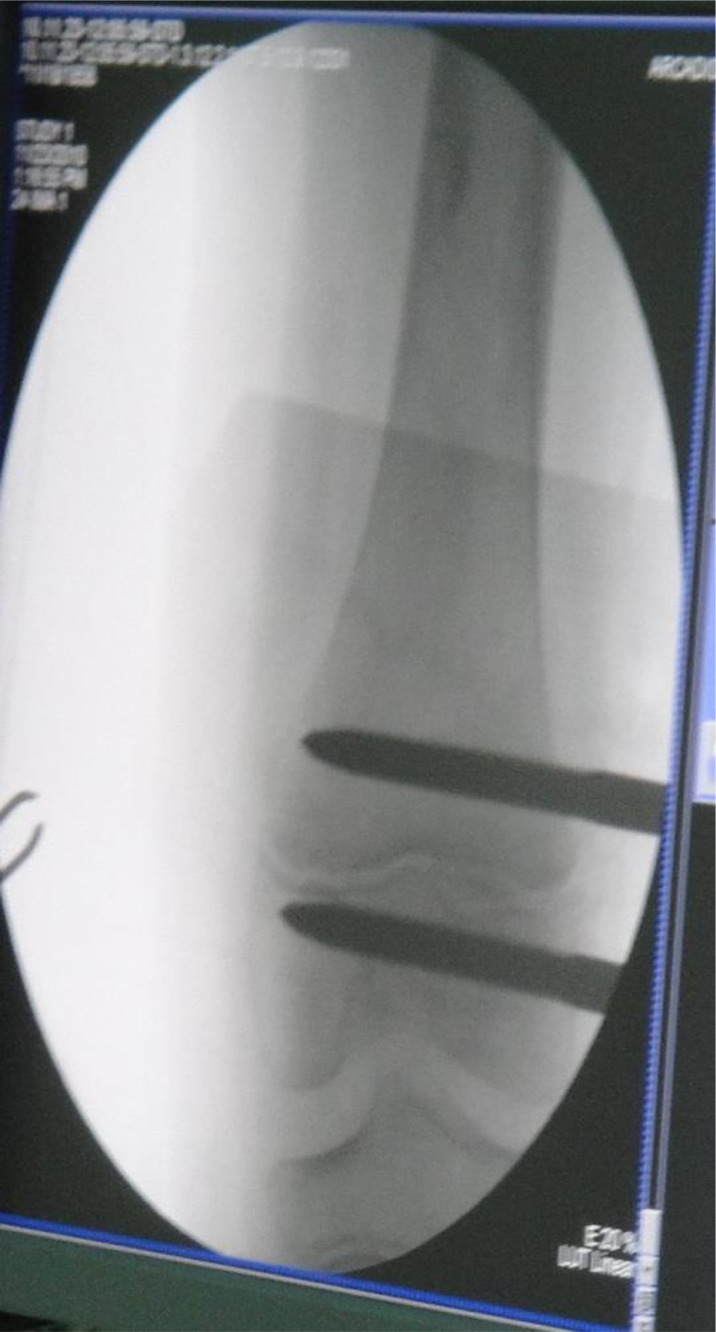
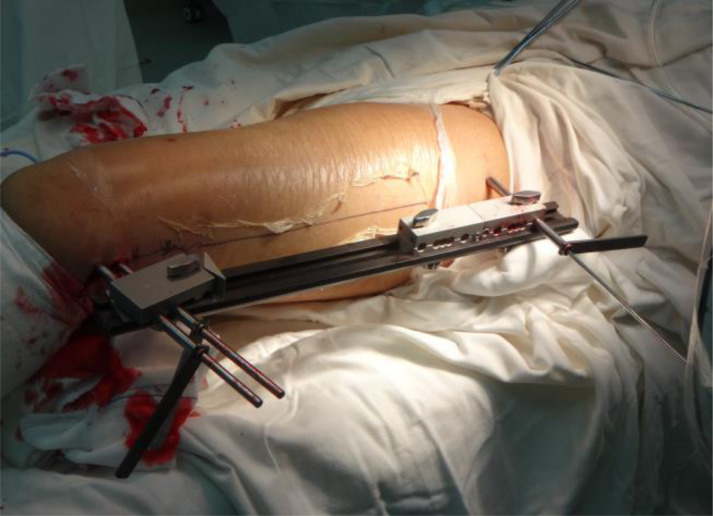
(1) Two or three locking pins were placed between the tuberosity or under the tuberosity in the proximal end, as well as at the metaphysis in the distal end of a long bone with tumor under the guidance of C-arm. The locking pins should not across the epiphyseal line and articular surface (Fig. 1). All locking pins were placed along the long axis of thighbone to ensure in the same plane both in the proximal end and the distal end (Fig. 2).
