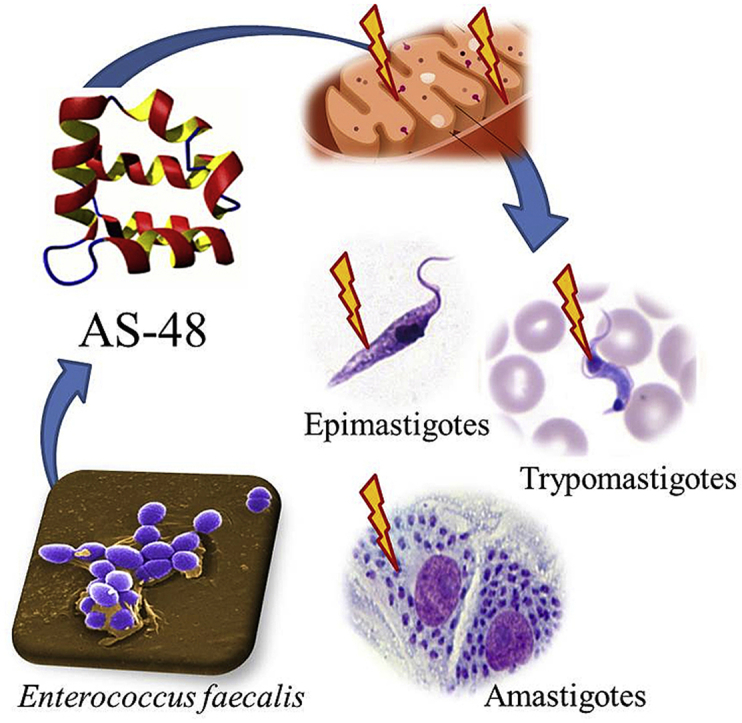
Abstract
Chagas disease caused by the protozoan parasite Trypanosoma cruzi represents a significant public health problem in Latin America, affecting around 8 million cases worldwide. Nowadays is urgent the identification of new antichagasic agents as the only therapeutic options available, Nifurtimox and Benznidazole, are in use for >40 years, and present high toxicity, limited efficacy and frequent treatment failures in the chronic phase of the disease. Recently, it has been described the antiparasitic effect of AS-48, a bacteriocin produced by Enterococcus faecalis, against Trypanosoma brucei and Leishmania spp. In this work, we have demonstrated the in vitro potential of the AS-48 bacteriocin against T. cruzi. Interesting, AS-48 was more effective against the three morphological forms of different T. cruzi strains, and displayed lower cytotoxicity than the reference drug Benznidazole. In addition, AS-48 combines the criteria established as a potential antichagasic agent, resulting in a promising therapeutic alternative. According to the action mechanism, AS-48 trypanocidal activity could be explained in a mitochondrion-dependent manner through a reactive oxygen species production and mitochondrial depolarization, causing a fast and severe bioenergetic collapse.
Keywords: AS-48, Antichagasic agent, Bacteriocin, Drug discovery, Trypanosoma cruzi
Graphical abstract
Highlights
-
•
AS-48 bacteriocin as drug discovery against Chagas Disease.
-
•
Improved trypanocidal activity against the three morphological forms of T. cruzi.
-
•
Activity against different T. cruzi strains, included those Benznidazole-resistant.
-
•
Trypanocidal activity could be explained in a mitochondrion-dependent manner.
-
•
AS-48 represents a significant advance in the search for new antichagas drugs.
1. Introduction
Chagas disease (CD) caused by the flagellated protozoan Trypanosoma cruzi and transmitted by a triatomine insect is a neglected tropical disease endemic in Latin America. It is estimated that around 8 million people are infected worldwide and more than 28 million are living at risk of infection (WHO, 2016). The parasites can adopt three different morphological forms along their life cycle depending on the interaction with the host: i) epimastigote (the proliferative insect vector born stage), ii) trypomastigote (the infective, non-proliferative form) and iii) amastigote (intracellular proliferative form) (Adade et al., 2013). The main drugs used for the treatment of CD are Benznidazole (BZN, Rochagan®, Radanil®, Roche) and Nifurtimox (NFX, Lampit®, Bayer), which are ineffective in the chronic phase of the disease and present significant side effects (Adade et al., 2013). To this must be added the existence of strains resistant to these drugs, that hinders the disease treatment (Wilkinson et al., 2008; Mejia et al., 2012; Campos et al., 2014). For this reason, it is urgent to identify and develop novel active drugs against new targets, with low toxicity and high tolerance in patients, being effective in both, acute and chronic phase of the infection.
Resistance to antimicrobial compounds is a global phenomenon headed by bacteria. Because of this, antimicrobial peptides (AMPs) have been rediscovered for their potential use in the treatment of multidrug-resistant bacteria (Yu et al., 2016; Mishra et al., 2017; Lázár et al., 2018). AMPs can be defined as a ubiquitous and diverse group of natural compounds produced by all living organisms as a part of their innate defense system, that display antimicrobial activity against pathogen organism (Adermann et al., 2014; Smith and Dyrynda, 2015; Chung and Khanum, 2017; Kubicek-Sutherland et al., 2017). They are gaining attention in recent years due to their fast mode of action against a broad spectrum of pathogen microorganism and low capacity to generate resistance. The AMPs produced by bacteria are called bacteriocins. They are ribosomally synthesized peptides with antimicrobial activities (Hassan et al., 2012; Cotter et al., 2013). The mechanism of action is primarily based on the disruption of the cell membranes, although other mechanisms of action have been described (Prince et al., 2016). Due to the high specificity that they generally present towards the membranes of bacteria, in general, they present low toxicity and have been proposed as promising candidates for clinical and veterinary use (Montalbán-López et al., 2011). Although their potential in the control of pathogenic bacteria is well documented, in the case of parasites not many studies are available. In fact, in the scientific literature there are some examples of AMPs with activity against T. cruzi either in vitro or in vivo (Amino et al., 2002; Jacobs et al., 2003; Fieck et al., 2010; Shin et al., 2016; Mello et al., 2017), however, to date, no bacteriocins with antiparasitic activity have been described.
One of the most exciting and well-known bacteriocin is the circular enterocin AS-48. It is a 70-residue, head-to-tail joined, amphipathic and cationic bacteriocin produced by different Enterococcus species (Maqueda et al., 2004) with a broad bactericidal activity against both Gram-positive and Gram-negative disease-associated pathogens (Gálvez et al., 1989; Aguilar-Pérez et al., 2018; Cebrián et al., 2018; Perales-Adán et al., 2018). The most distinctive structural feature of AS-48 is its circular structure and its amphipathicity, which contributes to a remarkable stability in a broad range of temperature and pH (Maqueda et al., 2004; Montalbán-López et al., 2011; Sánchez-Hidalgo et al., 2011). In addition, AS-48 exhibits very low immunogenicity and high resistance to exopeptidases (Maqueda et al., 1993).
The trypanocidal/leishmanicidal effect of AS-48 has been recently described. In both cases, the negative surface charge of the parasites has been related to the potent effect of AS-48. Although both parasites are from the same family, different action mechanisms have been described in each one (Abengózar et al., 2017; Martínez-García et al., 2018). The activity of AS-48 against T. brucei is the highest described until now, and it is because the high efficiency in the internalization of AS-48 by the flagellar pocket of the parasite. Although both parasites are from the same family, different action mechanisms have been described in each one (Abengózar et al., 2017; Martínez-García et al., 2018). In this study we report the in vitro activity of AS-48 against three different T. cruzi strains (including one BZN-resistant), investigating the putative mechanism of action. We have also demonstrated the low AS-48 cytotoxicity on uninfected Vero cells and the absence of pore-forming activity in both Vero cells and T. cruzi. Finally, AS-48 is a fast-acting cidal drug with an action mechanism that could be intermediate to those observed in T. brucei and Leishmania spp. In T. cruzi the trypanocidal activity could be mainly explained in a mitochondrion-dependent manner through the production of reactive oxygen species (ROS) and mitochondrial depolarization. In summary and according to with the positive results achieved in this work, AS-48 is shaping up as a promising therapeutic agent against Chagas disease, although it will be crucial to perform the in vivo studies, in order to know the effectiveness in both acute and chronic phases of the disease.
