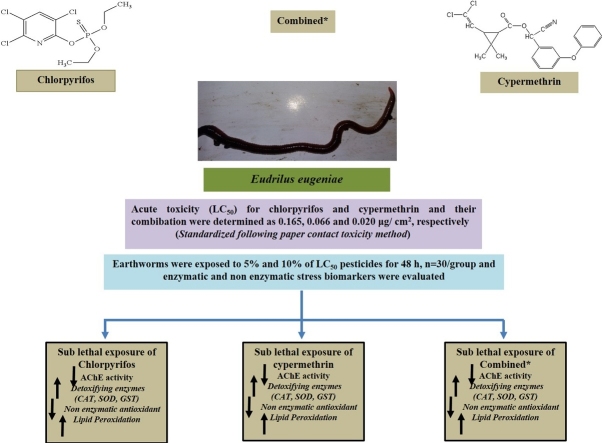
Graphical abstract
Keywords: AChE activity, Stress markers, Chlorpyrifos, Cypermethrin, Chlorpyrifos + Cypermethrin, Eudrilus eugeniae
Highlights
-
•
In the present study, co-exposed administered pesticides induced a higher level of toxicity to Eudrilus eugeniae.
-
•
Statistically significant changes were observed after 48 h exposure of CPF, cypermethrin and combination of the two, reflects the synergistic cumulative impact on the AChE and oxidative stress parameters in dose- dependent manner.
-
•
Significant changes were observed in different body segments (Pre-Clitellar, Clitellar and Post-Clitellar) of earthworm in tissue specific pattern.
Abstract
Recurrent application of chemical pesticides in the agricultural fields have adverse impact on flora and fauna of soil ecosystem. Earthworms immensely contribute in increasing the fertility of soil. They may act as a bioindicator for the ecotoxicological analysis of pesticide induced soil pollution. Earthworms, Eudrilus eugeniae were exposed to different concentrations of pesticides chlorpyrifos (OP), cypermethrin (a pyrethroid) and their combination for 48 h by paper contact toxicity method. The LC50 for commercial grade of chlorpyrifos, cypermethrin and combined pesticides were determined as 0.165, 0.066 and 0.020 μg/cm2, respectively. To assess the sub-lethal effect of these pesticides, E. eugeniae were exposed to 5% and 10% of LC50 of the pesticides for 48 h. Variation in morpho-behavioural changes such as coiling, clitellar swelling, mucus release, bleeding and body fragmentation in earthworms were observed after exposure of both pesticides and their combination. Various biochemical estimations such as specific activity of acetylcholinesterase (AChE), superoxide dismutase (SOD), catalase (CAT), glutathione -S-transferase (GST); levels of lipid peroxidation (LPO) and reduced glutathione (GSH) were carried out in different body segments. Significant changes in these stress markers were observed at low and high sub-acute concentration of pesticides exposed earthworm, Eudrilus eugeniae. Such changes indicate potential health risk to E. eugeniae if exposed to the high concentrations of these pesticides accumulated in soil.
1. Introduction
For the last few decades, an excessive use of pesticides and fertilizers in agriculture has polluted soil to an alarming level. This results change in aeration of soil and its fertility which further leads to an imbalance between flora and fauna residing the soil [1,2]. Soil is a complex mixture of minerals, organic matter and its flora and fauna. Therefore, the management of soil quality depends much on its fauna, being the main consumers and decomposers of soil ecosystem [3]. Earthworms are the excellent bioindicator for evaluating the health status of soil ecosystem. Earlier reports indicate that application of pesticides pose threat to their lives as they are exposed to pesticides contaminated soil [4]. They are much more susceptible and sensitive to the soil pollutants as compared to other soil inhabitants [5]. Therefore, earthworms are considered most suitable organisms for studying the impact of pesticides on their stress related biochemical parameters.
As pesticides of different chemical nature are applied simultaneously in agricultural field, earthworms get exposed to not only one group of pesticides but to their combination as well [6,7]. Since the potential toxicity of pesticide in combination may alter and have impact variably [8], assessment of ecological risk of pesticides applied individually or in combination becomes more relevant for a soil inhabitant, earthworms.
In the present study, two groups of pesticides namely chlorpyrifos (50%) [O, O-diethyl O-3, 5, 6-trichlor- 2-pyridyl phosphorothioate; CPF; PubChem CID: 2730] (an organophosphate; OP) and cypermethrin (5%) [RS-cyano (3 phenoxybenzyl) (1RS)-cis, trans-3–(2,2-dichlorovinyl)- 2,2-dimethylcyclopropane carboxylate; PubChem CID: 2912] (a pyrethroid) and their combination [chlorpyrifos (50%) + cypermethrin (5%)] were selected for acute and sub-acute toxicity evaluation in earthworm. Both Chlorpyrifos and cypermethrin are broad-spectrum insecticides used to control agricultural pests and have been listed as pesticides of potential concern by the US National Oceanic and Atmospheric Administration [9,10]. A variety of irreversible/reversible neurobehavioral and neurotoxic effects as well as physiological and morphological alterations have been reported in earthworms even at very low concentration of pesticide [11]. However, cypermethrin is more toxic regarding impact on physiological and morphological alterations as compared to neurobehavioral effect due to its stereochemistry and isomeric forms [12].
Studies on a number of oxidative stress biomarkers is of great importance as they exhibit alterations caused by pollutants in physiological health status of the organism. In terrestrial ecosystem, AChE inhibition in earthworm is regarded as an early warning of adverse effect of pesticides. However, impact of only a few pesticides has been studied in context to AChE on earthworms under both the laboratory and field conditions. Though most of the reports on such studies are available from southern part of India but are scanty from northern part of the country.
Reactive oxygen species (ROS) impedes the normal physiological functioning of cell in all living organisms. As ROSs mostly interact with all types of biomolecules and cells have ability to neutralize the numerous contaminants and endogenous metabolic byproducts mediating anti-oxidative enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione -S- transferase (GST), a detoxifying enzyme [13,14]. However, the increase in the level of ROS results into oxidative stress which in turn enhances lipid peroxidation causing the cellular damage and death of organisms [[15], [16], [17]]. Therefore, the aforementioned biomarkers such as SOD, CAT, GST and levels of LPO and GSH have been studied to evaluate the impact of contaminants on earthworms. Paper contact toxicity method (OECD guidelines, 207) [18] was applied to assess the impact of these pesticides in earthworm, Eudrilus eugeniae for evaluation of acute toxicity and biochemical changes.
2. Materials and methods
2.1. Chemicals
Formula grade pesticides namely Bilbo (50% EC chlorpyrifos, Bharat Insecticides Limited, New Delhi, India), Bolt Super (25% EC cypermethrin, Pharma Agro Chemical, Haru Nagla, Bareilly, India) and Bilbo (50% EC chlorpyrifos + 5% EC cypermethrin, Bharat Insecticides Limited, New Delhi, India) were used for acute as well as subacute toxicological study. All the chemicals used in the present study such as Acetylthiocholine iodide (AtChI; PubChem CID: 74629), 5,5-dithiobis (2-nitrobenzoic acid; DTNB; PubChem CID: 6254), Bovine Serum Albumin; BSA (PubChem CID: 16132389), thiobarbituric acid (PubChem CID: 2723628), pyrogallol (PubChem CID: 1057), 1-chloro-2,4-dinitrobenzene; CDNB (PubChem CID: 6), hydrogen peroxide; H2O2 (PubChem CID: 784), reduced glutathione; GSH (PubChem CID: 124886), dichromate (PubChem CID: 24502), glacial acetic acid (PubChem CID: 176) and tris-HCl (PubChem CID: 129821084) for biochemical analyses, were of analytical grades and were procured from Sigma- Aldrich (St. Louis, USA) and Merck (Germany).
2.2. Animals and maintenance
Earthworms, Eudrilus eugeniae [19] were procured from the School of Life Science, Charak Garden, Anupam Nagar Extn., Jiwaji University, Gwalior, Madhya Pradesh, 474001. They were carefully brought to laboratory along with mother culture and moist soil. Before experimentation, the earthworms were acclimatized for 15 days into rearing tank (95 × 55 × 75 cm3), containing 10 cm layer of uncontaminated cow dung with moist soil (1:1). A thin layer of leaves and dried moist grass were used for shade and moisture.