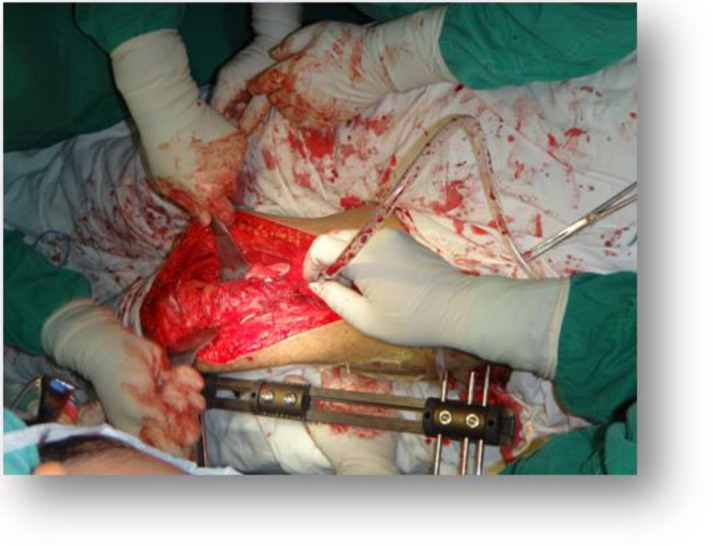
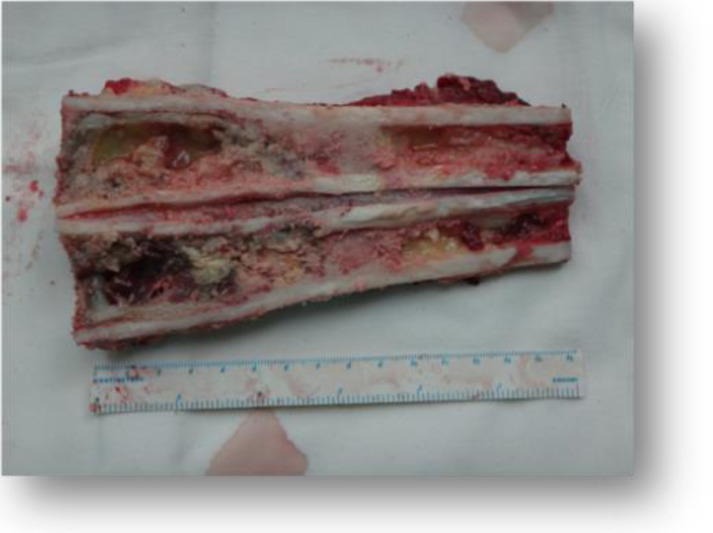
(2) The surgical tumor osteotomy plane was initially identified based on the invasive region of the intramedullary OS identified in MRI images. The security boundary of surgical resection was defined at 2–3 cm of tumor border in the T1 weighted image, and more than 3 cm of soft tissue border. The tumor was excised until at least 1 cm beyond the edema zone of soft tissue in the MRI T2 weighted image. All the femur cases had a resection of the vastus intermedius muscle together with the tumor (Figs. 3 and 4).
(3) The transported bone should be long enough so that the tumor osteotomy plane was at least 3 cm from the nearest locking pin of the external fixator. Two or three locking pins were placed in the transported bone and supporting structure, then the resection was performed (Fig. 5).
4) The resection should be carried out under the external periosteum. It was necessary to keep the complete discontinuity of the bone cortex and the continuity of external periosteum. Then, all outriggers were immobilized and the surgical incision was closed after placing the drainage.
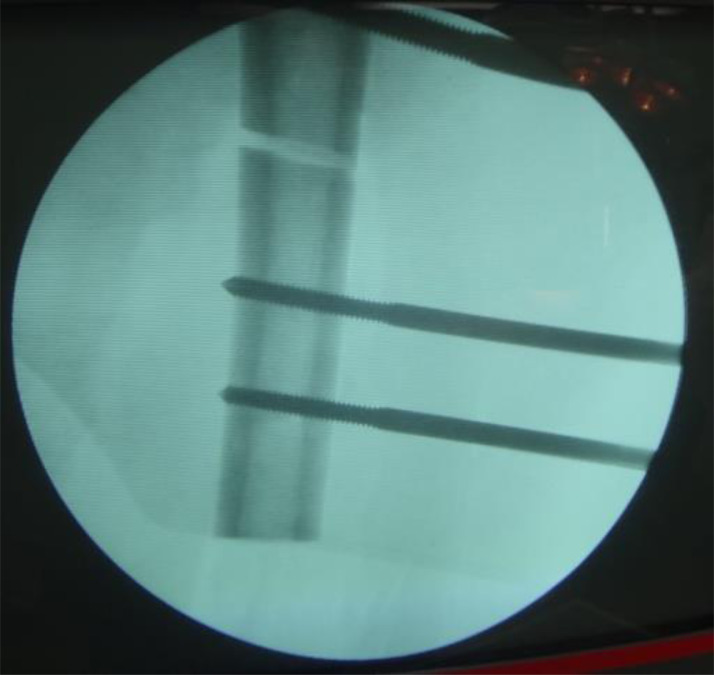
Fig. 1.