2. Material and methods
2.1. AS-48 purification
Bacteriocin AS-48 was purified to homogeneity from cultures of the Enterococcus faecalis UGRA10 strain (Ananou et al., 2008; Cebrián et al., 2012) by chromatographic passes (cationic interchange and reversed-phase high-performance liquid chromatography, RP-HPLC) as has been previously described (Cebrián et al., 2015). The protein concentration of the purified AS-48 was determined by measuring UV absorption at 280 nm a Nanodrop 2000 (Thermo Fisher Scientific. Waltham, MA, USA).
2.2. Drug susceptibility assays against Vero cells and T. cruzi forms
2.2.1. Vero cells culture and cytotoxicity tests
Vero cells (EACC number 84113001) were cultured in RPMI (Gibco®) with 10% (v/v) FBS heat-inactivated, at 37 °C in humidified 95% air and 5% CO2 atmosphere. Cytotoxicity tests were assessed using the method previously described (Martín-Escolano et al., 2018) at dosages of 1000 to 1 μM of AS-48 and BZN. Briefly, cytotoxicity was tested using 96-well microtiter plates by seeding the cells at 1.25 × 104 mL−1. After 24 h of incubation, the cells were treated with AS-48 and BZN in 0.2 mL volumes in RPMI (Gibco®) with 1% (v/v) FBS heat-inactivated for 48 h. Subsequently, resazurin sodium salt (Sigma-Aldrich) was added, the plates were incubated for a further 24 h, and finally the trypanocidal activity was determined by absorbance measurements. The trypanocidal effect, using GraphPad Prism 6, was expressed as the inhibition concentration 50 (IC50), i.e., the concentration required to result in 50% inhibition.
2.2.2. Epimastigote forms culture and in vitro activity assays
Three different T. cruzi strains were assessed: SN3 (IRHOD/CO/2008/SN3); Arequipa (MHOM/Pe/2011/Arequipa); and Tulahuen (TINF/CH/1956/Tulahuen), belonging to three discrete typing units (DTUs), I, V and VI, respectively. Epimastigote forms were cultured at 28 °C in RPMI (Gibco®) with 10% (v/v) FBS heat-inactivated, 0.03 M hemin and 0.5% (w/v) trypticase (BBL) (Kendall et al., 1990).
For the trypanocidal test, epimastigote forms were centrifuged in the exponential growth phase at 400 g for 10 min. Trypanocidal activity was determined according to the method previously described (Martín-Escolano et al., 2018) at dosages ranged from 50 to 0.02 μM of AS-48 and BZN. Briefly, tests were carried out by seeding the parasites at 5 × 105 mL-1 after adding AS-48 and BZN in 0.2 mL volumes in 96-well microtiter plates at 28 °C for 48 h. Finally, resazurin sodium salt (Sigma-Aldrich) was added, and after 24 h of incubation, the same procedure as described in 2.2.1. Section was performed.
2.2.3. In vitro activity assays against intracellular amastigote forms
Trypanocidal activity against amastigote forms and the infectivity index were determined according to the method previously described (Martín-Escolano et al., 2018) at dosages of 50 to 0.02 μM of each drug. Briefly, tests were performed in 24-well microtiter plates with rounded coverslips by seeding the Vero cells at 1 × 104 well−1 in RPMI (Gibco®) with 10% (v/v) FBS heat-inactivated, at 37 °C in humidified 95% air and 5% CO2 atmosphere. After 24 h of incubation, the cells were infected with Vero cell-derived trypomastigotes at a multiplicity of infection (MOI) ratio of 1:10. After 24 h, non-phagocyted parasites were washed away, and after adding AS-48 and BZN in 0.5 mL volumes in RPMI (Gibco®) with 1% (v/v) FBS heat-inactivated. After 72 h of incubation, the trypanocidal activity was assessed by analyzing 500 host cells in methanol-fixed and Giemsa-stained preparations. The trypanocidal effect was determined using GraphPad Prism 6, as mentioned in 2.2.1. Section.
2.2.4. In vitro activity assays against extracellular bloodstream trypomastigote forms
Epimastigote forms maintained at 28 °C were induced to transform into metacyclic forms using Grace's Insect Medium (Gibco®), TAU and TAU3AGG mediums following the method previously described (Martín-Escolano et al., 2018). The obtained metacyclic trypomastigote forms were used to infect Vero cells, and the obtained Vero cell-derived trypomastigotes were used to infect BALB/c mice. Finally, bloodstream trypomastigotes (BTs) were obtained by cardiac puncture during the parasitaemia peak.
Trypanocidal activity was determined as described previously (Martín-Escolano et al., 2018) in 96-well microtiter plates by seeding the parasites at 2 × 106 mL−1 in RPMI (Gibco®) with 10% (v/v) FBS heat-inactivated, and after addition of AS-48 and BZN at dosages of 100 to 0.2 μM in 0.2 mL volumes at 37 °C in humidified 95% air and 5% CO2 atmosphere. After 24 h of incubation, resazurin sodium salt (Sigma-Aldrich) was added, and after another 4 h of incubation, the same process as described in 2.2.1. Section was performed.
2.3. Permeability tests of the plasma membrane of Vero cells by trypan blue
The permeability of Vero cells was determined using 24-well microtiter plates by seeding the cells at 2 × 105 mL−1 after adding AS-48 at dosages of 5-, 10- and 25-fold higher than IC50 in 0.5 mL volumes at 37 °C in humidified 95% air and 5% CO2 atmosphere for 10, 30 and 60 min. Untreated Vero cells and Vero cells exposed to 0.1% (v/v) and 0.01% (v/v) Triton X-100 (TX-100) were also included as negative and positive controls, respectively. After incubations, Vero cells were collected, and the plasma membrane permeability was determined by adding trypan blue dye (Sigma-Aldrich) at 0.2% (final concentration) to the cell suspension and counting using an optical microscope and a hemacytometer counter.
2.4. Studies of the action mechanism
2.4.1. Determination of plasma membrane permeabilization by flow cytometry using propidium iodide (PI)
Alterations in membrane permeability of T. cruzi Arequipa epimastigote forms were detected by PI nucleic acid stain (Tiuman et al., 2014; Bustos et al., 2017). Membrane permeability was determined in 1.5 mL microtubes by inoculating the parasites at 1 × 107 mL−1 after adding AS-48 at dosages of 10-, 20- and 50-fold higher than IC50 in 0.5 mL at 28 °C for 10, 30 and 60 min. After incubations, epimastigote forms were centrifuged, washed with PBS, and stained with 2 μg/mL PI dye (Sigma-Aldrich) in 0.5 mL at 28 °C for 10 min (Tiuman et al., 2014). Non-stained parasites, untreated parasites and parasites exposed to 0.01% (v/v) and 0.1% (v/v) TX-100 as negative and positive controls were also included. Finally, the parasites were collected, washed twice with cold PBS, and resuspended in 1 mL of cold PBS for fluorescence measurements by flow cytometry in a BECTON DICKINSON FACSAria III flow cytometer using a BECTON DICKINSON FACSDiva v8.01 software (2350 Qume Drive, San Jose, Palo-Alto, California) (λEXC = 488 nm, λEM = 620 nm).
2.4.2. Metabolite excretion test by proton nuclear magnetic resonance (1H NMR)
Cultures of epimastigote forms (5 × 105 mL−1) in cell culture flasks were added of AS-48 at IC25 concentrations and then maintained at 28 °C for 72 h. Non-treated parasites (negative controls) were also included. The supernatants were then collected by centrifugation at 800g for 10 min to determine the excreted metabolites by 1H NMR using a VARIAN DIRECT DRIVE 400 MHz Bruker spectrometer with an AutoX probe using D2O as previously described (Fernández-Becerra et al., 1997). The binning, normalizations and analyses were obtained using Mestrenova 9.0 software.