2.3. Determination of median lethal concentration (LC50)
The acute toxicity experiments were carried out by paper contact toxicity method for 48 h using filter paper method (Whatman filter paper no. 1, J. S. Enterprises, New Delhi) recommended by OECD Guideline-207, Organization for Economic Co-operation and Development; OECD, 1984) [18]. The filter paper was cut into 8 × 3 cm in diameter covering the sides of flat bottom glass vials of 8 cm in length and 3 cm in diameter without overlapping. The pesticides were dissolved in acetone (PubChem CID: 180) solvent for the preparation of different concentrations and were loaded on filter paper with 1 ml of solution. The vials were rotated for uniform distribution of the toxicant. The treated paper was allowed to dry by using a slow stream of compressed air. Control vials were also run in parallel with the pesticide solvent. Deionized water (1 ml) was used to moist the filter paper in each vial after drying. The well-developed clitellate adult worms (fasted 3 h, on moist filter paper) were selected, washed and dried for testing. The earthworms were randomly divided into groups of 10 each and they were exposed (one earthworm per vial) to different concentrations of pesticides for 48 h.
Thereafter, chlorpyrifos was added as per the following concentrations 0.0670, 0.0894, 0.1120, 0.1400, 0.1680, 0.1960 and 0.2240 μg/cm2 to respective vials. At an interval of 12 h, the filter paper was changed and different concentrations of chlorpyrifos were restored afresh as mentioned above. The solution of different concentrations of chlorpyrifos used in this study was prepared afresh. The earthworm kept into the carrier solvent medium served as a control since pesticides were solubilized in acetone. Similarly, the same procedure was followed for cypermethrin and their combination (chlorpyrifos + cypermethrin) exposure using following concentrations 0.0028, 0.0056, 0.0112, 0.0168, 0.0224, 0.0279, 0.0335, 0.0391 μg/cm2 for cypermethrin and 0.035, 0.045, 0.055, 0.065, 0.075, 0.085, 0.095, 0.100 μg/cm2 for combination, respectively. The test was performed for 48 h treatment period and the number of dead earthworms per group was recorded against the concentration for each pesticide in a tabular form. The 48 h LC50 value of pesticides were calculated using arithmetic method of Karber as adopted by Dede & Kaglo [20] and confidence interval was calculated by using computer program USEPA, 1993 (version 1.5) [21]. The morpho-behavioral changes were observed and photographed during experimentation.
2.4. Sample collection
Animals were sacrificed immediately after exposure to the 5% and 10% of LC50 doses of the pesticides. Tissues from pre-clitellar, clitellar and post-clitellar regions were collected and processed for biochemical study and protein estimation.
2.5. Assay of enzymes
2.5.1. Acetylcholinesterase (AChE)
2.5.1.1. Preparation of tissue homogenates
Ten worms from each concentration in replicates along with control were chosen (n = 10). Among them four - six animals from each group (control, 5% and 10% of LC50 of both the pesticides) were randomly chosen and first three to five segments (above the pharynx, which consists of the paired supraesophageal ganglion or brain as pre-clitellar region), clitellar segments and post-clitellar regions were dissected for estimation of protein and assay of AChE activity. The tissues were weighed and homogenized (10%, w/v) in 50 mM sodium phosphate buffer (pH 8.0) containing 0.1% Triton X-100 (PubChem CID: 5590) using Potter–Elvehjam homogenizer fitted with a Teflon-coated pestle under ice cold condition. The homogenates were kept in cold with intermittent stirring and centrifuged at 4 °C for 30 min at 10,000 g in a refrigerated centrifuge (Model- 3K30 Sigma, St. Louis). The corresponding supernatants were used for the assay of AChE activity.
2.5.1.2. AChE activity
The activity of acetylcholinesterase (AChE, EC 3.1.1.7) was assessed by the method described by Ref. [22]. Measurements were made in triplicate for each tissue homogenate. Simultaneously, two blanks were also used. One blank contained phosphate buffer, DTNB, and ATI but not enzyme protein to determine the spontaneous hydrolysis of ATI, and the second blank contained phosphate buffer, DTNB, and enzyme protein but no substrate (ATI) to correct for any non-AChE-dependent formation of thionitrobenzoic acid (TNB). One unit of AChE activity was expressed as nmoles substrate hydrolyzed/min/mg protein under specified experimental conditions. The extinction coefficient of the yellow anion (1.36 × 104 M−1 cm−1) was employed for calculating the enzyme activity
2.5.2. Superoxide dismutase (SOD)
The activity of Superoxide Dismutase (SOD, EC 1.15.1.1) was assessed by the method described by Ref. [25]. The assay mixture for the enzyme contained 2 ml Tris-HCl buffer pH 8.2, 0.5 ml of 2 mM pyrogallol, aliquots of the enzyme preparation and water to give a final volume of 4 ml. The rate of inhibition of pyrogallol auto-oxidation after the addition of the enzyme was noted. The percentage inhibition in the auto-oxidation of pyrogallol in the presence of cell extract was converted to units of inhibition. The amount of enzyme required to give 50% inhibition of pyrogallol auto-oxidation is considered as 1 unit of enzyme activity. It is a spectrophotometric measurement of optical density of colored complex involving pyrogallol auto-oxidation at 412 nm for 3 min at the interval of 30 secs with or without the enzyme protein.
2.5.3. Catalase (CAT)
The activity of catalase (EC 1.11.1.6) was assayed by the method of Ref. [26]. The assay mixture contained 0.5 ml H2O2, 1 ml buffer and 0.4 ml water, 0.1 ml of tissue extract was added to initiate the reaction. 2 ml dichromate-acetic acid reagent was added after 15, 30 45 and 60 secs, to arrest the reaction. To the control tube the enzyme was added after the addition of the dichromate-acetic acid reagent. The tubes were then heated for 10 min and allowed to cool. The green color developed was read at 570 nm.
2.5.4. Glutathione- S-Transferase (GST)
This enzyme was assayed by the method of Ref. [27] with the extinction coefficient 9.6 mM−1. Reaction mixture contained 0.4 ml buffer, 0.1 ml tissue extract, 1.2 ml water and 0.1 ml CDNB were added and incubated in a water bath at 37 °C for 10 min. After incubation, 0.1 ml of reduced glutathione was added. The change in optical density was measured against a reagent blank at 340 nm at 30 seconds interval for initial 3 min. The GST activity was expressed as nM CDNB Conjugates/min/mg protein.
2.5.5. Estimation of Lipid peroxidation (LPO)
2.5.5.1. Preparation of cell-free extract
The tissues were excised, rinsed in isotonic ice-cold NaCl (0.9%) solution, blotted dry, and weighed. A 10% (w/v) homogenate was prepared in phosphate buffer (100 mM, pH 7.4) containing 150 mM KCl. The homogenate was centrifuged at 9000 g for 30 min. The pellet was discarded and cell-free supernatant used for estimation of lipid peroxidation.
2.5.5.2. LPO
Lipid peroxidation (LPO) was determined in tissue homogenates by colorimetric estimation of malondialdehyde (MDA)/thiobarbituric acid reactive substances (TBARS) formed using the method of Ref. [23]. The results were expressed as nM MDA released/min/mg protein using the extinction coefficient of 1.56 × 105 M −1 cm-1.
2.5.6. Estimation of Reduced Glutathione (GSH)
2.5.6.1. Preparation of cell free extract
The tissues were excised, rinsed in isotonic ice-cold NaCl (0.9%) solution, blotted dry,and weighed. A 10% (w/v) homogenate was prepared in phosphate buffer (100 mM, pH 7.4). The homogenate was centrifuged at 9000 g for 30 min. The pellet was discarded and cell-free supernatant used for estimation of reduced glutathione, GSH.
2.5.6.2. GSH
GSH was assayed by the method described by Ref. [24]. The reaction mixture contained sodium phosphate buffer (100 mM pH 7.4), DTNB (0.2 mM) and supernatant (S9). 1 ml of 10% (w/v) homogenate was taken and 1 ml of 5% TCA was added to it. The suspension was left for 30 min then it was centrifuged at 2500 rpm for 15 min. 0.5 ml of supernatant was taken and 2.5 ml of DTNB was added. The suspension was shaken thoroughly and read at 412 nm. The results were expressed as nmol per mg protein using respective formulae.
2.6. Determination of protein
Protein was estimated by method of Ref. [28]. Protein contents in supernatants of earthworm tissue homogenates were determined, using different concentrations of bovine serum albumin (BSA) as the standard.