Intraoperative C-arm fluoroscope showed the location of the locking pins before tumor resection. The locking pins should not across the epiphyseal line and articular surface.
Fig. 2.
Under the guidance of C-arm, two or three locking pins were placed in the in the distal end (at the metaphysis) and a proximal end (between the tuberosity or under the tuberosity) of the long bone with a tumor, respectively. All locking pins were placed along the long axis of thighbone to ensure in the same plane both in the proximal end and the distal end.
Fig. 3.
Completion the en bloc resection in the surgery.
Fig. 4.
The range of resection should be at least 2–3 cm beyond the edge of the tumor to minimize the possibility of relapse.
Fig. 5.
The osteotomy design of bone graft and the transported bone should be as long as possible with the tumor osteotomy plane at least 3 cm from the nearest locking pin of the external fixator. Two or three locking pins were placed in the transported bone and supporting structure, then the resection was performed. The integrity of the periosteum was preserved but the cortical bone was completely cut off.
2.5. The adjustment and removal of Ilizarov external fixator
The bone graft was conducted along the bone defect zone at 7 days post-operation by adjusting the screws of the external fixator with a speed of 0.25 mm/12 h. The anteroposterior and lateral radiograph was taken once a month to evaluate the situations of alignment and counterpoint. The extension cord of transported bone should be consistent with the three-dimensional of extension cord of heterolateral broken bone in the distal end. Otherwise, the second or even multiple operations should be performed to adjust the position of transported bone under the guidance of C-arm fluoroscopy.
For all patients, the fastening nails were gradually (1–2 nails each time) dismantled at 3–6 months after complete contact between the transported end and fixed bone end. At dismantling the last two fastening nails, the external fixator was removed together.
2.6. Follow-up and functional assessment
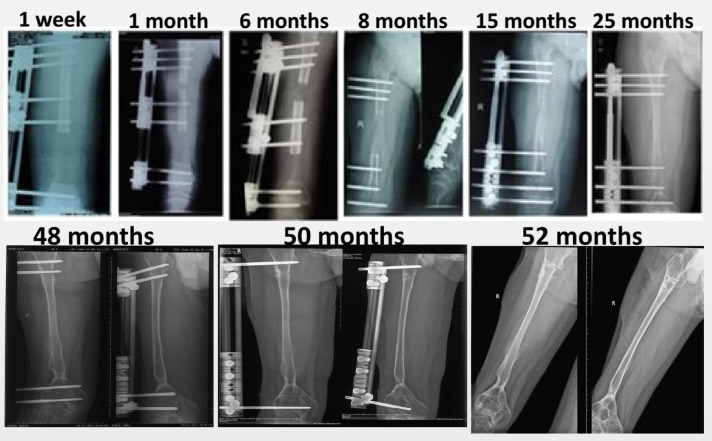
After surgery, the radiograph was conducted every 6 months to check the bone union or local tumor relapse. Alkaline phosphatase and thoracic CT were performed every 6 months for 5 years. To evaluate the bone defect extension, the patients were followed-up once a month for the first 6 months post-operation, and then once every 1–3 month before removal of the external fixator in case of poor line of force. Radiograph of surgical limbs was conducted in all patients. The radiographs (case 3) of complete transfer process at different follow-up periods (1 week to 52 months) were shown in Figure 6. Adverse events induced by operation or Ilizarov external fixator were recorded and classified into problems, obstacles or true complications as previously described [16]. Problems were the postoperative issues not requiring operative intervention (i.e., superficial pin site infections). Obstacles were the issues requiring operative intervention, but could be resolved (i.e., contracture release). True complications occurred intra-operatively or could not be resolved by operative intervention.
Fig. 6.
The complete transfer process at different follow-up periods in Case 3. the external fixator was removed at 52 months post-operation.
MSTS and ASAMI systems were employed to evaluate the bone union and functional recovery at the time and after removal of Ilizarov external fixator [16]. The item of bone union/ delayed union time in the ASAMI-function scoring system was omitted as the bone union in OS patients was relatively slow.