2.4.3. Mitochondrial dysfunction tests
2.4.3.1. Rhodamine 123 (Rho) and acridine orange (AO) assays by flow cytometry
The treated T. cruzi Arequipa epimastigote forms described in 2.4.2. Section were centrifuged, washed twice with PBS, and stained with 10 μg/mL Rho or AO dyes (Sigma-Aldrich) in 0.5 mL at 28 °C for 20 min (Sandes et al., 2014). Non-stained parasites and untreated parasites were also included as controls. After that, the parasites were collected, washed twice with cold PBS, and resuspended in 1 mL of cold PBS for fluorescence measurements by flow cytometry in a BECTON DICKINSON FACSAria III flow cytometer using a BECTON DICKINSON FACSDiva v8.01 software (2350 Qume Drive, San Jose, Palo-Alto, California). The fluorescence intensities for Rho (mitochondrial membrane potential) and AO (nucleic acids) were measured as described elsewhere (Sandes et al., 2014).
2.4.3.2. MitoSOX™ Red assays by flow cytometry
Cultures of T. cruzi Arequipa epimastigote forms (1 × 107 mL−1) in 1.5 mL microtubes were stained with 0.5 μM MitoSOX™ Red dye (Invitrogen) in 0.5 mL at 28 °C for 30 min (Abengózar et al., 2017), and washed twice with PBS before the assay (Piacenza et al., 2007). AS-48 was added at 5 μM (Abengózar et al., 2017) at 28 °C for 10, 30 and 60 min. Non-stained parasites, untreated parasites and parasites exposed to 200 μM hydrogen peroxide were also included. Finally, epimastigote forms were processed as described in 2.4.1. Section to measure the fluorescence intensity for oxMitoSOX (ROS production) (λEXC = 510 nm, λEM = 580 nm) (Piacenza et al., 2007).
2.5. Statistical analyses
Data were recorded on a Microsoft Excel spreadsheet, and statistical analyses were performed by using IBM SPSS Statistics software (v. 21). The t-test for paired samples was used to verify whether there were differences between the assays used, with p < 0.05 considered statistically significant and with a 95% confidence level. Also statistical studies based on contingency tables (prevalence) were conducted, together with the × 2 test of the relationship between variables.
3. Results and discussion
3.1. AS-48 effects over T. cruzi growth in vitro
Currently, the genetic diversity of T. cruzi is extensively known being the parasite classified into seven DTUs that show different evolutionary relationships, epidemiological and ecological associations, tropism, pathogenesis, genotype, phenotype, and drug resistance (Zingales, 2018). Different concentrations of purified AS-48 bacteriocin (50–0.02 μM) were assayed against extracellular epimastigote, trypomastigote and intracellular amastigote forms of three strains of T. cruzi belonging to different DTUs – SN3, Tc I; Arequipa, Tc V; and Tulahuen, Tc VI – to evaluate its inhibitory activity using BZN as the reference. The IC50 values are presented in Table 1. Toxicity of AS-48 and BZN against uninfected Vero cells was also tested in parallel (Table 1), and the selectivity index (SI) (SI = IC50 Vero cells/IC50 extra- and intracellular forms) (Table 2) against T. cruzi was calculated.
Table 1.
Trypanocidal activity of AS-48 and BZN on the three developmental forms of T. cruzi strains. Toxicity on Vero cells.
| Comp. | Activity IC50 (μM)a |
Activity IC50 (μM)a |
Activity IC50 (μM)a |
Toxicity IC50/72 h (μM) Vero cell |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arequipa strain |
SN3 strain |
Tulahuen strain |
||||||||
| E/72 h | A/72 h | T/24 h | E/72 h | A/72 h | T/24 h | E/72 h | A/72 h | T/24 h | ||
| BZN | 16.86 ± 1.81 | 8.25 ± 0.72 | 12.35 ± 1.14 | 36.51 ± 2.44 | 16.62 ± 1.46 | 36.09 ± 3.12 | 19.72 ± 1.75 | 9.96 ± 0.84 | 15.09 ± 1.30 | 80.35 ± 7.08 |
| AS-48 | 0.76 ± 0.11 | 0.99 ± 0.13 | 0.17 ± 0.04 | 1.16 ± 0.18 | 6.81 ± 0.89 | 0.11 ± 0.02 | 0.82 ± 0.11 | 1.98 ± 0.22 | 0.19 ± 0.03 | 93.06 ± 5.67 |
IC50 = concentration (μM) required to inhibit 50% population, calculated using GraphPad Prism 6. Results are averages of three separate determinations ± standard deviation. E, epimastigote forms. A, amastigote forms. T, trypomastigote forms.
Table 2.
Selectivity Index for AS-48 and BZN on extra- and intracellular forms of T. cruzi strains.
| Comp. | Selectivity index |
Selectivity index |
Selectivity index |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Arequipa straina |
SN3 straina |
Tulahuen straina |
|||||||
| E | A | T | E | A | T | E | A | T | |
| BZN | 4.77 | 9.73 | 6.51 | 2.20 | 4.83 | 2.23 | 4.07 | 8.07 | 5.32 |
| AS-48 | 122.45 | 94.00 | 547.41 | 80.22 | 13.67 | 846.00 | 113.49 | 47.00 | 489.79 |
| AS-48/BZNb | 26 | 10 | 84 | 36 | 3 | 379 | 28 | 6 | 92 |
Selectivity index (SI) = IC50 Vero cells/IC50 extracellular and intracellular form of parasite. bNumber of times that AS-48 exceeds the reference drug SI (on extracellular and intracellular forms of T. cruzi). E, epimastigote forms. A, amastigote forms. T, trypomastigote forms.
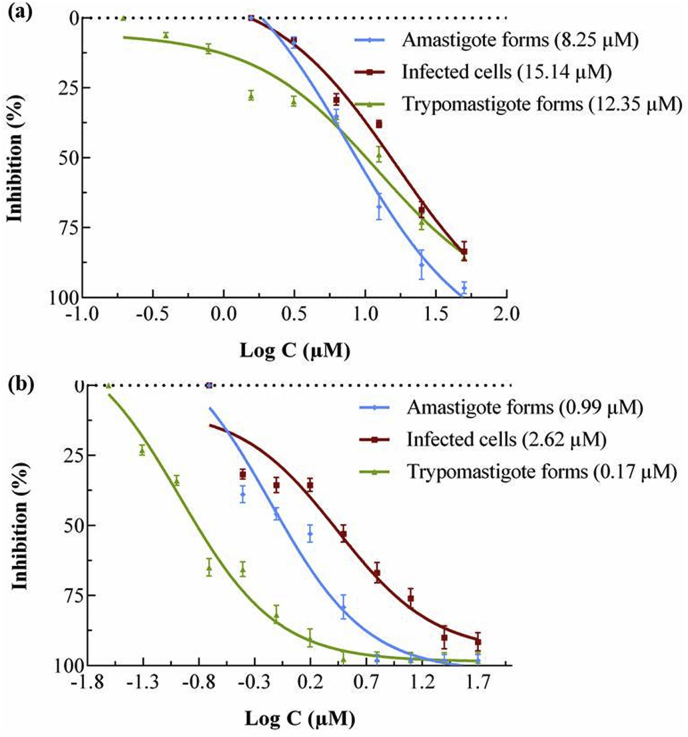
In the case of the epimastigote forms, the IC50/72 h values ranged between 0.76 μM for the Arequipa strain and 1.16 μM for the BZN resistant SN3 strain. These values were 22, 31 and 24-fold lower for Arequipa, SN3 and Tulahuen strains (respectively), than the values obtained for BZN.
Regarding the amastigote forms the relationship between the IC50/72 h of BZN/AS-48 was lower (8, 2 and 5-fold) due to the minor susceptibility of these forms. The strains Arequipa and Tuhaluen were the most sensitive to AS-48 (0.99 and 1.98 μM respectively) while the strain SN3 showed a higher resistance (6.81 μM). We suggest that this could be due to AS-48 has to cross many membranes to reach the parasite, and the intracellular environment may not be the most appropriate for the AS-48 activity.