2.7. Statistical analyses
LC50 was calculated by Karber method as adopted by Dede & Kaglo [20] and 95% confidence limits were calculated by a computer program (USEPA, version 1.5) [21]. All data were represented as mean ± SEM (n = 30) and were analyzed by one-way analysis of variance (one-way ANOVA) on Microsoft Office Excel work sheet. Statistical significances of the differences among the treatments were calculated by use of one-way analysis of variance and covariance (ANOVA) and least significant difference test were performed using SPSS (version 16.0). The results were considered significant at p ≤ 0.05 and p ≤ 0.01 levels (confidence levels 95% and 99%, respectively). All experiments were repeated thrice and analyzed statistically in accordance to Ref. [29].
3. Results and discussion
3.1. Acute toxicity determination (LC50) for chlorpyrifos, cypermethrin and their combination
The earthworms in the control vial were healthy and normal and without any mortality during experimentation. In chlorpyrifos treated vials, no mortality was recorded at 0.0670 μg/cm2 concentration after 48 h exposure. However, the percent mortality was found to be 10%, 20%, 30%, 50%, 70% and 100% at 0.0894, 0.1120, 0.1400, 0.1680, 0.1960 and 0.2240 μg/cm2 concentrations, respectively. The LC50 of chlorpyrifos for Eudrilus eugeniae following 48 h exposure was found to be 0.165 μg/cm2 (confidence interval: 0.134- 0.186) (Table 1). Similarly, earthworms were exposed to cypermethrin for 48 h and percent mortality for the same was recorded as 10%, 30%, 50%, 70%, 80%, 90% and 100% at concentrations 0.045, 0.055, 0.065, 0.075, 0.085, 0.095 and 0.100 μg/cm2, respectively. Accordingly, the LC50 for the cypermethrin was found to be 0.066 μg/cm2 (confidence interval: 0.056- 0.074) (Table 2). When earthworms were exposed to a combination of CPF + cypermethrin, the percent mortality for 48 h exposure were recorded as 10%, 20%, 30%, 50%, 60%, 80% and 100% at 0.0056, 0.0112, 0.0168, 0.0224, 0.0279, 0.0335 and 0.0391 μg/cm2 concentrations, respectively. The LC50 value for combination of pesticide was found to be 0.020 μg/cm2 (confidence interval: 0.015- 0.025) (Table 3).
Table 1.
Lethal concentrations (LC1–99) of chlorpyrifos for earthworm, Eudrilus eugeniae (identified by well-defined clitellum, n = 10).
| Lethal | Chlorpyrifos | 95% Confidence limits |
|
|---|---|---|---|
| Concentration | (μg/cm2) | Lower | Upper |
| LC1 | 0.095 | 0.033 | 0.123 |
| LC5 | 0.112 | 0.051 | 0.137 |
| LC10 | 0.122 | 0.064 | 0.145 |
| LC15 | 0.129 | 0.074 | 0.151 |
| LC50 | 0.165 | 0.134 | 0.186 |
| LC85 | 0.211 | 0.187 | 0.296 |
| LC90 | 0.223 | 0.196 | 0.342 |
| LC95 | 0.243 | 0.209 | 0.425 |
| LC99 | 0.286 | 0.233 | 0.646 |
| Slope ± SEM | 9.74 ± 3.10 | ||
| Intercept ± SEM | 12.62 ± 2.37 | ||
| Χ2 value | 3.02 | ||
| P | <0.05 | ||
Control group (theoretical spontaneous response rate) = 0.000.
Table 2.
Lethal concentrations (LC1–99) of cypermethrin for earthworm, Eudrilus eugeniae (identified by well-defined clitellum, n = 10).
| Lethal | Cypermethrin | 95% Confidence limits |
|
|---|---|---|---|
| Concentration | (μg/cm2) | Lower | Upper |
| LC1 | 0.037 | 0.019 | 0.048 |
| LC5 | 0.044 | 0.027 | 0.054 |
| LC10 | 0.048 | 0.032 | 0.057 |
| LC15 | 0.051 | 0.035 | 0.060 |
| LC50 | 0.066 | 0.056 | 0.074 |
| LC85 | 0.086 | 0.077 | 0.104 |
| LC90 | 0.091 | 0.082 | 0.115 |
| LC95 | 0.100 | 0.088 | 0.135 |
| LC99 | 0.118 | 0.100 | 0.185 |
| Slope ± SEM | 9.29 ± 2.28 | ||
| Intercept ± SEM | 15.94 ± 2.61 | ||
| Χ2 value | 1.48 | ||
| P | <0.05 | ||
Control group (theoretical spontaneous response rate) = 0.000.
Table 3.
Lethal concentrations (LC1–99) of combined pesticide (chlorpyrifos + cypermethrin) for earthworm, Eudrilus eugeniae (identified by well-defined clitellum, n = 10).
| Lethal | Combination pesticidea | 95% Confidence limits |
|
|---|---|---|---|
| Concentration | (μg/cm2) | Lower | Upper |
| LC1 | 0.004 | 0.001 | 0.007 |
| LC5 | 0.006 | 0.002 | 0.010 |
| LC10 | 0.008 | 0.004 | 0.012 |
| LC15 | 0.010 | 0.005 | 0.013 |
| LC50 | 0.020 | 0.015 | 0.025 |
| LC85 | 0.040 | 0.030 | 0.072 |
| LC90 | 0.047 | 0.035 | 0.096 |
| LC95 | 0.061 | 0.042 | 0.147 |
| LC99 | 0.097 | 0.059 | 0.331 |
| Slope ± SEM | 3.35 ± 0.747 | ||
| Intercept ± SEM | 10.72 ± 1.27 | ||
| Χ2 value | 4.43 | ||
| P | <0.05 | ||
Control group (theoretical spontaneous response rate) = 0.000.
(Chlorpyrifos + Cypermethrin).
Earthworms are considered as the ideal model for studying the soil contaminants. In soil ecosystem, mostly pesticides are present in the mixed form that may have either synergistic or antagonistic adverse impact on the health of earthworms [[30], [31], [32]]. There are plenty of reports available related to metal toxicity to these organisms. Information regarding the toxic impact of pesticides applied individually [33] is also available, however, studies on the impact of combination of pesticides are scanty mainly from India. Pesticides affect the earthworms adversely either through skin/dermal contact or by feeding the pesticide contaminated soil. Present study shows that paper contact toxicity of pesticides through skin of Eudrilus eugeniae, increases with the increase in pesticide concentrations. The LC50 value of chlorpyrifos and cypermethrin for 48 h treatment period in different species of earthworms have been reported as follows: For CPF, 3.2 μg/cm2 for Eisenia fetida [34], 14.19 μg/cm2 for Eisenia fetida [35] and for cypermethrin: 10.63 μg/cm2 for Eisenia fetida [35], 0.35 μg/cm2 for Pheretima peguana [36], 0.30 μg/cm2 for Metaphire posthuma and 3.84 μg/cm2 for Eisenia fetida [37]. While LC50 value for a combination of various pesticides in different earthworm species have been reported as follows: 6931.1 mg/ L of Butachlor + Chlorpyrifos mixture and 3699.3 mg/ L of Imidacloprid + Chlorpyrifos for Eisenia fetida [38], 35.06 mg/kg of Chlorpyrifos and cypermethrin mixture for Eisenia fetida andrei [39]. Almost similar observations have also been made by Refs. [40,41,31]. Increased percent mortality was observed in dose dependent manner in domestic hen exposed to combination of pesticides (chlorpyrifos + cypermethrin) [42].
3.2. Morphological study
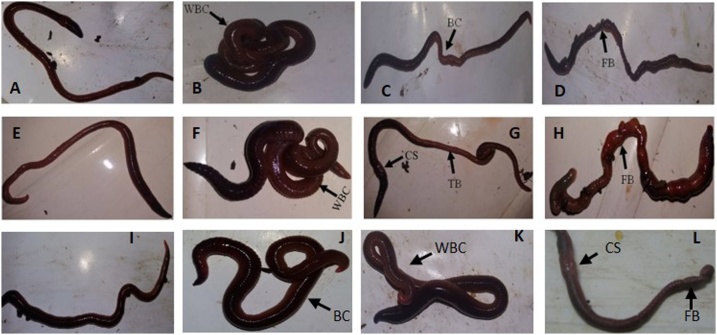
. A varying degree of morphological changes like coiling, clitellar swelling, mucus release, and bleeding followed by segmentation of body were observed in chlorpyrifos treated earthworms with respect to its increasing concentrations. However, more prominent morphological changes for cypermethrin were recorded as compared to chlorpyrifos while effect of co- exposed pesticide on morphological changes was found intermediate (Fig. 1). There were no morphological alterations observed in control group of earthworms during the experimentation.