2.7. Statistical analysis
Continuous data were expressed as the mean ± standard deviation (SD). Categorical data were presented as number and percentage (%). All analyses were performed using IBM SPSS Version 17 (SPSS Statistics V17, IBM Corporation, Somers, New York).
3. Results
3.1. Surgical treatment and outcome assessments
Surgical treatment and outcome of all patients were summarized in Table 2. The 10 patients started to transfer at 7th days post-operation with a speed of 0.25 mm/12 h. The mean length of transported fragment was 14 cm and the mean transport distance of the affected limb was 14 cm. The mean time in the external fixator was 34.2 ± 11.2 months (range: 16–47 months) and the mean external fixation index (EFI) was 75 days/ cm.
Table 2.
Surgical treatment and outcome.
| Case no. | Adverse events | Treatment for the adverse events | External fixator type | Length of transported fragment (cm) | Tumor in the surgical margina | External fixator duration (months) | External fixation index (days/cm) | Follow-up (months) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Knee-joint stiffness | Surgical release | Unilateral external | 15 | No | 20 | 40 | 100 | Survive without tumor |
| 2 | Angulation deformity; Poor alignment nonunion bone | Surgical adjustment; autologous bone graft | Unilateral external | 21 | No | 36 | 52 | 40 | Survive without tumor |
| 3 | Joint function disturbance | Functional training | Unilateral external | 12 | No | 52 | 131 | 58 | Survive without tumor |
| 4 | Relapse | Amputation | Unilateral external | 15 | No | 47 | 95 | 96 | Survive without tumor |
| 5 | Poor alignment; poor skin healing | Surgical adjustment; fibula remove; autologous bone graft | Unilateral external | 13 | No | 38 | 88 | 103 | Survive without tumor |
| 6 | Pin tract crossing infection; unconnected bone | Debridement, autologous bone graft | Unilateral external | 12 | No | 28 | 70 | 37 | Survive without tumor |
| 7 | Nonunion bone; low activity of joints | Bong graft surgery; functional training | Unilateral external | 13 | No | 34 | 78 | 44 | Survive without tumor |
| 8 | Pin tract infection (superficial) | Wound care | Unilateral external | 15 | No | 41 | 82 | 54 | Survive without tumor |
| 9 | Joint function disturbance | Functional training | Unilateral external | 12 | No | 30 | 75 | 60 | Survive without tumor |
| 10 | None | / | circular external | 14 | No | 16 | 35 | 94 | Survive without tumor |
Postoperative pathological finding.
The mean follow-up time was 68.6 ± 26.6 months (range: 37–103 months). Seven out of 10 patients underwent a second or multiple operations to treat the complications, including the malunion (n = 3), poor alignment of the line of force (n = 2) and pin tract infection (n = 2). The mean number of operation was 1.7 times. Only one patient (Case 4) underwent amputation at 47 months post-operation due to cancer relapse. The limb-salvage rate was 90% (9/10). All patients achieved tumor-free survival. Some patients had limbs length discrepancy, which was resolved by using a heel lift.
The bone union and functional recovery were assessed by the ASAMI-bone score, ASAMI-function score, and MSTS score (Table 3). At the time of external fixator removal, ASAMI-bone score was good in 66.7% (6/9) of the patients and fair in 33.3% (3/9) cases. ASAMI-function score was fair in 66.7% (6/9) cases and good in 33.3% (3/9) cases. The mean MSTS score was 18.6 ± 3.2 (n = 9). At 6 months after fixator removal, both the ASAMI-bone score and ASAMI-function score were excellent in 50% (4/8) and good in 50% (4/8) of the cases, respectively. The mean MSTS score was improved to 24.3 ± 1.82 (n = 8). At 10 months after fixator removal, both the ASAMI-bone score and ASAMI-function score were excellent in 80% (4/5) and good in 20% (1/5) of the cases, respectively. The mean MSTS score was further improved to 27.2 ± 1.11 (n = 5).
Table 3.
