Abstract
Non-O1, non-O139 Vibrio cholerae (NOVC) are genetically diverse strains that are generally non-pathogenic in healthy hosts. In immunocompromised patients or those with liver disease, NOVC have been shown to cause gastroenteritis, wound infections or sepsis and are often associated with high mortality rates. We report a case of a patient with liver cirrhosis and chronic venous insufficiency who was found to have NOVC bacteremia. The patient had recently visited Florida, USA but had no seafood consumption or exposure to aquatic environments. The patient was managed with antimicrobials, with a favorable outcome.
Introduction
Vibrio cholerae are gram negative, comma-shaped bacilli that inhabit aquatic environments and are classically associated with cholera epidemics. There are currently over 200 known serotypes of Vibrio cholerae, each distinguished by their surface lipopolysaccharide O-antigens. Cholera pandemics are most closely associated with serogroups O1 and O139, both of which produce the toxin responsible for severe gastroenteritis experienced during outbreaks [1]. Recent decades have seen the emergence of infections caused by non-O1, non-O139 Vibrio cholerae (NOVC). Most of these infections comprise of self-remitting gastroenteritis and until recently, their clinical significance has not been a major topic of discussion [2].
NOVC bacteremia is an uncommon infection, especially in immunocompetent individuals. However, a select patient population, such as those with liver cirrhosis, appear to be particularly susceptible. There have been few reports worldwide describing this entity, and new cases have been identified in certain regions within the United States [3]. We report a case of NOVC bacteremia in a patient with liver cirrhosis and chronic venous insufficiency detected in Detroit, Michigan which became of particular interest as our academic institution had not encountered such a case before.
Case report
A 62-year-old African American male with a past medical history of chronic venous insufficiency and chronic hepatitis C with liver cirrhosis (Child-Pugh Class B), presented to a Detroit emergency department with a painful right leg swelling associated with fatigue and chills of one-week duration. The patient had traveled by airplane to Florida two weeks prior to presentation but denied any recent trauma or bug bites.
On presentation, the patient was afebrile. His heart rate was 116 beats per minute, blood pressure was 127/77 mmHg and oxygen saturation was 96% on room air. He was alert and oriented to time, place and person. His physical examination was remarkable for right leg swelling. Additionally, multiple small superficial ulcers with serosanguinous drainage were noted over the right leg and thigh. The ulcers were chronic with minimal tenderness and no surrounding warmth or erythema. The left leg was also minimally swollen, with signs of venous insufficiency. A complete blood count revealed a hemoglobin of 11.4 g/dl, a white blood cell count of 8000 cells/mm3 (75% neutrophils) and a platelet count of 83,000 cells/mm3. Lactate in the blood was 3.5 mmol/L. Other laboratory blood tests showed a total bilirubin 4.1 mg/dL (direct bilirubin 3.6 mg/dL), albumin 2.3, INR 1.55, ALT 36 U/L, AST 54 U/L, creatinine 1.4 mg/dL and blood urea nitrogen 14 mg/dL. A venous duplex ultrasound of the lower extremities revealed a nonobstructive deep venous thrombosis of the femoral vein at the level of the mid-thigh.
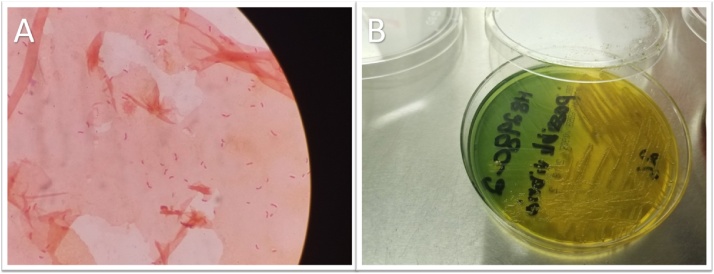
Two different sets of blood cultures drawn in the emergency department grew gram-negative, oxidase-positive bacilli in all four bottles (Fig. 1A). The Verigene assay was negative for all tested gram-negative organisms (Acinetobacter spp., Citrobacter spp., Enterobacter spp., Proteus spp., Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Pseudomonas aeruginosa) and to all tested resistance markers. The patient was started empirically on cefepime. When cultured on the thiosulfate-citrate-bile salts-sucrose (TCBS) agar, the organism formed large yellow colonies (Fig. 1B). The organism was identified as V. cholerae (API: 99.9%) and V. cholerae non O1 (MALDI ToF, Score: 1.84 RUO).