Fig. 1.
Morphological changes in earthworm E. eugeniae exposed to different concentrations of chlorpyrifos, cypermethrin and their combination (A–L). A: Control and B: 0.112 μg/cm2, C: 0.196 μg/cm2, D: 0.224 μg/cm2 concentrations of chlorpyrifos; E: Control and F: 0.0168 μg/cm2, G: 0.0279 μg/cm2, H: 0.0391 μg/cm2 concentrations of cypermethrin and I: Control and J: 0.055 μg/cm2 K: 0.075 μg/cm2 L: 0.095 μg/cm2 concentrations of their combination (chlorpyrifos + cypermethrin). WBC: Whole Body Coiling, BC: Body Constriction, FB: Fragmentation of Body, TB: Thinning of Body, CS: Clitellar Shrinkage, BC: Body coiling.
3.3. Impact of pesticides on enzyme activity (AChE, SOD, CAT and GST) and level of LPO & GSH
3.3.1. AChE activity
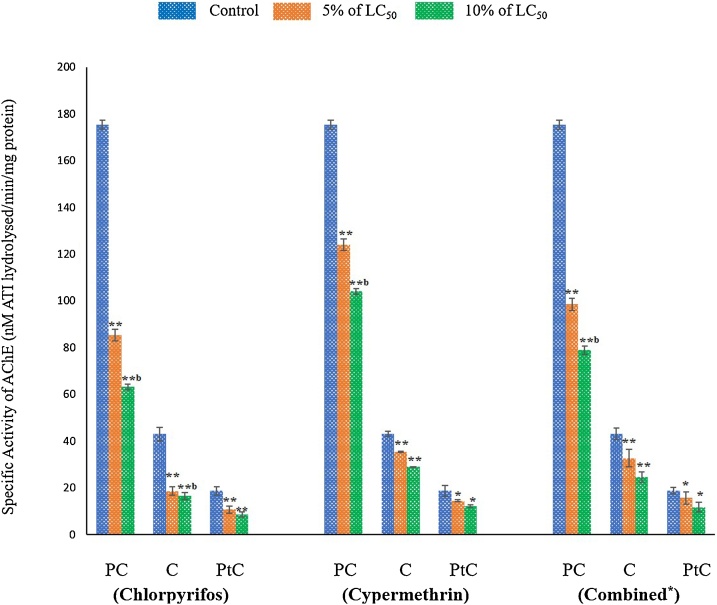
Significant inhibition in the specific activity of AChE in different body segments (pre- clitellar, clitellar and post-clitellar) of earthworm exposed to chlorpyrifos, cypermethrin and their combination (chlorpyrifos + cypermethrin) were observed. Maximum inhibition in AChE activity was observed in 10% of LC50 of combination of pesticides in all the segments studied which was followed by chlorpyrifos and cypermethrin. Specific activity of AChE (nM ATI hydrolyzed/min/mg protein) in different body segments (Pre-Clitellar, Clitellar and Post-Clitellar) of earthworm upon pesticide- chlorpyrifos, cypermethrin and their combination exposure are shown in Fig. 2. The decrease in the AChE activity was region wise and in dose dependent manner. The reason for maximum decrease in the AChE activity in pre-clitellar followed by other regions may be due to the fact that brain/ganglionic structures (dorsal brain) are situated in the prostomium of earthworm [43,44]. The difference in the level of inhibition of these pesticides may be due to their different mechanism of action. Chlorpyrifos, an organophosphate inhibits the enzyme activity by covalently phosphorylating the serine residue within active site group. The irreversible inhibition of AChE results in the excess accumulation of acetylcholine causing hyperactivity and consequently impairment of neuronal and muscular system [45]. However, pyrethroid (cypermethrin) blocks sodium channels and affect the function of GABA-receptors of nerve filaments resulting into the inhibition of AChE activity [46,47]. The other reason for difference in level of AChE inhibition might be due to accumulation or interaction of pesticide and their metabolite in different organs [48,49]. Effect of treatment of combined pesticide on AChE activity is more pronounced than the single pesticide treatment [39]. Our results corroborate the earlier similar findings [50,51].
Fig. 2.
For chlorpyrifos treated group, data represents mean ± SEM. ** p < 0.01 control vs 5% (8.4 ng/cm2) and 10% (16.8 ng/cm2.) of LC50. For cypermethrin exposed group, data represents mean ± SEM. * p < 0.05 control vs 5% (1.0 ng/cm2) and 10% (2.0 ng/cm2) of LC50, ** p < 0.01 control vs 5% and 10% of LC50 and for the earthworms exposed to combination of pesticide, data represents mean ± SEM * p < 0.05 control vs 5% and 10% of LC50, ** p < 0.01 control vs 5% (3.3 ng/cm2) and 10% (6.6 ng/cm2) of LC50. Here, PC: Pre-clitellar, C: Clitellar and PtC: Post- clitellar regions of earthworm.
3.3.2. Lipid peroxidation (LPO)
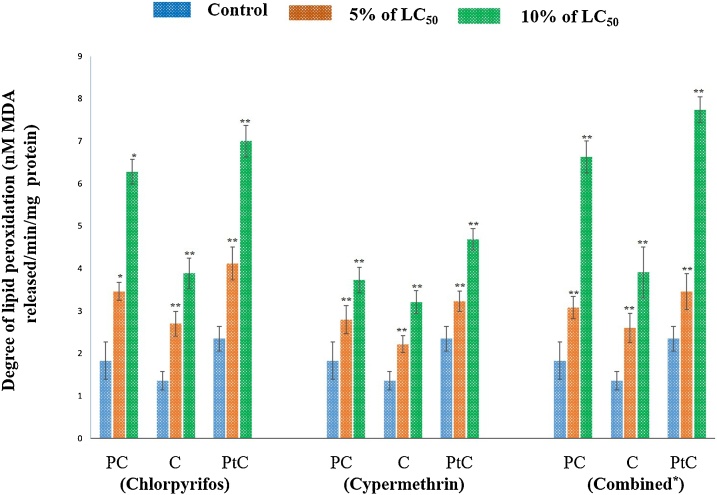
The content of MDA in pesticides (chlorpyrifos, cypermethrin and their combination) exposed earthworms was used to assess the impact on lipid peroxidation. Level of MDA (nM MDA released/min/mg protein) in different body segments (Pre-Clitellar, Clitellar and Post-Clitellar) of earthworm upon pesticide- chlorpyrifos, cypermethrin and their combination exposure are shown in Fig. 3. A dose dependent elevation in the level of LPO was observed in different body segments of earthworm (pre- clitellar, clitellar and post clitellar) after 48 h pesticides exposure. The maximum increase in lipid peroxidation was recorded at 10% of LC50 for 48 h in combination of pesticides followed by cypermethrin and chlorpyrifos individually/separately in post clitellar, pre- clitellar and clitellar regions, respectively. The results reflect the involvement of organ/tissue in detoxification of free radicals overproduced via activation of cytochrome P450 isozyme in pesticides exposed earthworms. This may further results in damage of cellular/ lipid membranes. Hence, MDA may be used as an indicator of pesticide pollution in earthworm as reports of earlier researchers also corroborates it [52,53].
Fig. 3.
For chlorpyrifos treated group, data represents mean ± SEM. ** p < 0.01 control vs 5% (8.4 ng/cm2) and 10% (16.8 ng/cm2.) of LC50. For cypermethrin exposed group, data represents mean ± SEM. * p < 0.05 control vs 5% (1.0 ng/cm2) and 10% (2.0 ng/cm2) of LC50, ** p < 0.01 control vs 5% and 10% of LC50 and for the earthworms exposed to combination of pesticide, data represents mean ± SEM * p < 0.05 control vs 5% and 10% of LC50, ** p < 0.01 control vs 5% (3.3 ng/cm2) and 10% (6.6 ng/cm2) of LC50. Here, PC: Pre-clitellar, C: Clitellar and PtC: Post- clitellar regions of earthworm.