The bone union and functional recovery were assessed by the ASAMI and MSTS score.
| Case no. | Assessment score | Time points |
||
|---|---|---|---|---|
| External fixator removal | Six months after fixator removal | More than 10 months after fixator removal | ||
| 1 | ASAMI-bone | Good | Good | Excellent |
| ASAMI-function | Fair | Excellent | Excellent | |
| MSTS | 19 | 26 | 27 | |
| 2 | ASAMI-bone | Fair | – | – |
| ASAMI-function | Fair | – | – | |
| MSTS | 15 | – | – | |
| 3 | ASAMI-bone | Fair | Good | – |
| ASAMI-function | Fair | Good | – | |
| MSTS | 18 | 21 | – | |
| 4 | ASAMI-bone | – | – | – |
| ASAMI-function | – | – | – | |
| MSTS | – | – | – | |
| 5 | ASAMI-bone | Fair | Good | Good |
| ASAMI-function | Fair | Good | Good | |
| MSTS | 17 | 24 | 26 | |
| 6 | ASAMI-bone | Good | Excellent | – |
| ASAMI-function | Good | Excellent | – | |
| MSTS | 21 | 24 | – | |
| 7 | ASAMI-bone | Good | Excellenta | – |
| ASAMI-function | Fair | Good | – | |
| MSTS | 16 | 24 | – | |
| 8 | ASAMI-bone | Good | Good | Excellent |
| ASAMI-function | Fair | Good | Excellent | |
| MSTS | 15 | 23 | 27 | |
| 9 | ASAMI-bone | Good | Excellent | Excellent |
| ASAMI-function | Good | Excellent | Excellent | |
| MSTS | 22 | 26 | 27 | |
| 10 | ASAMI-bone | Good | Excellent | Excellent |
| ASAMI-function | Good | Excellent | Excellent | |
| MSTS | 24 | 26 | 29 | |
4. Discussion
In this study, we investigated the safety and effectiveness of the Ilizarov method for bone transport in limb-salvage treatment for OS. The mean length of transported fragment and the mean transport distance of the affected limb were both 14 cm. The mean time in the external fixator was 34.2 ± 11.2 months (range: 16–47 months) and the mean EFI was 75 days/cm. The mean follow-up time was 68.6 ± 26.6 months (range: 37–103 months). Seven patients underwent second or multiple operations to treat the postoperative complications, and the mean number of operation was 1.7 times. Only one patient underwent amputation due to tumor relapse and all patients survived without tumor. The limb-salvage rate was 90%. At the time of external fixator removal, the ASAMI-bone score was good in 66.7% (6/9) of the patients and the ASAMI-function score was fair in 66.7% (6/9) cases. The mean MSTS score was 18.6 ± 3.2 (n = 9). At 10 months after fixator removal, both the ASAMI-bone score and ASAMI-function score were both excellent in 80% (4/5) and good in 20% (1/5) cases, and the mean MSTS score was further improved to 27.2 ± 1.11 (n = 5). Taken together, these results suggested that the Ilizarov method for bone transport can achieve good therapeutic effectiveness in the limb-salvage treatment for OS patients.
It has been shown that neoadjuvant chemotherapy can significantly improve the prognosis of OS patients [17], [18]. In this study, the therapeutic effect of chemotherapy was good except for one case with tumor necrosis rate <70%. Previous studies show that chemotherapy affects bone formation [19], [20]. In this study, the fastening nails were gradually dismantled at 3–6 months after complete contact between the transported end and fixed bone end. This gradually dismantling strategy can decrease the fracture risk of the transported bone. The mean transport distance of the affected limb was 14 cm within the 35-month with holder. The mean EFI was 75 days/cm, which is considerably higher than that in trauma or benign tumor bone defect and a unique characteristic of bone graft following chemotherapy [21], [22].
It has been reported that the recurrence rate following OS limb-salvage operation is about 10–30% [23], and the quality of the surgical margin is an influential factor associated with recurrence [23], [24]. In this study, no tumor was found in the surgical margin in all cases, and the shortest distance from tumor was 1.5 cm (averagely 2.3 cm). However, there was still one patient with relapse at 47 months post-operation and received amputation surgery after the second chemotherapy. The recurrence rate was 10% (1/10), and the limb-salvage rate was 90%. In the tumor resection, it is crucial to completely resect the tumor and the muscle around the tumor in the range of safe boundary. If the distance from the tumor osteotomy plane to joint end is smaller than 5 cm, prosthetic replacement/ bone allograft surgery should be considered. The transported bone should be as long as possible to provide enough line of force and allow more vascular branches to provide blood circulation.
ASAMI and MSTS standards are extensively used to assess the limb function following bone graft [21]. The bone delayed union time is limited to 6 months in ASAMI scoring system. Considering the interference of chemotherapy, the item of bone union/ delayed union time was omitted in ASAMI scoring system. This study revealed that the functional score was gradually improved among the time points of fixator removal, 3 months after of fixator removal and 10 months after fixator removal. This phenomenon should be attributed to the fact that long-term holder-wearing around knee joint led to insufficient joint activity. The knee joint function of all patients was significantly improved after removing the external fixator and conducting positively functional rehabilitation. Bone self-healing and reconstruction remained constantly proceeding after removing the fixator, which is one of the advantages of bone graft surgery. Meanwhile, joint functional rehabilitation can achieve satisfactory results at 3 months after holder-removing because the operation did not involve in the knee joint.