Finally, the trypomastigote forms were also susceptible to AS-48 treatment with the lower IC50/24 h: 0.17, 0.11 and 0.19 μM against Arequipa, SN3 and Tulahuen strains, respectively, while BZN needed 12.35, 36.09 and 15.09 μM, respectively. Interesting, these forms were more the most resistant to BZN (not in the case of Arequipa) (Table 1) with an IC50/24 h BZN/AS-48 ratio of 73, 328, and 79. The high AS-48 activity observed in these forms could be due to the characteristics of their membranes, which are composed mostly of phosphatidyl-choline and to a lesser extent of phosphatidyl-ethanol-amine (Agusti et al., 2000).
It is remarkable that BTs and intracellular amastigote forms are the most interesting forms from a clinical point of view (González et al., 2005) being in all cases sensitive to a low micromolar range of AS-48. In general, the IC50 observed for these forms were lower than the activity observed in the case of Leishmania spp. (IC50 of 4 μM and 10.2 μM for L. donovani and L. pifanoi promastigotes and amastigotes respectively). However, these IC50 were higher than in the case of T. brucei (1.7–3.12 nM) probably due to the fact that AS-48 is not able to enter inside T. cruzi as fast as in T. brucei. In fact, in the procyclic form of T. brucei or in the blood forms at 4 °C (when the parasite endocytic-membrane activity is stopped), the IC50 obtained for T. brucei were closed to the values obtained for T. cruzi (Martínez-García et al., 2018). These data make us think that the mechanism of action, in this case, could be intermediate between Leishmania spp. and T. brucei.
Furthermore, the toxicity towards Vero cells was also assayed. In this case, the IC50/72 h was higher for AS-48 (93.06 μM) than for BZN (80.35 μM) (Table 1). The calculated AS-48 IC50 toward these cells confirmed, together with the IC50 values against the different forms of the parasite, a high selectivity index for the different morphological forms of T. cruzi tested, much better than the selectivity index observed for BZN (Table 2).
Moreover, to obtain accurate information about the AS-48 trypanocidal activity, in vitro infection on Vero cells and treatment with AS-48 was performed. The rate of infection of T. cruzi Arequipa strain was measured by counting infected cells after 72 h of exposure at different concentrations of AS-48. The data are shown in Fig. 1, together with the data of amastigote and trypomastigote forms. It was found that the rates of infected cells decreased as the concentrations of AS-48 increased, with an IC50/72 h of 2.62 μM (6-fold lower than for BZN). Likewise, the total number of amastigote and trypomastigote forms decreased more for AS-48-treated cultures than for those treated with BZN.
Fig. 1.
Reduction of the infection of T. cruzi Arequipa strain regarding the decrease of amastigote and trypomastigote forms and infected cells treated with (a) BZN and (b) AS-48. Values are the means of three separate experiments ± standard deviation. In brackets: IC50 value, calculated using GraphPad Prism 6.
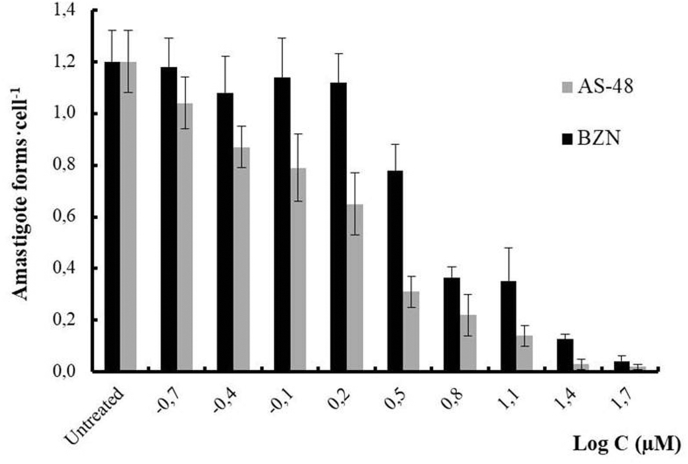
The average number of amastigote forms per cell was also measured at different concentrations (Fig. 2), giving an idea of the killing rate. The data show that AS-48 is faster acting than BZN, which is considered until now as a fast-acting compound (Chatelain, 2015). These data show that AS-48 not only inhibits the parasite multiplication (static compound) but also causes its death (cidal compound). These features are additional advantages since fast-acting trypanocidal drugs can eliminate the parasite in a few doses (Rycker et al., 2012).
Fig. 2.
Reduction in the number of amastigote forms of T. cruzi Arequipa strain per Vero cell treated with BZN and AS-48 for 72 h. Values are the means of three separate experiments ± standard deviation. Significant differences between the BZN and AS-48 for α = 0.05.
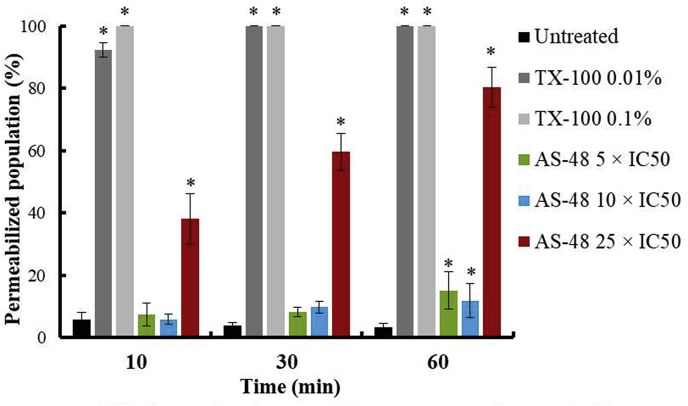
It is noticeable that in amastigote forms AS-48 has to go inside the cell to kill the parasite crossing the decks that surround it. This is the first time that has been reported activity for AS-48 inside of non-immune cells (different to macrophage in the case of Leishmania spp). In order to elucidate how AS-48 can enter into the Vero cells, a permeability membrane assay was performed at different AS-48 concentrations and exposure times, using TX-100 as control (Fig. 3 and SFig. 1). The tested AS-48 concentrations were higher than the IC50 obtained (Table 1) because the incubation times were shorter and a higher number of Vero cells were used for the test. The data show that AS-48 did not disrupt the Vero cells membranes at concentrations as high as 5- and 10-fold higher than IC50 – 191 and 382 μM, respectively – at 10 and 30 min and barely at 60 min, suggesting that AS-48 does not cause pores in spite of the high concentrations tested. Only those concentration very high tested (25-fold higher than IC50, which is clote to 1 mM) seem to alter the Vero cells membranes, and never at the concentrations tested – 50 to 0.2 μM – in the activity assays against intracellular amastigote forms. With these results, the mechanism by which AS-48 can enter in the cell, without affect the integrity of the membrane barrier, and being able to kill the parasite, remains uncertain. However, one possibility is the cellular depolarization mechanism induced by cationic peptides when they are used at relevant concentrations, facilitating their entry through the membranes and activity on internal targets (Hale and Hancock, 2007).
Fig. 3.
Permeabilization of the plasma membrane of Vero cells treated with TX-100 and AS-48 for 10 min, 30 min and 60 min. Values are the means of the three separate determinations ± standard deviation. * Significant differences between untreated and treated Vero cells for α = 0.05.
Overall, these in vitro assays showed that AS-48 is a fast-acting and cidal agent that presents an excellent trypanocidal activity independent of the form and strain of the T. cruzi used, with higher activity and lower cytotoxicity than BZN. Moreover, AS-48 meets the in vitro criteria to be considered as a potential antichagasic agents: IC50 ≤ 10 μM, and SI > 10 (Don and Ioset, 2014); or IC50 < 5 μM, SI > 10, and the majority of the in vitro criteria of the target product profile (TPP) (Chatelain, 2015).