3.3.3. Effect of pesticides on antioxidant enzymes
Pesticides induce oxidative stress in earthworm either by increased production of free radicals or by affecting the antioxidant defense mechanism such as change in rate of detoxification of the xenobiotics (pesticides) and scavenging enzyme activity [54]. The oxidative stress plays an important role in toxicity of different groups of pesticide such as organophosphorous [55] and pyrethroids [56,51,57]. α- cypermethrin induces increase in oxidative stress level significantly in rats in dose dependent manner [58]. Chlorpyrifos in combination with cypermethrin may have synergistic cumulative effect which is confirmed by the result of co- exposed groups [59].
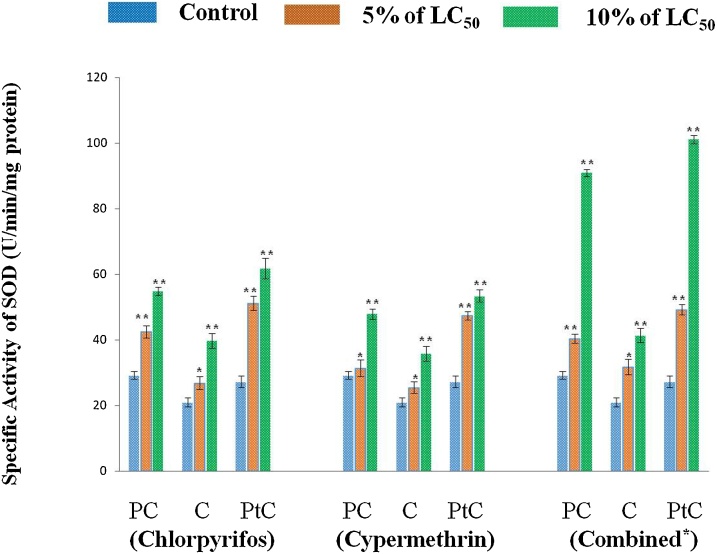
Superoxide dismutase (SOD), an important enzyme of cellular anti-oxidative defense system plays crucial role in the dismutation of free hydroxyl radical resulting in the formation of hydrogen peroxide (H2O2). The specific activity of SOD in the different region (pre- clitellar, clitellar and post clitellar) of earthworms exposed to pesticides (chlorpyrifos, cypermethrin and their combination) exhibited significant increase as compared to control for 48 h of exposure. Specific activity of SOD (U/min/mg protein)) in different body segments (Pre-Clitellar, Clitellar and Post-Clitellar) of earthworm upon pesticide- chlorpyrifos, cypermethrin and their combination exposure are shown in Fig. 4. The increase in the SOD activity was more pronounced in case of exposure to combination of pesticides in comparison to exposure of individual pesticides in a concentration dependent manner. So, the maximum elevation in the SOD activity was recorded in post- clitellar region followed by pre- clitellar and clitellar region at higher concentration (10% of LC50) of combined, chlorpyrifos and cypermethrin, respectively. Thus, the results show that the combination of pesticides may have synergistic cummulative effect on SOD activity over individual exposure. The increase in the SOD activity indicates that the exposure of pesticides to earthworm at higher concentration induce the overproduction of reactive oxygen species (ROS) and the difference in the chemical structure and in functional group as well as mechanism of action between the two groups of pesticide namely, organophosphates and pyrethroids may be responsible for the difference in the SOD activity. The organophosphorous particularly, chlorpyrifos is easily hydrolysable to its primary metabolite, TCP (3, 5, 6 – trichloro- 2 - pyridinol). The toxic potential of TCP is higher than that of chlorpyrifos which is possibly supposed to increase the level of ROS production. This in turn causes an increase in the activity of SOD more in combined case as well as chlorpyrifos exposure than cypermethrin [60,61,35,62].
Fig. 4.
For chlorpyrifos treated group, data represents mean ± SEM. ** p < 0.01 control vs 5% (8.4 ng/cm2) and 10% (16.8 ng/cm2.) of LC50. For cypermethrin exposed group, data represents mean ± SEM. * p < 0.05 control vs 5% (1.0 ng/cm2) and 10% (2.0 ng/cm2) of LC50, ** p < 0.01 control vs 5% and 10% of LC50 and for the earthworms exposed to combination of pesticide, data represents mean ± SEM * p < 0.05 control vs 5% and 10% of LC50, ** p < 0.01 control vs 5% (3.3 ng/cm2) and 10% (6.6 ng/cm2) of LC50. Here, PC: Pre-clitellar, C: Clitellar and PtC: Post- clitellar regions of earthworm.
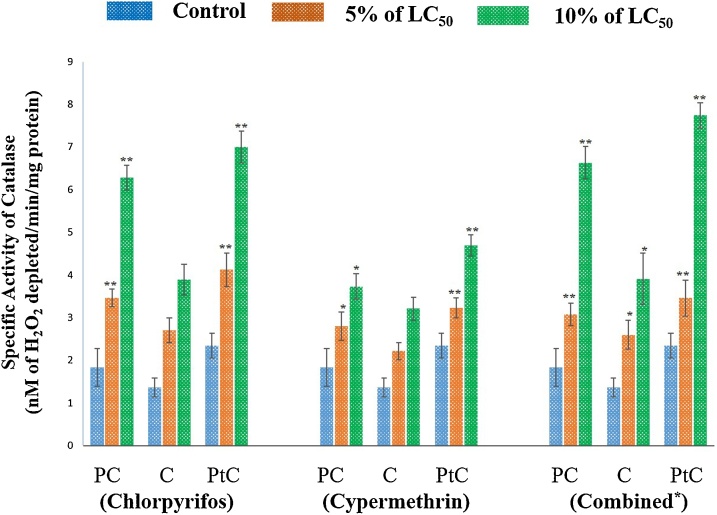
Catalase (CAT) is the peroxisomal enzyme which is responsible for scavenging the major metabolite, hydrogen peroxide (H2O2) produced from SOD activity [63,64]. Thus, H2O2 is further detoxified by CAT into molecular oxygen and water. In the present study, the changes observed in the CAT activity were in accordance with the SOD activity following 48 h exposure of pesticides. Specific activity of Catalase (nM H2O2 depleted/min/mg protein) in different body segments (Pre-Clitellar, Clitellar and Post-Clitellar) of earthworm upon pesticide- chlorpyrifos, cypermethrin and their combination exposure are shown in Fig. 5. A significant increase in the CAT activity was observed in concentration dependent manner in different regions of body (pre- clitellar, clitellar and post-clitellar) exposed to pesticide for 48 h. The elevation in the CAT activity was greater in co-exposed pesticide group followed by a higher concentration of chlorpyrifos and cypermethrin exposure (10% of LC50). This may be due to the increase in the level of ROS at higher concentration which may in turn increase the SOD activity followed by CAT. The enhanced activity of CAT attributed to an increase in the substrate concentration resulting in the maintenance of the H2O2 level and this is an adaptive mechanism against oxidative damage [53]. Thus, these anti-oxidative enzymes are involved in protection of cells against the adverse effects of the ROS produced due to higher concentration of the pesticide. Our results corroborate earlier reports [65,66,16,35].
Fig. 5.
For chlorpyrifos treated group, data represents mean ± SEM. ** p < 0.01 control vs 5% (8.4 ng/cm2) and 10% (16.8 ng/cm2.) of LC50. For cypermethrin exposed group, data represents mean ± SEM. * p < 0.05 control vs 5% (1.0 ng/cm2) and 10% (2.0 ng/cm2) of LC50, ** p < 0.01 control vs 5% and 10% of LC50 and for the earthworms exposed to combination of pesticide, data represents mean ± SEM * p < 0.05 control vs 5% and 10% of LC50, ** p < 0.01 control vs 5% (3.3 ng/cm2) and 10% (6.6 ng/cm2) of LC50. Here, PC: Pre-clitellar, C: Clitellar and PtC: Post- clitellar regions of earthworm.