Fig. 1.
(A) Gram stain (×1000) showing gram negative curved bacilli, (B) TCBS agar, a highly selective medium for Vibrio species, showing large yellow colonies indicating fermentation of sucrose.
The isolate was sent to the Michigan Department of Community Health where the organism in question was identified as V. cholerae, non-O1, non-O139. The isolate was then forwarded to the Centers for Disease Control and Prevention (CDC) where additional genetic testing revealed the presence of specific genes toxR and ampW and the absence of the cholera toxin ctxA gene and the toxin co-regulated pilus tcpA gene. The organism was susceptible to ceftriaxone, ciprofloxacin, trimethoprim/sulfamethoxazole and doxycycline. The patient’s antimicrobials were then switched to ceftriaxone 2 g once daily and doxycycline 100 mg every 12 h on day 3 of hospitalization.
Stool and wound cultures taken on the third day of admission while the patient was on antimicrobials were negative. On further questioning, the patient denied any recent or current diarrhea. He also denied any recent seafood consumption or exposure to aquatic environments while in Florida or after his return to Michigan. The patient’s symptoms improved over four days and he was discharged on a 14-day course of oral doxycycline 100 mg twice daily.
Discussion
NOVC are gram-negative, oxidase-positive curved bacilli that are typically found in warm aquatic environments such as sea shores, inland lakes and rivers [4]. Although NOVC organisms can be toxigenic, they are referred to as such to distinguish them from the O1 and O139 serogroups responsible for cholera pandemics [5]. NOVC infections can present as gastroenteritis, soft tissue or wound infections, otitis, biliary tract infections, urinary tract infections, pneumonia, meningitis or bacteremia, among others [6,7]. Chen et.al studied a series of 83 NOVC infections identified in two hospitals in Taiwan between 2009 to 2014. Out of these patients, 45 had acute gastroenteritis (54.2%), 12 had biliary tract infection (14.5%) and 11 had primary bacteremia (13.3%) [8]. The first case of NOVC bacteremia was reported in 1974 in the United States [9]. Since then, there have been over 350 cases of NOVC bacteremia reported in the literature worldwide [3]. Mortality in patients with NOVC bacteremia appears to exceed 25% [3,8].
Deshayer et al reviewed the literature on NOVC bacteremia, between 1974 and 2014. The review included 128 articles with 347 patients with primary and secondary bacteremia. The authors identified associated conditions in 333 (96%) of these patients. Liver cirrhosis was the most common associated condition (55%) followed by malignancy (20%). Additionally, 8% of the patients had skin wounds. The source of infection was identified in only 87 of these patients (25%). Among those patients, 54% reported seafood consumption, 30% were in contact with contaminated water and 11% drank contaminated water [3]. As noted by Petsaris et al, the majority of cases of NOVC bacteremia occurred in warmer seasons in patients with liver cirrhosis or hematologic malignancies who reported seafood consumption or contact with sea water [7]. A 2016 epidemiological study by Crowe et al identified 52 infections with NOVC in the United States over 30 years of surveillance (1984–2014). All isolates of NOVC were of the serogroups O75 (30 infections) or O141 (21 infections), except for one isolate which was non-typeable [10]. The majority of patients who contracted NOVC through seafood consumption had consumed raw oysters from Florida or clams from New Jersey. In addition, NOVC infections through freshwater exposure occurred mostly in Arizona, Michigan, Missouri and Texas, and it is suggested that this occurs through the accidental ingestion of copepods colonized by V. cholerae [10]. It is important to note that most patients with NOVC infections have no history of raw seafood consumption or aquatic exposure, as was the case for our patient.
NOVC is believed to access the bloodstream by spreading from the small intestines or skin wounds into the blood and lymphatic system. Hemolysin expression has been suggested to play a role in the organism’s invasion. Patients with liver cirrhosis are more susceptible to NOVC bacteremia, perhaps due to increased iron. Other factors postulated to contribute to NOVC bacteremia include increased intestinal permeability, abnormal portal vein flow and immunosuppression [11,12].