3.2. Studies about the mechanism of action
3.2.1. AS-48 effects over T. cruzi plasma membrane pore-formation
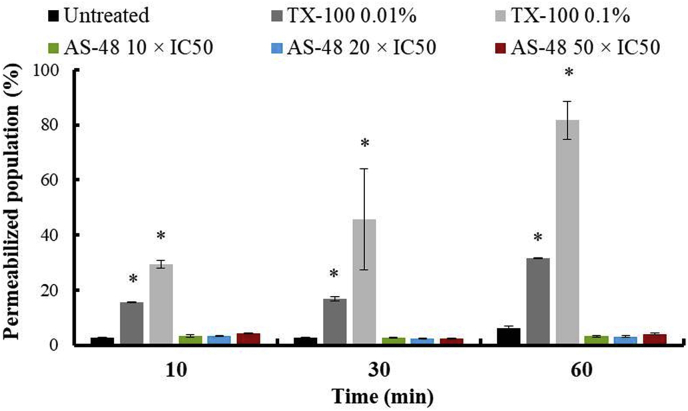
According to the bactericidal and leishmanicidal mechanism of action described for AS-48, the primary target is the cell membrane where AS-48 forms pores (Sánchez-Barrena et al., 2003; Abengózar et al., 2017). To confirm if the fast-acting cidal effect observed for AS-48 on T. cruzi is also due to the formation of pores in the membrane, permeability assay of the plasma membrane of T. cruzi was performed by flow cytometry. We used PI stain after treating the parasites at different AS-48 concentrations and times of exposure, and TX-100 as positive control (Fig. 4 and SFig. 2). The AS-48 concentrations tested were higher than the IC50 obtained (Table 1) because the incubation times were shorter and more epimastigote forms were used. Collectively the data indicate that AS-48 did not permeate the T. cruzi plasma membrane at any of the concentrations (5-, 20- and 50-fold higher than IC50 – 3.8, 15.2 and 38 μM, respectively –) and incubation times tested, and therefore AS-48 must subject T. cruzi to a fast-acting cell death mechanism that differs from the pore formation. This suggests that as in T. brucei, AS-48 should enter in the parasite by endocytosis that in T. cruzi is restricted to two specialized invaginations of the plasma membrane, the flagellar pocket, and the cytostome (De Souza, 2008). Unlike T. brucei bloodstream forms, in which the endocytic turnover of the surface is exceptionally high, in T. cruzi the endocytic rate is much lower (Porto-Carreiro et al., 2000). The lower endocytic level has been also observed in amastigote forms, which could also be related to the higher resistance to AS-48 (Batista et al., 2015). Besides, cytostome is not present in trypomastigote forms (De Souza, 2008; Vidal et al., 2016), that according to our results are the most susceptible form. Therefore, the main route of entry of AS-48 into the cell should be the flagellar pocket.
Fig. 4.
Permeabilization of the plasma membrane of epimastigote forms of T. cruzi Arequipa strain treated with TX-100 and AS-48 for 10 min, 30 min and 60 min. Values are the means of the three separate determinations ± standard deviation. * Significant differences between untreated and treated parasites for α = 0.05.
The study of the mechanism of action at the energy metabolism level was evaluated. Alteration of metabolites excretion (glycosomal level) and also mitochondrial dysfunction (mitochondrial level) were prompted for three reasons: a) the strong AS-48 fast-acting trypanocidal effect (in the absence of a plasma membrane permeabilization) that can be caused by a fast and severe bioenergetic collapse, b) the single mitochondrion of T. cruzi, a likely target for AS-48 due to its evolutionary resemblance with bacteria, and c) the presence of cardiolipin, a typical mitochondrial phospholipid that interacts strongly with AS-48 (Gálvez et al., 1989).
3.2.2. Metabolite excretion
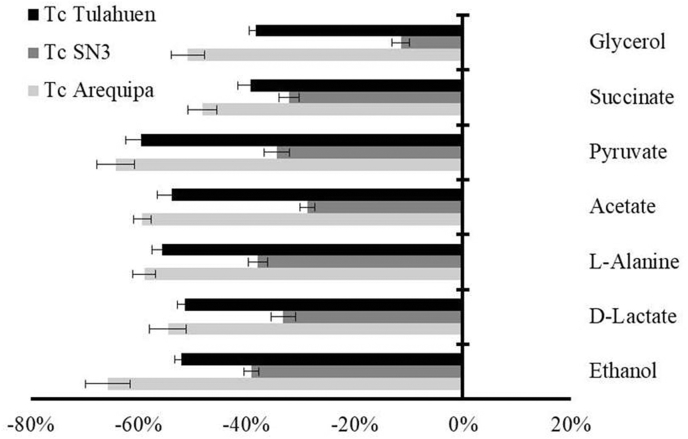
It is well-known that T. cruzi is unable to completely degrading glucose to CO2 in aerobic environments, catabolizes glucose at a high rate, and acidifies the medium owing to incomplete oxidation to acids like pyruvate, acetate and succinate, among others (Bringaud et al., 2006). Consistently, to know the effect of AS-48 on glucose metabolism, 1H NMR spectra of the medium of AS-48-treated epimastigote forms were qualitatively and quantitatively analyzed and compared with the spectra found for the untreated parasites (Fig. 5). The excretion of all metabolites in the three AS-48-treated T. cruzi strains was strikingly reduced concerning the corresponding untreated parasites. Therefore, a deficit in the production of catabolites by a blocking in the glycolytic pathway seems to be caused by AS-48. This alteration could be associated with an inhibition in the first enzymes involved in this pathway (glycosomal level) or to a mitochondrial dysfunction (mitochondrial level) that can produce an imbalance in the NADH/NAD+ and ATP/ADP ratios causing a blockade in the glycolytic pathway (Bringaud et al., 2006; Wen et al., 2010).
Fig. 5.
Variation (percentages) among peaks of catabolites excreted by T. cruzi epimastigote forms exposed to AS-48 at their IC25 in comparison to the control (untreated) incubated 72 h. Values are the means of three separate determinations ± standard deviation. Significant differences between the control and AS-48 for α = 0.05.
3.2.3. Mitochondrial dysfunction
Hence, to assess if the alteration in the glycolytic pathway is a consequence of a mitochondrial dysfunction, flow cytometry analysis of the membrane potential and ROS production from treated parasites was performed using Rho and MitoSOX™ Red as fluorescents, respectively (Fig. 6, Fig. 7 and SFig. 3).
Fig. 6.
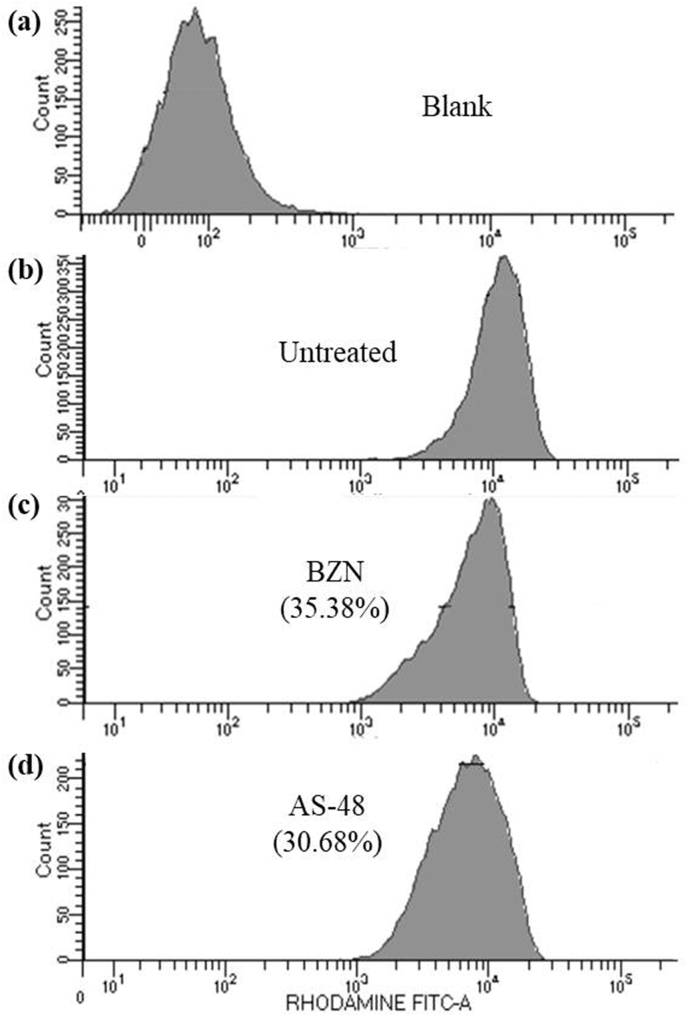
Cytometry analysis of the mitochondrial membrane potential from epimastigotes of T. cruzi Arequipa strain exposed to BZN and AS-48 at their IC25 in comparison to the control (untreated) incubated 72 h: (a) blank, (b) control (untreated), (c) BZN and (d) AS-48. Each drug was tested in three separate determinations. In brackets: reduction, in percentage, in the mitochondrial membrane potential with respect to untreated parasites. Significant differences between untreated parasites and parasites treated with BZN and AS-48 for α = 0.05.