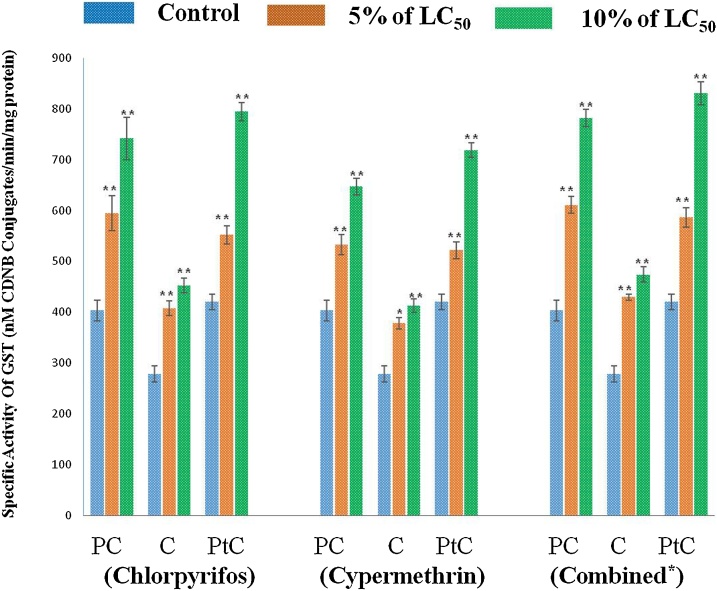
Glutathione -S- Transferase (GST) is a phase-II biotransforming enzyme is responsible for conversion of toxic compound (xenobiotics) into non-toxic in conjugation with an electrophilic substrate namely, glutathione [67]. Therefore, the increased level of GST may result into better protection against toxic effects of pesticides and serve as biomarker for pesticide pollution [68]. The activity of GST in 48 h exposed earthworms increased in a dose dependent manner. Specific activity of GST (nM CDNB Conjugates/min/mg protein) in different body segments (Pre-Clitellar, Clitellar and Post-Clitellar) of earthworm upon pesticide- chlorpyrifos, cypermethrin and their combination exposure are shown in Fig. 6. The GST activity also increased significantly in the various segments of the body of earthworm exposed to pesticide (chlorpyrifos, cypermethrin and their combination). The maximum increase was recorded in post-clitellar followed by pre- clitellar and clitellar regions of body of earthworms at 10% of LC50 which was further followed by 5% of LC50 as compared to control to mixture of pesticide, chlorpyrifos and cypermethrin, respectively in a dose dependent manner. The elevation in the GST activity may be due to increase in concentration of pesticide in the respective regions of body of earthworm which is transformed into non- toxic compounds with the help of enzyme, GST.
Fig. 6.
For chlorpyrifos treated group, data represents mean ± SEM. ** p < 0.01 control vs 5% (8.4 ng/cm2) and 10% (16.8 ng/cm2.) of LC50. For cypermethrin exposed group, data represents mean ± SEM. * p < 0.05 control vs 5% (1.0 ng/cm2) and 10% (2.0 ng/cm2) of LC50, ** p < 0.01 control vs 5% and 10% of LC50 and for the earthworms exposed to combination of pesticide, data represents mean ± SEM * p < 0.05 control vs 5% and 10% of LC50, ** p < 0.01 control vs 5% (3.3 ng/cm2) and 10% (6.6 ng/cm2) of LC50. Here, PC: Pre-clitellar, C: Clitellar and PtC: Post- clitellar regions of earthworm.
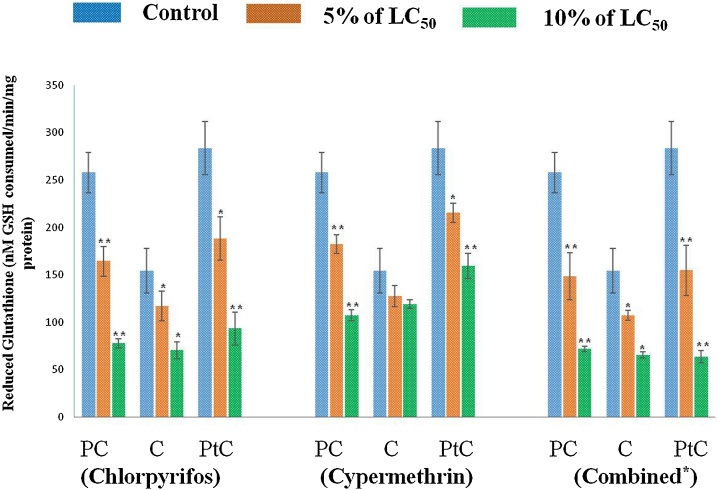
Reduced glutathione (GSH) is an electrophilic substance used in the process of biotransformation of pesticide into a non- toxic substance by GST enzyme. A marked decrease in the level of GSH was recorded in earthworm with the increase in the concentration of pesticide. Levels of GSH (nM GSH consumed/min/mg protein) in different body segments (Pre- Clitellar, Clitellar and Post-Clitellar) of earthworm upon pesticide- chlorpyrifos, cypermethrin and their combination exposure are shown in Fig. 7. The maximum decline was observed for combined pesticide followed by chlorpyrifos and cypermethrin in post-clitellar, pre- clitellar and clitellar regions of earthworm exposed for 48 h in a concentration dependent manner. Maximum decrease in the GSH level was recorded in 10% of LC50 exposed group followed by 5% of LC50 exposed group in comparison to control. Our result also corroborates with the other researchers [[69], [70], [71], [72]].
Fig. 7.
For chlorpyrifos treated group, data represents mean ± SEM. ** p < 0.01 control vs 5% (8.4 ng/cm2) and 10% (16.8 ng/cm2.) of LC50. For cypermethrin exposed group, data represents mean ± SEM. * p < 0.05 control vs 5% (1.0 ng/cm2) and 10% (2.0 ng/cm2) of LC50, ** p < 0.01 control vs 5% and 10% of LC50 and for the earthworms exposed to combination of pesticide, data represents mean ± SEM * p < 0.05 control vs 5% and 10% of LC50, ** p < 0.01 control vs 5% (3.3 ng/cm2) and 10% (6.6 ng/cm2) of LC50. Here, PC: Pre-clitellar, C: Clitellar and PtC: Post- clitellar regions of earthworm.
4. Conclusion
In natural environment, various pesticides are usually present in a mixed form and therefore their impact on the organisms may be synergistic, synergistic cumulative or antagonistic depending on their chemical nature, structure and mechanism of action. Such condition poses a serious threat to the life of these organisms. In the present study, these pesticides have altered the activity of AChE, oxidative stress related enzymes, LPO, GSH content and GST activity along with the morphological changes. This indicates that the toxic potential of these pesticides for the tested organism is greater when present in mixture than in individual. The effects observed were tissue specific and in dose dependent manner. Such changes in pesticide contaminated environment may adversely affect the survival of an eco-friendly non target organism, earthworm.
Author contributions
All the authors have contributed equally to this work.
Conflict of interest
The authors declare that they do not have any competing interests.
Acknowledgements
Authors express their gratitude to the Head of the Department of Zoology for providing Central Instrumental facility developed with the financial assistance from DST-FIST and UGC-SAP Phase I & II, New Delhi, India for carrying out this work. Financial assistance to the authors (RKT and SS) from UGC is gratefully acknowledged.