Compared to Ozaki et al.’s report [11], the incidence of complications was considerably reduced in the current study. The severe complications reported in Ozaki et al.’s study included poor bone formation, skin defect, pseudoarthrosis, talipes equinus, skin necrosis or infection; pin tract infection, thrombosis, osteomyelitis and varus deformity of femur, and non-union [11]. The common complications of bone transport include unconnected bone, lower limbs length discrepancy, pin tract infection and angulation deformity [11], [22], [25], [26], [27], [28]. In this study, patients with poor bone formation should undergo X-ray examination every month to timely adjust the speed of bone lengthening. If a cortical discontinuity was observed, distraction-compression (accordion maneuver) approach would be adopted to accelerate bone formation. Since bone transport is not a routine procedure for OS patients, the cases for the Ilizarov method for bone transport were strictly screened in this study, where the patients with poor skin condition were excluded. Therefore, there was no skin sacrifice during tumor resection. The pseudoarthrosis is caused by the insufficient strength of the lengthening bone. In this study, to improve the strength of the lengthening bone, autologous iliac bone grafting would be performed once the lengthening bone contacted with the contralateral host bone. Meanwhile, the fastening nails should be gradually dismantled at 3–6 months after complete contact between the transported end and fixed bone end. Talipes equinus is mainly caused by nerve damage or imbalance muscle strength. During the treatment, our patients did not have nerve damage and were guided for the training of lower limb strength and joint activity by rehabilitation physicians, thus did not develop talipes equinus. To avoid postoperative fracture, our patients should abduct within 1 month after external fixator removal. After that, if there was pain in the affected limb after exercise, the patient should continue to abduct and receive x-ray examination. Hence, there was no fracture after external fixator removal in our patients. In this study, ceftriaxone sodium was used for anti-inflammatory treatment in the perioperative period, and ubumex was used to improve the immunity during the whole treatment duration, so osteomyelitis did not occur in our patients. In this study, although 7 out of the 10 patients received additional surgery to treat the complications including the malunion, poor alignment of the line of force and pin tract infection, the patients can achieve good limb of extension on the basis of a high tumor-free survival rate. Three patients in this study suffered from unconnected bone, indicating that chemotherapy has an adverse effect on bone formation. The three patients with unconnected bone were all cured after receiving autologous iliac bone graft, and the function returned to normal without long-term complications. The autologous bone graft was performed at 3 months after completion of chemotherapy to minimize the effect of chemotherapy on bone formation. One patient (case 2) developed poor alignment of bone graft in the distal end of the femur at 6 months post-operation, which were resolved by adjusting the line of force and correctly identified the location of the distal end of the femur. Poor alignment of transported bone with the distal end of femur occurred in one patient (case 5) at 9 months post-operation, which was treated by adjusting the position of transported bone under the guidance of C-arm fluoroscopy. At 8 months after the second operation, the patient received the third surgery to readjust the position of transported bone again due to poor alignment. Bone resorption was observed at 3 months after the third operation, then the patient received autogenous fibula graft. At 2 months after the fourth operation, the patient received the fifth surgery to readjust the position of transported bone due to poor location. After 38 months with an external fixator, the fibula completely healed and the Ilizarov external fixator was removed. Pin tract infection occurred in 2 cases (20%) during the chemotherapy at 3 months post-operation, which should be attributed to the chemotherapy-induced decreased immunity. The incidence of infection was similar to previous studies [11], [25]. The pin tract infection rate could be reduced by extensively post-operative wound care. Bone transport method can truly achieve limb salvage with the long-term good function of the limb. In addition, its early complications can be resolved by timely treatments. Moreover, the effectiveness of the chemotherapy regimen guarantees the quality of life in our patients, which was markedly improved as compared with those in 1997. All the above reasons contribute to the improvement of Ilizarov method for tumor reconstruction in this study.
There are still some limitations of this study. There was still one patient receiving amputation due to tumor relapse, indicating that there is still room for improvement in the treatment strategy. In addition, this study was limited by its retrospective nature and small sample size. In the future, a prospective study with a large sample size should be conducted to further validate the findings of the current study. All these limitations should be addressed in the following study.
5. Conclusions
In summary, our results suggested that Ilizarov method for bone transport can achieve good therapeutic effectiveness in the limb-salvage treatment for OS patients with neoadjuvant chemotherapy as long as the complications can be timely recognized and well managed.
Acknowledgments
Acknowledgment
None.