Fig. 7.
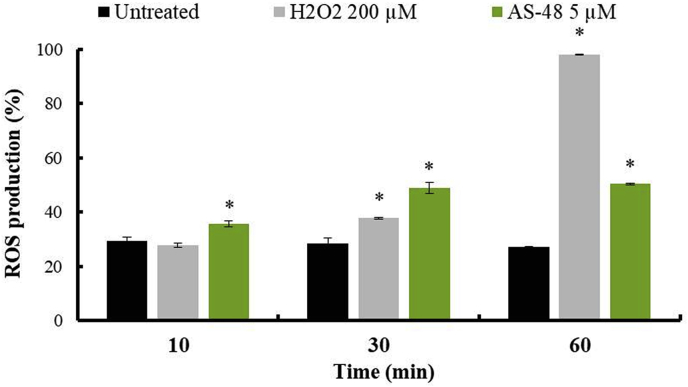
Production of mitochondrial ROS of epimastigote forms of T. cruzi Arequipa strain exposed to H2O2 and AS-48 at 200 μM and 5 μM, respectively, for 10 min, 30 min and 60 min. Values are the means of the three separate determinations ± standard deviation. * Significant differences between untreated and treated parasites for α = 0.05.
Regarding the mitochondrial membrane potential assay (Fig. 6), we confirmed that BZN-treated parasites showed a decrease in mitochondrial membrane potential (35.38%) because it is known that BZN kills T. cruzi through its transformation to highly reactive metabolites after reduction by type I nitroreductase activity (Hall and Wilkinson, 2012), causing, among others, respiratory chain inhibition. Interestingly, for AS-48, the treated parasites also showed a decrease in mitochondrial membrane potential (30.68%) despite having used a lower concentration (the IC25).
Regarding the ROS production assay (Fig. 7 and SFig. 3), AS-48 treated parasites at 5 μM showed significant ROS production from 10 min of exposure (35.65%), being even higher than the parasites treated with H2O2, and increasing to more than 50% at 60 min of exposure.
3.2.4. Nucleic acids levels
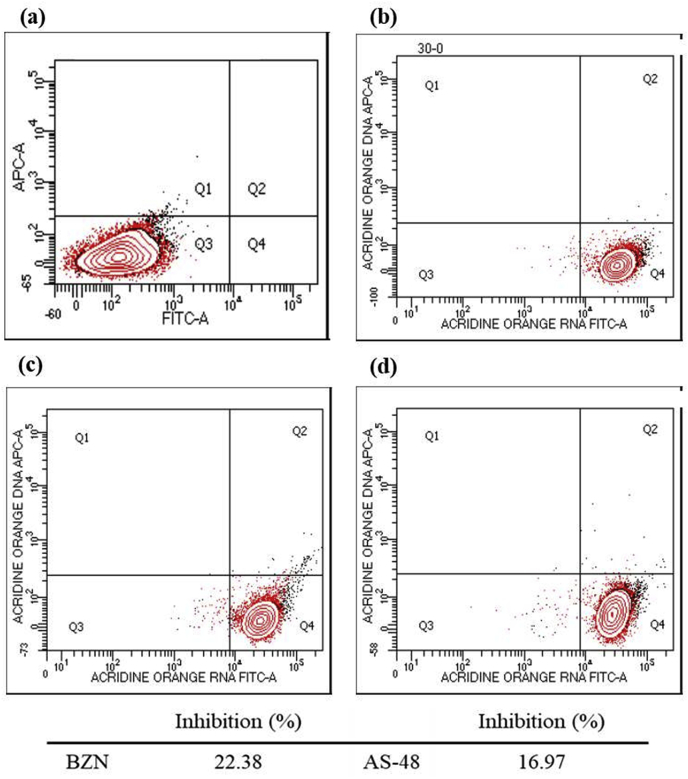
The mitochondrion plays a crucial role in cell death decisions, and alterations in the integrity and function of it lead to a decrease in ATP production and nucleic acids levels, inducing apoptosis or necrosis (Lee and Thévenod, 2006; Shang et al., 2009). Therefore, the alterations observed in the integrity and function of the mitochondrion can lead to a decrease in ATP production and nucleic acids levels, inducing apoptosis or necrosis (Lee and Thévenod, 2006; Shang et al., 2009). Consequently, to determine the nucleic acids levels, flow cytometry analyses were performed using AO as fluorescent (Fig. 8). Remarkably, BZN and AS-48-treated parasites showed similar decreases in the AO fluorescence intensity (22.38% and 16.97%, respectively), as a consequence of the reduction in the amount of nucleic acids. It must be noted that the decrease in nucleic acids levels is also due to random nucleic acids degradation as commonly attributed feature to cell necrosis, and not only because of the APT deficit (Verma et al., 2007).
Fig. 8.
Cytometry analysis showing the inhibition in the nucleic acids levels of epimastigotes of T. cruzi Arequipa strain exposed to BZN and AS-48 at their IC25 in comparison to the control (untreated) incubated 72 h: (a) blank, (b) control (untreated), (c) BZN, (d) AS-48. Each drug was tested in three separate determinations. Below: reduction, in percentage, in the nucleic acids levels with respect to untreated parasites. Significant differences between untreated parasites and parasites treated with BZN and AS-48 for α = 0.05.
Accordingly, the putative mechanism of action for AS-48 could be explained in a mitochondrion-dependent manner through a mitochondrial depolarization and ROS production that causes a fast collapse in the energy metabolism of T. cruzi, as has been described in Leishmania spp. (Abengózar et al., 2017). However, the AS-48 leishmanicidal activity is also produced by a partial plasma membrane permeabilization (Abengózar et al., 2017), a mechanism that does not happen in T. cruzi. We suggest that AS-48 should be endocytosed by the flagellar pocket. Moreover, the fact that intracellular amastigotes show a lower endocytosis activity than extracellular forms (Nogueira et al., 2015) also support their lower susceptibility of these to AS-48.
As AS-48 is a stable peptide that exhibits very low immunogenicity and high resistance to exopeptidases, it could be a promising drug against Chagas disease. Further validation of the usefulness of AS-48 in this field requires murine model assays to determine the in vivo trypanocidal activity of AS-48 during both the acute and chronic phases. Moreover, biochemical analysis will be performed in order to know the possible abnormalities associated with the treatments.