There are currently no definitive guidelines for the treatment of NOVC bacteremia. Because management primarily depends on antibiotic selection, obtaining an antimicrobial susceptibility profile is crucial when initiating treatment. NOVC has been shown to be generally susceptible to β-lactam antimicrobials, tetracyclines, fluoroquinolones and trimethoprim/sulfamethoxazole [3,13]. Single agent therapy is more commonly used in patients with NOVC gastroenteritis whereas dual-agent therapy is used in sicker patients with sepsis or septic shock and often in patients with NOVC bacteremia [14]. There is also no clear consensus on the duration of antibiotic therapy in patients with NOVC bacteremia, although Deshayes et al reported a median duration of 14 days [3]. Our patient received 3 days of empirical cefepime followed by 4 days of dual-agent therapy with ceftriaxone and doxycycline. He was then discharged to complete an additional 14 days of oral doxycycline. Five months later, he was readmitted to our institution with cellulitis of his lower extremity and had no evidence of recurrence of a NOVC infection.
Conflicts of interest
The author declare that there is no conflicts of interest.
References
- 1.Nelson E.J., Harris J.B., Morris J.G., Jr, Calderwood S.B., Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7(10):693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez R.M., Megli C.J., Taylor R.K. Growth and laboratory maintenance of Vibrio cholerae. Curr Protoc Microbiol. 2010 doi: 10.1002/9780471729259.mc06a01s17. Chapter 6:Unit 6A 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshayes S., Daurel C., Cattoir V., Parienti J.J., Quilici M.L., de La Blanchardiere A. Non-O1, non-O139 Vibrio cholerae bacteraemia: case report and literature review. Springerplus. 2015;4:575. doi: 10.1186/s40064-015-1346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker-Austin C., Trinanes J.A., Salmenlinna S., Lofdahl M., Siitonen A., Taylor N.G. Heat wave-associated vibriosis, Sweden and Finland, 2014. Emerg Infect Dis. 2016;22(7):1216–1220. doi: 10.3201/eid2207.151996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faruque S.M., Albert M.J., Mekalanos J.J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62(4):1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaki R., El-Hossary D., Jiman-Fatani A., Al-Ghamdi R. Non-O1/non-O139 Vibrio cholerae septicaemia in a Saudi man: a case report. JMM Case Rep. 2017;4(2) doi: 10.1099/jmmcr.0.005077. e005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petsaris O., Nousbaum J.B., Quilici M.L., Le Coadou G., Payan C., Abalain M.L. Non-O1, non-O139 Vibrio cholerae bacteraemia in a cirrhotic patient. J Med Microbiol. 2010;59(Pt 10):1260–1262. doi: 10.1099/jmm.0.021014-0. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.T., Tang H.J., Chao C.M., Lai C.C. Clinical manifestations of non-O1 Vibrio cholerae infections. PLoS One. 2015;10(1) doi: 10.1371/journal.pone.0116904. e0116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearrington E.L., Rand C.H., Jr, Mewborn A., Wilkerson J. Letter: non-cholera vibrio septicemia and meningoencephalitis. Ann Intern Med. 1974;81(3):401. doi: 10.7326/0003-4819-81-3-401_1. [DOI] [PubMed] [Google Scholar]
- 10.Crowe S.J., Newton A.E., Gould L.H., Parsons M.B., Stroika S., Bopp C.A. Vibriosis, not cholera: toxigenic Vibrio cholerae non-O1, non-O139 infections in the United States, 1984-2014. Epidemiol Infect. 2016;144(15):3335–3341. doi: 10.1017/S0950268816001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zmeter C., Tabaja H., Sharara A.I., Kanj S.S. Non-O1, non-O139 Vibrio cholerae septicemia at a tertiary care center in Beirut, Lebanon; a case report and review. J Infect Public Health. 2018;11(5):601–604. doi: 10.1016/j.jiph.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Restrepo D., Huprikar S.S., VanHorn K., Bottone E.J. O1 and non-O1 Vibrio cholerae bacteremia produced by hemolytic strains. Diagn Microbiol Infect Dis. 2006;54(2):145–148. doi: 10.1016/j.diagmicrobio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Lan N.P., Nga T.V., Yen N.T., Dung le T., Tuyen H.T., Campbell J.I. Two cases of bacteriemia caused by nontoxigenic, non-O1, non-O139 Vibrio cholerae isolates in Ho Chi Minh City, Vietnam. J Clin Microbiol. 2014;52(10):3819–3821. doi: 10.1128/JCM.01915-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couzigou C., Lacombe K., Girard P.M., Vittecoq D., Meynard J.L. Non-O:1 and non-O:139 Vibrio cholerae septicemia and pyomyositis in an immunodeficient traveler returning from Tunisia. Travel Med Infect Dis. 2007;5(1):44–46. doi: 10.1016/j.tmaid.2006.06.002. [DOI] [PubMed] [Google Scholar]