References
- 1.van Gestel C.A.M., Koolhaas J.E., Hamers T., van Hoppe M., van Roovert M., Korsman C., Reinecke S.A. Effects of metal pollution on earthworm communities in a contaminated floodplain area: linking biomarker, community and functional responses. Environ. Pollut. 2009;157:895–903. doi: 10.1016/j.envpol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Ali A.S., Naaz I. Earthworm biomarkers: the new tools of environmental impact assessment. Biosci. Biotechnol. Res. Commun. 2013;6:163–169. [Google Scholar]
- 3.Handrix P.F. Soil fauna. In: Sumner M.E., editor. Handbook of Soil Science. Section C. Soil Biology and Biochemistry. 2000. pp. C45–C85. Boca Raton-London-New York- Washington D C. [Google Scholar]
- 4.Jadhav S.S., David M. Effect of flubendiamide on morphology, avoidance behaviour and acetylcholinesterase activity in earthworm Eudrilus eugeniae. Int. J. Pharm. Pharm. Sci. 2017;9:233–238. [Google Scholar]
- 5.Chen J., Saleem M., Wang C., Liang W., Zhang Q. Individual and combined effects of herbicide tribenuron-methyl and fungicide tebuconazole on soil earthworm Eisenia fetida. Sci. Rep. 2018;8:2967. doi: 10.1038/s41598-018-21288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swarcewicz M.K., Gregorczyk A. The effects of pesticide mixtures on degradation of pendimethalin in soils. Environ. Monit. Assess. 2012;184:3077–3084. doi: 10.1007/s10661-011-2172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang D., Ql S.H., Zhang J.Q., Tan L.Z., Zhang J.P., Zhang Y., Xu F., Xing X.L., Hu Y., Chen W., Yang J.H., Xu M.H. Residues of organochlorine pesticides (OCPs) in agricultural Soils of Zhangzhou city, China. Pedosphere. 2012;22:178–189. [Google Scholar]
- 8.Hussain S., Siddique T., Saleem M., Arshad M., Khalid A. Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv. Agron. 2009;102:159–200. [Google Scholar]
- 9.Narra M.R., Begum G., Rajender K., Rao J.V. Toxic impact of two organophosphate insecticides on biochemical parameters of a food fish and assessment of recovery response. Toxicol. Ind. Health. 2011;28:343–352. doi: 10.1177/0748233711412423. [DOI] [PubMed] [Google Scholar]
- 10.Tiwari R.K., Singh S., Pandey R.S. Assessment of the acute toxicity of chlorpyrifos and cypermethrin to Heteropneustes fossilis and their impact on acetylcholinesterase activity. Drug Chem. Toxicol. 2017;20:1–8. doi: 10.1080/01480545.2017.1410171. [DOI] [PubMed] [Google Scholar]
- 11.Gambi N., Pasteris A., Fabbri E. Acetylcholinesterase activity in the earthworm Eisenia andrei at different conditions of carbaryl exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;145:678–685. doi: 10.1016/j.cbpc.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Lewis K.A., Tzilivakis J., Warner D.J., Green A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016;22:1050–1064. [Google Scholar]
- 13.Saint-Denis M., Narbonne J.F., Arnaud C., Ribera D. Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of lead acetate. Soil Biol. Biochem. 2001;33:395–404. [Google Scholar]
- 14.Tkalec M., Stambuk A., Srut M., Malaric K., Klobucar G.I.V. Oxidative and genotoxic effects of 900 MHz electromagnetic fields in the earthworm Eisenia fetida. Ecotox. Environ. Saf. 2013;90:7–12. doi: 10.1016/j.ecoenv.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Liu X.L., Sun Z.J., Chong W., Sun Z.T., He C.K. Growth and stress responses of the earthworm Eisenia fetida to Escherichia coli in an artificial soil. Microb. Pathog. 2009;46:266–272. doi: 10.1016/j.micpath.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Hu C.W., Li M., Cui Y.B., Li D.S., Chen J., Yang L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010;42:586–591. [Google Scholar]
- 17.Zhang Q., Zhang B., Wang C. Ecotoxicological effects on the earthworm Eisenia fetida following exposure to soil contaminated with imidacloprid. Environ. Sci. Pollut. Res. 2014;21:12345–12353. doi: 10.1007/s11356-014-3178-z. [DOI] [PubMed] [Google Scholar]
- 18.OECD . OECD Publishing; Paris: 1984. Test No. 207: Earthworm, Acute Toxicity Tests, OECD Guidelines for the Testing of Chemicals, Section 2. [Google Scholar]
- 19.Kinberg J.G.H. Annulata nova. Öfversigt af Königlich Vetenskapsakademiens förhandlingar. Stockholm. 1867;23:337–357. [Google Scholar]
- 20.Dede E.B., Kaglo H.D. Aqua-toxicological effects of water soluble fractions (WSF) of diesel fuel on O. Niloticus fingerlings. J. Appl. Sci. Environ. Manag. 2001;5:93–96. [Google Scholar]
- 21.USEPA . Methods for measuring the acute toxicity of effluents to freshwater and marine organisms. In: Weber C.I., editor. Environmental Monitoring Systems Laboratory. U.S. Environmental Protection Agency; Cincinnati, OH: 1993. p. 45268. EPA/600/4-90/027F. [Google Scholar]
- 22.Ellman G.L., Courtney K.D., Andres V., jr., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–90. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 23.Niehaus W.G., Jr, Samuelsson B. Formation of malondialdehyde from phospholipids archidonate during microsomal lipid peroxidation. Eur. J. Biochem. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 24.Ellman G.L., Fiches F.T. Quantitative determination of peptides by sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 26.Sinha A.K. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 27.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 28.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Bruning J.L., Knitz B.L. second edition. Scott, Foresman and Company; USA, Illinois: 1977. Computational Handbook of Statistics. [Google Scholar]
- 30.Phyu Y.L., Palmer C.G., Warne M.S., Hose G.C., Chapman J.C., Lim R.P. A comparison of mixture toxicity assessment: examining the chronic toxicity of atrazine, permethrin and chlorothalonil in mixtures to Ceriodaphnia cf. dubia. Chemosphere. 2011;85:1568–1573. doi: 10.1016/j.chemosphere.2011.07.061. [DOI] [PubMed] [Google Scholar]
- 31.Stepic S., Hackenberger B.K., Velki M., Loncaric Z., Hackenberger D.K. Effects of individual and binary-combined commercial insecticides endosulfan, temephos, malathion and pirimiphos-methyl on biomarker responses in earthworm Eisenia andrei. Environ. Toxicol. Pharmacol. 2013;36:715–723. doi: 10.1016/j.etap.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y., An X., Shen W., Chen L., Jiang J., Wang Q., Cai L. Individual and combined toxic effects of herbicide atrazine and three insecticides on the earthworm, Eisenia fetida. Ecotoxicol. 2016;25:991–999. doi: 10.1007/s10646-016-1656-4. [DOI] [PubMed] [Google Scholar]
- 33.Castellanos L.R., Hernandez T.C.S. Earthworm biomarkers of pesticide contamination: current status and perspectives. J. Pestic. Sci. 2007;32:360–371. [Google Scholar]
- 34.Rao J.V., Pavan Y.S., Madhavendra S.S. Toxic effects of chlorpyrifos on morphology and acetylcholinesterase activity in the earthworm, Eisenia foetida. Ecotoxicol. Environ. Saf. 2003;54:296–301. doi: 10.1016/s0147-6513(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 35.Wang J.H., Zhu L.S., Liu W., Wang J., Xie H. Biochemical Responses of Earthworm (Eisenia foetida) to the pesticides chlorpyrifos and fenvalerate. Toxicol. Mech. Meth. 2012;22:236–241. doi: 10.3109/15376516.2011.640718. [DOI] [PubMed] [Google Scholar]
- 36.Muangphra P., Tharapoom K., Euawong N., Namchote S., Gooneratne R. Chronic toxicity of commercial chlorpyrifos to earthworm Pheretima peguana. Environ. Toxicol. 2015;31:1450–1459. doi: 10.1002/tox.22150. [DOI] [PubMed] [Google Scholar]
- 37.Saxena P.N., Gupta S.K., Murthy R.C. Comparative toxicity of carbaryl, carbofuran, cypermethrin and fenvalerate in Metaphire posthuma and Eisenia fetida — a possible mechanism. Ecotoxicol. Environ. Saf. 2014;100:218–225. doi: 10.1016/j.ecoenv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Wang Y., Zhao X., Wang Q., Qian Y. Comparative and combined acute toxicity of butachlor, imidacloprid and chlorpyrifos on earthworm, Eisenia fetida. Chemosphere. 2014;100:111–115. doi: 10.1016/j.chemosphere.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Zhou S.P., Duan C.Q., Michelle W.H.G., Yang F.Z., Wang X.H. Individual and combined toxic effects of cypermethrin and chlorpyrifos on earthworm. J. Environ. Sci. 2011;23:676–680. doi: 10.1016/s1001-0742(10)60462-7. [DOI] [PubMed] [Google Scholar]