4. Conclusions
In conclusion, the trypanocidal properties of AS-48 were examined, and the experiments allowed us to present AS-48 as a promising alternative for the development of a new therapeutic agent against CD. AS-48 showed enhanced efficiency and lower toxicity than the reference drug BZN, and a broader spectrum of action. Finally, the action mechanism whereby AS-48 shows a fast-acting cidal effect against T. cruzi is mitochondrion-dependent through a ROS production and mitochondrial membrane depolarization, causing a fast and severe bioenergetic collapse. Interesting AS-48 has also displayed antiparasitic activity against amastigote forms (inside Vero cells) in a low micromolar range. This means that AS-48 has to enter in the cell to kill the parasite, being the first time that this kind of activity has been reported for a bacteriocin in non-defensive immune cells.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the Spanish Ministry of Economy and Competitiveness [grant numbers SAF2013-48971-C2-1-R, CSD2010-00065], both including funds from the European Regional Development Fundings (ERDF), and the Ministry of Education of Spain [RM-E, grant number FPU14/01537].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2019.03.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adermann K., Hoffmann R., Otvos L. IMAP 2012: antimicrobial peptides to combat (multi)drug-resistant pathogens. Protein Pept. Lett. 2014;21:319–320. doi: 10.2174/09298665113206660099. [DOI] [PubMed] [Google Scholar]
- Abengózar M.Á., Cebrián R., Saugar J.M., Gárate T., Valdivia E., Martínez-Bueno M., Maqueda M., Rivas L. Enterocin AS-48 as evidence for the use of bacteriocins as new leishmanicidal agents. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.02288-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adade C.M., Oliveira I.R.S., Pais J.A.R., Souto-Padrón T. Melittin peptide kills Trypanosoma cruzi parasites by inducing different cell death pathways. Toxicon. 2013;69:227–239. doi: 10.1016/j.toxicon.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Aguilar-Pérez C., Gracia B., Rodrigues L., Vitoria A., Cebrián R., Deboosère N., Song O.R., Brodin P., Maqueda M., Aínsa J.A. Synergy between circular bacteriocin AS-48 and ethambutol against. Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00359-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti R., Couto A.S., Alves M.J.M., Colli W., De Lederkremer R.M. Lipids shed into the culture medium by trypomastigotes of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz. 2000;95:97–102. doi: 10.1590/s0074-02762000000100016. [DOI] [PubMed] [Google Scholar]
- Amino R., Martins R.M., Procopio J., Hirata I.Y., Juliano M.A., Schenkman S. Trialysin, a novel pore-forming protein from saliva of hematophagous insects activated by limited proteolysis. J. Biol. Chem. 2002;277:6207–6213. doi: 10.1074/jbc.M109874200. [DOI] [PubMed] [Google Scholar]
- Ananou S., Muñoz A., Gálvez A., Martínez-Bueno M., Maqueda M., Valdivia E. Optimization of enterocin AS-48 production on a whey-based substrate. Int. Dairy J. 2008;18:923–927. [Google Scholar]
- Batista C.M., Kessler R.L., Eger I., Soares M.J. Trypanosoma cruzi intracellular amastigotes isolated by nitrogen decompression are capable of endocytosis and cargo storage in reservosomes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringaud F., Rivière L., Coustou V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol. Biochem. Parasitol. 2006;149:1–9. doi: 10.1016/j.molbiopara.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Bustos P.L., Volta B.J., Perrone A.E., Milduberger N., Bua J. A homolog of cyclophilin D is expressed in Trypanosoma cruzi and is involved in the oxidative stress-damage response. Cell Death Dis. 2017;3:16092. doi: 10.1038/cddiscovery.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M.C., Leon L.L., Taylor M.C., Kelly J.M. Benznidazole-resistance in Trypanosoma cruzi: evidence that distinct mechanisms can act in concert. Mol. Biochem. Parasitol. 2014;193:17–19. doi: 10.1016/j.molbiopara.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebrián R., Baños A., Valdivia E., Pérez-Pulido R., Martínez-Bueno M., Maqueda M. Characterization of functional, safety, and probiotic properties of Enterococcus faecalis UGRA10, a new AS-48-producer strain. Food Microbiol. 2012;30:59–67. doi: 10.1016/j.fm.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Cebrián R., Martínez-Bueno M., Valdivia E., Albert A., Maqueda M., Sánchez-Barrena M.J. The bacteriocin AS-48 requires dimer dissociation followed by hydrophobic interactions with the membrane for antibacterial activity. J. Struct. Biol. 2015;190:162–172. doi: 10.1016/j.jsb.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Cebrián R., Arévalo S., Rubiño S., Arias-Santiago S., Rojo M.D., Montalbán-López M., Martínez-Bueno M., Valdivia E., Maqueda M. Control of Propionibacterium acnes by natural antimicrobial substances: role of the bacteriocin AS-48 and lysozyme. Sci. Rep. 2018;8:11766. doi: 10.1038/s41598-018-29580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain E. Chagas Disease drug discovery: toward a new era. J. Biomol. Screen. 2015;20:22–35. doi: 10.1177/1087057114550585. [DOI] [PubMed] [Google Scholar]
- Chung P.Y., Khanum R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017;50:405–410. doi: 10.1016/j.jmii.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Cotter P.D., Ross R.P., Hill C. Bacteriocins - a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]
- De Souza W. An introduction to the structural organization of parasitic protozoa. Curr. Pharmaceut. Des. 2008;14:822–838. doi: 10.2174/138161208784041123. [DOI] [PubMed] [Google Scholar]
- Don R., Ioset J.R. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology. 2014;141:140–146. doi: 10.1017/S003118201300142X. [DOI] [PubMed] [Google Scholar]
- Fernández-Becerra C., Sanchez-Moreno M., Osuna A., Opperdoes F.R. Comparative aspects of energy metabolism in plant trypanosomatids. J. Eukaryot. Microbiol. 1997;44:523–529. [Google Scholar]
- Fieck A., Hurwitz I., Kang A.S., Durvasula R. Trypanosoma cruzi: synergistic cytotoxicity of multiple amphipathic anti-microbial peptides to T. cruzi and potential bacterial hosts. Exp. Parasitol. 2010;125:342–347. doi: 10.1016/j.exppara.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez A., Maqueda M., Martínez-Bueno M., Valdivia E. Bactericidal and bacteriolytic action of peptide antibiotic AS-48 against gram-positive and gram-negative bacteria and other organisms. Res. Microbiol. 1989;140:57–68. doi: 10.1016/0923-2508(89)90060-0. [DOI] [PubMed] [Google Scholar]
- González P., Marín C., Rodríguez-González I., Hitos A.B., Rosales M.J., Reina M., Díaz J.G., González-Coloma A., Sánchez-Moreno M. In vitro activity of C20-diterpenoid alkaloid derivatives in promastigotes and intracellular amastigotes of Leishmania infantum. Int. J. Antimicrob. Agents. 2005;25:136–141. doi: 10.1016/j.ijantimicag.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Hale J.D.F., Hancock R.E.W. Alternative mechanism of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti Infect. Ther. 2007;5:951–959. doi: 10.1586/14787210.5.6.951. [DOI] [PubMed] [Google Scholar]
- Hall B.S., Wilkinson S.R. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 2012;56:115–123. doi: 10.1128/AAC.05135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M., Kjos M., Nes I.F., Diep D.B., Lotfipour F. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012;113:723–736. doi: 10.1111/j.1365-2672.2012.05338.x. [DOI] [PubMed] [Google Scholar]