Conflicts of interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jbo.2019.100224.
Appendix. Supplementary materials
References
- 1.Katonis P., Datsis G., Karantanas A., Kampouroglou A., Lianoudakis S., Licoudis S., Papoutsopoulou E., Alpantaki K. Spinal osteosarcoma. Clin. Med. Insights. Oncol. 2013;7:199–208. doi: 10.4137/CMO.S10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison D.J., Geller D.S., Gill J.D., Lewis V.O., Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018;18:39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari S., Ruggieri P., Cefalo G., Tamburini A., Capanna R., Fagioli F., Comandone A., Bertulli R., Bisogno G., Palmerini E., Alberghini M., Parafioriti A., Linari A., Picci P., Bacci G. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. J. Clin. Oncol. 2012;30:2112–2118. doi: 10.1200/JCO.2011.38.4420. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y., Han L., He Z., Li X., Yang S., Yang J., Zhang Y., Li D., Yang Z. Advances in limb salvage treatment of osteosarcoma. J. Bone Oncol. 2018;10:36–40. doi: 10.1016/j.jbo.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamplot J.D., Denduluri S., Qin J., Li R., Liu X., Zhang H., Chen X., Wang N., Pratt A., Shui W., Luo X., Nan G., Deng Z.-L., Luo J., Haydon R.C., He T.-C., Luu H.H. The current and future therapies for human osteosarcoma. Curr. Cancer Ther. Rev. 2013;9:55–77. doi: 10.2174/1573394711309010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gosheger G., Gebert C., Ahrens H., Streitbuerger A., Winkelmann W., Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin. Orthop. Relat. Res. 2006;450:164–171. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 7.Shekkeris A.S., Hanna S.A., Sewell M.D., Spiegelberg B.G.I., Aston W.J.S., Blunn G.W., Cannon S.R., Briggs T.W.R. Endoprosthetic reconstruction of the distal tibia and ankle joint after resection of primary bone tumours. J. Bone Joint Surg. Br. 2009;91:1378–1382. doi: 10.1302/0301-620X.91B10.22643. [DOI] [PubMed] [Google Scholar]
- 8.Campanacci L., Manfrini M., Colangeli M., Alí N., Mercuri M. Long-term results in children with massive bone osteoarticular allografts of the knee for high-grade osteosarcoma. J. Pediatr. Orthop. 2010;30:919–927. doi: 10.1097/BPO.0b013e3181fa7981. [DOI] [PubMed] [Google Scholar]
- 9.Su A.W., Chen W.-M., Chen C.-F., Chen T.-H. Innovative trident fixation technique for allograft knee arthrodesis for high-grade osteosarcoma around the knee. Jpn. J. Clin. Oncol. 2009;39:739–744. doi: 10.1093/jjco/hyp108. [DOI] [PubMed] [Google Scholar]
- 10.Gubin A.V., Borzunov D.Y., Malkova T.A. The Ilizarov paradigm: thirty years with the Ilizarov method, current concerns and future research. Int. Orthop. 2013;37:1533–1539. doi: 10.1007/s00264-013-1935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozaki T., Nakatsuka Y., Kunisada T., Kawai A., Dan'ura T., Naito N., Inoue H. High complication rate of reconstruction using Ilizarov bone transport method in patients with bone sarcomas. Arch. Orthop. Trauma Surg. 1998;118:136–139. doi: 10.1007/s004020050333. [DOI] [PubMed] [Google Scholar]
- 12.Messerschmitt P.J., Garcia R.M., Abdul-Karim F.W., Greenfield E.M., Getty P.J. Osteosarcoma. J. Am. Acad. Orthop. Surg. 2009;17:515–527. doi: 10.5435/00124635-200908000-00005. http://www.ncbi.nlm.nih.gov/pubmed/19652033 [DOI] [PubMed] [Google Scholar]; (accessed January 21, 2018).
- 13.He X., Zhang H.-L., Hu Y.-C. Limb salvage by distraction osteogenesis for distal tibial osteosarcoma in a young child: a case report. Orthop. Surg. 2016;8:253–256. doi: 10.1111/os.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang B., Yi C., Zhang H., Zhang Q., Li Y., Wei Q., He W., Zeng Z. [Combined epiphyseal preservation and autograft bone transfer in treatment of children osteosarcoma] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:45–49. http://www.ncbi.nlm.nih.gov/pubmed/23427491 [PubMed] [Google Scholar]; (accessed January 21, 2018).