- 40.Lydy M.J., Linck S.L. Assessing the impact of triazine herbicides on organophosphate insecticide toxicity to the earthworm Eisenia fetida. Arch. Environ. Contam. Toxicol. 2003;45:343–349. doi: 10.1007/s00244-002-0218-y. [DOI] [PubMed] [Google Scholar]
- 41.Belden J.B., Lydy M.J. Effects of atrazine on acetylcholinesterase activity in midges (Chironomus tentans) exposed to organophosphorus insecticides. Chemosphere. 2001;44:1685–1689. doi: 10.1016/s0045-6535(00)00519-1. [DOI] [PubMed] [Google Scholar]
- 42.Sharma S., Uggini G.K., Patel V., Desai I., Balakrishnan S. Exposure to sub-lethal dose of a combination insecticide during early embryogenesis influences the normal patterning of mesoderm resulting in incomplete closure of ventral body wall of chicks of domestic hen. Toxicol. Rep. 2018;5:302–308. doi: 10.1016/j.toxrep.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rault M., Mazzia C., Capowiez Y. Tissue distribution and characterization of cholinesterase activity in six earthworm species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007;147:340–346. doi: 10.1016/j.cbpb.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Calisi A., Lionetto M.G., Schettino T. Biomarker response in the earthworm Lumbricus terrestris, exposed to chemical pollutants. Sci. Total Environ. 2011;409:4456–4464. doi: 10.1016/j.scitotenv.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO) Organophosphorus Insecticides: A General Introduction. WHO; Geneva: 1986. Metabolism and mode of action; pp. 39–48. [Google Scholar]
- 46.Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 1996;78:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 47.Dobsikova R., Velisek J., Wlasow T., Gomulka P., Svobodova Z., Novotny L. Effects of cypermethrin on some haematological, biochemical and histopathological parameters of common carp (Cyprinus carpio L) Neuro Endocrinol. Lett. 2006;27(Suppl. 2):91–95. [PubMed] [Google Scholar]
- 48.World Health Organization . World Health Organization; Geneva: 1989. EHC. No. 82: Cypermethrin. [Google Scholar]
- 49.Singh S., Tiwari R.K., Pandey R.S. Evaluation of acute toxicity of triazophos and deltamethrin and their inhibitory effect on AChE activity in Channa punctatus. Toxicol. Rep. 2018;5:85–89. doi: 10.1016/j.toxrep.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schreck E., Geret F., Gontier L., Trilhou M. Neurotoxic effect and metabolic responses induced by a mixture of six pesticides on the earthworm Aporrectodea caliginosa nocturna. Chemosphere. 2008;71:1832–1839. doi: 10.1016/j.chemosphere.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Velki M., Hackenberger B.K. Biomarker responses in earthworm Eisenia andrei exposed to pirimiphos-methyl and deltamethrin using different toxicity tests. Chemosphere. 2013;90:1216–1226. doi: 10.1016/j.chemosphere.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 52.Lin D.S., Zhou Q.X., Xie X.J., Liu Y. Potential biochemical and genetic toxicity of triclosan as an emerging pollutant on earthworms (Eisenia fetida) Chemosphere. 2010;81:1328–1333. doi: 10.1016/j.chemosphere.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Liu S., Zhou Q.X., Wang Y.Y. Ecotoxicological responses of the earthworm Eisenia fetida exposed to soil contaminated with HHCB. Chemosphere. 2011;83:1080–1086. doi: 10.1016/j.chemosphere.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 54.Abdollahi M., Ranjbar A., Shadnia S., Nikfar S., Rezaiee A. Pesticides and oxidative stress: a review. Med. Sci. Monit. 2004;10:144–147. [PubMed] [Google Scholar]
- 55.Ranjbar A., Pasalar P., Sedighi A., Abdollahi M. Induction of oxidative stress in paraquat formulating workers. Toxicol. Lett. 2002;131:191–194. doi: 10.1016/s0378-4274(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 56.Kale M., Rathore N., John S., Bhathagar D. Lipid peroxidative damage on pyrethroid exposure and alteration in antioxidant status in rat erythrocytes. A possible involvement of reactive oxygen species. Toxicol. Lett. 1999;105:197–205. doi: 10.1016/s0378-4274(98)00399-3. [DOI] [PubMed] [Google Scholar]
- 57.Ilyushina N., Goumenou M., Stivaktakis P.D., Vardavas A.I., Masaltsev G., Averianova N., Dmitricheva O., Revazova Y., Tsatsakis A.M., Rakitskii V. Maximum tolerated doses and erythropoiesis effects in the mouse bone marrow by 79 pesticides’ technical materials assessed with the micronucleus assay. Toxicol. Rep. 2019;6:105–110. doi: 10.1016/j.toxrep.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hongsibsong S., Stuetz W., Sus N., Prapamontol T., Grune T., Frank J. Dietary exposure to continuous small doses of α-cypermethrin in the presence or absence of dietary curcumin does not induce oxidative stress in male Wistar rats. Toxicol. Rep. 2014;1:1106–1114. doi: 10.1016/j.toxrep.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vardavas A.I., Stivaktakis P.D., Tzatzarakis M.N., Fragkiadaki P., Vasilaki F., Tzardi M., Datseri G., Tsiaoussis J., Alegakis A.K., Tsitsimpikou C., Rakitskii V.N., Carvalho F., Tsatsakis A.M. Long-term exposure to cypermethrin and piperonyl butoxide cause liver and kidney inflammation and induce genotoxicity in New Zealand white male rabbits. Food Chem. Toxicol. 2016 doi: 10.1016/j.fct.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 60.Racke K.D., Steele K.P., Yoder R.N., Dick W.A., Avidov E. Factors affecting the hydrolytic degradation of chlorpyrifos in soil. J. Agric. Food Chem. 1996;44:1582–1592. [Google Scholar]
- 61.Baskaran S., Kookana R.S., Naidu R. Contrasting behaviour of chlorpyrifos and its primary metabolite, TCP (3,5,6-trichloro-2-pyridinol), with depth in soil profiles. Aust. J. Soil Res. 2003;41:749–760. [Google Scholar]
- 62.Zhang Q., Zhu L., Wang J., Xie H., Wang J., Han Y., Yang J. Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ. Sci. Pollut. Res. 2013;20:201–208. doi: 10.1007/s11356-012-0962-5. [DOI] [PubMed] [Google Scholar]
- 63.Hegedüs A., Erdei S., Horváth G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci. 2001;160:1085–1093. doi: 10.1016/s0168-9452(01)00330-2. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W., Song Y.F., Sun T.H., Song X.Y., Zhou Q.X., Zheng S.L. Effects of phenanthrene and pyrene on Cytochrome P450 and antioxidant enzymes of earthworms (Eisenia fetida) Environ. Chem. 2007;26:202–207. (in Chinese) [Google Scholar]
- 65.Laszczyca P., Augustyniak M., Babczynska A., Bednarska K., Kafel A., Migula P., Wilczek G., Witas I. Profiles of enzymatic activity in earthworms from zinc, lead and cadmium polluted areas near Olkusz (Poland) Environ. Int. 2004;30:901–910. doi: 10.1016/j.envint.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Song Y., Zhu L.S., Wang J., Wang J.H., Liu W., Xie H. DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol. Biochem. 2009;41:905–909. [Google Scholar]
- 67.Bernard F., Brulle F., Dumez S., Lemiere S., Platel A., Nesslany F., Cuny D., Deram A., Vandenbulcke F. Antioxidant responses of annelids, brassicaceae and fabaceae to pollutants: a review. Ecotox. Environ. Saf. 2015;114:273–303. doi: 10.1016/j.ecoenv.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 68.Oruc E.O., Sevgiler Y., Uner N. Tissue- specific oxidative stress responses responses in fish exposed to 2,4-D and azinphosmethyl. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;137:43–51. doi: 10.1016/j.cca.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Valavanidis A., Vlahogianni T., Dassenakis M., Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox. Environ. Saf. 2006;64:178–189. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 70.Tiwari R.K., Singh S., Pandey R.S., Sharma B. Enzymes of earthworm as indicators of pesticide pollution in soil. Adv. Enzyme Res. 2016;4:113–124. [Google Scholar]
- 71.Marcano L., Hernandez J., Zapata-Vivenes E., Leon A. Effects of contaminated natural soil by Glyphosan®SL on biochemical responses of the earthworm Eisenia sp. J. Toxicol. Environ. Health Sci. 2017;9:92–97. [Google Scholar]
- 72.Vardavas A.I., Fragkiadaki P., Alegakis A.K., Dimitrios K., Goutzourelas N., Tsiaoussis J., Tsitsimpikou C., Stivaktakis P.D., Carvalho F., Tsatsakis A.M. Downgrading the systemic condition of rabbits after long term exposure to cypermethrin and piperonyl butoxide. Life Sci. 2016;145:114–120. doi: 10.1016/j.lfs.2015.12.026. [DOI] [PubMed] [Google Scholar]