- Jacobs T., Bruhn H., Gaworski I., Fleischer B., Leippe M. NK-lysin and its shortened analog NK-2 exhibit potent activities against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2003;47:607–613. doi: 10.1128/AAC.47.2.607-613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall G., Wilderspin A.F., Ashall F., Miles M.A., Kelly J.M. Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase does not conform to the “hotspot” topogenic signal model. EMBO J. 1990;9:2751–2758. doi: 10.1002/j.1460-2075.1990.tb07462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek-Sutherland J.Z., Lofton H., Vestergaard M., Hjort K., Ingmer H., Andersson D.I. Antimicrobial peptide exposure selects for Staphylococcus aureus resistance to human defence peptides. J. Antimicrob. Chemother. 2017;72:115–127. doi: 10.1093/jac/dkw381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázár V., Martins A., Spohn R., Daruka L., Grézal G., Fekete G., Számel M., Jangir P.K., Kintses B., Csörgő B., Nyerges Á., Györkei Á., Kincses A., Dér A., Walter F.R., Deli M.A., Urbán E., Hegedűs Z., Olajos G., Méhi O., Bálint B., Nagy I., Martinek T.A., Papp B., Pál C. Antibiotic-resistant bacteria show widespread collateral sensitivity to antimicrobial peptides. Nat. Microbiol. 2018;3:718–731. doi: 10.1038/s41564-018-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Thévenod F. A role for mitochondrial aquaporins in cellular life-and-death decisions? AJP Cell Physiol. 2006;291:C195–C202. doi: 10.1152/ajpcell.00641.2005. [DOI] [PubMed] [Google Scholar]
- Maqueda M., Gálvez A., Martínez-Bueno M., Guerra I., Valdivia E. Neutralizing antibodies against the peptide antibiotic AS-48: immunocytological studies. Antimicrob. Agents Chemother. 1993;37:148–151. doi: 10.1128/aac.37.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqueda M., Gálvez A., Bueno M.M., Sanchez-Barrena M.J., González C., Albert A., Rico M., Valdivia E. Peptide AS-48: prototype of a new class of cyclic bacteriocins. Curr. Protein Pept. Sci. 2004;5:399–416. doi: 10.2174/1389203043379567. [DOI] [PubMed] [Google Scholar]
- Martín-Escolano R., Moreno-viguri E., Santivañez-Veliz M., Martín-Montes Á., Medina-Carmona E., Paucar R., Marín C., Azqueta A., Cirauqui N., Pey A.L., Pérez-Silanes S., Sánchez-Moreno M. Second generation of Mannich base-type derivatives with in vivo activity against Trypanosoma cruzi. J. Med. Chem. 2018;61:5643–5663. doi: 10.1021/acs.jmedchem.8b00468. [DOI] [PubMed] [Google Scholar]
- Martínez-García M., Bart J.M., Campos-Salinas J., Valdivia E., Martínez-Bueno M., González-Rey E., Navarro M., Maqueda M., Cebrián R., Pérez-Victoria J.M. Autophagic-related cell death of Trypanosoma brucei induced by bacteriocin AS-48. Int. J. Parasitol. Drugs Drug Resist. 2018;8:203–212. doi: 10.1016/j.ijpddr.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia A.M., Hall B.S., Taylor M.C., Gómez-Palacio A., Wilkinson S.R., Triana-Chávez O., Kelly J.M. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J. Infect. Dis. 2012;206:220–228. doi: 10.1093/infdis/jis331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C.P., Lima D.B., Menezes R.R., Bandeira I.C.J., Tessarolo L.D., Sampaio T.L., Falcão C.B., Rádis-Baptista G., Martins A.M. Evaluation of the antichagasic activity of batroxicidin, a cathelicidin-related antimicrobial peptide found in Bothrops atrox venom gland. Toxicon. 2017;130:56–62. doi: 10.1016/j.toxicon.2017.02.031. [DOI] [PubMed] [Google Scholar]
- Mishra B., Reiling S., Zarena D., Wang G. Host defense antimicrobial peptides as antibiotics: design and application strategies. Curr. Opin. Chem. Biol. 2017;38:87–96. doi: 10.1016/j.cbpa.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalbán-López M., Sanchez-Hidalgo M., Valdivia E., Martinez-Bueno M., Maqueda M. Are bacteriocins underexploited? NOVEL applications for OLD antimicrobials. Curr. Pharmaceut. Biotechnol. 2011;12:1205–1220. doi: 10.2174/138920111796117364. [DOI] [PubMed] [Google Scholar]
- Nogueira P.M., Ribeiro K., Silveira A.C., Campos J.H., Martins-Filho O.A., Bela S.R., Campos M.A., Pessoa N.L., Colli W., Alves M.J., Soares R.P., Torrecilhas A.C. Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J. Extracell. Vesicles. 2015;4:28734. doi: 10.3402/jev.v4.28734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales-Adán J., Rubiño S., Martínez-Bueno M., Valdivia E., Montalbán-López M., Cebrián R., Maqueda M. LAB bacteriocins controlling the food isolated (Drug-Resistant) staphylococci. Front. Microbiol. 2018;9:1143. doi: 10.3389/fmicb.2018.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza L., Irigoín F., Alvarez M.N., Peluffo G., Taylor M.C., Kelly J.M., Wilkinson S.R., Radi R. Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem. J. 2007;403:323–334. doi: 10.1042/BJ20061281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Carreiro I., Attias M., Miranda K., De Souza W., Cunha-e-Silva N. Trypanosoma cruzi epimastigote endocytic pathway: cargo enters the cytostome and passes through an early endosomal network before storage in reservosomes. Eur. J. Cell Biol. 2000;79:858–869. doi: 10.1078/0171-9335-00112. [DOI] [PubMed] [Google Scholar]
- Prince A., Sandhu P., Ror P., Dash E., Sharma S., Arakha M., Jha S., Akhter Y., Saleem M. Lipid-II independent antimicrobial mechanism of nisin depends on its crowding and degree of oligomerization. Sci. Rep. 2016;6:37908. doi: 10.1038/srep37908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycker M.D., O'Neill S., Joshi D., Campbell L., Gray D.W., Fairlamb A.580H. A static-cidal assay for Trypanosoma brucei to aid hit prioritisation for progression into drug discovery programmes. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001932. e1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Barrena M.J., Martínez-Ripoll M., Gálvez A., Valdivia E., Maqueda M., Cruz V., Albert A. Structure of bacteriocin AS-48: from soluble state to membrane bound state. J. Mol. Biol. 2003;334:541–549. doi: 10.1016/j.jmb.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Sánchez-Hidalgo M., Montalbán-López M., Cebrián R., Valdivia E., Martínez-Bueno M., Maqueda M. AS-48 bacteriocin: close to perfection. Cell. Mol. Life Sci. 2011;68:2845–2857. doi: 10.1007/s00018-011-0724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandes J.M., Fontes A., Regis-da-Silva C.G., Brelaz De Castro M.C.A., Lima-Junior C.G., Silva F.P.L., Vasconcellos M.L.A.A., Figueiredo R.C.B.Q. Trypanosoma cruzi cell death induced by the Morita-Baylis-Hillman Adduct 3-Hydroxy-2-Methylene-3-(4-Nitrophenylpropanenitrile) PLoS One. 2014;9 doi: 10.1371/journal.pone.0093936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang X.J., Yao G., Ge J.P., Sun Y., Teng W.H., Huang Y.F. Procyanidin induces apoptosis and necrosis of prostate cancer cell line PC-3 in a mitochondrion-dependent manner. J. Androl. 2009;30:122–126. doi: 10.2164/jandrol.108.005629. [DOI] [PubMed] [Google Scholar]
- Shin J.M., Gwak J.W., Kamarajan P., Feno J.C., Rickard A.H., Kapila Y.L. Biochemical applications of nisin. J. Appl. Microbiol. 2016;120:1449–1465. doi: 10.1111/jam.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V.J., Dyrynda E.A. Antimicrobial proteins: from old proteins, new tricks. Mol. Immunol. 2015;68:383–398. doi: 10.1016/j.molimm.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Tiuman T.S., Ueda-Nakamura T., Alonso A., Nakamura C.V. Cell death in amastigote forms of Leishmania amazonensis induced by parthenolide. 2014;14:152. doi: 10.1186/1471-2180-14-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N.K., Singh G., Dey C.S. Miltefosine induces apoptosis in arsenite-resistant Leishmania donovani promastigotes through mitochondrial dysfunction. Exp. Parasitol. 2007;116:1–13. doi: 10.1016/j.exppara.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Vidal J.C., Alcantara C.L., de Souza W., Cunha-E-Silva N.L. Loss of the cytostome-cytopharynx and endocytic ability are late events in Trypanosoma cruzi metacyclogenesis. J. Struct. Biol. 2016;196:319–328. doi: 10.1016/j.jsb.2016.07.018. [DOI] [PubMed] [Google Scholar]
- Wen J.-J., Gupta S., Guan Z., Dhiman M., Condon D., Lui C., Garg N.J. Phenyl-α-tert-butyl-nitrone and Benzonidazole treatment controlled the mitochondrial oxidative stress and evolution of cardiomyopathy in chronic chagasic rats. J. Am. Coll. Cardiol. 2010;55:2499–2508. doi: 10.1016/j.jacc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S.R., Taylor M.C., Horn D., Kelly J.M., Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. U.S.A. 2008;105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2016. Chagas Disease (American Trypanosomiasis)http://www.who.int/mediacentre/factsheets/fs340/en/ (accessed 5.30.18) [Google Scholar]
- Yu G., Baeder D.Y., Regoes R.R., Rolff J. Combination effects of antimicrobial peptides. Antimicrob. Agents Chemother. 2016;60:1717–1724. doi: 10.1128/AAC.02434-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B. Trypanosoma cruzi genetic diversity: something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.