- 15.Futani H., Fukunaga S., Tsukamoto Y., Terada N., Ono J., Okamoto N., Otsuka Y., Tanizawa T., Tomatsuri M., Yoshiya S. Small cell osteosarcoma successfully treated by high-dose ifosfamide and methotrexate, combined with carboplatin and pirarubicin. Anticancer Res. 2012;32:965–971. http://www.ncbi.nlm.nih.gov/pubmed/22399618 [PubMed] [Google Scholar]; (accessed January 21, 2018).
- 16.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin. Orthop. Relat. Res. 1990:81–104. [PubMed] [Google Scholar]
- 17.Fuchs N., Bielack S.S., Epler D., Bieling P., Delling G., Körholz D., Graf N., Heise U., Jürgens H., Kotz R., Salzer-Kuntschik M., Weinel P., Werner M., Winkler K. Long-term results of the co-operative German–Austrian–Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1998;9:893–899. doi: 10.1023/a:1008391103132. http://www.ncbi.nlm.nih.gov/pubmed/9789613 [DOI] [PubMed] [Google Scholar]; (accessed January 18, 2018).
- 18.Kim M.S., Cho W.H., Song W.S., Lee S.-Y., Jeon D.-G. Time dependency of prognostic factors in patients with stage II osteosarcomas. Clin. Orthop. Relat. Res. 2007;463:157–165. doi: 10.1097/BLO.0b013e318142b27d. [DOI] [PubMed] [Google Scholar]
- 19.Morcuende J.A., Gomez P., Stack J., Oji G., Martin J., Fredericks D.C., Buckwalter J.A. Effect of chemotherapy on segmental bone healing enhanced by rhBMP-2. Iowa Orthop. J. 2004;24:36–42. http://www.ncbi.nlm.nih.gov/pubmed/15296204 [PMC free article] [PubMed] [Google Scholar]; (accessed January 20, 2018).
- 20.Tung T.H. Successful distraction osteogenesis across a growing cranial suture without an osteotomy. Plast. Reconstr. Surg. 1999;103:362–370. doi: 10.1097/00006534-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 21.McCoy T.H., Kim H.J., Cross M.B., Fragomen A.T., Healey J.H., Athanasian E.A., Rozbruch S.R. Bone tumor reconstruction with the Ilizarov method. J. Surg. Oncol. 2013;107:343–352. doi: 10.1002/jso.23217. [DOI] [PubMed] [Google Scholar]
- 22.Song H.R., Cho S.H., Koo K.H., Jeong S.T., Park Y.J., Ko J.H. Tibial bone defects treated by internal bone transport using the Ilizarov method. Int. Orthop. 1998;22:293–297. doi: 10.1007/s002640050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picci P., Sangiorgi L., Bahamonde L., Aluigi P., Bibiloni J., Zavatta M., Mercuri M., Briccoli A., Campanacci M. Risk factors for local recurrences after limb-salvage surgery for high-grade osteosarcoma of the extremities. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1997;8:899–903. doi: 10.1023/a:1008230801849. http://www.ncbi.nlm.nih.gov/pubmed/9358942 [DOI] [PubMed] [Google Scholar]; (accessed January 19, 2018).
- 24.Rozeman L.B., Cleton-Jansen A.M., Hogendoorn P.C.W. Pathology of primary malignant bone and cartilage tumours. Int. Orthop. 2006;30:437–444. doi: 10.1007/s00264-006-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuchiya H., Morsy A.F., Matsubara H., Watanabe K., Abdel-Wanis M.E., Tomita K. Treatment of benign bone tumours using external fixation. J. Bone Joint Surg. Br. 2007;89:1077–1083. doi: 10.1302/0301-620X.89B8.19132. [DOI] [PubMed] [Google Scholar]
- 26.Eralp L., Kocaoglu M., Bilen F.E., Balci H.I., Toker B., Ahmad K. A review of problems, obstacles and sequelae encountered during femoral lengthening: uniplanar versus circular external fixator. Acta Orthop. Belg. 2010;76:628–635. [PubMed] [Google Scholar]
- 27.Tsuchiya H., Shirai T., Morsy A.F., Sakayama K., Wada T., Kusuzaki K., Sugita T., Tomita K. Safety of external fixation during postoperative chemotherapy. J. Bone Jt. Surg. - Br. 2008;90:924–928. doi: 10.1302/0301-620X.90B7.20674. –B. [DOI] [PubMed] [Google Scholar]
- 28.Hazan E.J., Hornicek F.J., Tomford W., Gebhardt M.C., Mankin H.J. The effect of adjuvant chemotherapy on osteoarticular allografts. Clin. Orthop. Relat. Res. 2001;385:176–181. doi: 10.1097/00003086-200104000-00027. http://www.ncbi.nlm.nih.gov/pubmed/11302311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.